Abstract
Background
Cataract surgery is the most common ambulatory incisional surgery performed in the USA. Cystoid macular edema (CME), the accumulation of fluid in the central retina due to leakage from dilated capillaries, is the most common cause of vision impairment following cataract surgery. Acute CME, defined as CME of less than four months' duration, often resolves spontaneously. CME that persists for four months or longer is termed chronic CME. Non‐steroidal anti‐inflammatory drugs (NSAIDs) have been used to treat CME. This update adds new evidence and analyses to the previously published review.
Objectives
To examine the effectiveness of NSAIDs in the treatment of CME following cataract surgery.
Search methods
We searched the CENTRAL (2022, Issue 3); Ovid MEDLINE; Embase; PubMed; LILACS; mRCT (discontinued in 2014, last searched August 2011), ClinicalTrials.gov, and WHO ICTRP databases. We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on 20 March 2022.
Selection criteria
We included randomized controlled trials evaluating the effects of NSAIDs for CME following cataract surgery.
Data collection and analysis
Two review authors independently screened all titles and abstracts, reviewed full‐text publications against eligibility criteria, independently extracted data from newly included trials and assessed risk of bias for each included trial. We contacted trial authors for clarification or to request missing information. We provided a narrative synthesis of all included trials and their results. For continuous and dichotomous outcomes, we separately performed pooled analysis and reported mean difference (MD) and risk ratio (RR) as well as the associated 95% confidence interval (CI) whenever feasible. Two review authors independently graded the overall certainty of the evidence for each outcome using the GRADE approach.
Main results
We included nine trials with a total of 390 participants (393 eyes). Study participants' mean age was 72.2 years (interquartile range [IQR] 68.8 to 73.6) and 72% were women (IQR 69% to 74%). Three trials included participants with acute CME, and four included participants with chronic CME; the remaining two trials enrolled both participants with acute and chronic CME or participants with unknown CME duration. We assessed trials as having unclear (33%) or high risk of bias (67%).
Visual improvement of two or more lines at the end of treatment
Data from one trial in participants with acute CME show no treatment effect of topical ketorolac compared to placebo (RR 2.00, 95% CI 0.46 to 8.76; 22 participants). Data from a three‐arm trial in participants with acute CME demonstrate that, when compared with topical prednisolone, topical ketorolac (RR 1.33, 95% CI 0.58 to 3.07; 17 participants) or topical ketorolac and prednisolone combination therapy (RR 1.78, 95% CI 0.86 to 3.69; 17 participants) may have little or no effect on visual improvement. Results of subgroup analysis from two studies in participants with chronic CME suggest that, after treatment for 90 days or longer, NSAIDs may increase participants' likelihood of visual improvement by 1.87 fold (RR 2.87, 95% CI 1.58 to 5.22; I2 = 33%; 2 trials, 121 participants) relative to placebo. However, there was no evidence of treatment effects in the subgroup with two months of treatment or less (RR 0.72, 95% CI 0.30 to 1.73; P = 0.19, I2 = 41%; 2 trials, 34 participants). Overall, this evidence is very low certainty.
A single‐study estimate in patients with mixed CME indicates that topical diclofenac may increase the likelihood of visual improvement by 40% when compared to topical ketorolac (RR 1.40, 95% CI 1.02 to 1.94; 68 participants). However, the same trial reported no difference between the groups in mean final visual acuity in Snellen lines (MD 0.40, 95% CI −0.93 to 1.73). A three‐arm trial in patients with mixed CME reporting visual changes in ETDRS letters in comparisons between ketorolac and diclofenac (34 participants) or bromfenac (34 participants) suggests no evidence of effects. Overall, NSAIDs may slightly improve visual acuity in participants with mixed CME but the evidence is very uncertain.
Persistence of improvement of vision one month after discontinuation of treatment
One trial of participants with chronic CME tested oral indomethacin (RR 0.40, 95% CI 0.10 to 1.60; 20 participants) and the other compared topical ketorolac to placebo (RR 4.00, 95% CI 0.51 to 31.1; 26 participants). While there is no evidence of treatment effects, evidence suggests substantial between‐group heterogeneity (P = 0.07, I2 = 69.9%; very low‐certainty evidence). None of the trials in patients with acute or mixed CME reported this outcome.
Proportion of participants with improvement in leakage on fundus fluorescein angiography
One three‐arm trial in participants with acute CME shows that, when compared with topical prednisolone, there is no treatment benefit of topical ketorolac (RR 1.11, 95% CI 0.45 to 2.75; 17 participants) or topical ketorolac and topical prednisolone combination therapy (RR 1.56, 95% CI 0.72 to 3.38; 17 participants). This evidence is very low certainty.
The combined estimate from two trials in participants with chronic CME indicates NSAIDs have little to no effect over placebo on improving leakage (RR 1.93, 95% CI 0.62 to 6.02; 40 participants; very low‐certainty evidence). Neither of the trials in patients with mixed CME reported this outcome.
Proportion of participants with improved contrast sensitivity
Very low‐certainty evidence from one trial in participants with acute CME shows no treatment benefit of ketorolac (RR 1.11, 95% CI 0.45 to 2.75; 17 participants) or ketorolac and prednisolone combination therapy (RR 1.78, 95% CI 0.86 to 3.69; 17 participants) compared with topical prednisolone. None of the trials in patients with chronic or mixed CME reported this outcome.
Proportion of participants with improved central macular thickness on optical coherence tomography; measures of quality of life
No included trial reported these outcomes.
Adverse effects
Most trials observed no differences in ocular adverse events, such as corneal toxicity or elevated intraocular pressure, between comparison groups.
Authors' conclusions
Evidence on effects of NSAIDs in patients with CME is very uncertain and further investigation is warranted. Our findings are limited by small sample sizes, and heterogeneity in interventions, assessments, and reporting of clinically important outcomes.
Keywords: Aged; Female; Humans; Male; Anti-Inflammatory Agents, Non-Steroidal; Anti-Inflammatory Agents, Non-Steroidal/therapeutic use; Cataract; Cataract/complications; Diclofenac; Diclofenac/therapeutic use; Ketorolac; Ketorolac/therapeutic use; Macular Edema; Macular Edema/drug therapy; Macular Edema/etiology; Prednisolone; Prednisolone/therapeutic use; Quality of Life
Plain language summary
What are the benefits of NSAIDs for treating fluid accumulation in the central retina (macula) after cataract surgery?
Key messages
• We updated the evidence on the effects of non‐steroidal anti‐inflammatory drugs (NSAIDs, such as ibuprofen) among individuals with acute or chronic fluid accumulation in the central retina (macula) after cataract surgery.
• There was only very uncertain evidence that NSAIDs could improve vision or reduce fluid accumulation in the macula.
• More research is warranted to provide quality evidence.
What is cystoid macular edema (CME)?
Cataract surgery is the most common outpatient surgery performed in the USA. Sometimes after cataract surgery, inflammation inside the eye leads to leakage of fluid from retinal blood vessels, which results in the accumulation of fluid in cyst‐like spaces and swelling of the central part of the retina (macula). This is called 'cystoid macular edema', or CME, and may cause reduced or distorted vision. CME is one of the most frequent complications following cataract surgery.
How is CME treated?
CME after cataract surgery is often treated with NSAIDs or other medications to reduce inflammation inside the eye. NSAIDs are usually applied directly to the eye (topical) as eyedrops; they may be given alone or with another medication, such as a topical steroid or another NSAID.
What did we want to find out? We wanted to know whether NSAIDs, given alone or combined with other medications, can help people with CME following cataract surgery to see more clearly.
What did we do? We searched for studies that randomly assigned adults with CME after cataract surgery to be treated with NSAIDs, alone or with other medications. Studies compared NSAIDs with placebo (a dummy medicine with no active ingredients) or other active treatment (steroid or another NSAID). We compared and summarized the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We included 9 studies that involved a total of 390 people with a mean age of 72 years; 72% were women. Three studies enrolled exclusively people with acute CME (fluid accumulation in the macula of less than 4 months' duration) after cataract surgery, 4 studies enrolled people with chronic CME (fluid presence in the macula which persisted for 4 months or longer) after cataract surgery, and the remaining 2 studies included people with varying durations of post‐surgical fluid accumulation in the macula.
• People with acute CME: NSAIDs might improve vision or reduce retinal swelling compared to placebo or a topical steroid (3 studies).
• People with chronic CME: 2 studies that treated people with chronic CME with NSAIDs for two months or less showed no effects on vision improvement. In contrast, the other two studies suggested that NSAIDs might improve vision after 90 to 120 days of treatment. We did not find any evidence about the effects of NSAIDs on other outcomes, such as persistent improvement of vision after treatment stopped or reduced fluid accumulation in the macula.
• Length of time with CME not known: topical NSAID might have little or no effects on vision compared to a different NSAID.
Most of the included studies either did not report data on unwanted effects or did not report differences in unwanted effects between groups receiving different treatments.
What are the limitations of the evidence?
Our confidence in the evidence was limited because studies did not include many participants, and they used different medications in different amounts for different durations. The studies did not examine all the outcomes of interest. The findings were also limited by the fact that the trials were published from 1977 to 2006, and many advances in cataract surgery techniques have occurred throughout and since that time.
How up to date is this evidence? The evidence is up to date as of March 2022.
Summary of findings
Summary of findings 1. NSAID versus placebo or active control in acute cystoid macular edema.
| Non‐steroidal anti‐inflammatory drug vs placebo or active control for acute cystoid macular edema following cataract surgery | ||||||
|
Patient population: participants with acute CME (<
4 months) following cataract surgery Settings: private clinics or medical centers in the USA Intervention: ketorolac, diclofenac Comparison: placebo, prednisolone, ketorolac + prednisolone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
No. of participants (RCTs) |
Certainty of evidence (GRADE) |
Comments | |
|
Assumed risk* Placebo or prednisolone |
Corresponding risk NSAID ± prednisolone |
|||||
| Visual improvement at end of treatment, as defined by two or more Snellen lines | Placebo 18 per 100 |
Ketorolac 36 (8 to 100) per 100 |
RR 2.00 (0.46 to 8.76) | 22 (1 RCT) |
⊕⊝⊝⊝ Very lowa |
RR > 1 is better. Besides Flach 1998 (22 participants) and Heier 2000 (26 participants), Rho 2003 (34 participants) compared mean final VA between participants receiving ketorolac with those receiving diclofenac (20/50 vs 20/49) and concluded no overall differences |
|
Prednisolone 50 per 100 |
Ketorolac 67 (29 to 100) per 100 |
RR 1.33 (0.58 to 3.07) |
17 (1 RCT) |
|||
|
Ketorolac + prednisolone 89 (43 to 100) per 100 |
RR 1.78 (0.86 to 3.69) |
17 (1 RCT) |
||||
| Persistence of visual improvement one month after discontinuation of treatment | Not measured by any included trial |
|||||
|
Improvement in leakage on FFA |
Prednisolone 50 per 100 |
Ketorolac 56 (23 to 100) per 100 |
RR 1.11 (0.45 to 2.75) |
17 (1 RCT) |
⊕⊝⊝⊝ Very lowa |
RR > 1 is better. Data from the same 3‐arm trial (Heier 2000) |
|
Ketorolac + prednisolone 78 (36 to 100) per 100 |
RR 1.56 (0.72 to 3.38) |
17 (1 RCT) |
||||
| Improvement in central macular thickness on OCT | Not measured by any included trial |
|||||
|
Improved contrast sensitivity |
Prednisolone 50 per 100 |
Ketorolac 56 (23 to 100) per 100 |
RR 1.11 (0.45 to 2.75) |
17 (1 RCT) |
⊕⊝⊝⊝ Very lowa |
RR > 1 is better Data from the same 3‐arm trial (Heier 2000) |
|
Ketorolac + prednisolone 89 (43 to 100) per 100 |
RR 1.78 (0.86 to 3.69) |
17 (1 RCT) |
||||
| Change in QoL | Not measured by any included trial |
|||||
| *The risk in the intervention group (and its 95% CI) is based
on the assumed risk in the comparison group and the relative
effect of the intervention (and the associated 95% CI). CI: confidence interval; CME: cystoid macular edema; FFA: fundus fluorescein angiography (angiogram); NSAID: non‐steroidal anti‐inflammatory drug; OCT: optical coherence tomography; QoL: quality of life; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded for risk of bias (‐1), imprecision (‐1) and heterogeneity (‐1).
Summary of findings 2. NSAID versus placebo in chronic cystoid macular edema.
| Non‐steroidal anti‐inflammatory drug vs active control for chronic cystoid macular edema following cataract surgery | ||||||
|
Patient population: participants with chronic CME (≥ 4
months) following cataract surgery Settings: medical centers in the USA Intervention: topical ketorolac, topical fenoprofen, oral indomethacin Comparison: placebo (vehicle or saline) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
No. of participants (RCTs) |
Certainty of evidence (GRADE) |
Comments | |
|
Assumed risk* Placebo |
Corresponding risk NSAID |
|||||
|
Visual improvement at end of treatment, as defined by two
or more Snellen lines |
Treatment duration ≤ 2 months 44 per 100 |
32 (13 to 76) per 100 |
RR 0.72 (0.30 to 1.73) |
34 (2 RCTs) |
⊕⊝⊝⊝ Very lowa |
RR > 1 is better. When excluding Yannuzzi 1977, which examined oral NSAID, there was no evidence of subgroup differences and the combined RR was 2.33 (95% CI 1.17 to 4.66; 3 RCTs, 135 participants), favoring topical NSAIDs over placebo in visual improvement after at least 90 days of treatment. |
|
Treatment duration 90‐120 days 18 per 100 |
52 (28 to 94) per 100 |
RR 2.87 (1.58 to 5.22) |
121 (2 RCTs) |
|||
| Persistence of visual improvement one month after discontinuation of treatment | 26 per 100 | 30 (11 to 80) per 100 |
RR 1.17 (0.44 to 3.07) |
46 (2 RCTs) |
⊕⊝⊝⊝ Very lowb |
|
|
Improvement in leakage on FFA |
14 per 100 | 27 (9 to 84) per 100 |
RR 1.93 (0.62 to 6.02) |
40 (2 RCTs) |
⊕⊝⊝⊝ Very lowb |
RR > 1 is better. |
| Improvement in central macular thickness on OCT | Not measured by any included trial | |||||
| Improved contrast sensitivity | Not measured by any included trial | |||||
| Change in QoL | Not measured by any included trial | |||||
| *The risk in the intervention group (and its 95% CI) is based
on the assumed risk in the comparison group and the relative
effect of the intervention (and the associated 95% CI). CI: confidence interval; CME: cystoid macular edema; FFA: fundus fluorescein angiography (angiogram); NSAID: non‐steroidal anti‐inflammatory drug; OCT: optical coherence tomography; QoL: quality of life; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded for risk of bias (‐1), imprecision (‐1) and heterogeneity (‐1). bDowngraded for risk of bias (‐1) and extreme imprecision (‐2).
Summary of findings 3. NSAID versus active control in mixed cystoid macular edema.
| Non‐steroidal anti‐inflammatory drug vs active control for cystoid macular edema following cataract surgery | ||||||
|
Patient population: participants with acute or chronic
CME following cataract surgery Settings: private clinics in the USA or medical centers in Canada Intervention: bromfenac, diclofenac ± prednisolone, ketorolac ± prednisolone Comparison: ketorolac ± prednisolone | ||||||
| Outcomes |
Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
No. of participants (RCTs) |
Certainty of evidence (GRADE) |
Comments | |
|
Assumed risk* Active control |
Corresponding risk NSAID |
|||||
| Visual improvement at end of treatment, as defined by two or more Snellen lines |
Diclofenac + prednisolone 59 per 100 |
Ketorolac + prednisolone 83 (60 to 100) per 100 |
RR 1.40 (1.02 to 1.94) |
68 (1 RCT) |
⊕⊝⊝⊝ Very lowa |
RR > 1 is better. Rho 2004 also reported this outcome in Snellen lines (MD 0.40, 95% CI −0.93 to 1.73; 68 participants) Rho 2006 reported in ETDRS letters (MD 0.90, 95% CI −4.31 to 6.11; 34 participants) considering data from the diclofenac group or (MD 1.20, 95% CI −4.58 to 6.98; 34 participants) considering data from the bromfenac group |
| Persistence of visual improvement one month after discontinuation of treatment | Not measured by any included trial |
|||||
| Improvement in leakage on FFA | Not measured by any included trial |
|||||
| Improvement in central macular thickness on OCT | Not measured by any included trial |
|||||
| Improved contrast sensitivity | Not measured by any included trial | |||||
| Change in QoL | Not measured by any included trial | |||||
| *The risk in the intervention group (and its 95% CI) is based
on the assumed risk in the comparison group and the relative
effect of the intervention (and the associated 95% CI). CI: confidence interval; CME: cystoid macular edema; ETDRS: Early Treatment Diabetic Retinopathy Study; FFA: fundus fluorescein angiography (angiogram); NSAID: non‐steroidal anti‐inflammatory drug; OCT: optical coherence tomography; QoL: quality of life; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded for risk of bias (‐1), imprecision (‐1) and inconsistency (‐1).
Background
Cystoid macular edema (CME) is the presence of excess fluid in the extracellular space of the neurosensory retina presenting clinically with cyst‐like spaces and abnormal thickening of the central part of the retina (the macula). Cystoid macular edema following cataract surgery was first described by Irvine in 1953 (Irvine 1953), and Gass and Norton published the first detailed microscopic and fluorescein angiographic description of this condition in 1966 (Gass 1966); the entity later became known as Irvine‐Gass syndrome or pseudophakic CME. Cataract surgery is the most common ambulatory incisional surgery performed in the USA (Steiner 2014), with CME being one of its most frequent postoperative complications.
Description of the condition
Pseudophakic CME impairing patients’ vision has been reported to occur in 1% to 2% of patients, with a peak incidence occurring six weeks postoperatively, but subclinical CME may be observed on fluorescein angiography in about 30% of patients, and on optical coherence tomography in up to 40% of patients (Henderson 2007; Perente 2007; Shelsta 2010). Despite the many advances in cataract surgery techniques, clinically significant CME following modern cataract surgery has been reported to occur in about 2% of cases (Kessel 2014; Malwankar 2022; Taipale 2019).
Presentation and diagnosis
Pseudophakic CME may be asymptomatic or present with decreased or distorted vision, or both, with a peak incidence six weeks after cataract surgery. Slit‐lamp biomicroscopy may demonstrate retinal thickening; a blunted, or loss of, foveal depression; a yellowish appearance of the central macula; and a honeycomb appearance at the macula. Clinical findings may be best observed using a fundus contact lens, and red‐free light may facilitate detection of cystic changes. Fluorescein angiography may demonstrate perifoveal retinal telangiectasis, dilated capillaries, and perifoveal leakage in a 'petaloid’ pattern. Optic nerve staining may also be observed and helps to distinguish pseudophakic CME from other causes of CME. On optical coherence tomography, CME is characterized by a loss of the foveal depression, retinal thickening, intraretinal cystic hyporeflective lesions and, in some cases, hyporeflectivity under the central macula, indicating the presence of subretinal fluid.
Description of the intervention
Although the pathogenesis of pseudophakic CME remains incompletely understood, it is believed that inflammation leading to a breakdown of the blood‐retinal barrier plays a major role. It has been hypothesized that surgical manipulation causes significant release, by the anterior uveal tissue, of prostaglandins and other inflammatory mediators; the latter increase permeability of the perifoveal capillaries, leading to accumulation of fluid and cystic changes in the outer plexiform and inner nuclear layers of the retina (Miyake 2002). Corticosteroids and non‐steroidal anti‐inflammatory drugs (NSAIDs) have been used both pre‐ and postoperatively to try to prevent and treat pseudophakic CME (Brandsdorfer 2019; Grzybowski 2016; Orski 2021).
How the intervention might work
It is hypothesized that inflammation plays a major role in the pathogenesis of CME. Since NSAIDs inhibit cyclooxygenase, a rate‐limiting enzyme in the production of prostaglandins, NSAIDs may be effective in treating CME by reducing the production of inflammatory factors.
Why it is important to do this review
This is an update of a review last published in 2012, which examined the effects of topical NSAIDs on acute CME (3 trials) and chronic CME (4 trials), and concluded that the effects of NSAIDs in both acute and chronic CME remained unclear (Sivaprasad 2012). Therefore, we attempted to update the evidence base by including newly available trials evaluating treatment effects of topical NSAIDs on either acute or chronic CME, and by including additional analyses.
Objectives
To examine the effectiveness of NSAIDs in the treatment of CME following cataract surgery.
Methods
Criteria for considering studies for this review
Types of studies
This review included randomized controlled trials (RCTs).
Types of participants
Participants in the trials were adults who developed CME following cataract surgery in an eye with no history of ocular inflammatory disease, trauma or previous intraocular surgery. Surgery and the postoperative course of cataract are different in children and adults, so we excluded children from the review. We classified CME as either acute, defined as edema of less than four months' duration, or chronic, defined by CME that had persisted for four months or longer. In the current update, we also considered inclusion of trials that did not specify types of CME in their inclusion criteria (see Differences between protocol and review).
Types of interventions
We included trials that compared NSAIDs in any form or dosage to placebo, no treatment or another treatment modality with the aim of treating CME following cataract surgery. There was no minimum or maximum limit on the duration of treatment. To be consistent with the previous reviews (Sivaprasad 2004; Sivaprasad 2009; Sivaprasad 2012), we also considered trials that compared different NSAIDs as eligible.
Types of outcome measures
Primary outcomes
Critical outcomes
Improvement of visual acuity, defined by two or more lines in Snellen visual acuity or equivalent, at the end of treatment
Persistence of visual improvement one month after discontinuation of treatment
Secondary outcomes
Important outcomes
-
Improvement in CME, quantified by proportion of participants with:
improvement in leakage on fundus fluorescein angiography (FFA) as defined by the original study;
improvement in central macular thickness on optical coherence tomography (OCT) as defined by the original study; or
improvement in contrast sensitivity.
Change in quality of life as measured by validated questionnaires such as the National Eye Institute Visual Function Questionnaire (NEI‐VFQ 25; Mangione 2001) or the 36‐Item Short Form Health Survey (SF‐36; Hays 2001).
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for randomized controlled trials and controlled clinical trials. There were no language or publication year restrictions. The electronic databases were last searched on 20 March 2022.
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 3) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 20 March 2022) (Appendix 1).
MEDLINE Ovid (1946 to 20 March 2022) (Appendix 2).
Embase.com (1947 to 20 March 2022) (Appendix 3).
PubMed (1946 to 20 March 2022) (Appendix 4).
Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to 20 March 2022) (Appendix 5).
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com; discontinued in 2014; last searched August 2011) (Appendix 6).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 20 March 2022) (Appendix 7).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 20 March 2022) (Appendix 8).
Searching other resources
We handsearched reference lists of all identified trials and previous reviews for additional trials but did not identify eligible studies. We did not handsearch conference abstracts as they are already included in CENTRAL.
Data collection and analysis
Selection of studies
The Information Specialist updated the search strategies and performed the searches. We imported separate search results to the web‐based review management software, Covidence, to automatically identify and remove duplicates. Two review authors independently screened the titles and abstracts and classified each record as 'relevant (yes)', 'maybe relevant', or 'not relevant (no)' according to the eligibility criteria. We then retrieved full‐text articles that were classified as 'relevant' or 'maybe relevant' for additional review for inclusion. Two review authors independently assessed the full‐text articles and resolved any disagreements by discussion.
We also contacted trial authors to request clarification about trial design and the availability of full‐text publications besides the conference abstracts that were available to the review authors. If the trial authors did not respond within two weeks, we used the information available to determine eligibility whenever feasible. Specifically, we classified trials as 'awaiting classification' if they were completed but no trial results were publicly available on the trial registry website or in any indexed databases.
Data extraction and management
Two review authors independently extracted data in Covidence using data abstraction forms developed by Cochrane Eyes and Vision. We extracted information regarding the trial setting; countries where participant recruitment and intervention took place; sample size; trial duration and treatment duration; trial design elements (unit of randomization, unit of analysis); method of analysis (intent‐to‐treat or complete case analysis); sources of funding and potential conflicts of interests; characteristics of participants; intervention medications and dosage; and study outcomes (domain, specific measurement and metric, method of aggregation, and time points of reporting). Two review authors compared the extracted data, resolving discrepancies by discussion. One review author entered data into RevMan Web 2022 and a second review author verified the data entered.
Assessment of risk of bias in included studies
Two review authors independently assessed the quality of each trial identified for this update and we achieved consensus via discussion. We applied risk of bias assessment following the guidance in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Specific domains for consideration included the following.
Selection bias: were the randomization procedure and the allocation concealment adequate?
Performance bias: were the participants and people administering the treatment masked to the intervention?
-
Attrition bias:
were withdrawals and dropouts completely described?
was the analysis by intent‐to‐treat or 'available case' analysis?
Detection bias: were outcome assessors masked to the intervention?
Reporting bias: was there selective outcome reporting?
We graded trials as having low risk, high risk or, when the information reported was insufficient to make an assessment, unclear risk of bias. We documented supporting statements for our consensus assessments and presented these overall assessment results in a risk of bias summary figure (Higgins 2011). In the current update, we did not exclude trials based on the assessed levels of risk of bias.
Measures of treatment effect
We compared mean differences (MD) for continuous outcomes, such as mean improvement in visual acuity at the end of treatment in lines of Snellen acuity or Early Treatment Diabetic Retinopathy Study (ETDRS) letters. We estimated risk ratios (RR) for comparing dichotomous outcomes, such as proportions of participants with improvement in leakage on FFA or improved contrast sensitivity. We also reported 95% confidence intervals (CI) to express the uncertainty surrounding the point estimates.
Unit of analysis issues
All included trials enrolled participants with only one affected eye, except for Yannuzzi 1977, in which the investigators recruited three participants whose eyes were both treated with the same intervention. All trials reported outcomes at the individual, not the eye, level, including one cross‐over trial (Flach 1998).
Dealing with missing data
We did not impute any missing data. When the quality or specific metric of the available data prevented any statistical pooling, we excluded the trial from quantitative analyses and reported the data in a narrative manner when appropriate.
Assessment of heterogeneity
We examined the overall characteristics of the included trials, in particular the types of participants, types of interventions, and study design, to assess the extent to which the studies were sufficiently similar to permit a meaningful meta‐analysis of review outcomes. We considered the size and direction of intervention effects, and took into account the amount of heterogeneity as quantified by the I² statistic (Higgins 2002). As suggested in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022), we used the following thresholds to interpret I² values:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We did not assess small‐study effects via funnel plots, which would also signal for potential publication bias, given that fewer than 10 trials contributed data to any meta‐analysis. We assessed selective outcome reporting as part of the risk of bias assessment.
Data synthesis
We performed pooled analysis following the guidance in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions for data synthesis and analysis (McKenzie 2022). We applied the fixed‐effect model to synthesize data from fewer than three trials reporting the same outcome data. Additionally, we provided a narrative synthesis of all included trials and their results.
We compared NSAIDs with placebo or another active treatment (alone or in combination) and reported treatment effects in RR and the associated 95% CI. In some comparisons, we switched the intervention and comparator groups in the forest plot to present comparison results versus the same comparator (ketorolac 0.5%).
Subgroup analysis and investigation of heterogeneity
Previous reviews did not plan for any subgroup analysis. We performed post‐hoc subgroup analysis to explore potential sources of heterogeneity, such as treatment duration.
Sensitivity analysis
Previous reviews did not specify any sensitivity analysis. We did not perform any sensitivity analysis either because of the small numbers of trials reporting on the same review outcomes in the pooled analysis.
Summary of findings and assessment of the certainty of the evidence
For the current update, we prepared summary of findings tables presenting relative or absolute risks (Schünemann 2022a). Two review authors independently graded the overall certainty of the evidence for each outcome using the GRADE approach (Schünemann 2013).
Proportion of participants with improvement of two or more lines in Snellen visual acuity or equivalent at end of treatment
Proportion of participants with persistence of vision improvement one month after discontinuation of treatment
Proportion of participants with improvement in leakage on FFA
Proportion of participants with improvement in central macular thickness on OCT
Proportion of participants with improvement in contrast sensitivity
Change in quality of life
We graded the certainty of the evidence as 'high,' 'moderate,' 'low,' or 'very low' according to
risk of bias among included trials;
indirectness of evidence;
unexplained heterogeneity or inconsistency of results;
low precision of results; and
risk of publication bias (Schünemann 2013).
We downgraded certainty of the evidence by two levels when contextual considerations suggested extreme imprecision according to the recently published guidance by Schünemann and others (Schünemann 2022b).
We resolved discrepancies between the two review authors by discussion.
Results
Description of studies
Results of the search
In the first version of the review (Sivaprasad 2004), review authors screened 382 records and included seven eligible trials involving 266 participants (Burnett 1983; Flach 1987; Flach 1991; Flach 1998; Heier 2000; Rho 2003; Yannuzzi 1977). Additional updated searches in 2006, 2008, and 2011 resulted in 138, 56, and 77 records, but the review authors did not identify any new eligible trials for the published updates (Sivaprasad 2009; Sivaprasad 2012).
From the updated searches performed in March 2022, we screened 1560 records and excluded 1547 titles and abstracts. We assessed 13 full‐text articles for eligibility. From these, we found three trials that were already included in the previous review (Flach 1991; Heier 2000; Rho 2003), and we excluded five trials with reasons documented for exclusion (Aaronson 2020; Ginsburg 1994; NCT00438243; NCT01769352; Wolfensberger 1999).
In total, we included two new trials for the current update (Rho 2004; Rho 2006; Figure 1). We also classified two trials as 'awaiting classification' (NCT00595543; Yüksel 2017) because of unavailability of trial results or unclear information about study design despite our attempts to contact trial authors.
1.
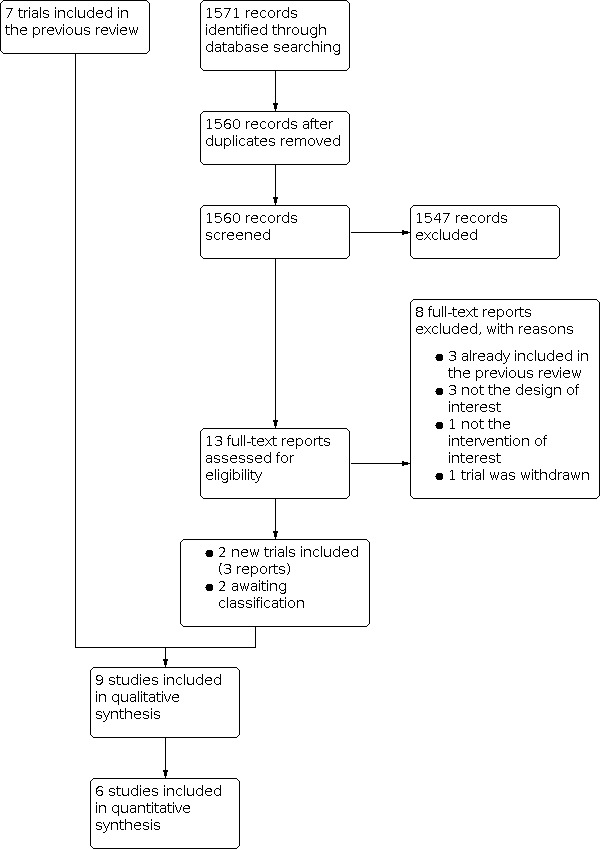
Study flow diagram
Included studies
In total, we included nine trials (390 participants, 393 eyes) in this review for qualitative synthesis and six in meta‐analyses that each included two to four trials (Figure 1).
Types of studies and setting
All trials included were of parallel‐group design, except for Flach 1998, which randomized the study participants initially and then again at the end of the first 30‐day treatment period. Despite the reported two‐week washout period, the authors of Flach 1998 did not provide separate outcome data for the second treatment period; we decided to extract and use only outcome data reported at the end of the first 30‐day period.
Seven of the trials were two‐arm trials. The remaining two trials were three‐arm trials, which compared the effects of a topical NSAID with two other active treatments (Heier 2000; Rho 2006). All of the included trials were conducted in the USA. Two trials were conducted in a single medical center (Yannuzzi 1977; Burnett 1983) and one in a private clinic (Rho 2003); investigators of three other trials reported multi‐site involvement (Flach 1987; Flach 1991; Heier 2000). The remaining three trials did not report trial setting (Flach 1998; Rho 2004; Rho 2006).
Types of participants
According to the post‐surgical intervals that study participants had from the latest cataract surgery or the duration with which the study participants had been diagnosed with CME before study enrollment, study participants of these nine trials could be classified into those with acute CME, defined as CME of less than four months' duration (Flach 1998; Heier 2000; Rho 2003), those with chronic CME, defined as CME which persisted for four months or longer (Burnett 1983; Flach 1987; Flach 1991; Yannuzzi 1977), or those with unspecified CME type (Rho 2004; Rho 2006). Only a few trials provided information about the CME duration when recruiting participants at baseline, which ranged from 4.1 weeks to 7 weeks for participants with acute CME (Heier 2000; Rho 2003), and 6 to 24 months for those with chronic CME (Burnett 1983). On average, each trial enrolled a median number of 30 participants (interquartile range [IQR] 22 to 52) or 30 eyes (IQR 23 to 52). Authors of four trials did not report sex distributions (Flach 1991; Flach 1998; Rho 2004; Rho 2006) or age compositions (Flach 1991) of their study participants. Based on other trials that reported age data, the average age of the study participants was 72.2 years (IQR 68.8 to 73.6); 72% were women (IQR 69% to 74%).
Types of interventions
We included trials in which NSAIDs in any form or dosage were compared to placebo, no treatment or another therapeutic modality with the aim of treating CME after cataract surgery (previous reviews also included comparisons of different types of NSAIDs [Sivaprasad 2004; Sivaprasad 2009; Sivaprasad 2012]). We did not specify a minimum or maximum duration of treatment.
In the three trials that enrolled patients with acute CME:
Flach 1998 compared the effect of topical ketorolac tromethamine 0.5% with placebo;
Heier 2000 compared topical ketorolac tromethamine 0.5% with topical prednisolone acetate 1% as well as with a combination of topical ketorolac tromethamine 0.5% and topical prednisolone acetate 1%; and
Rho 2003 compared topical ketorolac tromethamine 0.5% with topical diclofenac sodium 0.1%.
In the four trials that enrolled patients with chronic CME:
Yannuzzi 1977 compared the effect of oral indomethacin with placebo;
Burnett 1983 compared topical fenoprofen 1% with placebo; and
Flach 1987 and Flach 1991 compared topical ketorolac tromethamine 0.5% with placebo.
Two trials included participants with CME of unclear duration (Rho 2004, Rho 2006), and compared NSAID with one or more active treatment:
Rho 2004 reported comparison results of topical ketorolac tromethamine 0.5% versus topical diclofenac sodium 1%; participants in both groups were also treated with topical prednisolone acetate 1%;
Rho 2006, a three‐arm trial, compared the effect of topical bromfenac 0.09% with topical diclofenac sodium 0.1% and with topical ketorolac tromethamine 0.5%.
Across these trials, topical ketorolac tromethamine 0.5% was the most frequently used interventional NSAID (Flach 1987; Flach 1991; Rho 2004; Rho 2006), followed by topical diclofenac sodium 1% (Rho 2004; Rho 2006), topical bromfenac 0.09% (Rho 2006), topical fenoprofen sodium 1% (Burnett 1983), and oral indomethacin (Yannuzzi 1977). Besides placebo (or vehicle), active treatments under comparison included another NSAID (Rho 2003; Rho 2004; Rho 2006), and topical prednisolone acetate 1% alone (Heier 2000) or in combination with another NSAID (Heier 2000).
Types of outcomes
Critical outcomes
Improvement of two or more lines in Snellen visual acuity or equivalent at end of treatment
Six trials reported an improvement in Snellen visual acuity of two or more lines at the end of treatment (Flach 1987; Flach 1991; Flach 1998; Heier 2000; Rho 2004; Yannuzzi 1977). Heier 2000 and Rho 2004 also reported mean final visual acuity in Snellen lines, whereas Rho 2006 reported visual acuity in ETDRS letters.
Persistence of improvement of vision one month after discontinuation of treatment
Three trials reported persistent visual improvement at one month following cessation of treatment (Flach 1987; Flach 1991; Yannuzzi 1977).
Important outcomes
Proportion of participants with improvement in leakage on FFA
Six trials performed FFA as a diagnostic or monitoring tool (Burnett 1983; Flach 1987; Flach 1991; Heier 2000; Rho 2003; Yannuzzi 1977), although not all participants received repeat FFA at follow‐up in Rho 2003. Only three trials reported decreased leakage on FFA as an outcome measure (Burnett 1983; Flach 1987; Heier 2000).
Proportion of participants with improvement in central macular thickness on OCT
None of the included trials measured this outcome.
Proportion of participants with improved contrast sensitivity
Only one trial assessed contrast sensitivity (Heier 2000).
Change in quality of life
None of the included trials measured this outcome.
Excluded studies
The previous review (Sivaprasad 2004), excluded nine trials (Ahluwalia 1988; Azzolini 1986; IDSG 1997; Jampol 1994; Kraff 1985; Miyake 1995; Rossetti 1996; Singal 2004; Stark 1984) because they either tested NSAIDs for prophylactic purposes or tested efficacy of prednisolone, rather than an NSAID. The prior review update (Sivaprasad 2012) further excluded four trials (Asano 2008; Mathys 2010; Miyake 2007; Warren 2010).
For the current update, we excluded three trials that were not RCTs (Aaronson 2020; Ginsburg 1994; Wolfensberger 1999); one that examined effects of topical steroid (NCT01769352); and one trial that was withdrawn before enrolling participants (NCT00438243). Overall, we excluded 18 trials, as documented in the Characteristics of excluded studies.
Risk of bias in included studies
In this update, we applied the Cochrane risk of bias tool to assess the quality of the two newly‐added trials (Rho 2004; Rho 2006), as well as the seven trials included in the previous review (Sivaprasad 2012). We did not deem any of the included nine trials as low risk across all bias domains (Figure 2). We assessed three trials (Flach 1987; Flach 1991; Flach 1998) as having unclear risk of bias overall (33%) and the other six as having high (67%) risk of bias (see Figure 3 for risk of bias items presented as percentages).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Random sequence generation
Three trials used computer‐generated random sequences for the randomization process, which we deemed at low risk of bias (Flach 1987; Flach 1991; Flach 1998). One trial used centralized randomization by a pharmacy, but did not state their method of sequence generation, so we deemed it at unclear risk of bias (Heier 2000). The trial authors of the remaining five trials stated that the groups were randomly allocated, but the method of treatment assignment was unclear, so we deemed these trials at unclear risk of bias (56%; Burnett 1983; Rho 2003; Rho 2004; Rho 2006; Yannuzzi 1977).
Allocation concealment before assignment
Flach 1998 distributed treatment "in a double‐masked fashion", so we deemed this trial to be at low risk of bias (11%). In Heier 2000, the "randomization was performed by the pharmacy that supplied the premasked medications", suggesting that the pharmacy might have had the knowledge of the corresponding sequences of randomization and medications; we deemed this trial at high risk of bias (11%). The other seven trials did not describe how they concealed allocation, and we deemed them at unclear risk of bias (78%; Burnett 1983; Flach 1987; Flach 1991; Rho 2003; Rho 2004; Rho 2006; Yannuzzi 1977).
Blinding
Masking of participants and personnel
All of the included trials, except Rho 2003, Rho 2004 and Rho 2006, were double‐masked and we deemed them to be at low risk of bias (67%). Rho 2003 and Rho 2006 did not report on masking, and we deemed them at unclear risk of bias (22%). In Rho 2004, the medication bottles were unmasked; we deemed this trial at high risk of bias for this domain (11%).
Masking of outcome assessment
Four of the nine trials were reportedly "double‐masked" for both study participants and clinical investigators or examiners; we deemed these to be at low risk of bias (50%; Flach 1987; Flach 1991; Flach 1998; Heier 2000). In Burnett 1983 and Yannuzzi 1977, it was not clear whether or not all examiners and trial personnel performing and interpreting ocular examinations at follow‐up visits were masked; thus, we deemed these two trials at unclear risk of bias. Rho 2003 and Rho 2006 did not report on masking and we also deemed them at unclear risk of bias (44%). In Rho 2004, the medication bottles were unmasked; we deemed this trial at high risk of bias for this domain (11%).
Incomplete outcome data
Seven of the included nine trials reported outcome data for all or nearly all randomized participants; we deemed these trials at low risk of bias (78%; Burnett 1983; Flach 1987; Flach 1998; Heier 2000; Rho 2003; Rho 2006; Yannuzzi 1977). We deemed Flach 1991 and Rho 2004 as having unclear risk of bias: Flach 1991 excluded substantial proportions of participants (25% in the intervention group and 17% in the placebo group) from the analysis due to loss to follow‐up; Rho 2004 did not report the group‐specific follow‐up rates nor the planned study duration.
Selective reporting
We deemed five trials to be at low risk of bias because they reported all of their prespecified outcomes (56%; Burnett 1983; Flach 1987; Flach 1991; Heier 2000; Rho 2003). We judged three trials to be at unclear risk of bias (Flach 1998; Rho 2004; Rho 2006) because no trial protocol or detailed methods description was available for assessment. We also deemed Yannuzzi 1977 to be at unclear risk of bias because the authors did not clearly report FFA results in a conventional way and the unit of analysis was the eye, while the unit of randomization was the person.
Other potential sources of bias
We deemed five of the nine trials to be at unclear risk of bias (56%; Burnett 1983; Flach 1991; Flach 1998; Heier 2000; Yannuzzi 1977). Although the respective trials were supported, at least in part, by industry, the trial authors did not report or disclose relevant conflicts of interest. We also deemed Rho 2003 to be at unclear risk of bias although the author reported that he had no financial or proprietary interest "in any material or method mentioned"; the author was on the speaker's bureau of a company that made one of the drugs investigated in the trial. Similarly, we deemed Flach 1987 to be at unclear risk of bias because the first author served as a consultant for the same company that funded the trial. We deemed Rho 2004 and Rho 2006 to be at high risk of bias because the first author of these publications reported a commercial relationship with a company that made one of the drugs investigated in each of these trials.
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison 1: NSAID versus placebo or active control in participants with acute CME
Three of the nine included trials enrolled participants whose CME had been diagnosed within four months before study entry (Flach 1998; Heier 2000; Rho 2003). Flach 1998 compared topical ketorolac with placebo whereas the other two trials (Heier 2000; Rho 2003) compared topical ketorolac with one or more active treatments. See Table 1 showing findings of trials comparing NSAIDs with placebo or active control in participants with acute CME.
Critical outcomes
Improvement of two or more lines in Snellen visual acuity or equivalent at end of treatment
Two of the three trials reported on improvement in Snellen visual acuity of two or more lines at the end of treatment (Flach 1998; Heier 2000).
In Flach 1998, after the first 30‐day treatment period in this cross‐over study (22 participants), a two‐line visual acuity improvement was achieved in 4 of 11 (36%) participants treated with topical ketorolac tromethamine 0.5% and 2 of 11 (18%) participants in the placebo group, suggesting that there was no treatment benefit of ketorolac on visual improvement compared with placebo (RR 2.00, 95% CI 0.46 to 8.76; Figure 4). The authors also reported seven‐year outcomes of the 22 participants included in the study, demonstrating improved vision without additional medical or surgical treatment at the time of their last examination in all but two participants, whose decreased vision was unrelated to CME (anterior ischemic optic neuropathy in one participant, glaucoma in the other).
4.

Forest plot of comparison1: NSAID versus placebo or active control in acute CME, outcome 1.1 Proportion of participants with visual improvement
In the three‐arm trial (Heier 2000), participants were treated until the CME resolved or for three months before tapering. When compared with topical prednisolone acetate 1.0%, topical ketorolac tromethamine 0.5% may have little or no effect on visual acuity improvement (RR 1.33, 95%: 0.58 to 3.07; 17 participants). Similarly, ketorolac 0.5% and prednisolone 1.0% combination therapy may not increase or decrease the likelihood of improved visual acuity when compared with prednisolone alone (RR 1.78, 95% CI 0.86 to 3.69; 18 participants; Figure 4). The trial authors further compared visual acuity improvement in Snellen lines (Analysis 1.2), but found inconsistent results. Ketorolac had little or no effect on visual improvement (MD 0.80 Snellen lines, 95% CI 0.08 to 1.52; 18 participants) when compared with prednisolone alone. In contrast, the combination therapy was associated with an improvement in visual acuity of 2.70 more lines (95% CI 1.99 to 3.41; 17 participants) than was observed with prednisolone alone at three months.
1.2. Analysis.

Comparison 1: NSAID versus placebo or active control in acute CME, Outcome 2: Improvement in VA, Snellen lines*
Instead of reporting visual acuity improvement from baseline, Rho 2003 found no differences within 26 weeks in the mean final visual acuity between the ketorolac and diclofenac groups among all study participants (20/58 versus 20/49, P = 0.74; 34 participants), in the subgroup of participants who experienced CME reduction (20/30 versus 20/30, P = 1.0), and in the subgroup of participants with complete resolution of CME (20/25 versus 20/25, P = 1.0).
Overall, when compared with placebo or active treatment, the evidence on the treatment effects of ketorolac 0.5% on visual acuity improvement at the end of treatment is very uncertain. The certainty level of the evidence for this outcome was very low after we downgraded it for risk of bias, imprecision, and inconsistency.
Persistence of improvement of vision one month after discontinuation of treatment
None of the included trials reported this outcome.
Important outcomes
Proportion of participants with improvement in leakage on FFA
Heier 2000 observed improvement in leakage on FFA in 5 of 9 (55%) participants treated with ketorolac tromethamine 0.5%, 4 of 8 participants (50%) treated with prednisolone acetate 1.0%, and 7 of 9 participants (77%) treated with ketorolac and prednisolone combination therapy. When compared with prednisolone alone (RR 1.11, 95% CI 0.45 to 2.75; 17 participants) or with combination therapy (RR 1.56, 95% CI 0.72 to 3.38; 17 participants), we found no evidence that ketorolac had additional treatment benefits (Analysis 1.3). The certainty of the evidence for this outcome was very low after we downgraded it for risk of bias, imprecision, and heterogeneity.
1.3. Analysis.

Comparison 1: NSAID versus placebo or active control in acute CME, Outcome 3: Proportion of participants with improved FFA
Proportion of participants with improvement in central macular thickness on OCT
None of the included trials reported this outcome.
Proportion of participants with improved contrast sensitivity
Heier 2000 found improvement in contrast sensitivity in 5 of 9 participants (55%) treated with ketorolac tromethamine 0.5%, 4 of 8 participants (50%) treated with prednisolone acetate 1.0%, and 8 of 9 of participants (89%) treated with ketorolac and prednisolone combination therapy. When compared with prednisolone alone (RR 1.11, 95% CI 0.45 to 2.75; 17 participants) or with combination therapy (RR 1.78, 95% CI 0.86 to 3.69; 17 participants), there was no evidence that ketorolac had additional treatment benefits (Analysis 1.4). The certainty of the evidence for this outcome was very low after we downgraded it for risk of bias, imprecision, and heterogeneity.
1.4. Analysis.

Comparison 1: NSAID versus placebo or active control in acute CME, Outcome 4: Proportion of participants with improved contrast sensitivity
Change in quality of life
None of the included trials reported this outcome.
Other outcomes reported by the included trials
Improvement or reduction of CME by biomicroscopic examination
Rho 2003 repeatedly assessed participants for severity of CME by Goldmann contact lens biomicroscopy during the follow‐up period. FFA "was repeated if clinical CME persisted". At the end of the 26‐week treatment, there was no evidence that diclofenac may have effects on completely resolving CME (RR 1.04, 95% CI 0.71 to 1.51; 34 participants) when compared to ketorolac (Analysis 1.5). The authors also concluded that reported time (in weeks) to CME reduction was comparable in the ketorolac (mean 8.0, standard deviation [SD] 1.8) and diclofenac groups (mean 7.5, SD 1.5).
1.5. Analysis.

Comparison 1: NSAID versus placebo or active control in acute CME, Outcome 5: Proportion of participants with complete resolution*
Adverse events
Flach 1998 reported that "there were no complaints about burning or stinging following eyedrop instillation and "none of the patients developed any signs of ocular toxicity during the study." Rho 2003 found no adverse events of superficial punctate keratitis, allergic reaction, or other ocular changes "that prevented [patients] from completing the study". Heier 2000 did not mention any adverse events associated with the intervention treatments.
Comparison 2: NSAID versus placebo in participants with chronic CME
Four of the nine included trials enrolled participants whose CME had been diagnosed for at least four months prior to the study entry (Burnett 1983; Flach 1987; Flach 1991; Yannuzzi 1977). See Table 2 for review findings of trials comparing NSAIDs versus placebo in participants with chronic CME.
Critical outcomes
Improvement of two or more lines in Snellen visual acuity or equivalent at end of treatment
All four trials reported an improvement in Snellen visual acuity of two or more lines at the end of treatment.
Burnett 1983 compared topical fenoprofen sodium 1% (6 participants) with placebo (8 participants). At the end of the eight‐week treatment period, an improvement of two or more lines in Snellen visual acuity was similarly achieved in the fenoprofen group compared with the placebo group (RR 1.33, 95% CI 0.40 to 4.43).
Flach 1987 randomized 30 eyes of 30 patients to treatment with either topical ketorolac tromethamine 0.5% or placebo, four times daily for no more than 90 days. Four participants failed to complete the study: one participant was lost to follow‐up and another developed an ocular allergy in the ketorolac group; two participants in the placebo group did not complete the study due to poor compliance and myocardial infarction, respectively. Eight of 13 participants (62%) In the ketorolac group and 1 of 13 participants (8%) in the placebo group, achieved an improvement of two or more lines of Snellen visual acuity at day 60, showing an increased likelihood of visual improvement when treated with NSAIDs versus placebo (RR 8.00, 95% CI 1.16 to 55.2; 26 participants).
Flach 1991 randomized 120 eyes of 120 patients to either topical ketorolac tromethamine 0.5% or placebo, four times a day for no more than 120 days. However, only 46 of the 61 participants (75%) enrolled in the ketorolac group and 49 of the 59 participants (83%) enrolled in the placebo group were examined at day 90 or day 120, depending on results of distance visual acuity at day 90. By three months of treatment, 48% and 20% of the returning participants in the ketorolac group and placebo group, respectively, had a visual acuity improvement of two lines or more (RR 2.34, 95% CI 1.25 to 4.40; 95 participants).
Yannuzzi 1977 randomized 20 participants (23 eyes) to receive either 25 mg of orally administered indomethacin three times per day (10 participants, 10 eyes) or placebo three times per day (10 participants, 13 eyes) for six weeks. Participants were re‐assessed at weeks 3, 6, and 10. At the end of treatment (week 6), 2 of 10 (20%) eyes in the indomethacin group and 4 of 13 (31%) eyes in the placebo group had achieved an improvement of two or more lines in Snellen visual acuity; the estimated treatment effect at the person level was RR 0.40 (95% CI 0.10 to 1.60; 20 participants) comparing indomethacin with placebo.
Because of evidence of subgroup differences (P = 0.01, I2 = 84.7%), we did not combine estimates from trials that had treated participants with different durations of therapy (Analysis 2.1). Results from subgroups suggested that NSAIDs may increase participants' likelihood of visual improvement by 1.87 fold (RR 2.87, 95% CI 1.58 to 5.22; I2 = 33%; 2 trials, 121 participants) relative to placebo after treatment of 90 days or longer; however, there was no evidence of treatment effect of NSAID in the subgroup with two months or shorter use of NSAID (RR 0.72, 95% CI 0.30 to 1.73; P = 0.19, I2 = 41%; 2 trials, 34 participants; Figure 5).
2.1. Analysis.

Comparison 2: NSAID versus placebo in chronic CME, Outcome 1: Proportion of participants with visual improvement
5.

Forest plot of comparison 2: NSAID versus placebo in chronic CME, outcome 2.1 Proportion of participants with visual improvement
In a post‐hoc sensitivity analysis that excluded a trial that had examined oral indomethacin (Yannuzzi 1977), the between‐group heterogeneity attenuated (P = 0.30, I2 = 7.9%), and the combined results suggested that topical NSAIDs may increase participants' visual improvement as compared to placebo (RR 2.33, 95% CI 1.17 to 4.66; I2 = 22%; 3 trials, 135 participants; Analysis 2.2).
2.2. Analysis.

Comparison 2: NSAID versus placebo in chronic CME, Outcome 2: Proportion of participants with visual improvement ‐ sensitivity analysis
Overall, the certainty level of the evidence for this outcome was very low after we downgraded it for risk of bias, imprecision, and heterogeneity.
Persistence of improvement of vision one month after discontinuation of treatment
Yannuzzi 1977 reported persistence of visual acuity at one month following cessation of treatment. Two eyes of two participants in the indomethacin group and five eyes of five participants in the placebo group that had improved by two or more lines of vision after 6 weeks of treatment still maintained at least a two‐line visual acuity improvement one month following cessation of treatment (at day 70). The single‐study estimate for oral NSAID was RR 0.40 (95% CI 0.10 to 1.60; 20 participants) when compared to placebo. Similarly, in Flach 1987, four of the eight participants in the ketorolac group and one participant in the placebo group who achieved visual improvement at the end of the 60‐day treatment period still maintained two lines or more of visual improvement on day 90. The single‐study estimate was equivocal (RR 4.00, 95% CI 0.51 to 31.13; 26 participants). Although we did not combine results of these two trials due to between‐group heterogeneity (P = 0.07, I2 = 69.9%), overall, the estimate of either subgroup suggests that NSAIDs had no effect on persistent visual improvement one month after the treatment ended (Figure 6). The certainty of the evidence for this outcome was very low, downgraded one level due to risk of bias and two levels due to extreme imprecision.
6.

Forest plot of comparison 2: NSAID versus placebo in chronic CME, outcome 2.2 Proportion of participants with persistent visual improvement one month after discontinuation of treatment
Important outcomes
Proportion of participants with improvement in leakage on FFA
Two of the four trials reported on this outcome (Burnett 1983; Flach 1987; Analysis 2.4). Burnett 1983 observed decreased leakage on FFA in three participants in each of the fenoprofen (6 participants, 50%) and placebo groups (8 participants, 38%), corresponding to a single‐study estimated RR of 1.33 (95% CI 0.40 to 4.43). Flach 1987 observed decreased leakage on FFA in two of the 13 participants (15%) receiving ketorolac and none in the placebo group (13 participants). The combined estimate of RR for improvement in leakage on FFA was 1.93 (95% CI 0.62 to 6.02, 40 participants), indicating that NSAIDs had little to no beneficial effect over placebo (I2 = 0%; Figure 7). The certainty level of the evidence for this outcome was very low after downgrading for risk of bias (−1) and extreme imprecision (−2).
2.4. Analysis.

Comparison 2: NSAID versus placebo in chronic CME, Outcome 4: Proportion of participants with improved FFA
7.

Forest plot of comparison 2: NSAID versus placebo in chronic CME, outcome 2.3 Proportion of participants with improved FFA
Proportion of participants with improvement in central macular thickness on OCT
None of the included trials reported this outcome.
Proportion of participants with improved contrast sensitivity
None of the included trials reported this outcome.
Change in quality of life
None of the included trials reported this outcome.
Other outcomes reported by the included trials
Proportion of participants with complete resolution of CME
Burnett 1983 reported that there was no leakage on FFA at the final study visit in one of the six participants (17%) in the fenoprofen group and one of the eight participants (13%) in the placebo group after eight weeks of treatment with fenoprofen 1% versus placebo (Analysis 2.5).
2.5. Analysis.

Comparison 2: NSAID versus placebo in chronic CME, Outcome 5: Proportion of participants with complete resolution
Adverse events
Across trials, one participant in Flach 1987 assigned to treatment with ketorolac did not complete the study due to ocular allergy. Flach 1991 reported that there were no statistically significant differences in the number or severity of adverse events between treatment groups.
Comparison 3: NSAID versus active control in participants with mixed duration of CME
Two trials did not specify the types (or durations) of CME in the inclusion criteria for the primary study (Rho 2004; Rho 2006). See Table 3 for review findings comparing NSAID versus active treatments in participants with probably mixed types of CME.
Critical outcomes
Improvement of two or more lines in Snellen visual acuity or equivalent at end of treatment
One trial reported an improvement in Snellen visual acuity of two or more lines at the end of treatment (Rho 2004). Rho 2004 was a two‐arm trial that randomized 68 eyes of 68 patients to topical diclofenac sodium 0.1% or topical ketorolac tromethamine 0.5%. Both comparison groups were also provided with topical prednisolone acetate 0.1%. All participants reportedly completed at least three months of treatment yet the exact follow‐up duration was not reported. Based on the single‐study estimate, diclofenac + prednisolone may increase participants' likelihood of visual improvement by 40% when compared to ketorolac + prednisolone (RR 1.40, 95% CI 1.02 to 1.94; 68 participants; Analysis 3.1).
3.1. Analysis.

Comparison 3: NSAID versus active control in mixed CME, Outcome 1: Proportion of participants with visual improvement
Rho 2004 also reported visual acuity improvement in Snellen lines (Analysis 3.2). However, the single‐study estimate showed no effect of diclofenac over ketorolac in increasing or decreasing Snellen lines of visual acuity at the end of treatment (MD 0.40, 95% CI −0.93 to 1.73; 68 participants).
3.2. Analysis.

Comparison 3: NSAID versus active control in mixed CME, Outcome 2: Improvement in VA, Snellen lines*
Similarly, Rho 2006 reported visual improvement in ETDRS letters when participants completed the study at three months. Rho 2006 randomized 52 eyes of 52 patients to one of three regimens: topical bromfenac 0.09% twice daily, topical diclofenac sodium 0.0.1% four times daily, or topical ketorolac tromethamine 0.05% four times daily. The single‐study estimates showed that either diclofenac (MD 0.90, 95% CI −4.31 to 6.11 ETDRS letters; 34 participants) or bromfenac alone (MD 1.20, 95% CI −4.58 to 6.98 ETDRS letters; 34 participants) had no effect on visual improvement (Analysis 3.3).
3.3. Analysis.

Comparison 3: NSAID versus active control in mixed CME, Outcome 3: Improvement in VA, ETDRS letters*
Overall, NSAID may slightly increase participants' likelihood of visual improvement but the evidence is very uncertain. The certainty of the evidence for this outcome was very low because we downgraded it for risk of bias, imprecision, and inconsistency (Table 3).
Persistence of visual improvement of vision one month after discontinuation of treatment
None of the included trials reported this outcome.
Important outcomes
Proportion of participants with improvement in leakage on FFA
None of the included trials reported this outcome.
Proportion of participants with improvement in central macular thickness on OCT
None of the included trials reported this outcome.
Proportion of participants with improved contrast sensitivity
None of the included trials reported this outcome.
Change in quality of life
None of the included trials reported this outcome.
Other outcomes reported by the included trials
Complete resolution of CME by unspecified measurement or examination
Rho 2004 reported complete resolution of CME without specifying how it was determined. Rho 2004 observed that 10 of 36 participants (28%) in the diclofenac group and 8 of 32 participants (25%) in the ketorolac group achieved complete resolution of CME. The single‐study estimate suggests that diclofenac had no or little effect on CME resolution (RR 1.11, 95% CI 0.50 to 2.47; 68 participants; Analysis 3.4) when compared with another NSAID (ketorolac).
3.4. Analysis.

Comparison 3: NSAID versus active control in mixed CME, Outcome 4: Proportion of participants with complete resolution*
Adverse events
Rho 2004 stated that none of the participants "showed signs of corneal toxicity or significant intraocular pressure rise during the treatment period." There was no mention of ocular adverse events in Rho 2006.
Discussion
Summary of main results
We based our findings on data from nine RCTs that investigated the effectiveness of any form or dosage of NSAIDs compared to placebo, no treatment or another active treatment modality, including different types of NSAIDs, with the aim of treating CME following cataract surgery. NSAIDs may not improve visual acuity at the end of treatment for any type of CME. Although there was evidence that NSAIDs may slightly increase the likelihood of visual acuity improvement with 90 days or longer of treatment for patients with chronic CME, the overall evidence was very uncertain (very low‐certainty evidence). We did not find evidence that NSAIDs had an effect on persistent visual improvement after treatment discontinuation (very low‐certainty evidence); improvement of leakage on FFA (very low‐certainty evidence); or improvement in contrast sensitivity (very low‐certainty evidence). None of the included trials reported on improvement in central macular thickness on OCT or change in quality of life. Thus, this review found that evidence on the effects of NSAIDs in acute or chronic CME is very uncertain and further investigation is warranted.
Overall completeness and applicability of evidence
The findings of this review may be applicable to patients with CME of various durations after cataract surgery. However, because the majority of the included primary studies excluded high‐risk patients (those with pre‐existing macular disease, poorly controlled diabetes or hypertension), our review findings are not generalizable to such individuals. In addition, our findings are limited by small sample sizes of primary studies, a deficient number of eligible trials reporting on the same outcomes, heterogeneity in interventions (varying treatment medications or durations) and different follow‐up durations.
Further, the publication dates of the trials included in this review range from 1977 to 2006, and there have been many advancements in cataract surgery techniques throughout and since that time. For example, cataract surgery has progressed from intracapsular cataract extraction to extracapsular cataract extraction to phacoemulsification cataract surgery; from large sutured wounds to small, nonsutured wounds; from scleral to corneal incisions; and from retrobulbar or peribulbar anesthesia to topical anesthesia (Davis 2016; Fichman 1996). Advancements in cataract surgery may lead to differences in the underlying risks of developing CME postoperatively over time. However, several published studies indicate that, even after the many advancements in cataract surgery techniques, the rate of clinically significant CME following modern cataract surgery is about 2% (Kessel 2014; Malwankar 2022; Taipale 2019). The lack of a placebo group across CME types prohibited us from inferring any overall treatment benefits of NSAIDs and from distinguishing treatment effect from spontaneous resolution of CME.
It should also be noted that most of the trials included in this review were performed before the widespread use of OCT (Fujimoto 2016). Due to its non‐contact and non‐invasive approach, quick image acquisition, high‐resolution images, and safety profile, OCT has become a leading diagnostic imaging modality for pseudophakic CME (Trichonas 2014), although in some cases, obtaining FFA imaging may be useful in differentiating pseudophakic CME from other conditions such as diabetic macular edema.
Moreover, standards of conducting and reporting intervention trials have evolved over time, particularly since the publication of the first CONSORT (Consolidated Standards of Reporting Trials) Statement in 1996 (Begg 1996). Inadequate reporting of participant characteristics and heterogeneity in the study design, such as the treatment duration, follow‐up duration, and frequency of outcome measurements (without clearly pre‐specified study endpoints), contributed to the generally low certainty of the evidence. In addition, the lack of consistent and valid methods for assessing and reporting outcomes created a barrier to synthesizing the evidence.
Certainty of the evidence
None of the included nine trials was deemed as low risk across all bias domains; we assessed the majority of the trials as high risk of bias. We assessed most of the trials to be at unclear or high risk of bias on the random sequence generation and allocation concealment domains, and five of the trials at unclear or high risk of bias on the blinding of outcome assessment domain. Concerns in multiple bias domains, including unmasking of participants or assessors, substantial dropout rates, and selective outcome reporting, further downgraded the certainty level of the evidence for most, if not all, review outcomes. The evidence in the nine trials was very low certainty due to such factors as small sample sizes, poorly reported outcomes, inconsistent results, and the inability to distinguish drug effect from spontaneous resolution of CME.
Quality of the evidence
[left for empty]
Potential biases in the review process
We applied the standard Cochrane methods to conduct this update and avoided potential biases in the processes of literature search, critical appraisal, data extraction and entry, data analysis and interpretation. Although not always successful, we also contacted trial authors and publication authors to request clarification or detailed information regarding study design and outcome in attempts to reduce information bias. To reduce inter‐rater biases, two review authors also independently re‐assessed risk of bias in the seven eligible trials of the previous review (Sivaprasad 2004).
Agreements and disagreements with other studies or reviews
A recently published systematic review, which investigated the effect of NSAIDs for acute and chronic CME (Orski 2021), included seven trials, two of which were RCTs, and reported visual acuity improvement and central macular thickness reduction. However, similar to our review, the trials in that review were constrained by small sample sizes, as well as heterogeneity in interventions, treatment durations, follow‐up intervals, and outcome assessments. Because of these study limitations, the authors of Orski 2021 also concluded that the available evidence is insufficient to formulate strong conclusions regarding NSAIDs for the treatment of either acute or chronic CME following cataract surgery.
Similarly, the American Academy of Ophthalmology (AAO) released an Ophthalmic Technology Assessment (OTA) regarding the use of topical NSAIDs after cataract surgery (Kim 2015), and concluded that there was a lack of level I evidence to support the long‐term benefit of topical NSAIDs to prevent vision loss from CME at three months or more after cataract surgery. This OTA was based on a literature search of PubMed and the Cochrane Library in January 2015.
Authors' conclusions
Implications for practice.
For chronic CME, topical ketorolac tromethamine 0.5% may improve visual acuity with at least three months of treatment but the evidence was very low certainty. Overall, there was no evidence that NSAIDs had clinically meaningful effects on any pre‐specified review outcomes in any type of CME; further investigation is warranted.
Implications for research.
Further investigation is needed to better understand the pathophysiology and risk factors of CME; such research would permit identification of those eyes most at risk for CME, and may elucidate strategies to prevent CME. In addition, research is needed to better elucidate the incidence, time course, and factors predictive of spontaneous resolution of acute CME versus those predictive of chronic CME; such research may permit targeted treatment to those individuals likely to need treatment for resolution of CME and optimal visual outcomes.
Larger trials with longer follow‐up are needed to assess the efficacy of NSAIDs in treating CME. Studies are also needed to compare the relative efficacy of various available topical NSAIDs for the treatment of acute and chronic CME following cataract surgery, and to determine the optimal duration of treatment. Despite the paucity and low certainty of evidence regarding the effectiveness of NSAIDs on clinically important outcomes of CME treatment, NSAIDs, with or without a topical steroid, remain a first‐line therapy for CME following cataract surgery (Orski 2021). Research is needed to compare the effectiveness of NSAIDs with alternative treatments such as steroids, anti‐vascular endothelial growth factor (anti‐VEGF) agents, and combination therapies such as an NSAID plus steroids. In addition, there is a need for comparative cost‐effectiveness research to compare the use of NSAIDs in the prevention versus treatment of CME following cataract surgery.
What's new
| Date | Event | Description |
|---|---|---|
| 22 June 2022 | New citation required but conclusions have not changed | Analyses updated to include evidence on both acute and chronic CME as well as CME of unclear duration. Conclusions not changed. |
| 30 March 2022 | New search has been performed | Updated searches conducted with two new studies identified; one eligible study identified from previously excluded studies. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 9 January 2012 | New citation required but conclusions have not changed | A new co‐author has joined the review team. The review has been amended to include new Cochrane methodology including completion of risk of bias tables for all included studies. |
| 9 January 2012 | New search has been performed | Issue 2 2012: Update searches yielded no new studies for inclusion in the review. |
| 4 November 2008 | New search has been performed | Issue 1 2009: Updated searches yielded no new trials. |
| 21 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Iris Gordon and Lori Rosman, Information Specialists for Cochrane Eyes and Vision (CEV) and for CEV@US, who created the electronic search strategies and executed the updated searches, separately.
We also thank Genie Han and Louis Leslie, Assistant Managing Editors for CEV@US; Anupa Shah, Managing Editor for CEV, for support and guidance in preparation of this review.
We thank Sobha Sivaprasad (Moorfields Eye Hospital and UCL, London), Catey Bunce (University College London, London), Roxanne Crosby‐Nwaobi (Moorfields Eye Hospital, London), Nishal Patel (East Kent Hospitals University, UK), and Sreedhar Jyothi (Leeds University, Leeds) for their contributions to earlier versions of this review. We acknowledge Adriaan van Sorge for registering the title for this Cochrane Review.
We would also like to thank the following peer reviewers for their comments: Shriji Patel (Vanderbilt University) and Kirk Hou (University of California Los Angeles) for the review manuscript.
This review update was managed by CEV@US and was signed off for publication by Tianjing Li and Gianni Virgili.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Macular Edema] explode all trees #2 MeSH descriptor: [Macula Lutea] explode all trees #3 (macula*) AND (edema OR oedema) #4 (cme OR cmo) #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees #7 nsaid* #8 (nonsteroid* AND antiinflammator*) OR (non NEXT/1 steroid* AND antiinflammator*) OR (nonsteroid* AND anti NEXT/1 inflammator*) OR (non NEXT/1 steroid* AND anti NEXT/1 inflammator*) #9 (cyclooxygenase NEXT/1 inhibitor*) OR ("cyclooxygenase 2" NEXT/1 inhibitor*) OR ("cox 2" NEXT/1 inhibitor*) OR coxib* #10 MeSH descriptor: [Diclofenac] explode all trees #11 diclofenac* OR cambia OR cataflam OR dichlofenal OR diclofenaco OR diclofenacum OR "diclonate p" OR diclophenac OR dicrofenac OR dyloject OR feloran OR flector OR "gp 45,840" OR "gp45,840" OR novapirina OR orthofen OR orthophen OR ortofen OR pennsaid OR "pro‐513" OR solarize OR "sr 38" OR "sr38" OR voltaren OR voltarol OR zipsor OR zorvolex OR "15307‐79‐6" OR "15307‐86‐5" #12 MeSH descriptor: [Fenoprofen] explode all trees #13 fenoprofen* OR nalgesic OR nalfon OR fenprofen OR fepron OR feprona OR "lilly 53858" OR phenoprofen OR "29679‐58‐1" OR "31879‐05‐7" OR "34691‐31‐1" #14 MeSH descriptor: [Flurbiprofen] explode all trees #15 flurbiprofen* OR flubiprofen OR fluriproben OR dobrofen OR "e 7869" OR e7869 OR ocufen OR strefen OR flugalin OR froben OR "neo artrol" OR "novo flurprofen" OR ansaid OR "bts 18322" OR bts18322 OR cebutid OR "froben sr" OR ocuflur OR "5104‐49‐4" #16 MeSH descriptor: [Indomethacin] explode all trees #17 indometacin* OR osmosin OR indocid OR metindol OR "indomet 140" OR "indomethacin hydrochloride" OR amuno OR Indocin OR indometacine OR flexin OR tivorbex OR "53‐86‐1" OR "74252‐25‐8" OR "7681‐54‐1" #18 MeSH descriptor: [Ketoprofen] explode all trees #19 ketoprofen* OR "benzoylhydratropic acid" OR profenid OR alrheumum OR orudis OR "19,583 RP" OR "RP, 19,583" OR "RP 19583" OR RP19583 OR alrheumat OR oruvail OR "22071‐15‐4" OR "57495‐14‐4" #20 MeSH descriptor: [Piroxicam] explode all trees #21 piroxicam* OR "CP 16171" OR CP16171 OR feldene OR "36322‐90‐4" #22 {OR #6‐#21} #23 #5 AND #22 in Trials
Appendix 2. MEDLINE (OVID) search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp macular edema cystoid/ 13. exp macula lutea/ 14. (macula* adj3 oedema).tw. 15. (macula* adj3 edema).tw. 16. (CME or CMO).tw. 17. or/12‐16 18. exp anti inflammatory agents non steroidal/ 19. nsaid*.tw. 20. (nonsteroid* anti‐inflammator* or nonsteroid* antiinflammator*).tw. 21. (non‐steroid* anti‐inflammator* or non‐steroid* antiinflammator*).tw. 22. ("cyclooxygenase inhibitor*" or "cyclooxygenase 2 inhibitor*" or "cox 2 inhibitor*" or coxib*).tw. 23. exp diclofenac/ 24. (diclofenac* or cambia or cataflam or dichlofenal or diclofenaco or diclofenacum or "diclonate p" or diclophenac or dicrofenac or dyloject or feloran or flector or "gp 45,840" or "gp45,840" or novapirina or orthofen or orthophen or ortofen or pennsaid or "pro‐513" or solarize or "sr 38" or "sr38" or voltaren or voltarol or zipsor or zorvolex or "15307‐79‐6" or "15307‐86‐5").tw. 25. exp fenoprofen/ 26. (fenoprofen* or nalgesic or nalfon or fenprofen or fepron or feprona or "lilly 53858" or phenoprofen or "29679‐58‐1" or "31879‐05‐7" or "34691‐31‐1").tw. 27. exp flurbiprofen/ 28. (flurbiprofen* or flubiprofen or fluriproben or dobrofen or "e 7869" or e7869 or ocufen or strefen or flugalin or froben or "neo artrol" or "novo flurprofen" or ansaid or "bts 18322" or bts18322 or cebutid or "froben sr" or ocuflur or "5104‐49‐4").tw. 29. exp indometacin/ 30. (indometacin* or osmosin or indocid or metindol or "indomet 140" or "indomethacin hydrochloride" or amuno or Indocin or indometacine or flexin or tivorbex or "53‐86‐1" or "74252‐25‐8" or "7681‐54‐1").tw. 31. exp ketoprofen/ 32. (ketoprofen* or "benzoylhydratropic acid" or profenid or alrheumum or orudis or "19,583 RP" or "RP, 19,583" or "RP 19583" or RP19583 or alrheumat or oruvail or "22071‐15‐4" or "57495‐14‐4").tw. 33. exp piroxicam/ 34. (piroxicam* or "CP 16171" or CP16171 or feldene or "36322‐90‐4").tw. 35. or/18‐34 36. 17 and 35 37. 11 and 36
The search filter for trials at the beginning of the strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. Embase.com search strategy
1. 'randomized controlled trial'/exp 2. 'randomization'/exp 3. 'double blind procedure'/exp 4. 'single blind procedure'/exp 5. random*:ab,ti 6. #1 OR #2 OR #3 OR #4 OR #5 7. 'animal'/exp OR 'animal experiment'/exp 8. 'human'/exp 9. #7 AND #8 10. #7 NOT #9 11. #6 NOT #10 12. 'clinical trial'/exp 13. (clin* NEAR/3 trial*):ab,ti 14. ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti 15. 'placebo'/exp 16. placebo*:ab,ti 17. random*:ab,ti 18. 'experimental design'/exp 19. 'crossover procedure'/exp 20. 'control group'/exp 21. 'latin square design'/exp 22. #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 23. #22 NOT #10 24. #23 NOT #11 25. 'comparative study'/exp 26. 'evaluation'/exp 27. prospective study'/exp 28. control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti 29. #25 OR #26 OR #27 OR #28 30. #29 NOT #10 31. #30 NOT (#11 OR #23) 32. #11 OR #24 OR #31 33. exp retina macula cystoid edema/ 34. exp eye edema/ 35. exp retina macula lutea/ 36. (macula* NEAR/3 oedema):ti,ab,kw 37. (macula* NEAR/3 edema):ti,ab,kw 38. (CME OR CMO):ti,ab,kw 39. #33 OR #34 OR #35 OR #36 OR #37 OR #38 40. exp nonsteroidal antiinflammatory agent/ 41. (nsaid*):ti,ab,kw 42. ((nonsteroid* AND 'anti inflammator*') OR (nonsteroid* AND antiinflammator*)):ab,ti,kw 43. (('non steroid*' AND 'anti inflammator*') OR ('non steroid*' AND antiinflammator*)):ab,ti,kw 44. ("cyclooxygenase inhibitor*" or "cyclooxygenase 2 inhibitor*" or "cox 2 inhibitor*" or coxib*):ab,ti,kw 45. (diclofenac* OR cambia OR cataflam OR dichlofenal OR diclofenaco OR diclofenacum OR "diclonate p" OR diclophenac OR dicrofenac OR dyloject OR feloran OR flector OR "gp 45,840" OR "gp45,840" OR novapirina OR orthofen OR orthophen OR ortofen OR pennsaid OR "pro‐513" OR solarize OR "sr 38" OR "sr38" OR voltaren OR voltarol OR zipsor OR zorvolex OR "15307‐79‐6" OR "15307‐86‐5"):ab,ti,kw 46. (fenoprofen* OR nalgesic OR nalfon OR fenprofen OR fepron OR feprona OR "lilly 53858" OR phenoprofen OR "29679‐58‐1" OR "31879‐05‐7" OR "34691‐31‐1"):ab,ti,kw 47. (flurbiprofen* OR flubiprofen OR fluriproben OR dobrofen OR "e 7869" OR e7869 OR ocufen OR strefen OR flugalin OR froben OR "neo artrol" OR "novo flurprofen" OR ansaid OR "bts 18322" OR bts18322 OR cebutid OR "froben sr" OR ocuflur OR "5104‐49‐4"):ab,ti,kw 48. (indometacin* OR osmosin OR indocid OR metindol OR "indomet 140" OR "indomethacin hydrochloride" OR amuno OR Indocin OR indometacine OR flexin OR tivorbex OR "53‐86‐1" OR "74252‐25‐8" OR "7681‐54‐1"):ab,ti,kw 49. (ketoprofen* OR "benzoylhydratropic acid" OR profenid OR alrheumum OR orudis OR "19,583 RP" OR "RP, 19,583" OR "RP 19583" OR RP19583 OR alrheumat OR oruvail OR "22071‐15‐4" OR "57495‐14‐4"):ab,ti,kw 50. (piroxicam* OR "CP 16171" OR CP16171 OR feldene OR "36322‐90‐4"):ab,ti,kw 51. #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 52. #39 AND #51 53. #32 AND #52
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. macula*[tw] AND (edema[tw] OR oedema[tw]) 3. (CME[tw] OR CMO[tw]) 4. #2 OR #3 5. nsaid*[tw] 6. (nonsteroid*[tw] AND "anti inflammator*"[tw]) OR (nonsteroid*[tw] AND antiinflammator*[tw]) 7. ("non steroid*"[tw] AND "anti inflammator*"[tw]) OR ("non steroid*"[tw] AND antiinflammator*[tw]) 8. ("cyclooxygenase inhibitor*"[tw] OR "cyclooxygenase 2 inhibitor*"[tw] OR "cox 2 inhibitor*"[tw] OR coxib*[tw]) 9. (diclofenac*[tw] OR cambia[tw] OR cataflam[tw] OR dichlofenal[tw] OR diclofenaco[tw] OR diclofenacum[tw] OR "diclonate p"[tw] OR diclophenac[tw] OR dicrofenac[tw] OR dyloject[tw] OR feloran[tw] OR flector[tw] OR "gp 45,840"[tw] OR "gp45,840"[tw] OR novapirina[tw] OR orthofen[tw] OR orthophen[tw] OR ortofen[tw] OR pennsaid[tw] OR "pro‐513"[tw] OR solarize[tw] OR "sr 38"[tw] OR "sr38"[tw] OR voltaren[tw] OR voltarol[tw] OR zipsor[tw] OR zorvolex[tw] OR "15307‐79‐6"[tw] OR "15307‐86‐5"[tw]) 10. (fenoprofen*[tw] OR nalgesic[tw] OR nalfon[tw] OR fenprofen[tw] OR fepron[tw] OR feprona[tw] OR "lilly 53858"[tw] OR phenoprofen[tw] OR "29679‐58‐1"[tw] OR "31879‐05‐7"[tw] OR "34691‐31‐1"[tw]) 11. (flurbiprofen*[tw] OR flubiprofen[tw] OR fluriproben[tw] OR dobrofen[tw] OR "e 7869"[tw] OR e7869[tw] OR ocufen[tw] OR strefen[tw] OR flugalin[tw] OR froben[tw] OR "neo artrol"[tw] OR "novo flurprofen"[tw] OR ansaid[tw] OR "bts 18322"[tw] OR bts18322[tw] OR cebutid[tw] OR "froben sr"[tw] OR ocuflur[tw] OR "5104‐49‐4"[tw]) 12. (indometacin*[tw] OR osmosin[tw] OR indocid[tw] OR metindol[tw] OR "indomet 140"[tw] OR "indomethacin hydrochloride"[tw] OR amuno[tw] OR Indocin[tw] OR indometacine[tw] OR flexin[tw] OR tivorbex[tw] OR "53‐86‐1"[tw] OR "74252‐25‐8"[tw] OR "7681‐54‐1"[tw]) 13. (ketoprofen*[tw] OR "benzoylhydratropic acid"[tw] OR profenid[tw] OR alrheumum[tw] OR orudis[tw] OR "19,583 RP"[tw] OR "RP, 19,583"[tw] OR "RP 19583"[tw] OR RP19583[tw] OR alrheumat[tw] OR oruvail[tw] OR "22071‐15‐4"[tw] OR "57495‐14‐4"[tw]) 14. (piroxicam*[tw] OR "CP 16171"[tw] OR CP16171[tw] OR feldene[tw] OR "36322‐90‐4"[tw]) 15. #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 16. #1 AND #4 AND #15 17. Medline[sb] 18. #16 NOT #17
Appendix 5. LILACS search strategy
(MH: C11.768.585.439.245$ OR MH:A09.371.729.522$ OR (macula$ edema) OR (macula$ oedema) OR CME OR CMO) AND (MH:D27.505.696.663.850.014.040.500$ OR MH:D27.505.954.158.030$ OR MH:D27.505.954.329.030$ OR nsaid$ OR (nonsteroid$ anti‐inflammator$) OR (nonsteroid$ antiinflammator$) OR (non‐steroid$ anti‐inflammator$) OR (non‐steroid$ antiinflammator$) OR (cyclooxygenase inhibitor$) OR ("cyclooxygenase 2" inhibitor$) OR ("cox 2" inhibitor$) OR coxib$ OR MH:D02.241.223.601.210$ OR diclofenac$ OR cambia OR cataflam OR dichlofenal OR diclofenaco OR diclofenacum OR "diclonate p" OR diclophenac OR dicrofenac OR dyloject OR feloran OR flector OR "gp 45,840" OR "gp45,840" OR novapirina OR orthofen OR orthophen OR ortofen OR pennsaid OR "pro‐513" OR solarize OR "sr 38" OR "sr38" OR voltaren OR voltarol OR zipsor OR zorvolex OR "15307‐79‐6" OR "15307‐86‐5" OR MH:D02.241.223.701.350$ OR fenoprofen$ OR nalgesic OR nalfon OR fenprofen OR fepron OR feprona OR "lilly 53858" OR phenoprofen OR "29679‐58‐1" OR "31879‐05‐7" OR "34691‐31‐1" OR MH:D02.241.081.751.161$ OR MH:D02.455.426.559.389.185.350$ OR Flurbiprofen$ OR flubiprofen OR fluriproben OR dobrofen OR "e 7869" OR e7869 OR ocufen OR strefen OR flugalin OR froben OR "neo artrol" OR "novo flurprofen" OR ansaid OR "bts 18322" OR bts18322 OR cebutid OR "froben sr" OR ocuflur OR "5104‐49‐4" OR MH:D03.633.100.473.420$ OR Indomethacin$ OR osmosin OR indocid OR metindol OR "indomet 140" OR "indomethacin hydrochloride" OR amuno OR Indocin OR indometacine OR flexin OR tivorbex OR "53‐86‐1" OR "74252‐25‐8" OR "7681‐54‐1" OR MH:D02.241.223.701.480$ OR ketoprofen$ OR "benzoylhydratropic acid" OR profenid OR alrheumum OR orudis OR "19,583 RP" OR "RP, 19,583" OR "RP 19583" OR RP19583 OR alrheumat OR oruvail OR "22071‐15‐4" OR "57495‐14‐4" OR MH:D02.886.665.500$ OR MH:D03.383.855.500$ OR Piroxicam$ OR "CP 16171" OR CP16171 OR feldene OR "36322‐90‐4")
Appendix 6. metaRegister of Controlled Trials search strategy
cystoid macular oedema
Appendix 7. ClinicalTrials.gov search strategy
(macular edema OR macula lutea OR CME OR CMO) AND (Anti‐Inflammatory Agents, Non‐Steroidal OR NSAID OR antiinflammatory OR "anti inflammatory" OR cyclooxygenase inhibitor OR cyclooxygenase 2 inhibitor OR cox 2 inhibitor OR coxib OR diclofenac OR fenoprofen OR flurbiprofen OR indomethacin OR ketoprofen OR piroxicam)
Appendix 8. ICTRP search strategy
macular edema AND anti‐Inflammatory OR macular edema AND antiinflammatory OR macular edema AND non‐steroidal OR macular edema AND nonsteroidal OR macular edema AND cyclooxygenase inhibitor OR macular edema AND cyclooxygenase 2 inhibitor OR macular edema AND cox 2 inhibitor OR macular edema AND coxib OR macular edema AND diclofenac OR macular edema AND fenoprofen OR macular edema AND flurbiprofen OR macular edema AND indomethacin OR macular edema AND ketoprofen OR macular edema AND piroxicam
Data and analyses
Comparison 1. NSAID versus placebo or active control in acute CME.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion of participants with visual improvement | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 Ketorolac 0.5% vs vehicle | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.2 Ketorolac 0.5% vs prednisolone 1.0% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.3 Ketorolac 0.5% & prednisolone 1.0% vs prednisolone 1.0% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Improvement in VA, Snellen lines* | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 Ketorolac 0.5% | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 Ketorolac 0.5% & Prednisolone 1.0% | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Proportion of participants with improved FFA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 Ketorolac 0.5% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.2 Ketorolac 0.5% & prednisolone 1.0% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Proportion of participants with improved contrast sensitivity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.1 Ketorolac 0.5% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.2 Ketorolac 0.5% & prednisolone 1.0% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Proportion of participants with complete resolution* | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1: NSAID versus placebo or active control in acute CME, Outcome 1: Proportion of participants with visual improvement
Comparison 2. NSAID versus placebo in chronic CME.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion of participants with visual improvement | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Chronic CME, treatment ≤ 2 months | 2 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.30, 1.73] |
| 2.1.2 Chronic CME, treatment 90‐120 days | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [1.58, 5.22] |
| 2.2 Proportion of participants with visual improvement ‐ sensitivity analysis | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [1.17, 4.66] |
| 2.2.1 Chronic CME, treatment ≤ 2 months | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.40, 4.43] |
| 2.2.2 Chronic CME, treatment 90‐120 days | 2 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [1.10, 8.74] |
| 2.3 Proportion of participants with persistent visual improvement one month after discontinuation of treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Topical | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.51, 31.13] |
| 2.3.2 Oral | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.60] |
| 2.4 Proportion of participants with improved FFA | 2 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.62, 6.02] |
| 2.5 Proportion of participants with complete resolution | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.3. Analysis.

Comparison 2: NSAID versus placebo in chronic CME, Outcome 3: Proportion of participants with persistent visual improvement one month after discontinuation of treatment
Comparison 3. NSAID versus active control in mixed CME.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proportion of participants with visual improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Improvement in VA, Snellen lines* | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 Improvement in VA, ETDRS letters* | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.3.1 Diclofenac 0.1% | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐4.31, 6.11] |
| 3.3.2 Bromofenac 0.09% | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐4.58, 6.98] |
| 3.4 Proportion of participants with complete resolution* | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Burnett 1983.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, RCT Study duration: NR Unit of randomization: person Masking: double‐masked (the same examiner was masked) Study visits and time points: baseline and then every 2 weeks for 8 weeks Follow‐up duration: 8 weeks Numbers of participants lost or excluded after randomization: none reported How missing data were handled: NA Power and sample size calculation: NR |
|
| Participants |
Countries: USA Setting: single‐center, university hospital Characteristics by intervention group
Inclusion criteria
Exclusion criteria
Baseline comparison: NR |
|
| Interventions |
1 drop, 4 times daily for 8 weeks |
|
| Outcomes |
*Angiograms were graded in 2 parameters: the area of fluorescein diffusion, and the intensity of leaked fluorescein |
|
| Notes | Funding source: NEI grants EY1903 and EY7038; research grant from Eli Lilly, Inc. Declaration of interest: NR Trial registry: NR Publication language: English Contact information: Mark O.M. Tso, MD; Department of Ophthalmology, University of Illinois Eye and Ear Infirmary, Chicago IL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial authors state this is a "double‐masked study" and in the methods it states "patients were willing to be placed in either the treatment or placebo group without prior knowledge as to which they would belong", suggesting that the patients would be masked and the patients acknowledged this before consenting. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Visual acuity examiners were masked to treatment assignment; whether the fluorescein angiogram graders were masked is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for visual acuity, FFA, and iris fluorescein angiography were reported for all 14 participants. |
| Selective reporting (reporting bias) | Low risk | Outcomes pre‐specified in the Methods section were all reported. |
| Other bias | Unclear risk | The study was supported in part by NIH grants EY1903 and EY7038, and a research grant from Eli Lilly, Inc. The trial authors did not provide or disclose information regarding conflicts of interest. |
Flach 1987.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, RCT Study duration: NR Unit of randomization: person Masking: double‐masked Study visits and time points: baseline, day 30 and 60 as well as day 90 ("therapy was discontinued at 60 days") and depending on their response to cessation of topical treatment 120 days after initiation of treatment* Follow‐up duration: 90 days Numbers of participants lost or excluded after randomization: 4 (2 in each group) How missing data were handled: complete‐case analysis Power and sample size calculation: NR *"At 90 days patients received another full ocular examination. If distance VA decreased by 2 or more lines as compared to day 60, therapy was re‐instituted from a fresh bottle of the same solution the patient had previously received in a double‐masked fashion." "No patient received a regimen of topical therapy with either ketorolac or vehicle placebo for more than a total of 90 days during the course of this study." |
|
| Participants |
Countries: USA Setting: 2 medical centers in California Characteristics by intervention group
Inclusion criteria Aphakic and pseudophakic patients with reduced VA (≤ 20/40) for ≥ 6 months associated with clinical and FA evidence of aphakic or pseudophakic CME Exclusion criteria: patients were excluded from this study if
Patients with a history of > 1 ocular operation (for example, secondary implantation of intraocular lens) were not excluded from this study. Baseline comparison: "The two groups of patients were comparable in terms of age, sex, eye involved, presence of an updrawn pupil, type of intraocular lens, presence of diabetes, presence of hypertension, initial visual acuities, and initial angiography grade." |
|
| Interventions |
1 drop (0.05 mL) 4 times daily for 60 days |
|
| Outcomes |
At baseline and each study visit, patients received an ocular examination that included visual acuity at distance with Snellen testing after refraction; Amsler grid testing of the central field; slit‐lamp examination of the peri‐ocular areas and external portions of the globe; a dilated retinal examination using direct and indirect opthalmoscopy; contact lens biomicroscopy was not performed but a Hruby lens examination of the macula was performed; IOP were measured by applanation tonometry. |
|
| Notes | Funding source: this study was funded by Syntex Corporation. Declaration of interest: the trial authors reported they have no proprietary interest in ketorolac or Syntex Corporation. The publication states that Dr. Flach is an ophthalmic clinical pharmacologic consultant for Syntex Corporation. Trial registry: NR Publication language: English Contact information: Allan Flach, MD; Department of Ophthalmology, Veterans Administration Hospital and Medical Center, San Francisco CA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a "Computer‐generated predetermined randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were masked to treatment assignment (ketorolac and placebo were packaged in identical containers) |
| Blinding of outcome assessment (detection bias) | Low risk | Visual acuity examiner and fluorescein angiogram grader were masked to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 of the 30 participants (13%) enrolled, 2 in each group, "failed to complete this study" and were "not discussed in this report". The dropout rate was comparable between the 2 groups, with dropout reasons being reported. |
| Selective reporting (reporting bias) | Low risk | Data of VA and angiography were reported for the 26 participants included in the analysis. |
| Other bias | Unclear risk | The study was funded by Syntex Corp. "Dr. Flach is an ophthalmic clinical pharmacologic consultant for Syntex Corp." The trial authors have no proprietary interest in ketorolac or Syntex Corporation. |
Flach 1991.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, RCT Study duration: NR Unit of randomization: person Masking: double‐masked Study visits and time points: baseline, day 30, 60, 90, and 120 after the initiation of treatment and depending on their response to cessation of topical treatment* Follow‐up duration: up to 150 days (treatment discontinued at 90 days) Numbers of participants lost or excluded after randomization: 4 (2 in each group) How missing data were handled: complete‐case analysis Power and sample size calculation: NR *"At 120 days patients received another full ocular examination. If distance VA decreased by 2 or more lines as compared to day 90, therapy was re‐instituted from a fresh bottle of the same solution the patient had previously received in a double‐masked fashion." "No patient received either solution for more than a total of 120 days during this study." |
|
| Participants |
Countries: USA Setting: 10 medical centers Characteristics by intervention group
Inclusion criteria Aphakic and pseudophakic patients with reduced VA (distance VA of ≥ 20/40) for ≥ 6 months associated with clinical and FA evidence of aphakic or pseudophakic CME Exclusion criteria
Baseline comparison: "The two treatment groups were comparable in terms of age, gender, ethic origin, concurrent systemic disease, initial visual acuity, baseline fluorescein angiography grade, pupil shape, intraocular lenses, the presence of vitreous humor in the anterior chamber or to the surgical wound, and the presence of synechia." |
|
| Interventions |
1 drop (0.05 mL) 4 times daily for 90 days |
|
| Outcomes |
At baseline and each study visit, participants received an ocular examination that included VA at distance with Snellen testing after refraction; slit‐lamp examination of the peri‐ocular areas and external eye; a dilated retinal examination using direct and indirect opthalmoscopy; contact lens biomicroscopy was not performed but a Hruby lens examination of the macula was performed; IOP were measured by Goldmann applanation tonometry or its equivalent; FA was performed at baseline and day 90 |
|
| Notes | Funding sources: this study was supported by Syntex Research Declaration of interest: NR Trial registry: NR Publication language: English Contact information: Allan Flach, MD; Department of Ophthalmology, Veterans Administration Hospital and Medical Center, San Francisco CA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was assigned by a "computer‐generated predetermined randomization schedule". |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients with reduced visual acuity ...and evidence of cystoid macular edema were randomly assigned, in a double‐masked fashion to treatment with either ketorolac or placebo‐vehicle solution". |
| Blinding of outcome assessment (detection bias) | Low risk | "Visual acuity determination was the primary task of one person. This person was unaware of treatments, angiograms, and details of the clinical examination". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 out of 61 participants in the drug group (25%) and 10 out of 59 in the placebo group (17%) did not return at the end of the 90‐day treatment period. The proportions of participants with improved visual acuity at that visit was statistically higher in the drug group than in the placebo group. Although the attrition rates appeared statistically non‐significant (post‐hoc P < 0.05), it was unclear whether the missingness of outcome data led to over‐ or under‐estimation of the intervention effect on VA improvement. |
| Selective reporting (reporting bias) | Low risk | Proportion of patients with improvement in VA (≥ 2 Snellen lines) was reported for each group. |
| Other bias | Unclear risk | "This study was supported by Syntex Research". The trial authors did not report or disclose additional conflicts of interests. |
Flach 1998.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, cross‐over,
RCT Study duration: 1988‐1989 (18 months) Unit of randomization: person "Each patient included in the study initially received 30 days of treatment with either 0.5% ketorolac ophthalmic solution or vehicle solution of the same pH and tonicity packaged in an identical container" "Following completion of this initial treatment regimen, the patient was given the remaining treatment regimen in a randomized, double‐masked fashion for the subsequent 30 days." Masking: double‐masked Study visits and time points: baseline, 30 days, 60 days Follow‐up duration: 2 months Numbers of participants lost or excluded after randomization: 2 (1 in each group) How missing data were handled: complete‐case analysis Power and sample size calculation: NR |
|
| Participants |
Countries: USA Setting: NR Characteristics
Inclusion criteria "350 patients underwent planned ECCEs with the implantation of an intraocular lens. These patients were screened for the presence of clinical (vision less than 20/40 by Snellen testing) CME at monthly intervals during the first 4 months following their cataract surgery." Pseudophakic cystoid macular oedema was confirmed by FA Exclusion criteria Patients were excluded from the study if:
Baseline comparison: "These 2 groups (the drug‐treated first group and the placebo‐treated first group) of patients are comparable in terms of age; sex; eye involved; presence of pupil abnormalities; type of intraocular lens; presence of diabetes mellitis, systemic hypertension, or arteriosclerotic vascular disease; initial visual acuities; and initial angiography grades as summarized in Table XII" (Flach 1998) |
|
| Interventions |
1 drop 4times daily for 30 days |
|
| Outcomes |
|
|
| Notes |
Funding sources: supported by a Merit Review
grant from the Department of Veterans Affairs; a
grant from That Man May See, Inc; a departmental
core grant from the NIH‐UCSF, Department of
Ophthalmology; a research grant from Syntex, Palo
Alto, CA; an unrestricted research grant from
Allergan; and a grant from Research to Prevent
Blindness (general description, not specific to the
study itself) Declaration of interest: NR Trial registry: NR Publication language: English Contact information: Allan J Flach, MD; Department of Ophthalmology and Veterans Affairs, UCSF Medical Center |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each eligible patient received either 0.5% ketorolac or vehicle solution of the same pH and tonicity packaged in an identical container and distributed with a computer‐generated predetermined randomization schedule in a double‐masked fashion." |
| Allocation concealment (selection bias) | Low risk | The treatment was "distributed ... in a double‐masked fashion." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients received either 0.5% ketorolac or vehicle in an identical container "in a double‐masked" fashion". |
| Blinding of outcome assessment (detection bias) | Low risk | "The investigator was unaware of the treatment regimen." "The examiner was unaware of results of fluorescein angiograms and slit‐lamp observations." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of the 24 participants were "eliminated from the study during the first 10 days", 1 in each group; "these patients are not included in this report". |
| Selective reporting (reporting bias) | Unclear risk | It was unclear what study outcomes were pre‐specified. The study was not previously published, and the study findings were described in a narrative review, along with other previously published study findings. |
| Other bias | Unclear risk | "Supported by a Merit Review grant from the Department of Veterans Afairs; a grant from That Man May See, Inc; a departmental core grant from the National Institutes of Health‐University of California, San Francisco, Department of Ophthalmology; a research grant from Syntex, Palo Alto, California; an unrestricted research grant from Alergan; and a grant from Research to Prevent Blindness". The trial authors did not provide or disclose conflicts of interest. |
Heier 2000.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, RCT Study duration: NR Unit of randomization: person Masking: double‐masked (participants and examiners) Study visits and time points: baseline and then monthly Follow‐up duration: 3 months (treatment started to taper); at least 2 months of additional follow‐up (considered "outside of the study") Numbers of participants lost or excluded after randomization: 2 (1 in the ketorolac and 1 in the combination therapy group) How missing data were handled: complete‐case analysis Power and sample size calculation: NR |
|
| Participants |
Countries: USA Setting: multi‐center Characteristics by intervention group
Inclusion criteria Acute clinical CME (angiographic evidence of CME with VA of 20/40 or worse 21‐90 days after uncomplicated cataract extraction and lens implantation) occurring after phacoemulsification and posterior chamber lens implantation Exclusion criteria
Baseline comparison: not described but appeared comparable in Table 1 (Heier 2000). |
|
| Interventions |
1 drop 4 times daily for a maximum of 3 months "If CME was resolved on funduscopic and angiographic examination, the medications were tapered at the rate of one drop per week. If CME was still present, then medications were continued. If treatment was continued for 3 months, then medications were tapered as above." |
|
| Outcomes |
|
|
| Notes |
Funding sources: supported by an unrestricted
research grant from Allergan Pharmaceuticals Declaration of interest: NR Trial registry: NR Publication language: English Contact information: Jeffrey S. Heier, MD; Ophthalmic Consultants of Boston, Boston, MA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | High risk | The pharmacy prepared the randomization (scheme) and "supplied the premasked medications", indicating that the pharmacy had the knowledge of which medication and the sequence of randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study medications were masked to both patients and examiners". |
| Blinding of outcome assessment (detection bias) | Low risk | "The study medications were masked to both patients and examiners". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 out of 28 participants, 1 in ketorolac group and the other in the combination group, were excluded from the study after randomization due to the detection of AMD and retinal vein occlusion during the treatment period. Outcome data for the remaining 26 participants were reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcome data were analysed and reported. |
| Other bias | Unclear risk | The study was "supported by an unrestricted research grant from Allergan Pharmaceuticals." The trial authors did not report or disclose conflicts of interests. |
Rho 2003.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, RCT Study duration: 1995‐1999 Unit of randomization: person Masking: open‐label, unmasked Study visits and time points: baseline and then every 2‐4 weeks for 26 weeks Follow‐up duration: 26 weeks Numbers of participants lost or excluded after randomization: NR (NA) How missing data were handled: NA Power and sample size calculation: NR |
|
| Participants |
Countries: USA Setting: at a private vitreoretinal practice Characteristics by intervention group
Inclusion criteria Patients with acute CME as confirmed by FA "As most patients had received some form of perioperative or postoperative corticosteroid or NSAID, all completed a washout period of at least 14 days before beginning treatment in the study." Exclusion criteria "Patients with a history of intraocular surgery before cataract surgery, vitreous loss during cataract surgery, CME, uveitis, or vitreoretinal pathology were excluded from the study." Baseline comparison: "There were no significant differences between the groups in baseline characteristics" |
|
| Interventions |
1 drop 4 times a day for 26 weeks |
|
| Outcomes |
|
|
| Notes |
Ethics approval: "Institutional review board
approval was not necessary as the patients were
referrals to a private vitreoretinal practice." Funding sources: NR Declaration of interest: NR Trial registry: NR Publication language: English Contact information: David S. Rho, MD; Soll Eye Associates |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR |
| Blinding of outcome assessment (detection bias) | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Data of all patients presenting with CME who were treated" were included for analysis. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of interest, including VA, time to improvement, and time to resolution were reported. |
| Other bias | Unclear risk | "The author has no financial or proprietary interest in any material or method mentioned. The author is a member of the Novartis Ophthalmics speaker’s bureau." |
Rho 2004.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, RCT Study duration: NR Unit of randomization: person Masking: NR Study visits and time points: NR Follow‐up duration: at least 3 months Numbers of participants lost or excluded after randomization: NR How missing data were handled: NR Power and sample size calculation: NR |
|
| Participants |
Countries: USA Setting: NR Characteristics by intervention group
Inclusion criteria Patients with CME following uncomplicated phacoemulsification cataract removal and posterior chamber intraocular lens implantation surgery Exclusion criteria Patients with complicated surgery with posterior capsule rupture and vitreous loss, intraocular surgery before or after cataract surgery, CME > 1 year duration, uveitis history, and pre‐existing retinal pathology such as macular hole, epiretinal membrane, macular scar, macular edema or vein occlusion Baseline comparison: NR |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding source: NR Declaration of interest: D.S.Rho reported support from Novartis Ophthalmics R and S.M.Soll, None Trial registry: NR Publication language: English Contact information: D.S.Rho, MD; University of Medicine and Dentistry of New Jersey, Camden NJ; Wills Eye Hospital, Philadelphia PA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of whether or not study personnel were masked, but the treatment (medication) bottles were unmasked. |
| Blinding of outcome assessment (detection bias) | High risk | The bottle was unmasked and both participants and examiners were likely unmasked too. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants completed "at least 3 months of treatment" but the mean and range of follow‐up duration in each group were not provided. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available for evaluation. Primary study outcome (VA) was reported as in conventional metrics; unclear if the time point reported was pre‐specified |
| Other bias | High risk | First author was supported by the industry. |
Rho 2006.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, RCT Study duration: NR Unit of randomization: eyes (1 eye per person) Masking: NR Study visits and time points: baseline and then monthly for 3 months Follow‐up duration: 3 months Numbers of participants lost or excluded after randomization: NR How missing data were handled: NR Power and sample size calculation: NR |
|
| Participants |
Countries: USA Setting: NR Characteristics by intervention group
Inclusion criteria Eyes with acute pseudophakic CME of < 1 year duration after uncomplicated cataract surgery with phacoemulsification and posterior chamber intraocular lens implantation (it appeared that enrolled patients had 1 affected eye each) Exclusion criteria Intra‐operative vitreous loss or iris trauma, CME < 1 year duration, pre‐existing macular pathology, retinal vasculopathy, prior ipsilateral ophthalmic surgery, and uveitis history Baseline comparison: "The two groups of patients were comparable in terms of age, sex, eye involved, presence of an updrawn pupil, type of intraocular lens, presence of diabetes, presence of hypertension, initial visual acuities, and initial angiography grade." |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding source: NR Declaration of interest: D.S. Rho was supported by Ista Pharmaceuticals, Inc.; S.M. Soll, none; B.J. Markovitz, none Trial registry: NR Publication language: English Contact information: D.S. Rho, MD; University of Medicine and Dentistry of New Jersey, Camden, NJ; Wills Eye Hospital, Philadelphia, PA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NR |
| Blinding of outcome assessment (detection bias) | Unclear risk | NR |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants (eyes) were reportedly randomized and included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol was available for assessment. |
| Other bias | High risk | First trial author was supported by the industry |
Yannuzzi 1977.
| Study characteristics | ||
| Methods |
Study design: parallel‐group, RCT Study duration: NR Unit of randomization: person (unit of analysis: eye) Masking: NR Study visits and time points: baseline, week 3 and 6, 1 month after week 6 Follow‐up duration: 1 more month after completion of treatment Numbers of participants lost or excluded after randomization: NR How missing data were handled: NA Power and sample size calculation: NR |
|
| Participants |
Countries: USA Setting: single center Characteristics by intervention group:
Inclusion criteria "Patients with reduced vision and clinical and fluorescein angiographic evidence of CME four months or more after cataract surgery" Exclusion criteria: Patients who
Baseline comparison: NR |
|
| Interventions |
3 times a day for 6 weeks |
|
| Outcomes |
|
|
| Notes |
Funding sources: supported by a grant from
Merck, Sharpe and Dohme Research Laboratories Declaration of interest: NR Trial registry: NR Publication language: English Contact information: Lawrence A. Yannuzzi, MD; Fluorescein Clinic, Manhattan Eye, Ear, and Throat Hospital, New York, New York |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Unclear risk | "Twenty patients with reduced vision and clinical and fluorescein angiographic evidence of cystoid macular edema four months or more after cataract surgery were randomly assigned to the indomethacin or the placebo group." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was described as "double‐masked" but it was unclear who were masked in addition to study participants. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial authors state this was a "double‐masked study" but whether or not outcome assessors were masked was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Ten eyes of ten patients were in the indomethacin group; 13 eyes in ten patients were in the placebo group." |
| Selective reporting (reporting bias) | Unclear risk | FFA and VA were followed at each study visit. Results of FFA were reported as the presence (or absence) but not with the details about improvement or worsening. Also, the unit of randomization was person but the unit of analysis for VA was eye. |
| Other bias | Unclear risk | The study was supported by Merck, Sharpe and Dohme Research Laboratories. There was no information about authors' conflicts of interest or financial disclosure. |
AMD: age‐related macular degeneration; BCVA: best‐corrected visual acuity; CME: cystoid macular edema; ECCE: extracapsular cataract extraction; FA: fluorescein angiography; FFA: fundus fluorescein angiography; IOP: intraocular pressure; n: number; NA: not applicable; NEI: National Eye Institute; NIH: National Institutes of Health; NR: not reported; NSAID: non‐steroidal anti‐inflammatory drug; RCT: randomized controlled trial; SD: standard deviation; VA: visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaronson 2020 | Not the design of interest |
| Ahluwalia 1988 | Not the intervention of interest (for prevention) |
| Asano 2008 | Not the intervention of interest (for prevention) |
| Azzolini 1986 | Not the intervention of interest (for prevention) |
| Ginsburg 1994 | Not the design of interest |
| IDSG 1997 | Not the intervention of interest (for prevention) |
| Jampol 1994 | Not the design of interest (review) |
| Kraff 1985 | Not the intervention of interest (for prevention) |
| Mathys 2010 | Not the intervention of interest (for prevention) |
| Miyake 1995 | Not the design of interest (review) |
| Miyake 2007 | Not the intervention of interest (for prevention) |
| NCT00438243 | Trial withdrawal (before enrollment) |
| NCT01769352 | Not the intervention of interest (for prevention) |
| Rossetti 1996 | Not the intervention of interest (for prevention) |
| Singal 2004 | Not the intervention of interest (topical prednisolone) |
| Stark 1984 | Not the intervention of interest (for prevention) |
| Warren 2010 | Not the population of interest (previously treated with intravitreal steroid) |
| Wolfensberger 1999 | Not the design of interest |
Characteristics of studies awaiting classification [ordered by study ID]
NCT00595543.
| Methods | Parallel‐group, open‐label RCT |
| Participants | Age limitation: ≥ 18 years Gender: all eligible for study Inclusion criteria
Exclusion criteria
|
| Interventions | Target sample size: 166 participants
1 drop in the affected eye for 3 months |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Notes | Sources of financial support: Bp Consulting, Inc Date of first enrollment: January 2008 Contacts: David Rho, MD; Soll Eye Associates Contact efforts made: emailed to Dr. Rho twice but no responses received within 2 weeks Contact reasons: no trial results available |
Yüksel 2017.
| Methods | Single‐center, parallel‐group, randomized, interventional clinical trial |
| Participants | Age limitation: NR Gender: NR Inclusion criteria "All patients had clinically significant pseudophakic CME, which was defined as central macular thickness (CRT) >250 μm, presence of cysts on fundus biomicroscopy, and logMAR best‐corrected visual acuity (BCVA) ≥0.4. Fluorescein angiography (FA) was performed to confirm the diagnosis in six patients from the TA group and eight patients from nepafenac group." Exclusion criteria "Patients with a vitreomacular traction or epiretinal membrane were excluded. Drop‐outs from follow‐up were excluded." |
| Interventions | Sample size: 48 in total, 24 in each group
|
| Outcomes | Primary study outcomes
At months 1, 2, 3, and 6 |
| Notes | Sources of financial support: NR; the authors report no conflicts of interest Date of first enrollment: January 2013 Contacts: Umut Duygu Uzunel, MD; Turkey Contact efforts made: emailed to Dr. Uzunel twice without receiving responses within 2 weeks Contact reasons: mechanism of randomization needed clarification |
BCVA: best‐corrected visual acuity; CME: cystoid macular edema; CRT: central macular thickness; NR: not reported; RCT: randomized controlled trial; VA: visual acuity
Differences between protocol and review
Eligibility criteria for study selection was expanded to consider inclusion of trials that enrolled participants with cystoid macular edema (CME; formerly CMO) but did not specify types of CME in their inclusion criteria
-
Outcome modifications
A new 'Important outcome' was added in lieu of the current practice: 'proportion of participants with improvement in central macular thickness on optical coherence tomography'
One of the 'Important outcomes', quality of life, was redefined as 'changes in quality of life as measured by validated questionnaires'
-
Different outcome metrics of visual improvement as reported by the included trials were considered in the following order to be consistent with the protocol:
proportion of participants with two or more lines in Snellen visual acuity or equivalent (default)
visual improvement in Snellen lines
visual improvement in ETDRS letters
Headings and descriptions of review outcomes were updated and clarified.
-
Subsections of Methods were updated to the current standard by adding information in the following subsections:
Measures of treatment effect
Unit of analysis issues
Dealing with missing data
Assessment of heterogeneity
Data synthesis
Subgroup analysis and investigation of heterogeneity
Sensitivity analysis
Summary of findings and assessment of the certainty of the evidence
-
Other changes previously made since the publication of the protocol (Sivaprasad 2001)
Trials that compared different non‐steroidal anti‐inflammatory agents were allowed and included for evidence synthesis in Sivaprasad 2004.
Primary outcome: 'persistence of poor visual outcome due to CME (CMO) at one year defined as best‐corrected vision of less than 6/60 or equivalent in the operated eye' was rephrased as 'persistence of improvement of vision one month after discontinuation of treatment' in Sivaprasad 2004
Secondary outcome: 'proportion of participants with improved contrast sensitivity' was added in Sivaprasad 2004.
Contributions of authors
AW, JL and JS conceived the review question. AW and JL screened potential studies resulting from the searches, extracted data and assessed risk of bias for all studies. SL verified extracted data and performed data analysis. AW and SL wrote drafts of the review. JL and JS critically reviewed the draft. AW, SL, JL, and JS responded to peer review comments and comments from the editorial base.
Sources of support
Internal sources
-
None, Other
No internal source of support.
External sources
-
National Eye Institute, National Institutes of Health, USA
Cochrane Eyes and Vision US Project, supported by grant UG1EY020522 (PI: Tianjing Li, MD, MHS, PhD)
-
Public Health Agency, UK
The HSC Research and Development (R&D) Division of the Public Health Agency funds the Cochrane Eyes and Vision editorial base at Queen's University Belfast.
-
Queen's University Belfast, UK
The work of Gianni Virgili, Co‐ordinating Editor for Cochrane Eyes and Vision, is funded by the Centre for Public Health, Queen’s University of Belfast, Northern Ireland.
Declarations of interest
Andreas Wingert: none Su‐Hsun Liu: reports a grant from the National Eye Institute, National Institutes of Health, USA; payment to institution John Lin: none Jayanth Sridhar: consultant for Alcon, Genentech, DORC, Regeneron, and Apellis
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Burnett 1983 {published data only}
- Burnett J, Tessler H, Isenberg S, Tso MO. Double-masked trial of fenoprofen sodium: treatment of chronic aphakic cystoid macular edema. Ophthalmic Surgery 1983;14(2):150-2. [PubMed] [Google Scholar]
Flach 1987 {published data only}
- Flach AJ, Dolan BJ, Irvine AR. Effectiveness of ketorolac tromethamine 0.5% ophthalmic solution for chronic aphakic and pseudophakic cystoid macular edema. American Journal of Ophthalmology 1987;103(4):479-86. [DOI] [PubMed] [Google Scholar]
Flach 1991 {published data only}
- Flach AJ, Jampol LM, Weinberg D, Kraff MC, Yannuzzi LA, Campo RV, et al. Improvement in visual acuity in chronic aphakic and pseudophakic cystoid macular edema after treatment with topical 0.5% ketorolac tromethamine. American Journal of Ophthalmology 1991;112(5):514-9. [DOI] [PubMed] [Google Scholar]
- Flach AJ. Visual improvement in patients with chronic aphakic and pseudophakic cystoid macular edema (ACME) following a topically applied cyclooxygenase inhibitor (COI)-ketorolac tromethamine 0.5% ophthalmic solution. Report of a multi-center study. Investigative Ophthalmology & Visual Science 1991;32:ARVO abstract 91. [Google Scholar]
Flach 1998 {published and unpublished data}
- Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Transactions of the American Ophthalmological Society 1998;96:557-634. [PMC free article] [PubMed] [Google Scholar]
Heier 2000 {published data only}
- Heier JS, Topping TM, Baumann W, Dirks MS, Chern S, Flach AJ. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology 2000;107(11):2034-9. [DOI] [PubMed] [Google Scholar]
Rho 2003 {published data only}
- Rho DS. Treatment of acute pseudophakic cystoid macular edema: diclofenac versus ketorolac. Journal of Cataract and Refractive Surgery 2003;29(12):2378-84. [DOI] [PubMed] [Google Scholar]
Rho 2004 {published data only}
- Rho DS, Soll SM. Combination therapy for pseudophakic cystoid macular edema: diclofenac sodium 0.1% and prednisolone acetate 1% versus ketorolac tromethamine 0.5% and prednisolone acetate 1%. Investigative Ophthalmology & Visual Science 2004;45:ARVO E‐abstract 2030. [Google Scholar]
- Rho DS, Soll SM. Combination therapy for pseudophakic cystoid macular edema: diclofenac sodium 0.1% and prednisolone acetate 1% vs. ketorolac tromethamine 0.5% and prednisolone acetate 1%. American Academy of Ophthalmology 2004:AAO-PO-201.
Rho 2006 {published data only}
- Rho DS, Soll SM, Markovitz BJ. Bromfenac 0.09% versus diclofenac sodium 0.1% versus ketorolac tromethamine 0.5% in the treatment of acute pseudophakic cystoid macular edema. Investigative Ophthalmology & Visual Science 2006;47:ARVO E‐abstract 5211. [Google Scholar]
Yannuzzi 1977 {published data only}
- Yannuzzi LA, Klein RM, Wallyn RH, Cohen N, Katiz I. Ineffectiveness of indomethacin in the treatment of chronic cystoid macular edema. American Journal of Ophthalmology 1977;84(4):517-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aaronson 2020 {published data only}
- Aaronson A, Achiron A, Tuuminen R. Clinical course of pseudophakic cystoid macular edema treated with nepafenac. Journal of Clinical Medicine 2020;9(9):3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahluwalia 1988 {published data only}
- Ahluwalia BK, Kalra SC, Parmar IP, Khurana AK. A comparative study of the effect of antiprostaglandins and steroids on aphakic cystoid macular oedema. Indian Journal of Ophthalmology 1988;36(4):176-8. [PubMed] [Google Scholar]
Asano 2008 {published data only}
- Asano S, Miyake K, Ota I, Sugita G, Kimura W, Sakka Y, et al. Reducing angiographic cystoid macular edema and blood-aqueous barrier disruption after small incision phacoemulsification and foldable intraocular lens implantation: multicenter prospective randomized comparison of topical diclofenac 0.1% and betamethasone 0.1%. Journal of Cataract and Refractive Surgery 2008;34(1):57-63. [DOI] [PubMed] [Google Scholar]
Azzolini 1986 {published data only}
- Azzolini C, Bressan P, Dorigo MT. Medical treatment of cystoid macular oedema in aphakia patients. Minerva Oftalmologica 1986;28(1):15-20. [Google Scholar]
Ginsburg 1994 {published data only}
- Ginsburg AP, Cheetham JK, DeGryse RE, Abelson M. The effects of flurbiprofen and indomethacin on acute cystoid macular edema: functional vision and contrast sensitivity. Investigative Ophthalmology & Visual Science 1994;35:Abstract number 1153. [Google Scholar]
IDSG 1997 {published data only}
- Italian Diclofenac Study Group. Efficacy of diclofenac eyedrops in preventing postoperative inflammation and long-term cystoid macular edema. Journal of Cataract and Refractive Surgery 1997;23(8):1183-9. [DOI] [PubMed] [Google Scholar]
Jampol 1994 {published data only}
- Jampol LM, Jain S, Pudzisz B, Weinreb RN. Nonsteroidal anti-inflammatory drugs and cataract surgery. Archives of Ophthalmology 1994;112(7):891-4. [DOI] [PubMed] [Google Scholar]
Kraff 1985 {published data only}
- Kraff MC, Sanders DR, Jampol LM, Lieberman HL. Factors affecting pseudophakic cystoid macular edema: five randomized trials. American Intra-Ocular Implant Society Journal 1985;11:380-5. [DOI] [PubMed] [Google Scholar]
Mathys 2010 {published data only}
- Mathys KC, Cohen KL. Impact of nepafenac 0.1% on macular thickness and postoperative visual acuity after cataract surgery in patients at low risk for cystoid macular oedema. Eye 2010;24(1):90–6. [DOI] [PubMed] [Google Scholar]
Miyake 1995 {published data only}
- Miyake K. Nonsteroidal anti-inflammatory agents in cataract intraocular lens surgery. Current Opinion of Ophthalmology 1995;6(1):62-5. [PubMed] [Google Scholar]
Miyake 2007 {published data only}
- Miyake K, Nishimura K, Harino S, Ota I, Asano S, Kondo N, et al. The effect of topical diclofenac on choroidal blood flow in early postoperative pseudophakias with regard to cystoid macular edema formation. Investigative Ophthalmology and Visual Science 2007;48(12):5647–52. [DOI] [PubMed] [Google Scholar]
NCT00438243 {published data only}
- NCT00438243. Pilot study of the effect of topical bromfenac ophthalmic solution 0.09%in patients with acute post-operative cystoid macular edema. ClinicalTrials.gov/show/NCT00438243 (first received 22 February 2007).
NCT01769352 {published data only}
- NCT01769352. Treatment of post-surgical cystoid macular edema with topical steroids trial (TEMPEST-1). ClinicalTrials.gov/show/NCT01769352 (first received 16 January 2013).
Rossetti 1996 {published data only}
- Rossetti L, Bujtar E, Castoldi D, Torrazza C, Orzalesi N. Effectiveness of diclofenac eyedrops in reducing inflammation and the incidence of cystoid macular edema after cataract surgery. Journal of Cataract and Refractive Surgery 1996;22 Suppl 1:794-9. [DOI] [PubMed] [Google Scholar]
Singal 2004 {published data only}
- Singal N, Hopkins J. Pseudophakic cystoid macular edema: ketorolac alone vs. ketorolac plus prednisolone. Canadian Journal of Ophthalmology 2004;39(3):245-50. [DOI] [PubMed] [Google Scholar]
Stark 1984 {published data only}
- Stark WJ Jr, Maumenee AE, Fagadau W, Datiles M, Baker CC, Worthen D, et al. Cystoid macular edema in pseudophakia. Survey of Ophthalmology 1984;28 Suppl:442-51. [DOI] [PubMed] [Google Scholar]
Warren 2010 {published data only}
- Warren KA, Bahrani H, Fox JE. NSAIDS in combination therapy for the chronic pseudophakic cystoid macular edema. Retina 2010;30(2):260–6. [DOI] [PubMed] [Google Scholar]
Wolfensberger 1999 {published data only}
- Wolfensberger TJ, Herbort CP. Treatment of cystoid macular edema with non-steroidal anti-inflammatory drugs and corticosteroids. Documenta Ophthalmologica. Advances in Ophthalmology 1999;97(3-4):381-6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00595543 {published data only (unpublished sought but not used)}
- NCT00595543. Treatment of acute pseudophakic cystoid macular edema: bromfenac 0.09% versus diclofenac sodium 0.1% versus ketorolac tromethamine 0.5%. Clinicaltrials.gov/show/NCT00595543 (first received 16 January 2008).
Yüksel 2017 {published data only}
- Yüksel B, Uzunel UD, Kerci SG, Sağban L, Küsbeci T, Örsel T. Comparison of subtenon triamcinolone acetonide injection with topical nepafenac for the treatment of pseudophakic cystoid macular edema. Ocular Immunology and Inflammation 2017;25(4):513‐19. [DOI] [PubMed] [Google Scholar]
Additional references
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637-9. [DOI] [PubMed] [Google Scholar]
Brandsdorfer 2019
- Brandsdorfer A, Patel SH, Chuck RS. The role of perioperative nonsteroidal anti-inflammatory drugs use in cataract surgery. Current Opinion in Ophthalmology 2019;30(1):44-9. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 18 April 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Davis 2016
- Davis G. The evolution of cataract surgery. Missouri Medicine 2016;113(1):58-62. [PMC free article] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Fichman 1996
- Fichman RA. Use of topical anesthesia alone in cataract surgery. Journal of Cataract and Refractive Surgery 1996;22(5):612-4. [DOI] [PubMed] [Google Scholar]
Fujimoto 2016
- Fujimoto J, Swanson E. The development, commercialization, and impact of optical coherence tomography. Investigative Ophthalmology & Visual Science 2016;57:OCT1-OCT13. [DOI: 10.1167/iovs.16-19963] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gass 1966
- Gass JD, Norton EW. Cystoid macular edema and papilledema following cataract extraction: a fluorescein fundoscopic and angiographic study. Archives of Ophthalmology 1966;76(5):646-61. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Grzybowski 2016
- Grzybowski A, Sikorski BL, Ascaso FJ, Huerva V. Pseudophakic cystoid macular edema: update 2016. Clinical Interventions in Aging 2016;9(11):1221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hays 2001
- Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Annals of Medicine 2001;33:350-7. [DOI] [PubMed] [Google Scholar]
Henderson 2007
- Henderson BA, Kim JY, Ament CS, Ferrufino-Ponce ZK, Grabowska A, Cremers SL. Clinical pseudophakic cystoid macular edema: risk factors for development and duration after treatment. Journal of Cataract and Refractive Surgery 2007;33(9):1550-8. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Irvine 1953
- Irvine SR. A newly defined vitreous syndrome following cataract surgery. American Journal of Ophthalmology 1953;36(5):599-619. [DOI] [PubMed] [Google Scholar]
Kessel 2014
- Kessel L, Tendal B, Jørgensen KJ, Erngaard D, Flesner P, Andresen JL, et al. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: a systematic review. Ophthalmology 2014;121(10):1915-24. [DOI] [PubMed] [Google Scholar]
Kim 2015
- Kim SJ, Schoenberger SD, Thorne JE, Ehlers JP, Yeh S, Bakri SJ. Topical nonsteroidal anti-inflammatory drugs and cataract surgery: a report by the American Academy of Ophthalmology. Ophthalmology 2015;122(11):2159-68. [DOI] [PubMed] [Google Scholar]
Malwankar 2022
- Malwankar J, Son HS, Chang DF, Dun C, Woreta F, Prescott C, et al. Trends, factors, and outcomes associated with immediate sequential bilateral cataract surgery among Medicare beneficiaries. Ophthalmology 2022;129(5):478-87. [DOI] [PubMed] [Google Scholar]
Mangione 2001
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Archives of Ophthalmology 2001;119(7):1050-8. [DOI] [PubMed] [Google Scholar]
McKenzie 2022
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Chapter 9: Summarizing study characteristics and preparing for synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Miyake 2002
- Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Survey of Ophthalmology 2002;47 Suppl 1:S203-18. [DOI] [PubMed] [Google Scholar]
Orski 2021
- Orski M, Gawęcki M. Current management options in Irvine-Gass syndrome: a systemized review. Journal of Clinical Medicine 2021;10(19):4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perente 2007
- Perente I, Utine CA, Ozturker C, Cakir M, Kaya V, Eren H, et al. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Current Eye Research 2007;32(3):241-7. [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.13.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2022a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Schünemann 2022b
- Schünemann HJ, Neumann I, Hultcrantz M, Brignardello-Petersen R, ZengL, Murad MH, et al, for the GRADE Working Group. GRADE Guidance article 35: Update on rating imprecision for assessing contextualized certainty of evidence and making decisions. Journal of Clinical Epidemiology (in press). [DOI: ] [DOI] [PubMed]
Shelsta 2010
- Shelsta HN, Jampol LM. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina 2011;31(1):4-12. [DOI] [PubMed] [Google Scholar]
Steiner 2014
- Steiner CA, Karaca Z, Moore BJ, Imshaug MC, Pickens G. Surgeries in hospital-based ambulatory surgery and hospital inpatient settings, 2014. 2017 May [Updated 2020 Jul 20]. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb. Statistical Brief #223. Available from www.ncbi.nlm.nih.gov/books/NBK442035/ (accessed after July 2020). [PubMed]
Taipale 2019
- Taipale C, Holmström EJ, Ilveskoski L, Tuuminen R. Incidence of pseudophakic cystoid macular edema in eyes with and without pupil expansion device. Acta Ophthalmologica 2019;97(7):688-94. [DOI] [PubMed] [Google Scholar]
Trichonas 2014
- Trichonas G, Kaiser PK. Optical coherence tomography imaging of macular oedema. British Journal of Ophthalmology 2014;98:ii24-ii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sivaprasad 2001
- Sivaprasad S, Van SO, Van Sorge AA. Non-steroidal anti-inflammatory agents for treating cystoid macular oedema following cataract surgery (Protocol). Cochrane Database of Systematic Reviews 2001, Issue 1. Art. No: CD004239. [DOI: 10.1002/14651858.CD004239] [DOI] [PubMed] [Google Scholar]
Sivaprasad 2004
- Sivaprasad S, Bunce C, Jyothi S. Non-steroidal anti-inflammatory agents for treating cystoid macular oedema following cataract surgery. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD004239. [DOI: 10.1002/14651858.CD004239.pub2] [DOI] [PubMed] [Google Scholar]
Sivaprasad 2009
- Sivaprasad S, Bunce C, Jyothi S. Non-steroidal anti-inflammatory agents for treating cystoid macular oedema following cataract surgery. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD004239. [DOI: 10.1002/14651858.CD004239.pub2] [DOI] [PubMed] [Google Scholar]
Sivaprasad 2012
- Sivaprasad S, Bunce C, Crosby-Nwaobi R. Non-steroidal anti-inflammatory agents for treating cystoid macular oedema following cataract surgery. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No: CD004239. [DOI: 10.1002/14651858.CD004239.pub3] [DOI] [PubMed] [Google Scholar]


