Maintenance of epithelial barrier function requires dynamic repair and remodeling of tight junctions. In this issue, Higashi et al. demonstrate that the proteolytic cleavage of EpCAM by membrane-anchored serine proteinases releases Claudin-7 to join tight junctions, suggesting a novel mechanism that couples sensing with repair of damaged tight junctions.
Abstract
Maintenance of epithelial barrier function requires dynamic repair and remodeling of tight junctions. In this issue, Higashi et al. (2022. J. Cell Biol. https://doi.org/10.1083/jcb.202204079) demonstrate that the proteolytic cleavage of EpCAM by membrane-anchored serine proteinases releases Claudin-7 to join tight junctions, suggesting a novel mechanism that couples sensing with repair of damaged tight junctions.
Epithelial cells are attached by intercellular junctions, thereby forming a physical barrier that interface distinct internal and external environments. The apical most intercellular junction, referred to as the tight junction (TJ), plays a critical role in regulating barrier function by controlling the movement of ions, solutes, and water across the epithelium. Claudins, a family of tetraspan proteins, oligomerize in TJ strands between cells and are critical for the formation and maintenance of barrier function. Claudin family comprises 26 and 27 Claudin family members in humans and mice, respectively, which are differentially expressed in TJs to control barrier properties of epithelial cells. Though Claudins localize in TJs, a few Claudin family members, including Claudin-7, additionally localize in the lateral membrane of epithelial cells (1, 2). However, the functional contribution of lateral membrane associated Claudins to epithelial barrier function is not well understood.
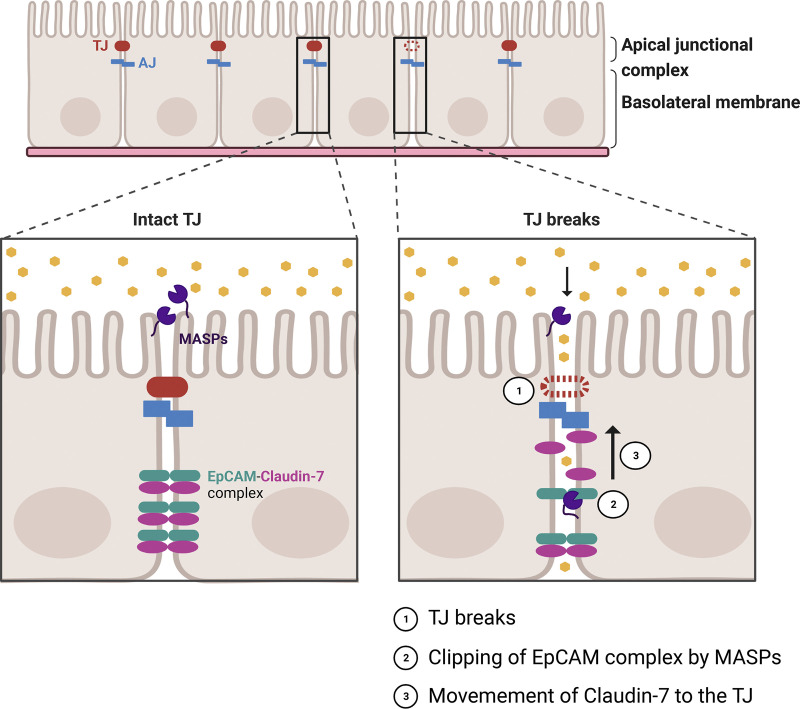
TJs are highly dynamic structures that are reorganized in response to physiological and pathological stimuli (3). For instance, intestinal epithelial cells with a high turnover rate actively remodel their junctions as they divide and differentiate before extrusion into the lumen in less than a week. Thus, from a barrier point of view, it is remarkable that cells maintain an intact barrier while remodeling cell-cell contacts during the life cycle of epithelial cells. In this context, there are several unanswered questions pertaining to barrier leaks at sites of junctional remodeling and stopgap measures that come into play to repair these leaks. Indeed, Stephenson et al. (4), found that local barrier leaks at sites of junctional remodeling are rapidly repaired by calcium-dependent Rho GTPase flares (4, 5). These leaks begin to repair before the Rho activation (4), indicating the existence of other mechanisms that restore the barrier function. In this issue of JCB, Higashi et al. (1), identified a new stopgap measure whereby epithelial cell adhesion molecule (EpCAM) is cleaved by membrane-anchored serine proteinases (MASPs) to release Claudin-7 from an EpCAM-Claudin-7 protein complex to strengthen the TJ breaks (Fig. 1; 6).
Figure 1.
Schematic of MASPs mediated clipping of EpCAM at sites of epithelial barrier leaks. Top: 2D view of epithelial cells connected to their neighbors via an apical junctional complex. Bottom: Side view of cell-cell junctions with intact tight junction (left) or a tight junction break (right). In epithelial cells with intact tight junction, Claudin-7 is sequestered in the lateral membrane by forming a complex with EpCAM and MASPs are localized to the apical membrane. In the event of tight junction breaks (1), EpCAM in the lateral membrane is cleaved by MASPs to release Claudin-7 (2), Claudin-7 moves to the tight junction (3) to restore barrier function in epithelial cells. TJ, tight junction; AJ, adherens junction; EpCAM, epithelial cell adhesion molecule; MASPs, membrane-anchored serine proteinases.
During the dynamic remodeling of TJ strands, unpolymerized Claudins are added at the basolateral ends of the polymerized TJ strands to strengthen the barrier (7). In this paper, Higashi et al. (6), asked if the pool of Claudin-7 in the basolateral membrane is used as a reserve to repair TJs during their dynamic remodeling (6). If so, what is the mechanism by which Claudin-7 is released from the basolateral membrane to the TJ? It is known that epithelial Claudin-7 interacts with EpCAM (8), protease treatment cleaves EpCAM in epithelial cells (9), and protease treatment induces TJ strand assembly (10). Thus, the authors determined if proteolytic cleavage of EpCAM could be relevant to TJ remodeling in a Claudin-7-dependent manner. The effect of trypsin on the localization of Claudin-7 and cleavage of EpCAM in model epithelial cells was examined. Interestingly, trypsin treatment increased the localization of Claudin-7 at TJs, which was associated with an increased number of TJ strands, and cleaved EpCAM. EpCAM was observed to sequester Claudin-7 at the basolateral membrane, and upon trypsin treatment, Claudin-7 dissociated from EpCAM to transition from detergent-soluble to detergent-insoluble fraction associated with the TJ. In short, proteolytic cleavage of EpCAM releases Claudin-7 from the EpCAM-Claudin-7 protein complex in the lateral membrane to be incorporated into TJ strands and strengthen the epithelial barrier.
In a screen to identify the proteinase responsible for EpCAM cleavage, the authors observed that camostat, an inhibitor specific to the MASP family, functions to cleave EpCAM, thereby controlling barrier function. Studies performed to further narrow down which MASP family members are responsible for EpCAM cleavage and Claudin protein incorporation in TJs identified TMPRSS1, 4, 14, and PRSS8. To further investigate barrier function dynamics during TJ remodeling in MASP-qKO cells, the authors used an elegant zinc-based ultrasensitive microscopic barrier assay (ZnUMBA) (4). The size, duration, and frequency of barrier leaks were increased in MASP-qKO cells compared to control cells. These studies not only highlighted how TJ breaks are repaired during junction remodeling but also identified an unexpected role for MASP family members in these biological events.
Given that TJs reside between the apical and basolateral membrane and contribute to their polarized separation (fence function), one can speculate that the EpCAM-Claudin-7 protein complex is protected from cleavage when the TJ is intact. However, following TJ breaks, apical proteases have access to basolateral proteins such as the EpCAM-Claudin-7 protein complex, thereby releasing Claudin-7, which can then incorporate into TJs and facilitate repair. Accordingly, spatial analysis of EpCAM and MASP proteins by surface biotin labeling of epithelial cells identified that PRSS8 and the cleaved EpCAM are localized in the apical membrane of epithelial cells.
In summary, the findings reported by Higashi et al. (6) provide novel insight into how TJ leaks are repaired in order to control the critical epithelial barrier function. How do TJ leaks activate MASPs, and if this mechanism is conserved across epithelial bi-cellular and tri-cellular TJs, will need to be addressed in future studies. In the broader biological context, it will be important to understand how TJs are remodeled and repaired in physiologic tissue homeostasis, during morphogenesis, and in pathologic states of inflammation and cancer.
Acknowledgments
We apologize to our colleagues’ work for not being discussed and cited due to space limitations.
Our work is supported by a grant from the National Institutes of Health to A. Nusrat. (R01 DK055679, R01 DK129214). A. Raya-Sandino is supported by the Crohn’s and Colitis Foundation Research Fellowship (623536).
References
- 1.Li, W.Y., et al. 2004. Am. J. Physiol. Renal Physiol. 10.1152/ajprenal.00384.2003 [DOI] [PubMed] [Google Scholar]
- 2.Mendoza-Rodríguez, C.A., et al. 2005. Cell Tissue Res. 10.1007/s00441-004-1010-7 [DOI] [PubMed] [Google Scholar]
- 3.Nusrat, A., et al. 2000. Am. J. Physiol. Gastrointest. Liver Physiol. 10.1152/ajpgi.2000.279.5.G851 [DOI] [PubMed] [Google Scholar]
- 4.Stephenson, R.E., et al. 2019. Dev. Cell. 10.1016/j.devcel.2019.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varadarajan, S., et al. 2022. J. Cell Biol. 10.1083/jcb.202105107 [DOI] [Google Scholar]
- 6.Higashi, T., et al. 2023. J. Cell Biol. 10.1083/jcb.202204079 [DOI] [Google Scholar]
- 7.Van Itallie, C.M., et al. 2019. Mol. Biol. Cell. 10.1091/mbc.E19-01-0008 [DOI] [Google Scholar]
- 8.Ladwein, M., et al. 2005. Exp. Cell Res. 10.1016/j.yexcr.2005.06.013 [DOI] [PubMed] [Google Scholar]
- 9.Wu, C.J., et al. 2017. J. Clin. Invest. 10.1172/JCI88428 [DOI] [Google Scholar]
- 10.Lynch, R.D., et al. 1995. Eur. J. Cell Biol. 66:257–267. [PubMed] [Google Scholar]