Abstract
Objectives
To investigate the impact of the SAR-Cov-2 pandemic and lockdown on individuals with bipolar disorder in comparison to healthy controls.
Methods
A longitudinal study of 560 participants including 147 healthy controls was conducted between April 30 and May 30, 2020 during a state-wide lockdown. Bi-weekly measures included the Coronavirus Impact Scale, the Pittsburg Sleep Quality Index, the Patient Health Questionnaire, 9-item, and the Generalized Anxiety Disorder scale, 7-item. Generalized estimating equations method was used to examine the longitudinal change of the measures within the lockdown and the change from pre-pandemic period to pandemic period.
Results
All participants reported an impact of lockdown. Individuals with bipolar disorder reported greater impact from the stay-at-home orders with disruptions in routines, income/employment, social support and pandemic related stress. While these measures improved over time, healthy controls recovered quicker and with greater magnitude than persons with bipolar disorder. Comparing mood symptom severity measures in mid-March through May 2020 to the same time window in 2015–2019 (pre- verses post-pandemic), there were no significant differences among individuals with bipolar disorder, whereas healthy controls showed a significant, albeit transient, increase in mood symptoms.
Conclusion
Everyone was impacted by the SARs-CoV pandemic; however, those with bipolar disorder experienced more life impacting changes from the stay-at-home orders vs healthy controls. These disruptions improved over time but much more slowly than healthy controls.
Pre- vs post-pandemic comparisons show a modest but significant increase in mood severity in the healthy controls which was not observed in those with bipolar disorder.
Keywords: Bipolar disorder, Covid-19, Lockdown, Pandemic, Mood, Sleep
Significant Outcomes.
Persons with bipolar disorder were more impacted by the Covid-19 pandemic related stay-at-home orders. While distress was experienced in both healthy controls and those with bipolar disorders, healthy controls recovered faster. Comparing the pre-pandemic and pandemic time period, people with bipolar disorder showed a lesser increase in distress than healthy controls, possibly indicative of an ongoing level of higher chronic mood symptomology leading to resiliency among those with bipolar.
Alt-text: Unlabelled box
Limitations.
This study is a self-report survey and may be influenced by respondent biases. It focused on a short time frame of one month within a longer stay at home order. Additional long-term evaluation of these measures will determine the pattern of effects over time. Finally, this study was performed in a single geographic location with a homogenous ethnic and racial population.
Alt-text: Unlabelled box
1. Introduction
The World Health Organization (WHO) declared Covid-19 a global pandemic on March 11, 2020 in recognition of an unfolding massive world-wide crisis.(Bavel et al., 2020) The Governor of the State of Michigan declared an executive order to stay-at-home (SAH) and self-isolate as independent households on March 24, 2020.(State of Michigan, 2008) From an epidemiological and population health perspective, such an executive order was considered an important step in controlling disease by reducing population movement that would decrease the spread of infection.(Moreland et al., 2020) Within 4-weeks after the executive order, physical distancing was shown to slow the spread of SARS-CoV-2, the virus that causes Covid-19, “flattening the curve” of infected individuals and thereby reducing strain on health care systems.(Lee et al., 2020; Matrajt and Leung, 2020) Subsequently, on June 1, 2020, the SAH order was lifted (Executive Order No. 2020–59). However, the mental health related consequences, both direct and indirect, of the SAH are unknown and of considerable concern in those who live with chronic mental health conditions.
Social support is among the primary factors for sustaining health and well-being, and social isolation often results in compromised mental and physical health.(Leigh-Hunt et al., 2017) Lockdowns disrupt social engagements, amplify the perceived risk of Covid-19 disease, decrease access to food or health care, and result in loss of employment or income, with an inherent risk of significant stress. People who live with chronic mental illness frequently experience first-hand limited social support and social isolation.(Leigh-Hunt et al., 2017) Those living with bipolar disorders are often acutely sensitive to factors that disrupt biological and social rhythms(Giglio et al., 2010; Matias C.A. Melo et al., 2017; Matias Carvalho Aguiar Melo et al., 2020), and measures considered necessary to curtail the spread of SARS-CoV-2, such as home confinement, social distancing, lockdowns and quarantine, have the capability to disrupt daily routines such as sleep schedules (Saltzman et al., 2020), inhibit access to health care, and exacerbate many medical conditions.(Lazzerini et al., 2020) There are urgent concerns for mental health during natural disasters, and the inherent instability of mood among those with bipolar disorders makes them highly susceptible to problems.(Esterwood and Saeed, 2020) Further, the subsequent economic volatility and financial insecurity felt by all could perpetuate or amplify mood and anxiety related symptoms.
There have been a number of studies (Anmella et al., 2020; Cullen et al., 2020; González-Sanguino et al., 2020; Melamed et al., 2020; Moore et al., 2020; Rajkumar, 2020; Saltzman et al., 2020; Sher, 2020; Zhu et al., 2020) specifically analyzing the effect of Covid-19 public health measures, like the Michigan SAH order, on people with and without mental health disorders in the United States and globally. Herein, we compared individuals with bipolar disorder and healthy controls during the one-month Michigan SAH mandate. The participants were part of an established longitudinal study of bipolar disorder which allowed us to compare their illness patterns to those prior to the pandemic.(McInnis et al., 2018a) We measured and compared the personal impact of the SAH using on-line self-report assessments of access to vital services, sleep, mood, and anxiety symptoms among those living with bipolar disorder to healthy controls. Given the longitudinal nature of the study we were able to compare pandemic-related symptoms to a pre-pandemic period.
2. Material and methods
2.1. Participants
Participants were enrolled as part of the Prechter Bipolar Longitudinal Cohort (McInnis et al., 2018b), an observational and open cohort study that recruits individuals with bipolar disorder and healthy controls at the University of Michigan. All active participants (n = 898) of the Prechter cohort were invited by email to participate, of which 560 enrolled (62% of the parent Longitudinal Cohort). This current study was approved by the University of Michigan IRB and electronically captured informed consent was obtained. While there is and continues to be incentive payment for their participation in the Longitudinal Cohort study, no additional incentives were offered for participation in the current Covid-19 pandemic study.
2.2. Design
For this pandemic impact study of the 560 participants enrolled, 68% were female and 7% reporting as Hispanic or Latino, with an average age of 49 (min = 20, max = 88) years at enrollment in 2020. These participants were diagnosed with Bipolar I (41%), Bipolar II (14%), Bipolar, NOS (5%), Recurrent Major Depression Disorder (2%), Schizoaffective Bipolar (2%), Non-Affective Disorder (3%) or Other Affective Disorder (4%). Twenty-six percent are healthy controls. Approximately 4% do not yet have diagnosis category confirmed by our practice of two in-house physicians. The sample that responded is reflective of the larger cohort with regard to proportion of sex and diagnosis. All bipolar diagnoses including Bipolar I, Bipolar II, Bipolar, NOS, Schizoaffective Bipolar were collapsed into the category of bipolar disease (BP; n = 345) and compared to the healthy control (HC; n = 147). Participation rates for each survey and time point are found in Table 1 while stratification of participants by diagnosis, sex and age are provided in Table 2 . Supplemental Figure 1 shows the age distribution for all different diagnoses stratified by sex. In this analysis, age was dichotomized to ≥ 60 years old or <60 years old.
Table 1.
Summary statistics for CIS, PHQ-9, GAD-7, and PSQI measures at the three survey time points based on the total 560 participants in the cohort.
| April 30 | May 14 | May 28 | |||
|---|---|---|---|---|---|
| CIS | N = 435 | N = 409 | N = 336 | ||
| Change in routines | Yes | 74.94% | 75.79% | 70.83% | |
| No | 25.06% | 24.21% | 29.17% | ||
| Change in family income/employment | Yes | 21.15% | 18.34% | 15.48% | |
| No | 78.85% | 81.66% | 84.52% | ||
| Change in food access | Yes | 6.44% | 8.07% | 5.65% | |
| No | 93.10% | 91.69% | 94.05% | ||
| NA | 0.46% | 0.24% | 0.30% | ||
| Change in medical health care access | Yes | 31.72% | 33.99% | 29.17% | |
| No | 68.05% | 65.77% | 69.94% | ||
| NA | 0.23% | 0.24% | 0.89% | ||
| Change in mental health treatment access | Yes | 11.72% | 11.98% | 12.50% | |
| No | 87.59% | 87.53% | 86.90% | ||
| NA | 0.69% | 0.49% | 0.60% | ||
| Change in access to social supports | Yes | 42.30% | 39.36% | 36.61% | |
| No | 57.70% | 60.39% | 63.39% | ||
| NA | / | 0.24% | / | ||
| Experiencing pandemic related stress | Yes | 44.14% | 44.25% | 37.80% | |
| No | 55.40% | 55.75% | 61.90% | ||
| NA | 0.46% | / | 0.30% | ||
| Stress and discord in the family | Yes | 9.89% | 8.56% | 10.12% | |
| No | 89.20% | 91.20% | 89.88% | ||
| NA | 0.92% | 0.24% | / | ||
| MOOD | PHQ-9 | N = 415 | N = 289 | N = 263 | |
| Mean=6.53 SD=6.06 | Mean=6.28 SD=6.23 | Mean=5.81 SD=6.18 | |||
| GAD-7 | N = 408 | N = 288 | N = 261 | ||
| Mean = 5.65 SD = 5.60 | Mean = 5.54 SD = 5.73 | Mean = 5.38 SD = 5.64 | |||
| SLEEP | N = 339 | N = 244 | N = 201 | ||
| Time it takes to fall asleep | <= 30 min | 73.45% | 73.77% | 79.60% | |
| >30 min | 26.55% | 26.23% | 20.40% | ||
| Sleep quality | Good | 71.09% | 72.13% | 76.12% | |
| Bad | 28.91% | 27.87% | 23.88% | ||
| Taking sleep medications | No | 58.41% | 56.97% | 62.19% | |
| Yes | 41.59% | 43.03% | 37.81% | ||
| Number of hours of asleep per day | Mean = 7.30 SD = 1.72 | Mean = 7.23 SD = 1.45 | Mean = 7.24 SD = 1.42 | ||
Table 2.
Summary statistics within each of the CIS, PHQ-9, GAD-7, and PSQI surveys based on 147 healthy controls and 345 participants with bipolar disorder (including Bipolar I, Bipolar II, Bipolar NOS, Schizoaffective Bipolar) at the three survey time points.
| April 30 | May 14 | May 28 | |||
|---|---|---|---|---|---|
| CIS | N = 374 | N = 360 | N = 293 | ||
| Diagnosis | BP | 69.25% | 68.89% | 66.89% | |
| HC | 30.75% | 31.11% | 33.11% | ||
| Sex | Male | 31.55% | 32.50% | 34.81% | |
| Female | 68.45% | 67.50% | 65.19% | ||
| Age | <60 | 71.39% | 69.17% | 68.26% | |
| >=60 | 28.61% | 30.83% | 31.74% | ||
| MOOD: PHQ-9 | N = 357 | N = 252 | N = 230 | ||
| Diagnosis | BP | 69.19% | 69.05% | 64.78% | |
| HC | 30.81% | 30.95% | 35.22% | ||
| Sex | Female | 69.19% | 67.06% | 63.91% | |
| Male | 30.81% | 32.94% | 36.09% | ||
| Age | <60 | 71.99% | 67.06% | 67.83% | |
| >=60 | 28.01% | 32.94% | 32.17% | ||
| MOOD: GAD-7 | N = 351 | N = 251 | N = 228 | ||
| Diagnosis | BP | 68.66% | 69.32% | 64.47% | |
| HC | 31.34% | 30.68% | 35.53% | ||
| Sex | Female | 69.23% | 67.33% | 64.04% | |
| Male | 30.77% | 32.67% | 35.96% | ||
| Age | <60 | 72.08% | 67.73% | 67.98% | |
| >=60 | 27.92% | 32.27% | 32.02% | ||
| SLEEP | N = 293 | N = 214 | N = 174 | ||
| Diagnosis | BP | 72.35% | 71.96% | 64.37% | |
| HC | 27.65% | 28.04% | 35.63% | ||
| Sex | Female | 69.28% | 68.22% | 64.94% | |
| Male | 30.72% | 31.78% | 35.06% | ||
| Age | <60 | 70.31% | 66.36% | 66.09% | |
| >=60 | 29.69% | 33.64% | 33.91% |
2.3. Measures
All self-report measures were collected digitally using REDCap electronic data capture tools hosted at University of Michigan.(Harris et al., 2019) REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
The Coronavirus Impact Scale (CIS) (Stoddard, Joel, 2020) contains 11 questions asking participants to rate the degree of change (none, mild, moderate and severe) in routines, family income/employment, access to food, medical care, and mental health treatment, access to extended family and social supports, experiences in stress related to the pandemic, and stress and discord in the family. Additionally, it asks whether the Participant personally diagnosed themselves with Covid-19, their symptoms, if any, and the number of immediate and extended family/household members have positively diagnosed with Covid-19. For those participants that answered a personal diagnosis of infection, or rated moderate or higher symptoms of COVID-19, this diagnosis was confirmed or denied within the University of Michigan Health System RDW-DataDirect: a self-serve tool for data retrieval.(Spector-Bagdady et al., 2020)
The CIS was designed to specifically rate the impact of the Covid-19 pandemic, be of low burden to the user, and provide comprehensive coverage of the types of adversities experienced due to the pandemic. It has not been validated, given the rapidity with which it was developed to respond to the clinical and research needs resulting from the Covid-19 pandemic. The CIS is registered with the NIH OBSSR suite of common instruments, and is included in the PhenxToolkit. (Hamilton et al., 2011).
The Patient Health Questionnaire (PHQ-9) is a self-administered module to measure depression, which scores each of the 9 DSM-IV criteria as “0″ (not at all) to “3″ (nearly every day). These responses are summed for a total score per measurement.(Kroenke et al., 2001) The Generalized Anxiety Disorder Scale (GAD-7) is also designed to be a self-administered instrument to measure severity and screen for the four most common anxiety disorders; Generalized Anxiety Disorder, Panic Disorder, Social Phobia and Post Traumatic Stress Disorder.(Spitzer et al., 2006) The GAD-7 assigns scores of 0, 1, 2, and 3 to the response categories “not at all,” “several days,” “more than half the days,” and “nearly every day,” respectively. These responses are also summed for a total score. Finally, the Pittsburg Sleep Quality Index (PSQI) is a self-reported, subjective measure of sleep quality and patterns.(Smyth, 1999) It assesses seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction over the last month. Scoring of the answers is based on a 0 to 3 scale, whereby 3 reflects the most disruption. In this study, four item level questions were used from the PSQI. Three of these item questions were collapsed into binary categories: 1) how long, in minutes, does it take to fall asleep was collapsed into either > or < = 30 min, 2) overall sleep quality was collapsed into either good or bad, 3) using sleep medications was collapsed into yes or no, and 4) how many hours of sleep per night on average experienced.
The CIS, PHQ-9, GAD-7, and PSQI were surveyed every two weeks on April 30, May 14, and May 28, 2020, providing 3 potential observations for each measurement and participant.
2.4. Statistical analysis
Analyses were completed using the R software (https://cran.r-project.org/). Mood measures were transformed into logarithm before formal model fitting to make them more normally distributed. Longitudinal analysis was conducted using the generalized estimating equations (GEE)(Liang and Zeger, 1986) to study the longitudinal change both within the 2020 pandemic period and between the pre-pandemic (5-year prior to 2020) and post-pandemic 2020.
3. Results
3.1. General description
While the CIS questionnaire included 4-category answers, ‘none’, ‘mild’, ‘moderate’ and ‘severe’, in our analyses, we condensed “Yes” to be inclusive of ‘moderate’ and ‘severe’ and “No” to ‘none’ and ‘mild’. The counts of participants in each of these 4-category as well as the percentage of “Yes” indicating a moderate or severe disruption, stratified by diagnosis, are illustrated in Supplemental Figure 2 and Supplemental Figure 3, respectively, for the first 8 items of the survey. The final 3 questions in the CIS ask whether; 1) participants themselves, 2) an immediate family member and 3) if extended family members have been diagnosed with or felt symptoms of Covid-19. On April 30th, 88% of the participants reported no symptoms or personal diagnosis of Covid-19 while 11% report mild symptoms and 1% report moderate symptoms. These aggregate proportions and specific participant responses largely did not change throughout the three time points, with the exception of one participant that moved from a moderate to severe category on April 30 to May 14th and stayed in the severe category on May 28th. These results are reported in Supplemental Table 1.
Summary statistics for the CIS, sleep and mood measures at the three survey time points, April 30, May 14 and May 28, 2020, for the 560 participants are given in Table 1. The SAH order caused routine disruptions in 75% of responding participants. Twenty-one percent of respondents reported a disruption in family income/employment at the initial assessment but by the end of study this disruption dropped to 15%. Disruption to food access occurred in 7% of the respondents on average over the three time points. Access to medical care was compromised in 32% of the respondents, whereas access to mental health care was disrupted in12% of the respondents. At the beginning of the observation period, access to social supports was disrupted in 42% of the respondents while 44% reported pandemic related stress. These numbers trended downward to 37% and 38%, respectively, by the end of May 2020. Family discord and related stress was reported in about 10% of the respondents on average across the three time points.
Table 2 shows the response counts and demographic information for the 345 individuals with bipolar disorder and 147 healthy control participants within the CIS, mood and sleep surveys for each of the three time points. There was a modest decay in the response rate over the one-month observational period as is commonly seen in longitudinal studies. For all surveys across all time points, the respondents are 66% female, 32% ≥ 60-years of age and 70% diagnosed with bipolar disorder.
3.2. Longitudinal analysis within the pandemic
Longitudinal analysis, using the generalized estimating equations method, focused on a subset of the CIS measures: changes in routines, family income/employment, access to social support, and the self-report of pandemic related stress, due to their initial large proportion of disruption. The percentages of respondents reporting difficulty accessing food, experiencing family discord, or experiencing symptoms of Covid-19 were initially low and showed little change over the month of study, thus were not included in the longitudinal analyses. The analysis results are in Table 3 .
Table 3.
Longitudinal Analysis Results within the Covid-19 period, May 2020. For responses CIS_1,2,3,4 and SLEEP_1,2,3 the effects are odds ratios, and for the rest responses the effects are linear in the log scale.
| BP vs HC at April 30 | HC Longitudinal Change Compared to April 30 | BP Longitudinal Change Compared to April 30 | SEX1 | AGE602 | |||
|---|---|---|---|---|---|---|---|
| May 14 | May 28 | May 14 | May 28 | ||||
| CIS_13 | 0.7158 (p = 0.1933) | 0.6083 (p = 0.0372) | 0.4552 (p = 0.0018) | 1.3117 (p = 0.1240) | 1.0425 (p = 0.8153) | 0.5486 (p = 0.0009) | 0.5811 (p = 0.0029) |
| CIS_24 | 1.2662 (p = 0.3838) | 0.5010 (p = 0.0027) | 0.5062 (p = 0.0087) | 0.9929 (p = 0.9649) | 0.7635 (p = 0.1218) | 1.2017 (p = 0.4102) | 0.7667 (p = 0.2557) |
| CIS_35 | 1.4259 (p = 0.1191) | 0.5724 (p = 0.0256) | 0.6785 (p = 0.1594) | 1.0461 (p = 0.7555) | 0.8393 (p = 0.2670) | 0.6969 (p = 0.0354) | 0.8125 (p = 0.2216) |
| CIS_46 | 3.7352 (p<0.0001) | 0.9095 (p = 0.7220) | 0.4737 (p = 0.0095) | 1.0825 (p = 0.5168) | 0.8766 (p = 0.3169) | 0.6124 (p = 0.0090) | 0.6751 (p = 0.0379) |
| PHQ-97 | 0.8693 (p<0.0001) | −0.2299 (p = 0.0002) | −0.2439 (p = 0.0004) | −0.0231 (p = 0.5602) | −0.1000 (p = 0.0752) | −0.1588 (p = 0.0541) | −0.1463 (p = 0.0872) |
| GAD-78 | 0.8540 (p<0.0001) | −0.1679 (p = 0.0141) | −0.2088 (p = 0.0064) | −0.0306 (p = 0.5256) | −0.0480 (p = 0.4261) | −0.1047 (p = 0.2336) | −0.2786 (p = 0.0021) |
| SLEEP_19 | 3.5538 (p = 0.0003) | 1.5969 (p = 0.1902) | 1.0193 (p = 0.9384) | 0.8514 (p = 0.2518) | 0.7121 (p = 0.0504) | 0.8347 (p = 0.4517) | 0.7059 (p = 0.1737) |
| SLEEP_210 | 2.6398 (p = 0.0027) | 0.9156 (p = 0.8028) | 0.9639 (p = 0.9008) | 0.9428 (p = 0.6739) | 0.9026 (p = 0.5855) | 1.0030 (p = 0.9897) | 0.6172 (p = 0.0522) |
| SLEEP_311 | 5.3612 (p<0.0001) | 0.9628 (p = 0.9038) | 0.9005 (p = 0.5700) | 1.0513 (p = 0.5575) | 0.9156 (p = 0.4811) | 1.2567 (p = 0.3179) | 0.7982 (p = 0.3353) |
| SLEEP_412 | 0.2269 (p = 0.1812) | −0.0911 (p = 0.3474) | 0.1330 (p = 0.1365) | −0.0358 (p = 0.7422) | −0.1453 (p = 0.2221) | 0.2359 (p = 0.1696) | 0.4672 (p = 0.0042) |
Female as the reference group.
Age>=60 versus Age<60 years old, with Age<60 as the reference group.
Having a change in routines, with “No” as the reference group.
Having a change in family income/employment, with “No” as the reference group.
Having a change in access to social supports, with “No” as the reference group.
Experiencing pandemic related stress, with “No” as the reference group.
A log transformation is applied: Y=log(PHQ+1).
A log transformation is applied: Y=log(GAD+1).
Time it takes to fall asleep, >30 min versus <=30 min, with <=30 min as the reference group.
Sleep quality, bad versus good, with good as the reference group.
Taking sleep medications, yes versus no, with “No” as the reference group.
Number of hours of asleep per day.
3.2.1. Bipolar vs healthy control at start of study
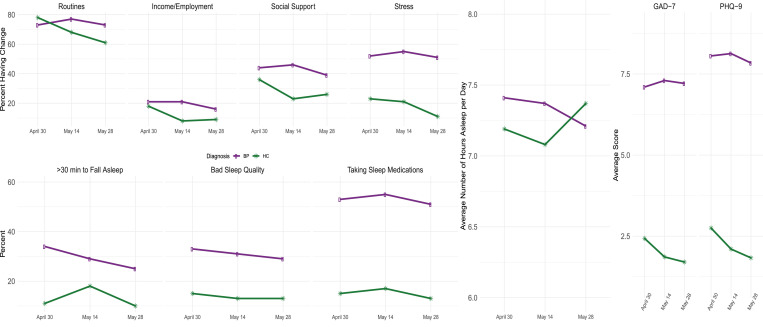
At the beginning of this study, 5-weeks into the state-wide lockdown in Michigan, the bipolar individuals were more disadvantaged as measured by their responses across all questionnaires compared to the healthy controls, adjusting for age and sex. Those with bipolar disorder were more likely to experience pandemic related stress (OR = 3.74, p < 0.0001), more likely to take more than 30 min to fall asleep (OR = 3.55, p = 0.0003), more likely to have had bad sleep quality (OR = 2.64, p = 0.0027), more likely to report taking sleep medications (OR = 5.36, p < 0.0001), and had reported higher PHQ-9 score (138% higher, p < 0.0001) and higher GAD-7 scores (134%, p < 0.0001) as shown in Fig. 1 . Data for these mean values from Fig. 1 are printed in Supplemental Table 2.
Fig. 1.
Longitudinal mean profiles, stratified by diagnosis, for (a) CIS, (b) item level responses for PSQI, (c) sleep duration and (d) mood measures GAD-7 and PHQ-9.
3.2.2. Longitudinal change for healthy controls
Disruption, as measured by the CIS, improved significantly in the healthy controls over the SAH time period, adjusting for sex and age. The odds of having a disruption in routines, in family income/employment, in access to social supports, and experiencing pandemic related stress all declined (i.e. an overall improvement) among the healthy controls at each subsequent time point over the study. For example, the odds ratio of reporting a disruption in routines was 0.61 (p- value = 0.04) at the middle of May and 0.46 (p = 0.002) by the end of May, compared to the beginning of study. The mood and anxiety symptoms for the healthy controls also improved significantly over the three times points in the study. The PHQ-9 scores, anchored by the initial scores in April declined, indicating a clinical improvement, by 21% (p = 0.0002) at mid-May and remained constant through to the end of May. Additionally, the GAD-7 score declined, also an indication of clinical improvement, by 16% (p = 0.01) at mid-May from April 30th and by 19% (p = 0.006) at the end of May from April 30th. For sleep responses, there was no significant longitudinal change for the healthy controls adjusting for sex and age.
3.2.3. Longitudinal change for individuals with bipolar disorder
Unlike the healthy controls, by the end of SAH orders, the bipolar participants showed no significant improvements in the CIS measured disruption questions and experienced sustained higher levels of mood and anxiety symptoms. The disruptions in routines experienced by those those with bipolar disorder persisted throughout May 2020, with an odds ratio, adjusting for sex and age, 1.31 (p = 0.12) comparing mid-May to end of April and 1.04 (p = 0.82) comparing end of May to beginning of study. For the other CIS responses, the odds of experiencing a disruption were slightly less by the end of study vs. beginning of study, but none were statistically significant. For example, the odds ratios for experiencing disruptions in family income/employment, access to social supports, and experiencing pandemic related stress were 0.76 (p = 0.12), 0.84 (p = 0.27) and 0.88 (p = 0.32), respectively, comparing the end of SAH to the beginning of study. These trends can also be seen in the mean profiles shown in Fig. 1 and Supplemental Table 2. Results show that mean profiles of the individuals with bipolar disorder remain constant, indicating sustained stress and disruption, compared to improving pattern of the healthy controls.
Measures of mood and sleep disruption remained elevated with little change or improvement over the observation period. Adjusting for sex and age, the PHQ-9 scores declined by 9.5% (p = 0.075) and GAD-7 scores declined by 5% (p = 0.43) over the study observation period. These minor declines in scores do not indicate significant clinical improvement. The odds ratios for taking > 30 min to fall asleep, having bad sleep quality, and taking sleep medications were 0.71 (p = 0.05), 0.90 (p = 0.59), and 0.92 (p = 0.48), respectively, over the study duration. For completeness of information, Supplemental Table 3 contains additional summary statistics about the participants with bipolar disorder stratified by whether their symptoms improve, stay the same, or worsen, for the pandemic related stress question in the CIS, the PHQ-9, and GAD-7 aggregate scores.
Sex and age were treated as confounders for the main research aims in this study and thus were adjusted for in the analysis of longitudinal change comparing diagnostic groups. However, Table 3 shows that adjusting for time, diagnosis and age, males are less likely to experience a disruption in routines compared to females (OR = 0.55, p = 0.0009) during the SAH, and are less likely to experience a change in access to social supports (OR = 0.70, p = 0.0354). Men were less likely to experience pandemic related stress (OR = 0.61, p = 0.0090), and had a 15% lower PHQ-9 score (p = 0.0541). Similarly, adjusting for time, diagnosis and sex, people older than 60 are less likely to experience changes in all the four CIS questions, had lower PHQ-9 and GAD-7 scores, and had better scores on measures of sleep quality.
3.3. Aggregate comparison of 2020 verses 2015–2019
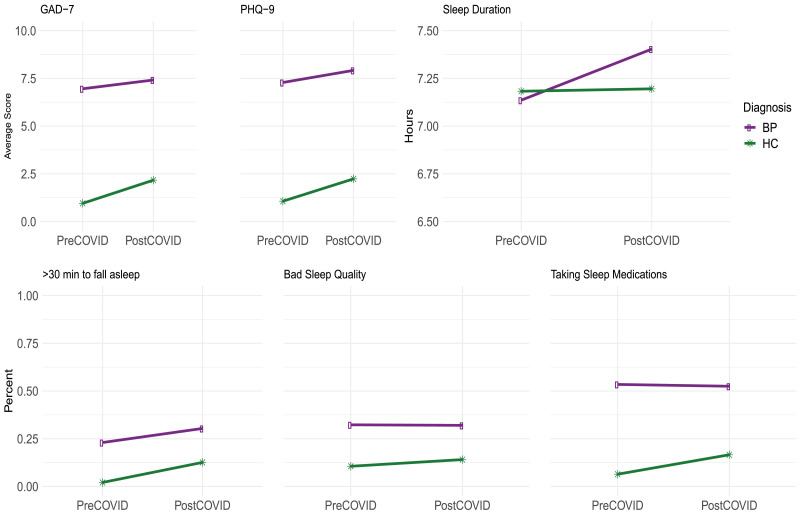
We compared measures from the current pandemic era to those from the five years prior. The participants from this study are actively engaged in the Prechter Longitudinal Study of Bipolar Disorder with extensive clinical outcomes data available for comparison.(McInnis et al., 2018b) However, the frequency of measurement is less (every two months) in the Prechter Longitudinal Study, and thus, for any particular year prior to 2020, within the same months as the SAH period in 2020 there might only be a few measurements available. Therefore, we aggregated data from 5 years prior to 2020 to make the pre-pandemic and post- pandemic comparison. Further, for both time periods, we included all measurements between March 15 and May 30 to be more inclusive of the broader SAH timeframe. The average of each measure of interest, pre and post-pandemic, is shown in Fig. 2 and the results of the comparison adjusting for sex and age are detailed in Table 4 .
Fig. 2.
Comparison of pre-pandemic and post-pandemic measures. (a) average mood measures, (b) sleep duration, and (c) item level responses for PSQI.
Table 4.
Longitudinal Analysis Results comparing pandemic period, March 15 to May 30, in 2020 to pre-pandemic period, March 15 to May 30, in 2015–2019. For responses SLEEP_1,2,3 the effects are odds ratios, and for the rest responses the effects are linear in the log scale.
| Post-pandemic to Pre-pandemic comparison for HC | Post-pandemic to Pre-pandemic comparison for BP | SEX | AGE60 | |
|---|---|---|---|---|
| PHQ-913 | 0.3346 (p<0.0001) | 0.0489 (p = 0.1512) | −0.0715 (p = 0.3059) | −0.1190 (p = 0.1046) |
| GAD-714 | 0.3975 (p<0.0001) | 0.0365 (p = 0.3246) | −0.0876 (p = 0.2544) | −0.2722 (p = 0.0006) |
| SLEEP_1 | 6.0761 (p = 0.0002) | 1.4556 (p = 0.0104) | 0.7769 (p = 0.2744) | 0.8373 (p = 0.4558) |
| SLEEP_2 | 1.3844 (p = 0.3892) | 0.9738 (p = 0.8725) | 0.9343 (p = 0.7435) | 0.6996 (p = 0.0954) |
| SLEEP_3 | 2.4854 (p = 0.0106) | 0.9672 (p = 0.8191) | 1.2195 (p = 0.3240) | 0.7944 (p = 0.2906) |
| SLEEP_4 | −0.0039 (p = 0.9750) | 0.2725 (p = 0.0198) | 0.1834 (p = 0.2450) | 0.3830 (p = 0.0129) |
A log transformation is applied: Y = log(PHQ+1).
A log transformation is applied: Y = log(GAD+1).
The healthy controls, typically with very low measurements for mood symptoms, showed an increase in these symptoms in the pandemic era. Compared to pre-pandemic era, adjusting for sex and age, the average PHQ-9 score for healthy controls is 39% higher (p < 0.0001) (2.23 vs. 1.06 without adjusting for sex and age), the average GAD-7 score is 49% higher (p < 0.0001) (2.17 vs. 0.95 without adjusting for sex and age), the odds for taking longer than 30 min to fall asleep are significantly higher (OR = 6.08, p = 0.0002), and the odds of taking sleep medications are significantly higher (OR = 2.49, p = 0.0106). All of these indicate a detrimental post-pandemic effect on these healthy controls.
For individuals with bipolar disorder, the change in these measures from the already elevated pre-pandemic disordered symptomology to the pandemic era is less. Compared to pre-pandemic era, adjusting for sex and age, the average PHQ-9 score is 5% higher (p = 0.15) (7.92 vs. 7.28 without adjusting for sex and age), the average GAD-7 score is 4% higher (p = 0.32) (7.41 vs. 6.95 without adjusting for sex and age), the odds ratio for taking longer than 30 min to fall asleep is 1.46 (p = 0.01), and the odds ratio for taking sleep medications is 0.97 (p = 0.82). The lesser change in these responses compared to the healthy controls is largely due to the fact that the healthy controls had very low measurements in the pre-pandemic era, whereas those with bipolar disorder experienced an ongoing and chronic level of symptoms prior to the pandemic. This is shown in Fig. 2, data found in Supplemental Table 4. Participants with bipolar disorder had higher values for all measures during the pre-pandemic era and those values continued into the pandemic era at the chronically eleveated level.
There is no significant difference in sex for all the responses in Table 4, adjusting for time, diagnosis and age. Investigating age however, people older than 60 had a 24% lower average GAD-7 score (p = 0.0006) and sleep 0.38 h longer (p = 0.0129) than people younger than 60, adjusting for time, diagnosis and sex. The difference in age for all the other responses considered was not found significant.
4. Discussion
Our main findings show that the self-reported psychological distress of being under SAH orders affects both those with and without bipolar disorders independent of age or sex, corroborating results seens in China late in 2019 and Australia in 2020.(Hao et al., 2020; Van Rheenen et al., 2020) Our study extends previous work by showing those with bipolar experience a greater and sustained level of disruption than healthy controls and that healthy controls exhibit a clear decrease in their response to pandemic related disruptions and mood measurements over the length of the SAH orders. Bipolar disorder participants either remained at their high levels of distress or decreased with a magnitude that was much smaller compared to the healthy controls. Neither the participants with bipolar disorder nor the healthy controls showed a significant longitudinal change in sleep quality over the SAH observation period. However, a trend towards increased sleep duration was seen in the participants with bipolar disorder with a corresponding decrease in sleep duration in healthy controls. Consistent with previous studies (Hou et al., 2020; Nivoli et al., 2011) men were less effected during the pandemic era compared to women (independent of diagnosis) as indicated by a lower level of disruption and mood measures among men during SAH orders.
Of our complete cohort of participants, there were 50 who responded with ‘1’ (mild) or commented on symptoms consistent with a respiratory illness on the CIS. Specific comments challenged the designation of ‘mild’ and the participant noted that the symptoms were ‘anything but mild’. There were nine who provided a response of moderate (hospitalized) or severe (required respiratory assistance). At least two respondents commented with statements that suggested the response reflected an overall frustration with the pandemic, marking many items as moderate or severe. Two participants were seen in the University of Michigan Health System, admitted with Covid-19 like symptoms but only one tested positive on the SARS-CoV-2 qRT-PCR analytical test. The other individual that was hospitalized with symptoms experienced loss of a close family member from the disease but tested negative. It is tempting to hypothesize a traumatic psychotic episode, or suggest a potential false negative test result. But that discussion is outside of this body of work. Of the participants who reported mild symptoms of Covid-19 over the observation period many were denied a SARS-CoV-2 detection test and sent home. This is consistent with known difficulties in obtaining diagnostic testing and hospitalization was reserved only for the critically ill during the early months of the pandemic. We are aware that individuals categorized as ‘mild’ and managed at home have experienced ongoing neurological symptoms including fatigue and ‘brain fog’ consistent with long-term clinical phenomenon associated with Covid-19 convalesence.(Chopra et al., 2020) (Chopra et al., 2020)
When comparing measures from pre-pandemic period, 5 years prior to 2020, to the pandemic period in 2020, the healthy controls showed a larger change in PHQ-9 scores, GAD-7 scores, the time it takes to fall asleep and the amount of taken sleep medications than the participants with bipolar disorder. People with bipolar disorder commonly experience ongoing and chronic mood symptoms. The Prechter longitudinal study cohort, from which our sample was derived, finds that the average PHQ-9 score at entry into the larger study is between 7 – 12 for any affected mood phenotype but 1.4 for the unaffected healthy controls.(McInnis et al., 2018b) The healthy and unaffected controls experienced minimal, if any, pre-pandemic symptoms and therefore were likely to take note of the stress more than the individuals with bipolar disorder who live with chronic symptoms. The additional pandemic related stress among those with bipolar did not significantly alter the chronic mood and anxiety symptoms captured in the PHQ-9 and GAD-7. The reason for this is not clear. It is possible to speculate that living with the stressors of chronic mental illness dulls the relative impact of additive stressors or perhaps it is the lack of a finer gradation in the instruments. The CIS may be better at quantifying the impact of the additional stresses that are not captured on the mood measures. It is noteworthy that the pre-pandemic to post-pandemic change in sleep quality and sleep duration is not significant for either the healthy controls or the individuals with bipolar disorder.
There are several limitations in this study. Sadly, two of our participants died by suicide in the summer of 2020 with no indication apparent from the patterns in self-reported measurements captured herein. Secondly, our study received an overall response rate of 62% of those invited. Those that did not respond may very well represent those that are experiencing the most emotional distress. However, the demographic distribution of this study cohort matchs closely to the larger longitudinal cohort with regard to sex, age and diagnosis. Thirdly, the state of Michigan was under SAH orders from March 24, 2020, whereas the data collection for this study started on April 30, 2020, a full 5-weeks later. Therefore, we were not able to study the pandemic and SAH impacts earlier, at least for the pandemic related responses captured in the CIS. Lastly, our participant population focuses solely on those that reside in the state of Michigan and under state of Michigan SAH orders. It remains to be seen if these differences can be repeated in a geographically different, racially more diverse population. Data analysis-wise, for the pre-pandemic and pandemic comparison, we aggregated measurements collected between March 15 and May 30 separately from 2015–2019 and from 2020 and used their respective means for each participant as the responses for our models. Such an analysis ignores the within subject variation both during pre-pandemic and the post-pandemic periods. The analysis was done in this manner to avoid different data collection frequency and data collection times of these two periods. In future studies, we will account for this within-subject variation and try to further understand the true infection rate of our participants.
Funding
This research was supported by Heinz C Prechter Bipolar Research Fund at the University of Michigan Depression Center and the Richard Tam Foundation. With gratitude, we acknowledge The University of Michigan Prechter Bipolar Longitudinal Research Participants who graciously provided information on their well-being in the current covid-19 pandemic.
Institutional review
This current study was approved by the University of Michigan IRB and electronically captured informed consent was obtained. While there is and continues to be incentive payment for their participation in a larger longitudinal study undertake at the University of Michigan, there was no additional incentives paid for participation in this study.
Additional acknowledgement
The authors also acknowledge the University of Michigan Medical School Research Data Warehouse and DataDirect for providing data aggregation, management, and distribution services in support of the research reported in this publication.
Data availability
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available because of privacy restrictions.
CRediT authorship contribution statement
Anastasia K. Yocum: Conceptualization, Data curation, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Yuqi Zhai: Data curation, Formal analysis, Methodology. Melvin G. McInnis: Resources, Funding acquisition, Writing - review & editing. Peisong Han: Conceptualization, Formal analysis, Methodology, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
All authors declare no competing or conflicting interest.
Acknowledgements
This research was supported by Heinz C Prechter Bipolar Research Fund at the University of Michigan Depression Center and the Richard Tam Foundation. With gratitude, we acknowledge The University of Michigan Prechter Bipolar Longitudinal Research participants who graciously provided information on their well-being in the current Covid-19 pandemic. The authors also acknowledge the University of Michigan Medical School Research Data Warehouse and DataDirect for providing data aggregation, management, and distribution services in support of the research reported in this publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2021.01.028.
Appendix. Supplementary materials
References
- Anmella G., Arbelo N., Fico G., Murru A., Llach C.D., Madero S., …, Pintor L. COVID-19 inpatients with psychiatric disorders: real-world clinical recommendations from an expert team in consultation-liaison psychiatry. J. Affect. Disord. 2020;274(April):1062–1067. doi: 10.1016/j.jad.2020.05.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavel J.J.V., Baicker K., Boggio P.S., Capraro V., Cichocka A., Cikara M., …, Willer R. Using social and behavioural science to support COVID-19 pandemic response. Nat. Human Behav. 2020;4(5):460–471. doi: 10.1038/s41562-020-0884-z. [DOI] [PubMed] [Google Scholar]
- Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H. Sixty-day outcomes among patients hospitalized with COVID-19. Ann. Intern. Med. 2020;(November):1–3. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen W., Gulati G., Kelly B.D. Mental health in the Covid-19 pandemic. N. Engl. J. Med. 2020;383:510–512. doi: 10.1093/qjmed/hcaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterwood E., Saeed S.A. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future. Psychiatr. Q. 2020;91(4):1121–1133. doi: 10.1007/s11126-020-09808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio L.M.F., Magalhães P.V.S., Andersen M.L., Walz J.C., Jakobson L., Kapczinski F. Circadian preference in bipolar disorder. Sleep Breath. 2010;14(2):153–155. doi: 10.1007/s11325-009-0301-3. [DOI] [PubMed] [Google Scholar]
- González-Sanguino C., Ausín B., Castellanos M.Á., Saiz J., López-Gómez A., Ugidos C., Muñoz M. Mental health consequences during the initial stage of the 2020 Coronavirus pandemic (COVID-19) in Spain. Brain Behav. Immun. 2020;87(May):172–176. doi: 10.1016/j.bbi.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton C.M., Strader L.C., Pratt J.G., Maiese D., Hendershot T., Kwok R.K., …, Haines J. The PhenX toolkit: get the most from your measures. Am. J. Epidemiol. 2011;174(3):253–260. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao F., Tan W., Jiang L., Zhang L., Zhao X., Zou Y., …, Tam W. Do psychiatric patients experience more psychiatric symptoms during COVID-19 pandemic and lockdown? A case-control study with service and research implications for immunopsychiatry. Brain Behav. Immun. 2020;87(April):100–106. doi: 10.1016/j.bbi.2020.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., …, Duda S.N. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 2019;95(May) doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F., Bi F., Jiao R., Luo D., Song K. Gender differences of depression and anxiety among social media users during the COVID-19 outbreak in China:a cross-sectional study. BMC Public Health. 2020;20(1):1–11. doi: 10.1186/s12889-020-09738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9, validity of a brief depression severity measure. JGIM. 2001;16(02):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzerini M., Barbi E., Apicella A., Marchetti F., Cardinale F., Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc. Health. 2020;4(5):e10–e11. doi: 10.1016/S2352-4642(20)30108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K., Han, S., & Jeong, Y. (2020). COVID-19, flattening the curve, and Benford's law. (January). [DOI] [PMC free article] [PubMed]
- Leigh-Hunt N., Bagguley D., Bash K., Turner V., Turnbull S., Valtorta N., Caan W. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health. 2017;152:157–171. doi: 10.1016/j.puhe.2017.07.035. [DOI] [PubMed] [Google Scholar]
- Liang K.-Y., Zeger S.L. Longitudinal data analysis using GLM. Biometrika. 1986;73(1):13–22. http://www.biostat.jhsph.edu/~fdominic/teaching/bio655/references/extra/liang.bka.1986.pdf Retrieved from. [Google Scholar]
- Matrajt L., Leung T. Evaluating the effectiveness of social distancing interventions to delay or flatten the epidemic curve of coronavirus disease. Emerg. Infect. Dis. 2020;26(8):1740–1748. doi: 10.3201/eid2608.201093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis M.G., Assari S., Kamali M., Ryan K., Langenecker S.A., Saunders E.F.H., …, Williams A. Cohort profile: the Heinz C. Prechter longitudinal study of bipolar disorder. Int. J. Epidemiol. 2018;47(1):28. doi: 10.1093/ije/dyx229. 28n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis M.G., Assari S., Kamali M., Ryan K., Langenecker S.A., Saunders E.F.H., …, Williams A. Cohort profile: the Heinz C. Prechter longitudinal study of bipolar disorder. Int. J. Epidemiol. 2018;47(1):28. doi: 10.1093/ije/dyx229. 28n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed O.C., Hahn M.K., Agarwal S.M., Taylor V.H., Mulsant B.H., Selby P. Physical health among people with serious mental illness in the face of COVID-19: concerns and mitigation strategies. Gen. Hosp. Psychiatry. 2020;66(May):30–33. doi: 10.1016/j.genhosppsych.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo M.C.A., Abreu R.L.C., Linhares Neto V.B., de Bruin P.F.C., de Bruin V.M.S. Chronotype and circadian rhythm in bipolar disorder: a systematic review. Sleep Med. Rev. 2017;34:46–58. doi: 10.1016/j.smrv.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Melo M.C.A., Garcia R.F., de Araújo C.F., Luz J.H., de Bruin P.F., de Bruin V.M. Chronotype in bipolar disorder: an 18-month prospective study. Braz. J. Psychiatry. 2020;42(1):68–71. doi: 10.1590/1516-4446-2019-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R.C., Colin, Depp A., Harvey P.D., Pinkham A.E. Assessing the real-time mental health challenges of COVID-19 in individuals with serious mental illnesses: protocol for a quantitative study. JMIR Res. Protoc. 2020;9(5) doi: 10.2196/19203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland A., Herlihy C., Tynan M.A., Sunshine G., McCord R.F., Hilton C., …, Popoola A. Timing of state and territorial COVID-19 stay-at-home orders and changes in population movement — United States, March 1–May 31, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(35):1198–1203. doi: 10.15585/mmwr.mm6935a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivoli A.M.A., Pacchiarotti I., Rosa A.R., Popovic D., Murru A., Valenti M., …, Colom F. Gender differences in a cohort study of 604 bipolar patients: the role of predominant polarity. J. Affect. Disord. 2011;133(3):443–449. doi: 10.1016/j.jad.2011.04.055. [DOI] [PubMed] [Google Scholar]
- Rajkumar R.P. Bipolar disorder, COVID-19, and the risk of relapse. Bipolar Disord. 2020:bdi.12947. doi: 10.1111/bdi.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman L.Y., Hansel T.C., Bordnick P.S. Loneliness, isolation, and social support factors in post-COVID-19 mental health. Psychol. Trauma. 2020;12:55–57. doi: 10.1037/tra0000703. [DOI] [PubMed] [Google Scholar]
- Sher L. The impact of the COVID-19 pandemic on suicide rates. QJM. 2020:1–17. doi: 10.1093/qjmed/hcaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. The Pittsburgh Sleep Quality Index (PSQI) J. Gerontol. Nurs. 1999;25(12):10–11. doi: 10.3928/0098-9134-19991201-10. [DOI] [PubMed] [Google Scholar]
- Spector-Bagdady K., Hutchinson R., O'Brien Kaleba E., Kheterpal S. Sharing health data and biospecimens with industry - A principle-driven, practical approach. N. Engl. J. Med. 2020;382(22):2072–2075. doi: 10.1056/NEJMp1915298. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- State of Michigan Office of the Governor. 2008;5(307) http://gov.ca.gov/news.php?id=11036 Executive Order S-13-08. Retrieved from. [Google Scholar]
- Stoddard, Joel K.J. Coronavirus Impact Scale FAQ. TBD. 2020 [Google Scholar]
- Van Rheenen, T.E., Meyer, D., Neill, E., Phillipou, A., Tan, E.J., Lin Toh, W., & Rossell, S.L. (2020). Mental health status of individuals with a mood-disorder during the COVID-19 pandemic in Australia: initial results from the COLLATE project. 10.1016/j.jad.2020.06.037. [DOI] [PMC free article] [PubMed]
- Zhu Z., Liu Q., Jiang X., Manandhar U., Luo Z., Zheng X., …, Zhang B. The psychological status of people affected by the COVID-19 outbreak in China. J. Psychiatr. Res. 2020;129(April):1–7. doi: 10.1016/j.jpsychires.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available because of privacy restrictions.