Abstract
Background
Consistent with pathophysiological models of psychosis, temporal disturbances in schizophrenia spectrum populations may reflect abnormal cortical (e.g., prefrontal cortex) and subcortical (e.g., striatum) cerebellar connectivity. However, few studies have examined associations between cerebellar connectivity and timing dysfunction in psychosis populations, and none have been conducted in youth at clinical high-risk (CHR) for psychosis. Thus, it is currently unknown if impairments in temporal processes are present in CHR youth or how they may be associated with cerebellar connectivity and worsening of symptoms.
Methods
A total of 108 (56 CHR/52 controls) youth were administered an auditory temporal bisection task along with a resting state imaging scan to examine cerebellar resting state connectivity. Positive and negative symptoms at baseline and 12 months later were also quantified.
Results
Controlling for alcohol and cannabis use, CHR youth exhibited poorer temporal accuracy compared to controls, and temporal accuracy deficits were associated with abnormal connectivity between the bilateral anterior cerebellum and a right caudate/nucleus accumbens striatal cluster. Poor temporal accuracy accounted for 11% of the variance in worsening of negative symptoms over twelve months.
Conclusions
Behavioral findings suggest CHR youth perceive durations of auditory tones as shortened compared to objective time, which may indicate a slower internal clock. Poorer temporal accuracy in CHR youth was associated with abnormalities in brain regions involved in an important cerebellar network implicated in prominent pathophysiological models of psychosis. Lastly, temporal accuracy was associated with worsening of negative symptoms across 12 months, suggesting temporal dysfunction may be sensitive to illness progression.
Background
It is becoming increasingly well-evidenced that patients with schizophrenia exhibit impairments in the perception and processing of temporal information (Ciullo et al., 2015, Stanghellini et al., 2015, Thoenes and Oberfeld, 2017). Consistent with a cognitive dysmetria theory (Andreasen et al., 1998, Andreasen and Pierson, 2008), these deficits may reflect cerebellar circuit abnormalities. Given the prevalence of research implicating the cerebellum in temporal processes (Breska and Ivry, 2016, Coull et al., 2011), as well as evidence demonstrating cerebellar structural and functional connectivity abnormalities across the schizophrenia spectrum (Bernard et al., 2014, Bernard and Mittal, 2014, Dean et al., 2014, Mittal et al., 2013a, Parker et al., 2014), it is surprising that there have been relatively few studies examining associations between timing dysfunction and cerebellar networks in schizophrenia spectrum populations, and none in the putative prodromal stage of the illness (i.e., the period immediately preceding the onset of psychosis). As a result, it is currently unknown if impairments in temporal processes are present before illness onset or how they may be associated with the pathophysiology and progression of the disorder. The present study investigates deficits in temporal processing in young adults diagnosed with a prodromal syndrome (i.e., at clinical high-risk; CHR) and aims to determine if impairments in timing are associated with abnormal cerebellar resting state functional connectivity and worsening of symptoms across 12 months.
Recent research has corroborated early 20th century phenomenological and autobiographical accounts of disturbances in the perception and experience of time in schizophrenia spectrum populations (Freedman, 1974, Minkowski, 1927, Stanghellini et al., 2015). For example, compared to controls, schizophrenia patients are less precise when judging the duration of auditory stimuli (Bolbecker et al., 2014, Carroll et al., 2008, Carroll et al., 2009b) and are less coordinated in the timing of motor behaviors (Carroll et al., 2009a). Furthermore, a recent meta-analysis demonstrated that psychosis patients, depending on the task, perceive time as both lengthened and shortened compared to objective time, which was proposed to reflect an abnormal internal time keeper (Thoenes and Oberfeld, 2017). Notably, similar findings in individuals with trait-level psychotic-like experiences (e.g., magical thinking, suspiciousness) suggest that deficits in temporal processes may reflect a fundamental vulnerability for the disorder (Lee et al., 2006, Penney et al., 2005, Reed and Randell, 2014).
The cerebellum is considered to be a critical region within the cerebello-thalamo-striato-cortical network that is thought to underlie this poor temporal coordination (Andreasen and Pierson, 2008, Barch, 2014, Schmahmann and Pandya, 2008). To date, the limited neuroimaging work in this area has predominately examined timing processes using temporal durations greater than one second (i.e., supra-second) (Ojeda et al., 2002, Ortuño et al., 2011, Volz et al., 2001). For example, compared to controls, patients with schizophrenia exhibit decreased activity in the prefrontal cortex and caudate nucleus when determining if pairs of supra-second auditory stimuli differ in duration (Volz et al., 2001). However, evidence suggests that processing of supra-second temporal stimuli requires less cerebellar involvement (Breska and Ivry, 2016, Buhusi and Meck, 2005, Meck, 2005), whereas the cerebellum is critical for temporal processing in the sub-second (i.e., less than one second) stimulus range (Breska and Ivry, 2016, Casini and Ivry, 1999, Coull et al., 2011, Harrington et al., 2004, Ivry and Keele, 1989, Lewis and Miall, 2003a, Lewis and Miall, 2003b). Taken together, given the cerebellum is critically involved in processing temporal information in the sub-second range and is considered a crucial region involved in the posited cognitive dysmetria seen in psychosis, the relative contributions of the cerebellum to deficits in temporal processing remain relatively understudied.
Furthermore, to our knowledge, the only extant sub-second timing neuroimaging work in psychosis focused on a single cerebellar region (i.e., the vermis) and did not observe abnormal activation during an auditory discrimination task (Davalos et al., 2011). Yet, robust evidence from task-based and resting state functional connectivity magnetic resonance imaging (fcMRI) studies in both healthy and schizophrenia spectrum populations indicates the cerebellum is comprised of functionally distinct topographical regions involved in cortical and subcortical circuitry associated with a wide range of functions that may underlie or contribute to timing processes (Bernard and Mittal, 2014, Schmahmann, 2018, Stoodley, 2012). Regional specificity is particularly important as distinct topographical regions are thought to be involved in both automatic timing processes (i.e., sub-second) (Kawashima et al., 2000, Salman, 2002), as well as higher order cognitive functions thought to contribute to temporal processing, particularly in supra-second timing (i.e., working memory/attention) (Buhusi and Meck, 2005, Meck, 2005, Merchant et al., 2013). For example, the anterior lobe of the cerebellum exhibits resting state connectivity with cortical motor regions (Schmahmann, 2018, Stoodley, 2012), and may be necessary for motor and perceptual timing (Jueptner et al., 1995, Kawashima et al., 2000, Lutz et al., 2000, Salman, 2002). This has been interpreted as evidence for cerebellar involvement in an automatic neural “time keeper” that provides a precise representation of temporal information (Kawashima et al., 2000, Lutz et al., 2000, Salman, 2002). In contrast, the posterior lobe (particularly Crus I) demonstrates connectivity with cortical regions (e.g., prefrontal cortex) involved in higher-order cognitive processes thought to contribute to temporal processing, such as attention and working memory (Bernard et al., 2012, Schmahmann, 2018, Stoodley et al., 2012). Thus, using a regional cerebellar approach may reveal distinct relationships between cerebellar topography and disturbances in temporal processing across the psychosis spectrum.
The use of fcMRI to investigate these questions in youth at CHR for psychosis may be particularly informative. For instance, fcMRI affords an examination of the intrinsic communication between specific brain regions without the potential confounds of functional tasks (Gupta et al., 2016, Whitfield-Gabrieli and Ford, 2012). Furthermore, evidence suggests that both decreased and increased resting state functional connectivity is present in CHR populations and is associated with worsening of positive and negative symptoms and conversion status (Anticevic et al., 2015, Bernard et al., 2014, Bernard et al., 2017, Dandash et al., 2013, Pelletier-Baldelli et al., 2018). Crucially, many of the same subcortical and cortical regions exhibiting abnormal connectivity in CHR youth overlap with regions implicated in timing (e.g., dorsal striatum, cerebellum, supplementary motor area (SMA), prefrontal cortex) (Coull et al., 2011). For example, Cao and colleagues (2018) observed hyperconnectivity in a network comprised of both subcortical (i.e., cerebellum, striatum, thalamus) and cortical areas (e.g., SMA), superior and medial frontal gyri) in CHR youth; hyperconnectivity across this network was associated with shorter time to conversion and disorganized symptoms. Given these same regions are implicated in timing and exhibit abnormalities in CHR youth, examining how potential deficits in timing are associated with cerebellar resting state functional connectivity stands to inform the field’s understanding of how these processes are implicated in psychosis risk.
The present study used a sub-second temporal bisection task and fcMRI to better understand timing dysfunction and cerebellar connectivity in CHR youth. We used sub-second temporal stimuli because timing in the sub-second range is more strongly implicated in cerebellar connectivity (Breska and Ivry, 2016). Moreover, we chose the temporal bisection task (Allan and Gibbon, 1991, Church and Deluty, 1977) because of its well-known psychophysical properties and extensive use in schizophrenia patients (see Thoenes and Oberfeld, 2017). Deficits in the temporal bisection task are typically interpreted with regards to pacemaker-accumulator models of timing (Carroll et al., 2008, Gibbon et al., 1984), which posit that the onset of a sensory event (e.g., an auditory tone) triggers an internal pacemaker that emits pulses that are stored and then summed in an accumulator for comparison to previously encoded temporal durations held in long-term memory (Carroll et al., 2008, Carroll et al., 2009b, Coull et al., 2011, Thoenes and Oberfeld, 2017). Previous studies have shown that patients with schizophrenia exhibit higher bisection points on the temporal bisection task which is thought to reflect a slower internal clock where fewer pulses are emitted during the clock stage, resulting in a greater proportion of intermediate tone durations perceived as shorter (Lee et al., 2006, Reed and Randell, 2014, Thoenes and Oberfeld, 2017). In addition, patients also show more variability (i.e., higher difference limens) in the bisection task when classifying stimuli (Bolbecker et al., 2014, Carroll et al., 2008).
In line with previous research in schizophrenia populations (Elvevåg et al., 2003, Lee et al., 2006, Reed and Randell, 2014, Thoenes and Oberfeld, 2017), we hypothesized that CHR participants would exhibit deficits in both temporal accuracy and precision compared to healthy controls. Given it is thought that sub-second timing preferentially recruits motor circuitry (Lewis and Miall, 2003b), we hypothesized that deficits in temporal accuracy and precision would be associated with abnormal anterior cerebellar resting state connectivity with cortical and/or subcortical motor regions (e.g., SMA, striatum) in CHR participants. To examine associations between temporal dysfunction and baseline symptoms and worsening of symptoms, we conducted exploratory analyses on positive and negative symptoms at baseline, as well as changes in positive and negative symptoms over a 12-month period.
Methods
Participants
Data for the present study were obtained from 56 CHR and 52 healthy control (HC) youth (total N=108; 16–21 years old, M age=19.01, SD age=1.44). Exclusion criteria for both groups included any neurological disorder, history of head injury, life-time substance dependence, or any past or current psychotic disorder (e.g., schizophrenia). For HCs, the presence of a psychotic disorder in a first-degree relative and any past or current Axis I disorders were additional exclusionary criteria. We experienced 30% attrition at 12-month follow-up which is comparable to other studies in this area (Bernard et al., 2017, Mittal et al., 2013a, Mittal et al., 2008). Informed consent was obtained in accordance with the protocol approved by the University Institutional Review Board.
Clinical Interviews
The Structured Interview for Psychosis Risk Syndromes (SIPS; McGlashan et al., 2010, Miller et al., 1999) was administered to diagnose a CHR syndrome at the baseline assessment and track symptom change at follow-up. Criteria for a prodromal syndrome included one or more of the following: (1) progression or recent onset of attenuated positive symptoms, (2) the presence of a first-degree relative with a psychotic disorder accompanied by a recent decline in global functioning, or (3) a decline in global functioning with the presence of schizotypal personality disorder (Miller et al., 1999). In addition, the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID; First et al., 1995) was administered in order to rule out a psychotic disorder and to note the occurrence of any comorbid conditions. Clinical interviews were administered by advanced doctoral students with inter-rater reliabilities that exceeded the minimum study criterion of Kappa ≥ 0.80.
Because alcohol and cannabis use can affect both cerebellar function and temporal processes (Deshmukh et al., 2002, Hicks et al., 1984, Lieving et al., 2006, Solowij et al., 2011, Tinklenberg et al., 1976), frequency of use was assessed using the Alcohol Use Scale (AUS) and Drug Use Scale (DUS) (Drake et al., 1996). These ratings were numerically coded from 0 (never) to 5 (almost daily) for use in our statistical analyses.
Temporal Bisection Task
The bisection task requires participants to classify intermediate tone durations as either “short” or “long” depending on their similarity to two previously learned anchor tones (880 Hz), a “short” 300 ms tone and a “long” 600 ms tone. The task was comprised of training, practice, and test phases (see Supplementary Material (SM) for training/practice details). During the test phase, participants were presented with the 300 ms and 600 ms anchor tones, and five arithmetically spaced intermediate tone durations (350 ms, 400 ms, 450 ms, 500 ms, and 550 ms) and were instructed to judge whether each tone was “short” or “long”. Participants completed three test blocks consisting of 35 trials each (i.e., five presentations per stimulus duration).
For each participant, the proportion of “long” responses for both the anchor and intermediate stimuli were plotted as a function of stimulus duration and used to quantify the accuracy and precision of an individual’s temporal perception. This method yields a psychometric response curve for each participant that is typically sigmoidal (S-shaped; Figure 1a), resulting in the proportion of tone durations near the short anchor (300 ms) classified as “long” to be near zero and tone durations near the long anchor (600 ms) classified as “long” to be near one. The bisection point is the duration value at which intermediate durations are equally probable to be classified as “short” or “long”. Bisection points will typically fall closer to the geometric mean of the anchor durations when the ratio of “long” to “short” anchor durations are small (e.g., 600/300 ms) (Kopec and Brody, 2010). Thus, depending on the anchor ratio, deviations from the geometric or arithmetic mean reflect the temporal accuracy of an individual’s temporal perception (Thoenes and Oberfeld, 2017). The slope of the plotted function is referred to as the difference limen and reflects temporal precision (i.e., variability). Smaller difference limens indicate steeper slopes, and thus, greater temporal precision when classifying the anchor and intermediate tones (Carroll et al., 2008, Thoenes and Oberfeld, 2017). See SM for derivations of the two temporal measures.
Figure 1.
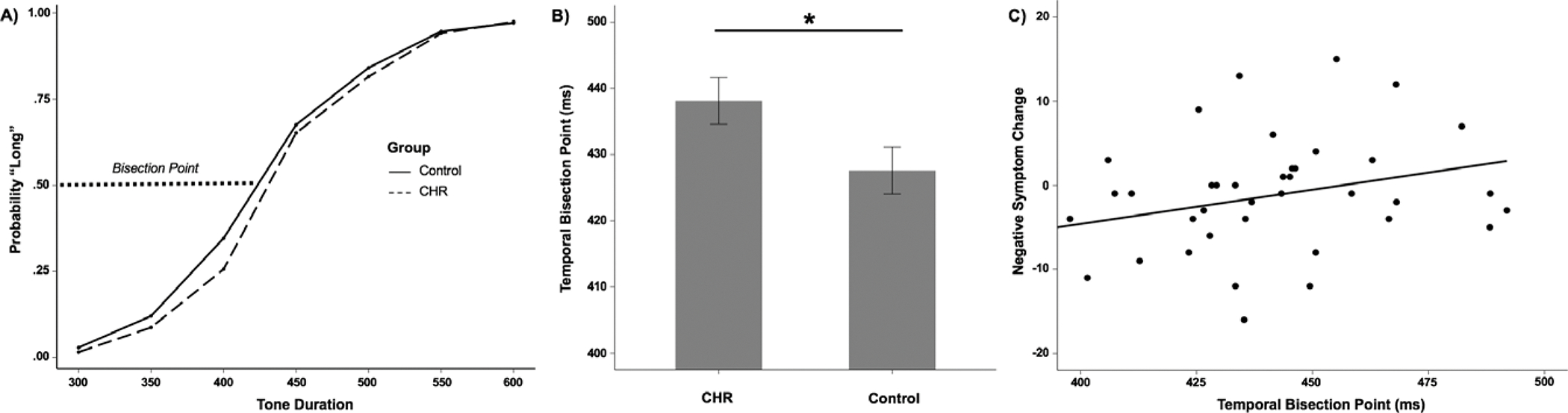
A) The proportion of long responses as a function of stimulus duration. The dotted line at 0.50 on the y-axis represents the bisection point. B) Group differences in the bisection point (Note: * = p < .05). C) Scatter plot representing relationship between baseline temporal bisection point and negative symptoms at 12-month follow-up.
MRI Scanning Procedure
Structural and resting state functional scans were acquired using a 3-Tesla Siemens Tim Trio MRI scanner (Siemens AG, Munich, Germany) using a standard 12-channel head coil. Structural images were acquired with a T1-weighted 3D magnetization prepared rapid gradient multi-echo sequence (MPRAGE; sagittal plane; repetition time (TR)=2530 ms; echo times (TE)=1.64 ms, 3.5 ms, 5.36 ms, 7.22 ms, 9.08 ms; GRAPPA parallel imaging factor of 2; 1 mm3 isomorphic voxels, 192 interleaved slices; FOV=256 mm; flip angle=7°; time=6:03 min). A 5-minute 34 s resting state blood-oxygen-level dependent (BOLD) scan was acquired with a T2-weighted echo-planar functional protocol (number of volumes=165; TR=2000 ms; TE=29 ms; matrix size=64 × 64 × 33; FA=75°; 3.8 × 3.8 × 3.5 mm3 voxels; 33 slices; FOV=240 mm). See SM for more information regarding parameters for identifying incidental pathology and participant scanning procedures.
fcMRI Data Preprocessing
Data were preprocessed in FSL (v. 5; http://fsl.fmrib.ox.ac.uk/fsl), which involved motion correction, brain extraction, high-pass filtering (100 s), and spatial smoothing (6 mm FWHM). Next, functional images were aligned to the MNI 2-mm brain template with a two-step procedure. First, the resting state scan was aligned to the high-resolution MPRAGE using a linear boundary-based registration method, which relies on white matter boundaries (Greve and Fischl, 2009, Jenkinson et al., 2002, Jenkinson and Smith, 2001). Second, the MPRAGE was nonlinearly aligned to the template and the two registrations were then combined to align the fcMRI scan to the template. To account for motion-related artifacts, temporal derivative regressors were calculated with the Artifact Rejection Toolbox (ART; http://www.nitrc.org/projects/artifact_detect/). The resultant motion regressors were entered into the model as a temporal derivative nuisance covariate at the subject level (see SM for details on motion-artifact control).
Functional Connectivity - Cerebellar Seed to Voxel Connectivity Approach
Functional connectivity analyses were conducted using data from 51 CHR and 48 HC participants (1 CHR and 4 HC participants did not complete the scanning portion of the study) in the CONN toolbox v. 17.f (Whitfield-Gabrieli and Nieto-Castanon, 2012). The data were band-pass filtered from 0.008 to 0.09 Hz. Lobular seed regions-of-interest (ROIs) within the bilateral anterior cerebellum (Lobules I – V), bilateral Crus I, and bilateral Lobule X were defined based on the SUIT atlas (Diedrichsen, 2006, Diedrichsen et al., 2009) as described by Bernard and colleagues (Bernard and Seidler, 2013). The anterior cerebellum and Crus I were used to examine if specific associations between topographical regions involved in motor processes (anterior cerebellum) and higher-order cognition (Crus I) were associated with temporal dysfunction. Lobule X, which is primarily involved in vestibular functions (Baumann et al., 2015), was used as a control region in order to demonstrate specificity across the cerebellar lobular ROIs. The mean time-series, averaged across all voxels within each seed ROI (anterior cerebellum, Crus I, and Lobule X) were used as regression parameters, and correlated with all other voxels in the brain in separate seed-to-voxel connectivity analyses. Anatomical images were segmented into gray matter, white matter, and CSF with SPM8 in order to create masks for signal extraction. The CONN toolbox uses principal components analysis to extract five temporal components from the segmented CSF and white matter, which were entered as confound regression in the subject-level general linear model (GLM). This approach corrects for confounds of motion and physiological noise without regressing out global signal, providing equivalent global signal reduction (Chai et al., 2011, Murphy et al., 2009). As previously mentioned, the composite motion metric from the ART toolbox was included as a confound regressor.
Connectivity Analyses Approach
The study examined group differences in seed connectivity, and also tested for interactions to investigate if associations between cerebellar resting state connectivity and temporal measures (performed outside of the scanner) were different by group. In order to interpret any significant interactions, we extracted connectivity weights for significant seed-to-voxel clusters to compare temporal measures between groups. Consistent with the behavioral statistical approach, we limited fcMRI analyses to timing variables with significant group differences. Data in tables and statistical maps were first thresholded at the voxel-level at puncorr < .001 and then corrected at the cluster-level to a false-discovery rate (FDR) of p < .05 (Chumbley and Friston, 2009). Because antipsychotic medications are dopamine (DA) antagonist, and evidence suggests that DA affects temporal processing in both animals and humans (Buhusi and Meck, 2005, Coull et al., 2011), connectivity weights for significant seed-to-voxel clusters were examined with and without individuals receiving antipsychotics (N=7 CHR).
Behavioral Analysis Approach
Chi-square tests and independent t-tests were employed to examine group differences in demographic variables (Table 1). To test for group differences between CHR and HC young adults in the bisection point, difference limen, and anchor tone accuracy we used analysis of covariance (ANCOVA) controlling for alcohol consumption and cannabis use. Outliers were defined as ± 3 standard deviations from the mean on any temporal measure. A single outlier was identified for each of the timing variables, resulting in the removal of one CHR participant from each analysis. In addition, three CHR participants were excluded from all analyses, two for extreme response times (i.e., multiple responses > 5 sec) on the temporal bisection task, and one for alcohol consumption and cannabis use not being assessed. Thus, the final sample consisted of 52 CHR and 52 HC participants (total N=104) for all ANCOVA analyses.
Table 1.
Demographics characteristics with group comparisons
| CHR | HC | Statistic | P | |
|---|---|---|---|---|
|
| ||||
| Age | ||||
| Mean (SD) | 18.85 (1.35) | 19.12 (1.54) | t (102) = −0.95 | ns. |
| Gender | ||||
| Male | 35 | 23 | ||
| Female | 17 | 29 | χ2 (1) = 5.61 | .02 |
| Education (yr.) | ||||
| Mean (SD) | 12.51 (1.50) | 13.12 (1.62) | t (102) = −1.98 | ns. |
| Parent education | ||||
| Mean (SD) | 15.32 (2.44) | 15.18 (3.12) | t (102) = 0.25 | ns. |
Note: Clinical high-risk (CHR); Healthy controls (HC); Statistic reflects test of group differences for each demographic variable; Non-significant (ns.).
To avoid inflating the experiment-wise Type 1 error rate, examination of associations between temporal measures and symptoms were limited to temporal variables with significant group differences. Partial correlations, controlling for alcohol consumption and cannabis use, were employed to examine associations between temporal measures and baseline positive and negative symptoms. To examine relationships between timing variables and worsening of symptoms, we used a hierarchical linear regression approach predicting change in symptoms at 12-month follow-up compared to baseline from timing variables controlling for baseline symptoms, alcohol consumption, and cannabis use. Change scores were computed by subtracting baseline positive and negative symptoms from 12-month follow-up positive and negative symptoms, respectively. Longitudinal analyses were conducted on the 39 CHR and 39 HC participants (N=78) retained in the sample. Two-tailed tests with α=.05 were used for all analyses. Consistent with fcMRI statistical approach, behavioral analyses were examined with and without CHR youth receiving antipsychotics.
Results
There were no group differences in regards to age, education, or parent education (Table 1). There was a significant group difference in the distribution of sex at baseline and a significant correlation between sex and temporal bisection points within the control group. We controlled for sex in all models that included group and temporal bisection point; results remained unchanged, thus we reported results without controlling for sex (see SM for statistics controlling for sex). See Table 2 for descriptive statistics for temporal measures and symptoms. With the exception of symptom change (see below), behavioral and fcMRI findings did not change with the removal of CHR participants receiving antipsychotics.
Table 2.
Descriptive Statistics for Temporal Measures and Symptoms at baseline and 12-month Follow-up
| N | Mean | SD | Skew | |
|---|---|---|---|---|
|
| ||||
| Temporal Bisection | ||||
| CHR/HC | 52/52 | 438.11/427.58 | 25.36/25.60 | 0.14/−0.27 |
| Difference Limen | ||||
| CHR/HC | 52/52 | 42.66/43.30 | 17.48/15.23 | 1.98/.87 |
| Negative Symptoms (baseline) | ||||
| CHR/HC | 52/52 | 10.01/0.42 | 7.02/1.05 | 0.26/3.55 |
| Positive Symptoms (baseline) | ||||
| CHR/HC | 52/52 | 12.08/0.44 | 4.12/1.11 | −0.56/2.52 |
| Negative Symptoms (12-month follow-up) | ||||
| CHR/HC | 39/39 | 8.49/0.54 | 7.48/1.35 | 0.61/3.53 |
| Positive Symptoms (12-month follow-up) | ||||
| CHR/HC | 39/39 | 9.41/0.36 | 5.77/0.67 | 0.20/1.67 |
Note: Clinical high-risk (CHR); Healthy control (HC); Standard deviation (SD); Social Communication Questionnaire (SCQ)
Group Differences in Temporal Bisection
CHR participants had significantly poorer temporal accuracy (i.e., higher bisection points) compared to HC participants, F(1, 100)=6.14, p=.02, ηp2=.06 (Figure 1b). There was not a significant group difference in temporal precision, F(1, 100)=.41, p=.52, ηp2=.004. Accuracy rates (i.e., percent correct) for the anchor tones were comparable between CHR (98%) and HC (98%) participants, F(1, 100)=.14, p=.71, ηp2=.001.
Resting State Functional Connectivity Results
There were no significant group differences in connectivity when using the anterior cerebellum, Crus I, or Lobule X as seed regions. In the anterior cerebellum, there was a significant interaction between group and temporal accuracy in the seed-to-voxel analysis, FDRcorrected p < .05 (Cluster Size: 173; MNI Coordinates [XYZ]: 14, 26, 8; t=5.01; p = .0001) (Figure 2a). Specifically, higher bisection points were associated with increased resting state connectivity between the anterior cerebellum and a right caudate/nucleus accumbens (NAcc) striatal cluster at rest in the CHR group (Figure 2b). Healthy controls showed the opposite effect, wherein higher bisection points were associated with decreased resting state connectivity between the anterior cerebellum and the right caudate/NAcc cluster. There was not a significant interaction between group and temporal accuracy for either Crus I or Lobule X. There were no significant group differences in brain activation or motion outliers (See SM for group comparison statistics).
Figure 2.
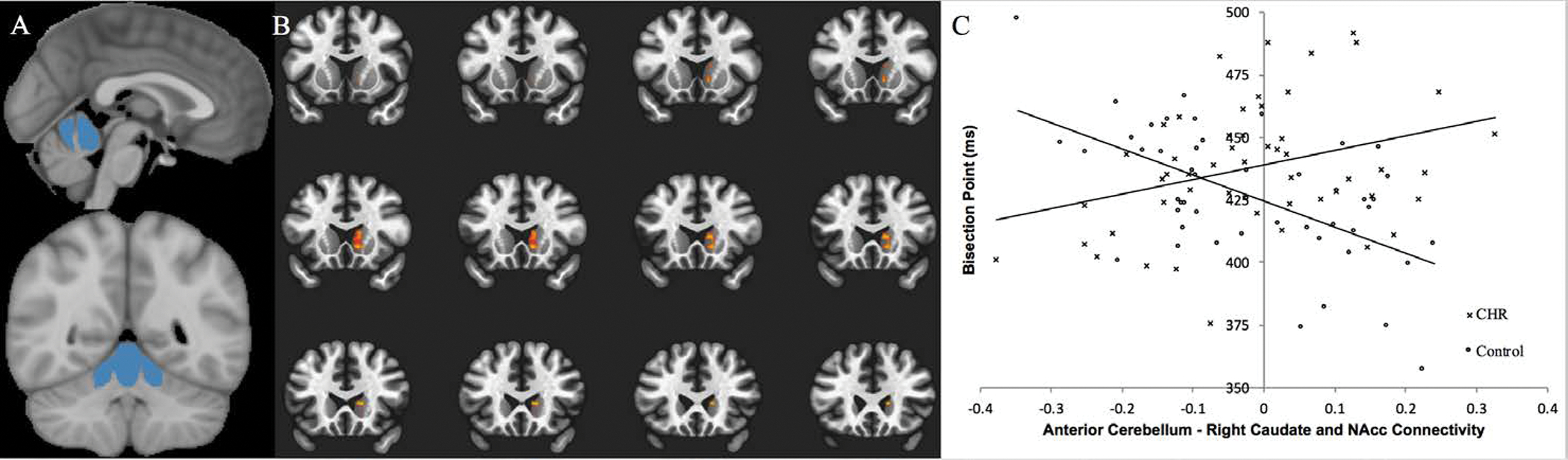
A) Left Panel: Depicts the bilateral anterior cerebellum seed region. Right Panel: Depicts the contiguous striatal cluster in the right caudate and nucleus accumbens (NAcc) which showed a significant group by bisection interaction (FDR corrected p<.05). B) Connectivity between the regions was extracted and plotted by group and bisection score.
Temporal Bisection Point and Worsening of Symptoms
Within the CHR group, temporal accuracy was not significantly associated with either baseline positive (rpartial=.01, p=.92) or baseline negative symptoms (rpartial=.09, p=.53). Hierarchal linear regression was used to examine if baseline temporal accuracy was associated with symptoms at follow-up (Table 3). Higher temporal bisection points (i.e., poorer temporal accuracy) accounted for 9% of the variance in worsening of positive symptoms at 12-month follow-up (β=.31, p=.06). Higher temporal bisection points accounted for 11% of the variance in worsening of negative symptoms at 12-month follow-up (β=.35, p=.03) (Figure 1c). Notably, the models were improved with the removal of CHR youth receiving antipsychotic treatment (N = 5 at follow-up). After removal, higher temporal bisection points accounted for 18% of the variance in worsening of positive symptoms (β=.45, p=.01), and 18% of the variance in worsening of negative symptoms (β=.45, p=.007) at 12-month follow-up.
Table 3.
Hierarchical Linear Regressions Results (Full Sample)
| 12-Month Symptom Change | Block I: Baseline Symptoms, Alcohol Consumption, Cannabis Use | Block II: Baseline Symptoms, Alcohol Consumption, Cannabis Use, Temporal Bisection Point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| R 2 | df | F | βsymptoms | βalcohol | βcannabis | Δ R2 | Δ df | Δ F | βsymptoms | βalcohol | βcannabis | βbisection | |
|
|
|||||||||||||
| Positive symptoms | .30 | 3,35 | 1.19 | −.05 | −.05 | .30 † | .09 † | 1, 34 | 3.77 | −.08 | −.06 | .39 * | .31 † |
| Negative symptoms | .45 * | 3,35 | 2.93 | −.45 ** | −.03 | .03 | .11 * | 1, 34 | 5.43 | −.47 ** | −.04 | .12 | .35 * |
Note:
for p < .01
for p < .05
for p < .08.
Conclusions
To our knowledge, this is the first study to examine temporal dysfunction and its association with cerebellar resting state connectivity and worsening of symptoms in individuals at CHR for psychosis. As predicted, CHR participants were less accurate (i.e., higher bisection points) in their temporal perception when classifying auditory tone durations compared to healthy controls. Broadly, this suggests that, similar to patients with schizophrenia (Thoenes and Oberfeld, 2017), CHR individuals perceive time as shortened compared to objective time. In contrast to our prediction, impairments in temporal precision were not observed in the current study. Additionally, poorer temporal accuracy was associated with increased resting state connectivity between the anterior cerebellum and a right caudate/NAcc striatal cluster in the CHR group. This suggests that timing dysfunction in CHR youth is associated with abnormalities in brain regions involved in an important cerebellar network implicated in prominent etiological models of psychosis (Andreasen et al., 1999, Andreasen and Pierson, 2008, Kendler and Schaffner, 2011). Lastly, poorer temporal accuracy was also associated with worse negative symptom severity at 12-month follow-up, suggesting deficits in temporal processing are sensitive to symptom progression in CHR youth.
Poorer temporal accuracy in the CHR group is consistent with existing literature demonstrating that patients with schizophrenia exhibit deficits in temporal accuracy on auditory bisection tasks (Elvevåg et al., 2003, Thoenes and Oberfeld, 2017). In contrast to studies with psychosis populations, we did not observe impairment in temporal precision in the CHR group. This may suggest that deficits in temporal precision develop later in the course of psychosis or may be a result of long-term antipsychotic use. However, given there is considerable variation in illness trajectories in CHR samples (Addington et al., 2018), lack of an observed deficit in temporal precision in the current study may also reflect sample heterogeneity. Moreover, given that individuals with genetic vulnerability for psychosis (i.e., first-degree relatives) exhibit less temporal precision than healthy controls (Penney et al., 2005), the lack of group differences in the current study may reflect phenotypic differences between high-risk populations. Consistent with this notion, evidence from genetic contributions to timing have shown that polymorphisms known to modulate D2 density in the striatum (DRD2/ANKK1-Taq1a; (Jönsson et al., 1999) are associated with sub- but not supra-second temporal perception (Wiener et al., 2011), which may suggest temporal dysfunction could be an endophenotype for schizophrenia and high-risk populations.
Consistent with predictions, higher bisection points were associated with abnormal anterior cerebello-striatal connectivity in the CHR group. Although we did not make a priori predictions regarding hypo- or hyperconnectivity in regards to cerebellar resting state connectivity, increased cerebello-striatal connectivity in the CHR group is consistent with past work demonstrating hyperconnectivity in cerebello-striatal connectivity in CHR and schizophrenia populations (Cao et al., 2018, Zhuo et al., 2018). Relationships between abnormal cerebellar connectivity and temporal accuracy were not observed for either Crus I or Lobule X seed regions, suggesting there may be regional specificity between distinct cerebellar topographical regions and temporal dysfunction in CHR youth. These findings are generally consistent with the small number of neuroimaging investigations of temporal perception in patients with schizophrenia demonstrating abnormal striatal activity (but not cerebellar) in both supra- and sub-second temporal discrimination tasks (Davalos et al., 2011, Volz et al., 2001). However, these studies either did not include the cerebellum as an ROI in analyses (Volz et al., 2001) or limited cerebellar ROIs to the vermis (Davalos et al., 2011). However, it will be important to replicate these findings in larger CHR and schizophrenia samples.
When considering the present findings from a pacemaker-accumulator model of timing, they may suggest individuals at CHR for psychosis have a slower internal clock, resulting in poorer temporal. This interpretation is further supported by the observed associations between poorer temporal accuracy and abnormal cerebello-striatal connectivity, regions that are recruited in the automatic processing of sub-second temporal stimuli (Lewis and Miall, 2003b). Consistent with this assertion, evidence suggests that the dorsal striatum may serve as either the pacemaker (i.e., pulse emitter) (Coull et al., 2011) and/or accumulator (i.e., encoding) (Harrington et al., 2004, Rao et al., 2001) for the neural representation of temporal information, with the cerebellum contributing a similar or complimentary role when sub-second timing is required (Kunimatsu et al., 2018, Teki et al., 2012). Moreover, imaging studies suggest that the caudate nucleus in particular is critical for perception of temporal durations in both supra- and sub-section stimulus ranges (Harrington et al., 2004, Meck et al., 2008, Pouthas et al., 2005, Rao et al., 2001, Tregellas et al., 2006). An alternative or complimentary explanation is that the observed temporal accuracy deficits may be emblematic of a more pervasive information processing disturbance (Nieman et al., 2013, Thomas et al., 2017) that may interact with or explain putative internal clock slowing. Similarly, auditory processing deficits are evident in both CHR populations and schizophrenia patients (Corcoran et al., 2015, Javitt and Sweet, 2015, Mathalon et al., 2018, Perez et al., 2014, Turetsky et al., 2009), and work on the potential effects of auditory processing deficits on auditory temporal accuracy will be an important area of future research.
Interestingly, temporal accuracy was not associated with baseline symptoms, but did predict worsening of negative symptoms across twelve months. When examining these associations without CHR youth receiving antipsychotic medication, temporal accuracy was also associated with worsening of positive symptoms, suggesting that antipsychotic medication may mask underlying effects. Given the demonstrated associations between motor circuitry and negative symptom worsening in CHR youth (Bernard et al., 2014, Dean and Mittal, 2015, Mittal et al., 2013b), and the current evidence for an association between poorer temporal accuracy and abnormal cerebello-striatal connectivity, associations with worsening of negative symptoms is not surprising. Intriguingly, transcranial magnetic stimulation (TMS) stimulation of the cerebellum has been shown to modulate temporal accuracy in healthy controls (Fierro et al., 2007, Grube et al., 2010, Koch et al., 2007, Lee et al., 2007), suggesting cerebellar stimulation may be a viable early intervention for CHR individuals to mitigate illness progression. Indeed, similar stimulation methods have been shown to improve negative symptoms in schizophrenia populations (Brady Jr et al., 2019, Demirtas-Tatlidede et al., 2010).
There are several limitations to the current study. First, the current findings are correlational in nature and cannot provide conclusive evidence that similar regions would be activated during task-based fMRI, requiring further task-based work to replicate the current findings. Similarly, methods for identifying the functional boundaries of the cerebellum are rapidly advancing (see Guell et al., 2019, King et al., 2019), and it will be important for future well-powered studies to validate and incorporate newer parcellation approaches in CHR populations. This may further aid in clarifying the specific cerebellar topographical abnormalities associated with timing dysfunction in CHR youth. Second, although the sample for the current study is comparable to other research in CHR and schizophrenia populations, larger samples may reveal interesting subgroups. For example, given the heterogenous presentation characteristic of psychotic disorders, deficits in temporal precision may be present in a yet undetermined subgroup of CHR individuals.
Supplementary Material
Financial Support:
This work was supported by the National Institutes of Health (V.A.M., grant numbers R01MH094650, R21/R33MH103231).
Footnotes
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Addington J, Stowkowy J, Liu L, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ & Tsuang MT (2018). Clinical and functional characteristics of youth at clinical high-risk for psychosis who do not transition to psychosis. Psychological Medicine, 1–8. [DOI] [PubMed] [Google Scholar]
- Allan LG & Gibbon J (1991). Human bisection at the geometric mean. Learning and Motivation 22, 39–58. [Google Scholar]
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T & Flaum M (1999). Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biological Psychiatry 46, 908–920. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S & O’leary DS (1998). “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin 24, 203–218. [DOI] [PubMed] [Google Scholar]
- Andreasen NC & Pierson R (2008). The role of the cerebellum in schizophrenia. Biological Psychiatry 64, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J & Goodyear B (2015). Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry 72, 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM (2014). Cerebellar-thalamic connectivity in schizophrenia. Oxford University Press US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, Leggio M, Mattingley JB, Molinari M & Moulton EA (2015). Consensus paper: the role of the cerebellum in perceptual processes. The Cerebellum 14, 197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Dean DJ, Kent JS, Orr JM, Pelletier-Baldelli A, Lunsford-Avery JR, Gupta T & Mittal VA (2014). Cerebellar networks in individuals at ultra high-risk of psychosis: Impact on postural sway and symptom severity. Human Brain Mapping 35, 4064–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA & Mittal VA (2014). Cerebellar-motor dysfunction in schizophrenia and psychosis-risk: the importance of regional cerebellar analysis approaches. Frontiers in Psychiatry 5, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Orr JM & Mittal VA (2017). Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. NeuroImage: Clinical 14, 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA & Seidler RD (2013). Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. The Cerebellum 12, 721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, Jaeggi SM, Buschkuehl M, Monk CS & Jonides J (2012). Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Frontiers in Neuroanatomy 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolbecker AR, Westfall DR, Howell JM, Lackner RJ, Carroll CA, O’Donnell BF & Hetrick WP (2014). Increased timing variability in schizophrenia and bipolar disorder. PloS One 9, e97964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO Jr, Gonsalvez I, Lee I, Öngür D, Seidman LJ, Schmahmann JD, Eack SM, Keshavan MS, Pascual-Leone A & Halko MA (2019). Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia. American Journal of Psychiatry, appi. ajp. 2018.18040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breska A & Ivry RB (2016). Taxonomies of timing: where does the cerebellum fit in? Current Opinion in Behavioral Sciences 8, 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV & Meck WH (2005). What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience 6, 755. [DOI] [PubMed] [Google Scholar]
- Cao H, Chén OY, Chung Y, Forsyth JK, McEwen SC, Gee DG, Bearden CE, Addington J, Goodyear B & Cadenhead KS (2018). Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nature Communications 9, 3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CA, Boggs J, O’Donnell BF, Shekhar A & Hetrick WP (2008). Temporal processing dysfunction in schizophrenia. Brain and Cognition 67, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CA, O’donnell BF, Shekhar A & Hetrick WP (2009a). Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain and Cognition 71, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CA, O’Donnell BF, Shekhar A & Hetrick WP (2009b). Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain and Cognition 70, 181–190. [DOI] [PubMed] [Google Scholar]
- Casini L & Ivry RB (1999). Effects of divided attention on temporal processing in patients with lesions of the cerebellum or frontal lobe. Neuropsychology 13, 10. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Castanón AN, McCarthy JM, Cohen BM & Öngür D (2011). Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology 36, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley JR & Friston KJ (2009). False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage 44, 62–70. [DOI] [PubMed] [Google Scholar]
- Church RM & Deluty MZ (1977). Bisection of temporal intervals. Journal of Experimental Psychology: Animal Behavior Processes 3, 216. [DOI] [PubMed] [Google Scholar]
- Ciullo V, Spalletta G, Caltagirone C, Jorge RE & Piras F (2015). Explicit time deficit in schizophrenia: systematic review and meta-analysis indicate it is primary and not domain specific. Schizophrenia Bulletin 42, 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Keilp J, Kayser J, Klim C, Butler P, Bruder G, Gur R & Javitt D (2015). Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychological Medicine 45, 2959–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Cheng R-K & Meck WH (2011). Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 36, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandash O, Fornito A, Lee J, Keefe RS, Chee MW, Adcock RA, Pantelis C, Wood SJ & Harrison BJ (2013). Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophrenia Bulletin 40, 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos DB, Rojas DC & Tregellas JR (2011). Temporal processing in schizophrenia: effects of task-difficulty on behavioral discrimination and neuronal responses. Schizophrenia Research 127, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Bernard JA, Orr JM, Pelletier-Baldelli A, Gupta T, Carol EE & Mittal VA (2014). Cerebellar morphology and procedural learning impairment in neuroleptic-naive youth at ultrahigh risk of psychosis. Clinical Psychological Science 2, 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ & Mittal VA (2015). Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. NPJ schizophrenia 1, 14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, Seidman LJ, Schmahmann JD & Pascual-Leone A (2010). Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophrenia Research 124, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh A, Rosenbloom MJ, Pfefferbaum A & Sullivan EV (2002). Clinical signs of cerebellar dysfunction in schizophrenia, alcoholism, and their comorbidity. Schizophrenia Research 57, 281–291. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J (2006). A spatially unbiased atlas template of the human cerebellum. Neuroimage 33, 127–138. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E & Ramnani N (2009). A probabilistic MR atlas of the human cerebellum. Neuroimage 46, 39–46. [DOI] [PubMed] [Google Scholar]
- Drake R, Mueser K & McHugo G (1996). Clinician rating scales: alcohol use scale (AUS), drug use scale (DUS), and substance abuse treatment scale (SATS). Outcomes assessment in clinical practice, 113–116. [Google Scholar]
- Elvevåg B, McCormack T, Gilbert A, Brown G, Weinberger D & Goldberg T (2003). Duration judgements in patients with schizophrenia. Psychological Medicine 33, 1249–1261. [DOI] [PubMed] [Google Scholar]
- Fierro B, Palermo A, Puma A, Francolini M, Panetta M, Daniele O & Brighina F (2007). Role of the cerebellum in time perception: a TMS study in normal subjects. Journal of the Neurological Sciences 263, 107–112. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M & Williams JB (1995). Structured clinical interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute. [Google Scholar]
- Freedman BJ (1974). The subjective experience of perceptual and cognitive disturbances in schizophrenia: A review of autobiographical accounts. Archives of General Psychiatry 30, 333–340. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Church RM & Meck WH (1984). Scalar timing in memory. Annals of the New York Academy of Sciences 423, 52–77. [DOI] [PubMed] [Google Scholar]
- Greve DN & Fischl B (2009). Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M, Lee K-H, Griffiths TD, Barker AT & Woodruff PW (2010). Transcranial magnetic theta-burst stimulation of the human cerebellum distinguishes absolute, duration-based from relative, beat-based perception of subsecond time intervals. Frontiers in Psychology 1, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X, Schmahmann JD, Gabrieli JD & Ghosh SS (2018). Functional gradients of the cerebellum. Elife 7, e36652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T, Silverstein SM, Bernard JA, Keane BP, Papathomas TV, Pelletier-Baldelli A, Dean DJ, Newberry RE, Ristanovic I & Mittal VA (2016). Disruptions in neural connectivity associated with reduced susceptibility to a depth inversion illusion in youth at ultra high risk for psychosis. NeuroImage: Clinical 12, 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M & Rao SM (2004). Neural representation of interval encoding and decision making. Cognitive Brain Research 21, 193–205. [DOI] [PubMed] [Google Scholar]
- Hicks RE, Gualtieri T, Mayo JP Jr & Perez-Reyes M (1984). Cannabis, atropine, and temporal information processing. Neuropsychobiology 12, 229–237. [DOI] [PubMed] [Google Scholar]
- Ivry RB & Keele SW (1989). Timing functions of the cerebellum. Journal of Cognitive Neuroscience 1, 136–152. [DOI] [PubMed] [Google Scholar]
- Javitt DC & Sweet RA (2015). Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nature Reviews Neuroscience 16, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis 5, 143–156. [DOI] [PubMed] [Google Scholar]
- Jönsson E, Nöthen M, Grünhage F, Farde L, Nakashima Y, Propping P & Sedvall G (1999). Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry 4, 290. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Rijntjes M, Weiller C, Faiss J, Timmann D, Mueller S & Diener H (1995). Localization of a cerebellar timing process using PET. Neurology 45, 1540–1545. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Okuda J, Umetsu A, Sugiura M, Inoue K, Suzuki K, Tabuchi M, Tsukiura T, Narayan SL & Nagasaka T (2000). Human cerebellum plays an important role in memory-timed finger movement: an fMRI study. Journal of Neurophysiology 83, 1079–1087. [DOI] [PubMed] [Google Scholar]
- Kendler KS & Schaffner KF (2011). The dopamine hypothesis of schizophrenia: an historical and philosophical analysis. Philosophy, Psychiatry, & Psychology 18, 41–63. [Google Scholar]
- King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB & Diedrichsen J (2019). Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nature Neuroscience 22, 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Salerno S, Gerfo EL & Caltagirone C (2007). Repetitive TMS of cerebellum interferes with millisecond time processing. Experimental Brain Research 179, 291–299. [DOI] [PubMed] [Google Scholar]
- Kopec CD & Brody CD (2010). Human performance on the temporal bisection task. Brain and Cognition 74, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimatsu J, Suzuki TW, Ohmae S & Tanaka M (2018). Different contributions of preparatory activity in the basal ganglia and cerebellum for self-timing. Elife 7, e35676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K-H, Dixon JK, Spence SA & Woodruff PW (2006). Time perception dysfunction in psychometric schizotypy. Personality and Individual Differences 40, 1363–1373. [Google Scholar]
- Lee K-H, Egleston PN, Brown WH, Gregory AN, Barker AT & Woodruff PW (2007). The role of the cerebellum in subsecond time perception: evidence from repetitive transcranial magnetic stimulation. Journal of Cognitive Neuroscience 19, 147–157. [DOI] [PubMed] [Google Scholar]
- Lewis PA & Miall RC (2003a). Brain activation patterns during measurement of sub-and supra-second intervals. Neuropsychologia 41, 1583–1592. [DOI] [PubMed] [Google Scholar]
- Lewis PA & Miall RC (2003b). Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Current Opinion in Neurobiology 13, 250–255. [DOI] [PubMed] [Google Scholar]
- Lieving LM, Lane SD, Cherek DR & Tcheremissine OV (2006). Effects of marijuana on temporal discriminations in humans. Behavioural Pharmacology 17, 173–183. [DOI] [PubMed] [Google Scholar]
- Lutz K, Specht K, Shah NJ & JaÈncke L (2000). Tapping movements according to regular and irregular visual timing signals investigated with fMRI. Neuroreport 11, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Roach BJ, Ferri JM, Loewy RL, Stuart BK, Perez VB, Trujillo TH & Ford JM (2018). Deficient auditory predictive coding during vocalization in the psychosis risk syndrome and in early illness schizophrenia: the final expanded sample. Psychological Medicine, 1–8. [DOI] [PubMed] [Google Scholar]
- McGlashan T, Walsh B & Woods S (2010). The psychosis-risk syndrome: handbook for diagnosis and follow-up. Oxford University Press. [Google Scholar]
- Meck WH (2005). Neuropsychology of timing and time perception. Brain and Cognition 58, 1–8. [DOI] [PubMed] [Google Scholar]
- Meck WH, Penney TB & Pouthas V (2008). Cortico-striatal representation of time in animals and humans. Current Opinion in Neurobiology 18, 145–152. [DOI] [PubMed] [Google Scholar]
- Merchant H, Harrington DL & Meck WH (2013). Neural basis of the perception and estimation of time. Annual Review of Neuroscience 36, 313–336. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R & Davidson L (1999). Symptom assessment in schizophrenic prodromal states. Psychiatric Quarterly 70, 273–287. [DOI] [PubMed] [Google Scholar]
- Minkowski E (1927). La schizophrénie. Psychopathologie des schizoïdes et des schizophrénes.
- Mittal VA, Dean DJ, Bernard JA, Orr JM, Pelletier-Baldelli A, Carol EE, Gupta T, Turner J, Leopold DR & Robustelli BL (2013a). Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophrenia Bulletin 40, 1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Bernard JA, Orr JM, Pelletier-Baldelli A, Carol EE, Gupta T, Turner J, Leopold DR & Robustelli BL (2013b). Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophrenia Bulletin, sbt199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Neumann C, Saczawa M & Walker EF (2008). Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Archives of General Psychiatry 65, 165–171. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB & Bandettini PA (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman DH, Ruhrmann S, Dragt S, Soen F, van Tricht MJ, Koelman JH, Bour LJ, Velthorst E, Becker HE & Weiser M (2013). Psychosis prediction: stratification of risk estimation with information-processing and premorbid functioning variables. Schizophrenia Bulletin 40, 1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda N, Ortuno F, Arbizu J, Lopez P, Martí-Climent JM, Penuelas I & Cervera-Enguix S (2002). Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Human Brain Mapping 17, 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuño F, Guillén-Grima F, López-García P, Gómez J & Pla J (2011). Functional neural networks of time perception: challenge and opportunity for schizophrenia research. Schizophrenia Research 125, 129–135. [DOI] [PubMed] [Google Scholar]
- Parker KL, Narayanan NS & Andreasen NC (2014). The therapeutic potential of the cerebellum in schizophrenia. Frontiers in Systems Neuroscience 8, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier-Baldelli A, Andrews-Hanna JR & Mittal VA (2018). Resting state connectivity dynamics in individuals at risk for psychosis. Journal of Abnormal Psychology 127, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney TB, Meck WH, Roberts SA, Gibbon J & Erlenmeyer-Kimling L (2005). Interval-timing deficits in individuals at high risk for schizophrenia. Brain and Cognition 58, 109–118. [DOI] [PubMed] [Google Scholar]
- Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH & Mathalon DH (2014). Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biological Psychiatry 75, 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouthas V, George N, Poline JB, Pfeuty M, VandeMoorteele PF, Hugueville L, Ferrandez AM, Lehéricy S, LeBihan D & Renault B (2005). Neural network involved in time perception: an fMRI study comparing long and short interval estimation. Human Brain Mapping 25, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Mayer AR & Harrington DL (2001). The evolution of brain activation during temporal processing. Nature Neuroscience 4, 317. [DOI] [PubMed] [Google Scholar]
- Reed P & Randell J (2014). Altered time-perception performance in individuals with high schizotypy levels. Psychiatry Research 220, 211–216. [DOI] [PubMed] [Google Scholar]
- Salman MS (2002). Topical review: the cerebellum: it’s about time! but timing is not everything-new insights into the role of the cerebellum in timing motor and cognitive tasks. Journal of Child Neurology 17, 1–9. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD (2018). The cerebellum and cognition. Neuroscience Letters. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD & Pandya DN (2008). Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex 44, 1037–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Yücel M, Respondek C, Whittle S, Lindsay E, Pantelis C & Lubman D (2011). Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychological Medicine 41, 2349–2359. [DOI] [PubMed] [Google Scholar]
- Stanghellini G, Ballerini M, Presenza S, Mancini M, Raballo A, Blasi S & Cutting J (2015). Psychopathology of lived time: abnormal time experience in persons with schizophrenia. Schizophrenia Bulletin 42, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ (2012). The cerebellum and cognition: evidence from functional imaging studies. The Cerebellum 11, 352–365. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM & Schmahmann JD (2012). Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59, 1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teki S, Grube M & Griffiths TD (2012). A unified model of time perception accounts for duration-based and beat-based timing mechanisms. Frontiers in Integrative Neuroscience 5, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenes S & Oberfeld D (2017). Meta-analysis of time perception and temporal processing in schizophrenia: Differential effects on precision and accuracy. Clinical Psychology Review 54, 44–64. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Green MF, Hellemann G, Sugar CA, Tarasenko M, Calkins ME, Greenwood TA, Gur RE, Gur RC & Lazzeroni LC (2017). Modeling deficits from early auditory information processing to psychosocial functioning in schizophrenia. JAMA Psychiatry 74, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinklenberg JR, Roth WT & Kopell BS (1976). Marijuana and ethanol: differential effects on time perception, heart rate, and subjective response. Psychopharmacology 49, 275–279. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB & Rojas DC (2006). Effect of task difficulty on the functional anatomy of temporal processing. Neuroimage 32, 307–315. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG & Gur RE (2009). Profile of auditory information-processing deficits in schizophrenia. Psychiatry Research 165, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz H-P, Nenadic I, Gaser C, Rammsayer T, Häger F & Sauer H (2001). Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport 12, 313–316. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S & Ford JM (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology 8, 49–76. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S & Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Wiener M, Lohoff FW & Coslett HB (2011). Double dissociation of dopamine genes and timing in humans. Journal of Cognitive Neuroscience 23, 2811–2821. [DOI] [PubMed] [Google Scholar]
- Zhuo C, Wang C, Wang L, Guo X, Xu Q, Liu Y & Zhu J (2018). Altered resting-state functional connectivity of the cerebellum in schizophrenia. Brain Imaging and Behavior 12, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


