Abstract
The effects of spaceflight on the infectious disease process have only been studied at the level of the host immune response and indicate a blunting of the immune mechanism in humans and animals. Accordingly, it is necessary to assess potential changes in microbial virulence associated with spaceflight which may impact the probability of in-flight infectious disease. In this study, we investigated the effect of altered gravitational vectors on Salmonella virulence in mice. Salmonella enterica serovar Typhimurium grown under modeled microgravity (MMG) were more virulent and were recovered in higher numbers from the murine spleen and liver following oral infection compared to organisms grown under normal gravity. Furthermore, MMG-grown salmonellae were more resistant to acid stress and macrophage killing and exhibited significant differences in protein synthesis than did normal-gravity-grown cells. Our results indicate that the environment created by simulated microgravity represents a novel environmental regulatory factor of Salmonella virulence.
Environmental signals regulate the expression of virulence determinants in pathogenic bacteria. Specifically, in Salmonella spp. osmolarity, starvation, stress, pH, and growth phase have all been shown to affect the expression of numerous virulence parameters of this organism (7, 21). Among Salmonella serotypes, Salmonella enterica serovar Typhimurium is among the leading causes of human disease according to the database of The National Center for Infectious Diseases, and it has therefore been extensively studied. The most commonly recognized clinical syndrome caused by serovar Typhimurium is gastroenteritis (15); however, this organism also has the potential to cause systemic disease in humans, particularly in immunocompromised individuals (39). In mice, serovar Typhimurium causes a lethal systemic infection that is similar to human typhoid fever, and thus is used as a model for studying systemic Salmonella infections (20). Results presented in this study indicate that altered gravitational forces and/or low shear conditions should be added to the list of environmental signals implicated in the regulation of virulence attributes in serovar Typhimurium.
The effect of spaceflight on the infectious disease process has only been investigated at the level of host susceptibility. Numerous studies have suggested that spaceflight results in a blunting of the immune system in both humans and animals (25, 28, 35). These results suggest an increased risk of infectious disease events occurring during spaceflight. While it is clear that the susceptibility of the host is important in the ability to resist infection, of equal importance are the virulence attributes of the pathogen. Assessment of the ability of microgravity to elicit changes in bacterial virulence is essential in determining microbial risks and options for reducing those risks to crew members during space flight missions.
Several effects on microorganisms during spaceflight have been reported, including changes in bacterial growth and antibiotic resistance (24, 36); however, no studies have been published regarding the effect of spaceflight on bacterial virulence. As the duration and frequency of space missions increase, the potential of infectious diseases occurring in-flight becomes a critical issue. This creates an urgent need to investigate the potential change in bacterial virulence caused by prolonged conditions of microgravity.
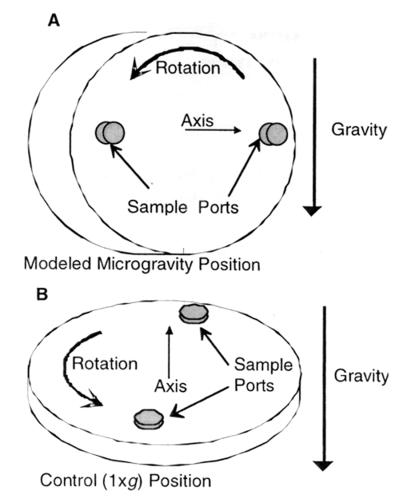
The task of investigating the effect of microgravity on cellular reactions has been enhanced by the use of the high-aspect-ratio vessel (HARV; Synthecon, Inc., Houston, Tex.), a rotating bioreactor designed at the Johnson Space Center, (Houston, Tex.) (32). The HARV bioreactor produces an environmental condition in which the gravitational vectors are randomized over the surface of the cells, resulting in an overall-time-averaged gravitational vector of 10−2 × g (37). This reduction in gravity creates a sustained low-shear environment for cell growth and is intended to model in the laboratory the effects of weightlessness or microgravity on cells (11, 33). Figure 1 shows how the HARV bioreactors are oriented to grow cells under conditions of modeled microgravity (Fig. 1A) or normal gravity (1 × g) (Fig. 1B). In this study, we used the HARV bioreactor to examine the effects of modeled microgravity on the pathogenicity of, and protein synthesis in, the enteric pathogen S. enterica serovar Typhimurium.
FIG. 1.
High-aspect-ratio rotating-wall vessel bioreactor (HARV). A HARV bioreactor in the MMG orientation (A) and in the normal gravity “control” position (B) is shown. When completely filled with liquid so that gas bubbles cannot cause turbulence, a HARV, with its axis of rotation perpendicular to gravity, simulates microgravity by nullifying the downward gravity vector. When the HARV is placed in a vertical “control” position (axis of rotation parallel to gravity vector), the gravity vector is no longer nullified (1).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All studies were performed using wild-type serovar Typhimurium χ3339 (12), which is an animal-passaged isolate of the virulent SL1344 wild-type (14). Bacterial cells were first grown in Lennox broth (17) (L broth) as static overnight cultures at 37°C. Cultures were then inoculated at a dilution of 1:200 into 50 ml of L broth and subsequently introduced into the HARV. Care was taken to ensure that the reactor was completely filled with culture medium (zero headspace). The reactor vessel was oriented to grow cells under conditions of modeled microgravity (Fig. 1A) or normal gravity (Fig. 1B). All incubations in the HARV (i.e., MMG and normal gravity) were done at 37°C with a rotation rate of 25 rpm. Cell density was measured as viable counts plated on L agar for CFU per milliliter. Both MMG and normal-gravity-grown salmonellae exhibited very similar growth profiles (data not shown). All studies were performed using salmonellae cultured in the bioreactors for 10 h as described above, since this time period corresponded to mid-log phase growth.
Mouse virulence assays.
Virulence in 8-week-old female BALB/c mice (Charles River Laboratories, Wilmington, Mass.) was determined by the oral administration of serial dilutions of MMG or normal-gravity-grown χ3339. Bacteria were grown as described above and were harvested as described previously (26). Animal inoculations for the determination of the 50% lethal dose (LD50) values were performed as described previously (26). The data represent an average of three trials, with five mice per dose. The viability was evaluated for 10 days for LD50 studies and 30 days for time-to-death studies. The median lethal dose was determined by the method of Reed and Muench (30).
Enumeration of bacteria in mouse tissues.
The effect of modeled microgravity on the tissue distribution of serovar Typhimurium strain χ3339 in mice was assessed in vivo by peroral inoculation into 8-week-old female BALB/c mice. Bacteria were grown as described above and were harvested as described previously (26). Quantitation of viable serovar Typhimurium in tissues and organs was performed as described previously (26) from two groups of five mice each in two independent trials.
Intracellular survival assays.
To examine the effect of MMG on the intracellular survival of serovar Typhimurium χ3339 in J774 cells (29), an in vitro intracellular survival assay was performed as described previously (26).
Acid stress survival assay.
Salmonellae grown under MMG or normal gravity were evaluated for their ability to survive acid stress. To determine sensitivity to acid, salmonellae were grown as described above and then subjected to acidic conditions by adding a citrate buffer adjusted to pH 3.5. Cells were incubated statically immediately upon induction of acid stress. Samples were removed immediately (t0) and at timed intervals. At each time point, cells were diluted in buffered saline and plated on L agar to determine the CFU per milliliter.
Two-dimensional analysis of serovar Typhimurium protein patterns.
Two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of cellular proteins was performed, in duplicate, using a modified version of the O'Farrell technique (27) as described previously by Burns-Keliher et al. (2). Serovar Typhimurium χ3339 was grown under MMG or normal gravity and labeled with Trans35S Label (150 μCi/ml) (ICN Radiochemicals, Irvine, Calif.) at 8.5 h after inoculation into the bioreactors. Labeling continued for 2.5 h. At 30-min intervals following the addition of label, the bacterial samples were harvested and prepared for protein isolation as described previously (2). Gels were loaded with 535,000 or 557,000 dpm of preparations obtained from serovar Typhimurium cultured for 10 h under MMG or normal gravity, respectively. Gels were treated with Amplify (Amersham International, Arlington Heights, Ill.) for 30 min, dried, and exposed to Kodak X-Omat AR X-ray film (Eastman Kodak Co., Rochester, N.Y.) at −70°C for 5 weeks before development. A description of the equipment and software used for image acquisition and analysis of protein patterns has been given previously (2).
RESULTS
Effect of MMG on serovar Typhimurium virulence.
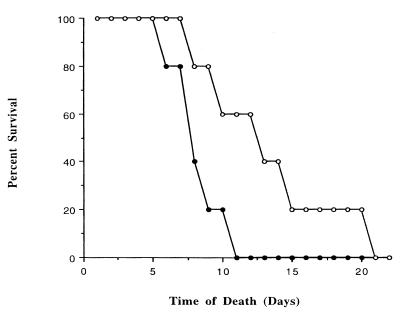
To determine whether altered gravitational vectors play a role in the pathogenesis of serovar Typhimurium, we examined the mouse virulence of this bacterium after growth under MMG or normal gravity. The oral lethal dose required to kill 50% of the animals (i.e., the LD50) (30) for serovar Typhimurium grown under conditions of modeled microgravity was 5.2 times lower than the LD50 for the same strain grown under normal gravity: 4.3 × 106 versus 2.2 × 107 CFU, respectively. In addition, mice inoculated with 106 CFU of MMG-grown cells exhibited a decrease in average time to death compared to mice given similar doses of cells grown under normal gravity (Fig. 2). The results presented in Fig. 2 correspond to representative data from a percent survival assay of mice inoculated perorally with 1.9 × 106 CFU of MMG or normal-gravity-grown cells, respectively. Six days postinfection with MMG-grown χ3339, the survival rate of mice was lower than that of mice infected with normal-gravity-grown cells (Fig. 2). This difference was more pronounced at 10 days postinfection, with 20% of mice infected with MMG-cultured χ3339 surviving at this time point compared to a 60% survival rate of normal-gravity-grown cells (Fig. 2). The difference in virulence between MMG and normal-gravity-grown cells was abrogated when bacterial inoculum titers reached 108 CFU or greater, since no animals survived 10 days postinfection with either MMG or normal-gravity-grown cells (data not shown). The lack of an observed difference in mouse virulence between MMG and normal-gravity-grown serovar Typhimurium at bacterial titers of >108 CFU may be a result of death by overwhelming bacterial growth in the murine reticuloendothelial tissues.
FIG. 2.
Survival of mice after oral infection with serovar Typhimurium χ3339 grown under MMG or normal gravity. Serovar Typhimurium χ3339 grown under MMG (●) or normal gravity (○) was administered perorally as individual infections to 8-week-old female BALB/c mice at inoculum titers of 1.9 × 106 and 1.9 × 106 CFU, respectively. The percent survival is defined as the percentage of mice infected with MMG- or normal-gravity-grown χ3339 organisms surviving at the indicated number of days postinfection.
Tissue distributions of MMG and normal-gravity-grown serovar Typhimurium following oral infection of mice.
Results from animal infectivity experiments indicated that, at bacterial inoculum titers between 106 and 107 CFU, MMG-grown serovar Typhimurium was more effective at causing a systemic infection of mice following oral infection than its normal-gravity-grown counterpart. Therefore, we determined the tissue distribution for MMG or normal-gravity-grown serovar Typhimurium χ3339 in the murine spleen and liver 6 days after oral inoculation, a time at which wild-type salmonellae are capable of conferring a systemic infection in most mice. After oral infection of either MMG or normal-gravity-grown cells at 106 CFU, the MMG-grown χ3339 exhibited an enhanced ability to colonize the murine spleen (27-fold) and liver (12.5-fold) compared to the normal-gravity-grown strain (Table 1). This finding is in agreement with the virulence data, which indicated that, when administered by the oral route at inoculum titers of approximately 106 CFU, χ3339 grown under MMG was significantly more virulent than when grown under normal gravity. Conversely, at 6 days postinfection with 109 CFU, there was no significant difference in the abilities of MMG and normal-gravity-grown cells to colonize murine spleens and livers (data not shown). These results demonstrate that MMG significantly enhanced the virulence of serovar Typhimurium for mice following oral infection at low doses.
TABLE 1.
Tissue distribution of MMG and normal-gravity-grown S. enterica serovar Typhimurium χ3339 in mice after peroral infectiona
| Tissue | No. of bacteria (CFU/g of tissue) ± SEM
|
Fold difference | |
|---|---|---|---|
| MMG-grown χ3339 | 1 × g grown χ3339 | ||
| Spleen | 1.0 × 104 ± 6.9 × 103 | 3.7 × 102 ± 2.1 × 102 | 27.0 |
| Liver | 7.6 × 102 ± 1.1 × 102 | 6.1 × 101 ± 6.1 × 101 | 12.5 |
MMG- and normal-gravity-grown χ3339 organisms (1.9 × 106 and 1.9 × 106 CFU, respectively) were administered perorally as individual infections to 8-week-old BALB/c mice. The spleen and liver were excised 6 days after peroral infection. Five mice were euthanized at each time point. The statistical difference was calculated as the standard error of the mean.
MMG-cultured serovar Typhimurium is more resistant to acid stress than when grown under normal gravity.
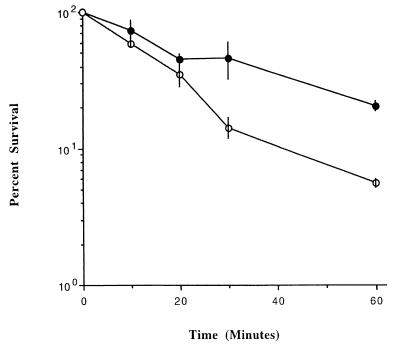
The enhanced virulence of salmonellae cultured under conditions of modeled microgravity may be due, in part, to increased resistance to the acid stress the bacteria encounter within macrophages and during passage through the stomach. To test this hypothesis, we examined the ability of serovar Typhimurium grown under MMG to survive acid stress compared to the same strain grown under normal gravity. We observed an enhanced ability (threefold) of MMG-grown χ3339 to survive acid stress in comparison to normal-gravity-grown χ3339 (Fig. 3). These data suggest that MMG-grown serovar Typhimurium may be better adapted to survive the acid stress conditions encountered during the natural course of a systemic Salmonella infection.
FIG. 3.
Survival of MMG and normal-gravity-grown serovar Typhimurium at pH 3.5. Cells were grown under conditions of modeled microgravity (●) or normal gravity (○) and quantitated as described in Materials and Methods. Results represent averages of two trials. Error bars represent the standard error of the mean.
Survival of MMG and normal-gravity-grown serovar Typhimurium within macrophages.
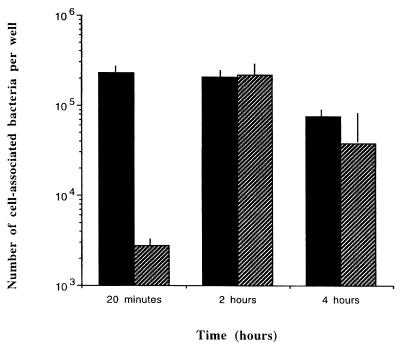
To determine if there was a difference in sensitivity to macrophage killing between MMG and normal-gravity-grown χ3339, we measured the intracellular survival capacities of these strains within the murine macrophage-like cell line J774 (29) (Fig. 4). Representative survival curves presented in Fig. 4 show that the survival rate of MMG-grown cells was significantly higher (81-fold) than those for normal-gravity-grown cells during the first 20 min after infection of J774 monolayers. However, at 2 and 4 h after infection of the J774 monolayers, there was no significant difference in intracellular survival between MMG and normal-gravity-grown serovar Typhimurium. This indicates that, in the absence of MMG, the enhanced survival of serovar Typhimurium within J774 cells decreases over time. This may reflect an adaptation to the intracellular environment by bacteria cultured under each tested condition, with gradually decreased differences in survival levels observed at later time points.
FIG. 4.
Survival of MMG- and normal-gravity-cultured serovar Typhimurium χ3339 within J774 macrophage-like cells. A total of 2 × 105 J774 cells were infected with MMG-grown (solid bars) (2.3 × 105) or normal-gravity-grown (hatched bars) (7.0 × 105) χ3339 at a multiplicity of infection of between 10 and 30. The experimental protocol was performed as described previously (26). Bacteria were recovered at the time points indicated and then quantitated by plating for CFU on L agar medium. Data are expressed as an average of three wells plus the standard deviation.
To further delineate the physiological basis for the difference in mouse virulence observed between MMG and normal-gravity-cultured serovar Typhimurium, we examined the ability of MMG-grown χ3339 to adhere to and invade tissue culture cells relevant to those it would encounter during the normal course of a systemic infection (using a human intestinal epithelial cell line [Int-407] and a human colon cell line [CaCo-2]). MMG-grown serovar Typhimurium produced flagella and exhibited similar adherence and invasion profiles into tissue culture cells compared to cultures grown under normal gravity (data not shown). Based on these data, the enhanced virulence of MMG-cultured χ3339 may not be attributable to an enhanced ability to colonize and penetrate epithelial cells of the murine gastrointestinal tract.
Analysis of serovar Typhimurium proteins synthesized in response to MMG.
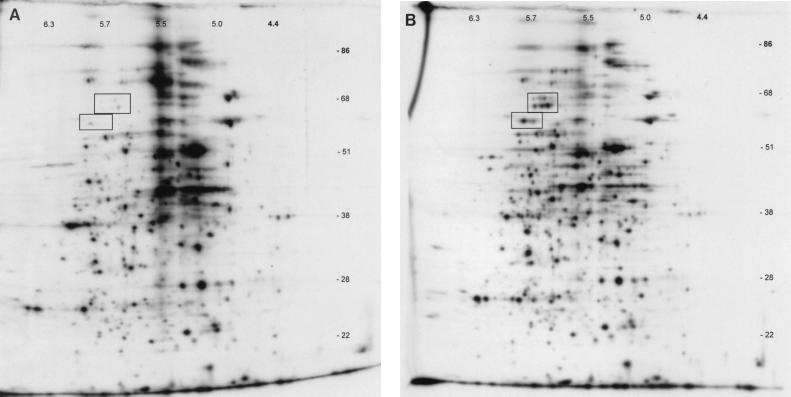
In an effort to address the extent to which MMG affects protein synthesis in serovar Typhimurium, we used two-dimensional gel electrophoresis to examine total protein synthesis during growth of χ3339 under conditions of MMG or normal gravity. Studies of the proteins synthesized by serovar Typhimurium in response to MMG revealed significant differences compared to the pattern of proteins synthesized in response to normal gravity. This analysis revealed that there were 38 proteins downregulated threefold or more during growth in MMG compared to the growth in normal gravity. Among them was a group of proteins which were downregulated ≥10-fold, and these have been highlighted in Fig. 5.
FIG. 5.
Two-dimensional SDS-PAGE and autofluorography of χ3339 whole-cell proteins synthesized in the presence of Trans35S Label. (A) Whole-cell proteins synthesized by χ3339 during growth under MMG. (B) Whole-cell proteins synthesized by χ3339 during growth under normal gravity. Rectangles indicate the locations of proteins which are missing or reduced during growth under MMG compared to under normal gravity.
DISCUSSION
The success of a microbe during pathogenesis relies upon its ability to sense and respond to a myriad of environments during infection of the host. Salmonella spp. are a prime example of this concept, as during their pathogenic lifestyle these organisms must respond to a wide variety of host environmental stresses, including nutrient limitation, oxygen limitation, acidic pH, elevated temperature, and toxic oxidative products (7, 21). Indeed, S. enterica serovar Typhimurium carefully regulates the expression of its virulence genes in response to the diverse environments encountered during the infection process through the activation and/or repression of groups of genes, each designed to confer a selective advantage under the specific environmental constraints (7, 21). We evaluated here the effect of MMG on the virulence of serovar Typhimurium following oral infection in mice. Our results indicate that serovar Typhimurium cultured under environmental conditions of MMG, compared to conditions of normal gravity, exhibited enhanced virulence in mice following oral infection. The recovery of increased numbers of MMG-grown serovar Typhimurium from the murine liver and spleen following oral infection with low doses of bacteria supports this hypothesis. We should note that there is a difference in the overall LD50 between bacteria cultured in L broth in the bioreactors and those cultured in L broth as standard aerated flasks. Specifically, the LD50 for serovar Typhimurium χ3339 is higher for the bacteria cultured in the HARV (both under MMG and normal gravity) compared to those cultured in standard shake flasks (3, 9, 12, 23). This may be the result of a difference in aeration and/or motion between the bacteria cultured in the HARV and those grown in a shake flask (4, 16). However, to our knowledge, this study provides the first direct evidence of a role for MMG and/or low-shear stress in microbial virulence.
The underlying physiological mechanism(s) for the enhanced virulence of serovar Typhimurium observed during growth under MMG are not known. However, the difference in sensitivity to acid pH between MMG-cultured salmonellae and that of the same strain grown under normal gravity suggests that increased resistance to the acidic conditions encountered by salmonellae during its natural course of infection may account, in part, for the increased virulence observed for the MMG-cultured bacteria. In particular, macrophage survival comparisons between MMG and normal-gravity-grown serovar Typhimurium suggest that MMG-cultured cells may be better able to withstand the antimicrobial defenses of host macrophages during the infection process. In addition, comparative analysis of the proteins synthesized by serovar Typhimurium during growth under MMG revealed significant differences in protein profiles compared to growth under normal gravity, suggesting that MMG and/or low shear are novel regulators of gene expression in serovar Typhimurium. The major difference in protein synthesis was shown to be a downregulation of groups of proteins during growth under MMG. This would seem to indicate that it is the absence, or decreased synthesis of, particular proteins which is contributing to the effects seen in MMG. It has been suggested previously that the absence of particular genes may contribute to bacterial pathogenicity (22). Our observation of the downregulation of proteins in response to MMG would appear to be in agreement with this finding.
The HARV bioreactor is designed to provide both a low-shear environment and a randomized gravitational vector over the surface of the cell (37). This type of rotating cylindrical culture vessel has had significant success in developing high-fidelity tissue assemblies for clinical research, and it has also been used for investigations into the growth, regulatory, and differentiation processes within normal and tumorigenic tissues (8, 10, 37). Reduced shear stress has been shown to be a critical component in the ability of mammalian tissues to differentiate into three-dimensional structures possessing many aspects of differentiated cells observed both in vivo and in organ models (11). Previous studies analyzing the growth of bacterial, viral, plant, and mammalian cells in modeled microgravity have indicated numerous changes in gene expression and physiology (5, 6, 10, 11, 13, 18, 19, 34). Recently, the use of microarray chip technology has been used to show that microgravity affects the expression of numerous genes in human kidney cells (13). Accordingly, results presented in this report indicate that the low-shear environment of modeled microgravity represents a novel environmental regulatory factor of Salmonella virulence. Alternatively, it is also possible that salmonellae are capable of detecting and responding to changes in gravity and/or shear via global regulators which respond to other environmental factors. It is tempting to speculate that a low-shear environment encountered in the host during the infection process may offer a partial explanation as to why bacterial infections initiated via human-to-human transmission often progress more rapidly than infections initiated from nonhuman sources (31). Presumably, this increased virulence is attributable to the physiological state of the bacteria, which have adapted to the diverse environmental niches encountered in the animal host and are thus “programmed” to produce the necessary virulence factors required to cause disease.
The virulence of bacterial cultures at low titers becomes critically important in individuals who are immunocompromised, as would appear to be the case during space flight (25, 35). The potential interaction of the crew with pathogenic bacteria in a self-contained environment is increased with the addition of proposed regenerative life support systems, including waste remediation (38). As the duration of the mission increases, enteric bacteria such as serovar Typhimurium will inevitably compose a large segment of the bacterial consortia in certain systems. The commencement of long-term missions that will use regenerative systems, such as the International Space Station, creates an urgent need to investigate potential changes in bacterial pathogenicity caused by prolonged conditions of microgravity. This becomes a significant issue to address, especially if, as appears to be the case, host defenses deteriorate during spaceflight (25, 35).
In conclusion, it will be important to determine whether changes in gravity and/or shear modulate virulence only in salmonellae or for a wide variety of microbial pathogens. The results of comparative studies will be instrumental in the determination of the mechanism(s) regulating enhanced virulence under conditions of modeled microgravity. We anticipate that this research, which is the first of its kind to examine the effect of modeled microgravity on microbial pathogenicity, will ultimately provide significant insights into the molecular basis of Salmonella virulence. As our knowledge of Salmonella virulence and the ability of this organism to survive in diverse environments increases, it can be anticipated that the means by which Salmonella infection can be controlled by the use of vaccines and other countermeasures will lessen the likelihood and therefore, the consequences of, Salmonella infections occurring during spaceflight and on Earth. Studies to identify and characterize MMG-regulated genes in S. enterica serovar Typhimurium are currently in progress in our laboratory. These studies should enhance our understanding of the role of MMG in Salmonella virulence.
ACKNOWLEDGMENTS
We thank Roy Curtiss III for critical review of the manuscript, Michael Schurr and Kent Buchanan for helpful discussions, and Jacqueline Terlonge for assistance with animal experiments.
This work was supported by the National Aeronautics and Space Administration (NASA) subcontract 111-20-30-06, NASA-Ames grant NAG 2-1378, and NIH grant AI-24533.
REFERENCES
- 1.Bouma J E, Pierson D L. Combined effects of simulated microgravity and multi-strain interactions on population dynamics of a constructed microbial community. SAE Technical Paper Series 981605. 28th International Conference on Environmental Systems. 1998. Danvers, Mass. [Google Scholar]
- 2.Burns-Keliher L, Portteus A, Curtiss R. Specific detection of Salmonella typhimurium proteins synthesized intracellularly. J Bacteriol. 1997;179:3604–3612. doi: 10.1128/jb.179.11.3604-3612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coynault C, Robbe-Saule V, Norel F. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in the expression of the RpoS (ςs) regulon. Mol Microbiol. 1996;22:149–160. doi: 10.1111/j.1365-2958.1996.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 4.Ernst R K, Dombrowski D M, Merrick J R. Anaerobiosis, type 1 fimbriae and growth phase are factors that affect invasion of HEp-2 cells by Salmonella typhimurium. Infect Immun. 1990;58:2014–2016. doi: 10.1128/iai.58.6.2014-2016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang A, Pierson D L, Mishra S K, Koenig D W, Demain A L. Effect of simulated microgravity and shear stress on microcin B17 production by Escherichia coli and on its excretion into the medium. Appl Environ Microbiol. 1997;63:4090–4092. doi: 10.1128/aem.63.10.4090-4092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang A, Pierson D L, Mishra S K, Koenig D W, Demain A L. Secondary metabolism in simulated microgravity: β-lactam production by Streptomyces clavuligerus. J Ind Microbiol. 1997;18:22–25. doi: 10.1038/sj.jim.2900345. [DOI] [PubMed] [Google Scholar]
- 7.Foster J W, Spector M P. How Salmonella survive against the odds. Annu Rev Microbiol. 1995;49:145–174. doi: 10.1146/annurev.mi.49.100195.001045. [DOI] [PubMed] [Google Scholar]
- 8.Freed L E, Langer R, Martin I, Pellis N R, Vunjak-Novakovic G. Tissue engineering of cartilage in space. Proc Natl Acad Sci USA. 1997;94:13885–13890. doi: 10.1073/pnas.94.25.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galan J E, Curtiss R., III Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb Pathog. 1989;6:433–443. doi: 10.1016/0882-4010(89)90085-5. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin T, Schroeder W F, Wolf D A, Moyer M P. Rotating-wall vessel coculture of small intestine as a prelude to tissue modeling: aspects of simulated microgravity. Proc Soc Exp Biol Med. 1992;202:181–192. doi: 10.3181/00379727-202-43525. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin T J, Prewett T L, Wolf D A, Spaulding G F. Reduced shear stress: a major component in the ability of mammalian tissues to form three-dimensional assemblies in simulated microgravity. J Cell Biochem. 1993;51:301–311. doi: 10.1002/jcb.240510309. [DOI] [PubMed] [Google Scholar]
- 12.Gulig P, Curtiss R. Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987;55:2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond T G, Lewis F C, Goodwin T G, Lennehan R M, Wolf D A, Hire K P, Campbell W C, Benes E, O'Reilly K C, Globus R K, Kaysen J H. Gene expression in space. Nat Med. 1999;4:359. doi: 10.1038/7331. [DOI] [PubMed] [Google Scholar]
- 14.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 15.Hook E W. Salmonella species (including typhoid fever) In: Mandell G L, Douglas R G, Bennette J E, editors. Principals and practice of infectious diseases. New York, N.Y: John Wiley & Sons; 1985. pp. 1258–1268. [Google Scholar]
- 16.Lee C A, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lennox E S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 18.Long J P, Pierson S, Hughes J H. Suppression of Epstein-Barr virus reactivation in lymphoblastoid cells cultured in simulated microgravity. In Vitro Cell Dev Biol Anim. 1999;35:49–54. doi: 10.1007/s11626-999-0043-3. [DOI] [PubMed] [Google Scholar]
- 19.Long J P, Pierson S, Hughes J H. Rhinovirus replication in HeLa cells cultured under conditions of simulated microgravity. Aviat Space Environ Med. 1998;69:851–856. [PubMed] [Google Scholar]
- 20.Mackaness G B, Blanden R V, Collins F M. Host-parasite relations in mouse typhoid. J Exp Med. 1966;124:573–600. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahan M J, Slauch J M, Mekalanos J J. Environmental regulation of virulence gene expression in Escherichia, Salmonella, and Shigella spp. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: ASM Press; 1996. pp. 2803–2816. [Google Scholar]
- 22.Maurelli A T, Fernandez R E, Bloch C A, Rode C K, Fasano A. Black holes and bacterial pathogenicity: a large genomic deletion that enhances the virulence of Shigella spp. and enteroinvasive Escherichia coli. Proc Natl Acad Sci USA. 1998;95:3943–3948. doi: 10.1073/pnas.95.7.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer P N, Wilmes-Riesenberg M R, Stathopoulos C, Curtiss R., III Virulence of a Salmonella typhimurium OmpD mutant. Infect Immun. 1998;66:387–390. doi: 10.1128/iai.66.1.387-390.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra S K, Pierson D L. Encyclopedia of microbiology. Vol. 4. San Diego, Calif: Academic Press, Inc.; 1992. Space flight, effects on microorganisms; pp. 53–60. [Google Scholar]
- 25.Nefedov Y U G, Yeremin A V, Drozdova V I, Skryabin A S, Guseva O A, Mukhina N N. Immunological reactivity and prediction of allergic complications in the crew of the second expedition of Salyut 4. Kosm Biol I Avikosm Med. 1978;12:15–29. [PubMed] [Google Scholar]
- 26.Nickerson C A, Curtiss R., III Role of sigma factor RpoS in initial stages of Salmonella infection. Infect Immun. 1997;65:1814–1823. doi: 10.1128/iai.65.5.1814-1823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Farrell P H. High resolution two-dimensional gel electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 28.Pellis N R, Goodwin T G, Risin D, McIntyre B W, Pizzini R P, Cooper D, Baker T L, Spaulding G F. Changes in gravity inhibit lymphocyte locomotion through type 1 collagen. In Vitro Cell Dev Biol. 1997;33:398–405. doi: 10.1007/s11626-997-0012-7. [DOI] [PubMed] [Google Scholar]
- 29.Ralph P, Nakoinz I. Phagocytosis by a macrophage tumour and its cloned cell line. Nature. 1975;257:393–394. doi: 10.1038/257393a0. [DOI] [PubMed] [Google Scholar]
- 30.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 31.Salyers A A, Whitt D D. Bacterial pathogenesis: a molecular approach. Washington, D.C.: ASM Press; 1994. Yersinia infections; pp. 216–217. [Google Scholar]
- 32.Schwarz, R. P., and D. A. Wolf. January 29, 1991. Rotating bioreactor cell culture apparatus. U.S. patent 4,988,623.
- 33.Schwarz R P, Goodwin T J, Wolf D A. Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J Tissue Culture Methods. 1992;14:51–58. doi: 10.1007/BF01404744. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Linden J C. Shear stress effects on plant cell suspension cultures in a rotating wall vessel bioreactor. J Ind Microbiol Biotechnol. 1999;22:44–47. [Google Scholar]
- 35.Taylor G R. Space microbiology. Annu Rev Microbiol. 1974;28:121–137. doi: 10.1146/annurev.mi.28.100174.001005. [DOI] [PubMed] [Google Scholar]
- 36.Tixador R, Richoilley G, Gassett G, Templier J, Bes J, Moatti N, Lapchine L. Study of minimum inhibitory concentration of antibiotics on bacteria cultivated in vitro in space (Cytos 2 experiment) Aviat Space Environ Med. 1985;56:748–751. [PubMed] [Google Scholar]
- 37.Unsworth B R, Lelkes P I. Growing tissues in microgravity. Nat Med. 1998;4:901–907. doi: 10.1038/nm0898-901. [DOI] [PubMed] [Google Scholar]
- 38.Waligora J M, Powell M R, Sauer R L. Spacecraft life-supported systems. In: Nicogossian A, Huntoon C, Pool S, editors. Space physiology and medicine. Philadelphia, Pa: Lea & Febiger; 1994. pp. 109–127. [Google Scholar]
- 39.Wilkins E G L, Roberts C. Extraintestinal salmonellosis. Epidemiol Infect. 1988;100:361–368. doi: 10.1017/s095026880006711x. [DOI] [PMC free article] [PubMed] [Google Scholar]