Abstract
Background
The impact of right ventricular dysfunction(RVD) on the prognosis of acute respiratory distress syndrome(ARDS) patients is controversial.
Objectives
The objectives of this systematic review and meta-analysis was to investigate whether RVD or pulmonary vascular dysfunction are associated with increased mortality in patients with ARDS.
Methods
We searched Pubmed, Embase, Cochrane Library, Wanfang Data, CNKI, and the WHO Clinical Trial Registry for studies of RVD or pulmonary vascular dysfunction in patients with ARDS.
Results
The presence of RVD or pulmonary vascular dysfunction in patients with ARDS was associated with an increase in mortality (OR = 1.68, 95% CI = 1.21–2.32, P = 0.069, I2 = 40.8%). Subgroup analyses obtained similar results. Funnel plots and the Egger's test indicated no publication bias, and sensitivity analyses determined that the results were stable.
Conclusion
The prognosis of patients with ARDS and RVD or pulmonary vascular dysfunction is worse than that of ARDS patients without RVD or pulmonary vascular dysfunction.
Keywords: Acute respiratory distress syndrome, Right ventricular dysfunction, Acute cor pulmonale, Pulmonary vascular dysfunction, Prognosis
Introduction
Acute respiratory distress syndrome is a type of respiratory failure characterized by refractory hypoxemia, progressive respiratory distress, and non-cardiogenic pulmonary edema. The introduction of lung-protective ventilation has led to a slight decrease in the mortality rates associated with ARDS. However, overall, mortality rates remain high despite continuous advances in pathophysiological knowledge and mechanical ventilation strategies. The main reasons for this persistently high mortality rate include the pathophysiological changes caused by ARDS and increases in right ventricular afterload caused by mechanical ventilation. As early as 1977, Zapol and Snider were the first to report that patients with ARDS have pulmonary vascular dysfunction. They found that pulmonary vascular resistance and the right ventricular work index were increased in patients with ARDS who underwent mechanical ventilation. Further, right ventricular dysfunction led to acute cor pulmonale (ACP) and refractory circulatory failure.1 Of the different forms of right ventricular dysfunction (e.g., pulmonary vascular dysfunction, right ventricular systolic dysfunction), ACP is the most severe. Studies have reported that the incidence of ACP in patients with ARDS is approximately 25%. However, studies regarding the impact of RVD on the prognosis of ARDS have yielded mixed findings. Some studies have shown that RVD may increase mortality in ARDS,2 while others have found that the presence of RVD has no effect on the mortality rate associated with ARDS.3 , 4 Thus, we conducted a systematic review and meta-analysis that aimed to investigate whether RVD influences the prognosis of ARDS. The findings of this work will help guide the early clinical identification of RVD and the adoption of measures to prevent RVD.
Methods
This review's protocol was developed prospectively, ahead of the conduct of the literature search. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guided the conduct and reporting of this work.5
Search strategy
The search strategy consisted of the following keywords: "right ventricular dysfunction," "acute respiratory distress syndrome," and "prognosis". It was run within PubMed, the Cochrane Library, and EMBASE. We also searched CNKI and Wanfang Data to further identify published literature without language restrictions, as well as the WHO Clinical Trial Registry to identify unpublished literature and ongoing studies. There were no language restrictions, but studies had to be published between January 1st, 2000 and April 3rd, 2020. Appendix 1 contains further information about the search strategy [e-Appendix 1]. The reference lists of all included studies were also manually searched to identify additional eligible studies. Authors were contacted in the event of any confusion surrounding the data that they presented in their studies.
Selection of studies: inclusion and exclusion criteria
The inclusion criteria were as follows: (1) cohort studies or cross-sectional studies of lung-protective ventilation strategies, (2) studies in which the mortality rate associated with pulmonary vascular dysfunction or RVD (among patients with ARDS) could be calculated or extracted, and (3) independent studies on adult patients diagnosed with RVD or pulmonary vascular dysfunction (for repetitive studies, we used the most recently published data).
Meeting abstracts and summaries or studies focusing on specific subgroups of the population (e.g., children, pregnant women) were excluded.
Data extraction
Data were extracted from included studies using a standardized form. The following data were extracted independently by two investigators: (1) first author, as well as year and country or area of publication, (2) sample size, (3) number of patients in the exposed group (i.e., ARDS patients with RVD or pulmonary vascular dysfunction), (4) number of patients in the control group (i.e., ARDS patients without RVD or pulmonary vascular dysfunction), (5) number of deaths in the exposed group, (6) number of deaths in the control group, (7) definition of right heart dysfunction, (8) method used to diagnose right ventricular dysfunction, (9) study design, and (10) outcome. We extracted or calculated mortality (95% confidence interval, CI) from available data. Two authors independently screened the articles for inclusion, conducted quality assessments, extracted the data, and determined eligibility for inclusion in the pooled data analyses. A third reviewer checked the primary reviewers’ article selection, data extraction, and risk of bias assessment. She was blinded to their decisions. Any disagreements were resolved through discussion until consensus was reached. Articles by the same author were carefully investigated to avoid duplicate research being included in the analyses.
Risk of bias assessment
The methodological quality of included studies was independently evaluated by two researchers using the Newcastle-Ottawa Scale (NOS). Both cohort and pre-post studies were assessed using the NOS, which uses a semiquantitative star system. The maximum possible score is nine stars, and the evaluation criteria assess study selection, comparability, and outcomes. A score ≥ 7 stars indicates good quality, 5–7 stars indicates intermediate quality, and ≤ 4 stars indicates poor quality.6 Disagreements between researchers were resolved through discussions. Further detail about the risk of bias assessment is provided in e-Appendix 2. Studies’ NOS scores are shown in e-Appendix 3.
Statistical methods
Statistical heterogeneity was assessed using Cochrane's Q and the I2 statistic. The results of these tests determined the choice of a fixed or random-effects model. Specifically, data were pooled using a random-effects model if significant heterogeneity was present (P < 0.1). Otherwise, a fixed-effects model was used. Pooled odds ratios (ORs) and 95% CIs were calculated. A 95% CI that did not include an OR of 1.0 was considered statistically significant. Publication bias was evaluated through funnel plots based on each study's log OR and the standard error of log OR. Statistical analyses were conducted using Stata (version 15.0, StataCorp, College Station, Texas). Subgroup analyses based on the types of RVD were performed if the number of included studies was sufficient. Sensitivity analysis used the leave-one-out method. Briefly, the robustness of the synthesized results was evaluated by investigating the effects of excluding individual studies on the main outcome. Publication bias was evaluated using a funnel plot when the number of included studies was > 10.
Results
Overview of included studies
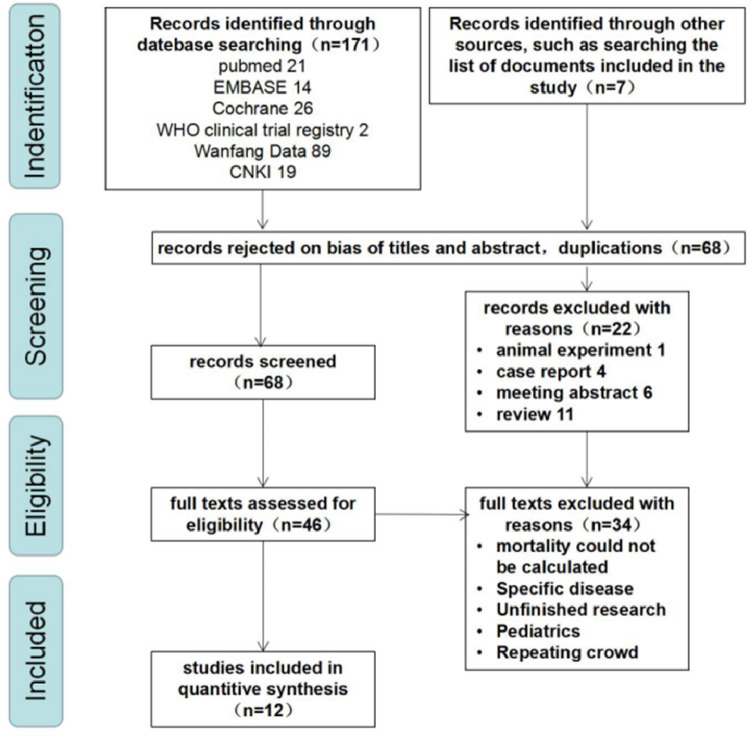
A total of 12 studies (two case-control studies7 , 8 and ten cohort studies) met the inclusion criteria.2, 3, 4 , 9, 10, 11, 12, 13, 14, 15 After pooling, 780 cases were in the group comprised of patients with ARDS and RVD or pulmonary vascular dysfunction, and 1539 cases were in the group comprised of patients with ARDS without RVD or pulmonary vascular dysfunction group. Fig. 1 presents the flow of studies into the review. Table 1 displays the characteristics of included studies.
Fig. 1.
Study flow diagram.
Table 1.
Characteristics of included studies.
| Study | Country | Sample size (n = ) | Patients with RVD or pulmonary vascular dysfunction (n=) | Type | Non-survivors in patients with RVD or pulmonary vascular dysfunction (n=) | Non-survivors in patients without RVD or pulmonary vascular dysfunction (n=) | Definition of RVD | Diagnosticmodality:TTE/TEE/PAC | outcome |
|---|---|---|---|---|---|---|---|---|---|
| Florence Boissier et al.2 | France | 226 | 49 | P | 28 | 63 | ACP | TEE | 28-day mortality |
| David Osman et al.3 | France | 145 | 14 | P | 9 | 73 | ACP | PAC | 28-day mortality |
| Antoine Vieillard-Baron et al.4 | France | 75 | 19 | P | 6 | 18 | ACP | TEE | 28-day mortality |
| C. Lazzeri et al.7 | Italy | 121 | 44 | P | 28 | 24 | other | TEE/TTE | ICU mortality |
| Zhang et al.8 | China | 21 | 6 | R | 5 | 4 | ACP | TEE | ICU mortality |
| Armand Mekontso Dessap et al.9 | France | 752 | 164 | P | 78 | 244 | ACP | TEE | Hospital mortality |
| Annick Legras et al.10 | France | 195 | 36 | P | 10 | 35 | ACP | TTE/TEE | 28-day mortality |
| Jer´ ome Fichet et al.11 | Canada | 50 | 15 | R | 3 | 12 | other | TTE | ICU Mortality |
| Gwenae¨lle Lhe´ritier et al.12 | France | 200 | 45 | P | 11 | 35 | ACP | TEE | 28-day mortality |
| Todd M. Bull et al.13 | USA | 475 | 349 | P | 103 | 24 | other | PAC | 60-day mortality |
| Manuela Bonizzoli et al.14 | Italy | 28 | 25 | P | 9 | 1 | other | TEE | ICU mortality |
| Wu et al.15 | China | 31 | 14 | P | 7 | 2 | ACP | TTE | 28-day mortality |
RVD: right ventricular dysfunction; ACP: acute cor pulmonale; TEE: transesophageal echocardiography; TTE: transthoracic echocardiography; PAC: pulmonary artery catheter; P:Prospective observational; R:Retrospective.
Meta-analysis of the effects of RVD or pulmonary vascular dysfunction on mortality
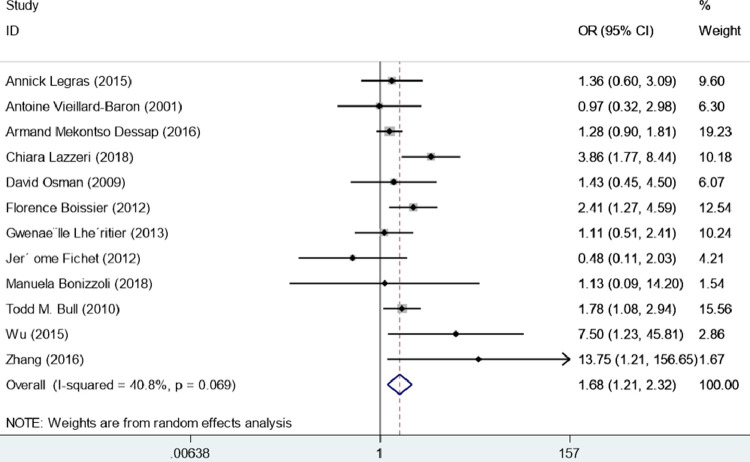
Among the 12 included studies involving 2319 patients with ARDS, the OR of mortality in patients with ARDS and RVD or pulmonary vascular dysfunction was 1.68 (95% CI = 1.21–2.32, I2 = 40.8%, P = 0.002), regardless of diagnostic methods or evaluation criteria. Given the presence of statistical heterogeneity, the data were analyzed using a random-effects model. The results of the overall meta-analysis indicated that the risk of death in patients with ARDS and RVD or pulmonary vascular dysfunction was significantly elevated, as compared with patients with ARDS and without RVD or pulmonary vascular dysfunction. The result of I2 = 40.8% suggested that there was no apparent heterogeneity among studies (Fig. 2 ).
Fig. 2.
Forest plot for odds ratio of mortality of right ventricular dysfunction or pulmonary vascular dysfunction in patients with acute respiratory distress syndrome undergoing lung protective ventilation.
Subgroup analysis
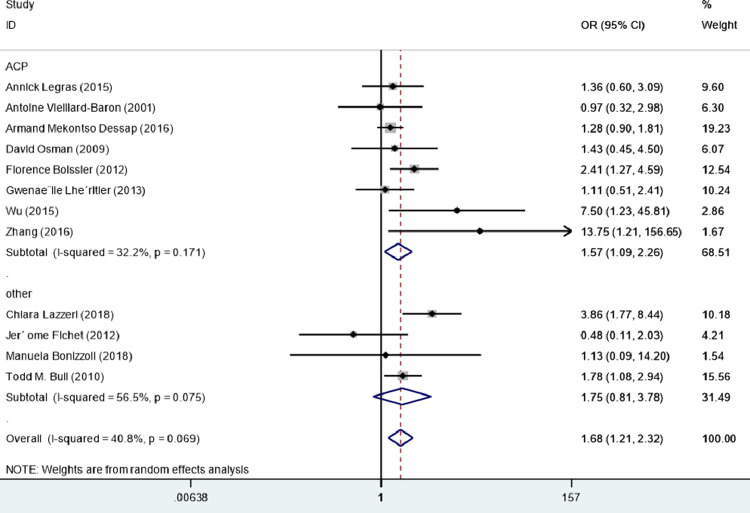
All 12 studies were included in subgroup analyses based on RVD types. Among them, eight studies enrolled patients with ACP2, 3, 4 , 8, 9, 10 , 12 , 15 and four studies enrolled patients with pulmonary vascular dysfunction or mild RVD.7 , 11 , 13 , 14 The types of RVD within the included studies are listed in e-Appendix 4. After pooling individual studies, ACP was significantly associated with death in patients with ARDS (OR = 1.57, 95% CI = 1.09−2.25, I2 = 32.2%, P = 0.015). In contrast, in the subgroup analysis, there was no association between pulmonary vascular dysfunction or mild RVD and death (OR = 1.75, 95% CI = 0.81–3.75, P = 0.165, I2 = 56.5%) (Fig. 3 ).
Fig. 3.
Pulmonary vascular dysfunction or mild right ventricular dysfunction and acute cor pulmonale.
Publication bias
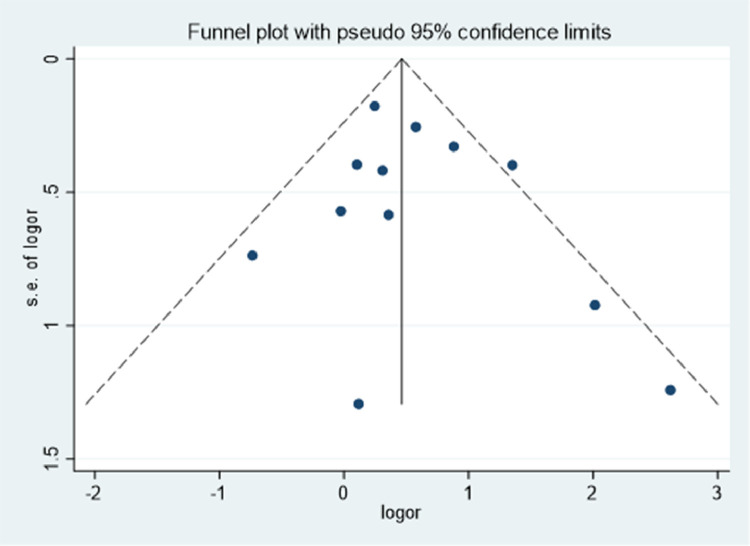
All 12 of the included studies were mainly distributed at the top and middle part of the funnel plot (Fig. 4 ), with a basic symmetric distribution that resembled an upside-down funnel. This implied that there was no significant publication bias. Further, Egger's test also indicated that there was no publication bias (P = 0.435).
Fig. 4.
Qualitative detection of publication bias via a funnel plot.
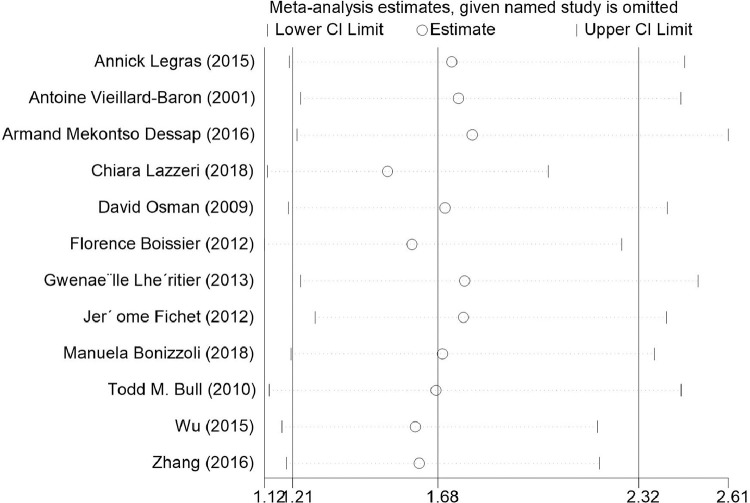
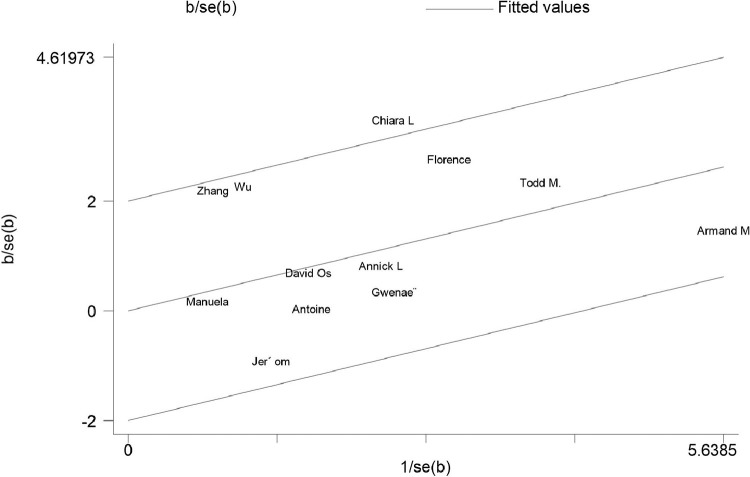
Sensitivity analysis
The sensitivity analysis suggested that the results were stable (Fig. 5 ). The homogeneity test confirmed that the 12 studies were suitable for obtaining a combined effect measure (Qh = 18.58, P = 0.069). The Galbraith plot (Fig. 6 ) showed that one study was outside the 95% confidence range. Consequently, there was not enough evidence to reject the homogeneity of the studies included in the meta-analysis.
Fig. 5.
The sensitivity analysis.
Fig. 6.
The Galbraith plot.
Discussion
As a disease commonly occurring in the ICU, ARDS is characterized by progressive dyspnea and bilateral pulmonary infiltration.16 Fifty years have passed since ARDS was first discovered. Yet, despite advances in lung-protective mechanical ventilation strategies and other treatments,17 , 18 ARDS incidence and mortality are still relatively high. A recent RCT comparing conservative and liberal oxygenation targets for patients with ARDS did not find significant differences in organ function or mortality between the two groups. This suggests that relatively short-term hypoxia has no evident adverse effects and that hypoxemia is not a leading cause of death from ARDS.19 In contrast, a strong correlation between hemodynamic instability and mortality has been found among patients with ARDS.20 Moreover, various factors such as vasospasm, acidosis, and inflammation induced by microthrombus, arterial remodeling, and hypoxia may elevate pulmonary vascular resistance and pulmonary artery pressure in patients with ARDS. Further, mechanical ventilation therapy can lead to ACP by increasing transpulmonary pressure and right heart afterload. Studies have found that the incidence rate of ACP is approximately 60% in the early stage of mechanical ventilation21 and declines to 20–30% after the use of lung-protective ventilation strategies.22 Since the left ventricle and right ventricle share an interventricular septum and a pericardial cavity, increases in the volume and pressure of the right ventricle restrict the diastole of the left ventricle.23 This in turn affects the cardiac output of the left ventricle and leads to further circulatory deterioration. Therefore, ACP is considered to be an important factor influencing the prognosis of patients with ARDS. Right ventricular dysfunction is a marker of the severity of ARDS and exerts no direct effect on prognosis. To illustrate, our landmark study observed that ACP had no impact on mortality in patients with ARDS.4 This may be attributed to prone position ventilation, which controls the plateau pressure and partial pressure of carbon dioxide (PaCO2). This intervention is more common in patients with ARDS and ACP, thus reducing their mortality. Lhéritier et al. also reported no differences in mortality between ARDS patients with and without ACP. They also found that more patients with ARDS and ACP were treated with nitric oxide (NO) inhalation and prone position ventilation than patients without ACP.12 In contrast, Boissier et al. found that ACP was independently associated with mortality.12 Moreover, similar results were obtained by Osman et al.3 when studying the influence of flow-directed pulmonary artery catheter (PAC) on the prognosis of patients with ARDS and pulmonary vascular dysfunction. Hence, the purpose of this review was to conduct a meta-analysis that investigated the prognosis of patients with ARDS and pulmonary vascular dysfunction or RVD to determine whether RVD was associated with mortality. The findings of this work can help with the rapid recognition of RVD and to lower the mortality of ARDS by providing evidence to support the earliest possible use of a right ventricle-protective ventilation strategy or prone position ventilation. This study summarized the published research on the prognosis of patients with ARDS and RVD. Twelve independent studies containing mortality rate data were included and subjected to meta-analysis. We found that mortality was notably higher in patients with ARDS and RVD than in patients with ARDS and without RVD. This suggests that RVD in patients with ARDS is associated with a poorer prognosis. Subgroup analyses revealed that mortality rates were similar among patients with ARDS and pulmonary vascular dysfunction or mild RVD. A significantly higher mortality rate was only present among patients with ARDS and ACP.
The included studies used many different definitions of RVD, including pulmonary vascular dysfunction and ACP. To determine whether RVD type was related to prognosis among patients with ARDS, we conducted a subgroup analysis by definition of RVD. According to the American Society of Echocardiography, quantitative parameters of right ventricular function below the lower limits of corresponding normal values indicate RVD. Right ventricular dysfunction is defined as the condition in which the right ventricle is unable to supply adequate blood flow for pulmonary circulation under normal central venous pressure (CVP).23 ACP refers to the acute dilation and/or dysfunction of the right heart in the context of acute lung disease, accompanied by pulmonary vascular dysfunction.24 As a form of RVD, ACP is caused by the acute exacerbation of right heart afterload and can result in right heart failure. The types of RVD within the included studies are listed in e-Appendix 4. Different forms of RVD, including early pulmonary vascular dysfunction and severe ACP, were included in the present study.
A strength of this paper is that it included more recent studies. A previous meta-analysis investigated incidence and mortality rates among patients with ARDS and ACP. However, it only included seven studies, with 1250 patients, published before 2016.25 The results of this meta-analysis indicated that ACP did not have an influence on mortality (OR = 1.16, 95% CI = 0.80–1.67). In the present meta-analysis, we included additional studies conducted over the last four years and obtained different results. Second, all of the included studies’ participants were patients who underwent lung-protective ventilation strategies, thereby enhancing the reliability of our results. Third, our study included different forms of RVD. In doing so, we determined that only ACP is associated with a poor prognosis. The presence of pulmonary vascular dysfunction alone did not increase the risk of death. Fourth, we included studies that used different methods to detect RVD. Pulmonary artery catheterization is the "gold standard" for the detection of pulmonary vascular dysfunction and ACP. However, high costs and invasiveness, as well as equipment, technology, and operator requirements of hemodynamic monitoring, seriously limit its clinical application. Critical care echocardiography is rapidly becoming the primary method for right ventricular function assessment. Fifth, our literature search was conducted in a wide range of databases (n = 6), including Pubmed, Embase, and Cochrane library; CNKI and Wanfang Data for the identification of literature published in languages other than English; and the WHO Clinical Trial Registry (who.int/ictrp) for identification of unpublished literature and ongoing studies.
This study also has some limitations. First, statistically significant differences in baseline data (including age and SOFA score) among some studies may undermine the comparability of results between studies. Second, the heterogeneity present in our study may be due to the following reasons: different causes of ARDS, the potentially higher mortality rate among patients with ARDS due to pulmonary disease, different diagnostic criteria for ARDS, different echocardiographic examination times, the use of adjuvant therapies such as nitric oxide inhalation or prone position ventilation in some patients, and publication bias. Third, the presence of some poor quality studies with small sample sizes may have also affected the results. Fourth, we did not perform subgroup analyses for transesophageal echocardiography and transthoracic echocardiography since some research has indicated that transthoracic echocardiography may be restricted by poor reecho. Moreover, Lhéritier et al. found that a transesophageal echocardiogram is superior to transthoracic echocardiography in diagnosing RVD in patients with moderate or serious ARDS requiring mechanical ventilation. Therefore, it is unclear whether transthoracic echocardiography reduces the diagnostic rate of RVD.
Conclusion
This study found that patients with ARDS and RVD have an increased risk of mortality. Consequently, critical ultrasonography should be routinely used to assess the right ventricular function of patients with ARDS. If necessary, flow-directed PAC may also be used to continuously assess and closely monitor right ventricular function, including pulmonary vascular function and systolic and diastolic function of the right ventricle. This will support the early detection of RVD and the adoption of interventions that reduce right ventricular load and improve cardiac function. Recommended strategies for the treatment of ARDS include right heart-protective ventilation,26 , 27 circulation management with right heart protection, lung recruitment maneuvers, and prone position ventilation. In addition, extracorporeal membrane oxygenation is recommended for patients with moderate and severe ARDS to decrease pulmonary vascular resistance and minimize the effects on the right ventricle.
Future
Some questions remain unanswered about RVD in patients with ARDS. For example, the association between indicators of RVD and ARDS prognosis, or how the administration of a pulmonary vasodilator at the early stage of the disease affects the clinical prognosis of patients with ARDS who are at risk for RVD. Future research should focus on validating clinical risk scoring systems for RVD, immediate assessment by echocardiography, and implementing therapeutic measures that may improve the prognosis of early-stage ARDS. Echocardiographic indicators (e.g., TAPSE and S’ wave velocity) can be used to predict early RVD and guide interventions according to changes in right ventricular function over time. Finally, right ventricle-protective ventilation strategies should be further investigated, including the ascertainment of optimal PEEP and PEEP titration, to determine whether they can decrease mortality in patients with ARDS.
Declaration of Competing Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgments
Consent for publication
All authors read and approved the final manuscript for publication.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ statement
The authors hereby confirm that neither the manuscript nor any part of it has been published or is being considered for publication elsewhere. We acknowledge that all authors participated sufficiently in the work and take public responsibility for its content.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.hrtlng.2021.04.011.
Appendix. Supplementary materials
References
- 1.Warren M., Zapol M.D.M.T. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med. 1977;296(9):476–480. doi: 10.1056/NEJM197703032960903. [DOI] [PubMed] [Google Scholar]
- 2.Boissier F., et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intens Care Med. 2013;39(10):1725–1733. doi: 10.1007/s00134-013-2941-9. [DOI] [PubMed] [Google Scholar]
- 3.Osman D., et al. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intens Care Med. 2009;35(1):69–76. doi: 10.1007/s00134-008-1307-1. [DOI] [PubMed] [Google Scholar]
- 4.Vieillard-Baron A., et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med. 2001;29(8):1551–1555. doi: 10.1097/00003246-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Knobloch K., Yoon U., Vogt P.M. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Cranio-MaxilloFacial Surg. 2011;39:91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Wells G.A., Shea B.J., O'Connell D., et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of Non-Randomized studies in meta-analysis. Appl Eng Agric. 2012;18:727–734. [Google Scholar]
- 7.Lazzeri C., et al. Lactate and echocardiography before Veno-Venous extracorporeal membrane oxygenation support. Heart, Lung Circul. 2018;27(1):99–103. doi: 10.1016/j.hlc.2017.02.006. .doi. [DOI] [PubMed] [Google Scholar]
- 8.Zizhou W.J.H.J., Fengfeng Z.B. The characteristics of acute cor pulmonale in critically ill patients with H7N9 influenza virus infection. Chin Crit Care Med. 2016;28(9):822–826. [Google Scholar]
- 9.Mekontso Dessap A., et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intens Care Med. 2016;42(5):862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 10.Legras A., et al. Acute respiratory distress syndrome (ARDS)-associated acute cor pulmonale and patent foramen ovale: a multicenter noninvasive hemodynamic study. Crit Care. 2015;19:174. doi: 10.1186/s13054-015-0898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fichet J., et al. Feasibility of right ventricular longitudinal systolic function evaluation with transthoracic echocardiographic indices derived from tricuspid annular motion: a preliminary study in acute respiratory distress syndrome. Echocardiography. 2012;29(5):513–521. doi: 10.1111/j.1540-8175.2011.01650.x. [DOI] [PubMed] [Google Scholar]
- 12.Lhéritier G., et al. Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: a multicenter study. Intens Care Med. 2013;39(10):1734–1742. doi: 10.1007/s00134-013-3017-6. [DOI] [PubMed] [Google Scholar]
- 13.Bull T.M., et al. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2010;182(9):1123–1128. doi: 10.1164/rccm.201002-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonizzoli M C.S.L.C. Speckle tracking echocardiography and right ventricle dysfunction in acute respiratory distress syndrome: a pilot study. Echocardiography. 2018;35(12):1982–1987. doi: 10.1111/echo.14153. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q. 2015. The value of right ventricular function in evaluation of disease severity and prognosis in patients with acute respiratory distress. [Google Scholar]
- 16.Ashbaugh DG B.D.P.T. Acute respiratory distress in adults. Lancet North Am Ed. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 17.Amato MB B.C.M.D. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 18.NETWORK T.A.R.D. Ventilation with lower tidal volumes as compared with traditional didal volumes for acute lung injury and the acute respiratory distress. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 19.Mezidi M., Claude G. Conservative versus liberal oxygenation targets for mechanically ventilated patients a pilot multicenter randomized controlled trial. J Thorac Dis. 2016;8(3):307–310. doi: 10.21037/jtd.2016.02.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xavier Repesse C.C.A.A. Acute respiratory distress syndrome: the heart side of the moon. Curr Opin Crit Care. 2016;22(1):38–44. doi: 10.1097/MCC.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 21.Jardin F G.P.D.O. Two-dimensional echocardiographic evaluation of right ventricular size and contractility in acute respiratory failure. Crit Care Med. 1985;13(11):952–956. doi: 10.1097/00003246-198511000-00035. [DOI] [PubMed] [Google Scholar]
- 22.Page B., et al. Low stretch ventilation strategy in acute respiratory distress syndrome: eight years of clinical experience in a single center. Crit Care Med. 2003;31(3):765–769. doi: 10.1097/01.CCM.0000055402.68581.DC. [DOI] [PubMed] [Google Scholar]
- 23.CR. G. Pathophysiology of right ventricular failure. Crit Care Med. 2008;36:S57–S65. doi: 10.1097/01.CCM.0000296265.52518.70. 1 Suppl. [DOI] [PubMed] [Google Scholar]
- 24.Jardin F D.O.B.J. Echocardiographic pattern of acute cor pulmonale. Chest. 1997;111(1):209–217. doi: 10.1378/chest.111.1.209. [DOI] [PubMed] [Google Scholar]
- 25.Das Saurabh Kumar. Incidence proportion of acute cor pulmonale in patients with acute respiratory distress syndrome subjected to lung protective ventilation: a systematic review and meta-analysis. Indian J Crit Care Med. 2017;21(6):364–375. doi: 10.4103/ijccm.IJCCM_155_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieillard-Baron A. Experts' opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intens Care Med. 2016;42(5):739–749. doi: 10.1007/s00134-016-4326-3. [DOI] [PubMed] [Google Scholar]
- 27.Alexis Paternot M.D. Rationale and description of right ventricle-protective ventilation in ARDS. Respir Care. 2016;61(10):1391–1396. doi: 10.4187/respcare.04943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.