Abstract
Objective:
To review, analyze, and synthesize the literature on endothelial dysfunction in critically ill children with multiple organ dysfunction syndrome and to develop a consensus biomarker-based definition and diagnostic criteria.
Data Sources:
Electronic searches of PubMed and EMBASE were conducted from January 1992 to January 2020, using a combination of medical subject heading terms and text words to define concepts of endothelial dysfunction, pediatric critical illness, and outcomes.
Study Selection:
Studies were included if they evaluated critically ill children with endothelial dysfunction, evaluated performance characteristics of assessment/scoring tools to screen for endothelial dysfunction, and assessed outcomes related to mortality, functional status, organ-specific outcomes, or other patient-centered outcomes. Studies of adults or premature infants (≤36 weeks gestational age), animal studies, reviews/commentaries, case series with sample size ≤10, and non-English language studies with inability to determine eligibility criteria were excluded.
Data Extraction:
Data were abstracted from each eligible study into a standard data extraction form along with risk of bias assessment.
Data Synthesis:
We identified 62 studies involving 84 assessments of endothelial derived biomarkers indirectly linked to endothelial functions including leukocyte recruitment, inflammation, coagulation, and permeability. Nearly all biomarkers studied lacked specificity for vascular segment and organ systems. Quality assessment scores for the collected literature were low.
Conclusions:
The Endothelial Subgroup concludes that there exists no single or combination of biomarkers to diagnose endothelial dysfunction in pediatric MODS. Future research should focus on biomarkers more directly linked to endothelial functions and with specificity for vascular segment and organ systems.
Summary:
We critically review the literature on endothelial dysfunction in critically ill children to develop a consensus biomarker-based definition and diagnostic criteria.
Introduction
Long considered to be the organ system of the intensivist, the endothelial system is the largest organ in the human body with a cumulative surface area estimated to be 1,000 to 4,000 square meters in adults.1 First identified in the 1800s, endothelial cells (ECs) were initially thought to be inert cells lining the inner lumen of blood vessels. The endothelium is now known to be a highly active biological system with unique segmental and organ-specific functions2 that forms a continuous lining of the inner surface of all blood and lymphatic vessels. ECs actively regulate flow, maintain fluidity of blood and lymph, contain fluid and solutes, and facilitate macromolecule exchange with tissues. The endothelium also participates extensively in the immune response, controls the recruitment and activation of circulating immune cells and helps to regulate both local and systemic inflammatory responses.3 Given these critical functions, maintaining endothelial homeostasis is essential for the normal function of every organ system. Endothelial dysfunction is defined as the loss or acquisition of aberrant cellular functions that propagate pathological processes (disruption of vascular homeostasis), which has been identified as key to the onset and evolution of multiple critical illness states.
The endothelium extends to within micrometers of all tissue parenchymal cells and is involved in virtually all pathologic processes, suggesting potential importance in multiple organ dysfunction syndrome (MODS). Functions of ECs are highly variable across different organs (e.g. alveolar, renal, hepatic capillaries) and vascular segments within different organs (e.g. artery, capillary, vein). Furthermore, ECs function in leukocyte recruitment, modulation of permeability, inflammation, coagulation and blood flow change in response to disease progression and resolution.4, 5 These factors make precise measure of the contribution of endothelial dysfunction to MODS challenging.
Many have identified circulating biomarkers that purportedly offer clues about endothelial activation, which is defined as the acquisition of new cellular functions to restore homeostasis and resolve dysfunction. However, many of these markers are typically indirect measures of a single EC function, and they lack temporal, segmental and organ specificity. Ultimately, these markers inadequately reflect the systemic vascular response to critical illness.6 An ideal EC biomarker for MODS should both account for the functional differences of ECs in different organs and segments as well as evaluate the relative contribution to restoring or disrupting organ function. Such a biomarker, or group of biomarkers, would be invaluable clinical tools to aid in identification, risk stratification, and treatment approaches to pediatric MODS.
To address this knowledge gap, the Endothelial Subgroup was formed within the Pediatric Organ Dysfunction Information Update Mandate (PODIUM) Collaborative to investigate endothelial dysfunction in critically ill children. We sought to review current literature in pediatric endothelial dysfunction to develop a consensus biomarker-based definition and diagnostic criteria for MODS.
Methods
The PODIUM Collaborative sought to develop evidence-based criteria for organ dysfunction in critically ill children. The present manuscript reports on the systematic review on endothelial dysfunction scoring tools performed as part of PODIUM, provides a critical evaluation of the available literature, and proposes recommendations for future research. The PODIUM Executive Summary details Population, Interventions, Comparators, and Outcomes (PICO) questions, search strategies, study inclusion and exclusion criteria, and processes for risk of bias assessment, data abstraction and synthesis, and for drafting and developing agreement for criteria indicating endothelial dysfunction.7 A quantitative meta-analysis was not performed for several reasons: 1) relative estimates of effect could not be calculated for studies lacking control groups; 2) studies included patients with a variety of etiologies of critical illness; 3) heterogeneity existed in the hemodynamic parameters and timing of assessments relative to disease onset or biomarker evaluation. Therefore, all studies were qualitatively analyzed.
Results
Of 1,071 unique citations identified between 1992 and 2020, 156 articles met criteria for full text review. Less than half (n=62 studies) measured 36 unique biomarkers which were included in the final analysis, as shown in the PRISMA flowchart (Fig. 1). Data tables (Supplemental Tables 1 and 2) and risk of bias assessment summaries (Supplemental Fig. 1) are detailed in the Data Supplement.
Figure 1.
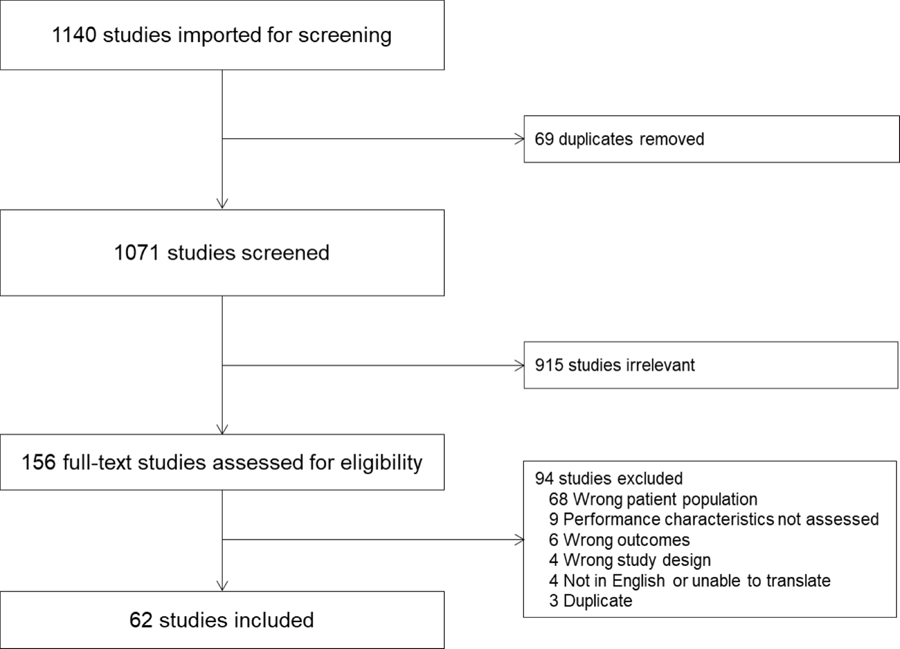
Study flow diagram according to the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols recommendations.
Of the 62 studies included, 51% (32/62) were prospective cohort investigations, 8% (5/62) were retrospective cohort investigations, 16% (10/62) were case-control studies, and 18% (11/62) were case series. Some studies incorporated more than one design. Twenty-six percent were conducted in the United States (16/62), 24% (15/62) in Asia, and 21% (13/62) in Africa. Protein biomarkers measured from the serum or plasma were examined in the majority of studies (92%, 57/62) to predict outcomes including mortality (35%, 22/62), functional status (11%, 7/62), or organ-specific outcomes including morbidity (10%, 6/62). The quality of collected evidence for identifying an endothelial biomarker for MODS is poor. Risk of bias for the collected literature was high in 44% (27/62) of studies assessed, moderate in 24% (15/62) and low in 24% (15/62) (Supplemental Fig. 1). The collected studies enrolled low numbers of subjects, with heterogeneous diagnoses and highly variable severity of illness. Of the identified studies, most 42% (26/62) focused on sepsis including 8 studies of children with malaria-induced MODS. Acute lung injury was the second most studied diagnosis and reflected 15% (9/62) of studies, followed by neurologic injury (infection, trauma and stroke) and cardio-pulmonary bypass-related vascular dysfunction (both 13% (8/62)) of the reviewed studies. Finally, congenital heart disease, including pulmonary hypertension was involved in 7% (4/62) of studies and necrotizing enterocolitis, lupus, bone marrow transplantation, extracorporeal life support, hemolytic uremic syndrome, scorpion bite and disseminated intravascular coagulation were each the focus of a single study.
Many studies surveyed biomarkers relative to more than one EC function, resulting in 84 assessments of different EC functions across the 62 studies. Of the measured biomarkers, 33% (28/84) involved leukocyte recruitment, 21% were markers of permeability (18/84), 14% were involved in inflammation (12/84), 14% described coagulation (12/82) and 13% assessed markers of blood flow (11/84). Notably, no studies investigated endothelial junctional proteins such as claudin-5 or VE-cadherin, which are selectively expressed in ECs.
Discussion
Our comprehensive literature review revealed that no single biomarker of endothelial dysfunction in critically ill pediatric patients with MODS has been identified. The endothelium supports many functions essential for homeostasis and extends to close proximity (microns) of all organ parenchymal cells. Disruption of EC functions, such as systemic inflammation (leukocyte recruitment and activation), capillary leak (permeability), coagulopathies (coagulation) and capillary shunting (heterogenous microvascular blood flow), have all been associated with MODS. It is of critical importance to identify endothelial biomarkers related to MODS diagnosis, progression and resolution. The lack of sufficient prospective trials, use of multiple exposures and outcomes, and lack of biomarker correlation with EC function, resulted in insufficient evidence for a consensus biomarker-based definition of endothelial dysfunction in pediatric MODS.
Biomarkers of leukocyte adhesion, including ICAM1, VCAM1 and E, L and P-selectin, are the most commonly surveyed biomarkers of EC dysfunction in MODS.8 In intact ECs, these molecules are upregulated in response to inflammatory cytokines like TNF or IL-1. Upon stimulation, these molecules collect on the luminal surface of EC where they facilitate leukocyte adhesion and transmigration. However, all studies reviewed measured the soluble forms of these molecules after release from the EC which may represent fragments of dead or sloughed EC. These markers do not provide information on specific organ dysfunction and are not EC specific as many molecules are expressed on circulating immune cells. While soluble adhesion molecules were the most commonly investigated in our survey of existing literature, these may be poor biomarkers of EC dysfunction.
In addition to alterations in leukocyte recruitment, vascular leak can be significantly deranged during endothelial dysfunction in pediatric MODS. The majority of studies focusing on EC permeability investigated the angiopoietin-TIE2 axis.9 Angiopoietin-1 (Angpt-1) is a pleotropic glycoprotein that promotes vascular quiescence and stabilizes of EC barrier function. Angiopoietin-2 (Angpt-2) predominately functions as anl antagonist of Angpt-1 at the receptor tyrosine kinase, TIE2. However, Angpt-2 has several other independent, context-specific functions in vasculogenesis.10 Angpt-2 is produced by EC and stored in Weibel Palade bodies, along with E-selectin, von Willebrand factor (vWF) and other proteins11, which are exocytosed in response to inflammation, hypoxemia, and alterations in blood flow.12 Recently, Angpt-2 has been shown to alter vascular permeability in a concentration-dependent fashion, which differs in vascular segments and organs.10 A direct link between Angpt-2 concentrations and the progression or resolution of capillary leak is not established. Indeed, Angpt-2 does not affect permeability of tight junction architecture in cultured cells.13 Specifically, there appears to be no interaction with Angpt-2 and capillary tight junction proteins like claudin-5 or ZO-1, or with adherens junction proteins like VE-cadherin. Furthermore, there are currently no studies investigating the role of molecules specific for EC junctions, such as claudin-5 or VE-cadherin, in pediatric MODS.
Interleukins modulate the activity of recruited immune cells and are elevated in critical illness.14 These can be secreted by endothelial cells and may recruit adhesion proteins to areas of injury.15 However, the effects of these cytokines on EC function are poorly defined and these molecules are unlikely to represent autocrine signaling. For example, soluble Fas (sFas) is thought to be anti-apoptotic while its ligand (sFasL) is pro-apoptotic16, although these functions are not unique to ECs.
Several studies investigated damage to the endothelial glycocalyx, a glycoprotein monolayer important in vascular integrity and permeability selectivity.17–21 It is not clear from the current literature which factors induce sloughing of the glycocalyx and what consequences ensue from glycocalyx exposure. It is also not clear which vascular segments are most affected when this occurs. More research is needed to answer these important questions.
Vascular endothelial growth factor (VEGF) and its receptor, sFLT1, are thought to aid in growth and repair of the vascular intima.22 Several studies suggest the association between increased circulating levels and critical illness states, but there has not been a documented association with MODS.
Regulation of blood flow is a critical measure of EC function and of clear importance to the intensivist. Investigations of EC blood flow regulation predominately focus on reactive nitrogen species (RNS) and particularly reflect the production, and therefor action, of nitric oxide (NO), a potent vasodilator that is locally-acting and extremely short-lived.23 However, RNS levels may be altered by numerous other cell types and are not clearly linked to abundance or reginal activity of NO. Systemic levels of NO degradation byproducts (nitrite/nitrate) do not accurately reflect regulation of local blood flow. Similarly, inhibitors of NO production have not been demonstrated to relate to EC function.
The regulation of blood fluidity and coagulation is intimately linked to inflammation and predominately regulated by ECs.24 Several studies investigated levels of EC derived coagulation factors.25 Many of these proteins are fairly specific to ECs. Secreted markers, such as vWF and Factor VIII, are released by activated EC, but are not differentiated between EC in different vascular segments or organs. Other markers, such as fibrinogen, may be secreted from EC and other parenchymal cells.26 Some of the surveyed markers of dysregulated coagulation, such as the extensively studied activated protein C (also thrombomodulin, tissue factor pathway inhibitor and plasminogen activator inhibitor) are bound to EC membranes and the significance of soluble concentrations of these proteins is not clear. Functional markers, such as INR, PT, PTT and thromboelastography (TEG) could be coupled with levels of specific EC derived molecules and accurately reflect vascular function in this domain. More investigation would be required to link functional coagulation markers to MODS.
Finally, a single study found endothelial-derived (endoglin/CD105, PECAM1/CD31, VEGFR2/CD309, and MadCAM1 positive) microvesicles were increased in the blood of neonates with MODS requiring extracorporeal life support.21 Microvesicles (MV), also known as ectosomes or microparticles, are released from ECs in response to a variety of stimulation.27 MVs contain microRNA, protein and lipid signaling molecules and are coated with surface markers providing information of their cells of origin. The identified study only quantified EC derived MVs in MODS and did not investigate differences in MV contents. An important area of future research will be to categorize surface protein expression on MV that reflect vascular segments or organ specific EC function and decipher the signaling cargo within MV. If surface markers can be correlated with cellular origin and their contents to function, MV may provide specificity for vascular segment and organ- EC function.
Conclusions
The Endothelial Subgroup concludes that there are no single or combination of endothelial-derived biomarkers for pediatric MODS. Nearly all biomarkers studied lacked vascular segment and organ specificity. Most biomarkers indirectly surveyed EC functions or relied on circulating markers of unknown biologic significance. Scientific priorities for future research include further study of the endothelial glycocalyx, which may provide vascular segment and organ specificity, and MVs, which may also provide specificity with additional insights into vascular function.
Supplementary Material
Supplemental Figure 1. Risk of Bias Assessment Summary for Studies Included in the PODIUM Endothelial Dysfunction Systematic Review (n=62 studies)
Supplemental Table 1. Studies Included in the PODIUM Endothelial Dysfunction Systematic Review (n=62 studies)
Supplemental Table 2. Performance Characteristics for Assessment Tools and Scores for Endothelial Dysfunction in Critically Ill Children (n=62 studies)
Footnotes
Conflict of interest: none relevant to this research.
The guidelines/recommendations in this article are not American Academy of Pediatrics policy, and publication herein does not imply endorsement.
References
- 1.Jaffe EA. Cell biology of endothelial cells. Hum Pathol Mar 1987;18(3):234–9. [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Endothelium as an organ system. Crit Care Med May 2004;32(5 Suppl):S271- [DOI] [PubMed] [Google Scholar]
- 3.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res Feb 2 2007;100(2):158–73. [DOI] [PubMed] [Google Scholar]
- 4.Wong BW, Marsch E, Treps L, Baes M, Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. Embo j Aug 1 2017;36(15):2187–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eelen G, de Zeeuw P, Simons M, Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ Res Mar 27 2015;116(7):1231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce RW, Giuliano JS Jr., Pober JS. Endothelial Cell Function and Dysfunction in Critically Ill Children. Pediatrics Jul 2017;140(1) e20170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bembea MM, Agus M, Akcan-Arikan A, et al. Pediatric organ dysfunction information update mandate (PODIUM) contemporary organ dysfunction criteria: executive summary. Pediatrics 2022;149(suppl 1):e2021052888B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhart K, Bayer O, Brunkhorst F, Meisner M. Markers of endothelial damage in organ dysfunction and sepsis. Crit Care Med May 2002;30(5 Suppl):S302–12. [DOI] [PubMed] [Google Scholar]
- 9.Melendez E, Whitney JE, Norton JS, et al. Systemic Angiopoietin-1/2 Dysregulation in Pediatric Sepsis and Septic Shock. Int J Med Sci 2019;16(2):318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019;8(5):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. Article. Journal of Clinical Investigation 1973;52(11):2745–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rondaij MG, Bierings R, Kragt A, Van Mourik JA, Voorberg J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Short Survey. Arteriosclerosis, Thrombosis, and Vascular Biology 2006;26(5):1002–1007. [DOI] [PubMed] [Google Scholar]
- 13.Pierce RW, Zahr RA, Kandil S, Faustino EVS, Pober JS. Sera From Children After Cardiopulmonary Bypass Reduces Permeability of Capillary Endothelial Cell Barriers. Pediatr Crit Care Med Jul 2018;19(7):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Article. Annals of Internal Medicine 1993;119(8):771–778. [DOI] [PubMed] [Google Scholar]
- 15.Pober JS. Cytokine-mediated activation of vascular endothelium. Physiology and Pathology. Article. American Journal of Pathology 1988;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Suda T, Haze K, et al. Fas ligand in human serum. Article. Nature Medicine 1996;2(3):317–322. [DOI] [PubMed] [Google Scholar]
- 17.De Melo Bezerra Cavalcante CT, Castelo Branco KM, Pinto Júnior VC, et al. Syndecan-1 improves severe acute kidney injury prediction after pediatric cardiac surgery 2016;152(1):178–186. [DOI] [PubMed] [Google Scholar]
- 18.Erdman LK, Dhabangi A, Musoke C, et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: a retrospective case-control study 2011;6(2):e17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura D, Saravia J, Rovnaghi CR, et al. Plasma Biomarker Analysis in Pediatric ARDS: Generating Future Framework from a Pilot Randomized Control Trial of Methylprednisolone: A Framework for Identifying Plasma Biomarkers Related to Clinical Outcomes in Pediatric ARDS 2016;4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaikh H, Boudes E, Khoja Z, Shevell M, Wintermark P. Angiogenesis dysregulation in term asphyxiated newborns treated with hypothermia 2015;10(5): e0128028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitkova V, Panek M, Janec P, et al. Endothelial Microvesicles and Soluble Markers of Endothelial Injury in Critically Ill Newborns 2018;2018:1975056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sela S, Itin A, Natanson-Yaron S, et al. A novel human-specific soluble vascular endothelial growth factor receptor 1: Cell type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Article. Circulation Research 2008;102(12):1566–1574. [DOI] [PubMed] [Google Scholar]
- 23.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Article. Nature 1987;327(6122):524–526. [DOI] [PubMed] [Google Scholar]
- 24.Levi M, Van Der Poll T. Inflammation and coagulation. Review. Critical Care Medicine 2010;38(SUPPL. 2):S26–S34. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Kakuuchi M, Maruyama I. Endotheliopathy in septic conditions: mechanistic insight into intravascular coagulation. Crit Care Mar 8 2021;25(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiRito JR, Hosgood SA, Reschke M, et al. Lysis of cold-storage-induced microvascular obstructions for ex vivo revitalization of marginal human kidneys. Am J Transplant Jul 5 2020;21(1):161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. Review. Journal of Cell Biology 2013;200(4):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Risk of Bias Assessment Summary for Studies Included in the PODIUM Endothelial Dysfunction Systematic Review (n=62 studies)
Supplemental Table 1. Studies Included in the PODIUM Endothelial Dysfunction Systematic Review (n=62 studies)
Supplemental Table 2. Performance Characteristics for Assessment Tools and Scores for Endothelial Dysfunction in Critically Ill Children (n=62 studies)


