Abstract
Importance
Paediatric inflammatory multisystem syndrome, temporally associated with SARS-CoV-2 (PIMS-TS) is a novel disease first identified in 2020. Recent cohort studies have described the complex presentation and symptomatology. This paper provides detailed description of the dysphagia and dysphonia symptoms, management, and outcome.
Objective
To describe dysphagia and dysphonia in PIMS-TS.
Design
Retrospective cohort study.
Setting
Single tertiary and quaternary children's hospital.
Participants
All 50 children treated for paediatric multisystem inflammatory disease between April and June 2020 were included in this study.
Main Outcome(s) and Measure(s)
Dysphonia: GRBAS Perceptual Severity Scores, Vocal Handicap Index scores and the Vocal Tract Discomfort Scale. Dysphagia: Functional Oral Intake Scale.
Results
Fifty children met the diagnostic criteria for PIMS-TS. 33 (66%) were male. Median age was 10 years (range: 1–17). 36 (72%) were of Black, Asian or minority ethnic background. Nine (18%) required specialist assessment and management of dysphagia and/or dysphonia. Five (55%) were male with a median age of 9 years 7 months (range: 1–15 years). Symptoms typically resolved within three months. Two children presented with persisting dysphonia three months post-presentation. Neurological, inflammatory, and iatrogenic causes of dysphagia and dysphonia were identified.
Conclusions and Relevance
Dysphonia and dysphagia are present in children with PIMS-TS. Further data is required to understand pathophysiology, estimate incidence, and determine prognostic factors. This preliminary data highlights the need for dysphagia and dysphonia screening and timely referral for specialist, multidisciplinary assessment and treatment to ensure short-term aspiration risk is managed and long-term, functional outcomes are optimised.
Keywords: Dysphonia, Dysphagia, PIMS-TS, SARS-CoV-2
Abbreviations: ENT, Ear nose and throat; FEES, Fibreoptic endoscopic evaluation of swallowing; MDT, Multidisciplinary; NIHR, National institute of health research; PIMS-TS, Paediatric Inflammatory Multisystem Response Syndrome associated with Covid-19; PROMS, Patient reported outcome measures; SLT, Speech and language therapist/therapy; TPN, Total parenteral nutrition; UCL, University College London; UK, United Kingdom; VFSS, Videofluoroscopic swallow study
1. Introduction
COVID-19 is a novel disease caused by the coronavirus SARS-CoV-2, first identified in Wuhan, China at the end of 2019. Evidence from the first wave of the pandemic in the UK suggested children generally presented with milder disease [1], rarely requiring hospital treatment and case-rate mortality was <0.5% [2]. In April 2020 however, it was recognised that increasing numbers of children were presenting with a severe inflammatory response requiring specialist inpatient care across the UK. This triggered a rapid review by the Royal College of Paediatrics and Child Health (RCPCH) and a new condition, now known as Paediatric Inflammatory Multisystem Response Syndrome temporally associated with SARS-Cov-2 (PIMS-TS) was defined. This syndrome has similar but distinct features to other inflammatory conditions such as hemophagocytic lymphohistiocytosis (HLH), Kawasaki disease, toxic shock syndrome and macrophage activation syndrome [3]. It has since been defined by the RCPCH as involving a persistent fever, inflammatory response and single or multi-organ dysfunction not explained by other microbial causes [4]. Whilst the majority of these cases present with a negative SARS-CoV-2 PCR, the children often present with antibodies consistent with previous SARS-CoV-2 with or without a known epidemiologic link [3]. The delayed post-viral onset and clinical manifestations of PIMS-TS are distinct to that of primary respiratory phenotypes of SARS-CoV [5]. Although children may present with respiratory features such as cough, this is not a prominent feature of the disease.
Increasing numbers of cohort studies have highlighted the complex and multi-system nature of the condition [6,7] and defined the need for multi-specialist management including cardiology, critical care, infectious diseases, and rheumatology [8].
A recent paediatric cohort study outlined the otolaryngologic manifestations of a cohort of children with PIMS-TS [9] and identified dysphonia and dysphagia as the primary reason for in-person follow up by an otolaryngologist following hospital admission for PIMS-TS. However, to date there have been no studies detailing the specific dysphagia and dysphonia presentations and outcomes of these children.
The aim of this paper is to present a detailed description of the dysphagia and dysphonia symptoms, management and outcome in the cohort of children first described by Cheong et al. [9], We suggest key time points and a care pathway for dysphonia and dysphagia assessment and intervention in children with PIMS-TS.
2. Methods
2.1. Patients
Patients meeting the RCPCH criteria for PIMS-TS [4] presenting to a tertiary children's hospital between April and June 2020 were included in this cohort study. Patient demographics, SARS-CoV-2 serology and nasopharyngeal PCR, date of presentation to hospital and intubation history were collated via retrospective review of electronic patient records. Ethnicity was classified and reported in accordance with UK Government standard groups [10]. This study was registered as a service evaluation project at Great Ormond Street Hospital (registration number 3051).
2.2. Data collection
The functional oral intake scale (FOIS) [11] was used as the primary outcome measure for dysphagia. Outcomes were recorded at initial assessment and at three months post-presentation. Clinical data regarding swallow function, including instrumental assessment results, were gathered from medical records by a specialist speech and language therapist (SLT).
Clinical voice measures included the GRBAS Perceptual Severity Scores, Vocal Handicap Index (VHI) scores and the Vocal Tract Discomfort Scale (VTDS) [[12], [13], [14]]. Measures were recorded by a specialist SLT in paediatric voice at initial and three month follow-up voice clinic appointments.
Due to the small cohort numbers, descriptive statistics are reported.
3. Results
50 patients with a confirmed diagnosis of PIMS-TS were admitted between April–June 2020. The median age was 10 years (range: 1–17). 33/50 were male and 36/50 were of Black, Asian or minority ethnic background. 12/50 of these cases had positive PCR test results detecting SARS-CoV-2 and 42/50 of these cases presented with positive IgG antibodies against SARS-CoV-2. 38/50 children required paediatric intensive care (PICU) admission and 18/50 required intubation and mechanical ventilation. No children received a tracheostomy.
As previously published, 18/50 (36%) presented with parent reported dysphonia and 18/50 (38%) with parent reported dysphagia during their acute admission [9]. Of those 50 patients, nine were identified as requiring specialist assessment for dysphonia and/or dysphagia; one dysphagia only, three dysphagia and dysphonia and five isolated dysphonia. The median age of these patients was 9 years 7 months (range: 1–15 years). 5/9 (55%) were male. The mean time to referral for those identified with a dysphagia ± dysphonia was 13.5 days and for those with an isolated dysphonia was 71.6 days post presentation.
3.1. Dysphonia
Eight children (16%) required follow-up SLT assessment for persisting dysphonia post-discharge from hospital. The median age of the children was 10 years 8 months (range: 8–15 years). 5/8 children were intubated for a median number of 4 days (range: 1–12 days). One child had a history suggestive of a pre-existing dysphonia exacerbated by PIMS-TS symptomology. For the children presenting with co-existing dysphagia, the dysphonia was often documented in the clinical notes during admission but referral for in-depth assessment of the dysphonia was not made at the time.
3.2. Assessment and management of dysphonia
The overall voice quality severity score in the eight children with persisting clinical dysphonia ranged from mild to severe with the most prominent features being asthenia and breathiness. In 2/8 cases additional adaptive strain/muscle tension was identified. Challenges with pitch control were noted in two patients and subsequently confirmed to be associated with unilateral neurological weakness. Multidisciplinary team (MDT) assessment including laryngoscopy with stroboscopy was carried out on all 8 cases (with the MDT Paediatric Voice Clinic re-established in early June). Findings included generalised neurological pharyngeal and laryngeal weakness (3/8), unilateral vocal fold weakness (2/8), generalised inflammatory pharyngeal and laryngeal response (2/8) and one patient showed no organic changes. One case had a co-occurring candida infection associated with prolonged steroid management. 4/8 children reported symptoms of vocal tract discomfort, consistent with laryngeal findings, including soreness, tightness, and dryness. Self-reported psychosocial impact as evaluated using the VHI varied between minimal and severe impact and was not related to the level of perceptual voice severity. Table 1 outlines dysphonia presentation at initial outpatient appointment and associated dysphonia management.
Table 1.
Recorded reason for initial referral and voice outcome measures at initial voice outpatient clinic.
| Patient | Reason for referral | Days from SLT/ENT screen to initial voice outpatient clinic appointment | GRBAS* | VHI** | VTDS Measures*** | Intervention Type |
|---|---|---|---|---|---|---|
| 1 | “Breathy, asthenic voice quality ranging from mild dysphonia to some periods of complete aphonia” | 45 | G2 R1-2 B1-2 A2 S1 | 20/120 | Mild consistent dryness, mild occasional tightness and aching | Medication Voice therapy |
| 2 | “Moderate-severe dysphonia” | 66 | G1 R0-1 B1 A1 S0 diplophonia in upper pitch range | 67/120 | Occasional dryness | Injection medialisation Voice therapy |
| 3 | “Hoarse voice” | 17 | G1-2 R1-2 B1-2 A1 S1-2 | Ø | Previous Soreness. | Voice therapy |
| Incomplete as difficulties being heard via remote clinic | ||||||
| 4 | “Dysphonia” | 10 | G1-2 R0 B1 A1-2 S0 | 55/120 | Frequent tickling and irritability in the larynx. | Voice therapy |
| 5 | “Altered voice that has persisted” | 17 | NAD | 7/20 WNL | NAD | Prednisolone treatment in acute phase |
| Voice care advice and discharge | ||||||
| 6 | “++quiet. Hoarse, harsh quality” | 37 | G0-1 R0-1 B0-1 A0 S0-1 | Ø | Ø | Voice care advice |
| Not completed as via remote clinic | Not completed as via remote clinic | |||||
| 7 | “Aphonia” | 72 | G0-1 R0-1 B0-1 A0-1 S0 | 7/20 WNL | Frequent mild tickling | Voice care advice and discharge |
| 8 | “Severe dysphonia” | 31 | G0 R0 B0 A1 S0 | 48/120 | Tightness, aching, soreness, a lump in the throat and dryness | Discharge no follow up |
| Results impacted by co-existing features of social communication disorder. |
Management was specific to the child's own psychosocial report of voice problems, their communication environment, vocal requirements and the confirmed aetiology. Management included voice care and conservation advice and physiological voice exercises. One child had a vocal fold medialisation injection and post-operative direct voice therapy. 6/8 cases were discharged with resolution of symptoms by six months but in 2/8 cases the dysphonia persisted.
3.3. Dysphagia
Four children (8%) required specialist SLT assessment and intervention for dysphagia. The median age of these children on admission was 10 years 5 months (range: 1–15 years). All required intubation and one patient required ECMO. The median number of days intubated was 6.5 days (range: 3–13 days). None of the children had any pre-existing dysphagia.
3.4. Assessment and management of dysphagia
All four children received total parenteral nutrition (TPN) or nasogastric tube feeds whilst intubated. 3/4 of the children were referred directly to SLT following observed concerns of aspiration by the nursing staff. Of these children, two were initially able to tolerate a modified texture diet alongside nasogastric tube feeds and one child was placed nil oral. The median time to resolution of symptoms was 45.5 days (range: 28–127 days).
Bedside clinical assessment of swallowing was completed for all four children. Two of the children received a videofluoroscopic swallow study (VFSS) and one child a fiberoptic endoscopic evaluation of swallowing assessment (FEES) to characterise swallow physiology and guide management of aspiration risk. Table 2 outlines feeding outcomes, hypothesised causes of the dysphagia and management for children assessed.
Table 2.
Dysphagia presentation and outcome.
| Patient | FOIS at initial assessment (appendix 1) | FOIS 3 months post assessment | Instrumental assessment | Key findings from instrumental ax | Hypothesised cause of dysphagia |
|---|---|---|---|---|---|
| 1 | 1 | 6 | Videofluoroscopy swallow study | Reduced soft palate elevation leading to escape of contrast to the nasal cavity. | Cranial neuropathy |
| Partial epiglottic inversion. | |||||
| Diminished pharyngeal stripping wave and reduced base of tongue retraction. | |||||
| Reduced duration of pharyngoesophageal opening leading to partial obstruction of the bolus through the PES. | |||||
| Collection of residue within the pharyngeal structures. | |||||
| Aspiration of pharyngeal residue. | |||||
| 2 | 1 | 6 | Fibreoptic endoscopic evaluation of swallowing | Base of tongue candida and diffuse inflammation throughout the pharynx. | Myopathy |
| Swallow initiation prompt with good vestibular closure and epiglottic inversion. | |||||
| Images indicate mild left sided weakness. Vallecular residue was observed with assessment duration. | |||||
| 3 | 2 | 7 | No | N/A | Myopathy (myopathic changes on EMG) |
| 4 | 5 | 7 | Videofluoroscopy swallow study | Swallow initiation at the level of the pyriform sinus. | Iatrogenic laryngeal nerve injury |
| Partial approximation of arytenoids to epiglottic petiole. | |||||
| Incomplete laryngeal vestibule closure. | |||||
| Persistent Laryngeal penetration. |
4. Discussion
This paper describes the dysphonia and dysphagia outcomes of a cohort of children admitted to a tertiary level hospital with a diagnosis of PIMS-TS. In our experience, children with dysphagia require early management of aspiration risk but have good potential for spontaneous recovery within three months, regardless of severity at initial presentation. Children may present with persisting dysphonia post hospital discharge, but this also typically resolves with targeted intervention, within six months.
Causes of dysphonia and dysphagia in our cohort were varied. All of the children intubated in this cohort (36%) presented with moderate-severe impairment in physical functioning post extubation, as determined by a physiotherapist. Whilst this impairment in physical functioning may have resulted from critical illness myopathy or as an outcome of the inflammatory process in PIMS-TS, it is reasonable to assume a proportion of children presenting with dysphonia or dysphagia did so as a result of their intubation and critical illness. Post extubation dysphagia is well documented in the literature [[15], [16], [17]].
It is also plausible that the dysphonia and dysphagia were caused by the disease itself. A small number of studies report dysphagia as a complication of inflammatory conditions, including Kawasaki disease [[18], [19], [20]] and HLH [21]. Dysphonia in our cohort was not limited to those who became critically ill or required intubation, suggesting the inflammatory process itself played a causal role. In the context of dysphagia, one child presented with multiple cranial neuropathies including velopharyngeal dysfunction as an early symptom of PIMS-TS. This was characterised by food and fluids coming out of his nose when eating and drinking. Neurological impairment in PIMS-TS has been associated with higher systemic inflammatory markers [22].
In some children presentations were also associated with a secondary response to treatment methods, for example diffuse laryngeal candida secondary to the use of high dose steroidal treatments or laryngeal nerve injury as an iatrogenic consequence of treatment.
There is limited evidence of dysphonia occurring secondary to pulmonary complications such as reduced breath support or cough as was predicted given the differences in disease mechanism between respiratory phenotype of SARS-CoV-2 and the delayed inflammatory response of PIMS-TS [5]. Efforts should be made to identify the underlying physiology and aetiology of the dysphagia and/or dysphonia in order to develop targeted interventions.
To do this, instrumental assessment is required, involving radiological examination (VFSS) or endoscopic evaluation (laryngoscopy, stroboscopy or FEES). These were identified as aerosol generating procedures (AGPs) early in the COVID-19 pandemic and were not recommended due to the high risk of disease transmission [23]. Specific guidance on AGPs and disease transmission in children was not available and remains limited. At our centre, we utilised comprehensive risk assessment with appropriate personal protective equipment (PPE) [24] to carry out VFSS throughout this period and endoscopic assessments from June 2020. Given the limited data available for this novel disease and varied dysphonia and dysphagia presentations described in our cohort, instrumental assessment is vital to identify appropriate management.
Higher numbers of dysphonia and dysphagia were identified using parent reported outcome measures (previously reported) [9]. This may be due to the transient nature of presenting dysphonia or dysphagia. Alternatively the PROM tool used identified those with changes to their eating and drinking which were not a true dysphagia, such as requiring non-oral feeding while intubated. Discrepancy between PROMs and clinician reported outcomes have also been reported previously [25] however, there is a possibility that a number of children who required SLT assessment were not referred. Therefore, screening of all patients is recommended.
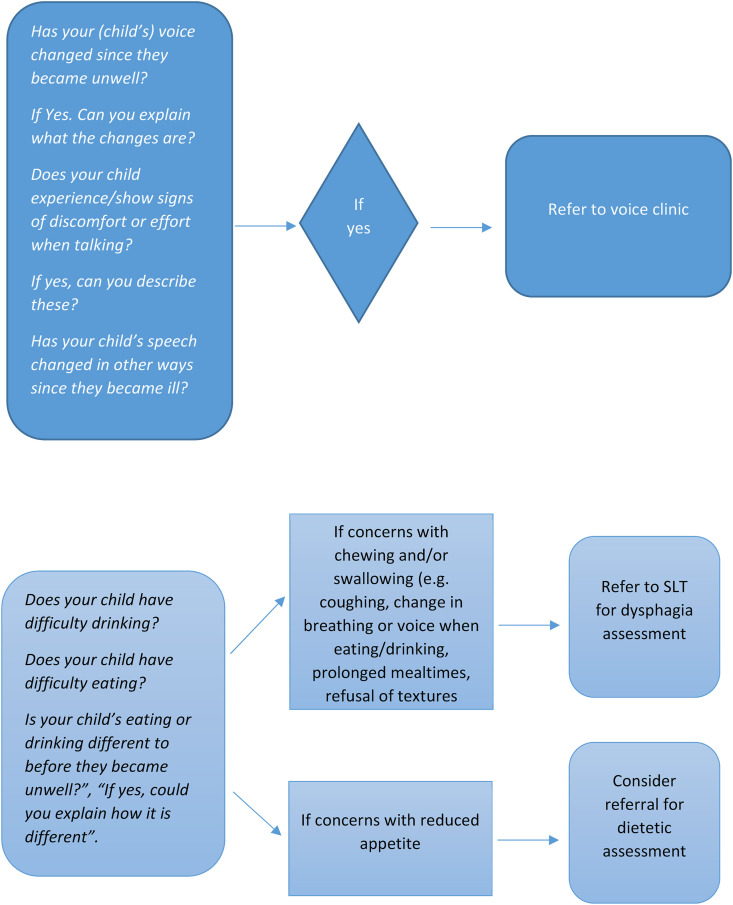
Children with dysphagia were referred to SLT on average 58 days sooner than children presenting with dysphonia alone. Dysphagia is often associated with increased length of hospital admission due to potential complications such as aspiration pneumonia and dehydration [26]. Therefore, timely identification and management of dysphagia is required in order to minimise complications [27], aid return to oral feeding where possible, and support decisions surrounding the need for supplementary feeding methods such as nasogastric tube feeds. We recommend dysphagia is considered in children with PIMS-TS requiring intubation and ventilation. Where intubation has not been necessary, screening questions for dysphagia are outlined (Fig. 1 ).
Fig. 1.
Recommended dysphonia/dysphagia screening questions during acute ward admission (parent proxy).
Dysphonia assessment and management occurred following hospital discharge. Only one child was seen on intensive care to support their dysphonia. Intervention involved supporting functional communication in an open-plan ward environment where mask and visor use additionally impacted. Comprehensive dysphonia assessment during hyperacute and acute stage treatment was not a priority for patients and their families. Often, dysphonia was only recognised as a priority by children and their parents when the child returned home or to school. We therefore recommend that screening for dysphonia occurs as part of multidisciplinary PIMS-TS follow up clinics with access to ENT/SLT voice clinic assessment where necessary.
4.1. Limitations
A limitation of this study is that it includes data from a single centre. Where outcome measures allowed, data was collected from retrospective review of the notes rather than prospective assessment. This means that there are some omissions of outcome measures or variability in the time-frame in which initial outcome measures were collected. Restrictions on outpatient appointments at time of data collection, including the use of virtual web-based clinics, also prevented some outcome measures being collected.
4.2. Further study
This paper outlines the possible dysphonia and dysphagia consequences of PIMS-TS. To date there has been no reported outcomes of the dysphagia and dysphonia consequences of adolescents presenting with COVID-19 respiratory presentations, despite the high incidence of both seen in adult populations [28,29]. This is a possible area of further study.
5. Conclusions
Children with PIMS-TS may present with dysphonia and/or dysphonia arising from a range of different causes. Specialist ENT and SLT assessment of voice and swallowing is required at different time points along the patient journey. Prompt SLT swallow assessment supports safe progression with oral intake within the acute and initial community step-down phases. Joint ENT and SLT outpatient clinics are necessary for the longer term management of dysphonia.
Funding
Rhiannon Halfpenny is currently undertaking a pre-doctoral clinical academic fellowship funded by the National Institute for Health Research (NIHR) (award reference: NIHR300391).
Alexandra Stewart is currently undertaking a PhD funded by the National Institute for Health Research (NIHR) (award reference: ICA-CDRF-2018-04-ST2-042).
Research at Great Ormond Street Hospital for Children NHS Trust is supported by the NIHR GOS/ICH Biomedical Research Centre (BRC). This paper presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgements
We would like to acknowledge the following for their support in data collection and reviewing of this work:
Justin Penner, MD, FRCPC, MRCPCH, MSc. DTMH.
Nicola Gorb, BA (Hons) PGdip MRCSLT, HCPC.
We thank the ENT and Speech and Language Therapy Departments at Great Ormond Street Hospital.
References
- 1.Swann O.V., Holden K.A., Turtle L., Pollock L., Fairfield C.J., Drake T.M., Seth S., Egan C., Hardwick H.E., Halpin S., Girvan M., Donohue C., Pritchard M., Patel L.B., Ladhani S., Sigfrid L., Sinha I.P., Olliaro P.L., Nguyen-Van-Tam J.S., Horby P.W., Merson L., Carson G., Dunning J., Openshaw P.J.M., Baillie J.K., Harrison E.M., Docherty A.B., Semple M.G. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:5. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladhani S.N., Amin-Chowdhury Z., Davies H.G., Aiano F., Hayden I., Lacy J., Sinnathamby M., De Lusignan S., Demirjian A., Whittaker H., Andrews N., Zambon M., Hopkins S., Ramsay M.E. COVID-19 in children: analysis of the first pandemic peak in England. Arch. Dis. Child. 2020;105 doi: 10.1136/archdischild-2020-320042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G., Johnson M., Griffiths B., du Pré P., Mohammad Z., Deep A., Playfor S., Singh D., Inwald D., Jardine M., Ross O., Shetty N., Worrall M., Sinha R., Koul A., Whittaker E., Vyas H., Scholefield B.R., Ramnarayan P. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc. Heal. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS) - guidance for clinicians. https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance RCPCH, (n.d.) (accessed March 5, 2021)
- 5.Penner J., Abdel-Mannan O., Grant K., Maillard S., Kucera F., Hassell J., Eyre M., Berger Z., Hacohen Y., Moshal K., Group G.P.M. A new disease with unknown sequelae: six-month multidisciplinary follow-up and outcomes of paediatric inflammatory multisystem syndrome (PIMS-TS) patients at a UK tertiary paediatric centre. SSRN Electron. J. 2021;4642:1–10. doi: 10.2139/ssrn.3798557. [DOI] [PubMed] [Google Scholar]
- 6.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., Ramnarayan P., Fraisse A., Miller O., Davies P., Kucera F., Brierley J., McDougall M., Carter M., Tremoulet A., Shimizu C., Herberg J., Burns J.C., Lyall H., Levin M. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA, J. Am. Med. Assoc. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felsenstein S., Willis E., Lythgoe H., McCann L., Cleary A., Mahmood K., Porter D., Jones J., McDonagh J., Chieng A., Varnier G., Hughes S., Boullier M., Ryan F., Awogbemi O., Soda G., Duong P., Pain C., Riley P., Hedrich C.M. Presentation, treatment response and short-term outcomes in paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS) J. Clin. Med. 2020;9:3293. doi: 10.3390/jcm9103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harwood R., Allin B., Jones C.E., Whittaker E., Ramnarayan P., Ramanan A.V., Kaleem M., Tulloh R., Peters M.J., Almond S., Davis P.J., Levin M., Tometzki A., Faust S.N., Knight M., Kenny S., Agbeko R., Aragon O., Baird J., Bamford A., Bereford M., Bharucha T., Brogan P., Butler K., Carroll E., Cathie K., Chikermane A., Christie S., Clark M., Deri A., Doherty C., Drysdale S., Duong P., Durairaj S., Emonts M., Evans J., Fraser J., Hackett S., Hague R., Heath P., Herberg J., Ilina M., Jay N., Kelly D., Kerrison C., Kraft J., Leahy A., Linney M., Lyall H., McCann L., McMaster P., Miller O., O'Riordan S., Owens S., Pain C., Patel S., Pathan N., Pauling J., Porter D., Prendergast A., Ravi K., Riorden A., Roderick M., Scholefield B.R., Semple M.G., Sen E., Shackley F., Sinha I., Tibby S., Verganano S., Welch S.B., Wilkinson N., Wood M., Yardley I. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc. Heal. 2021;5:133–141. doi: 10.1016/S2352-4642(20)30304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheong R.C.T., Jephson C., Frauenfelder C., Cavalli L., Moshal K., Butler C.R., Wyatt M.E. Otolaryngologic manifestations in pediatric inflammatory multisystem syndrome temporally associated with COVID-19. JAMA Otolaryngol. Head Neck Surg. 2021 doi: 10.1001/jamaoto.2020.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.List of ethnic groups - GOV.UK. https://www.ethnicity-facts-figures.service.gov.uk/style-guide/ethnic-groups n.d. (accessed March 6, 2021)
- 11.Crary M.A., Carnaby Mann G.D., Groher M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005;86:1516–1520. doi: 10.1016/j.apmr.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 12.Hirano M., McCormick K.R. Clinical examination of voice by minoru hirano. J. Acoust. Soc. Am. 1986;80:1273. doi: 10.1121/1.393788. 1273. [DOI] [Google Scholar]
- 13.Jacobson B.H., Johnson A., Grywalski C., Silbergleit A., Jacobson G., Benninger M.S., Newman C.W. The voice Handicap Index (VHI): development and validation. Am. J. Speech Lang. Pathol. 1997;6:66–69. doi: 10.1044/1058-0360.0603.66. [DOI] [Google Scholar]
- 14.Mathieson L., Hirani S.P., Epstein R., Baken R.J., Wood G., Rubin J.S. Laryngeal manual therapy: a preliminary study to examine its treatment effects in the management of muscle tension dysphonia. J. Voice. 2009;23:353–366. doi: 10.1016/j.jvoice.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmeister J., Zaborek N., Thibeault S.L. Postextubation dysphagia in pediatric populations: incidence, risk factors, and outcomes. J. Pediatr. 2019;211:126–133.e1. doi: 10.1016/j.jpeds.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Brodsky M.B., Huang M., Shanholtz C., Mendez-Tellez P.A., Palmer J.B., Colantuoni E., Needham D.M. Recovery from dysphagia symptoms after oral endotracheal intubation in acute respiratory distress syndrome survivors: a 5-year longitudinal study. Ann. Am. Thorac. Soc. 2017;14:376–383. doi: 10.1513/AnnalsATS.201606-455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rassameehiran S., Klomjit S., Mankongpaisarnrung C., Rakvit A. Postextubation dysphagia. Baylor Univ. Med. Cent. Proc. 2015;28:18–20. doi: 10.1080/08998280.2015.11929174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson R.M., Little J.R., Storch G.A. Kawasaki-like syndromes associated with human immunodeficiency virus infection. Clin. Infect. Dis. 2001;32:1628–1634. doi: 10.1086/320523. [DOI] [PubMed] [Google Scholar]
- 19.Kontopoulou T., Kontopoulos D.G., Vaidakis E., Mousoulis G.P. Adult Kawasaki disease in a European patient: a case report and review of the literature. J. Med. Case Rep. 2015;9:1–7. doi: 10.1186/s13256-015-0516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sève P., Stankovic K., Smail A., Durand D.V., Marchand G., Broussolle C. Adult Kawasaki Disease: report of two cases and literature review. Semin. Arthritis Rheum. 2005;34:785–792. doi: 10.1016/j.semarthrit.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Radmanesh F., Rodriguez-Pla A., Pincus M.D., Burns J.D. Severe cerebral involvement in adult-onset hemophagocytic lymphohistiocytosis. J. Clin. Neurosci. 2020;76:236–237. doi: 10.1016/j.jocn.2020.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sa M., Mirza L., Carter M., Carlton Jones L., Gowda V., Handforth J., Hedderly T., Kenny J., Lascelles K., Lin J.P., Lumsden D., McDougall M., Miller O., Rossor T., Shivamurthy V., Siddiqui A., Singh R., Tang S., White M., Byrne S., Lim M. Systemic inflammation is associated with neurologic involvement in pediatric inflammatory multisystem syndrome associated with SARS-CoV-2. Neurol. Neuroimmunol. Neuroinflammation. 2021;8 doi: 10.1212/NXI.0000000000000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolton L., Mills C., Wallace S., Brady M.C. Aerosol generating procedures, dysphagia assessment and COVID‐19: a rapid review. Int. J. Lang. Commun. Disord. 2020;55:629–636. doi: 10.1111/1460-6984.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.COVID-19 and Speech and Language Therapy – Guidance. https://www.rcslt.org/learning/rcslt-guidance/ RCSLT, (n.d.) (accessed March 15, 2021)
- 25.Rouhani M.J., Clunie G., Thong G., Lovell L., Roe J., Ashcroft M., Holroyd A., Sandhu G., Al Yaghchi C. Laryngoscope; 2020. A Prospective Study of Voice, Swallow, and Airway Outcomes Following Tracheostomy for COVID-19. [DOI] [PubMed] [Google Scholar]
- 26.Attrill S., White S., Murray J., Hammond S., Doeltgen S. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: a systematic review. BMC Health Serv. Res. 2018;18:594. doi: 10.1186/s12913-018-3376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman K.W., Yu G.P., Schaefer S.D. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch. Otolaryngol. Head Neck Surg. 2010;136:784–789. doi: 10.1001/archoto.2010.129. [DOI] [PubMed] [Google Scholar]
- 28.Archer S.K., Iezzi C.M., Gilpin L. Swallowing and voice outcomes in patients hospitalised with COVID-19: an observational cohort study. Arch. Phys. Med. Rehabil. 2021 doi: 10.1016/j.apmr.2021.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson C., Capewell R., Ellis S., Matthews S., Adamson S., Wood M., Fitch L., Reid K., Shaw M., Wheeler J., Pracy P., Nankivell P., Sharma N. Dysphagia presentation and management following coronavirus disease 2019: an acute care tertiary centre experience. J. Laryngol. Otol. 2020;134:981–986. doi: 10.1017/S0022215120002443. [DOI] [PMC free article] [PubMed] [Google Scholar]