Abstract
Synthesis of α,β-unsaturated-γ-lactams continue to attract attention due to the importance of this structural motif in organic chemistry. Herein, we report the development of a visible-light-induced excited-state copper-catalyzed [4+1] annulation reaction for the preparation of a wide range of γ-H, -OH, and -OR substituted α,β-unsaturated-γ-lactams using acrylamides as the 4-atom unit and aroyl chlorides as the 1-atom unit. This modular synthetic protocol features mild reaction conditions, broad substrate scope, and high functional group tolerance. The reaction is amenable to late-stage diversification of complex molecular architectures, including derivatives of marketed drugs. The products of the reaction can serve as versatile building blocks for further derivatization. Preliminary mechanistic studies suggest an inner-sphere catalytic cycle involving photoexcitation of the Cu(BINAP) catalyst, single-electron transfer, and capture of radical intermediates by copper species followed by reductive elimination or protonation to give the desired γ-functionalized α,β-unsaturated-γ-lactams.
Graphical Abstract

INTRODUCTION
α,β-Unsaturated-γ-lactams are core structural elements in natural and synthetic organic compounds possessing a wide diversity of important biological activities.1 For example, natural products, such as Ascosali Pyrrolidinone A, and marketed drugs, including Imrecoxib and Glimepiride, are used as antimalarial, anti-inflammatory, and anti-diabetic agents, respectively (Scheme 1A). The α,β-unsaturated-γ-lactam fragment also serves as a versatile synthetic building block and has been used in the preparation of functionalized γ-lactams2 and pyrroles.3 Consequently, considerable effort has been devoted to the synthesis of α,β-unsaturated-γ-lactam scaffolds, leading to the development of many attractive stoichiometric and catalytic synthetic strategies.4,5 For example, Wang et al. reported the Fe-catalyzed intramolecular cyclization of tertiary enamides followed by an intriguing 1,3-OH shift to afford γ-hydroxy α,β-unsaturated-γ-lactam derivatives (Scheme 1B).5d More recently, Kleji and co-workers developed an elegant Pd-catalyzed domino synthesis of γ-hydrogen α,β-unsaturated-γ-lactams.5i Despite these advances, a catalytic protocol for the preparation of γ-alkoxyl α,β-unsaturated-γ-lactam derivatives is rare.6 As such, the development of an operationally simple, modular, and unified method to access a diverse range of γ-alkoxyl, γ-hydroxy, and γ-hydrogen α,β-unsaturated-γ-lactams is highly desirable.
Scheme 1.

Applications and synthesis of α,β-unsaturated-γ-lactams.
Visible light-induced excited-state catalysis is becoming a versatile tool in organic synthesis because it allows access to an excited-state reaction landscape for the discovery of novel chemical transformations.7 The use of ecologically benign and cost-effective copper complexes (Cu: US$0.512/mol)8 replacing precious metal RuII polypyridine (Ru: US$2,015/mol) or cyclo-metallated IrIII complexes (Ir: US$30,282/mol) as photocatalysts has recently attracted increasing attention.9,10,11 In addition to the economic benefits of their use, copper photocatalysts have dual catalytic reactivity and can serve as both photocatalysts and coupling catalysts,11 participating in inner-sphere catalysis through direct engagement with radical intermediates. We have a continuing interest in advancing fundamental knowledge in excited-state and radical chemistry,12 expanding the reaction profile of Cu catalysis,12g and exploring synthetic applications of the Umpolung reactivity of acyl radicals.12b–d, 12g Accordingly, we questioned if a copper catalyst could capture a radical intermediate generated from the addition of a nucleophilic acyl radical to acrylamide and undertake a reaction pathway distinct from the Ir-photocatalyst-mediated reaction (Scheme 1C).13 Herein, we report the realization of this goal leading to the first excited-state Cu-catalyzed [4+1] annulation reaction for the construction of a broad array of valuable γ-functionalized α,β-unsaturated-γ-lactams.
RESULTS AND DISCUSSION
We began our investigation with the evaluation of a broad set of conditions, including different catalysts, ligands, and solvents (Tables S1–S3, SI). Upon exposing N,2-diphenylacrylamide (1a), 4-fluorobenzoyl chloride (2a), and n-hexanol (3r) to visible light from 100 W blue light-emitting diodes (LED) in the presence of Cu2O (10.0 mol%) and rac-BINAP (20 mol%) in a 1:1 mixture of p-xylene:DCE (0.100 M) at room temperature, we obtained the desired γ-hexyloxy α,β-unsaturated-γ-lactam 4a in 98% NMR yield (Table S4, SI).14 Control experiments showed that copper catalyst, the rac-BINAP ligand, and light are essential for the reaction, and the absence of any one of these afforded no desired product. It is worth noting that conventional Ru- and Ir-based photocatalysts failed to generate the desired product (Tables S1, SI), demonstrating the distinct reactivity of the copper photocatalyst.
With the optimized reaction conditions established, we explored the scope of the reaction by examining a variety of (hetero)aroyl chlorides, enamides, and alcohols (Table 1). The reaction tolerated a range of (hetero)aroyl chlorides with different electronic properties and functional groups (Table 1A). For example, electron-deficient aroyl chlorides substituted with halogen, nitrile, ester, trifluoromethoxyl, and trifluoromethyl groups reacted smoothly, furnishing the desired products (4a-4i) in 68–92% yield. Electron-neutral (4j) and electron-rich aroyl chlorides (4k-4l) are viable substrates. Although alkyl acyl chlorides failed, aroyl chlorides with extended π-conjugation such as a β-napthyl ring (4m) and heteroaroyl chlorides, including isoxazolyl (4n) and benzothiophenyl rings (4o), tolerate the reaction conditions and afford the corresponding γ-hexyloxy α,β-unsaturated-γ-lactams in 70–85% yield.
Table 1.
Scope of aroyl chlorides, acrylamides, and alcohols for the synthesis of γ-alkoxy, γ-hydroxy α,β-unsaturated-γ-lactamsa

|
See Supporting Information for experimental detailss. Standard conditions A: 1 (1.00 equiv), 2 (2.00 equiv), 3 (5.00 equiv), Cu2O (10.0 mol%), rac-BINAP (20.0 mol%), p-xylene:DCE (1:1, 0.100 M), 100 W blue LED, rt, 22 h. Cited yields are of isolated material following chromatography.
N,2-diphenylacrylamide (1a) was used as a coupling partner in presence of hexanol (3r).
4-Br-benzoyl chloride (2c) was used as a coupling partner in presence of 3r.
1a and 2c were used as the standard reactants.
Benzoyl chloride (2j) was used as a coupling partner.
The reaction proceeded well with a broad array of acrylamide derivatives with simple or complex molecular skeletons (Table 1B). Acrylamides bearing α-aromatic substituents with different electronic properties and substitution patterns reacted under the standard reaction conditions, affording the desired products 5a-5f in 71–84% yield. Investigations into medicinally relevant heterocyclic scaffolds demonstrated that carbazole, dibenzothiophene, and benzothiophene-derived acrylamides could be employed to generate the corresponding products (5g-5i) in good yields. α-Alkyl-substituted acrylamides (5j-5l) are also viable substrates under this protocol. Both N-aryl- and N-alkyl-protected acrylamides reacted smoothly, furnishing the corresponding products (5m, 5n) in 87% and 85% yield, respectively. Late-stage modifications of biologically active molecules are often a key to the identification of medicinal agents.15 To demonstrate the applicability of this excited-state copper-catalyzed [4+1] annulation reaction in late-stage diversification, derivatives of bio-relevant molecules, such as ibuprofen, adapalene, and piperazine were subjected to the standard reaction conditions, and delivered the desired products (5o-5q) in good yields. Thus, our strategy can increase structural diversity, synthetic efficiency, and is compatible with biologically-active pharmaceutical scaffolds.
We next sought to examine the generality of this transformation by exploring the scope of the alcohol coupling partner. As outlined in Table 1C, a wide variety of hydroxy compounds reacted efficiently in this excited-state copper-catalyzed cross-coupling protocol. Water can be used as a coupling partner to furnish the γ-hydroxy α,β-unsaturated γ-lactam (6a). A series of primary and secondary alcohols bearing trichloromethane, cyclopropane, adamantane, N-Boc piperidine, cyclohexane, a phenyl ring, and thiophene are compatible under the standard conditions, affording the desired products (6b-6m) with up to 94% yield. It is noteworthy that alkyne and alkene functionalities survive the reaction intact (6n-6o), providing useful handles for further synthetic elaboration. Naturally occurring alcohols, such as nerol and (−)-citronellol, can be used directly to furnish the corresponding products 6p and 6q in 51% and 81% yield, respectively, further demonstrating the synthetic utility of the transformation. The structure of the coupling products were unambiguously confirmed by single-crystal X-ray analysis of compound 6e (Table 1).
During the exploration of the substrate scope, 2-trifluoromethyl benzoyl chloride afforded the desired γ-hexyloxy α,β-unsaturated-γ-lactam in only 11% yield. Interestingly, careful examination of the reaction mixture led to the identification of γ-hydrogen α,β-unsaturated-γ-lactam 7a (Table 2). Fine-tuning the reaction conditions by modifying the catalysts, solvents, and removal of alcohol additives furnished the desired product 7a in 91% yield (Tables S5–S8, SI). The reaction proved to be general and tolerated (hetero)aroyl chlorides with diverse electronic and substitution patterns (Table 2A). Both electron-rich and electron-poor aroyl chlorides were converted into the corresponding α,β-unsaturated-γ-lactams (7a-7q) in good to excellent yields. Ortho-, meta-, and para-substituents on the ring are tolerated (7d-7f), although the reaction of the para-substituted substrate required the addition of 0.50 equiv of PhSiH3 to give the desired product in high yield. Our protocol was also efficient toward substrates with the pharmaceutically relevant heteroarenes (7r-7t), trifluoromethyl (7a, 7j), fluoro (7b, 7j, 7k), and trifluoromethoxyl (7l, 7m) groups. Functional groups such as nitrile (7h), chloride (7c, 7g, 7k, 7r, and 7t), or bromide (7d-7f, 7i) that are frequently reactive in typical late transition-metal catalysis are also compatible. These groups are very valuable for further orthogonal manipulations and build-up of molecular complexity.
Table 2.
Scope of aroyl chlorides and acrylamides for the synthesis of γ-hydrogen α,β-unsaturated-γ-lactamsa

|
See Supporting Information for experimental details. Standard conditions B: 1 (1.00 equiv), 2 (2.00 equiv), CuI (10.0 mol%), rac-BINAP (20.0 mol%), p-xylene (0.100 M), 100 W blue LED, rt, 22 h. Cited yields are of isolated material following chromatography.
N,2-diphenylacrylamide (1a) was used as a coupling partner.
0.50 equiv of PhSiH3 was added, 36h.
The reaction time was extended to 36 h.
2-Br-benzoyl chloride (2d) was used as a coupling partner.
The scope with respect to the acrylamides was also investigated (Table 2B). In general, acrylamides with (i) α-aryl (8a-8f, 8i, 8j), α-heteroaryl (8g-8h, 8m), and α-alkyl (8k, 8l) substituents, (ii) aryl (8a-8i, 8k-8m) or alkyl (8j) N-protecting groups, and (iii) derivatives of bioactive molecules (8m) were competent under the optimized reaction conditions, affording the desired products with up to 91% yield. It is of note that 80 of the 82 compounds reported here have not been prepared previously, demonstrating that our protocol allows access to unexplored chemical space, thus aiding the discovery of new pharmaceuticals, agrochemicals, and functional materials.
To demonstrate the synthetic utility of the α,β-unsaturated-γ-lactam products toward useful molecular scaffolds, a series of transformations were carried out (Scheme 2). Treatment of the α,β-unsaturated-γ-lactam product 7g with Pd/C in the presence of H2 (1 atm) gave the corresponding γ-lactam 9a in 68% yield with >20:1 diastereoselectivity. Epoxidation of 7g using hydrogen peroxide at 60 °C formed a compound 9b with an oxa-azabicyclohexan-2-one ring in 61% yield. Product 7g could be converted to a TBS-protected pyrrole 9c in 89% yield using tert-butyldimethylsilyl trifluoromethanesulfonate (TBSOTf) at room temperature.
Scheme 2.

Post functionalization of α,β-unsaturated-γ-lactam 7g.
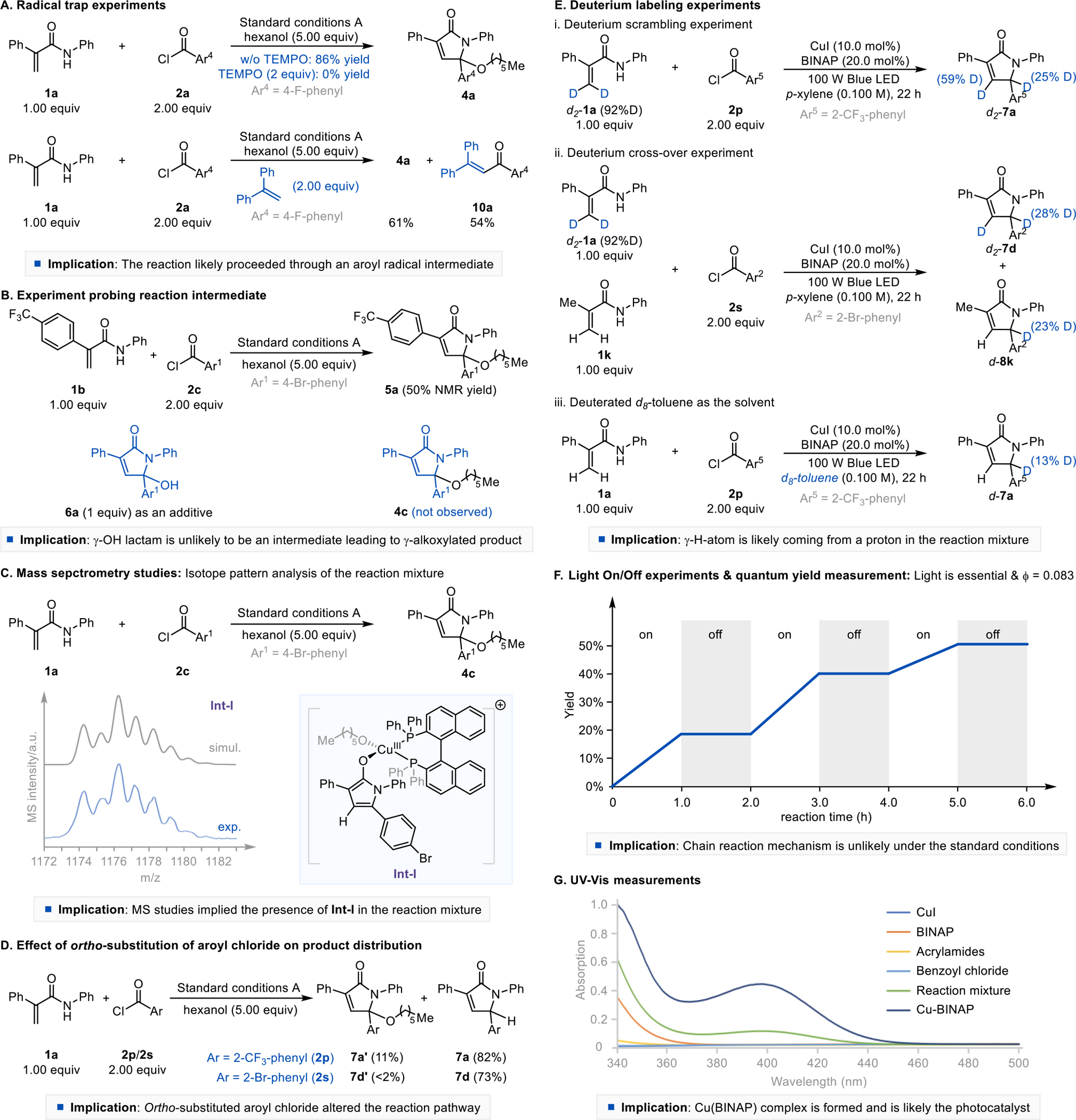
A series of mechanistic studies were conducted to gain a better understanding of the reaction mechanism. The addition of a radical scavenger, (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO), to the standard three-component reaction was found to inhibit the formation of the desired product 4a (Scheme 3A). Although we could not isolate the TEMPO-trapped adduct, we were able to obtain adduct 10a in 54% yield when 1,1-diphenylethylene was used as a radical trap (Scheme 3A). These experiments imply that the reaction is likely a radical process involving an aroyl radical intermediate. To determine whether the γ-alkoxylated product is formed through an N-acyliminium ion intermediate generated from the corresponding γ-hydroxy adduct such as 6a,6e we carried out a standard reaction using 1b and 2c as substrates with 6a as an additive (Scheme 3B). This reaction afforded product 5a only and none of the product 4c derived from 6a, indicating that γ-hydroxy adduct is unlikely to be an intermediate leading to the γ-alkoxylated product. To identify potential reaction intermediates, we also conducted a time-dependent mass spectrometry analysis of the three-component reaction using 1a and 2c as starting materials (Scheme 3C). Analysis of isotope patterns and collision-induced fragmentation of the reaction mixture implied the formation of copper complex Int-I (Scheme 3C, Fig. S5, SI). We envisioned that the copper(III) catalyst in Int-I migrates to the γ-position and subsequent reductive elimination liberates the desired product 4c, regenerating the Cu(I)BINAP catalyst.11x, 11y, 16 However, when an ortho-substituted aroyl chloride is used as the substrate, this reaction pathway is disfavored by a steric interaction between the phosphine ligand and the γ-aryl ring of Int-I. In this case, Int-I is more likely to be protonated and then tautomerized, forming γ-hydrogenated products such as 7a and 7d (Scheme 3D).
Scheme 3. Mechanistic studies.
See Supporting Information for experimental details.
The formation of the γ-hydrogenated product is very intriguing, and we performed a series of deuterium labeling experiments to investigate the origin of the γ-hydrogen atom in the product (Scheme 3E). Deuterated compound d2-1a was used as a starting material, and in this case, we obtained the desired product d2-7a with 25% deuterium incorporation at the γ-position. Also, a cross-over experiment using d2-1a and 1k as starting materials afforded the products d2-7a and d-8k with 28% and 23% γ-deuterium incorporation, respectively. GCMS analysis of the reaction mixture revealed the formation of chlorinated p-xylene (Fig. S6, SI).17 Using d8-toluene as the solvent, we obtained a product d-7a with 13% γ-deuteration. Collectively, these results suggest that the γ-hydrogen atom originates from both acrylamide and the solvent.
Light On/Off experiments showed that the reaction stops in the absence of light (Scheme 3F). The quantum yield of the standard reaction forming the γ-hydrogenated product is 0.083 (Fig. S8, SI), implying that an extended radical chain process is unlikely. The UV-Vis absorption spectra of the CuI-BINAP complex shows a peak at 397 nm with a tail into the visible region, which resembles the UV-Vis absorption spectra of the standard reaction mixture (Scheme 3G). This indicates the involvement of the CuI-BINAP photoactive complex in the reaction medium. A preformed CuI-BINAP complex was catalytically competent and afforded the desired product in 91% yield (Fig. S10, SI). Finally, 4-fluorobenzoyl chloride (2a) was shown to quench the luminescence of the CuI-BINAP complex. (Fig. S11, SI).
Based on these mechanistic insights, a plausible reaction mechanism was proposed and is depicted in Scheme 4. Blue-light photoexcitation of CuI-BINAP complex I generates excited *[CuI-BINAP] complex II, which reduces the aroyl chloride 2 (Ep of benzoyl chloride = –1.02 V vs. SCE)13c to form copper complex III, liberating aroyl radical 2’. The addition of 2’ to the alkene of acrylamide 1 affords tertiary alkyl radical IV, which is captured by III to form intermediate V. Intramolecular condensation of V furnishes intermediate VI. In the presence of alcohol, VI undergoes ligand exchange and migration of the copper center to the γ-position followed by reductive elimination, liberating the desired γ-alkoxylated product 4 and regenerating copper catalyst I. In the absence of alcohols or if an ortho-substituted aroyl chloride is used, complex VI will proceed through protonation and tautomerization to form γ-hydrogenated product 7 and copper species VIII. Reduction of VIII by p-xylene affords copper catalyst I,17 closing the catalytic cycle.
Scheme 4.

Proposed reaction mechanisms.
SUMMARY
In summary, we report the development of an excited-state copper-catalyzed [4+1] annulation reaction. This provides a unified and modular strategy for the preparation of a wide array of γ-hydrogen, -hydroxy, and -alkoxy α,β-unsaturated γ-lactams. The reaction features mild reaction conditions, has broad substrate scope, and tolerates complex molecular architecture, including derivatives of marketed drugs. The α,β-unsaturated-γ-lactam products can serve as versatile building blocks for the synthesis of useful heterocycles. Preliminary mechanistic studies suggest an inner-sphere catalytic cycle that involves photoexcitation of the Cu(BINAP) catalyst, single-electron transfer, and trapping of radical intermediates by copper species followed by reductive elimination or protonation to give the desired coupling products. Further studies of the reaction mechanism and expansion of the substrate scope as well as the asymmetric process are currently underway.
Supplementary Material
ACKNOWLEDGMENT
The research reported in this publication was partially supported by the National Institutes of General Medical Sciences (R35-GM119652 to M.-Y.N.), the National Science Foundation [CHE-1905172 to C.J.J.] The Shimadzu UPLC/MS used for portions of this work were purchased with funds from NIGMS equipment administrative supplement (R35-GM119652–04S1), Shimadzu Scientific Instruments grant, and Office of the Vice President for Research at Stony Brook University. We thank Dr Vincent M. Lynch (University of Texas, Austin) for the X-ray data of 6e. We also want to thank anonymous reviewer 2 for careful reading of them manuscript and for the comprehensive comments and suggestions.
Footnotes
ASSOCIATED CONTENT
Experimental details and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Accession Codes
CCDC2202589 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by email to data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Any additional relevant notes should be placed here.
REFERENCES
- (1).(a) Fenteany G; Standaert RF; Lane WS; Choi S; Corey EJ; Schreiber SL Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 1995, 268, 726–731. [DOI] [PubMed] [Google Scholar]; (b) Singh SB; Goetz MA; Jones ET; Bills GF; Giacobbe RA; Herranz L; Stevens-Miles S; Williams DL Jr. Oteromycin: A Novel Antagonist of Endothelin Receptor. J. Org. Chem. 1995, 60, 7040–7042. [Google Scholar]; (c) Kakeya H; Onozawa C; Sato M; Arai K; Osada H Neuritogenic Effect of Epolactaene Derivatives on Human Neuroblastoma Cells Which Lack High-Affinity Nerve Growth Factor Receptors. J. Med. Chem. 1997, 40, 391–394. [DOI] [PubMed] [Google Scholar]; (d) Micheli F; Pasquarello A; Tedesco G; Hamprecht D; Bonanomi G; Checchia A; Jaxa-Chamiec A; Damiani F; Davalli S; Donati D; et al. Diaryl substituted pyrrolidinones and pyrrolones as 5-HT2C inhibitors: Synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2006, 16, 3906–3912. [DOI] [PubMed] [Google Scholar]; (e) Feng Z; Chu F; Guo Z; Sun P Synthesis and anti-inflammatory activity of the major metabolites of imrecoxib. Bioorg. Med. Chem. Lett. 2009, 19, 2270–2272. [DOI] [PubMed] [Google Scholar]; (f) Zhu G-Y; Chen G; Liu L; Bai L-P; Jiang Z-H C-17 Lactam-Bearing Limonoids from the Twigs and Leaves of Amoora tsangii. J. Nat. Prod. 2014, 77, 983–989. [DOI] [PubMed] [Google Scholar]; (g) Caruano J; Muccioli GG; Robiette R Biologically active γ-lactams: synthesis and natural sources. Org. Biomol. Chem. 2016, 14, 10134–10156. [DOI] [PubMed] [Google Scholar]
- (2).(a) Hughes G; Kimura M; Buchwald SL Catalytic Enantioselective Conjugate Reduction of Lactones and Lactams. J. Am. Chem. Soc. 2003, 125, 11253–11258. [DOI] [PubMed] [Google Scholar]; (b) Xie Y; Zhao Y; Qian B; Yang L; Xia C; Huang H Enantioselective N-H Functionalization of Indoles with α,β-Unsaturated γ-Lactams Catalyzed by Chiral Bronsted Acids. Angew. Chem. Int. Ed. 2011, 50, 5682–5686. [DOI] [PubMed] [Google Scholar]; (c) Shao C; Yu H-J; Wu N-Y; Tian P; Wang R; Feng C-G; Lin G-Q Asymmetric Synthesis of β-Substituted γ-Lactams via Rhodium/Diene-Catalyzed 1,4-Additions: Application to the Synthesis of (R)-Baclofen and (R)-Rolipram. Org. Lett. 2011, 13, 788–791. [DOI] [PubMed] [Google Scholar]
- (3).Song T; Arseniyadis S; Cossy J Asymmetric Synthesis of α-Quaternary γ-Lactams through Palladium-Catalyzed Asymmetric Allylic Alkylation. Org. Lett. 2019, 21, 603–607. [DOI] [PubMed] [Google Scholar]
- (4).For selected examples of stoichiometric synthesis, see: Howard E; Lindsey R Jr; Theobald C Synthesis of 3-substituted 5-hydroxy-3-pyrrolin-2-ones. J. Am. Chem. Soc. 1959, 81, 4355–4358.Gaviña F; Costero A; Andreu M; Carda M; Luis S 2-Aza-2, 4-cyclopentadienone. Existence and reactivity. J. Am. Chem. Soc. 1988, 110, 4017–4018.Snider BB; Neubert BJ A novel biomimetic route to the 3-acyl-5-hydroxy-3-pyrrolin-2-one and 3-acyl-3, 4-epoxy-5-hydroxypyrrolidin-2-one ring systems. J. Org. Chem. 2004, 69, 8952–8955.Coleman RS; Walczak MC; Campbell EL Total synthesis of lucilactaene, a cell cycle inhibitor active in p53-inactive cells. J. Am. Chem. Soc. 2005, 127, 16038–16039.Adib M; Mahdavi M; Noghani MA; Bijanzadeh HR Reaction between isocyanides and chalcones: an efficient solvent-free synthesis of 5-hydroxy-3, 5-diaryl-1, 5-dihydro-2H-pyrrol-2-ones. Tetrahedron Lett. 2007, 48, 8056–8059.
- (5).For selected examples of catalytic synthesis, see: Morimoto T; Chatani N; Murai S The first catalytic carbonylative [4+ 1] cycloaddition using a 1, 3-conjugated system. A new transformation of α, β-unsaturated imines to unsaturated γ-lactams catalyzed by Ru3 (CO) 12. J. Am. Chem. Soc. 1999, 121, 1758–1759.Kang S-K; Kim K-J; Yu C-M; Hwang J-W; Do Y-K Ru-Catalyzed Cyclocarbonylation of α-and β-Allenic Sulfonamides: Synthesis of γ-and δ-Unsaturated Lactams. Org. Lett. 2001, 3, 2851–2853.Ma S; Xie H Steric Hindrance-Controlled Pd (0)-Catalyzed Coupling−Cyclization of 2, 3-Allenamides and Organic Iodides. An Efficient Synthesis of Iminolactones and γ-Hydroxy-γ-lactams. J. Org. Chem. 2002, 67, 6575–6578.Yang L; Lei C-H; Wang D-X; Huang Z-T; Wang M-X Highly Efficient and Expedient Synthesis of 5-Hydroxy-1 H-pyrrol-2-(5 H)-ones from FeCl3-Catalyzed Tandem Intramolecular Enaminic Addition of Tertiary Enamides to Ketones and 1, 3-Hydroxy Rearrangement. Org. Lett. 2010, 12, 3918–3921.Zhou Z; Liu G; Lu X Regiocontrolled coupling of aromatic and vinylic amides with α-allenols to form γ-lactams via rhodium (iii)-catalyzed C–H activation. Org. Lett. 2016, 18, 5668–5671.Mardjan MID; Parrain JL; Commeiras L Copper (I)-Catalysed Multicomponent Reaction: Straightforward Access to 5-Hydroxy-1H-pyrrol-2 (5H)-ones. Adv. Synth. Catal. 2016, 358, 543–548.Chang J; Liu B; Yang Y; Wang M Pd-Catalyzed C–S Activation/Isocyanide Insertion/Hydrogenation Enables a Selective Aerobic Oxidation/Cyclization. Org. Lett. 2016, 18, 3984–3987.Roy SJS; Mukherjee S “On water” catalytic enantioselective sulfenylation of deconjugated butyrolactams. Org. Biomol. Chem. 2017, 15, 6921–6925.Xie J; Xue S; Escudero-Adán EC; Kleij AW Domino Synthesis of α, β-Unsaturated γ-Lactams by Stereoselective Amination of α-Tertiary Allylic Alcohols. Angew. Chem. Int. Ed. 2018, 57, 16727–16731.Wang SG; Liu Y; Cramer N Asymmetric Alkenyl C-H Functionalization by Cp(x) Rh(III) forms 2H-Pyrrol-2-ones through [4+1]-Annulation of Acryl Amides and Allenes. Angew. Chem. Int. Ed. 2019, 58, 18136–18140.Chen M; Dong G Copper-catalyzed desaturation of lactones, lactams, and ketones under pH-neutral conditions. J. Am. Chem. Soc. 2019, 141, 14889–14897.Yoo W-J; Chen W; Nguyen TV; Kobayashi S One-Pot Synthesis of α, β-Unsaturated γ-Lactones and Lactams via a Sequential trans-Hydroalumination and Catalytic Carboxylation of Propargyl Alcohols and Amines with Carbon Dioxide. Org. Lett. 2020, 22, 2328–2332.
- (6).For selected examples of stoichiometric synthesis of γ-alkoxyl α,β-unsaturated-γ-lactams, see: Clark AJ; Dell CP; McDonagh JM; Geden J; Mawdsley P Oxidative 5-endo cyclization of enamides mediated by ceric ammonium nitrate. Org. Lett. 2003, 5, 2063–2066.Dias-Jurberg I; Gagosz F; Zard SZ Unusual approach to branched 3-alkynylamides and to 1, 5-dihydropyrrol-2-ones. Org. Lett. 2010, 12, 416–419.Howard JK; Hyland CJ; Just J; Smith JA Controlled oxidation of pyrroles: synthesis of highly functionalized γ-lactams. Org. Lett. 2013, 15, 1714–1717. For examples of catalytic synthesis from protected or free γ-hydroxy α,β-unsaturated-γ-lactams, see: Cuiper AD; Kellogg RM; Feringa BL Enantioselective palladium catalyzed allylic substitution of acyloxypyrrolinones by alcohols. Chem. Commun. 1998, 655–656.Maity AK; Roy S A Multimetallic Piano-Stool Ir–Sn3 Catalyst for Nucleophilic Substitution Reaction of γ-Hydroxy Lactams through N-Acyliminium Ions. J. Org. Chem. 2012, 77, 2935–2941.
- (7).For selected reviews on photoredox catalysis in organic synthesis, see: Albini A; Fagnoni M Green chemistry and photochemistry were born at the same time. Green Chem. 2004, 6, 1–6.Hoffmann N Photochemical reactions as key steps in organic synthesis. Chem. Rev. 2008, 108, 1052–1103.Houmam A Electron transfer initiated reactions: Bond formation land bond dissociation. Chem. Rev. 2008, 108, 2180–2237.Narayanam JMR; Stephenson CRJ Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113.Prier CK; Rankic DA; MacMillan DWC Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363.Schultz DM; Yoon TP Solar synthesis: prospects in visible light photocatalysis. Science 2014, 343, 1239176.Romero NA; Nicewicz DA Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166.Shaw MH; Twilton J; MacMillan DWC Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926.Skubi KL; Blum TR; Yoon TP Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074.Twilton J; Le C; Zhang P; Shaw MH; Evans RW; MacMillan DWC The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1.Matsui JK; Lang SB; Heitz DR; Molander GA Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development. ACS Catal. 2017, 7, 2563–2575.Stephenson CR; Yoon TP; MacMillan DW Visible Light Photocatalysis in Organic Chemistry; John Wiley & Sons, 2018.Chuentragool P; Kurandina D; Gevorgyan V Catalysis with Palladium Complexes Photoexcited by Visible Light. Angew. Chem. Int. Ed. 2019, 58, 11586–11598.Kancherla R; Muralirajan K; Arunachalam S; Rueping M Visible Light-Induced Excited-State Transition-Metal Catalysis. Trends Chem. 2019, 1, 510–523.Kurandina D; Chuentragool P; Gevorgyan V Transition-Metal-Catalyzed Alkyl Heck-Type Reactions. Synthesis 2019, 51, 985.Zhou W-J; Cao G-M; Zhang Z-P; Yu D-G Visible Light-induced Palladium-catalysis in Organic Synthesis. Chem. Lett. 2019, 48, 181–191.Cheng W-M; Shang R Transition Metal-Catalyzed Organic Reactions under Visible Light: Recent Developments and Future Perspectives. ACS Catal. 2020, 10, 9170–9196.Chan AY; Perry IB; Bissonnette NB; Buksh BF; Edwards GA; Frye LI; Garry OL; Lavagnino MN; Li BX; Liang Y; et al. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122, 1485–1542.Cheung KPS; Sarkar S; Gevorgyan V Visible Light-Induced Transition Metal Catalysis. Chem. Rev. 2022, 122, 1543–1625.Grosskopf J; Kratz T; Rigotti T; Bach T Enantioselective Photochemical Reactions Enabled by Triplet Energy Transfer. Chem. Rev. 2022, 122, 1626–1653.Genzink MJ; Kidd JB; Swords WB; Yoon TP Chiral Photocatalyst Structures in Asymmetric Photochemical Synthesis. Chem. Rev. 2022, 122, 1654–1716.Pitre SP; Overman LE Strategic Use of Visible-Light Photoredox Catalysis in Natural Product Synthesis. Chem. Rev. 2022, 122, 1717–1751.Lechner VM; Nappi M; Deneny PJ; Folliet S; Chu JCK; Gaunt MJ Visible-Light-Mediated Modification and Manipulation of Biomacromolecules. Chem. Rev. 2022, 122, 1752–1829.Corbin DA; Miyake GM Photoinduced Organocatalyzed Atom Transfer Radical Polymerization (O-ATRP): Precision Polymer Synthesis Using Organic Photoredox Catalysis. Chem. Rev. 2022, 122, 1830–1874.Capaldo L; Ravelli D; Fagnoni M Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C-H Bonds Elaboration. Chem. Rev. 2022, 122, 1875–1924.Holmberg-Douglas N; Nicewicz DA Photoredox-Catalyzed C-H Functionalization Reactions. Chem. Rev. 2022, 122, 1925–2016.Murray PRD; Cox JH; Chiappini ND; Roos CB; McLoughlin EA; Hejna BG; Nguyen ST; Ripberger HH; Ganley JM; Tsui E; et al. Photochemical and Electrochemical Applications of Proton-Coupled Electron Transfer in Organic Synthesis. Chem. Rev. 2022, 122, 2017–2291.Julia F; Constantin T; Leonori D Applications of Halogen-Atom Transfer (XAT) for the Generation of Carbon Radicals in Synthetic Photochemistry and Photocatalysis. Chem. Rev. 2022, 122, 2292–2352.Kwon K; Simons RT; Nandakumar M; Roizen JL Strategies to Generate Nitrogen-centered Radicals That May Rely on Photoredox Catalysis: Development in Reaction Methodology and Applications in Organic Synthesis. Chem. Rev. 2022, 122, 2353–2428.Chang L; An Q; Duan L; Feng K; Zuo Z Alkoxy Radicals See the Light: New Paradigms of Photochemical Synthesis. Chem. Rev. 2022, 122, 2429–2486.Tay NES; Lehnherr D; Rovis T Photons or Electrons? A Critical Comparison of Electrochemistry and Photoredox Catalysis for Organic Synthesis. Chem. Rev. 2022, 122, 2487–2649.Nevesely T; Wienhold M; Molloy JJ; Gilmour R Advances in the E --> Z Isomerization of Alkenes Using Small Molecule Photocatalysts. Chem. Rev. 2022, 122, 2650–2694.Allen AR; Noten EA; Stephenson CRJ Aryl Transfer Strategies Mediated by Photoinduced Electron Transfer. Chem. Rev. 2022, 122, 2695–2751.Buglioni L; Raymenants F; Slattery A; Zondag SDA; Noel T Technological Innovations in Photochemistry for Organic Synthesis: Flow Chemistry, High-Throughput Experimentation, Scale-up, and Photoelectrochemistry. Chem. Rev. 2022, 122, 2752–2906.Candish L; Collins KD; Cook GC; Douglas JJ; Gomez-Suarez A; Jolit A; Keess S Photocatalysis in the Life Science Industry. Chem. Rev. 2022, 122, 2907–2980.
- (8).Aug, 2022. https://www.dailymetalprice.com/ as of.
- (9).For selected reviews on excited-state copper catalysis, see: Paria S; Reiser O Copper in photocatalysis. ChemCatChem 2014, 6, 2477–2483.Reiser O Shining light on copper: unique opportunities for visible-light-catalyzed atom transfer radical addition reactions and related processes. Acc. Chem. Res. 2016, 49, 1990–1996.Larsen CB; Wenger OS Photoredox catalysis with metal complexes made from earth-abundant elements. Chem. - Eur. J. 2018, 24, 2039–2058.Wenger OS Photoactive complexes with earth-abundant metals. J. Am. Chem. Soc. 2018, 140, 13522–13533.Hockin BM; Li C; Robertson N; Zysman-Colman E Photoredox catalysts based on earth-abundant metal complexes. Catal. Sci. Technol. 2019, 9, 889–915.Hossain A; Bhattacharyya A; Reiser O Copper’s rapid ascent in visible-light photoredox catalysis. Science 2019, 364.Nicholls TP; Bissember AC Developments in visible-light-mediated copper photocatalysis. Tetrahedron Lett. 2019, 60, 150883.Kojima M; Matsunaga S The merger of photoredox and cobalt catalysis. Trends Chem. 2020, 2, 410–426.Zhong M; Pannecoucke X; Jubault P; Poisson T Recent advances in photocatalyzed reactions using well-defined copper (I) complexes. Beilstein J. Org. Chem. 2020, 16, 451–481.Sandoval-Pauker C; Molina-Aguirre G; Pinter B Status report on Copper (I) Complexes in Photoredox Catalysis; Photophysical and Electrochemical Properties and Future Prospects. Polyhedron 2021, 115105.
- (10).For selected examples of excited-state copper catalyzed reactions, see: Creutz SE; Lotito KJ; Fu GC; Peters JC, Photoinduced Ullmann C–N Coupling: Demonstrating the Viability of a Radical Pathway. Science 2012, 338, 647–651;Zhao W; Wurz RP; Peters JC; Fu GC, Photoinduced, Copper-Catalyzed Decarboxylative C–N Coupling to Generate Protected Amines: An Alternative to the Curtius Rearrangement. J. Am. Chem. Soc. 2017, 139, 12153–12156;Oh SH; Malpani YR; Ha N; Jung Y-S; Han SB, Vicinal Difunctionalization of Alkenes: Chlorotrifluoromethylation with Cf3so2cl by Photoredox Catalysis. Org. Lett. 2014, 16, 1310–1313;Liu Z; Chen H; Lv Y; Tan X; Shen H; Yu H-Z; Li C, Radical Carbofluorination of Unactivated Alkenes with Fluoride Ions. J. Am. Chem. Soc. 2018, 140, 6169–6175;Rawner T; Lutsker E; Kaiser CA; Reiser O, The Different Faces of Photoredox Catalysts: Visible-Light-Mediated Atom Transfer Radical Addition (Atra) Reactions of Perfluoroalkyl Iodides with Styrenes and Phenylacetylenes. ACS Catal. 2018, 8, 3950–3956;Hossain A; Engl S; Lutsker E; Reiser O, Visible-Light-Mediated Regioselective Chlorosulfonylation of Alkenes and Alkynes: Introducing the Cu (Ii) Complex [Cu (Dap) Cl2] to Photochemical Atra Reactions. ACS Catal. 2018, 9, 1103–1109;Han B; Li Y; Yu Y; Gong L, Photocatalytic Enantioselective Α-Aminoalkylation of Acyclic Imine Derivatives by a Chiral Copper Catalyst. Nat. Commun. 2019, 10, 1–9;Zhang H; Huang C; Yuan XA; Yu S, Photoexcited Chiral Copper Complex-Mediated Alkene E --> Z Isomerization Enables Kinetic Resolution. J. Am. Chem. Soc. 2022, 144, 10958–10967.
- (11).For selected examples of copper catalysts served as both photocatalysts and cross-coupling catalysts, see: Uyeda C; Tan Y; Fu GC; Peters JC A new family of nucleophiles for photoinduced, copper-catalyzed cross-couplings via single-electron transfer: Reactions of thiols with aryl halides under mild conditions (O° C). J. Am. Chem. Soc. 2013, 135, 9548–9552.Sagadevan A; Ragupathi A; Hwang KC Photoinduced Copper-Catalyzed Regioselective Synthesis of Indoles: Three-Component Coupling of Arylamines, Terminal Alkynes, and Quinones. Angew. Chem. Int. Ed. 2015, 54, 13896–13901.Bagal DB; Kachkovskyi G; Knorn M; Rawner T; Bhanage BM; Reiser O Trifluoromethylchlorosulfonylation of Alkenes: Evidence for an Inner-Sphere Mechanism by a Copper Phenanthroline Photoredox Catalyst. Angew. Chem. Int. Ed. 2015, 54, 6999–7002.Kainz QM; Matier CD; Bartoszewicz A; Zultanski SL; Peters JC; Fu GC Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science 2016, 351, 681–684.Sagadevan A; Charpe VP; Ragupathi A; Hwang KC Visible Light Copper Photoredox-Catalyzed Aerobic Oxidative Coupling of Phenols and Terminal Alkynes: Regioselective Synthesis of Functionalized Ketones via C≡C Triple Bond Cleavage. J. Am. Chem. Soc. 2017, 139, 2896–2899.Matier CD; Schwaben J; Peters JC; Fu GC Copper-catalyzed alkylation of aliphatic amines induced by visible light. J. Am. Chem. Soc. 2017, 139, 17707–17710.Lei W-L; Wang T; Feng K-W; Wu L-Z; Liu Q Visible-light-driven synthesis of 4-alkyl/aryl-2-aminothiazoles promoted by in situ generated copper photocatalyst. ACS Catal. 2017, 7, 7941–7945.Ahn JM; Peters JC; Fu GC Design of a photoredox catalyst that enables the direct synthesis of carbamate-protected primary amines via photoinduced, copper-catalyzed N-alkylation reactions of unactivated secondary halides. J. Am. Chem. Soc. 2017, 139, 18101–18106.Xiao P; Li C-X; Fang W-H; Cui G; Thiel W Mechanism of the visible-light-mediated copper-catalyzed coupling reaction of phenols and alkynes. J. Am. Chem. Soc. 2018, 140, 15099–15113.Yu XY; Zhao QQ; Chen J; Chen JR; Xiao WJ Copper-Catalyzed Radical Cross-Coupling of Redox-Active Oxime Esters, Styrenes, and Boronic Acids. Angew. Chem. Int. Ed. 2018, 57, 15505–15509.Minozzi C; Caron A; Grenier-Petel JC; Santandrea J; Collins SK Heteroleptic Copper (I)-Based Complexes for Photocatalysis: Combinatorial Assembly, Discovery, and Optimization. Angew. Chem. Int. Ed. 2018, 57, 5477–5481.Li Y; Zhou K; Wen Z; Cao S; Shen X; Lei M; Gong L Copper (II)-catalyzed asymmetric photoredox reactions: enantioselective alkylation of imines driven by visible light. J. Am. Chem. Soc. 2018, 140, 15850–15858.Hossain A; Vidyasagar A; Eichinger C; Lankes C; Phan J; Rehbein J; Reiser O Visible-Light-Accelerated Copper (II)-Catalyzed Regio-and Chemoselective Oxo-Azidation of Vinyl Arenes. Angew. Chem. Int. Ed. 2018, 57, 8288–8292.He J; Chen C; Fu GC; Peters JC Visible-light-induced, copper-catalyzed three-component coupling of alkyl halides, olefins, and trifluoromethylthiolate to generate trifluoromethyl thioethers. ACS Catal. 2018, 8, 11741–11748.Sagadevan A; Pampana VKK; Hwang KC Copper Photoredox Catalyzed A3’Coupling of Arylamines, Terminal Alkynes, and Alcohols through a Hydrogen Atom Transfer Process. Angew. Chem. Int. Ed. 2019, 58, 3838–3842.Caron A; Morin E. m.; Collins SK Bifunctional copper-based photocatalyst for reductive pinacol-type couplings. ACS Catal. 2019, 9, 9458–9464.Hossain A; Bhattacharyya A; Reiser O Copper’s rapid ascent in visible-light photoredox catalysis. Science 2019, 364.Xia HD; Li ZL; Gu QS; Dong XY; Fang JH; Du XY; Wang LL; Liu XY Photoinduced Copper-Catalyzed Asymmetric Decarboxylative Alkynylation with Terminal Alkynes. Angew. Chem. Int. Ed. 2020, 59, 16926–16932.Zhang Y; Sun Y; Chen B; Xu M; Li C; Zhang D; Zhang G Copper-catalyzed photoinduced enantioselective dual carbofunctionalization of alkenes. Org. Lett. 2020, 22, 1490–1494.Crisenza GE; Faraone A; Gandolfo E; Mazzarella D; Melchiorre P Catalytic asymmetric C–C cross-couplings enabled by photoexcitation. Nat. Chem. 2021, 13, 575–580.Yan Q; Cui W; Song X; Xu G; Jiang M; Sun K; Lv J; Yang D Sulfonylation of Aryl Halides by Visible Light/Copper Catalysis. Org. Lett. 2021, 23, 3663–3668.Chen C; Peters JC; Fu GC Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity. Nature 2021, 1–11.Qi R; Wang C; Huo Y; Chai H; Wang H; Ma Z; Liu L; Wang R; Xu Z Visible Light Induced Cu-Catalyzed Asymmetric C (sp3)–H Alkylation. J. Am. Chem. Soc. 2021, 143, 12777–12783.Chen J; Liang Y-J; Wang P-Z; Li G-Q; Zhang B; Qian H; Huan X-D; Guan W; Xiao W-J; Chen J-R Photoinduced Copper-Catalyzed Asymmetric C–O Cross-Coupling. J. Am. Chem. Soc. 2021, 143, 13382–13392.Wang PZ; Wu X; Cheng Y; Jiang M; Xiao WJ; Chen JR Photoinduced Copper-Catalyzed Asymmetric Three-Component Coupling of 1,3-Dienes: An Alternative to Kharasch-Sosnovsky Reaction. Angew. Chem. Int. Ed. 2021, 60, 22956–22962.Treacy SM; Rovis T Copper catalyzed C (sp3)–H bond alkylation via photoinduced ligand-to-metal charge transfer. J. Am. Chem. Soc. 2021, 143, 2729–2735.Xu P; López-Rojas P; Ritter T Radical decarboxylative carbometalation of benzoic acids: a solution to aromatic decarboxylative fluorination. J. Am. Chem. Soc. 2021, 143, 5349–5354.Su W; Xu P; Ritter T Decarboxylative Hydroxylation of Benzoic Acids. Angew. Chem. Int. Ed. 2021, 60, 24012–24017.Li QY; Gockel SN; Lutovsky GA; DeGlopper KS; Baldwin NJ; Bundesmann MW; Tucker JW; Bagley SW; Yoon TP Decarboxylative cross-nucleophile coupling via ligand-to-metal charge transfer photoexcitation of Cu (II) carboxylates. Nat. Chem. 2022, 14, 94–99.Ueda Y; Masuda Y; Iwai T; Imaeda K; Takeuchi H; Ueno K; Gao M; Hasegawa J.-y.; Sawamura M Photoinduced Copper-Catalyzed Asymmetric Acylation of Allylic Phosphates with Acylsilanes. J. Am. Chem. Soc. 2022, 144, 2218–2224.
- (12). Lee KN; Ngai M-Y, Recent Developments in Transition-Metal Photoredox-Catalysed Reactions of Carbonyl Derivatives. Chem. Commun. 2017, 53, 13093–13112; Banerjee A; Lei Z; Ngai M-Y, Acyl Radical Chemistry Via Visible-Light Photoredox Catalysis. Synthesis 2019, 51, 303–333; Lei Z; Banerjee A; Kusevska E; Rizzo E; Liu P; Ngai MY, Β-Selective Aroylation of Activated Alkenes by Photoredox Catalysis. Angew. Chem. Int. Ed. 2019, 58, 7318–7323; Sarkar S; Banerjee A; Yao W; Patterson EV; Ngai M-Y, Photocatalytic Radical Aroylation of Unactivated Alkenes: Pathway to Β-Functionalized 1, 4-, 1, 6-, and 1, 7-Diketones. ACS Catal. 2019, 9, 10358–10364; Zhao G; Yao W; Kevlishvili I; Mauro JN; Liu P; Ngai M-Y, Nickel-Catalyzed Radical Migratory Coupling Enables C-2 Arylation of Carbohydrates. J. Am. Chem. Soc. 2021, 143, 8590–8596; Zhao G; Yao W; Mauro JN; Ngai MY, Excited-State Palladium-Catalyzed 1,2-Spin-Center Shift Enables Selective C-2 Reduction, Deuteration, and Iodination of Carbohydrates. J. Am. Chem. Soc. 2021, 143, 1728–1734; Banerjee A; Sarkar S; Shah JA; Frederiks NC; Bazan-Bergamino EA; Johnson CJ; Ngai MY, Excited-State Copper Catalysis for the Synthesis of Heterocycles. Angew. Chem. Int. Ed. 2022, 61, e202113841; Yao W; Zhao G; Wu Y; Zhou L; Mukherjee U; Liu P; Ngai M-Y, Excited-State Palladium-Catalyzed Radical Migratory Mizoroki–Heck Reaction Enables C2-Alkenylation of Carbohydrates. J. Am. Chem. Soc. 2022, 144, 3353–3359; Zhao G; Mukherjee U; Zhou L; Wu Y; Yao W; Mauro JN; Liu P; Ngai M-Y, C2-Ketonylation of Carbohydrates Via Excited-State Palladium-Catalyzed 1, 2-Spin-Center Shift. Chem. Sci. 2022, 13, 6276–6282.
- (13).(a) Bergonzini G; Cassani C; Wallentin CJ Acyl Radicals from Aromatic Carboxylic Acids by Means of Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2015, 54, 14066–14069. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bergonzini G; Cassani C; Lorimer-Olsson H; Hörberg J; Wallentin CJ Visible-Light-Mediated Photocatalytic Difunctionalization of Olefins by Radical Acylarylation and Tandem Acylation/Semipinacol Rearrangement. Chem. - Eur. J. 2016, 22, 3292–3295. [DOI] [PubMed] [Google Scholar]; (c) Xu S-M; Chen J-Q; Liu D; Bao Y; Liang Y-M; Xu P-F Aroyl chlorides as novel acyl radical precursors via visible-light photoredox catalysis. Org. Chem. Front. 2017, 4, 1331–1335. [Google Scholar]; (d) Fan X; Lei T; Chen B; Tung C-H; Wu L-Z Photocatalytic C–C bond activation of oxime ester for acyl radical generation and application. Org. Lett. 2019, 21, 4153–4158. [DOI] [PubMed] [Google Scholar]
- (14).Using (S)-BINAP or (R)-BINAP as ligand gave comparable yields. However, no enantiomeric excess (ee) was observed under the standard reaction conditions.
- (15).Cernak T; Dykstra KD; Tyagarajan S; Vachal P; Krska SW The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. [DOI] [PubMed] [Google Scholar]
- (16).For selected reviews on Cu(III) complexes in catalysis, see: Hickman AJ; Sanford MS High-valent organometallic copper and palladium in catalysis. Nature 2012, 484, 177–185.Casitas A; Ribas X The role of organometallic copper (III) complexes in homogeneous catalysis. Chem. Sci. 2013, 4, 2301–2318; For selected examples of C-O reductive elimination from Cu(III) center, see: references 11(x) and 11(y).
- (17).The exact mechanism of how the chlorinated p-xylene was formed is unclearly and is the subject of future studies. A possible mechanism involves comproportionation of Cu(I) and Cu(III) to form Cu(II), which undergoes LMCT under photo-irradiation to liberate Cu(I) species and Cl radical. The Cl radical abstracts benzylic hydrogen to generate benzylic radical that is captured by Cu(II)Cl followed by reductive elimination, affording benzyl chloride and the Cu(I) catalyst. For examples of LMCT process of copper catalysts, see refs. 11(z), 11(aa)–11(ac).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


