Abstract
Background and study aims Mucinous pancreatic cystic lesions (PCLs) have the potential for malignant transformation, for which the only accepted curative modality is surgery. A novel intracystic therapy with large surface area microparticle paclitaxel (LSAM-PTX) may treat PCLs without local or systemic toxicities. Safety and preliminary efficacy of LSAM-PTX for the treatment of PCLs administered by endoscopic ultrasound-guided fine-needle injection (EUS-FNI) was evaluated.
Patients and methods Ten subjects with confirmed PCLs (size > 1.5 cm) received intracystic LSAM-PTX via EUS-FNI at volumes equal to those aspirated from the cyst in sequential cohorts at 6, 10, and 15 mg/mL in a standard “3 + 3” dose-escalation protocol. The highest dose with acceptable safety and tolerability was taken into the confirmatory phase where nine additional subjects received two injections of LSAM-PTX 12 weeks apart. Subjects were followed for 6 months after initial LSAM-PTX treatment for endpoints including: adverse events (AEs), tolerability, pharmacokinetic analysis of systemic paclitaxel drug levels, and change in cyst volume.
Results Nineteen subjects completed the study. No dose-limiting toxicities, treatment-related serious AEs, or clinically significant laboratory changes were reported. Systemic paclitaxel concentrations did not exceed 3.5 ng/mL at any timepoint measured and fell below 1 ng/mL by Week 2, supporting the lack of systemic toxicity. By Week 24 a cyst volume reduction (10–78 %) was seen in 70.6 % of subjects.
Conclusions Intracystic injection of LSAM-PTX into mucinous PCLs resulted in no significant AEs, a lack of systemic absorption, and resulted in reduction of cyst volume over a 6 month period.
Introduction
Prevalence of pancreatic cystic lesions (PCLs) in an asymptomatic population is reported from 2.4 % to 13.5 %, increasing with age, including 10 % of the population over the age of 70 and 37 % over the age of 80 1 2 . Mucinous pancreatic neoplasms comprise approximately 61 % of PCLs divided into two categories: intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) 3 . IPMNs, the most common neoplastic PCLs, present as main duct or branch duct IPMNs, which have up to a 25 % risk of malignant transformation and are candidates for surveillance at routine intervals and surgical resection depending on change in clinical course 4 5 .
Because of concerns about mucin leakage, pancreatic fistulae formation, and recurrence, the standard surgical treatment for IPMNs and MCNs is partial pancreatectomy with lymph node dissection. Following surgical resection, the rate of recurrence can be as high as 20 % 6 . Untreated high-risk cysts may become malignant while under observation. It is difficult to identify cyst histology without resection with the discrepancy between pre-resection and post-resection diagnosis of PCLs reported as high as 33 % 7 . Current methods for determining malignancy lack specificity and sensitivity 8 9 10 11 12 . Cyst diameter is unreliable and does not rule out malignancy 13 14 . Evaluation of histological and morphological appearance, and assessment of molecular and genetic aspects, are more recently being used to assess risk of progression 15 16 . However, there still remains a need for less-invasive treatment of these lesions, given the morbidity, mortality, and risk associated with pancreatic surgery.
Several studies using endoscopic ultrasound (EUS)-guided ablation have been reported 17 18 19 20 21 22 23 . In a review pooling data from eight studies, complete cyst resolution with EUS-guided ablation following injection of paclitaxel-based regimens was seen in 63.6 % (221 of 347) with adverse events (AEs) seen in 15 % (52 of 347) of subjects 24 . In a review of 16 publications that included regimens of paclitaxel, alone and in combination with gemcitabine, with and without ethanol lavage, a complete response was seen in 50 % to 78 % of subjects in studies that ranged from 10 to 164 subjects with follow-up of 9 to 72 months 25 . Pancreatitis occurred in up to 14 % of subjects, abdominal pain was reported in all studies and intracystic hemorrhage and abscesses were reported in larger studies 20 .
A particle-engineered form of paclitaxel has been developed, resulting in large surface area microparticle paclitaxel (LSAM-PTX, NanoPac, CritiTech, Inc., Lawrence, Kansas, United States) with a mean particle size of 3 to 4 microns, surface area > 24 m 2 /g and containing approximately 2 billion paclitaxel molecules each, to address the limitation of short-term, intermittent systemic exposure to the cytotoxic agent when injected into lesions or tumors 26 27 . LSAM-PTX and LSAM-DTX (LSAM docetaxel) are undergoing clinical trials as intratumoral therapy for a number of solid carcinomas 27 28 .
This early-phase study evaluates the safety, tolerability, and preliminary efficacy of LSAM-PTX injected into mucinous PCLs.
Patients and methods
Study design
This prospective, multicenter open label clinical trial (ClinicalTrials.gov Identifier: NCT03188991, registered June 16, 2017) was approved by the Institutional Review Boards of all participating sites prior to subjects being consented and enrolled. Eligible subjects over the age of 18 years with a recently confirmed unilocular mucinous PCL ≥ 1.5 and ≤ 4 cm on computed tomography (CT) scan were included in the study. Recently confirmed mucinous cystic pancreatic neoplasms were confirmed by presence of mucin, cyst fluid carcinoembryonic antigen above 192 U/L, or other reliable diagnostic means such as endomicroscopy; KRAS analysis could also be performed at the discretion of the investigator.
Subjects were required to have normal hematologic, hepatic, and renal function. Exclusion criteria included positive cytology indicating malignancy, thrombotic or embolic events, known hypersensitivity to the study agent, known drug or alcohol abuse, and pregnant or breastfeeding women. Subjects were followed for 6 months after the first injection for clinical endpoints including safety and tolerability, pharmacokinetic (PK) analysis of systemic paclitaxel drug levels, and cyst volume changes (reported by CT scan at 3 and 6 months).
Interventional protocol
All subjects received parenteral antibiotic prophylaxis during the endoscopic procedure. The subject was positioned in the left lateral decubitus position and sedated using monitored anesthesia care or general anesthesia. A linear array echoendoscope was inserted via the mouth and advanced to the stomach or duodenum, whichever provided the better access to the cyst. The cyst was measured using electronic calipers and the size recorded. A 22-gauge fine-needle aspiration (FNA) needle was used to perform the intervention. Doppler ultrasound imaging was used to verify lack of intervening vascular structures in the path to the cyst. The needle tip was maintained in the cyst for the duration of the procedure. Using a syringe, the cyst fluid was aspirated, and the withdrawn volume documented. The syringe was replaced with one filled with LSAM-PTX at a volume at least equal to that aspirated from the cyst and this was injected into the cyst.
Treatment
LSAM-PTX was administered to subjects enrolled in sequential, escalating cohorts in a standard ‘3 + 3’ dose-escalation protocol (6, 10, or 15 mg/mL) at a volume equivalent to at least that removed from the cyst. Prior to administration, LSAM-PTX was reconstituted with 1 % polysorbate 80, NF in 0.9 % sodium chloride to form a suspension which is further diluted with 0.9 % sodium chloride to achieve the desired concentration. Subjects underwent endoscopic ultrasound-guided FNA (EUS-FNA) of cyst fluid followed by EUS-guided fine-needle injection (EUS-FNI) of LSAM-PTX directly into the cyst. The LSAM-PTX was not aspirated, creating a depot of paclitaxel allowing slow release of particles and prolonged exposure of the cyst epithelium to high drug concentrations ( Supplementary Fig. 1 ).
The highest dose determined to have a good safety and tolerability profile was taken into the second phase of the study, in which additional subjects received two injections of LSAM-PTX 12 weeks apart. At the 12 week visit, cyst fluid obtained by EUS-FNA was sent for paclitaxel evaluation, when possible, prior to the second injection.
Safety
Data including AEs, lab assessments, vital signs, and concomitant medications from all subjects in a cohort was evaluated by the Data Safety Monitoring Board (DSMB) to determine whether the dose received was considered safe, tolerable, and if dose escalation could proceed. The DSMB reviewed the data once subjects had completed the 2 week follow-up visit, assessing safety and tolerability based on the DSMB Charter, which included reference to dose-limiting toxicities (DLTs). Once the dose was deemed appropriate for expansion by the DSMB, additional subjects were enrolled at that dose level. In addition, subjects in this phase of the study were dosed on two occasions, 12 weeks apart, then followed for 6 months after initial injection for evaluation of safety and response to therapy.
Objectives
The primary objective was to evaluate the safety, including AEs, and assess tolerability of LSAM-PTX injected directly into mucinous PCLs by EUS-FNI. Secondary objectives were to describe the PK of LSAM-PTX administered into the cyst and to determine whether any LSAM-PTX concentrations (6, 10, or 15 mg/mL) showed signs of preliminary efficacy. For study purposes, ≥ 30 % reduction in cyst volume at 12 and 24 weeks was considered an indication of preliminary efficacy. Thirty percent was selected as an indication of response based on the RECIST 1.1 criteria for neoplasm response (diameter changes) in cancer patients 29 .
PK analysis
Plasma samples were taken on the day of injection at 1 and 2 hours after the procedure as well as at all other study visits, to characterize the PK of LSAM-PTX. The analysis of plasma paclitaxel concentration was performed by Covance Madison Laboratories Bioanalytical Group (Madison, Wisconsin, United States).
Cyst volume calculations
If more than one pancreatic cyst was present, the investigator selected a single target cyst for treatment. Magnetic resonance cholangiopancreatography (MRCP) or CT scan were used to estimate cyst volume in the dose-escalation phase. The same imaging modality as used for baseline assessments was repeated at the 3 and 6 month timepoints for comparison. CT scans were the required modality for the two-injection subjects. Cyst response was measured by change in the longest diameter of the cyst and calculated volume using the standard formula for an ellipsoid object 22 23 30 31 :
Volume = 1/6 × 3.14 × (d1 × d2 × d3), if three diameters were recorded.
Volume = 1/6 × 3.14 × (d1 × d2 2 ), if two diameters were recorded.
Volume = 1/6 × 3.14 × d1 3 , if only one diameter was recorded.
Data collection
The safety data (e. g., treatment-emergent AEs (TEAEs), DLTs, vital signs, laboratory values) were reviewed on an ongoing basis throughout the study by the Medical Monitor. All reported TEAEs were coded in MedDRA and summarized by system organ class and preferred term for each of the dose groups. Any serious TEAEs were summarized separately. Other data collected included physical exams, vital signs, and changes in laboratory parameters. Laboratory assessments were performed at the local laboratory for each site.
Statistical analysis
All calculations and analyses were performed using SAS version 9.4 or higher under the Windows Server 2012R2 operating system at McDougall Scientific, Ltd. (Toronto, Canada). Descriptive summaries of continuous data consisted of the mean, standard deviation (SD), median, minimum, and maximum. Categorical data was summarized with frequencies and percentages. All data were listed by subject and treatment. This study was not powered for inference, so no inferential statistical analyses were included.
There was no formal sample size calculation in this early-phase study; however, to provide a reference for the safety reviews of each cohort and the possible expansion of a cohort with safety concerns, nQuery Advisor (Version 6) employing the procedure “confidence interval for the probability of observing a rare event” determined that, for an event with an occurrence rate of 0.33, the probability of detecting it with three subjects is 69.9 % vs 91.0 % for six subjects, and for an event rate of 0.05 the probability of detecting the event was 14.3 % and 26.5 % for three and six subjects, respectively. Data was presented as observed, with no imputation for missing data.
Results
Study demographics
Twenty subjects were enrolled in the study. Three subjects were enrolled in each of the three cohorts (6, 10, and 15 mg/mL) during the dose-escalation phase of the study, and a further 11 subjects were enrolled at 15 mg/mL in the second phase of the study ( Fig. 1 ). In the second phase, one subject withdrew consent prior to dosing and, therefore, was excluded. Nineteen subjects were treated, the majority of whom were female (68.4 %), White (78.9 %), and not Hispanic/Latino (84.2 %). Seventeen of the 19 treated mucinous PCLs were branch duct IPMNs (89 %) and the remaining two were MCNs, with diameters averaging 2.71 cm ( Table 1 ). Of the 10 subjects who received LSAM-PTX in the second phase, eight received two injections at least 12 weeks apart and two received a single injection, one due to a serious AE (gastric outlet obstruction [GOO]), and one due to COVID restrictions.
Fig. 1.
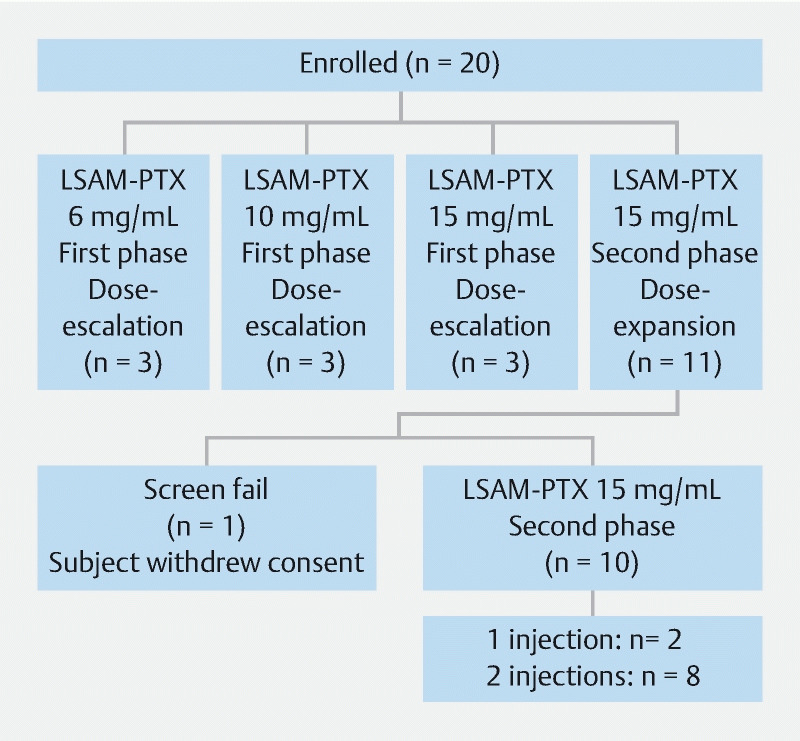
Flow diagram for subjects enrolled to receive large surface area microparticle paclitaxel (LSAM-PTX).
Table 1. Disposition at study entry of subjects enrolled to receive large surface area microparticle paclitaxel (LSAM-PTX).
| Escalation | Expansion |
Total
(n = 19) |
|||
| LSAM-PTX 6 mg/mL (n = 3) |
LSAM-PTX 10 mg/mL (n = 3) |
LSAM-PTX 15 mg/mL (n = 3) |
LSAM-PTX 15 mg/mL (n = 10) |
||
| Age, mean (SD), years | 66.3 (8.3) | 73.7 (11.5) | 59.7 (9.1) | 67.8 (6.6) | 67.2 (8.4) |
| Sex, female, n (%) | 3 (100) | 2 (66.7) | 2 (66.7) | 6 (60) | 13 (68.4) |
| Race | |||||
|
1 | 3 | 3 | 8 | 15 |
|
2 | 0 | 0 | 1 | 3 |
|
0 | 0 | 0 | 1 | 1 |
| Ethnicity | |||||
|
0 | 0 | 0 | 3 | 3 |
|
3 | 3 | 3 | 7 | 16 |
| ECOG | |||||
|
2 | 3 | 3 | 5 | 13 |
|
1 | 0 | 0 | 4 | 5 |
|
0 | 0 | 0 | 0 | 0 |
|
0 | 0 | 0 | 1 | 1 |
| Cyst type | |||||
|
0 | 0 | 0 | 2 | 2 |
|
3 | 3 | 3 | 8 | 17 |
| Cyst Longest Diameter, cm | |||||
|
2.5–2.8 | 1.6–2.8 | 2.3–3.9 | 0.9 * –4.9 | |
|
2.67 (0.15) | 2.17 (0.6) | 3.27 (0.85) | 2.74 (1.14) | |
|
2.7 | 2.1 | 3.6 | 2.85 | |
One subject during screening had a cyst with longest diameter of 1.3 cm and a family history of pancreatic cancer. Upon enrollment, the longest diameter of the cyst was 0.9 cm.
Adverse events
AEs were evaluated using MedDRA version 20.0. Overall, 18 of 19 subjects reported at least one TEAE. A total of 99 TEAEs were reported in all groups combined. No deaths or DLTs occurred. Seventy-four (74.75 %) TEAEs were mild, 17 (17.17 %) moderate, 4 (4.04 %) severe, and 4 (4.04 %) of unknown severity. The majority of TEAEs, 85 (85.86 %), were considered not related to LSAM-PTX, while 13 (13.13 %) and one (1.01 %) were considered possibly or probably related, respectively.
Across all treatment groups the most frequent TEAEs (n = 32 [32.32 % of all events]) reported were abdominal discomfort (6.1 %), peripheral edema (6.1 %), nausea (6.1 %), headache (5.1 %), and fatigue (5.1 %). Five serious TEAEs were reported in five subjects. Of these, three were severe (breast cancer, abdominal pain, organizing pneumonia), and two were of moderate severity (gastric outlet obstruction, hepatic encephalopathy). The GOO was considered probably related to study treatment as dosing occurred within 2 weeks of an endoscopic retrograde cholangiopancreatography (ERCP) procedure and stent placement; following hospitalization and naso-jejunal tube placement, resolution and discharge occurred within 3 days. The remaining four serious TEAEs were considered not related to LSAM-PTX.
Cyst response
Change in cyst diameter is the routine way of reporting response to treatment in pancreatic cysts. In subjects who demonstrated an increase in longest diameter (5 of 18) at Week 12, three of five subjects subsequently had a decrease in cyst diameter by Week 24, one receiving a single injection of 15 mg/mL and two receiving two 15 mg/mL injections 3 months apart. A reduction in the longest diameter was seen in 61 % (11 of 18) and 53 % of subjects (9 of 17) at Weeks 12 and 24, respectively. Of subjects demonstrating a reduction, a 30 % reduction (selected as the signal for preliminary efficacy) in longest diameter was seen in three of nine subjects by Week 24 ( Table 2 ). Two and three subjects showed no change at Weeks 12 and 24, respectively. Overall, there was no increase in cyst diameter in 72 % and 71 % of subjects at Weeks 12 and 24, respectively.
Table 2. Response to large surface area microparticle paclitaxel (LSAM-PTX); cyst volume and diameter changes.
| Change criteria * | Volume (cm 3 ) [all available diameters] | Longest diameter (cm) | ||
| Week 12 (n = 18) |
Week 24 (n = 17) |
Week 12 (n = 18) |
Week 24 (n = 17) |
|
| Increase in cyst size, n (%) | 8 (44.4) | 5 (29) | 5 (28) | 5 (29.4) |
| No change, n (%) | 1 (5.5) | 0 (0.0) | 2 (11.1) | 3 (17.6) |
| Decrease in cyst size, n (%): | 9 (50.0) | 12 (70.6) | 11 (61.1) | 9 (52.9) |
|
4 | 6 | 11 | 6 |
|
5 | 6 | 0 | 3 |
| Reduced/further reduced between Week 12 and Week 24, n (%) | 11 (64.7) | 11 (64.7) | ||
Of the 19 subjects enrolled, one did not have imaging data available at Week 12 and two did not have imaging data available at Week 24.
Overall reduction in volume, using the ellipsoid calculations described earlier, was seen in 50 % (9 of 18) and 71 % (12 of 17) at Weeks 12 and 24, respectively. Fifty percent of the subjects who had reduction following treatment reported an overall reduction in volume of 30 % or more at Weeks 12 and 24 when compared with baseline ( Table 2 ). In subjects for whom both Week 12 and 24 data were available (n = 16), 69 % showed a reduction in volume between Weeks 12 and 24. This occurred in five subjects after a single injection and in six subjects, after two injections. In the subjects who had an increase in calculated volume at Week 12, 75 % showed a relative decrease by Week 24, occurring in one subject who received one injection at the 15 mg/mL concentration and in another five after two injections at 15 mg/mL. The mean cyst volume calculated based on all available diameters measured using CT, magnetic resonance imaging, or EUS, and by number of injections for subjects receiving 15 mg/mL, are summarized ( Table 3 ).
Table 3. Mean (SD) cyst volume in subjects receiving large surface area microparticle paclitaxel (LSAM-PTX).
| Escalation | Expansion | Escalation and expansion | |||
| LSAM-PTX 6 mg/mL (n = 3) |
LSAM-PTX 10 mg/mL (n = 3) |
LSAM-PTX 15 mg/mL – Single Injection (n = 5) |
LSAM-PTX 15 mg/mL – Two Injections (n = 8) |
LSAM-PTX> 15 mg/mL (n = 13) |
|
| Mean (SD) cyst volume based on all available diameters 1 , cm 3 | |||||
|
7.77 (3.28) | 4.81 (5.11) | 15.57 (9.46) | 8.68 (5.80) | 11.33 (7.85) |
|
5.71 (3.89) | 3.45 (2.19) | 14.78 (12.78) 2 | 10.05 (8.98) | 11.63 (10.06) 3 |
|
4.14 (3.92) | 4.38 (0.52) | 15.02 (12.14) | 6.60 (8.14) | 10.43 (10.56) 4 |
| Mean (SD) cyst volume based on longest diameter, cm 3 | |||||
|
9.99 (1.68) | 6.16 (4.81) | 31.89 (20.04) | 15.75 (10.36) | 21.96 (16.23) |
|
8.28 (4.06) | 5.71 (0.94) | 26.73 (19.73) 2 | 16.69 (16.47) | 20.04 (17.41) 3 |
|
7.45 (7.10) | 8.36 (2.74) | 28.14 (16.21) | 13.87 (16.02) | 20.36 (17.00) 4 |
For cyst volume calculated from all available diameters, following rules are applied:
Volume = 1/6 × 3.14 × (d1 × d2 × d3), if 3 diameters were recorded.
Volume = 1/6 × 3.14 × (d1 × d2 2 ), if 2 diameters were recorded.
Volume = 1/6 × 3.14 × d1 3 , if 1 diameter was recorded.
n = 4.
n = 12.
n = 11.
PK analysis
The plasma paclitaxel concentrations are summarized by dose group ( Table 4 ). The mean plasma concentrations for the 6 mg/mL and 10 mg/mL cohorts were increased at the 2 hour timepoint compared to the 1 hour timepoint, whereas in the 15 mg/mL dose group the peak plasma concentration occurred 1-hour after injection. At lower doses and single injection cohorts at most timepoints, paclitaxel levels were below the level of quantitation (25 pg/mL). In the 15 mg/mL injection group, paclitaxel was detected at all measured timepoints with the peak occurring one week following injection in the single injection cohort, and in subjects who received two 15 mg/mL injections peaks were measured 1 week after the first injection and at 2 hours post-injection following the second injection ( Table 4 ). In four of eight subjects who received two injections of LSAM-PTX, cyst fluid was collected prior to the second injection for paclitaxel evaluation. Paclitaxel levels in the cyst 3 months following a single injection of LSAM-PTX were > 250 ng/mL, demonstrating retention of paclitaxel within the cyst for at least 3 months ( Table 4 ). The same four subject’s cyst fluid was also sent for pathological evaluation and there was evidence of necrotic debris in all samples.
Table 4. Paclitaxel concentration in subjects after large surface area microparticle paclitaxel (LSAM-PTX).
| Plasma paclitaxel concentration (pg/mL) – mean (SD) | |||
| Visit/timepoint | LSAM-PTX 6 mg/mL (n = 3) | LSAM-PTX 10 mg/mL (n = 3) | LSAM-PTX 15 mg/mL (n = 13) |
| Day 1 (1 hr post-injection) | 220.67 (213.86) | 115.00 (106.78) | 545.45 (1009.88) 1 |
| Day 1 (2 hr post-injection) | 282.00 (247.47) | 252.03 (191.02) | 324.29 (480.47) 2 |
| Week 1 | 15.33 (26.56) | 31.30 (28.48) | 476.18 (901.44) |
| Week 2 | 0.00 (0.00) 3 | 8.87 (15.36) | 294.02 (445.33) |
| Week 4 | 0.00 (0.00) 3 | 10.03 (17.38) | 62.67 (91.72) |
| Week 8 | 0.00 (0.00) 3 | 0.00 (0.00) 3 | 42.58 (62.00) |
| Week 12 | 0.00 (0.00) 3 | 0.00 (0.00) 3 | 40.25 (60.01) 4 |
| Week 12 (1 hr post-injection) | 213.66 (218.20) 5 | ||
| Week 12 (2 hr post-injection) | 274.28 (309.32) | ||
| Week 14 | 57.75 (111.53) | ||
| Week 16 | 24.20 (40.05) 6 | ||
| Week 24 /end of study | 0.00 (0.00) 3 | 0.00 (0.00) 3 | 26.28 (47.80) 5 |
| Cyst fluid paclitaxel concentration (ng/mL) | |||
| Sample 1 | 17,800 | ||
| Sample 2 | 270 | ||
| Sample 3 | 2,990 | ||
| Sample 4 7 | |||
n = 12
n = 11
All below the level of quantitation (i. e. < 25 pg/mL) paclitaxel concentration values are set to 0.
n = 4
n = 8
n = 5
The cyst fluid paclitaxel concentration for Sample 4 was above the upper limit of quantitation, thus a value could not be determined.
Discussion
This early-phase multicenter study was designed to evaluate various concentrations of LSAM-PTX injected into PCLs, the majority of which were branch duct IPMNs, not necessarily considered high-risk. Safety and tolerability were confirmed up to 15 mg/mL. AEs were generally mild and transient. No systemic toxicities were reported, and there were no reports of pancreatitis, intracystic bleeding or leakage. The preliminary data also suggest that LSAM-PTX was retained inside the cyst for at least 3 months, prolonging the duration of exposure of the epithelial cyst lining to the paclitaxel particles.
Following intracystic injection of LSAM-PTX, reductions in cyst volume were seen at the 6 month follow-up in 12 of 17 subjects, ranging from 10 % to 78 % ( Fig. 2 ). Interestingly, some patients in this study had a slight increase in cyst volume at Week 12, which could be explained by presence of LSAM-PTX for a prolonged period of time within the cyst causing a local inflammatory reaction associated with cyst wall destruction. Of eight subjects who demonstrated increases in calculated volume at Week 12 compared to baseline, six (75.0 %) showed a decrease in calculated volume at Week 24 compared to Week 12, one of which occurred after a single injection and five after two injections of the 15 mg/mL concentration of LSAM-PTX ( Fig. 2 ). Our data suggest that EUS-FNI of LSAM-PTX may offer an alternate therapy for patients who are not fit for surgery or who have multiple pancreatic cysts involving various parts of the pancreas, or for those patients who elect not to undergo surgery.
Fig. 2 .
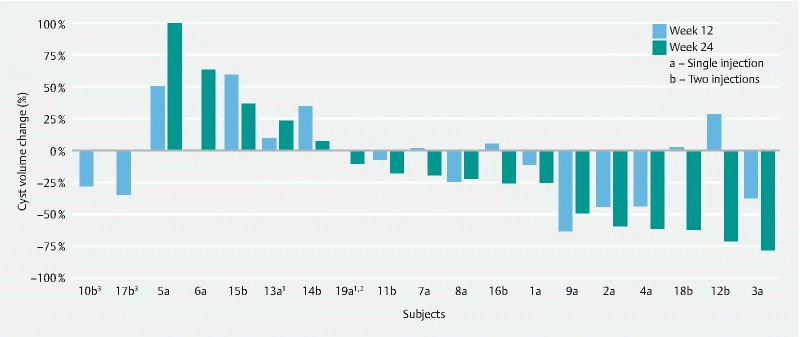
Change in cyst volume based on single or two injections of large surface area microparticle paclitaxel (LSAM-PTX) at Week 12 and 24. 1 Subject was enrolled in the two-injection group but received a single injection. 2 Subject did not have a Week 12 CT scan. 3 Subject did not have a Week 24 CT scan.
EUS-FNI of chemotherapy or alcohol for ablation of PCL has been reported and a recent review article 24 summarized six studies (207 patients) where alcohol lavage was performed and eight studies (347 patients) in which a paclitaxel-based regimen, with or without alcohol, was used. Results were pooled from all of the studies. In the ethanol-treated studies, complete resolution of the cyst was observed in 32.8 % of the patients with 21.2 % having complications following treatment, including abdominal pain in 14.5 %, pancreatitis in 2.4 %, and intracystic bleeding in one patient (0.5 %). In the paclitaxel-based treated group, complete resolution was observed in 63.6 % of the patients with 15 % having post-treatment complications, including abdominal pain in 4 %, pancreatitis in 5 %, and intracystic bleeding in two patients (0.5 %). These findings were also reported in another review article 25 in which the use of luaromacrogol was included for evaluation of ablative therapies. In the luaromacrogol study 32 , 29 subjects were followed for 9 months and reported a complete response in 37.9 % of the subjects. However, variability in study designs, the differences in defining complete response, length of follow-up, and the differences in the types of pancreatic cysts being treated make it difficult to draw formal comparisons.
Pancreatitis after ethanol injection is believed to be secondary to alcohol extravasation or the known exuberant inflammatory effects of alcohol on the pancreatic parenchyma or surrounding tissues 33 and after paclitaxel injection, possibly due to the Cremophor EL excipient in Taxol ® 34 , which is not present in LSAM-PTX used in this study. Although the majority of patients included in our study had branch duct IPMNs, no subjects treated with LSAM-PTX developed pancreatitis nor was there evidence of intracystic bleeding or leakage. The AE profile is markedly improved when compared with alcohol ablation, and therefore, injection of LSAM-PTX when cyst progression is anticipated may be a suitable alternative.
The American Gastroenterological Association published practice guidelines in 2015 on diagnosis and management of asymptomatic neoplastic pancreatic cysts, which established indications for performing EUS during the surveillance period 35 . Among these indications are change in cyst size or morphology, development of a solid component, or an increase in main pancreatic duct diameter. The American College of Gastroenterology published guidelines in 2018, which pointed out the management of pancreatic cysts is based on minimal evidence and the practicality of surgical resection and/or surveillance, and further stated that many new diagnostic aids such as the use of cyst fluid cytology, cyst ablation, and devices like needle micro-biopsy forceps can be helpful for identifying IPMN and MCN, but there is insufficient evidence to recommend these procedures for clinical practice 36 . Given the morbidity and mortality of pancreatic surgery, the unpredictable histology prior to surgery, and the uncertainty of observation, finding a less invasive treatment is desirable. Limitations of the current study include the small number of patients treated and the limited follow-up to only 6 months, along with the lack of histological or morphological data on cyst wall epithelium. PCLs are space-occupying lesions, and if there is destruction of the cyst lining by LSAM-PTX, there could be fibrotic healing rather than regeneration of pancreatic tissue. The preliminary signals in this single-arm safety study are encouraging based on the assessments performed.
Conclusions
In conclusion, in this dose-escalation early-phase safety study, the concentration of 15 mg/mL LSAM-PTX injected into mucinous PCLs was found to be safe and tolerable. Preliminary findings included reduction in cyst size over the 6 month study period without producing clinically significant TEAEs. Eight subjects received a second injection of their cyst in the second part of the study and following on from the study, four of them have continued to receive further treatments with LSAM-PTX under an expanded access program with cysts further reducing in volume 37 38 . Maintaining a state of partial remission, or arrested growth, of a cystic lesion may prevent progression of the cyst. Smaller cysts or those not meeting surgical criteria may be able to be managed with early intervention using EUS-FNI of LSAM-PTX, and in cysts where progression is anticipated, this treatment may provide an option for preventing the growth and transformation to a cancerous state. The prolonged residence time of LSAM-PTX within the cyst provides for continuous exposure to a therapy without increasing AEs, and if the cyst does not progress, the need for surgical intervention is avoided. Although cyst reduction was durable over the 6 month follow-up period, longer term studies with additional injections are needed to confirm the durability of cyst reduction following LSAM-PTX injection, and it will be important to determine what effect LSAM-PTX has on the cyst wall epithelium, and on markers associated with cyst destruction and malignant transformation.
Acknowledgments
NanoPac is a registered trademark of NanOlogy, LLC. This research was funded by NanOlogy, LLC. The authors would like to thank CritiTech, Inc. (Lawrence, KS) for investigational product supply, and NanOlogy, LLC for study funding. Additional thanks to Alyson Marin for assistance in data compilation and document preparation.
Footnotes
Competing interests Gere diZerega holds a consultant/advisory role, has stock ownership or receives funding from NanOlogy, LLC. Shelagh Verco, James Verco, and Alison Wendt are full-time employees of NanOlogy, LLC. Mohamed O Othman is a consultant for Abbvie, BSC, Olympus, Conmed, Apollo and Nestle and received grant funding from Abbvie, Lucid Diagnostics, Conmed and NanOlogy, LLC. Kalpesh Patel is a consultant for Conmed and Abbvie. Somashekar G. Krishna is PI of an investigator-initiated study in part funded by a grant to The Ohio State University Wexner Medical Center from Mauna Kea Technologies, Paris, France. The remaining authors declare that they have no conflict of interest.
Supplementary material :
References
- 1.Farrell J J. Prevalence, diagnosis and management of pancreatic cystic neoplasms: current status and future directions. Gut Liver. 2015;9:571–589. doi: 10.5009/gnl15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong K, Nio C Y, Hermans J J et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–811. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Valsangkar N P, Morales-Oyarvide V, Thayer S P et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4–12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheiman J M, Hwang J H, Moayyedi P. American Gastroenterological Association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824–848. doi: 10.1053/j.gastro.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Fernandez-Del Castillo C, Kamisawa T et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. doi: 10.1016/j.pan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Fernandez-del Castillo C, Adsay V et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.de Pretis N, Mukewar S, Aryal-Khanal A et al. Pancreatic cysts: Diagnostic accuracy and risk of inappropriate resections. Pancreatology. 2017;17:267–272. doi: 10.1016/j.pan.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Basar O, Yuksel O, Yang D J et al. Feasibility and safety of microforceps biopsy in the diagnosis of pancreatic cysts. Gastrointest Endosc. 2018;88:79–86. doi: 10.1016/j.gie.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 9.Blaszczak A M, Krishna S G. Endoscopic diagnosis of pancreatic cysts. Curr Opin Gastroenterol. 2019;35:448–454. doi: 10.1097/MOG.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 10.Tacelli M, Celsa C, Magro B et al. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: Systematic review with meta-analysis. Dig Endosc. 2020;32:1018–1030. doi: 10.1111/den.13626. [DOI] [PubMed] [Google Scholar]
- 11.Westerveld D R, Ponniah S A, Draganov P V et al. Diagnostic yield of EUS-guided through-the-needle microforceps biopsy versus EUS-FNA of pancreatic cystic lesions: a systematic review and meta-analysis. Endosc Int Open. 2020;8:E656–E667. doi: 10.1055/a-1119-6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacevic B, Klausen P, Rift C V et al. Clinical impact of endoscopic ultrasound-guided through-the-needle microbiopsy in patients with pancreatic cysts. Endoscopy. 2021;53:44–52. doi: 10.1055/a-1214-6043. [DOI] [PubMed] [Google Scholar]
- 13.Pitman M B, Genevay M, Yaeger K et al. High-grade atypical epithelial cells in pancreatic mucinous cysts are a more accurate predictor of malignancy than "positive" cytology. Cancer Cytopathol. 2010;118:434–440. doi: 10.1002/cncy.20118. [DOI] [PubMed] [Google Scholar]
- 14.Park J W, Jang J Y, Kang M J et al. Mucinous cystic neoplasm of the pancreas: is surgical resection recommended for all surgically fit patients? Pancreatology. 2014;14:131–136. doi: 10.1016/j.pan.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Pusateri A J, Krishna S G. Pancreatic cystic lesions: pathogenesis and malignant potential. Diseases. 2018;6:50. doi: 10.3390/diseases6020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishna S G. Endoscopic ultrasound-guided confocal endomicroscopy requires high-quality imaging and interpretation for diagnostic evaluation of pancreatic cystic lesions. Endosc Int Open. 2020;8:E310–E311. doi: 10.1055/a-1067-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh H C, Seo D W, Lee T Y et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636–642. doi: 10.1016/j.gie.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Oh H C, Seo D W, Kim S C et al. Septated cystic tumors of the pancreas: is it possible to treat them by endoscopic ultrasonography-guided intervention? Scand J Gastroenterol. 2009;44:242–247. doi: 10.1080/00365520802495537. [DOI] [PubMed] [Google Scholar]
- 19.Oh H C, Seo D W, Song T J et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–179. doi: 10.1053/j.gastro.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 20.DeWitt J M, Al-Haddad M, Sherman S et al. Alterations in cyst fluid genetics following endoscopic ultrasound-guided pancreatic cyst ablation with ethanol and paclitaxel. Endoscopy. 2014;46:457–464. doi: 10.1055/s-0034-1365496. [DOI] [PubMed] [Google Scholar]
- 21.Moyer M T, Dye C E, Sharzehi S et al. Is alcohol required for effective pancreatic cyst ablation? The prospective randomized CHARM trial pilot study. Endosc Int Open. 2016;4:E603–607. doi: 10.1055/s-0042-105431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez V, Takahashi N, Levy M J et al. EUS-guided ethanol lavage does not reliably ablate pancreatic cystic neoplasms (with video) Gastrointest Endosc. 2016;83:914–920. doi: 10.1016/j.gie.2015.08.069. [DOI] [PubMed] [Google Scholar]
- 23.Choi J H, Seo D W, Song T J et al. Long-term outcomes after endoscopic ultrasound-guided ablation of pancreatic cysts. Endoscopy. 2017;49:866–873. doi: 10.1055/s-0043-110030. [DOI] [PubMed] [Google Scholar]
- 24.Attila T, Adsay V, Faigel D O. The efficacy and safety of endoscopic ultrasound-guided ablation of pancreatic cysts with alcohol and paclitaxel: a systematic review. Eur J Gastroenterol Hepatol. 2019;31:1–9. doi: 10.1097/MEG.0000000000001297. [DOI] [PubMed] [Google Scholar]
- 25.Du C, Chai N L, Linghu E Q et al. Endoscopic ultrasound-guided injective ablative treatment of pancreatic cystic neoplasms. World J Gastroenterol. 2020;26:3213–3224. doi: 10.3748/wjg.v26.i23.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baltezor M, Farthing J, Sittenauer J Taxane particles and their use. U.S. Patent 9,814,685: Nov. 14, 2017
- 27.Verco S, Maulhardt H, Baltezor M et al. Local administration of submicron particle paclitaxel to solid carcinomas induces direct cytotoxicity and immune-mediated tumoricidal effects without local or systemic toxicity: preclinical and clinical studies. Drug Deliv Transl Res. 2021;11:1806–1817. doi: 10.1007/s13346-020-00868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maulhardt H, Verco S, Baltezor M et al. Local administration of large surface area microparticle docetaxel to solid carcinomas induces direct cytotoxicity and immune-mediated tumoricidal effects: preclinical and clinical studies. Drug Deliv Transl Res. 2022:In press. doi: 10.1007/s13346-022-01226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenhauer E A, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Rkein A M, Harrigal C, Friedman A C et al. Comparison of the accuracy of CT volume calculated by circumscription to prolate ellipsoid volume (bidimensional measurement multiplied by coronal long axis) Acad Radiol. 2009;16:181–186. doi: 10.1016/j.acra.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Chalian H, Seyal A R, Rezai P et al. Pancreatic mucinous cystic neoplasm size using CT volumetry, spherical and ellipsoid formulas: validation study. JOP. 2014;15:25–32. doi: 10.6092/1590-8577/1893. [DOI] [PubMed] [Google Scholar]
- 32.Linghu E, Du C, Chai N et al. A prospective study on the safety and effectiveness of using lauromacrogol for ablation of pancreatic cystic neoplasms with the aid of EUS. Gastrointest Endosc. 2017;86:872–880. doi: 10.1016/j.gie.2017.03.1525. [DOI] [PubMed] [Google Scholar]
- 33.Moyer M T, Sharzehi S, Mathew A et al. The Safety and efficacy of an alcohol-free pancreatic cyst ablation protocol. Gastroenterology. 2017;153:1295–1303. doi: 10.1053/j.gastro.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Mills K M, Johnson D M, Middlebrooks M et al. Possible drug-associated pancreatitis after paclitaxel-cremophor administration. Pharmacotherapy. 2000;20:95–97. doi: 10.1592/phco.20.1.95.34653. [DOI] [PubMed] [Google Scholar]
- 35.Vege S S, Ziring B, Jain R et al. American Gastroenterological Association Institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–822. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Elta G H, Enestvedt B K, Sauer B G et al. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464–479. doi: 10.1038/ajg.2018.14. [DOI] [PubMed] [Google Scholar]
- 37.Krishna S G, Hart P A, Papachristou G et al. Sa300 Tandem EUS-Guided fine needle injection of intracystic submicron particle paclitaxel (NanoPac) as treatment for Brach-duct IPMN: An interim safety, pharmacokinetic, and efficacy analysis. Gastroenterology. 2021;160:S475–S476. [Google Scholar]
- 38.Krishna S G, Ardeshna D R, Hart P A et al. The efficacy of intracystic injection of large surface area microparticle paclitaxel in the management of intraductal papillary mucinous neoplasms: results from an expanded access protocol. Gastrointestinal Endoscopy. 2022;95:AB531–AB532. doi: 10.1016/j.pan.2023.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


