Abstract
Mongolia is an expansive nation, dominated by agriculture with livestock under nomadic herder care contributing significantly to the economy. Mongolian veterinarians service these herder's livestock and dogs, and are often the first point of contact for animal health advice, including ectoparasite prophylaxis. Dogs are competent reservoir and sentinel hosts for several zoonotic vector-borne diseases (VBD). These diseases in dogs can be dependent on the presence of other sylvatic or domestic reservoir hosts, the abundance of competent vectors and supporting environmental and climatic conditions. Therefore, VBD present a true One Health challenge. The direct and close association of nomadic herders with livestock and livestock protection dogs coupled with frequent relocation (associated with nomadic lifestyles) places all three host groups (herders, livestock and livestock protection dogs) at risk of acquiring VBD. Our study set out to investigate the overall knowledge, perceptions and practices of Mongolian veterinarians towards canine vector-borne diseases (CVBD). A hardcopy questionnaire was delivered through the Mongolian Veterinary Medical Association to a cohort of veterinarians representing 39% of Mongolia's total veterinary workforce with a 53% response rate. A total of 297 participants were included in the final study. The bulk of participants were livestock veterinarians, followed by mixed animal veterinarians. Overall Mongolian veterinarians' knowledge of CVBD were scored as low (58%; 0–3 points) or medium (32%; 4–6 points) on a ten-point scale. There was a significant discrepancy between self-rated and actual knowledge. Females had 1.7 (95% CI 1.1, 2.8) times higher knowledge compared with males and those veterinarians who had 3–5 canine consultations per day were also found to have higher knowledge (odds ratio 1.4, 95% CI 0.4, 4.5). Most veterinarians utilised two or less resources to source information on CVBD over the previous 12 months. The potential of climate-induced emergence of vector populations and their associated pathogens makes it imperative that veterinarians in Mongolia have the necessary resources and knowledge to be on the forefront of CVBD preparedness and mitigation. This study identifies the knowledge gaps and addresses the need for further resources for Mongolian veterinarians to effectively engage in a One Health approach for negating CVBD in animals and humans.
Keywords: Dog, Mongolia, One Health, Vector, Veterinarian
Highlights
-
•
Canine vector borne disease (CVBD) knowledge of Mongolian veterinarians is low.
-
•
There is a discrepancy between self-rated CVBD knowledge and actual knowledge.
-
•
Female veterinarians have higher CVBD knowledge compared to males.
-
•
The majority of Mongolian veterinarians are livestock veterinarians.
1. Introduction
Mongolia is a geographically expansive and climatically diverse nation that contains 21 administrative provinces (aimags). The country is sparsely populated with approximately 3.4 million people [1]. While there is a consistent downward trend, approximately 26% of Mongolia's population remain traditionally nomadic [2], relocating their livestock herds each season and utilizing meat and dairy production as their principal source of income. These livestock play a critical role in Mongolia's economy [3] with an estimated 67 million head currently under herder care [1].
The majority of herders (66–88%) own a dog [4,5], usually with the principal intent of protecting their livestock from predators. It is this direct and close association of nomadic herders, livestock and their livestock protection dogs, which place them at risk of acquiring zoonotic canine vector-borne diseases (CVBD). Mongolian veterinarians typically train for five years at the Mongolian University of Life Sciences (MULS) and provide veterinary services to predominantly livestock in Mongolia. Veterinarians also engage in an inherent working relationship with nomadic herders, and on occasion, treat livestock herder dogs, often being the first point of contact for advice on ectoparasite control.
Canine vector-borne diseases are transmitted by arthropod hosts such as biting ticks, fleas and flies and can be disseminated across diverse geographical regions with movement of dogs [6,7] and other competent sylvatic and domestic hosts. They not only impact the health of dogs, but many can also infect and may cause disease in humans, through the bite of the same arthropod vectors. The role of dogs as either primary or secondary reservoirs for diversity of zoonotic CVBD across Eurasia and the Far East is becoming increasingly evident [8,9]. Several of these CVBD, namely tick-borne encephalitis virus (TBEV), granulocytic anaplasmosis, Bartonella, borreliosis and spotted fever group rickettsiae, have also been reported throughout Mongolia infecting multiple sylvatic and domestic hosts, as well as humans [[10], [11], [12], [13], [14], [15]]. The Mongolian medical authorities are attempting to monitor the Mongolian general public for TBEV, Lyme borreliosis and rickettsiosis, although their data has been collected sporadically in recent years and what has been collected appears to be incomplete [13]. Given the role of dogs as competent reservoir hosts for several of these zoonotic vector-borne agents, veterinarians play a critical role in combating CVBD in Mongolia. The purpose of this study was to investigate the overall knowledge and practices of Mongolian veterinarians and hence their ability to provide accurate advice to herders and the community on CVBD.
2. Materials and methods
2.1. Survey design
A paper-based questionnaire was designed to investigate the knowledge and practices of Mongolian veterinarians regarding CVBD and ectoparasite control (Supplementary material S1). The questionnaire was first drafted in English and then translated into Mongolian Cyrillic script by a bilingual researcher of the Institute of Veterinary Medicine (IVM) at the MULS. The questionnaire consisted of 30 questions and the time needed for completion was estimated to be 20 to 30 min depending on the level of detail provided. Data with regards to participants' demographics (gender, location, year of graduation, school of graduation), clinical practices (species encountered, average number of consultations per day, average number of canine consultations per day) and knowledge of CVBD were collected. Participants were asked whether they had ever heard of 13 different CVBD pathogens including Anaplasma phagocytophilum (granulocytic anaplasmosis), Babesia spp. (tick fever), Borrelia spp. (Lyme disease), Coxiella burnetii (Q fever), Dirofilaria immitis (heartworm), Leishmania spp. (kala-azar, cutaneous leishmaniosis) and TBEV. If participants responded positively, further questions were posed around the pathogen's presence in different host species in Mongolia, putative ectoparasite vectors and methods and frequency of diagnosis. Participants were asked to rate the impact of the pathogen on animal health in Mongolia. Further, the questionnaire included questions around client recommendations (e.g., ectoparasite prophylaxis and active ingredients recommended). The majority of questions were closed to remove ambiguity of responses, however a small number of open-ended questions were included to capture a wide range of options and perspectives (e.g. other reasons for not recommending ectoparasite prophylaxis).
2.2. Data collection
The target population for this survey were nationally registered Mongolian veterinarians in clinical practice across all 21 aimags of Mongolia. Between May 2018 and May 2019, hardcopies of the questionnaire were provided at mandatory Mongolian Veterinary Medical Association (MVMA) training sessions at the State Central Veterinary Laboratory in Ulaanbaatar. New licensing legislation introduced in 2018 stipulates that all practicing veterinarians complete training and licensing examinations within three years post-graduation, and thereafter every five years. Participation in this study was voluntary and two cash prizes (approx. $18.50 USD each) were offered through a lottery system per session as incentives.
The study was approved by the University of Melbourne Human Research Ethics Committee (ID number 1851192.1) and the MULS was notified of the approval prior to starting the research.
2.3. Data analysis
Hardcopy questionnaire responses were manually entered into a Research Electronic Data Capture (REDCap) database [16]. Free-text answers and comments were translated into English by a professional translator. Data were downloaded, checked and analysed using R version 4.0.0 and RStudio version 1.2.5001 including the packages ggplot2 [17], epitools [18], gmodels [19], MASS [20] and AICcmodavg [21]. Participants were excluded from the study if they did not answer at least 75% of the questions. Six variables were recategorized or collapsed. The continuous variable year of graduation was split into three equal time frames, 1970–1985, 1986–2001 and 2002–2018. Type of practice was divided into companion animal (dogs and cats only), mixed (cattle, camels, equids, goats, sheep, pigs, poultry, yaks that include dog or cat) and livestock (all production animals, including equids and camels) based on which species practitioners encountered in practice. Average number of consultations and average number of canine consultations per day were collapsed from ≤2, 3–5, 6–10, 11–15 and > 16 to ≤2, 3–5, 6–10 and > 10. Self-rated knowledge was recorded on a 5-point scale ranging from very poor to excellent and collapsed into three categories poor (incorporating very poor and poor), satisfactory and good (incorporating good and excellent). Resources utilised to source knowledge on CVBD within the last 12 months were re-categorised from individual sources such as books, journals and conference abstracts, direct discussion with other veterinarians, government health/public health websites/guidelines/newsletters, internet in general, social media, and other into the number of resources used ≤2, 3–5 and > 6 resources. The distribution of variables was assessed using descriptive statistics and frequency tables. Any variables with >12% missing data were excluded from further analyses.
The outcome variable was developed incorporating nine questions around the participants' knowledge of Babesia spp., Borrelia spp., Rickettsia spp. and TBEV given that these four pathogens may impact significantly on both human and canine health. Moreover, presence of these four pathogens in Mongolia has been reported for multiple species, although they are yet to be documented in Mongolian dogs [[10], [12], [13],[24], [25], [26]]. Participants received one point each for having heard of any of these four pathogens, one additional point if they were able to correctly identify the ectoparasite vector for each pathogen, and an additional point if they knew that Babesia spp. were present in livestock in Mongolia [27]. Points received were summed up resulting in a scale ranging from 0 (absent knowledge) to 10 (excellent knowledge). This scale was then collapsed into an ordinal variable with three levels: low (0–3 points), medium (4–6 points) and high level of knowledge (7–10 points). Contingency tables of explanatory variables with the three levels of knowledge were created and checked for small numbers.
Univariable ordinal logistic regression analyses were performed utilizing this outcome variable. Those variables that had a likelihood ratio chi-square test p-value of ≤0.25 were selected for inclusion in the multivariable analysis. All candidate variables were assessed pairwise for collinearity using Spearman's rank correlation coefficients or Pearson's chi-square test. Of those variables that were determined to be highly correlated (rs > 0.7 or p-value <0.05) only the variable with the lower univariable p-value was included into the multivariable analysis.
Multivariable ordinal logistic regression was performed using a manual backward stepwise elimination approach [28]. The variable with the highest p-value based on the likelihood ratio chi-square test was continually removed until all remaining variables were significant. Confounding was evaluated by comparing odds ratios of models with and without a potential confounding variable (> 25% change). Biologically meaningful two-way interactions in the final model were tested for significance. The Akaike information criterion was used to assess the overall model fit [29].
3. Results
3.1. Participant demographics
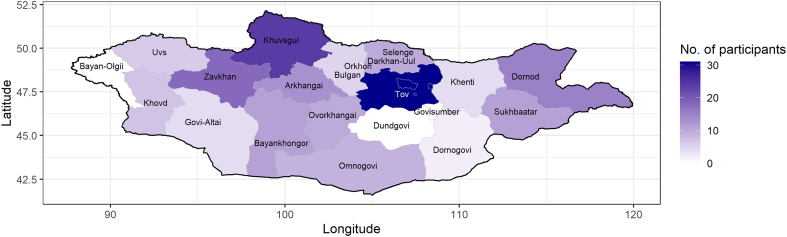
There are currently an estimated 1500 licensed veterinarians in private practice in Mongolia) and a total of 577 surveys were distributed during 11 mandatory licensing training sessions. Overall, 304 participants filled in the questionnaire resulting in a response rate of 53% and representing around 21% of Mongolia's veterinary workforce. Seven participants were excluded as their responses indicated that they did not work in clinical practice, resulting in 297 veterinarians included in this analysis. Overall, participants represented 17 of the 21 aimags of Mongolia, although a large proportion of participants did not state in which aimag they were currently practicing (39%). Fig. 1 shows the number of participants per aimag. The majority of participants came from Tov aimag, incorporating Ulaanbaatar (17%), closely followed by Khuvsgul (13%), and Zavkhan (10%). Table 1 provides an overview of the main demographic characteristics of the participants. Male and female veterinarians were represented equally, with the majority having graduated since 2002 (65%, 184/281). The bulk of participants were livestock veterinarians (52%, 153/297), followed by mixed animal veterinarians (44%, 130/297). Over 84% of participants stated that they frequently used less than two resources to enhance their CVBD knowledge and >90% of participants rated their self-knowledge of CVBD as satisfactory or above.
Fig. 1.
Choropleth map of Mongolia showing the number of participants per aimag.
Table 1.
Demographic characteristics of practicing veterinarians in Mongolia. Questionnaires were partially or fully completed by 297 veterinarians. Where incomplete data were recorded, the denominator used in the percentage calculation is indicated in parentheses after the variable name.
| Variable Categories | N | % |
|---|---|---|
| Gender (n = 296) | ||
| Male | 149 | 50.3 |
| Female | 147 | 49.7 |
| Year of graduation (n = 281) | ||
| 1970–1985 | 39 | 13.9 |
| 1986–2001 | 58 | 20.6 |
| 2002–2018 | 184 | 65.5 |
| Average number of total consultations seen per day | ||
| ≤ 2 | 99 | 33.3 |
| 3–5 | 120 | 40.4 |
| 6–10 | 45 | 15.2 |
| > 10 | 33 | 11.1 |
| Average number of canine consultations seen per day | ||
| ≤ 2 | 232 | 78.1 |
| 3–5 | 46 | 15.5 |
| 6–10 | 11 | 3.7 |
| > 10 | 8 | 2.7 |
| Species usually encountered in practice | ||
| Cats | 41 | 13.8 |
| Cattle/Yaks | 219 | 73.7 |
| Camels | 63 | 21.2 |
| Dogs | 140 | 47.1 |
| Horses/Donkeys/Mules | 166 | 55.9 |
| Pigs | 45 | 15.2 |
| Poultry | 19 | 6.4 |
| Sheep/Goats | 248 | 83.5 |
| Type of practice | ||
| Companion animal practice | 14 | 4.7 |
| Mixed animal practice | 130 | 43.8 |
| Livestock practice | 153 | 51.5 |
| Number of resources utilised in the last 12 months to source information on canine vector-borne diseases (CVBD) | ||
| ≤ 2 resources | 249 | 83.8 |
| 3–5 resources | 37 | 12.5 |
| > 6 resources | 11 | 3.7 |
| Self-rated knowledge of CVBD (n = 290) | ||
| Poor | 28 | 9.6 |
| Satisfactory | 178 | 61.4 |
| Good | 84 | 29.0 |
3.2. General CVBD awareness
Participants were assessed on their general CVBD awareness based on whether or not they had ever heard of 13 different CVBD pathogens as defined in Table 2. More than half of the participants had not heard of most CVBD pathogens listed with the exception of Babesia spp. (26%) and TBEV (42%). Of the participants who had heard of Babesia spp. and TBEV, most participants recognized that these pathogens infected livestock (81% and 72%, respectively) and the majority identified ticks as the vector (88% and 87%, respectively). A large proportion of veterinarians relied on pathognomonic clinical signs and history of ectoparasite exposure as the most common form of diagnosis.
Table 2.
Mongolian practicing veterinarians' responses to having ever heard of the following canine vector-borne pathogens.
| Variable Categories | Na | % |
|---|---|---|
| Anaplasma phagocytophilum (Granulocytic anaplasmosis) | ||
| Yes | 89 | 30.0 |
| No | 159 | 53.5 |
| Anaplasma platys (Cyclical thrombocytopenia) | ||
| Yes | 46 | 15.5 |
| No | 189 | 63.6 |
| Babesia spp. (Tick fever) | ||
| Yes | 188 | 63.3 |
| No | 76 | 25.6 |
| Bartonella spp. (Bartonellosis) | ||
| Yes | 25 | 8.4 |
| No | 234 | 78.8 |
| Borrelia spp. (Lyme disease) | ||
| Yes | 42 | 14.1 |
| No | 223 | 75.1 |
| Coxiella burnetii (Q fever) | ||
| Yes | 127 | 42.8 |
| No | 146 | 49.2 |
| Dirofilaria immitis (Heartworm) | ||
| Yes | 75 | 25.3 |
| No | 210 | 70.7 |
| Dirofilaria repens (Subcutaneous nodule worm) | ||
| Yes | 54 | 18.2 |
| No | 221 | 74.4 |
| Ehrlichia canis (Tropical canine pancytopenia) | ||
| Yes | 21 | 7.1 |
| No | 263 | 88.6 |
| Leishmania spp. (Kala-azar, Cutaneous leishmaniosis) | ||
| Yes | 46 | 15.5 |
| No | 235 | 79.1 |
| Neoehrlichia spp. | ||
| Yes | 12 | 4.0 |
| No | 268 | 90.2 |
| Rickettsia spp. (Spotted fever disease) | ||
| Yes | 67 | 22.6 |
| No | 204 | 68.7 |
| Tick-borne encephalitis virus | ||
| Yes | 153 | 51.5 |
| No | 126 | 42.4 |
Variables have up to 12% missing data adding up to N < 297 except for Bartonella spp. (13%) Anaplasma phagocytophilum (16%) and Anaplasma platys (21%).
Interestingly, more than half of the participating veterinarians reported recommending both flea (62%, 167/268) and tick (58%, 133/230) prophylaxis to their canine clients, whereas heartworm and sandfly prophylaxis were recommended less frequently (27%, 61/225, and 35% 78/221, respectively). The most common reason for not recommending flea, tick or heartworm prophylaxis stated was the belief that clients were not interested (65%, 96/147) followed by the participants being of the opinion that their practicing area was void of fleas, ticks and/or heartworm (52%, 76/147).
3.3. Regression analysis
Utilizing the outcome variable described above participants were categorized into low (n = 173), medium (n = 96) and high CVBD knowledge (n = 28). Table 3 shows Mongolian practicing veterinarian's univariable analysis results.
Table 3.
Univariable logistic regression results for 297 practicing veterinarians in Mongolia who responded to a hardcopy survey on canine vector-borne diseases (CVBD) between May 2018 and May 2019.
| Variable Categories | Regression coefficient b | SE (b) | Odds Ratio (95% CI) | P-value |
|---|---|---|---|---|
| Gender | 0.017 | |||
| Male | Ref. | |||
| Female | 0.55 | 0.23 | 1.72 (1.10, 2.74) | |
| Year of graduation | 0.442 | |||
| 1970–1985 | Ref. | |||
| 1986–2001 | 0.54 | 0.43 | 1.71 (0.75, 4.08) | |
| 2002–2018 | 0.37 | 0.38 | 1.38 (0.67, 3) | |
| Average number of total consultations seen per day | 0.124 | |||
| ≤ 2 | ||||
| 3–5 | −0.37 | 0.27 | 0.68 (0.40, 1.17) | |
| 6–10 | 0.41 | 0.34 | 1.50 (0.77, 2.93) | |
| > 10 | 0.05 | 0.39 | 1.05 (0.48, 2.24) | |
| Average number of canine consultations seen per day | 0.050 | |||
| ≤ 2 | Ref. | |||
| 3–5 | 0.84 | 0.3 | 2.32 (1.27, 4.21) | |
| 6–10 | 0.29 | 0.6 | 1.34 (0.39, 4.24) | |
| > 10 | 0.5 | 0.69 | 1.65 (0.39, 6.32) | |
| Encounters cats | 0.562 | |||
| No | Ref. | |||
| Yes | 0.19 | 0.33 | 1.20 (0.63, 2.27) | |
| Encounters cattle/yaks | 0.765 | |||
| No | Ref. | |||
| Yes | −0.08 | 0.26 | 0.93 (0.56, 1.55) | |
| Encounters camels | 0.138 | |||
| No | Ref. | |||
| Yes | −0.43 | 0.29 | 0.65 (0.36, 1.15) | |
| Encounters dogs | 0.362 | |||
| No | Ref. | |||
| Yes | 0.21 | 0.23 | 1.23 (0.79, 1.94) | |
| Encounters horses/donkeys/mules | 0.027 | |||
| No | Ref. | |||
| Yes | −0.51 | 0.23 | 0.60 (0.38, 0.95) | |
| Encounters pigs | 0.326 | |||
| No | Ref. | |||
| Yes | −0.32 | 0.33 | 0.73 (0.37, 1.37) | |
| Encounters poultry | 0.901 | |||
| No | Ref. | |||
| Yes | −0.06 | 0.46 | 0.94 (0.36, 2.29) | |
| Encounters sheep/goats | 0.025 | |||
| No | Ref. | |||
| Yes | −0.67 | 0.3 | 0.51 (0.29, 0.92) | |
| Type of practice | 0.726 | |||
| Companion animal practice | ||||
| Mixed animal practice | −0.17 | 0.53 | 0.85 (0.30, 2.49) | |
| Livestock practice | −0.32 | 0.53 | 0.72 (0.26, 2.13) | |
| Number of resources utilised in the last 12 months to source information on CVBD | 0.111 | |||
| ≤ 2 | Ref. | |||
| 3–5 | 0.44 | 0.34 | 1.54 (0.79, 2.98) | |
| > 6 | 1.12 | 0.64 | 3.06 (0.86, 10.82) | |
| Self-rated knowledge | 0.370 | |||
| Poor | Ref. | |||
| Satisfactory | −0.03 | 0.39 | 0.97 (0.46, 2.13) | |
| Good | −0.39 | 0.43 | 0.67 (0.29, 1.58) |
Seven variables were included in the multivariable analysis as they had p-values of <0.25: gender, average number of total consultations per day, average number of canine consultations per day, whether camels, horses/donkey/mule, sheep and goats were encountered, and total number of resources utilised over the previous 12 months. None of these variables were deemed to be highly correlated. The final multivariable model was based on a total of 296 responses (Table 4). High level knowledge of CVBD was significantly associated with both gender and average number of canine consultations seen per day. Female veterinarians were 1.7 (95% CI 1.1, 2.8) times more likely to have a higher level of knowledge compared to their male colleagues. Furthermore, veterinarians who averaged 3–5 canine consultations per day were significantly more likely to have higher CVBD knowledge than those who averaged ≤2 canine consultations per day (odds ratio 1.4, 95% CI 0.4, 4.5). In contrast, year of graduation, average number of total consultations per day, and encountering sheep and goats, horses, donkey and mules and camels were not significantly associated with the level of knowledge of Mongolian veterinarians. No biologically meaningful two-way interactions among variables in the final model were significant. Model fit to the data was assessed as being adequate (AIC 531.99).
Table 4.
Final multivariable ordinal logistic regression model for levels of knowledge of canine vector-borne diseases based on data from 296 practicing Mongolian veterinarians who responded to a hardcopy survey between May 2018 and May 2019.
| Variable Categories | Regression Coefficient b | Std Error (b) | OR (95% CI) | P-value |
|---|---|---|---|---|
| Intercept low knowledge | 0.79 | 0.19 | – | |
| Intercept medium knowledge | 2.78 | 0.26 | – | |
| Gender | 0.015 | |||
| Male | Ref. | |||
| Female | 0.57 | 0.23 | 1.76 (1.11, 2.80) | |
| Average number of daily canine consultations seen per day | 0.041 | |||
| ≤ 2 | Ref. | |||
| 3–5 | 0.88 | 0.31 | 2.40 (1.13, 4.39) | |
| 6–10 | 0.34 | 0.60 | 1.41 (0.40, 4.47) | |
| > 10 | 0.39 | 0.70 | 1.48 (0.35, 5.71) |
4. Discussion
This study provides an overview of the knowledge of Mongolian veterinarians towards CVBD and is the first comprehensive study of its kind. Overall CVBD knowledge was low, similar to that of Baltic countries, in which only 26% and 34% of veterinarians recognized the zoonotic potential of D. immitis and D. repens, respectively [30]. CVBD are typically associated with being highly endemic in the tropics as tropical environments permit diversity and abundance of ectoparasites some of which can transmit an extensive range of infectious agents [31]. The low knowledge among veterinarians on CVBD in the northern hemisphere may reflect the likelihood of these tick-borne diseases being less prevalent in temperate climates such as Mongolia. As Mongolia has subzero temperatures throughout winter, CVBD might not be considered as a pertinent risk to the health of companion and working dogs. This is interesting as the majority of participants identified ticks as the vector for both Babesia spp. and TBEV, and in turn suggests these ticks are highly adapted to continental climate and extreme winter temperatures. Moreover, the majority of dog owners in Mongolia come from socioeconomically disadvantaged communities and do not consider dogs as an integral part of the family, that is as companion animals. The demand and economic incentives placed on veterinarians to improve their knowledge on canine health is therefore limited. Over 95% of veterinarians in this study were livestock only or mixed practice veterinarians that occasionally tended to dogs. In total, only 15% of veterinarians reported attending to three or more canine consultations per day. This is in stark contrast to developed nations, such as the United States, where 67% of veterinarians in private practice are companion animal veterinarians and just 15% are food animal or mixed animal practitioners [32].
Having between three to five canine consultations per day was significantly associated with higher CVBD knowledge. It is likely that veterinarians with an increased interest in canine health and exposed to a wider range of canine health related issues are also more inclined to seek out related training and resources than veterinarians who only occasionally attend to dogs.
Interestingly, the questions relating to veterinarian's awareness of Babesia spp., its vector, host range and impacts to health were answered to a relatively high standard compared with the other pathogens. This is likely owing to the livestock focus of Mongolian veterinarians and frequency at which they encounter this highly endemic tick-borne piroplasm in cattle, with prevalence’s of 10% for Babesia bovis [33] and 9% for Babesia bigemina reported [27].
This study revealed a wide discrepancy between ‘actual’ knowledge and ‘perceived’ self-rated knowledge in Mongolian veterinarians. Only 9% of the 173 participants categorized as having low ‘actual’ knowledge on CVBD, self-rated their knowledge as low, with the majority (91%) self-rating their knowledge as satisfactory or high. There are no comparable published studies that investigate self-rated knowledge of CVBD in veterinarians. In a slightly broader context, this result is in contrast to a Finnish study that assessed the knowledge of veterinarians with regard to zoonotic diseases and found that only around 10% of veterinarians were confident that they had a good knowledge of zoonoses and their prevention [35].
In our study, females had 1.7 times higher knowledge compared to males. This finding is consistent with females showing higher levels of medical knowledge than males in several studies [[36], [37], [38]]. This result is encouraging as globally there is a gender transition in the veterinary industry from predominantly male to female. Females make up 80.5% of veterinary students in the USA [39] and > 80% of veterinarians in Finland, Latvia and Sweden [40]. Approximately 40% of veterinarians in Mongolia are female, which may shift in the future.
This study revealed that the majority of participants utilised two or less resources to source information on CVBD over the previous 12 months. As predominantly livestock veterinarians, they may not consider CVBD knowledge as essential to their everyday practice. For example, in Alberta, Canada, livestock veterinarians prioritized herd health and epidemiology training over internal medicine, animal welfare, and specialty diagnostics [41]. For veterinarians in New Zealand, the choice of continuing education (CE) activities was most commonly influenced by interest in the topic(s) and the desire to become more competent in practice [42].
There are some limitations to this study with both misclassification and selection bias potentially present. The questionnaire was completed after a long day of mandatory training and fatigue may have affected participant knowledge recall. Participation was voluntary, and this may have skewed towards those inclined to partake in surveys or had particular interests in the topic. Nevertheless, a good response rate of over 50% was reached and overall the final study population represented 21% of Mongolia's veterinarian workforce, all of whom were in clinical practice at the time of the study.
5. Conclusion
Veterinarians have a central role in the mitigation of CVBD across Mongolia at both the individual and population level spanning multiple hosts. The potential of climate-induced emergence of vector populations and their associated pathogens makes it imperative that veterinarians in Mongolia have access to the essential resources and knowledge which enable them to be on the forefront of CVBD awareness, preparedness and mitigation. Our study demonstrates the poor knowledge of Mongolian veterinarians on CVBD and the pressing need for the collaboration of government, non-government organisations, academia and industry to fund and support continuing veterinary education programs for Mongolian veterinarians. Client-driven demands for better standards of companion animal healthcare in future may drive the Mongolian veterinary industry to invest in research and training on the epidemiology, diagnosis, treatment and control of CVBD. Further to this, veterinarians should ideally develop a deep understanding of One Health concepts in the event they are confronted with emerging zoonotic diseases. This could be addressed by incorporating One Health within the MULS curriculum for undergraduate students, or added to the mandatory veterinary training conducted via the MVMA for graduates. At a national level, the development of an intersectoral One Health framework for the surveillance of ectoparasite vectors and their pathogens among animal populations that complements or merges with human disease surveillance would be a significant benefit [43]. A One Health surveillance and notification system would identify, analyse, and provide an early warning tool on emerging pathogens in both animals and humans [44]. This would allow all stakeholders - including veterinarians, medical practitioners and government - to be informed and allow joint decision making (the efficacy of which has been demonstrated on many occasions in other countries), to improve both animal and human health across Mongolia.
Funding source
This study was supported through funding from Bayer Animal Health GmbH, an Elanco Animal Health Company, Alfred-Nobel-Str. 50, 40,789 Monheim am Rhein, Germany and the Crawford Fund Student Award for recognition of the impact and benefit of international agricultural research of Australian research in developing countries. Davitt's MPhil scholarship was funded by the Noel Rudolf Hall bequest for canine related research through The University of Melbourne, Australia.
CRediT authorship contribution statement
Cassandra Davitt: Conceptualization, Methodology, Software, Writing – original draft, Investigation, Writing – review & editing. Rebecca Traub: Conceptualization, Methodology, Software, Data curation, Writing – original draft, Supervision, Writing – review & editing. Basan Batsukh: Conceptualization, Methodology, Software, Data curation. Banzragch Battur: Conceptualization, Methodology, Software. Martin Pfeffer: Conceptualization, Methodology, Software, Data curation, Supervision, Writing – review & editing. Anke K. Wiethoelter: Conceptualization, Methodology, Software, Data curation, Writing – original draft, Investigation, Supervision, Software, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors do not have any conflicts of interest to declare.
Acknowledgements
We acknowledge and would like to thank Mrs. Narantsatsral Sandagdorj of the Laboratory of Molecular Genetics at the Institute of Veterinary Medicine for translating the questionnaire.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2022.100458.
Appendix A. Supplementary data
Supplementary material Questionnaire to investigate knowledge of Mongolian veterinarians towards canine vector-borne disease.
Data availability
Data will be made available on request.
References
- 1.National Statistic Office of Mongolia . 2021. Mongolian Statistical Information Service. ([cited 2021 13/08/2021]; Available from: 1212.mn) [Google Scholar]
- 2.Papageorgiou S., et al. Detection and epidemiology of tick-borne pathogens in free-ranging livestock in Mongolia. Clin. Exp. Pathol. 2012:3. [Google Scholar]
- 3.Enkhtaivan B., et al. Molecular detection of Anaplasma ovis in small ruminants and ixodid ticks from Mongolia. Parasitol. Int. 2019;69:47–53. doi: 10.1016/j.parint.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Barnes A.N., et al. Knowledge and practices surrounding zoonotic disease among Mongolian herding households. Pastoralism: Research, Policy and Practice. 2020;10(1) [Google Scholar]
- 5.Barnes A.N., et al. Zoonotic enteric parasites in Mongolian people, animals, and the environment: using one health to address shared pathogens. PLoS Negl. Trop. Dis. 2021;15(7) doi: 10.1371/journal.pntd.0009543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright I., et al. Parasites Vectors. BMC; 2020. Parasites and vector-borne diseases disseminated by rehomed dogs; pp. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baneth G., et al. Vector-borne diseases - constant challenge for practicing veterinarians: recommendations from the CVBD world forum. Parasit. Vectors. 2012;5(1):55. doi: 10.1186/1756-3305-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansfield K., et al. Frontiers in Cellular and Infection Microbiology. Frontiers Media S.A; 2017. Emerging Tick-Borne Viruses in the Twenty-First Century. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hekrlova A., et al. Tick-borne encephalitis in dogs: application of “nested real-time RT-PCR” for intravital virus detection. BERLINER UND MUNCHENER TIERARZTLICHE WOCHENSCHRIFT. 2015;128(9–10):397–401. [PubMed] [Google Scholar]
- 10.Walder G., et al. Serological evidence for tick-borne encephalitis, borreliosis, and human granulocytic anaplasmosis in Mongolia. Int. J. Med. Microbiol. 2006;296(Supplement 1):69–75. doi: 10.1016/j.ijmm.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Khasnatinov M., et al. 76.005: tick-borne encephalitis virus in Mongolia. Int. J. Infect. Dis. 2010;14(Supplement 1):e372–e373. [Google Scholar]
- 12.von Fricken M.E., et al. Estimated seroprevalence of Anaplasma spp. and spotted fever group Rickettsia exposure among herders and livestock in Mongolia. Acta Trop. 2018;177:179–185. doi: 10.1016/j.actatropica.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Černý J., et al. Hard ticks and tick-borne pathogens in Mongolia—a review. Ticks Tick Borne Dis. 2019;10(6) doi: 10.1016/j.ttbdis.2019.101268. [DOI] [PubMed] [Google Scholar]
- 14.Chaorattanakawee S., et al. Tracking tick-borne diseases in Mongolian livestock using next generation sequencing (NGS) Ticks Tick Borne Dis. 2022;13(1) doi: 10.1016/j.ttbdis.2021.101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuzawa T., et al. PCR detection of Anaplasma phagocytophilum and Borrelia burgdorferi in Ixodes persulcatus ticks in Mongolia. Jpn. J. Infect. Dis. 2014;67(1):47–49. doi: 10.7883/yoken.67.47. [DOI] [PubMed] [Google Scholar]
- 16.Harris P., et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wickham H. Springer-Verlag; New York: 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- 18.Aragon T.J., et al. 2020. Epidemiology Tools, in Tools for Training and Practicing Epidemiologists Including Methods for Twoway and Multi-Way Contingency Tables. [Google Scholar]
- 19.Warnes G., et al. 2018. gmodels: Various R Programming Tools for Model Fitting. [Google Scholar]
- 20.Venables W., Ripley B. 4th ed. Springer; New York: 2002. Modern Applied Statistics with S. [Google Scholar]
- 21.Mazerolle M. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c) 2020. https://cran.r-project.org/package=AICcmodavg R package version 2.3–1:[Available from:
- 24.Muto M., et al. Isolation and characterization of tick-borne encephalitis virus from Ixodes persulcatus in Mongolia in 2012. Ticks Tick Borne Dis. 2015;6(5):623–629. doi: 10.1016/j.ttbdis.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Pulscher L.A., et al. A cross-sectional study of small mammals for tick-borne pathogen infection in northern Mongolia. Infect. Ecol. Epidemiol. 2018;8(1):1450591. doi: 10.1080/20008686.2018.1450591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karnath C., et al. Detection of Babesia venatorum, Anaplasma phagocytophilum and Candidatus Neoehrlichia mikurensis in Ixodes persulcatus ticks from Mongolia. Ticks Tick Borne Dis. 2016;7(2):357–360. doi: 10.1016/j.ttbdis.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Sivakumar T., et al. Genetic detection of Babesia bigemina from Mongolian cattle using apical membrane antigen-1 gene-based PCR assay. Vet. Parasitol. 2012;187(1–2):17–22. doi: 10.1016/j.vetpar.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Dohoo I.M., Stryhn H. 2nd ed. VER Inc.; Charlotte, USA: 2009. Veterinary Epidemiologic Research. [Google Scholar]
- 29.Bevans R. An introduction to the Akaike information criterion, When & How to Use It. 2020. https://www.scribbr.com/statistics/akaike-information-criterion/ Available from:
- 30.Tiškina V., Pikka J. Vector-borne parasitic infections in dogs in the Baltic and Nordic countries: a questionnaire study to veterinarians on canine babesiosis and infections with Dirofilaria immitis and Dirofilaria repens. Vet. Parasitol. 2017;244:7–11. doi: 10.1016/j.vetpar.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Huggins L.G., et al. Assessment of a metabarcoding approach for the characterisation of vector-borne bacteria in canines from Bangkok, Thailand. Parasit. Vectors. 2019;12(1):1–11. doi: 10.1186/s13071-019-3651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Veterinary Medical Association. U.S veterinarians 2018. https://www.avma.org/resources-tools/reports-statistics/market-research-statistics-us-veterinarians-2018 2018 [cited 2021; Available from:
- 33.Altangerel K., Battsetseg B., Battur B., Sivakumar T., Batmagnai E., Javkhlan G., Tuvshintulga B., Igarashi I., Matsumoto K., Inokuma H., Yokoyama N. The first epidemiological survey of Theileria orientalis infection in Mongolian cattle. Vet. Parasitol. 2011;182:343–348. doi: 10.1016/j.vetpar.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 35.Kinnunen P.M., et al. Veterinarians as a risk group for zoonoses: exposure, knowledge and protective practices in Finland. Saf. Health Work. 2022;13:78–85. doi: 10.1016/j.shaw.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teo T.Y., et al. Global Heart; 2016. PS309 Gender Differences in Knowledge, Attitudes and Practices Towards Cardiovascular Disease and its Treatment among Asian Patients. [PubMed] [Google Scholar]
- 37.Paul A., et al. Knowledge, attitudes, and practices toward the novel coronavirus among Bangladeshis: implications for mitigation measures. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0238492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan Y.H., et al. Knowledge, attitude and practice (KAP) survey of osteoporosis among students of a tertiary institution in Malaysia. Trop. J. Pharm. Res. 2014;13(1):155–162. [Google Scholar]
- 39.Association of American Veterinary Medical Colleges . 2017. Annual Data Report 2016-2017; pp. 1–39. [Google Scholar]
- 40.Federation of veterinarians of Europe . 2019. Survey of the veterinary profession of Europe; pp. 1–140. [Google Scholar]
- 41.Delver H.A. Continuing veterinary medical education needs and delivery preferences of Alberta veterinarians. J. Vet. Med. Educ. 2008;35(1):129–137. doi: 10.3138/jvme.35.1.129. [DOI] [PubMed] [Google Scholar]
- 42.Gates M.C. Practices, preferences, and opinions of New Zealand veterinarians towards continuing professional development. N. Z. Vet. J. 2021;69(1):27–37. doi: 10.1080/00480169.2020.1803156. [DOI] [PubMed] [Google Scholar]
- 43.Poh K.C., et al. All for one health and one health for all: considerations for successful citizen science projects conducting vector surveillance from animal hosts. Insects. 2022;13(6) doi: 10.3390/insects13060492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefrançois T., et al. Elsevier; England: 2022. After 2 Years of the COVID-19 Pandemic, Translating One Health into Action Is Urgent, in Lancet (London, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Questionnaire to investigate knowledge of Mongolian veterinarians towards canine vector-borne disease.
Data Availability Statement
Data will be made available on request.