Abstract
Purpose
To evaluate graft detachment after Descemet membrane endothelial keratoplasty (DMEK) in pseudophakic eyes and DMEK combined with cataract surgery (triple DMEK).
Design
Analysis of 3 single-center prospective cohort studies and 1 randomized controlled trial.
Participants
Participants with Fuchs’ endothelial corneal dystrophy.
Methods
A validated neural network for image segmentation quantified graft detachment on anterior segment OCT (AS-OCT) images 3 days after DMEK and at the 2-week postoperative visit. Area and volume of graft detachment were compared between DMEK only and triple DMEK using generalized estimating equation models and adjusting for participant age and the size of the air bubble.
Main Outcome Measures
Area and volume of DMEK graft detachment.
Results
Among 207 participants with 270 eyes included, 75 pseudophakic eyes had DMEK only and 195 eyes had triple DMEK. A total of 147 eyes had less than one third of detachment at day 3. In 139 of these eyes (95%), detachment was still less than one third at the 2-week scan, indicating that postoperative graft detachment at 2 weeks occurred mainly in eyes with early detachment. When superimposing all 3-dimensional maps from 2 weeks after surgery, the central graft was mainly attached and detachment was located at the graft margin. The mean area of graft detachment decreased from 28% in DMEK only and 38% in triple DMEK to 16% in DMEK only and 25% in triple DMEK at the 2-week postoperative visit. At 2 weeks, the mean area of detachment was 1.85-fold higher (95% confidence interval [CI], 1.34–2.56) and the mean volume was 2.41-fold higher (95% CI, 1.51–3.86) in triple DMEK compared with DMEK. A total of 46 eyes received rebubbling procedures, with 7 eyes (9%) in the DMEK group and 39 eyes (20%) in the triple DMEK group (adjusted risk ratio, 3.1; 95% CI, 1.3–7.1), indicating that rebubbling was more common in eyes undergoing triple DMEK.
Conclusions
Automated segmentation of AS-OCT images allowed precise quantification of graft detachment over time and identified DMEK combined with cataract surgery as a risk factor. Frequency of operative follow-up might be guided by extent of detachment in the first postoperative days after DMEK.
Keywords: Anterior segment OCT, Fuchs' endothelial corneal dystrophy, Incomplete donor graft attachment, Machine learning, Neural network, Rebubbling, Triple DMEK
Abbreviations and Acronyms: AS-OCT, anterior segment OCT; CI, confidence interval; DMEK, Descemet membrane endothelial keratoplasty; IQR, interquartile range
To treat clinically advanced Fuchs’ endothelial corneal dystrophy, Descemet membrane endothelial keratoplasty (DMEK) has become the gold standard over the past 15 years.1 Given excellent visual rehabilitation and graft survival after DMEK, the research focus has shifted on further improving refractive outcomes and minimizing complications such as the most frequent complication, incomplete graft attachment (i.e., detachment).2
Risk factors for graft detachment possibly include younger donor age3,4 resulting in tighter scrolls, older recipient age,5 intraoperative complications and surgical technique such as incomplete extraction of the patient’s Descemet’s membrane and overlap with the graft,5, 6, 7 dips in intraocular pressure after DMEK,8 and the use of air instead of gas for the intracameral tamponade.9,10 Whether the combination of DMEK with cataract surgery causes more or less graft detachment is incompletely understood.11, 12, 13
The definition of graft detachment and the indication and timing for repeat injecting of air or gas (rebubbling) vary between providers.2,14 Given the numerous definitions of detachment, the proportion of eyes with graft detachment ranges between 2% and 82% and the proportion of eyes requiring rebubbling ranges between 0% and 76%.1
A precise quantification of graft detachment is crucial for understanding risk factors and the impact of interventions on long-term outcomes. At present, the diagnosis is made clinically with slit-lamp biomicroscopy supported by anterior segment OCT (AS-OCT).2,15 To guide clinical decision making, we developed and validated a convolutional neural network to quantify the area and volume of graft detachment using a UNet++-based deep learning model for image segmentation of AS-OCT scans.16 Quantifications were valid and able to identify detachments missed by clinicians on slit-lamp exam alone.16
In the present study, we applied the neural network to precisely quantify graft detachment in the early postoperative period after DMEK in a pooled analysis of 4 prospective studies of participants with advanced Fuchs’ endothelial corneal dystrophy. We assessed whether concomitant cataract surgery was a risk factor for graft detachment after DMEK.
Methods
Study Design with Inclusion and Exclusion Criteria
This study included participants of 2 completed and 1 ongoing prospective single-center cohort studies (German Clinical Trials Register, DRKS00016996;17 DRKS00020945;18,19 DRKS00020946, ongoing) and of 1 completed randomized controlled trial (ClinicalTrials.gov, NCT04140422).20 Ethics Committee approval was obtained for all studies and this analysis. Written consent has been obtained from all patients. The study adhered to the principles of the Declaration of Helsinki.
Inclusion criteria of all studies were the diagnosis of clinically advanced Fuchs’ endothelial corneal dystrophy with an indication for DMEK. Participants with preexisting ocular diseases other than Fuchs’ dystrophy or cataract or previous ocular surgeries other than uncomplicated cataract surgery and YAG-capsulotomy, if needed, were not included. Participants who wore contact lenses regularly, took systemic or topical medications potentially influencing the cornea, or had systemic diseases potentially influencing the cornea were not included.
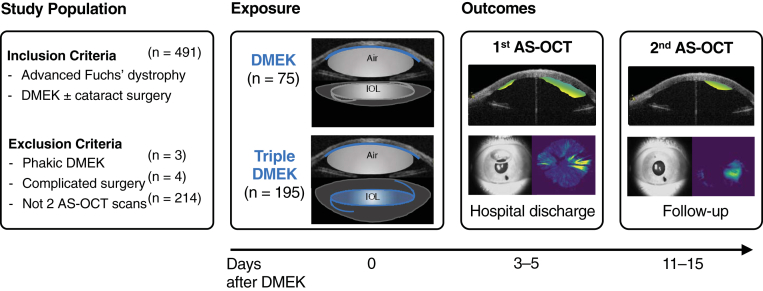
This study included only those eyes with a postoperative AS-OCT scan on the day of discharge from the hospital and a postoperative AS-OCT scan on the day of the first follow-up after discharge. Eyes with severe intraoperative complications such as intraoperative bleeding or severe floppy iris syndrome were excluded from this study (Fig 1).
Figure 1.
Study design. This study included participants with Fuchs’ dystrophy scheduled for Descemet membrane endothelial keratoplasty (DMEK). All eyes were pseudophakic before DMEK or underwent DMEK combined with cataract surgery (triple DMEK). Anterior segment OCT (AS-OCT) imaging was conducted twice after DMEK to determine the area and the volume of graft detachment using a validated neural network for image segmentation.16
Baseline Examination and Surgery
At baseline before DMEK, all participants received a complete ophthalmological examination including subjective refraction, vision and glare testing using ETDRS charts, a straylight meter measurement (C-Quant, Oculus), and slit-lamp biomicroscopy evaluation with grading of disease severity using the modified Krachmer scale.21 As a metric of disease severity, Scheimpflug imaging was performed,22,23 and edema resolution after DMEK was predicted on the basis of corneal thickness, standardized backscatter, posterior corneal elevation, and regularity of lines of equal corneal thickness (isopachs).24
Descemet membrane endothelial keratoplasty and cataract surgery were performed as described previously.3,13 After cataract surgery, if needed, surgical iridectomy was performed in all eyes using a diamond blade and an iris forceps before stripping the host’s Descemet membrane at the inferior cornea (5 surgeons) or at the 10 o’clock position (1 surgeon). Descemet’s membrane was stripped completely under air within the marked area of the graft’s diameter. Viscoelastic and air were removed completely over 2 paracenteses at the 10 and 2 o’clock positions using bimanual I/A (Centurion, Alcon). The endothelial graft was prepared by the corneal surgeons at the day of surgery. The graft was inserted using a uniplanar clear corneal incision at the 12 o’clock position, unfolded, attached, and centered by tapping at the corneal surface and by using saline and air injections. The incision was of the same size for DMEK as for triple DMEK. At the end of surgery, the anterior chamber was completely filled with filtered air, and the eye was pressurized to approximately 25 mmHg. After surgery, all surgeons recommended supine positioning for at least 24 hours. Intraocular pressure was routinely measured 1 to 4 hours and 4 to 8 hours after surgery or when symptomatic.
Quantification of Graft Detachment
The first routine AS-OCT was obtained on the day of discharge from the hospital. The second AS-OCT was obtained at the postoperative visit, approximately 2 weeks after DMEK, unless participants had to be seen for medical reasons or public holidays altered the schedule. Anterior segment OCT scans of the cornea were generated by trained personnel using a Casia-1 (Tomey). Each scan consisted of 256 cross-sectional images. In brief, the validated neural network automatically segmented the posterior corneal surface and the graft in each image to quantify the area, volume, and height of graft detachment.16 The area of detachment (range, 0%–100%; continuous) was determined with respect to the total graft surface (range of trephine sizes, 7–8 mm).
To allow comparison with an arbitrary cutoff for clinically relevant detachment of one third as proposed by others,15,25 the area of graft detachment was binarized at 33%. To determine whether the detachment was mainly flat or deep, mean graft detachment was calculated for each scan by dividing the volume by the area of detachment. The location of graft detachment was assessed by superimposing all centered 3-dimensional maps and color-coded by the number of eyes with detachment at each pixel with a maximum scale based on the first AS-OCT scan.
To quantify the postoperative air tamponade, 2 investigators (A.S.K., F.B.-D.) marked the cornea, the iris, and the air bubble in the en face overview image of the AS-OCT using QuPath.26 The size of the air bubble in the upright position during imaging was put in proportion to the size of the cornea.
Statistical Analysis
Means of detached area and volume were estimated using linear generalized estimating equations with robust standard errors, accounting for between-eye correlation within participants. By including terms for type of surgery (triple DMEK vs. DMEK only), time, and time · type of surgery, potentially mistimed measurements for the 2-week visit were accounted for, and marginal effects were predicted at 15 days postoperatively. Relative risks for a graft detachment area > 33% at the 2-week visit were estimated using Poisson generalized estimating equations with a log link and robust standard errors. Variance of graft detachment explained at the participant level was estimated as the between-participant intraclass correlation in linear mixed-effects models. All models were adjusted for participant age and the size of the air bubble at the first scan as linear terms; models including the 2-week visit were additionally adjusted for the exact day (continuous), as noted earlier; and models for between-participant variance of graft detachment were additionally adjusted for type of surgery (binary).
Results
Participant Characteristics and Surgery
The underlying prospective studies included 491 eyes. This study included 270 eyes of 80 men and 127 women with AS-OCT on the day of hospital discharge and at the 2-week visit (Figure 1). Eyes not included in this study had intraoperative complications (4 eyes), were phakic before and after DMEK (3 eyes), or had no AS-OCT at either time point (214 eyes). The main reasons that AS-OCT was unavailable were workflow-related and related to long-distance referrals with follow-up at local ophthalmologists. Eyes without both AS-OCT scans were not systematically different from eyes included in this study (Table S1, available at www.ophthalmologyscience.org).
Among eyes included in this study, 75 eyes (28%) were pseudophakic preoperatively and received DMEK only and 195 eyes received DMEK combined with cataract surgery (triple DMEK). In the DMEK only group, cataract surgery was performed at a median of 5.2 years (interquartile range [IQR], 2.9–9.1) before DMEK.
As expected, pseudophakic participants with DMEK only tended to be older with more advanced disease severity, as indicated by higher tomographically predicted edema resolution and higher modified Krachmer grading compared with phakic participants treated with triple DMEK. Median intraocular pressure within the first hours after surgery tended to be higher after triple DMEK compared with DMEK only. Intraocular pressure after surgery was severely elevated in 8 eyes in the triple DMEK group and in 1 eye in the DMEK only group, which required release of air because of pupillary block (Table 1).
Table 1.
Participant Characteristics by Type of Surgery: DMEK Only versus DMEK Plus Cataract Surgery (Triple DMEK)
| DMEK Only | Triple DMEK | |
|---|---|---|
| Participants, n (%) | 60 (29) | 147 (71) |
| Eyes, n (%) | 75 (28) | 195 (72) |
| Women, n (%) | 35 (58) | 92 (63) |
| Characteristics before surgery | ||
| Age, yrs | 76 (70–80) | 67 (60–74) |
| Best-corrected visual acuity, ETDRS letters∗ | 71 (63–78) | 74 (69–80) |
| Straylight, logS† | 1.41 (1.33–1.56) | 1.37 (1.26–1.54) |
| Modified Krachmer grades, n (%)‡ | ||
| Grade 4, confluent guttae < 5 mm diameter | 0 (0) | 9 (5) |
| Grade 5, confluent guttae, ≥ 5 mm diameter | 38 (58) | 100 (52) |
| Grade 6, visible corneal edema | 27 (42) | 58 (30) |
| Missing | 9 | 24 |
| Predicted corneal edema resolution, μm‡ | 84 (68–102) | 72 (57–87) |
| Central corneal thickness, μm | 625 (592–672) | 608 (571–635) |
| Anterior corneal backscatter, SU | 1965 (1736–2309) | 1728 (1501–1910) |
| Posterior corneal backscatter, SU | 1276 (1052–1438) | 1094 (926–1262) |
| Parallel isopachs, n (%) | 8 (12) | 58 (34) |
| Posterior corneal elevation, μm | 20 (13–31) | 14 (0–27) |
| Characteristics of surgery | ||
| Trephine and graft size, n (%) | ||
| 7.0 mm | 0 (0) | 1 (1) |
| 7.5 mm | 4 (5) | 6 (3) |
| 8.0 mm | 71 (95) | 188 (96) |
| Corneal abrasion, n (%) | 5 (7) | 4 (2) |
| Eye pressure directly after surgery, mmHg | 20 (16–24) | 22 (18–30) |
| Intervention to lower eye pressure, n (%) | 1 (1) | 8 (4) |
| Donor characteristics§ | ||
| Age at death, yrs | 77 (67–82) | 70 (63–79) |
| Women, n (%) | 25 (38) | 53 (31) |
| Pseudophakic eyes, n (%) | 17 (26) | 32 (19) |
| Endothelial cell density, cells/mm2 | 2263 (2117–2409) | 2336 (2117–2482) |
Continuous variables are presented as median (IQR), and categorical variables are presented as count (percentage).
DMEK = Descemet Membrane Endothelial Keratoplasty; SU = scatter units.
Best-corrected visual acuity was assessed using ETDRS charts in 260 eyes.
Disability straylight was assessed using a straylight meter in 225 eyes.
Disease severity was assessed using slit-lamp exam20 and tomographically in 240 eyes using a validated model for prediction of edema resolution based on corneal thickness, standardized corneal backscatter expressed in scatter units, regularity of lines of equal corneal thickness (isopachs), and posterior corneal elevation.23
Data available for 240 donor eyes.
Overall Graft Detachment in the First Two Weeks after Surgery
In the first AS-OCT at hospital discharge, on a median of 3 days after DMEK (IQR, 3–4), the median area of the graft detachment was 31% (IQR, 21–45). In 123 eyes (46%), more than one third of the graft surface was not attached (Table 2; Fig 2). The air bubble filled on a median of 25% (IQR, 11–37) of the anterior chamber.
Table 2.
Graft Detachment by Type of Surgery: DMEK Only versus DMEK Plus Cataract Surgery (Triple DMEK)
| DMEK Only |
Triple DMEK (DMEK Plus Cataract Surgery) |
|||||
|---|---|---|---|---|---|---|
| First AS-OCT | Second AS-OCT | Change First to Second |
First AS-OCT | Second AS-OCT | Change First to Second |
|
| Mean Area in % | 28.1 | 16.4 | –11.7 (–15.7 to –7.8) | 37.9 | 24.7 | –13.2 (–15.7 to –10.7) |
| Difference in Groups | 0 (ref) | 0 (ref) | 9.8 (4.1–15.5) | 8.3 (2.8–13.8) | ||
| Mean Volume in μl | 0.39 | 0.31 | –0.09 (–0.17 to –0.0) | 0.61 | 0.58 | –0.03 (–0.13 to 0.06) |
| Difference in Groups | 0 (ref) | 0 (ref) | 0.21 (0.07–0.37) | 0.27 (0.10–0.44) | ||
AS-OCT = anterior segment OCT; DMEK = Descemet membrane endothelial keratoplasty; ref = reference.
Estimated means and 95% confidence intervals (CIs) for slopes and differences in groups (triple DMEK minus DMEK) from generalized estimating equation models. All analyses were adjusted for age, percent air bubble, and exact date difference for the second AS-OCT imaging.
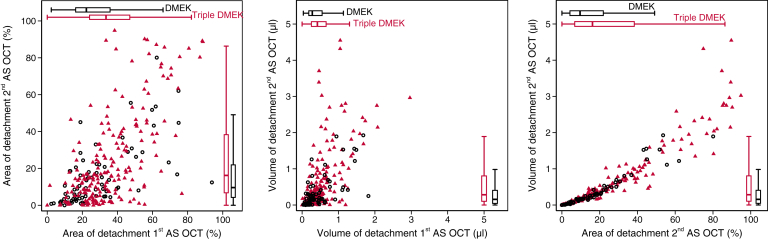
Figure 2.
Area and volume of graft detachment. Area (left) and volume (middle) of graft detachment were quantified at the first anterior segment OCT (AS-OCT) scan on day 3 and at the second AS-OCT at the 2-week visit after Descemet membrane endothelial keratoplasty (DMEK only; hollow circles) and DMEK combined with cataract surgery (triple DMEK; triangle). Boxes span the interquartile range (IQR), internal points indicate the median, and whiskers span 1.5 times the IQR. The association between area and volume for each eye is shown at the 2-week visit (right).
In the second AS-OCT scan at the 2-week postoperative visit (on a median of 15 days after DMEK, IQR, 13-18), the median area of the graft detachment was 15% (IQR, 5–33) and tended to be smaller than in the first scan (Table 2; Fig 2). In 139 of 147 eyes (95%) with less than one third of detachment in the first scan, graft detachment was still less than one third in the 2-week scan. In 63 of 123 eyes (51%) with more than one third of detachment in the first scan, graft detachment was less than one third in the 2-week scan, whereas in the remaining 60 eyes (49%), graft detachment was still more than one third of detachment (histogram as Fig S1, available at www.ophthalmologyscience.org).
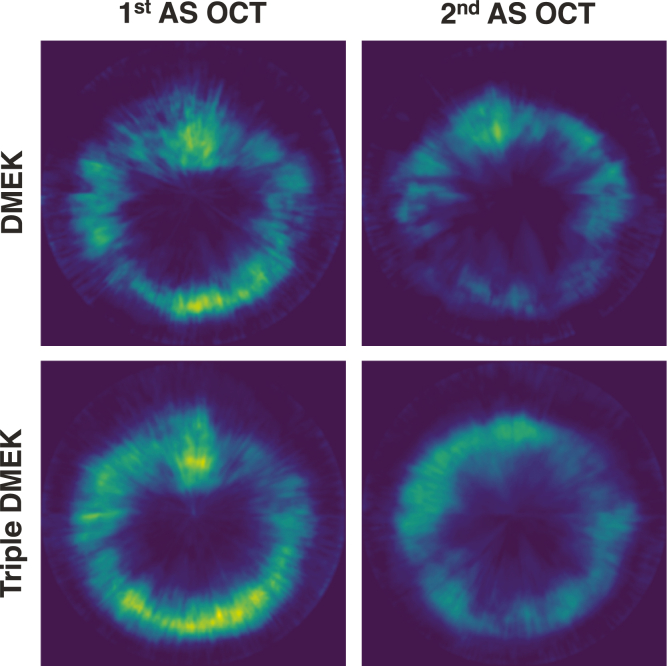
When superimposing all 3-dimensional maps from 2 weeks after surgery, the central graft was consistently attached. Areas of detachment were located at the graft margins, in particular the superior margin, suggesting that graft detachment was more common at the site of the clear corneal incision (Fig 3).
Figure 3.
Location of graft detachment of superimposed three-dimensional maps of graft detachment, using anterior segment OCT (AS-OCT) scans after Descemet membrane endothelial keratoplasty (DMEK) only or DMEK combined with cataract surgery (triple DMEK).16 The brighter and the more yellow the color at a given pixel, the higher the extent of detachment and the higher the number of eyes with graft detachment.
Participants with more detachment in 1 eye at the 2-week visit were more likely to have more detachment in the fellow eye. Some 21% of the variance in the area of detachment was explained by between-subject variance (intraclass correlation coefficient; 95% confidence interval [CI], 5–59) when adjusting for participant age, size of the air bubble, and type of DMEK, suggesting that additional patient characteristics could influence graft detachment.
Differences in Graft Detachment Between DMEK Only and Triple DMEK
In the first AS-OCT scan 3 days after surgery, the mean area of graft detachment was 1.29-fold higher (adjusted mean ratio; 95% CI, 1.08–1.53) in triple DMEK compared with DMEK only (Table 2). The mean volume of graft was 1.30-fold higher (95% CI, 0.94–1.80) in triple DMEK compared with DMEK only.
At the 2-week visit, the graft was fully attached with an area of less than 5% detachment in 42 eyes with triple DMEK (22%) and in 25 eyes with DMEK only (33%) (Fig S1). The mean area of graft detachment was 1.85-fold higher (95% CI, 1.34–2.56), the mean volume was 2.41-fold higher (95% CI, 1.51–3.86), and the mean detachment was 1.26-fold higher (95% CI, 1.09–1.45) in triple DMEK compared with DMEK only.
The risk of having more than one third of graft detachment 2 weeks after surgery was higher in eyes undergoing triple DMEK (59 eyes, 29%) compared with DMEK only (11 eyes, 15%; adjusted relative risk, 2.28; 95% CI, 1.25–4.12).
Interventions for Incomplete Graft Attachment
A total of 46 eyes received rebubbling procedures on a median of 15 days after surgery (IQR, 11–18) with 7 eyes (9%) in the DMEK group and 39 eyes (20%) in the triple DMEK group (adjusted risk ratio, 3.1; 95% CI, 1.3–7.1), indicating that rebubbling was more common in eyes undergoing DMEK combined with cataract surgery. Five of the eyes in the triple DMEK group required 2 or 3 rebubbling procedures.
Before rebubbling, the mean area of graft detachment was 51% in the DMEK group and 66% in the triple DMEK group, indicating that the area of graft detachment was larger when DMEK surgery was combined with cataract surgery (adjusted mean difference, 16 percentage points; 95% CI, 3–30). At the postoperative visit after rebubbling, the mean area of detachment was 9% in the DMEK only group and 13% in the triple DMEK group (n = 27).
Discussion
This study investigated graft detachment, the most common complication after endothelial keratoplasty over the first postoperative weeks, applying a validated neural network for AS-OCT image segmentation in a large cohort of participants with Fuchs’ dystrophy. In contrast to previous studies relying on conventional slit-lamp biomicroscopy or AS-OCT, the precise quantification of the area and volume of detachment allowed for longitudinal comparisons and demonstrated that attachment in the first days after DMEK predicted graft attachment 2 weeks later. Graft detachment was mainly flat and located at the graft margins, in particular at the superior cornea at the site of the clear corneal incision (Figure 3). Combining DMEK with cataract surgery resulted in a larger area and volume of detachment and more frequently rebubbling compared with DMEK only in already pseudophakic eyes.
Significant graft detachment at the 2-week visit is unlikely in eyes with low detachment in the first days after state-of-the-art DMEK, in line with previous findings of others.6,15,25 Interestingly, grafts that were intraoperatively presumably completely attached later detached despite an air tamponade, as previously suggested by Yeh et al,15 who observed complete attachment in 79% of eyes 1 hour after DMEK, but only in 49% of eyes at 1 week and in 65% of eyes at 1 month. In this study, even among grafts with large areas of detachment in the first postoperative days, every second graft reattached spontaneously within the next 2 weeks. Given this favorable and partially predictable natural history, postoperative protocols for supine positioning, which range from a few hours up to 7 days,25,27,28 and protocols for the frequency of clinical follow-up could be guided by the extent of early graft detachment.
In contrast to previous manual analyses of detachment pattern, which reported a diffuse overall detachment with a trend toward the inferior-nasal cornea,6,29 our automated, in-depth analysis suggested a directional pattern of graft detachment (Fig 2). Although most grafts were not completely attached at the inferior cornea in the first days after surgery, 2 weeks later, most grafts were completely attached at the site of the surgical iridectomy. Despite the beneficial effects of the air tamponade at the superior cornea in an upright position,10,25 graft attachment was incomplete at the superior margins of the graft in most eyes 2 weeks after surgery. Knowledge about the exact location of graft detachment may be informative for planning rebubbling procedures and may help prevent unintentional damage to the graft. Furthermore, it may help when evaluating surgical techniques such as identifying potential weak points at clear corneal incisions, the impact of sulfur hexafluoride tamponade instead of filtered air, or the use of laser iridotomies instead of surgical iridectomies.
Our prospectively enrolled participants with no ocular disease other than Fuchs’ dystrophy and cataract allowed analyzing cataract surgery as an independent risk factor for graft detachment. An obvious limitation of the current study is that the type of surgery (triple DMEK vs. DMEK only) was not assigned randomly, and such a trial may not be realistic to conduct. However, it is apparent that participants receiving DMEK only tended to be older because they had to have previously received cataract surgery alone. They also had more advanced disease severity and worse visual acuity at baseline despite a clear intraocular lens. Nevertheless, graft detachment was considerably worse in the triple DMEK group despite their more favorable baseline characteristics. These observations strongly support that the differences in detachment result from the type of surgery, although the true difference may be even larger than estimated.
More frequent and extensive detachment when combining DMEK with cataract surgery compared with DMEK only might be explained by the more instable iris-lens diaphragm, the longer duration of surgery with iris manipulation, or the circulation of tiny particles of viscoelastics, acetylcholine, or lenticular fragments from the bag or the sulcus. In our setting, it is unlikely that differences were due to the size of the incision or the size of the air bubble, which did not differ between DMEK only and triple DMEK. Whether the observed doubling in risk for > 33% detachment and an 8 to 9 percentage point greater area of detachment in triple DMEK compared with DMEK only at 2 weeks (Table 2) is reason enough to separate cataract surgery from DMEK needs to be carefully considered. Risks of each individual surgery, be it separate cataract surgery or rebubbling, may need to be considered.
Study Limitations
This study focused on graft detachment, a standardized outcome measure when using an objective, validated quantification method such as the AS-OCT–based neural network used here. The automated segmentation may help clinicians find areas of detachment faster and easier, especially when the detachment is small or the cornea is edematous. Compared with clinical slit-lamp–based experience, the median area of graft detachment at the first AS-OCT (31%) may seem relatively large, which is fully expected as the neural network identifies graft detachments missed by corneal specialists on slit-lamp exam.13,16 Further, it quantifies depth (mainly flat) and location (mainly graft margins) of detachment. These measures contrast with the usual rules of thumb, such as one third of area detached, which may not be nuanced enough as an indication for rebubbling. An alternative outcome, the rebubbling rate, is at the discretion of the surgeon and subject to potential constraints in healthcare access. Nevertheless, rebubbling was highly effective and was done 3 times as often after triple DMEK than DMEK only.
Conclusions
The automated segmentation and analysis of AS-OCT images quantified DMEK graft detachment and attachment patterns for participants with Fuchs’ dystrophy. For most participants, graft detachment decreased spontaneously during the first weeks after surgery without intervention, and most grafts were attached centrally. After DMEK with cataract surgery, graft detachment was approximately doubled compared with DMEK only in pseudophakic eyes. Understanding natural attachment and detachment patterns after DMEK will be useful for counseling patients, guiding intensity of clinical follow-up, and analyzing long-term consequences for graft survival and patient-reported outcomes.
Manuscript no. XOPS-D-22-00008.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): K.W.: Consultant – ProQR Therapeutics, The Netherlands, for projects unrelated to this work; Invented V-FUCHS, which is licensed by the Mayo Clinic to Aerie Pharmaceuticals, Inc, Iris Medicine Inc, and Santen, Inc.
K.W. and D.B.Z.: Invented the corneal edema prediction tool, which is licensed by the University of Freiburg to Oculus Optikgeräte GmbH.
Financial Support: Berta-Ottenstein-Program for Advanced Clinician Scientists and Program for Clinical Studies, Faculty of Medicine Freiburg, Germany (to K.W.), Deutsche Forschungsgemeinschaft (German Research Foundation – Project Number 440526480; to K.W.), thesis scholarship by the Deutsche Ophthalmologische Gesellschaft (German Ophthalmological Society; to D.B.Z.), and travel grant by the Wissenschaftliche Gesellschaft Freiburg (to A.-M.S.K.). The sponsor or funding organization had no role in the design or conduct of this research.
Paper presentation at: the Cornea and Eye Banking Forum of the Cornea Society and the Eye Bank Association of America, New Orleans, Louisiana, November 12, 2021.
HUMAN SUBJECTS: Human subjects were included in this study. Ethics Committee of the University of Freiburg approval was obtained for all studies and this analysis. The study adhered to the principles of the Declaration of Helsinki. Written consent has been obtained from all patients.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Kladny, Zander, Wacker
Data collection: Kladny, Zander, Lieberum, Glatz, Brandi-Dohrn, Wacker
Analysis and interpretation: Kladny, Zander, Lieberum, Glatz, Brandi-Dohrn, Reinhard, Wacker
Obtained funding: Wacker; Investigators were employees at the University of Freiburg.
Overall responsibility: Kladny, Zander, Lieberum, Glatz, Brandi-Dohrn, Reinhard, Wacker
Supplementary Data
References
- 1.Deng S.X., Lee W.B., Hammersmith K.M., et al. Descemet membrane endothelial keratoplasty: safety and outcomes: A Report by the American Academy of Ophthalmology. Ophthalmology. 2018;125:295–310. doi: 10.1016/j.ophtha.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Parekh M., Leon P., Ruzza A., et al. Graft detachment and rebubbling rate in Descemet membrane endothelial keratoplasty. Surv Ophthalmol. 2018;63:245–250. doi: 10.1016/j.survophthal.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Heinzelmann S., Huther S., Bohringer D., et al. Influence of donor characteristics on Descemet membrane endothelial keratoplasty. Cornea. 2014;33:644–648. doi: 10.1097/ICO.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 4.Hill J.R., Chen S.Y., Bauer A.J., et al. Younger donor tissue in Descemet membrane endothelial keratoplasty surgery: clinical outcomes. Cornea. 2021;40:1024–1030. doi: 10.1097/ICO.0000000000002582. [DOI] [PubMed] [Google Scholar]
- 5.Dunker S., Winkens B., van den Biggelaar F., et al. Rebubbling and graft failure in Descemet membrane endothelial keratoplasty: a prospective Dutch registry study. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2020-317041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tourtas T., Schlomberg J., Wessel J.M., et al. Graft adhesion in Descemet membrane endothelial keratoplasty dependent on size of removal of host's Descemet membrane. JAMA Ophthalmol. 2014;132:155–161. doi: 10.1001/jamaophthalmol.2013.6222. [DOI] [PubMed] [Google Scholar]
- 7.Brockmann T., Brockmann C., Maier A.K., et al. Clinicopathology of graft detachment after Descemet's membrane endothelial keratoplasty. Acta Ophthalmol. 2014;92:e556–e561. doi: 10.1111/aos.12419. [DOI] [PubMed] [Google Scholar]
- 8.Heinzelmann S., Bohringer D., Haverkamp C., et al. Influence of postoperative intraocular pressure on graft detachment after Descemet membrane endothelial keratoplasty. Cornea. 2018;37:1347–1350. doi: 10.1097/ICO.0000000000001677. [DOI] [PubMed] [Google Scholar]
- 9.Rickmann A., Szurman P., Jung S., et al. Impact of 10% SF6 gas compared to 100% air tamponade in Descemet's membrane endothelial keratoplasty. Curr Eye Res. 2018;43:482–486. doi: 10.1080/02713683.2018.1431286. [DOI] [PubMed] [Google Scholar]
- 10.Siebelmann S., Lopez Ramos S., Scholz P., et al. Graft detachment pattern after Descemet membrane endothelial keratoplasty comparing air versus 20% SF6 tamponade. Cornea. 2018;37:834–839. doi: 10.1097/ICO.0000000000001597. [DOI] [PubMed] [Google Scholar]
- 11.Chaurasia S., Price F.W., Jr., Gunderson L., Price M.O. Descemet's membrane endothelial keratoplasty: clinical results of single versus triple procedures (combined with cataract surgery) Ophthalmology. 2014;121:454–458. doi: 10.1016/j.ophtha.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Leon P., Parekh M., Nahum Y., et al. Factors associated with early graft detachment in primary Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2018;187:117–124. doi: 10.1016/j.ajo.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Fritz M., Grewing V., Gruber M., et al. Rotational alignment of corneal endothelial grafts and risk of graft detachment after Descemet membrane endothelial keratoplasty: a double-masked pseudo-randomized study. Acta Ophthalmol. 2021;99:e1334–e1339. doi: 10.1111/aos.14849. [DOI] [PubMed] [Google Scholar]
- 14.Ang M., Wilkins M.R., Mehta J.S., Tan D. Descemet membrane endothelial keratoplasty. Br J Ophthalmol. 2016;100:15–21. doi: 10.1136/bjophthalmol-2015-306837. [DOI] [PubMed] [Google Scholar]
- 15.Yeh R.Y., Quilendrino R., Musa F.U., et al. Predictive value of optical coherence tomography in graft attachment after Descemet's membrane endothelial keratoplasty. Ophthalmology. 2013;120:240–245. doi: 10.1016/j.ophtha.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Glatz A., Böhringer D., Zander D.B., et al. Three-dimensional map of Descemet membrane endothelial keratoplasty detachment: development and application of a deep learning model. Ophthalmol Sci. 2021;1 doi: 10.1016/j.xops.2021.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grewing V., Fritz M., Muller C., et al. [The German version of the Visual Function and Corneal Health Status (VFUCHS): a Fuchs dystrophy-specific visual disability instrument] Ophthalmologe. 2020;117:140–146. doi: 10.1007/s00347-019-0938-7. [DOI] [PubMed] [Google Scholar]
- 18.Fritz M., Grewing V., Maier P., et al. Diurnal variation in corneal edema in Fuchs endothelial corneal dystrophy. Am J Ophthalmol. 2019;207:351–355. doi: 10.1016/j.ajo.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 19.de Jong B., Brandi-Dohrn F., van der Meulen I.J.E., et al. Diurnal variation in straylight in patients with Fuchs endothelial corneal dystrophy and controls. Cornea. 2022 doi: 10.1097/ICO.0000000000003002. [DOI] [PubMed] [Google Scholar]
- 20.Zander D.B., Bohringer D., Fritz M., et al. Hyperosmolar eye drops for diurnal corneal edema in Fuchs' endothelial dystrophy: a double-masked, randomized controlled trial. Ophthalmology. 2021;128:1527–1533. doi: 10.1016/j.ophtha.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Louttit M.D., Kopplin L.J., Igo R.P., Jr., et al. A multicenter study to map genes for Fuchs endothelial corneal dystrophy: baseline characteristics and heritability. Cornea. 2012;31:26–35. doi: 10.1097/ICO.0b013e31821c9b8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaren J.W., Wacker K., Kane K.M., Patel S.V. Measuring corneal haze by using Scheimpflug photography and confocal microscopy. Invest Ophthalmol Vis Sci. 2016;57:227–235. doi: 10.1167/iovs.15-17657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun S.Y., Wacker K., Baratz K.H., Patel S.V. Determining subclinical edema in Fuchs endothelial corneal dystrophy. Revised classification using Scheimpflug tomography for preoperative assessment. Ophthalmology. 2019;126:195–205. doi: 10.1016/j.ophtha.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Zander D., Grewing V., Glatz A., et al. Predicting edema resolution after Descemet membrane endothelial keratoplasty for Fuchs dystrophy using Scheimpflug tomography. JAMA Ophthalmol. 2021;139:423–430. doi: 10.1001/jamaophthalmol.2020.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dirisamer M., van Dijk K., Dapena I., et al. Prevention and management of graft detachment in descemet membrane endothelial keratoplasty. Arch Ophthalmol. 2012;130:280–291. doi: 10.1001/archophthalmol.2011.343. [DOI] [PubMed] [Google Scholar]
- 26.Bankhead P., Loughrey M.B., Fernandez J.A., et al. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017;7 doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saethre M., Drolsum L. The role of postoperative positioning after DSAEK in preventing graft dislocation. Acta Ophthalmol. 2014;92:77–81. doi: 10.1111/j.1755-3768.2012.02560.x. [DOI] [PubMed] [Google Scholar]
- 28.Dapena I., Moutsouris K., Ham L., Melles G.R. Graft detachment rate. Ophthalmology. 2010;117:847–847e1. doi: 10.1016/j.ophtha.2009.09.054. [DOI] [PubMed] [Google Scholar]
- 29.Siebelmann S., Kolb K., Scholz P., et al. The Cologne rebubbling study: a reappraisal of 624 rebubblings after Descemet membrane endothelial keratoplasty. Br J Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2020-316478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.