Abstract
Background
The exact prevalence of Multisystem Inflammatory Syndrome in Adults (MIS-A) is largely unknown. Vague and multiple definitions and treatment options often add to the confusion on how to label the diagnosis with certainty.
Objectives
The objective of the study was to determine the demographic profile, clinical presentation, laboratory findings and outcomes of MIS-A in COVID-19.
Methods
A systematic review was conducted after registering with PROSPERO. Multiple databases were systematically searched to encompass studies characterizing MIS-A from 1st January 2020 up to 31st August 2021. The inclusion criteria were- to incorporate all published or in press peer-reviewed articles reporting cases of MIS-A. We accepted the following types of studies: case reports, case-control, case series, cross-sectional studies and letters to the editors that incorporated clinical, laboratory, imaging, as well as the hospital course of MIS-A patients. The exclusion criteria for the review were- articles not in English, only abstracts published, no data on MIS-A and articles which have focus on COVID-19, and not MIS-A. Two independent authors screened the articles, extracted the data, and assessed the risk of bias.
Results
A total of 53 articles were included in this review with a sample size of 79 cases. Majority of the patients were males (73.4%) with mean age of 31.67±10.02 years. Fever (100%) and skin rash (57.8%) were the two most common presenting symptoms. Echocardiographic data was available for 73 patients of whom 41 (73.2%) had reduced left ventricular ejection fraction. Cardiovascular system was most frequently involved (81%) followed by gastrointestinal (73.4%) and mucocutaneous (51.9%) involvement. Anti-inflammatory therapies used in treatment included steroids (60.2%), intravenous immunoglobulin (37.2%) and biologics (10.2%). Mean duration of the hospital stay was 11.67±8.08 days. Data regarding the outcomes was available for all 79 subjects of whom 4 (5.1%) died during course of hospital stay.
Conclusions
Emergence of MIS-A calls for further large-scale studies to establish standard case definitions and definite treatment guidelines.
Keywords: COVID-19, Multisystem inflammatory syndrome, Adult, Steroids
Introduction
Coronavirus disease 2019 (COVID-19), caused by novel coronavirus SARS CoV-2, has rapidly evolved into a pandemic leading to widespread morbidity and mortality. Multisystemic involvement has been one of the defining features in COVID-19 with the respiratory system being the most commonly affected.1 Systemic inflammation is the key pathophysiology of COVID-19 infection, especially in moderate and severe cases, with a host of pro-inflammatory cytokines being responsible for the cytokine surge.2 This inflammatory state often subsided during the convalescent phase. However, a post infectious hyperinflammatory phase termed as multisystem inflammatory syndrome in children (MIS-C) was first reported in the pediatric population in April 2020.3 , 4 This emerging clinical entity is usually seen among young children, weeks following infection with SARS-CoV-2, and tends to involve the cardiovascular and the gastrointestinal system.3, 4, 5 A similar multisystem hyperinflammatory state with a temporal association with COVID-19 has recently been described in adults and is termed as the multisystem inflammatory syndrome in adults (MIS-A).6
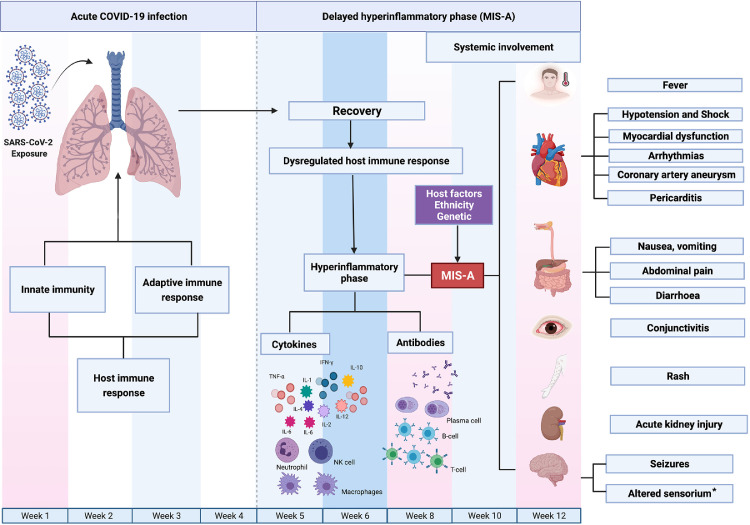
The exact pathophysiology of MIS-A remains unclear and is thought to occur due to the dysregulated immune response involving both the innate and the adaptive immune system occurring weeks after recovery from COVID-19 infection. Possible pathophysiological mechanisms include (i) formation of autoantibodies, (ii) antibody recognition of persistent viral antigens on infected cells, and (iii) hyperinflammatory response due to the viral super antigens. Additionally, gender, genetic predisposition and ethnicity may play a defining role in occurrence of MIS-A (Fig. 1 ).7 Case definitions for MIS-A have been put forth by the CDC which labels it as a hyper inflammatory syndrome with multiorgan (≥2) dysfunction in an adult (>21 years of age) having antecedent evidence of a SARS-CoV-2 infection.8 The exact prevalence of MIS-A is largely unknown due to limited data in the form of case reports and series describing the occurrence, clinical features and outcomes of this novel clinical entity. This systematic review was carried out to evaluate the clinical signs and symptoms, laboratory findings, imaging results, and outcomes of individuals with MIS-A.
Fig. 1.
Figure highlighting the pathophysiology of MIS-A. SARS-CoV-2 infection is characterized by an inflammatory immune response comprising both the innate as well as the adaptive immune system leading to recovery in majority of cases. However, in a fraction of cases following recovery, there develops a dysregulated immune response leading to a hyperinflammatory phase characterized by macrophage activation which leads to activation of innate as well as adaptive immune system comprising B-cells and T-cells with the production of inflammatory cytokines as well as antibodies. These inflammatory cytokines lead to multisystem inflammatory response and development of MIS-A. The exact cause for the dysregulated immune response following recovery is not known however, has been speculated to be due to super antigens or persistent viral antigens or even autoantibodies. Figure created by Biorender.com. * Altered sensorium implies decreased consciousness, altered mental status, altered awareness or confusion.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were adhered to while carrying out this systematic review (Fig. 2 ). The study protocol was registered with PROSPERO (International Prospective Register of Systematic Reviews) with the registration number CRD42021272912. A systematic search of the following databases viz. PubMed, Medline, Embase, Scopus, Cochrane Library, WHO Global COVID Literature Database and Google Scholar was carried out. Additionally, the references of included articles and reviews focusing on MIS-A were studied. All publications in the English language from 1st January 2020 up to 31st August 2021 were reviewed. The combination of the following keywords were used as the search strategy for literature search in the various databases: Age group [adults with age restriction (21 years)] AND Virus (COVID-19, novel coronavirus, SARS-CoV-2, 2019-nCoV, severe acute respiratory syndrome coronavirus 2) AND Condition [Adult inflammatory multisystem syndrome temporally associated with SARS-CoV-2 infection, multisystem inflammatory syndrome in adults (MIS-A), hyperinflammation, hyperinflammatory shock, macrophage activation syndrome, hemophagocytic lymphohistiocytosis (HLH)]. The diagnosis of MIS-A was based on the CDC case definition for MIS-A8 which is enumerated in supplementary Table 1.
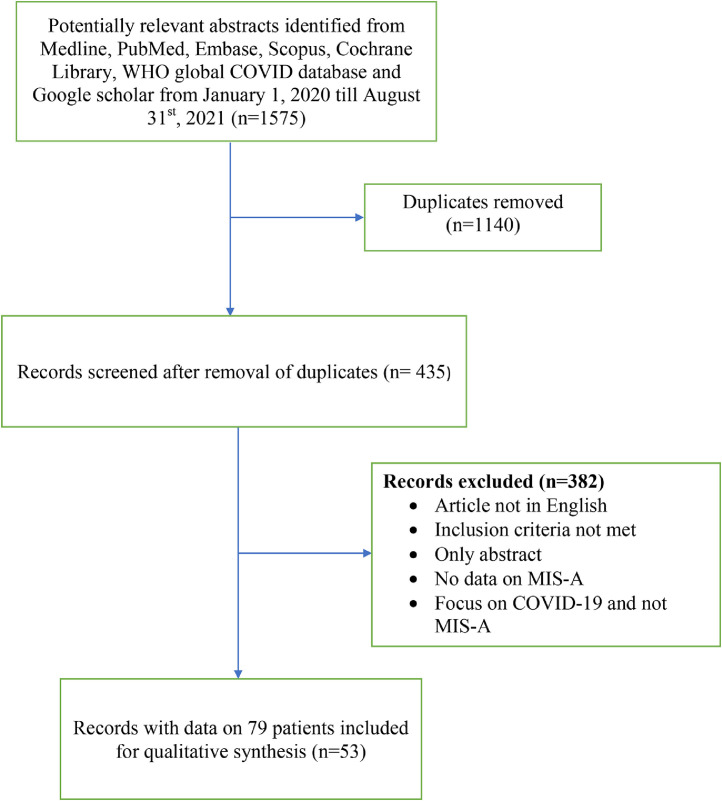
Fig. 2.
PRISMA flow diagram of the systematic review.
The inclusion criteria were to incorporate all published or in press peer-reviewed articles reporting cases of MIS-A. We accepted the following types of studies: case reports, case-control, case series, cross-sectional studies and letters to the editors that incorporated clinical, laboratory, imaging, as well as the hospital course of MIS-A patients. The exclusion criteria for the review were- articles not in English, only abstracts published, no data on MIS-A and articles which have focus on COVID-19 and not MIS-A.
Screening by the title and abstract was conducted independently by two investigators (NM, SK). A third investigator (PI) was consulted to resolve differences of opinion in either phase. Subsequent full-text review and data extraction was conducted by investigators (PI, SK, NM, PS, KG) using Google Sheets (Google, Mountain View, CA, USA). Our goals were to evaluate the clinical signs and symptoms, laboratory findings, imaging results, and outcomes of individuals with MIS-A.
The data collected from the studies included demographics, number of patients, clinical signs and symptoms, laboratory, hematological, inflammatory and cardiac markers, imaging in the form of echocardiography, cardiac magnetic resonance imaging (CMR), computed tomography (CT) of chest and abdomen, treatment modalities and outcomes. Only the initial laboratory values including inflammatory markers were recorded (e.g., at admission or first reported value). All signs and symptoms pre- hospitalization and during the patient's hospitalization were included. All echocardiograms were taken into consideration. Ejection fraction (EF), valvular dysfunction, pericardial effusion, coronary artery dilation, or aneurysm were recorded. Cardiac dysfunction was defined as an EF <50% and was categorized into mild (EF: 41–50%), moderate (EF: 31–40%) and severe (EF: ≤30%) left ventricular (LV) dysfunction.9 In all these patients, evidence of SARS-CoV-2 infection was based on either (a) positivity on RT-PCR or (b) positive antibody or antigen test. Data regarding the outcomes were also evaluated including intensive care unit (ICU) stay, need for mechanical ventilation, inotropic support and mortality. Risk of bias for observational studies was assessed using the quality assessment tool published by the National Institutes of Health.10 Risk of bias was assessed independently by two investigators (PI, SK) and disagreements were resolved by a third researcher (PI). Furthermore, the level of evidence was assessed according to Sackett.11 Continuous data were summarized as mean with standard deviation. Categorical data were summarized as counts with percent. The means, standard deviations, counts and percent were calculated using SPSS, version 24.0 (IBM Corp) for Mac.
Results
A total of 1575 potentially relevant abstracts were identified from Medline, PubMed, Embase, Scopus, Cochrane Library, WHO global COVID database and Google scholar from 1st January 2020 till 31st August 2021. Out of these, 1140 were removed as they were duplicates. Out of the 435 studies left, 382 were excluded due to various reasons including articles not in English, inclusion criteria not met, only abstract, no data on MIS-A, focus on COVID-19 and not MIS-A, review article and scientific letter with no patient data. Ultimately, 53 articles were included in this review with a total sample size of 79 MIS-A cases (Fig. 2: PRISMA flow diagram). The summary of the included studies is summarized in Table 1. 6 , 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63
Table 1.
Summary of the included MIS-A studies in the systematic review.
| S.No | First author | Age (years)/Gender | Publication type/ Number of cases | Symptoms | Laboratory/other system investigations | Inflammatory markers | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Varadaraj G et al. [12] | 21–30/ Male (3/3) | Case series (n = 3) Out of 4 reported, 3 included as the fourth one previously published by Dabas et al. [30] |
Fever (3/3), skin rash (1/3), lymphadenopathy (1/3) | Thrombocytopenia (1/3), ↑ creatinine (2/3), ↑ troponin (3/3) | ↑ Procalcitonin (3/3), ↑ CRP (3/3), ↑ LDH (3/3), ↑ ferritin (3/3), ↑ d-dimers (3/3) | Antibiotics (3/3), anticoagulants (3/3), inotropes (3/3) | Discharged (3/3) |
| 2 | Chung H et al. [13] | 28/Male | Case report (n = 1) | Fever, nausea, vomiting, diarrhea | ↑ BNP, Low EF | ↑ CRP, ↑ Ferritin, ↑ Procalcitonin, ↑ Fibrinogen |
Corticosteroid, IVIG, anticoagulant, antibiotic, inotrope | Discharged |
| 3 | Fiore M et al. [14] | 42/Male | Case report (n = 1) | Fever, diarrhea, conjunctivitis, confusion | Lymphopenia, Low EF | ↑ CRP | Corticosteroid, IVIG, antiplatelet, inotropes | Discharged |
| 4 | Razmi TM et al. [15] | 40/Female | Case report (n = 1) | Fever, rash, lymphadenopathy | Lymphopenia | ↑ ESR, ↑ CRP | Steroids | Discharged |
| 5 | Shaigany S et al. [16] | 45/Male | Case report (n = 1) | Fever, cough, nausea, vomiting, abdominal pain, skin rash, conjunctivitis, cracking of lips | Lymphopenia, ↑ troponin, ↑ NT-Pro-BNP |
↑ ESR, ↑ CRP, ↑ IL-6, ↑Ferritin, ↑D-dimer |
Anticoagulant, IVIG, tocilizumab | Discharged |
| 6 | Kerkerian G et al. [17] | 60/Male | Case report (n = 1) | Fever, skin rash, myalgia, lymphadenopathy, conjunctivitis, peripheral edema, glossitis | Lymphopenia, ↑ troponin, ↑NT-Pro-BNP | ↑ CRP, ↑D-dimer, ↑Ferritin | Corticosteroid, IVIG, antiplatelet | Discharged |
| 7 | Ahmad F et al. [18] | 26/Male | Case report (n = 1) | Fever, diarrhea, nausea, Abdominal pain, Skin rash | Severe LV dysfunction | ↑ LDH, ↑ CRP, ↑ d-dimer, ↑Ferritin |
Corticosteroid, IVIG, antiplatelet, anticoagulant, RRT, inotrope, anakinra | Discharged |
| 8 | Baruah R et al. [19] | 22/Male | Case report (n = 1) | Fever, nausea, vomiting, diarrhea, skin rash, conjunctivitis | ↑ NT-Pro-BNP, ↑ troponin |
↑ Procalcitonin, ↑ CRP, ↑Ferritin, ↑ d-dimer |
Corticosteroid, IVIG, antiplatelet, antibiotic, inotrope | Discharged |
| 9 | Yamada Y et al. [20] | 51/Male | Case report (n = 1) | Fever, conjunctivitis, peripheral edema, cervical lymphadenopathy, fatigue | ↑ NT-Pro-BNP | ↑Procalcitonin, ↑CRP, ↑IL-6, ↑Ferritin, ↑D-dimer, ↑Fibrinogen |
Corticosteroid, antibiotic, inotrope | Discharged |
| 10 | Razavi AC et al. [21] | 23/Male | Case report (n = 1) | Dyspnea, fever, diarrhea, fatigue, conjunctivitis, headache | Lymphopenia, thrombocytopenia | ↑ CRP, ↑ Ferritin, ↑ d-dimer, ↑ Fibrinogen |
Corticosteroid, antiplatelet, anticoagulant, IVIG antibiotic | Discharged |
| 11 | Salzman MB et al. [22] | 40/Male | Case report (n = 1) | Dyspnea, fever, abdominal pain, diarrhea, fatigue, headache | NR | ↑ CRP, ↑ Ferritin, ↑ d-dimer, ↑ Fibrinogen |
Corticosteroid, anticoagulant, antibiotic | Discharged |
| 12 | Bastug A et al. [23] | 40/Male | Case report (n = 1) | Fever, abdominal pain, diarrhea | Lymphopenia, ↑troponin, ↑ BNP | ↑ Procalcitonin, ↑ Ferritin, ↑ d-dimer |
Corticosteroid, anticoagulant, IVIG, antibiotic | Discharged |
| 13 | Pombo F et al. [24] | 24/Male | Case report (n = 1) | Dyspnea, fever, cough, abdominal pain, skin rash, diarrhea | ↑ NT-Pro-BNP | ↑ CRP, ↑Ferritin, ↑D-dimer, ↑Fibrinogen, ↑ESR | Corticosteroid | Discharged |
| 14 | Kofman AD et al. [25] | 25/Female | Case report (n = 1) | Dyspnea, fever, cough, vomiting, abdominal pain, diarrhea, conjunctivitis, lymphadenopathy, fatigue | Neutrophilia | ↑ CRP, ↑Ferritin, ↑D-dimer, ↑ ESR | IVIG, antiplatelet, antibiotic, inotrope | Discharged |
| 15 | Chau VQ et al. [26] | 24–42/Male (5/5) | Case series (n = 5) | Dyspnea (4/5), fever (5/5), cough (1/5), chest pain (2/5), vomiting (1/5), diarrhea (2/5), skin rash (3/5), lymphadenopathy (1/5), fatigue (1/5) | ↑ BNP (5/5) | ↑ CRP (5/5), ↑ IL-6 (5/5), ↑ Ferritin (5/5), ↑ d-dimer (5/5) |
Corticosteroid (5/5), anticoagulant (5/5), RRT (2/5), inotrope (5/5) | Discharged (5/5) |
| 16 | Ahsan T et al. [27] | 28/Male | Case report (n = 1) | Fever, confusion, nausea, vomiting, skin rash, conjunctivitis, Myalgia | Anemia, lymphocytosis | ↑ESR, ↑CRP | Corticosteroid | Discharged |
| 17 | Faller E et al. [28] | 23/Male | Case report (n = 1) | Fever, cough, vomiting, diarrhea, skin rash, conjunctivitis | Leukocytosis, ↑ Troponin |
↑ LDH, ↑ CRP, ↑ IL-6, ↑Ferritin, ↑D-dimer, |
Anticoagulant, inotrope | Discharged |
| 18 | Julius MA et al. [29] | 59/Female | Case report (n = 1) | Fever, skin rash, lymphadenopathy, myalgia | ↑ Troponin | ↑LDH, ↑CRP | Corticosteroid, inotropes, RRT | Died due to multiorgan failure including shock, respiratory, renal and fulminant hepatic failure |
| 19 | Morris SB et al. [6] | 21/Male 27/Male 42/Female |
Case series (n = 3/9) Out of 9 reported, 3 fulfilled the CDC criteria for MIS-A |
Fever (3/3), cough (1/3), nausea (1/3), vomiting (1/3), diarrhea (2/3), skin rash (1/3), myalgia (1/3), lymphadenopathy (1/3) | ↑ Troponin (3/3), Reduced EF (3/3) | ↑ CRP (3/3), ↑ IL-6 (1/3), ↑Ferritin (3/3) ↑D-dimer (3/3) |
Steroids (3/3), anticoagulation (3/3), inotrope (3/3), antiplatelet (1/3) | Discharged (3/3) |
| 20 | Dabas R et al. [30] | 22/Male | Case letter (n = 1) | Fever, nausea, abdominal pain, skin rash, conjunctivitis. Cracking of lips, myalgia, fatigue, joint pains | Transaminitis | ↑ ESR, ↑ CRP, ↑ LDH, ↑ IL-6, ↑ Ferritin, ↑ Procalcitonin |
Anticoagulant, antibiotic | Discharged |
| 21 | Veyseh M et al. [31] | 43/Female | Case report (n = 1) | Fever, abdominal pain, diarrhea, skin rash | Leukocytosis, low EF | ↑ CRP, ↑ LDH, ↑Ferritin, ↑ d-dimer | Steroids | Discharged |
| 22 | Hékimian G et al. [32] | 22–37/ Male(2/4), Female(2/4) | Case series (n = 4/11) Out of 11 reported, 4 fulfilled the CDC criteria for MIS-A |
Dyspnea (2/4), fever (4/4), cough (1/4), chest pain (1/4), abdominal pain (2/4), diarrhea (3/4), skin rash (1/4), conjunctivitis (1/4), lymphadenopathy (1/4), fatigue (4/4), joint pain (1/4), headache (2/4) | Lymphopenia (1/4), ↑creatinine (1/4), ↑ troponin (4/4), ↑ AST (2/4), ↑ ALT (3/4), ↑ NT-Pro-BNP (3/4) | ↑Procalcitonin (3/4), ↑LDH (2/4), ↑CRP (3/4), ↑Ferritin (4/4), ↑ d-dimer (4/4), ↑ Fibrinogen (4/4) |
Corticosteroid (1/4), IVIG (2/4), antibiotic (1/4), ECMO (1/4) | Discharged (4/4) |
| 23 | Bulut H et al. [33] | 20/Male | Case report (n = 1) | Fever, abdominal pain, diarrhea, skin rash | Anemia, thrombocytopenia, ↑ NT-Pro-BNP, Low EF | ↑ CRP, ↑ LDH, ↑ Ferritin | Corticosteroid, antiplatelet, anticoagulant, IVIG, antibiotic, inotrope | Discharged |
| 24 | Cogan E et al. [34] | 19/Female | Case report (n = 1) | Fever, skin rash, conjunctivitis, cracking of lips, peripheral edema | Low EF, ↑ troponin |
↑ CRP, ↑ LDH, ↑ IL-6, ↑ Ferritin, ↑ d-dimer |
Corticosteroids, IVIG, tocilizumab, inotrope | Discharged |
| 25 | Brown LN et al. [35] | 39/Male | Case report (n = 1) | Fever, dyspnea, vomiting, confusion, diarrhea, skin rash, Lymphadenopathy, myalgia | Thrombocytopenia | ↑ CRP, ↑ LDH, ↑ Ferritin, ↑Procalcitonin, ↑Fibrinogen |
Corticosteroid, IVIG, antiplatelet | Discharged |
| 26 | Gopalakrishnan M et al. [36] | 28/Male | Case report (n = 1) | Fever, skin rash, odynophagia | Thrombocytopenia, ↑ troponin | ↑ ESR, ↑ CRP, ↑ Ferritin | IVIG, antibiotic, inotropes | Died due to refractory shock and respiratory failure |
| 27 | Diakite S et al. [37] | 33/Male | Case report (n = 1) | Fever, dyspnea, chest pain, diarrhea, conjunctivitis, cracking of lips | Anemia, leukocytosis, ↑ troponin | ↑ CRP, ↑ d-dimer |
Steroid, IVIG, antiplatelet, O2, inotropes | Discharged |
| 28 | Alexandra OG et al. [38] | 22/Male | Case report (n = 1) | Fever, cough, abdominal pain, diarrhea, skin rash, inguinal lymphadenopathy, myalgia, odynophagia | Leukocytosis | ↑ CRP, ↑ d-dimer |
Steroid, IVIG, Cyclophosphamide, Rituximab, Tocilizumab, inotrope, Ventilatory support, ECMO | Discharged |
| 29 | Kinter CW et al. [39] | 32/Male | Case report (n = 1) | Fever, abdominal pain, skin rash, conjunctivitis, Lymphadenopathy, Neck pain | Leukocytosis, ↑ BNP, transaminitis, low EF |
↑ CRP, ↑ IL-6, ↑ Ferritin |
Steroid, antiplatelet, IVIG | Discharged |
| 30 | Shan Y et al. [40] | 34/Male | Case report (n = 1) | Fever, vomiting abdominal pain, diarrhea, skin rash, conjunctivitis, myalgia, headache | Leukocytosis, thrombocytopenia, ↑ troponin | ↑ LDH, ↑ CRP, ↑ IL-6, ↑ Ferritin, ↑ d-dimer |
Steroid, IVIG, RRT, O2, ventilatory support, inotrope | Discharged |
| 31 | Moghadam P et al. [41] | 21/Male | Case letter (n = 1) | Fever, diarrhea, skin rash, conjunctivitis, | Leukocytosis, ↑ troponin |
↑ CRP, ↑ Ferritin, ↑ Procalcitonin |
Antibiotics, inotrope | Discharged |
| 32 | Aggarwal A et al. [42] | 21/Male | Case report (n = 1) | Fever, abdominal pain, diarrhea, Headache | ↑ d-dimer, ↑ BNP | ↑ CRP, ↑ Ferritin, ↑ Procalcitonin |
Steroid, IVIG, Anakinra | Discharged |
| 33 | Toplu SA et al. [43] | 24/Female | Case report (n = 1) | Fever, abdominal pain, conjunctivitis, headache | Lymphopenia, thrombocytopenia, ↑ NT-Pro-BNP | ↑ Procalcitonin, ↑ CRP, ↑ LDH, ↑ IL-6, ↑Ferritin, ↑ d-dimer |
Corticosteroid, colchicine, antibiotic | Discharged |
| 34 | Chug L et al. [44] | 25/Male | Case report (n = 1) | Fever, confusion, diarrhea, conjunctivitis | NR | ↑ inflammatory markers (values not mentioned) | Corticosteroid, inotrope | Discharged |
| 35 | Brajkovic AV et al. [45] | 22/Male | Case report (n = 1) | Fever, cough, headache, sore throat | ↑ Troponin, ↑ NT-Pro-BNP, ↑AST, ↑ALT |
↑ ESR, ↑ Procalcitonin, ↑ CRP, ↑ IL-6, ↑ Ferritin, ↑ d-dimer, ↑ LDH |
Corticosteroid, antiplatelet, anticoagulant, IVIG, antibiotic, inotrope | Discharged |
| 36 | Mieczkowska K et al. [46] | 32/Male 43/Female |
Case series (n = 2) |
Fever (2/2), cough (1/2), diarrhea (1/2), skin rash (2/2), conjunctivitis (1/2), peripheral edema (1/2), lymphadenopathy (1/2), fatigue (1/2), headache (1/2) | ↑ Troponin (1/2), ↑ AST (2/2), ↑ ALT (1/2) |
↑ ESR (2/2), ↑ Procalcitonin (1/2), ↑ CRP (2/2), ↑ IL-6 (2/2), ↑ Ferritin (2/2), ↑ d-dimer (2/2), ↑ Fibrinogen (1/2) |
Corticosteroid (2/2), anticoagulant (2/2), antibiotic (2/2), inotrope (1/2) | Discharged |
| 37 | Uwaydah AK et al. [47] | 22/Male | Case report (n = 1) | Fever, cough, nausea, vomiting, abdominal pain, diarrhea, skin rash, conjunctivitis, fatigue, headache | Thrombocytopenia, ↑ troponin, ↑ NT-Pro-BNP, ↑ AST, ↑ ALT |
↑ Procalcitonin, ↑ CRP, ↑ IL-6, ↑ Ferritin, ↑ d-dimer |
Corticosteroid, antibiotic | Discharged |
| 38 | Fox SE et al. [48] | 31/Female | Case report (n = 1) | Fever, nausea, vomiting, conjunctivitis, lymphadenopathy | Anemia, ↑ serum creatinine, ↑ lactate, ↑ AST, ↑ ALT |
↑ CRP, ↑ d-dimer, ↑ Ferritin |
NR | Died due to shock and ventricular fibrillation. Autopsy revealed cardiac endothelitis and vasculitis. |
| 39 | Boudhabhay I et al. [49] | 46/Male | Case report (n = 1) | Fever, skin rash | Thrombocytopenia, ↑ serum creatinine, ↑ troponin |
↑ Ferritin, ↑ LDH, ↑ CRP |
Hemodialysis, eculizumab, inotrope | Discharged |
| 40 | Pasara V et al. [50] | 26/Male | Case report (n = 1) | Dyspnea, fever, cough, chest pain, diarrhea, headache, | ↑ troponin, ↑ NT-Pro-BNP |
NR | Corticosteroid, IVIG, antibiotic, inotrope | Discharged |
| 41 | Downing S et al. [51] | 51/Male | Case report (n = 1) | Dyspnea, fever, cough, fatigue, headache, | NR | NR | Corticosteroid, antiplatelet, colchicine | Discharged |
| 42 | Malangu B et al. [52] | 46/Male | Case report (n = 1) | Dyspnea, fever, cough, chest pain, skin rash, fatigue | Thrombocytopenia, ↑ serum creatinine, ↑ AST, ↑ ALT | ↑Ferritin, ↑ CRP, ↑ LDH, ↑ d-dimer, ↑ Fibrinogen, |
Anticoagulant, antibiotic | Discharged |
| 43 | Li M et al. [53] | 28/Male | Case report (n = 1) | Fever, lymphadenopathy, fatigue | ↑ troponin, ↑ BNP, ↑ AST, ↑ ALT | ↑ CRP, ↑ ferritin, |
Corticosteroid, IVIG, antibiotic, | Discharged |
| 44 | Lerner RK et al. [54] | 26/Male | Case report (n = 1) | Fever, abdominal pain | Anemia, leukocytosis, thrombocytopenia, transaminitis, ↑ troponin, low EF |
↑ LDH, ↑ Procalcitonin |
Corticosteroid, RRT, IVIG, ECMO, inotrope | Died due to myocardial dysfunction and shock |
| 45 | Viana-Garcia A et al. [55] | 24/Female | Case report (n = 1) | Fever, nausea, vomiting, abdominal pain, skin rash, lymphadenopathy, headache, odynophagia | Anemia, ↑ NT Pro-BNP |
↑ LDH, ↑ CRP, ↑ IL-6, ↑ Ferritin |
Corticosteroid, IVIG | Discharged |
| 46 | CattaneoP et al. [56] | 27/Male | Case report (n = 1) | Fever, chest pain, skin rash, conjunctivitis, lymphadenopathy, bilateral leg pain, headache, cracking of lips, | Thrombocytopenia, ↑ troponin | ↑ CRP, ↑ Ferritin, ↑ Procalcitonin |
Corticosteroid, anakinra, antibiotic | Discharged |
| 47 | Gulseran M et al. [57] |
31/Female |
Case report (n = 1) | Pregnant lady with fever and chest pain | Leukocytosis, ↑ troponin, ↑ BNP, transaminitis, global left ventricular dysfunction |
↑ CRP, ↑ IL-6, ↑ d-dimer, ↑ fibrinogen |
Steroid, IVIG, anticoagulant, antibiotic, immunosuppressant, inotrope | Discharged |
| 48 | Choudary A et al. [58] | 26/Male | Image (n = 1) | Fever, cough, abdominal pain, vomiting, diarrhea, myalgia | ↑ Troponin, reduced LV function | ↑ Ferritin, ↑ Procalcitonin, ↑ d-dimer |
Antiplatelet, antibiotic, inotrope | Discharged |
| 49 | Davogustto GE et al. [59] | 45 (mean)/Male (10/15), Female (5/15) | Case series (n = 15) | Symptoms: NR | Individual data: NR | Individual data: NR | Immunosuppressant n = 4; antibiotics n = 7; non-invasive ventilatory support n = 1 | Discharged (n = 15) |
| 50 | Cherif MY et al. [60] | 35/Female | Case report (n = 1) | Fever, cough, dyspnea, vomiting, diarrhea, skin rash, conjunctivitis, peripheral edema, cracking of lips, myalgia, hypogeusia | Lymphopenia, thrombocytopenia, ↑ troponin, ↑ NT Pro-BNP |
↑ LDH, ↑ CRP, ↑ Ferritin |
Hydroxychloroquine, antibiotics | Discharged |
| 51 | Jones I et al. [61] | 26/Male | Correspondence (n = 1) | Fever, abdominal pain, skin rash, conjunctivitis, lymphadenopathy, cracking of lips, constipation, anorexia | Lymphopenia | ↑ d-dimer, ↑ CRP, ↑ Ferritin |
Corticosteroid, IVIG, antiplatelets | Discharged |
| 52 | Sokolovsky S et al. [62] | 36/Female | Case report (n = 1) | Fever, vomiting, abdominal pain, diarrhea, skin rash, conjunctivitis, peripheral edema, lymphadenopathy, cracking of lips, joint pain | Anemia, leukocytosis | ↑ ESR, ↑ d-dimer, ↑ CRP | Corticosteroid, IVIG., antiplatelets | Discharged |
| 53 | Lidder A et al. [63] | 45/Male | Case report (n = 1) | Fever, diarrhea, skin rash, conjunctivitis, | Lymphopenia, ↑ troponin |
↑ ESR, ↑ CRP, ↑ IL-6, ↑ Ferritin |
Corticosteroid, IVIG, tocilizumab | Discharged |
Abbreviations: BNP: B-type natriuretic peptide, CRP: C reactive protein, NT Pro-BNP: N terminal Pro-BNP, IL-6: Interleukin-6, IVIG: Intravenous Immunoglobulin, EF: Ejection fraction, ESR: Erythrocyte sedimentation rate, LDH: Lactate dehydrogenase, ALT: Alanine transaminase, AST: Aspartate transaminase, ↑: raised; RRT: renal replacement therapy, NR: not reported.
Demographic features and clinical characteristics
Of the 79 cases included, majority of them were males (73.4%), with a mean age of 31.67±10.02 years. The data regarding the race/ethnicity was available for 55 (69.6%) individuals with subjects most belonging to the Asian (25.4%), Caucasian (23.6%) and the Hispanic (21.8%) ethnicity. The mean duration from symptom onset to hospital admission was 5.84±8.01 days. Fever (100%) and skin rash (57.8%) were the two most common presenting symptoms. Diarrhea (51.6%) and abdominal pain (40.6%) were the most common gastrointestinal manifestations and mimicked viral gastroenteritis or inflammatory bowel disease. Twenty-six (32.9%) adults diagnosed with MIS-A, had comorbidities, with hypertension and obesity being the most frequent. In patients with a prior COVID-19 infection, the mean duration between prior infection and symptom onset was 31.61±14.34 days. The demographic and clinical characteristics of subjects with MIS-A has been listed in Table 2 .
Table 2.
Demographic and clinical characteristics of subjects with MIS-A.
| Characteristics | Number of Patients (N = 79) | N (%) |
|---|---|---|
| Age [Mean ± SD] | Data available: 79 | 31.67±10.02 years |
| Gender | Data available: 79 | Males: 58 (73.4%) Females: 21 (26.6%) |
| Ethnicity Caucasian Hispanic Latin Asian African Afro-American |
Data available: 55/79 |
13 (23.6%) 12 (21.8%) 1 (1.8%) 14 (25.4%) 4 (7.3%) 11 (20%) |
| Clinical features Fever Dyspnea Cough Chest pain Nausea Vomiting Diarrhea Abdominal pain Skin rash Conjunctivitis Lymphadenopathy Confusion Peripheral Edema Myalgia Joint pain Headache Cracking of lips Sore throat Odynophagia |
Data available: 64/79 |
64 (100%) 20 (31.2%) 16 (25%) 8 (12.5%) 10 (15.6%) 16 (25%) 33 (51.6%) 26 (40.6%) 37 (57.8%) 26 (40.6%) 22 (34.3%) 4 (6.2%) 5 (7.8%) 25 (39.1%) 4 (6.2%) 16 (25%) 9 (14.1%) 2 (3.1%) 4 (6.2%) |
| Systemic involvement Cardiovascular Muco-cutaneous Gastrointestinal Musculoskeletal Renal Hematological Neurological Pulmonary |
Data available: 79/79 |
64 (81%) 41 (51.9%) 58 (73.4%) 24 (30.4%) 34 (43.1%) 33 (41.8%) 13 (16.4%) 23 (29.1%) |
| Comorbidities Hypertension Diabetes Dyslipidemia Obesity Coronary artery disease Asthma Malignancy Chronic kidney disease |
26 (32.9%) 10 6 2 17 1 2 4 3 |
|
| Duration of symptoms [Mean ± SD] | Data available: 58/79 | 5.84±8.01 days |
| Duration of hospital stay [Mean ± SD] | Data available: 59/79 | 11.67±8.08 days |
| Time between exposure and symptom onset [Mean ± SD] | Data available: 33/79 | 31.61±14.34 days |
| COVID-19 status Antibody positivity RTPCR positivity COVID-19 vaccination |
Data available: 68/79 Data available: 77/79 Data available: 79/79 |
58 (85.3%) 28 (36.4%) 2 (2.5%) |
Abbreviations: SD- Standard deviation, COVID-19- coronavirus disease, RTPCR- reverse transcriptase polymerase chain reaction.
Laboratory and radiological investigations
The details of various laboratory and radiological investigations have been summarized in Table 3 . Inflammatory markers were elevated in a majority of cases with leukocytosis reported in 36/44 (81.8%) and an elevated CRP in 56/57 (98.2%). Lymphopenia was observed in 27/40 (67.5%) of cases. Cardiac involvement was seen in a majority of cases where cardiac investigations and imaging were performed. An elevated cardiac troponin was reported in 43/50 (86%) while elevated Brain natriuretic peptide (BNP) and NT-pro BNP were observed in 16/17 (94.1%) and 14/15 (93.3%) patients each. Echocardiographic data was available for 73 patients of whom 41 (73.2%) had a reduced left ventricular ejection fraction (LVEF<50%) while 32 (43.8%) had a normal echocardiogram. Right ventricular dysfunction was present in one-fifth of the patients included in the study. Data regarding CMR was available for 18 patients of whom 6 (33.3%) had evidence of myocardial edema, 4 (22.2%) had late gadolinium enhancement and 2 (22.2%) had pericardial effusion. None of the patients had any evidence of coronary artery aneurysms on cardiac imaging. Evidence of current or past SARS-CoV-2 infection was based on RT-PCR positivity in 28/77 (36.4%) subjects and positive serology in 58/68 (85.3%) patients
Table 3.
Laboratory and radiological investigations in subjects with MIS-A.
| Investigations | Number of Patients (N = 79) | N (%) |
|---|---|---|
| Hematology Hemoglobin (g/dl) Total leucocyte count (per mm3) Absolute lymphocyte count (per mm3) Platelet count (per mm3) Thrombocytopenia Lymphopenia |
Data available: 26 Data available: 44 Data available: 35 Data available: 32 Data available: 35 Data available: 40 |
11.58±2.17 16,171.14±8288.58 1340.97±1685.32 185,062.5 ± 105,793.45 18/35 (51.4%) 27/40 (67.5%) |
| Organ functions: Serum creatinine (mg/dl) Cardiac troponin (ng/ml) Serum BNP (pg/ml) Serum NT-pro BNP (pg/ml) |
Data available: 29 Data available: 50 Data available: 17 Data available: 15 |
2.31±2.00 287.06±1435.491 3061.88±4738.37 13,400.27±12,843.65 |
| Inflammatory markers LDH (U/L) CRP (mg/dl) IL-6 (pg/ml) Ferritin (ng/ml) Procalcitonin (ng/ml) Positive procalcitonin (>0.5 ng/ml) ESR (mm/hr) |
Data available: 28 Data available: 57 Data available: 20 Data available: 53 Data available: 29 Data available: 45 Data available: 14 |
676.49±1182.34 165.39±152.31 219.04±327.05 3062.83±4169.16 24.21±58.44 43/45 (95.5%) 75.86±31.89 |
| Coagulation profile D-Dimer (ng/ml) Fibrinogen (mg/dl) |
Data available: 47 Data available: 24 |
3268.16±4570.20 654.39±313.70 |
| Imaging Echocardiogram - Baseline EF (%) - Normal LVEF (≥50%) - Mild LV dysfunction (LVEF: 40–49%) - Moderate LV dysfunction (LVEF: 30–39%) - Severe LV dysfunction (LVEF: <30%) - Reduced LVEF (<50%) - Improvement in LVEF - Right ventricular dysfunction - Pericardial effusion Cardiac MRI - LGE - Pericardial effusion - Myocardial edema CT abdomen - Terminal ileitis - Colitis - Hepatosplenomegaly - Mesenteric adenitis CT chest - GGOs - Pulmonary embolism - Pleural effusion - Consolidation - Lymphadenopathy |
Data available: 73 Data available: 18 Data available: 15 Data available: 40 |
39.09±14.12% 32 (43.8%) 13 (37.1%) 12 (34.2%) 10 (28.6%) 41 (73.2%) 23/41 (56.1%) 15 (20.5%) 8 (10.9%) 4 (22.2%) 2 (11.1%) 6 (33.3%) 3 (20%) 2 (13.3%) 2 (13.3%) 3 (20%) 7 (17.5%) 1 (2.5%) 11 (27.5%) 8 (20%) 2 (5%) |
Abbreviations: BNP- B-terminal natriuretic peptide, CRP- C reactive protein, NT Pro- BNP- N terminal Pro-BNP, IL-6- Interleukin-6, IVIG- Intravenous Immunoglobulin, EF- Ejection fraction, ESR- Erythrocyte sedimentation rate, LDH- Lactate dehydrogenase, ALT- Alanine transaminase, AST- Aspartate transaminase, LVEF-. Left ventricular ejection fraction, LGE- Late Gadolinium Enhancement, CT- computed tomography, GGO- Ground glass opacities.
Systemic involvement, treatment and outcomes
Cardiovascular system was the most frequently involved (81%) followed by gastrointestinal (73.4%) and mucocutaneous (51.9%) involvement (Table 2). On admission, 35/39 (89.7%) of cases had tachycardia while 43/72 (59.7%) were hypotensive. Forty-three (58.1%) of the adults diagnosed with MIS-A were admitted in the ICU. Shock was reported in 40/78 (51.3%) patients during the course of hospital stay mandating cardiovascular support in the form of inotropes (46.1%), intra-aortic balloon pump [IABP] (2.6%) or extracorporeal membrane oxygenation [ECMO] (3.8%). Acute kidney injury (AKI) requiring dialysis occurred in 6 (7.7%) patients. Respiratory dysfunction was reported in nearly one-third of patients with high flow humidified oxygen therapy used in 16 (20.5%), NIV support in 5 (6.4%) and mechanical ventilation in 12 (15.4%) patients Table 4. summarizes the information regarding the treatment administered and the outcomes. A variety of anti-inflammatory therapies were used for the treatment of MIS-A including steroids (60.2%), intravenous immunoglobulin (IVIG) [37.2%] and biologics (10.2%) such as Tocilizumab and Anakinra. Concomitant antibiotic therapy was administered in 60.2% patients while 32% of the subjects’ received anticoagulants. The mean duration of the hospital stay was 11.67±8.08 days. Data regarding the outcomes was available for all the 79 subjects of whom 4 (5.1%) died during the course of hospital stay while 75 (94.9%) were discharged from the hospital. These deaths were due to myocardial dysfunction leading to refractory shock in three and multiorgan failure in one. Only one of these four cases underwent autopsy (Table 1) which revealed cardiac endothelitis and vasculitis.
Table 4.
Therapies administered for MIS-A and clinical outcomes.
| Medications and Outcome | Number of Patients | N (%) |
|---|---|---|
| Medical treatment for MIS Steroids Anti-inflammatory other than steroids - Colchicine - Cyclophosphamide Biologics - Tocilizumab - Anakinra - Rituximab - Eculizumab IVIG Antibiotics Antiplatelets Anticoagulants HCQs |
Data available: 78 |
47 (60.2%) 3 (3.8%) 2 (2.6%) 1 (1.3%) 8 (10.2%) 4 (5.1%) 3 (3.8%) 1 (1.3%) 1 (1.3%) 29 (37.2%) 47 (60.2%) 16 (20.5%) 25 (32%) 1 (1.3%) |
| Shock Inotropes IABP ECMO Dialysis |
Data available: 78 Data available: 78 Data available: 78 Data available: 78 Data available: 78 |
40 (51.3%) 36 (46.1%) 2 (2.6%) 3 (3.8%) 6 (7.7%) |
| Oxygen support NIV IMV |
Data available: 78 Data available: 78 Data available: 78 |
16 (20.5%) 5 (6.4%) 12 (15.4%) |
| ICU stay | Data available: 74 | 43 (58.1%) |
| Outcome Died Discharged |
Data available: 79 |
4 (5.1%) 75 (94.9%) |
MIS-Multisystem inflammatory syndrome, IVIG- Intravenous immunoglobulin, HCQs-Hydroxychloroquine, IABP-Intra Aortic balloon pump, ECMO-Extracorporeal membrane oxygenation, NIV- Noninvasive ventilation. IMV-Invasive mechanical ventilation, ICU-Intensive care unit.
Discussion
The exact incidence of MIS-A is largely unknown; however, MIS-A as a distinct clinical entity following COVID-19 infection is increasingly being recognized in the past few months.64 This systematic review was carried out to determine the demographic profile, symptoms, systemic involvement, laboratory profile, treatment and outcome of patients diagnosed with MIS-A. MIS-C, a similar disease in the pediatric age group, is already a distinct entity with well-defined diagnostic criteria as well as treatment strategies.65 However, in terms of MIS-A, the findings of our systematic review reveal that despite being reported globally among various ethnic groups, the clinical profile and treatment strategies are variable and often individualized. Additionally, there is a lack of a consistent criteria adopted for establishing a diagnosis of MIS-A. In our systematic review, the diagnostic criterion adopted was the CDC case definition for MIS-A which includes any individual ≥21 years of age presenting with fever and at least three other clinical criteria including either cardiovascular involvement or rash and non-purulent conjunctivitis in presence of laboratory evidence of inflammation and antecedent SARS-CoV-2 infection.8 The other proposed criterion includes the Brighton Collaboration Case Definition for MIS-A, which classifies MIS-A cases into “definite”, “probable”, “possible”, and “insufficient evidence”.66 However, the Brighton Collaboration Case Definition has certain limitations including (a) absence of an age-based cutoff as manifestations of MIS-C and MIS-A are quite different, (b) greater stress has been laid on the disease activity which is measured primarily by cardiac investigations besides hematological tests and c) creating sublevels of diagnosis with “probable” and “possible” cases leading to diagnostic confusion without any overt therapeutic benefits.66
Majority of the patients with MIS-A in our review were young (mean age of 31 years) with a male predisposition. In absence of large datasets, it is unclear whether this observation is due to a selection bias or MIS-A is truly a predominant clinical entity among younger age groups. MIS-A has been reported among various ethnic profiles however, in our review Asians, Caucasians and Hispanics had greater frequency of MIS-A. Though gender and ethnic variations have been reported in COVID-19,67 it is still unclear whether this applies to MIS-A too. Additionally, one-third of our patients had comorbidities with hypertension and obesity being more common. Adults with MIS are more likely to have comorbidities with obesity being one of the possible risk factors for developing MIS-A as reported in patients with MIS-C too. Obesity often predisposes to a systemic inflammatory state due to accumulation of inflammatory cells within the fat tissue as well as the adipose tissue-associated cytokines which are often proinflammatory.68 However, the currently available data is limited and there is a need for large scale studies to identify potential host factors as determinants for developing MIS-A.
Though the exact pathophysiology is not clear, evidence suggests MIS-A associated with COVID-19 is a post-infectious hyperinflammatory response triggered by a dysfunctional immune response leading to systemic inflammation, endothelial dysfunction and procoagulant state (Fig. 1). This hyperinflammatory response is evident in terms of elevated acute inflammatory markers such as CRP, IL-6, ferritin, and ESR. Fever and rash were the most common presenting symptoms in patients with MIS-A. Since the initial clinical presentation can be non-specific mimicking acute infection, a high index of suspicion for underlying MIS-A should be maintained for all patients presenting with similar complaints 4–6 weeks following recovery from COVID-19. A similar clinical presentation can be seen in patients with severe COVID-19 with elevated inflammatory markers and systemic involvement. In our review, in a majority of patients, symptoms of MIS-A usually occurred within four weeks of prior COVID-19 infection whereas MIS-C has been reported to occur within 1–6 weeks following COVID-19 in a recent systematic review.69 The systemic involvement in MIS-A is often varied, with cardiovascular, gastrointestinal, mucocutaneous and musculoskeletal systems, being commonly affected. In our review of the 79 documented MIS-A cases, the cardiovascular system was most commonly affected followed by gastrointestinal and mucocutaneous involvement. Cardiac involvement in these patients often manifested as shock on initial presentation (51.3%) or left ventricular (LV) dysfunction (73.2%) on echocardiography. Cardiac imaging data revealed that a majority of patients had mild/moderate LV dysfunction which was reversible in 56.1% cases. Recovery of LVEF within a few weeks following MIS-A suggests that the LV dysfunction is usually a part of the systemic inflammatory response or acute stress rather than ischemic or a part of viral myocarditis. Cardiac MRI, an emerging imaging modality, was reported in a fraction of patients with diffuse myocardial edema and late gadolinium enhancement being predominant findings hereby suggesting underlying myocardial inflammation. Similar findings too have been reported in patients with MIS-C69 , 70 wherein the cardiovascular system was one of the most commonly affected organ systems. In contrast to MIS-C wherein 7.1% patients have been reported to have CAAs,69 none of the patients reported in our review had CAAs. Clinical presentation in MIS-A varies with the majority of them (58.1%) requiring ICU admission, a finding previously reported in MIS-C cases.69 , 70 Of the 79 included patients, 4 (5.1%) patients succumbed to the illness during index hospitalization. In comparison, a recent systematic review reported mortality in 1.7% of MIS-C cases69 thereby highlighting that patient with MIS-A have a higher mortality than MIS-C cases.
There is a lack of uniform treatment strategy for MIS-A with supportive therapy being used in the majority of cases. Treatment largely focuses on immunosuppression using steroids or other immuno-modulators. Supportive management strategies such as oxygen supplementation, mechanical ventilation, and even ECMO may be required in critically ill patients. In absence of large-scale clinical data and standard treatment protocols, treatment strategies in MIS-A are often based on therapies used for MIS-C. The American College of Rheumatology (ACR) guidelines on treatment of MIS-C recommends immunomodulatory therapies such as glucocorticoids and/or IVIG to be the first line treatment modality.71 Findings from our review too revealed that the immunomodulatory therapies including steroids (60.2%) and IVIG were the most common therapeutic modalities used in MIS-A followed by other immuno-suppressants and biologics. A significant proportion of patients (60.2%) were also administered concomitant antibiotics as the majority of patients present with acute febrile illness with systemic involvement mimicking bacterial infection. Anticoagulants were administered in 32% of patients with MIS-A. The ACR guidelines for MIS-C recommend anticoagulation in patients with (a) documented thrombosis, (b) moderate-severe LV dysfunction and (c) CAAs.71These findings reinforce the urgent need for standard treatment guidelines for MIS-A.
A previous review article on MIS-A by Patel et al72 in September 2021 included 221 patients from reported cases, voluntary reports to CDC of MIS-A and the patients aged 18–20 years in CDC surveillance for MIS-C. Our systematic review included all adults more than 21 years old as per the CDC definition for MIS-A. This is why the mean age in our systematic review was higher (31.67+10.02 years) along with 100% patients reporting fever (required as per CDC criteria for MIS-A) as compared to median age of 21 years and 96% patients having fever in review by Patel et al.
Around 73.2% in our systematic review had reduced left ventricular ejection fraction which was higher than the previous systematic review (54%). However, after excluding the CDC patients in the previous review, this incidence was nearly similar. Cardiac involvement was the most common followed by gastrointestinal manifestations in both the reviews. Steroids were most commonly used therapy in both. However, IVIG was more commonly used (55%) in the previous review as they included more younger patients many of whom had Kawasaki-like presentation (10 patients). The review by Patel et al. itself claims that none of the MIS-A reported to the CDC met the criteria for Kawasaki disease. Thus, the systematic review by Patel et al. had a few limitations including combining data from various sources and using cases from MIS-C surveillance system causing a reporting bias. The current review overcomes these limitations by strictly following the CDC case definition for MIS-A.
Limitations
Our systematic review on MIS-A had a few limitations. This study is mainly descriptive including primarily case reports and case series due to which the level of evidence is low. Additionally, due to inclusion of multiple studies, there is a risk of reporting bias. We stringently followed the CDC case definition of MIS-A and excluded reports which did not describe patients presenting with fever, a cardinal characteristic of MIS-A.
Conclusion
MIS-A was previously an unknown clinical entity in the early half of 2020 and has recently assumed a greater recognition following multiple waves of COVID-19 infection. There is a need for prompt recognition of MIS-A in order to limit the hyperinflammatory response and prevent development of severe organ dysfunction and poor outcomes. Though MIS-A is a rare clinical entity, its long-term sequelae is largely unknown. The emergence of MIS-A calls for harmonizing case definitions for establishing a correct diagnosis as well as definite treatment guidelines. This would largely be possible through wider research, collaborative efforts and development of data registries and clinical cohorts.
Financial and competing interests
No conflict of interests declared.
Informed consent
Not applicable
Contributors
SK, PI, NM involved in Conceptualization, literature search, writing the original draft of manuscript, literature search, planning, conduct and editing. SK, PI, NM, PS, KG involved in review and editing. All the authors have read and agreed with the submitted manuscript
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.hrtlng.2022.03.007.
Appendix. Supplementary materials
References
- 1.Kunal S., Gupta K., Sharma S.M., Pathak V., Mittal S., Tarke C. Cardiovascular system and COVID-19: perspectives from a developing country. Monaldi Arch Chest Dis. 2020;90(2) doi: 10.4081/monaldi.2020.1305. [DOI] [PubMed] [Google Scholar]
- 2.Ramos-Casals M., Brito-Zerón P., Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol. 2021;17:315–332. doi: 10.1038/s41584-021-00608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang L., Tang K., Levin M., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S., Malhotra N., Gupta N., Agrawal S., Ish P. The curious case of coronavirus disease 2019 (COVID-19) in children. J Pediatr. 2020;222:258–259. doi: 10.1016/j.jpeds.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris S.B., Schwartz N.G., Patel P., et al. Godfred-Cato S. case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection - United Kingdom and United States, march-august 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marzano A.V., Cassano N., Moltrasio C., Verdoni L., Genovese G., Vena G.A. Multisystem inflammatory syndrome in children associated with COVID-19: a review with an emphasis on mucocutaneous and kawasaki disease-like findings. Dermatology. 2021:1–9. doi: 10.1159/000515449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). Multisystem inflammatory syndrome in adults (MIS-A) case definition information for healthcare providers. Available at https://www.cdc.gov/mis/mis-a/hcp.html (accessed on 15th August 2021)
- 9.Hendel R.C., Budoff M.J., Cardella J.F., et al. ACC/AHA/ACR/ASE/ASNC/HRS/NASCI/RSNA/SAIP/SCAI/SCCT/SCMR/SIR 2008 key data elements and definitions for cardiac imaging: a report of the American college of cardiology/American heart association task force on clinical data standards (writing committee to develop clinical data standards for cardiac imaging) Circulation. 2009;119:154–186. doi: 10.1161/CIRCULATIONAHA.108.191393. doi: 10.1161/CIRCULATIONAHA. 108.191393. [DOI] [PubMed] [Google Scholar]
- 10.National Institutes of Health (2014). National heart, lung, and blood institute. quality assessment tool for observational cohort and cross-sectional studies. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 15th August 2021).
- 11.Sackett D.L. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95:2S–4S. [PubMed] [Google Scholar]
- 12.Varadaraj G., Sangeetha B., Sandhu S., Santhiya G. Four cases of multisystem inflammatory syndrome in adults associated with SARS-COV-2 infection - an overview of clinical features, diagnosis and treatment. J Assoc Physicians India. 2021;69:11–12. [PubMed] [Google Scholar]
- 13.Chung H., Seo H., Park S., et al. The first case of multisystem inflammatory syndrome in adult after COVID-19 in Korea. J Korean Med Sci. 2021;36:e181. doi: 10.3346/jkms.2021.36.e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiore M., Ryan B., Youssef G.B., James S., Zubair H. A case of multisystem inflammatory syndrome and shock after COVID-19 in an adult. Critical Care Medicine. 2021;49:37. doi: 10.1097/01.ccm.0000726312.25394.8f. [DOI] [Google Scholar]
- 15.Razmi T.M., Afra T.P., Mohammed T.P., Ashik P.T.M., Sukesh E. COVID-19-associated multisystem inflammatory syndrome in adults with Kawasaki disease-like cutaneous manifestations. Br J Dermatol. 2021;185:e35. doi: 10.1111/bjd.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaigany S., Gnirke M., Guttmann A., et al. An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. Lancet. 2020;396:e8–e10. doi: 10.1016/S0140-6736(20)31526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerkerian G., Vaughan S.D. Multisystem inflammatory syndrome in an adult after SARS-CoV-2 infection. CMAJ. 2021;193:E956–E961. doi: 10.1503/cmaj.210232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad F., Ahmed A., Rajendraprasad S.S., et al. Multisystem inflammatory syndrome in adults: a rare sequela of SARS-CoV-2 infection. Int J Infect Dis. 2021;108:209–211. doi: 10.1016/j.ijid.2021.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baruah R., Case Gupta R. Report on a young patient with multisystem inflammatory syndrome in adult (MIS-A) Ann Med Health Sci Res. 2021;11:1385–1387. [Google Scholar]
- 20.Yamada Y., Fujinami K., Eguchi T., Takefuji H., Mori N. Multisystem inflammatory syndrome in adults after mild SARS-CoV-2 infection. Japan. Emerg Infect Dis. 2021;27:1740–1742. doi: 10.3201/eid2706.210728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razavi A.C., Chang J.L., Sutherland A., Niyogi A., Ménard G.E. A 23-year-old man with multisystem inflammatory syndrome after mild COVID-19. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620974200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzman M.B., Huang C.W., O'Brien C.M., Castillo R.D. Multisystem inflammatory syndrome after SARS-CoV-2 infection and COVID-19 vaccination. Emerg Infect Dis. 2021;27:1944–1948. doi: 10.3201/eid2707.210594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastug A., Aslaner H., Aybar Bilir Y., et al. Multiple system inflammatory syndrome associated with SARS-CoV-2 infection in an adult and an adolescent. Rheumatol Int. 2021;41:993–1008. doi: 10.1007/s00296-021-04843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pombo F., Seabra C., Soares V., Sá A.J., Ferreira I., Mendes M. COVID-19-related multisystem inflammatory syndrome in a young adult. Eur J Case Rep Intern Med. 2021;8 doi: 10.12890/2021_002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kofman A.D., Sizemore E.K., Detelich J.F., Albrecht B., Piantadosi A.L. A young adult with COVID-19 and multisystem inflammatory syndrome in children (MIS-C)-like illness: a case report. BMC Infect Dis. 2020;20:716. doi: 10.1186/s12879-020-05439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chau V.Q., Giustino G., Mahmood K., et al. Cardiogenic shock and hyperinflammatory syndrome in young males with COVID-19. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007485. [DOI] [PubMed] [Google Scholar]
- 27.Ahsan T., Rani B. A case of multisystem inflammatory syndrome post-COVID-19 infection in an adult. Cureus. 2020;12:e11961. doi: 10.7759/cureus.11961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faller E., Barry R., O'Flynn O., Kearney P., Sadlier C. Kawasaki-like multisystem inflammatory syndrome associated with SARS-CoV-2 infection in an adult. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2020-240845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julius M.A., Cantrell D., Sharif S., Zelnik Yovel D., Rapoport M.J. The first fatal post-COVID-19 adult patient with multi-system inflammatory syndrome in Israel. Isr Med Assoc J. 2021;23:212–213. [PubMed] [Google Scholar]
- 30.Dabas R., Varadaraj G., Sandhu S., Bhatnagar A., Pal R. Kawasaki-like multisystem inflammatory syndrome associated with COVID-19 in an adult: a case report. Br J Dermatol. 2021 doi: 10.1111/bjd.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veyseh M., Webster P., Blanco I. COVID-19-associated inflammatory syndrome in an adult woman with unexplained multiple organ failure: staying vigilant for COVID-19 complications as the pandemic surges. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hékimian G., Kerneis M., Zeitouni M., et al. Coronavirus disease 2019 acute myocarditis and multisystem inflammatory syndrome in adult intensive and cardiac care units. Chest. 2021;159:657–662. doi: 10.1016/j.chest.2020.08.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulut H., Herbers A.H.E., Hageman I.M.G., et al. SARS-CoV-2-induced multisystem inflammatory syndrome in a young adult: case report. SN Compr Clin Med. 2021:1–7. doi: 10.1007/s42399-021-00998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cogan E., Foulon P., Cappeliez O., Dolle N., Vanfraechem G., De Backer D. Multisystem inflammatory syndrome with complete kawasaki disease features associated with SARS-CoV-2 infection in a young adult. a case report. Front Med (Lausanne) 2020;7:428. doi: 10.3389/fmed.2020.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown L.M., Semler M.W., Hansen M., Person A.K., Kelly S.G. Multisystem inflammatory syndrome in an adult with COVID-19. Infect Dis Clin Pract (Baltim Md) 2021;29:e174–e176. doi: 10.1097/IPC.0000000000000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gopalakrishnan M., Sekar D., Pradeep R., Aditya V., Thabah M.M. Fatal Multisystem inflammatory syndrome (MIS) in a young adult following a recent mild Covid-19. Journal of Medicine and Healthcare. 2021;3:1–2. [Google Scholar]
- 37.Diakite S., Bousdira N., Tachon G., Ackermann F., Groh M., Rohmer J. Regression of coronary aneurysms with intravenous immunoglobulins and steroids for COVID-19 adult multisystem inflammatory syndrome. JACC Case Rep. 2021;3:581–585. doi: 10.1016/j.jaccas.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Othenin-Girard A., Regamey J., Lamoth F., et al. Multisystem inflammatory syndrome with refractory cardiogenic shock due to acute myocarditis and mononeuritis multiplex after SARS-CoV-2 infection in an adult. Swiss Med Wkly. 2020;150:w20387. doi: 10.4414/smw.2020.20387. [DOI] [PubMed] [Google Scholar]
- 39.Kinter C.W., Saxon G.E., Ahmad M., Berhane H., Gensler L., Khosroshahi A. Multisystem inflammatory syndrome in an adult with involvement of the skin, lymph nodes, muscle, heart, liver, and kidneys. Rheumatology. 2021:keab426. doi: 10.1093/rheumatology/keab426. 10.1093/rheumatology/keab426. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Yizhi S., Vishal D., Ronald G.N., Michael B.R., Amanda L.T. Multisystem inflammatory syndrome in an adult after COVID-19. Infectious Diseases in Clinical Practice. 2020;28:e28–e29. doi: 10.1097/IPC.0000000000000938. [DOI] [Google Scholar]
- 41.Moghadam P., Blum L., Ahouach B., et al. Multisystem inflammatory syndrome with particular cutaneous lesions related to COVID-19 in a young adult. Am J Med. 2021;134:e36–e37. doi: 10.1016/j.amjmed.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abhimanyu A., Ezra C., Marisol F., et al. Multisystem inflammatory syndrome in an adult with COVID-19-A trial of anakinra. Infectious Diseases in Clinical Practice. 2021 doi: 10.1097/IPC.0000000000001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altunisik Toplu S., Ersoy Y., Bayindir Y., Kilic T., Bayazit V. Multisystem inflammatory syndrome in adults (MIS-A) associated with SARS-CoV-2 infection in a young adult case from Turkey. Medeni Med J. 2021;36:180–184. doi: 10.5222/MMJ.2021.95422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luis C., Nora Moron C., Julin M., Jill L., Luke B., Carlos S. Multisystem inflammatory syndrome in an adult associated with COVID-19. Critical Care Medicine. 2021;49:92. doi: 10.1097/01.ccm.0000726740.59021.c1. [DOI] [Google Scholar]
- 45.Vujaklija Brajković A., Zlopaša O., Gubarev Vrdoljak N., Goran T., Lovrić D., Radonić R. Acute liver and cardiac failure in multisystem inflammatory syndrome in adults after COVID-19. Clin Res Hepatol Gastroenterol. 2021;45 doi: 10.1016/j.clinre.2021.101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mieczkowska K., Zhu T.H., Hoffman L., et al. Two adult cases of multisystem inflammatory syndrome associated with SARS-CoV-2. JAAD Case Rep. 2021;10:113–115. doi: 10.1016/j.jdcr.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uwaydah A.K., Hassan N.M.M., Abu Ghoush M.S., Shahin K.M.M. Adult multisystem inflammatory syndrome in a patient who recovered from COVID-19 postvaccination. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox S.E., Lameira F.S., Rinker E.B. Vander Heide RS. Cardiac Endotheliitis and Multisystem Inflammatory Syndrome After COVID-19. Ann Intern Med. 2020;173:1025–1027. doi: 10.7326/L20-0882. doi: 10.7326/L20-0882. Epub 2020 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boudhabhay I., Rabant M., Roumenina L.T., et al. Case report: adult post-COVID-19 multisystem inflammatory syndrome and thrombotic microangiopathy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.680567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vedran P., Marko K., Maja Hrabak P., et al. New fever and acute heart failure weeks after COVID-19 – red flags for multisystem inflammatory syndrome in adults. Cardiologia Croatica. 2021;16:179. 179. [Google Scholar]
- 51.Downing S., Chauhan V., Chaudry I.H., Galwankar S., Sharma P., Stawicki S.P. Colchicine, aspirin, and montelukast - a case of successful combined pharmacotherapy for adult multisystem inflammatory syndrome in COVID-19. J Glob Infect Dis. 2020;12:221–224. doi: 10.4103/jgid.jgid_296_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malangu B., Quintero J.A., Capitle E.M. Adult inflammatory multi-system syndrome mimicking kawasaki disease in a patient with COVID-19. Cureus. 2020;12:e11750. doi: 10.7759/cureus.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M., Haque W., Vuppala S., Tobias E. Rare presentation of multisystem inflammatory syndrome in an adult associated with SARS-CoV-2 infection: unilateral neck swelling. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lerner R.K. Harlequin syndrome in a young adult with multi-system inflammatory syndrome post COVID-19. Clin Surg. 2021;4:1–4. [Google Scholar]
- 55.Viana-García A., Pina-Belmonte A., Salavert-Pamblanco S., Atienza-Garcia A. Multisystemic inflammatory syndrome in a young adult after SARS-CoV-2 infection: case report. J Med Virol. 2021;93:5243–5245. doi: 10.1002/jmv.27083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cattaneo P., Volpe A., Cardellino C.S., et al. Multisystem inflammatory syndrome in an adult (MIS-A) successfully treated with anakinra and glucocorticoids. Microorganisms. 2021;9:1393. doi: 10.3390/microorganisms9071393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gulersen M., Staszewski C., Grayver E., et al. Coronavirus disease 2019 (COVID-19)-related multisystem inflammatory syndrome in a pregnant woman. Obstet Gynecol. 2021;137:418–422. doi: 10.1097/AOG.0000000000004256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chowdhary A., Joy E., Plein S., Abdel-Rahman S.E. Multisystem inflammatory syndrome in an adult with SARS-CoV-2 infection. Eur Heart J Cardiovasc Imaging. 2021;22:e17. doi: 10.1093/ehjci/jeaa232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davogustto G.E., Clark D.E., Hardison E., et al. Characteristics associated with multisystem inflammatory syndrome among adults with SARS-CoV-2 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chérif M.Y., de Filette J.M.K., André S., Kamgang P., Richert B., Clevenbergh P. Coronavirus disease 2019-related Kawasaki-like disease in an adult: a case report. JAAD Case Rep. 2020;6:780–782. doi: 10.1016/j.jdcr.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones I., Bell L.C.K., Manson J.J., Last A. UCLH COVID response team. An adult presentation consistent with PIMS-TS. Lancet Rheumatol. 2020;2:e520–e521. doi: 10.1016/S2665-9913(20)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sokolovsky S., Soni P., Hoffman T., Kahn P., Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am J Emerg Med. 2021;39 doi: 10.1016/j.ajem.2020.06.053. 253.e1-253.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lidder A.K., Pandit S.A., Lazzaro D.R. An adult with COVID-19 kawasaki-like syndrome and ocular manifestations. Am J Ophthalmol Case Rep. 2020;20 doi: 10.1016/j.ajoc.2020.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chow E.J. The multisystem inflammatory syndrome in adults with SARS-CoV-2 infection-another piece of an expanding puzzle. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.10344. [DOI] [PubMed] [Google Scholar]
- 65.Clarke J. MIS-C clinical guidance released amid race to define the condition. Nat Rev Rheumatol. 2020;16:538. doi: 10.1038/s41584-020-0489-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogel T.P., Top K.A., Karatzios C., Hilmers D.C., Tapia L.I., Moceri P., et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037–3049. doi: 10.1016/j.vaccine.2021.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kopel J., Perisetti A., Roghani A., Aziz M., Gajendran M., Goyal H. Racial and gender-based differences in COVID-19. Front Public Health. 2020;8:418. doi: 10.3389/fpubh.2020.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ellulu M.S., Patimah I., Khaza'ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmed M., Advani S., Moreira A., et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaushik A., Gupta S., Sood M., Sharma S., Verma S. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J. 2020;39:e340–e346. doi: 10.1097/INF.0000000000002888. [DOI] [PubMed] [Google Scholar]
- 71.Henderson L.A., Canna S.W., Friedman K.G., et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol. 2021;73:e13–e29. doi: 10.1002/art.41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel P., DeCuir J., Abrams J., Campbell A.P., Godfred-Cato S., Belay E.D. Clinical characteristics of multisystem inflammatory syndrome in adults: a systematic review. JAMA Netw Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.26456. Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.