Abstract
Purpose
Clinical OCT angiography (OCTA) of the retinal microvasculature offers a quantitative correlate to systemic disease burden and treatment efficacy in sickle cell disease (SCD). The purpose of this study was to use the higher resolution of adaptive optics scanning light ophthalmoscopy (AOSLO) to elucidate OCTA features of parafoveal microvascular compromise identified in SCD patients.
Design
Case series of 11 SCD patients and 1 unaffected control.
Participants
A total of 11 eyes of 11 SCD patients (mean age, 33 years; range, 23–44; 8 female, 3 male) and 1 eye of a 34-year-old unaffected control.
Methods
Ten sequential 3 × 3 mm parafoveal OCTA full vascular slab scans were obtained per eye using a commercial spectral domain OCT system (Avanti RTVue-XR; Optovue). These were used to identify areas of compromised perfusion near the foveal avascular zone (FAZ), designated as regions of interest (ROIs). Immediately thereafter, AOSLO imaging was performed on these ROIs to examine the cellular details of abnormal perfusion. Each participant was imaged at a single cross-sectional time point. Additionally, 2 of the SCD patients were imaged prospectively 2 months after initial imaging to study compromised capillary segments across time and with treatment.
Main Outcome Measures
Detection and characterization of parafoveal perfusion abnormalities identified using OCTA and resolved using AOSLO imaging.
Results
We found evidence of abnormal blood flow on OCTA and AOSLO imaging among all 11 SCD patients with diverse systemic and ocular histories. Adaptive optics scanning light ophthalmoscopy imaging revealed a spectrum of phenomena, including capillaries with intermittent blood flow, blood cell stasis, and sites of thrombus formation. Adaptive optics scanning light ophthalmoscopy imaging was able to resolve single sickled red blood cells, rouleaux formations, and blood cell–vessel wall interactions. OCT angiography and AOSLO imaging were sensitive enough to document improved retinal perfusion in an SCD patient 2 months after initiation of oral hydroxyurea therapy.
Conclusions
Adaptive optics scanning light ophthalmoscopy imaging was able to reveal the cellular details of perfusion abnormalities detected using clinical OCTA. The synergy between these clinical and laboratory imaging modalities presents a promising avenue in the management of SCD through the development of noninvasive ocular biomarkers to prognosticate progression and measure the response to systemic treatment.
Keywords: Adaptive optics, OCT angiography, Oculomics, Retinal microvasculature, Sickle cell disease
Abbreviations and Acronyms: ADD, airy disk diameter; AOSLO, adaptive optics scanning light ophthalmoscopy; BCVA, best-corrected visual acuity; D, diopters; FA, fluorescein angiography; FAZ, foveal avascular zone; HbSC, hemoglobin SC; HbSS, hemoglobin SS; IOP, intraocular pressure; OCTA, OCT angiography; RBC, red blood cell; ROI, region of interest; SCD, sickle cell disease; SCR, sickle cell retinopathy
Sickle cell disease (SCD) has been present in areas endemic to malaria such as sub-Saharan Africa, the Middle East, and India for thousands of years, with some areas of Africa having a newborn incidence of up to 2% per year.1 In the present-day United States, SCD is the most common inherited blood disorder and has a population prevalence of approximately 100 000, with > 3 million people living with sickle cell trait.2 By 2050, the global number of people with SCD is expected to increase by 30%.3
Despite significant progress in diagnosis and management over the years, SCD continues to cause significant morbidity and mortality from a relatively young age.1 The median survival of affected individuals in high-income countries remains low at 43 years.4 Sickle cell disease is associated with substantial economic cost to society and imposes significant financial burden to the patient due to treatment costs and productivity loss. Because the disease is multifactorial and its effects on the body are complex, it is difficult to understand the full scope of the cost of illness, and there is still a gap in estimating the total healthcare expenditure in the United States and globally.5
As novel therapeutics are being developed for this debilitating disease, strong clinical end points are being sought after to aid in the assessment of disease severity and treatment efficacy.6 The intrinsic transparency of the ocular media and visibility of the retinal tissue provide a rare window into the human body, enabling noninvasive study of the living retinal microvascular milieu.7 OCT angiography (OCTA) is a recent clinical advance in noninvasive, contrast-free retinal microvascular imaging, providing higher lateral and axial resolution than conventional intravenous fluorescein angiography (FA).8 OCT angiography uses movement of red blood cells (RBCs) as intrinsic “contrast” to depict capillary perfusion within discrete retinal layers presented in the en face perspective.9
Many of the features of OCTA are particularly well suited for the study of sickle cell retinal disease.10 Its quantitative nature has allowed exploration of foveal avascular zone (FAZ) size, microvascular density, and vessel tortuosity.11,12 Cross-sectional OCTA studies have been used to detect the presence and stage of sickle cell maculopathy,13 and to correlate findings with genetics and systemic features.14 Zhou et al15 have added sequential OCTA imaging and the concept of an intermittent perfusion index, which reflects systemic disease burden and is able to measure response to systemic therapy.
Despite these clinical advances, OCTA is still limited in its ability to resolve the details of cellular flow and vessel wall features due to the optical aberrations of the eye.16 Adaptive optics scanning light ophthalmoscopy (AOSLO) can compensate for these optical limitations and has demonstrated the ability to image retinal features at a cellular level in vivo, including photoreceptors, retinal pigment epithelial cells, vascular mural cells, hyalocytes, and ganglion cells and their nerve fibers.17, 18, 19, 20, 21, 22, 23, 24
Backscattered light in AOSLO can be separated into confocal and nonconfocal components.25 Confocal AOSLO FA and reflectance imaging using singly scattered light have been used to visualize decreased macular microvascular density in eyes with sickle cell retinopathy (SCR).26 Nonconfocal imaging techniques using multiply scattered light have been shown to enhance edge contrast, improving the ability of AOSLO to reveal retinal vessel wall and blood flow features.21
Nonconfocal detection schemes include using offset detection,22,27 split-detection with the annular detection area split into 2 halves,28 quadrant-detection with the annular detection area split into 4 sections,25 or multi-offset detection.22,29, 30, 31 An important limitation of these techniques for a given image is that edge contrast enhancement is limited to the respective detector’s direction, leading to suboptimal visualization of edges that run parallel to the detector’s direction.32 Recently, Migacz et al24 addressed this orientation dependence by using quadrant-detection for image capture and a directional difference filtering approach using an emboss filter to process and merge quadrant images into a single image.
The purpose of this study was to use nonconfocal quadrant-detection AOSLO with a directional difference filtering approach using an emboss filter to investigate the dynamics of perfusion abnormalities detected by OCTA in patients with sickle cell disease, using its higher level of resolution to provide clinicians with a better understanding of OCTA findings. The secondary purpose of this study was to assess the utility of AOSLO vascular imaging as a clinical research tool for elucidating sickle cell microvascular pathophysiology, specifically blood cell–blood cell and blood cell–endothelial cell adhesion interactions, which are being targeted by novel therapies.33 Patients were imaged cross-sectionally and, in some cases, prospectively to study the natural history of thrombo-occlusive events and response to hydroxyurea therapy.
Methods
Study Population
This study was conducted at the New York Eye and Ear Infirmary of Mount Sinai. It followed the tenets of the Declaration of Helsinki, was Health Insurance Portability and Accountability Act compliant, and was approved by the Institutional Review Board of New York Eye and Ear Infirmary of Mount Sinai. Written informed consent was obtained from each research participant after an explanation of the nature and risks of the study.
This was a case series study, which was in part cross-sectional and in part prospective. A chart review was performed to identify appropriate SCD patients for imaging. Patients chosen for the study were imaged within 1 month of their last clinic visit. Historical data gathered from the clinical record included age, sex, past medical and ocular history, past and current medicine and systemic treatments, and ocular treatments. Eye examinations of participants included best-corrected visual acuity (BCVA), intraocular pressure (IOP) measured by Goldman applanation, slit-lamp examination, and retinal appearance on documented dilated fundus examination, macular OCT raster scans (Heidelberg Spectralis HRA+OCT, Heidelberg Engineering Inc), and intravenous FA (Optos California icg, Optos PLC).
Inclusion criteria for patients were BCVA of at least 20/30, IOP ≤ 25 mmHg, normal anterior segment, and clear media. Exclusion criteria included cataracts grade ≥ 3, according to the Lens Opacity Classification System III,34 myopia ≤ –6.00 diopters (D) or hyperopia ≥ +5.00 D, and any history of prior ocular surgery including cataract or refractive surgery. Patients were also excluded if they reported a history of additional systemic cardiovascular disease including diabetes mellitus and hypertension. For participants in whom both eyes met inclusion criteria, a single eye was randomly selected for inclusion in the study.
We imaged one 34-year-old unaffected man, with no past medical or ocular history, as a control for a picture of healthy microvasculature and blood flow using OCTA and AOSLO vascular imaging. Eleven eyes of 11 SCD patients with a mean age of 33 years (range, 23–44 years) were chosen for imaging from the chart review (Table 1 shows patient demographics and relevant histories). All patients were imaged once cross-sectionally at the initial visit. Patients 4 and 6 were imaged prospectively 2 months after the initial visit to study compromised capillary segments across time and with treatment.
Table 1.
Patient Demographics
| Patient | Age, yrs/Sex | Genotype | Sickle Cell Sequelae | Treatment | Eye | SCR Type |
|---|---|---|---|---|---|---|
| 1 | 44/F | HbSS | Pain crises, ACS, asthma, anemia | Hydroxyurea, FA, Vitamin D chronic RBC transfusions | OS | NP |
| 2 | 23/F | HbSS | Pain crises, ACS | Hydroxyurea, FA | OD | NP |
| 3 | 31/M | HbSC | PE | None (stopped warfarin 6 mos prior) |
OS | P/VH |
| 4 | 36/F | HbSC | Pain crises, anemia | FA, history of RBC transfusions during pregnancy | OD | P |
| 5 | 23/M | HbSS | Pain crises, history of cholecystectomy | Vitamin D | OD | NP |
| 6 | 31/F | HbSS | Anemia | None, history of RBC transfusions | OD | NP |
| 7 | 25/F | HbSS | Pain crises | FA, Vitamin B-12, Vitamin D, Vitamin E, history of RBC transfusions | OS | NP |
| 8 | 42/F | HbSS | Repaired mitral valve regurgitation | Hydroxyurea, FA, Vitamin D, ASA, metoprolol, levothyroxine | OD | P status post SLP |
| 9 | 39/F | HbSS | Pain crises | Hydroxyurea, FA, amlodipine, metoprolol | OS | NP |
| 10 | 37/F | HbSS | Pain crises, asthma | Hydroxyurea, FA, montelukast | OS | NP |
| 11 | 31/M | HbSS | Pain crises, PE, ACS, intubations ×6 | History of BMT 2 yrs prior, rivaroxaban, Vitamin D | OD | NP |
ACS = acute chest syndrome; ASA = acetylsalicylic acid; BMT = bone marrow transplant; F = female; FA = folic acid; HbSC = hemoglobin SC; HbSS = hemoglobin SS; M = male; NP = nonproliferative; OD = right eye; OS = left eye; P = proliferative; PE = pulmonary embolism; SLP = scatter laser photocoagulation; RBC = red blood cell; SCR = sickle cell retinopathy; VH = vitreous hemorrhage.
Imaging Protocol
Mydriasis and cycloplegia were induced at the beginning of each imaging session with 1 drop each of 2.5% phenylephrine hydrochloride ophthalmic solution (Bausch & Lomb Inc) and 1% tropicamide ophthalmic solution (Akorn Inc).
OCTA Image Acquisition
The OCTA imaging protocol was performed as previously described.15,35 Once the eye of interest was dilated, a commercial spectral domain OCT system (Avanti RTVue-XR, Optovue) was used to obtain 10 sequential 3 × 3 mm en face OCTA scans centered on the fovea. The 10 OCTA scans were collected over 10 minutes, with one scan collected approximately every minute. Imaging was performed using the standard device wavelength of 840 nm, bandwidth of 45 nm, and axial line rate of 70 kHz. The system’s split-spectrum amplitude decorrelation angiography algorithm was used to map the perfused vessels for each scan.9
The OCTA full vascular slab was segmented, extending from the inner limiting membrane to 9 μm below the posterior boundary of the outer plexiform layer. The 10 sequential OCTA scans were compared sequentially to identify regions of interest (ROIs) adjacent to the foveal avascular zone (FAZ) with poorly perfused capillary segments. Poorly perfused capillary segments were nonperfused or intermittently perfused. Intermittently perfused capillary segments were defined as vessels showing perfusion in ≥ 1 OCTA scan with nonfilling during a previous or subsequent scan. Nonperfused capillary segments, although not directly visible on OCTA scans, were inferred to be present in areas of grossly irregular FAZ borders and rarified surrounding capillary beds.
AOSLO Image Acquisition
The AOSLO imaging of ROIs was performed immediately after OCTA imaging. The custom-built AOSLO simultaneously collected confocal and nonconfocal quadrant-detection images using a circular pinhole ∼1 Airy disk diameter (ADD) and an annular pinhole with an inner diameter of 2 ADD and an outer diameter of 20 ADD.25 During AOSLO video capture of an ROI, frames from the 4 quadrant detectors were used to create 4 split-detection frames at 180°, 135°, 90°, and 45° as shown in the Supplementary Methods Figure (available at www.ophthalmologyscience.org) and as previously described.25
During imaging, each participant’s head was stabilized using a customized bite bar. Participants were instructed to direct their gaze to a green internal fixation target. The imaging light source was a superluminescent diode with a wavelength centered at 790 nm, and imaging was performed at a frame rate of 16.6 Hz. Each video recording segment contained approximately 200 frames. A few videos were taken at each ROI at each time point. Participants were given frequent breaks in between video segment acquisitions. During breaks, participants were encouraged to blink, and artificial tear drops were given as needed to help maintain good tear film coverage throughout the imaging session. The AOSLO imaging sessions lasted approximately 1 hour.
Ancillary Ocular Imaging
Color fundus photography (Topcon DRI OCT Triton, Topcon Medical Systems Inc) and macular OCT raster scans (Heidelberg Spectralis HRA+OCT, Heidelberg Engineering Inc) were performed on the day of imaging. OCT macula scans with ETDRS thickness measurements centered at the fovea were used to assess for macular thinning. Axial length measurements were taken using an IOLMaster (Carl Zeiss Meditec, Inc) and were used to adjust for the retinal magnification of the OCTA with the Littmann formula36 and of the AOSLO images as previously published.28
OCTA Image Processing
The 10 OCTA scans were registered and averaged into figures for presentation purposes using ImageJ (ImageJ, US National Institutes of Health).37 Additionally, the 10 sequential OCTA scans were assembled into video sequences for the demonstration of intermittent blood flow. All OCTA averaged images and videos presented in this article represent OCTA full vascular layer scans, except for those in Figures 7 and S11 (available at www.ophthalmologyscience.org) and Video 7, which represent OCTA superficial layer scans for better comparison with AOSLO. The OCTA superficial layer was defined as having boundaries from the inner limiting membrane to 10 μm above the posterior boundary of the inner plexiform layer.
Figure 7.
Right eye of patient 6. A, Standard color fundus photograph. Line represents horizontal OCT scan through fovea. White box represents parafoveal area imaged with OCT angiography (OCTA). B, Averaged OCTA of 10 scans at (B1) baseline and (B2) 2 months after initiation of treatment with hydroxyurea. Red A labels the associated arteriole, and blue V’s label the associated venules. Green boxes represent region of interest (ROI) imaged with adaptive optics scanning light ophthalmoscopy (AOSLO). Scale bar represents 200 μm. C1, C2, Single frames from confocal AOSLO videos from baseline taken 5 minutes apart, and (C3) a single frame from a confocal AOSLO video taken after 2 months of treatment. C4, C5, Single frames from nonconfocal AOSLO videos from baseline taken 5 minutes apart, and (C6) a single frame from a nonconfocal AOSLO video taken after 2 months of treatment. C1, C2, C4, C5, Yellow arrows point to thrombi containing blood cells. Red arrows point to a nonperfused capillary segment. C3, C6, Yellow arrows point to resolved thrombi. Red arrows point to a capillary segment with restored perfusion. Scale bar represents 20 μm. D, Macular OCT acquired at baseline. The associated Video 7 is available at www.ophthalmologyscience.org.
AOSLO Image Processing
Frames from confocal and nonconfocal quadrant-detection AOSLO videos were registered and processed offline to create averaged images and videos.38,39 To optimize edge contrast along any edge in the 4 split-detection frames per ROI, a directional difference filtering approach using an emboss filter was used, as illustrated in Supplementary Methods Figure A–D (available at www.ophthalmologyscience.org). This approach was similar to the one previously described by Migacz et al,24 which used a directional difference filtering approach with an emboss filter to enhance edge contrast of the cell perimeters of vitreous cortex hyalocytes, improving visualization of these cells compared with the original split-detection images. The directional difference filtering approach used in this article had the following differences: The kernel size used was 5 × 5 pixels, instead of 3 × 3 pixels, with pixel values of +1.0 and –1.0, instead of +1.85 and –1.85; the emboss filters were applied on single frames instead of averaged images; and the 4 emboss-filtered frames were merged using minimum intensity projection instead of maximum intensity projection. These parameters were decided in a subjective and qualitative manner, adapting the hyalocyte image processing method to optimize visualization of blood vessel and blood cell structures on individual frames (Supplementary Methods Figure D vs. E, available at www.ophthalmologyscience.org). For the remainder of this article, nonconfocal quadrant-detection AOSLO is referred to as “nonconfocal AOSLO.”
Results
Imaging results from each of the 12 participants (11 SCD patients and 1 unaffected control) are presented in a case series fashion. All participants were imaged once at the initial imaging visit, and 2 patients (patients 4 and 6) were imaged prospectively at baseline and 2 months later. We found evidence of abnormal blood flow patterns on OCTA and AOSLO in all imaged SCD patients. Results from patients 8 to 11 are shown in the Appendix (available at www.ophthalmologyscience.org). Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 to Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Fig S9 (unaffected control and patients 1–8) include accompanying Videos 1 to 9 (available at www.ophthalmologyscience.org). The frame rate of all videos (both OCTA and AOSLO) was 16 frames per second, which is similar to the original unregistered AOSLO videos recorded at a frame rate of 16.6 Hz.
Figure 1.
Left eye of a 34-year-old unaffected control. A, Standard color fundus photograph. Line represents horizontal OCT scan through fovea. White box localizes parafoveal area imaged with OCT angiography (OCTA). B, Averaged OCTA of 10 scans. Red A labels the associated arteriole, and blue V’s label the associated venules. Green box localizes the region of interest (ROI) imaged with adaptive optics scanning light ophthalmoscopy (AOSLO). Scale bar represents 200 μm. Single frames from (C1) a confocal and (C2) a nonconfocal AOSLO video. Scale bar represents 20 μm. D, Macular OCT. The associated Video 1 is available at www.ophthalmologyscience.org.
Figure 2.
Left eye of patient 1. A, Standard color fundus photograph. Line represents horizontal OCT scan through fovea. White box localizes the parafoveal area imaged with OCT angiography (OCTA). B, Averaged OCTA of 10 scans. Red A’s label the associated arterioles, and blue V labels the associated venule. Green box localizes the region of interest (ROI) imaged with adaptive optics scanning light ophthalmoscopy (AOSLO). Scale bar represents 200 μm. C1, Single frame from a confocal, and (C2, C3) 2 frames from a nonconfocal AOSLO imaging video. White arrow represents direction of blood flow in the capillary segment of interest. Red arrow points to a red blood cell (RBC) rouleau. Yellow arrow points to a single sickled RBC. Blue V labels the associated venule from (B). Scale bar represents 200 μm. C4, C5, Zoomed-in views from (C3) highlighting morphological features of the torpedo-shaped single sickled RBC (yellow arrow) and the RBC rouleau (red arrow). Scale bar represents 10 μm. D, Macular OCT. White arrow indicates temporal macular thinning. The associated Video 2 is available at www.ophthalmologyscience.org.
Figure 3.
Right eye of patient 2. A, Standard color fundus photograph. Line represents horizontal OCT scan through fovea. White box localizes parafoveal area imaged with OCT angiography (OCTA). B, Averaged OCTA of 10 scans. Red A’s label the associated arterioles, and blue V labels the associated venule. Green box localizes region of interest (ROI) imaged with adaptive optics scanning light ophthalmoscopy (AOSLO). Scale bar represents 200 μm. Single frames from (C1) a confocal and (C2) a nonconfocal AOSLO video. White arrows represent direction of blood flow in the capillary segments of interest. Yellow arrows represent area of blood cell stasis. Red arrows represent area of sluggish blood flow. Blue V labels the venule from (B). Scale bar represents 20 μm. D, Macular OCT. The associated Video 3 is available at www.ophthalmologyscience.org.
Figure 4.
Left eye of patient 3. A, Standard color fundus photograph. Line represents horizontal OCT scan through fovea. White box localizes parafoveal area imaged with OCT angiography (OCTA). B, Averaged OCTA of 10 scans. Red A’s label the associated arterioles, and blue V labels the associated venule. Green box localizes region of interest (ROI) imaged with adaptive optics scanning light ophthalmoscopy (AOSLO). Scale bar represents 200 μm. Single frames from (C1) a confocal and (C2) a nonconfocal AOSLO video. White arrows represent direction of blood flow in the capillary segments of interest. Yellow arrows point to an immobile thrombus containing blood cells. Red arrows point to an area of plasma with occasional tumbling red blood cells. Blue arrows represent a sclerotic capillary segment. Blue V’s label the venule from (B). Scale bar represents 20 μm. D, Macular OCT. White arrow indicates temporal macular thinning. The associated Video 4 is available at www.ophthalmologyscience.org.
Figure 5.
Right eye of patient 4, imaged 2 months apart without changes in disease management. A, Standard color fundus photograph. Line represents horizontal OCT scan through fovea. White box localizes parafoveal area imaged with OCT angiography (OCTA). B, Averaged OCTA of 10 scans at (B1) baseline and (B2) 2 months later. Red A’s label the associated arterioles, and blue V labels the associated venule. Green boxes localize region of interest (ROI) imaged with adaptive optics scanning light ophthalmoscopy (AOSLO). Scale bar represents 200 μm. C1, C3, Single frames from confocal AOSLO videos at baseline and 2 months later. White arrow indicates direction of blood flow. C2, C4, Single frames from nonconfocal AOSLO videos at baseline and 2 months later. Yellow arrows show the presence at baseline of a red blood cell blocking the capillary as it bifurcates from its feeder arteriole, and its absence 2 months later. Red arrows point to the segment with sparse blood cell flow at baseline which showed restored perfusion 2 months later. Blue V labels the venule from (B). Scale bar represents 20 μm. D, Macular OCT acquired at baseline. The associated Video 5 is available at www.ophthalmologyscience.org.
Figure 6.
Right eye of patient 5. A, Standard color fundus photograph. Line represents horizontal OCT scan through fovea. White box localizes parafoveal area imaged with OCT angiography (OCTA). B, Averaged OCTA of 10 scans. Red A labels the associated arteriole, and blue V labels the associated venule. Green box localizes region of interest (ROI) imaged with adaptive optics scanning light ophthalmoscopy (AOSLO). Scale bar represents 200 μm. C1, Single frame from a confocal AOSLO video. C2, C3, Single frames from a nonconfocal AOSLO video. White arrow indicates the direction of blood flow. Black arrows point to the nonperfused capillary segment. Yellow arrows point to blood cells with associated material forming a thrombus stuck to the capillary wall at the bifurcation of a capillary. Red arrows point to spillover and peristaltic back flow of blood cells into the nonperfused capillary segment from the perfused capillary segment. Blue arrow points to a red blood cell rouleau. Blue V labels the venule from (B). Scale bar represents 20 μm. D, Macular OCT. White arrow indicates nasal macular thinning. The associated Video 6 is available at www.ophthalmologyscience.org.
Figure 8.
Left eye of patient 7. A, Standard color fundus photograph. Line represents horizontal OCT scan through fovea. White box localizes parafoveal area imaged with OCT angiography (OCTA). B, Averaged OCTA of 10 scans. Red A’s label the associated arterioles, and blue V labels the associated venule. Green box localizes region of interest (ROI) imaged with adaptive optics scanning light ophthalmoscopy (AOSLO). Scale bar represents 200 μm. C1, Single frame from a confocal AOSLO video. White arrow indicates the direction of blood flow through the capillary segment of interest. C2, C3, Single frames from a nonconfocal AOSLO video. Yellow arrow points to the formation of a cellular thrombus and subsequent nonperfusion of the capillary segment. Red arrows point to an adjacent poorly perfused capillary segment. Blue V labels the venule from (B). Scale bar represents 20 μm. D, Macular OCT. White arrow indicates temporal macular thinning. The associated Video 8 is available at www.ophthalmologyscience.org.
Unaffected Control
Figure 1 shows the left eye of the 34-year-old unaffected control. Video 1 (available at www.ophthalmologyscience.org) demonstrates dynamic features of blood flow and the vessel walls. Identification of the arterioles and venules was based on correlation to fundus photographs and confirmed by the presence of periarteriolar capillary free zones within the superficial OCTA layers.
In the unaffected control, the color fundus photograph and macular OCT appeared normal (Fig 1A, D). The OCTA revealed an intact FAZ and normal parafoveal microvascular perfusion. An ROI was chosen with capillary and noncapillary blood vessels (Fig 1B; Video 1B) for AOSLO imaging, which confirmed normal vessel wall features and blood flow (Fig 1C; Video 1C). A confocal AOSLO image (Fig 1C1; Video 1C1) was included for comparison with a nonconfocal AOSLO image (Fig 1C2; Video 1C2) to show its limitations in revealing vascular details compared with nonconfocal imaging. Intravascular blood cells appeared as highly reflective objects under confocal AOSLO visualization (Fig 1C1; Video 1C1). With nonconfocal AOSLO, individual blood cell boundaries were more easily discernible (Fig 1C2; Video 1C2).
Patient 1
Figure 2 and Video 2 (available at www.ophthalmologyscience.org) show the left eye of patient 1, a 44-year-old woman with hemoglobin SS (HbSS) disease and nonproliferative SCR. The color fundus photograph revealed increased vascular tortuosity with angioid streaks (Fig 2A), and the macular OCT showed moderate thinning of the temporal macula (Fig 2D, white arrow). OCT angiography showed widening of the intercapillary spaces in the parafoveal region (Fig 2B; Video 2B), with an intermittently perfused capillary segment at the temporal border of the FAZ (Fig 2B; Video 2B, box).
Adaptive optics scanning light ophthalmoscopy imaging of the intermittently perfused capillary showed a pattern of poor forward flow followed by restoration of perfusion within the capillary segment (Video 2C). During an episode of slowed forward flow, the nonconfocal images and Video 2 revealed a single torpedo-shaped sickled RBC within the capillary segment (Fig 2C3, C4, yellow arrows; Video 2C2), and a trailing acellular plasma space preceded by an RBC rouleau (Fig 2C3, C5, red arrows; Video 2C2).
The sickled morphology of the isolated cell and the rouleau formation are consistent with the behavior of RBCs from SCD patients in the laboratory setting.40,41 A rouleau (plural is rouleaux) is a stack of RBCs that forms when RBCs stick to each other along their flat surfaces. Although nonspecific and not always pathological, the increased presence of rouleaux signals the presence of a pathologic state.42 In patient 1, the RBC rouleau appeared responsible for the congestion, moving forward haltingly as it navigated the surrounding capillary wall. Individual RBCs can be seen within the stack of the rouleau (Fig 2C5; Video 2C2).
Patient 2
Figure 3 and Video 3 (available at www.ophthalmologyscience.org) depict the right eye of patient 2, a 23-year-old woman with HbSS disease and nonproliferative SCR. The color fundus photograph and macula OCT appeared grossly normal (Fig 3A, D). OCT angiography revealed compromised perfusion along the superior-nasal and inferior border of the FAZ, raising suspicion for the presence of poor perfusion in this area (Fig 3B; Video 3B, box).
Confocal AOSLO imaging at the inferior border of the FAZ showed foci of RBC stasis (Fig 3C1; Video 3C1, yellow arrows) and sluggish blood cell flow (Fig 3C1; Video 3C1, red arrows) within a perivenular capillary segment. Nonconfocal AOSLO imaging was able to define the outlines of the static blood cells better than confocal AOSLO imaging.
Patient 3
Figure 4 and Video 4 (available at www.ophthalmologyscience.org) show the left eye of patient 3, a 31-year-old man with hemglobin SC (HbSC) disease and proliferative SCR. The fundus image demonstrated increased vascular tortuosity (Fig 4A), and the macular OCT showed severe thinning of the temporal macula (Fig 4D, white arrow). OCT angiography revealed an enlarged irregular FAZ surrounded by widened inter-capillary spaces, especially superior-temporally (Fig 4B; Video 4B). The irregular superior border of the FAZ was inferred to be due to nonperfused capillary segments in this area (Fig 4B; Video 4B, box).
Confocal AOSLO imaging revealed a dilated capillary segment containing multiple immobile reflective foci (Fig 4C1; Video 4C1, yellow arrows), suggesting a blood cell aggregate. Nonconfocal AOSLO imaging confirmed the presence of a wall-to-wall immobile blood cell thrombus (Fig 4C2; Video 4C2, yellow arrows). Nonconfocal AOSLO was able to resolve a plasma-filled capillary segment interrupted by tumbling RBCs spilling over from the nearby venule (Fig 4C2; Video 4C2, red arrows) and an adjacent sclerotic “ghost” capillary segment inferior to the dilated capillary segment (Fig 4C2; Video 4C2, blue arrows). The thrombus in this area appeared fixed in place throughout the imaging session.
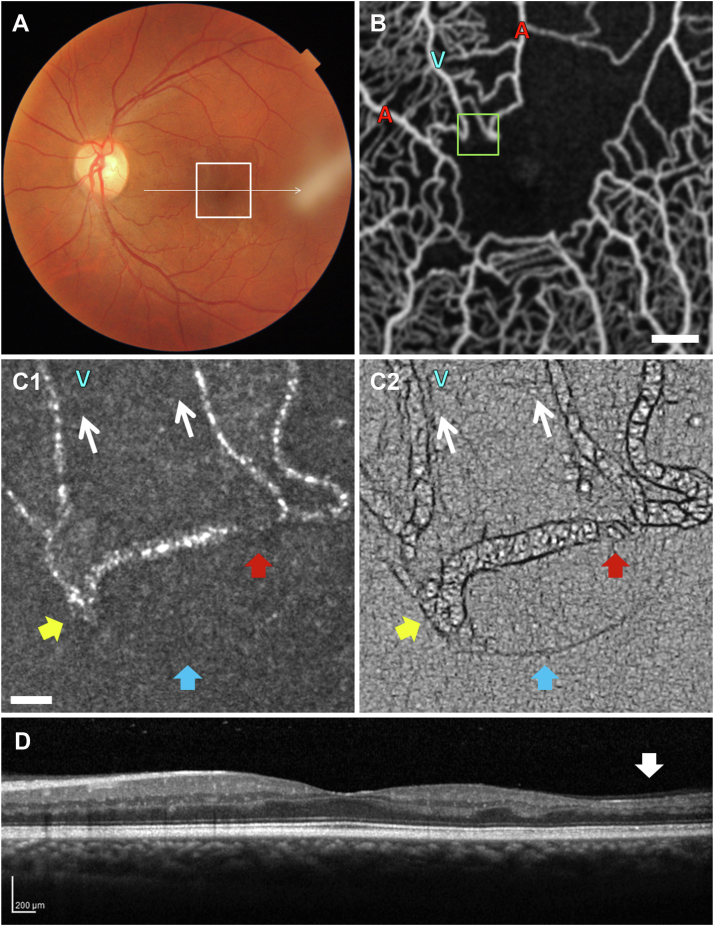
Patient 4
Figure 5 and Video 5 (available at www.ophthalmologyscience.org) show the right eye of patient 4, a 36-year-old woman with HbSC disease and proliferative SCR. The patient had no visual symptoms and no evidence of vitreous hemorrhage, prompting the decision to monitor her retina closely without laser treatment or changes to her systemic management. The patient’s images were taken at baseline and 2 months thereafter as a natural history survey of her microvascular occlusive activity. The color fundus photograph and macula OCT appeared grossly normal (Fig 5A, D), which remained unchanged between the 2 visits. OCT angiography showed a relatively stable capillary bed between the 2 visits, with some poorly perfused capillaries at baseline appearing well perfused 2 months later, and a few initially well-perfused capillaries appearing poorly perfused at the later imaging session (Fig 5B1 vs. B2).
At the baseline visit, OCTA revealed an intermittently nonperfused periarteriolar capillary segment at the temporal border of the FAZ (Fig 5B1, box; Video 5C2, inset). Confocal AOSLO imaging revealed a reflective intravascular mass blocking entry into the capillary segment as it bifurcated off its arteriole (Fig 5C1, yellow arrow). Nonconfocal AOSLO imaging was able to resolve the mass as an RBC caught against the vessel wall (Fig 5C2; Video 5C2, yellow arrows). Blockage of the capillary lumen was only partial, as sparse RBC flow was visualized (Video 5C2, red arrow).
At the 2-month visit, OCTA showed restored perfusion through the capillary segment (Fig 5B2, box; Video 5C4, inset). Adaptive optics scanning light ophthalmoscopy imaging explained the OCTA findings, confirming the presence of restored capillary perfusion, which was better visible in the nonconfocal AOSLO Video (Video 5C4, yellow and red arrows) compared with confocal and nonconfocal single frames (Fig 5C3, C4). This case demonstrates the dynamic changes that can occur naturally from month to month in sickle cell disease.
Patient 5
Figure 6 and Video 6 (available at www.ophthalmologyscience.org) show the right eye of patient 5, a 23-year-old man with HbSS disease and nonproliferative SCR. Fundus appearance was grossly normal (Fig 6A), and OCT revealed a broad foveal depression and moderate thinning of the nasal macula (Fig 6D, white arrow). OCT angiography showed an enlarged FAZ with widened parafoveal intercapillary spaces (Fig 6B; Video 6B, box).
Adaptive optics scanning light ophthalmoscopy confocal imaging revealed the presence of a nonperfused capillary segment not visible on OCTA (Fig 6C1; Video 6C1, black arrows). A reflective immobile intravascular mass was visible at its bifurcation from an upstream feeder capillary (Fig 6C1; Video 6C1, yellow arrows). Nonconfocal AOSLO imaging confirmed that there were no blood cells flowing through the capillary segment of interest (Fig 6C2, C3; Video 6C2, black arrows). Nonconfocal AOSLO imaging also revealed that the immobile mass at the capillary bifurcation consisted of a cellular thrombus stuck to the capillary wall (Fig 6C2, C3; Video 6C2, yellow arrows). Pulsatile spillover and backflow of blood cells into the nonperfused capillary segment from a downstream perfused capillary segment was also visible (Fig 6C2, C3; Video 6C2, red arrows). In the parallel perfused capillary segment, a transient RBC rouleau was visible (Fig 6C3, Video 6C2, blue arrows). The lack of blood cell flow through the capillary segment of interest was consistent throughout AOSLO imaging.
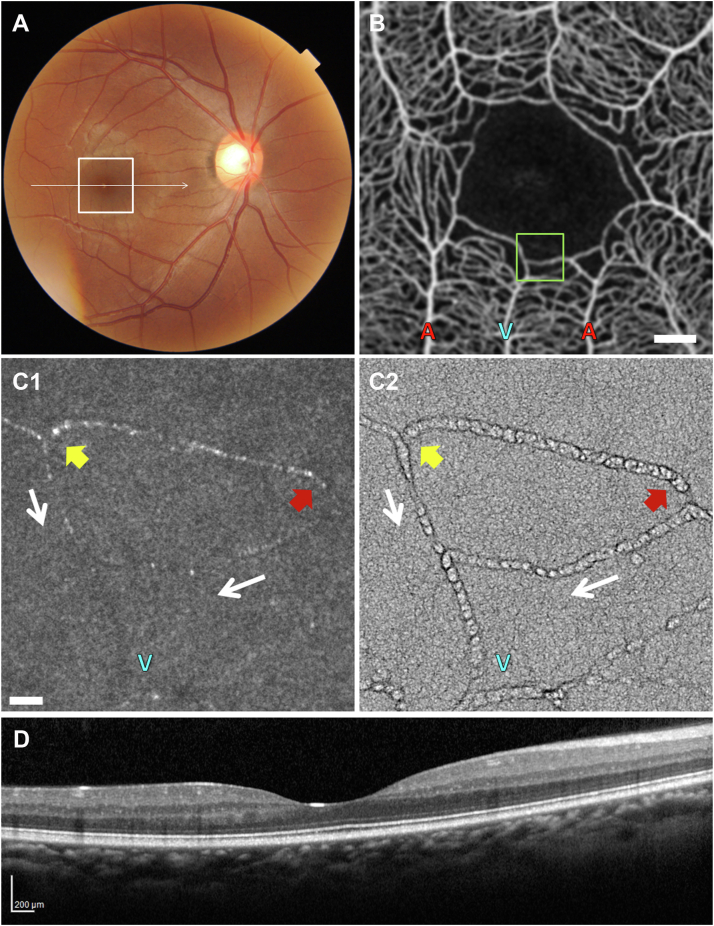
Patient 6
Figure 7 and Video 7 (available at www.ophthalmologyscience.org) show the right eye of patient 6, a treatment-naïve 31-year-old woman with HbSS disease and nonproliferative SCR, at baseline and 2 months after initiation of oral hydroxyurea therapy. Fundus exam at the initial visit showed increased arteriolar tortuosity (Fig 7A), and macular OCT showed a broad foveal depression (Fig 7D). OCT angiography revealed widened parafoveal capillary spacing (Fig 7B1; Video 7B). There was an intermittently nonperfused capillary segment near the superior-temporal border of the FAZ (Fig 7B1; Video 7B, box). Patient 6 started oral hydroxyurea therapy a few days after her initial imaging visit. Two months after initiation of therapy, her dilated fundus exam and macular OCT appeared unchanged. However, OCTA revealed quantitative improvement in overall perfusion of the macula (Zhou et al,15 Fig 6). With treatment, many of the intermittently nonperfused capillary segments identified on the initial OCTA images were now consistently perfused (Fig 7B2).
At the initial baseline visit, confocal AOSLO imaging demonstrated blood cells within thrombi at 2 consecutive capillary bifurcations (Fig 7C1, C2, yellow arrows) and a poorly perfused capillary segment (Fig 7C1, C2, red arrows). Nonconfocal AOSLO imaging revealed more detail within the thrombi (Fig 7C4, C5; Video 7C4, C5, yellow arrows). Nonconfocal AOSLO also showed enlargement of a thrombus over a 5-minute interval (Fig 7C4, C5; Video 7C4, C5, yellow arrows).
In images from the second visit, 2 months after initiation of oral hydroxyurea therapy, AOSLO showed resolution of the thrombi (Fig 7C3 and C6, yellow arrows) and restored perfusion through the previously nonperfused capillary segments (Fig 7C3, C6, red arrows). The resolution of thrombi and restored perfusion are better visualized on the nonconfocal AOSLO Video (Video 7C6, yellow and red arrows).
Patient 7
Figure 8 and Video 8 (available at www.ophthalmologyscience.org) show the left eye of patient 7, a 25-year-old woman with HbSS disease and nonproliferative SCR. Fundus exam revealed peripapillary and macular atrophic pigmentary changes (Fig 8A). Macular OCT showed severe global macular thinning (Fig 8D, white arrows). OCT angiography demonstrated widening of inter-capillary spaces (Fig 8B), with intermittently nonperfused perivenular capillary segments near the inferior-temporal border of the FAZ (Video 8B, box).
Confocal and nonconfocal AOSLO imaging captured the formation of a cellular thrombus in a capillary segment (Fig 8C; Video 8C1, C2, yellow arrows). Nonconfocal AOSLO imaging revealed an adjacent poorly perfused capillary segment (Fig 8C2, C3; Video 8C2, red arrows).
Discussion
In this series of case examples, AOSLO imaging provided an in vivo histological correlate to the compromised capillary perfusion events recorded with clinical OCTA in SCD patients, revealing dynamic cellular details. Adaptive optics scanning light ophthalmoscopy combined with OCTA imaging provided novel insights into the details of sickle cell disease pathophysiology. The combination of these imaging modalities has the potential to enable a more individualized and more effective approach to diagnosing, prognosticating, and treating this devastating disease.
Sickle cell disease shifts the perfusion equilibrium toward cycles of microvascular vaso-occlusion and hemolytic anemia.43 These events cause ischemia–reperfusion injury and complete infarctions, leading to painful crises, progressive organ damage, and significant morbidity and mortality in relatively young patients.44 The traditional concept of sickled RBCs blocking microvascular blood flow is now understood to represent only a fraction of a far more complex and multifactorial disease process with acute-on-chronic aspects.45
Because of its multifactorial nature, perhaps the best way to appreciate the complexity of SCD is through in vivo observations within the physiological environment of the human vascular system. Traditionally, ocular and fundus examinations, and imaging such as fundus photography and OCT, have been used to provide insights into the pathophysiology of SCD.46 For example, the lower degree of anemia resulting in greater blood viscosity in HbSC disease compared with HbSS disease appears to explain the higher incidence of retinopathy in HbSC disease.47
Clinical OCTA imaging as a noninvasive in vivo method has provided previously unachievable quantitative insights into sickle cell pathophysiology. OCT angiography has revealed that temporal retinal thinning correlates to decreased microvascular density48 and that microvascular insults likely precede and cause structural thinning.49 OCT angiography has also confirmed that the majority of capillary pruning appears to be periarteriolar,50 supporting certain prior studies46 and refuting other animal studies implicating post-capillary venules,51 underscoring the importance of in vivo human study.
In our study, AOSLO imaging provided visual evidence that blood cell–blood cell and blood cell–endothelial interactions are at least partially responsible for microvascular occlusive events in SCD. A spectrum of phenomena at the cellular level was revealed with AOSLO imaging, including capillaries with intermittent blood cell flow, blood cell stasis, and sites of thrombus formation containing cellular material. It is apparent that AOSLO imaging has enough resolution to visualize single sickled RBCs and RBC rouleaux formations. Figure 2 and Video 2 represent one of a few instances where we were able to conclusively distinguish a single sickled RBC flowing through a capillary segment based on its classic morphology. There was an RBC rouleau trailing behind that sickled RBC, forming a space of plasma in front and behind the sickled RBC, and slowing forward flow, making it possible to adequately visualize the cell’s morphology. There were likely multiple sickled RBCs captured in other patients within rapidly moving RBC columns, RBC rouleaux, and within thrombus formations. However, it was often difficult to conclusively identify cells as sickled RBCs in these instances.
Longitudinal imaging of patient 4 (Fig 5, Video 5) with AOSLO allowed study of the natural history of thrombo-occlusive events, showing that a thrombus may resolve on its own with time and lead to the return of normal capillary perfusion. Longitudinal OCTA imaging of patient 6 (Fig 7, Video 7) showed that macular perfusion improves with oral hydroxyurea therapy. As shown by longitudinal AOSLO imaging in patient 6, the improvement in perfusion with treatment was at least in part related to the resolution of cell-containing thrombi.
Grading systemic disease burden in SCD, especially at early stages of the disease, has proven challenging for clinicians. Development of therapeutic interventions has run into some challenges as well, especially in determining how effective a medication is for a given individual. OCT angiography and AOSLO imaging offer a powerful combination for evaluating disease activity and response to systemic therapeutic interventions. The intermittent perfusion index biomarker, based on serial OCTA imaging as described by Zhou et al,15 is able to quantify the ischemic burden of the retina in SCD patients. Intermittent perfusion index shows potential as a quantifiable surrogate for measuring systemic disease burden and response to systemic therapy.
As a tradeoff to its magnification capability, AOSLO imaging provides a much smaller field of view compared with that of OCTA, limiting its clinical utility for global quantitative analysis. The level of magnification and enhanced resolution provided, however, is most helpful for explaining OCTA findings from a cellular perspective. This use of AOSLO imaging may help reclassify SCD patients according to predominant mechanisms of microvascular occlusion, including RBC sickling, thrombus formation, and blood cell–vessel wall interactions, allowing for a more individualized therapeutic approach.
Study Limitations
Limitations in this study included the subjective method of optimizing the directional difference filtering approach for blood vessel and blood cell visualization. Future studies are warranted to systematically assess emboss filter parameters for visualization of various retinal structures. Furthermore, although the apparent sizes and shapes of blood cells and vascular structures in the merged emboss filtered frames looked comparable to those in the corresponding split-detection frames, future quantitative studies rigorously exploring the effect of directional difference filtering on apparent size and shape are needed.
Limitations in this study also included the small sample size of patients with sickle cell and the mostly cross-sectional nature of the study. Because of the small sample size, we were unable to differentiate AOSLO findings in SCD patients with nonproliferative versus proliferative SCR or between HbSC and HbSS patients. Future studies in planning will expand on the participant numbers and longitudinal observations, and will aim toward development of quantitative metrics of blood flow patterns to improve assessment of disease activity and response to therapies. Hopefully, useful correlation of these ocular metrics to current clinical and laboratory findings in SCD can be achieved.
Conclusions
Sickle cell disease is a peculiar disease process that has provided several significant opportunities for advancement of medical knowledge, including in the fields of molecular and genetic disease.52,53 We hope that our exploration of in vivo retinal microvascular features in SCD contributes in a small way to the blossoming field of oculomics. We hope to advance the understanding of this systemic disease process through the insights gained from combining AOSLO and OCTA retinal imaging.
Acknowledgment
In Memory of Peter N. Gillette, MD (1932-2020). Dr. Gillette lived a life of service. He was a pioneer and an advocate in the sickle cell community, and devoted his entire life to the care of his patients. Dr. Gillette’s life is well captured by Sir William Osler, who said in 1907, “To serve the art of medicine as it should be served, one must love his fellow man.”
Manuscript no. XOPS-D-22-00034.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): A.P.: Employee – Tesseract Health.
R.B.R.: Consultant – OptoVue, Boehringer-Ingelheim, Ocusciences, CellView, Topcon; Personal financial interest – Opticology, Guardion.
J.G.: Consultant – Global Blood Therapeutics, CSL Behring, Novartis; Contracted Research – Pfizer; Advisory Board – Synforma synteract DSMB.
N.S.: Paid researcher – Stanford University
A.D.: Patent Nos. 20120327368, 20080007693, 20190235624
Supported by the National Institutes of Health under Award Numbers R01HL159116, R01EY027301, R01EY032147, R01EY032669, R01EY031360, and P30EY026877. Additional funding for this research was provided by the New York Eye and Ear Infirmary Foundation Grant, the Marrus Family Foundation, a Challenge Grant, departmental awards from Research to Prevent Blindness, and the Jorge N. Buxton Microsurgical Foundation. The sponsors and funding organizations had no role in the design or conduct of this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
HUMAN SUBJECTS: Human subjects were included in this study. This study followed the tenets of the Declaration of Helsinki, was HIPAA compliant, and was approved by the Institutional Review Board of New York Eye and Ear Infirmary of Mount Sinai. Written informed consent was obtained from each participating research subject after an explanation of the nature and risks of the study.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Pinhas, Migacz, Zhou, Gillette, Sredar, Dubra, Glassberg, Rosen, Chui
Data collection: Pinhas, Migacz, Zhou, Castanos Toral, Otero-Marquez, Israel, Sredar, Dubra, Rosen, Chui
Analysis and interpretation: Pinhas, Migacz, Zhou, Castanos Toral, Otero-Marquez, Israel, Sun, Gillette, Sredar, Dubra, Glassberg, Rosen, Chui
Obtained funding: Rosen, Chui, Glassberg, Dubra; Study was performed as part of the authors' regular employment duties. No additional funding was provided.
Overall responsibility: Pinhas, Migacz, Zhou, Castanos Toral, Otero-Marquez, Israel, Sun, Gillette, Sredar, Dubra, Glassberg, Rosen, Chui
In Memory of Peter N. Gillette, M.D 1932-2020 Dr. Gillette’s life was a life of service. He was a pioneer and an advocate in the sickle cell community, and devoted his entire life to the care of his patients. Dr. Gillette’s life is well captured by Sir William Osler, who said in 1907, “To serve the art of medicine as it should be served, one must love his fellow man.”
Supplementary Data
VidClip of left eye of a 34-year-old unaffected control. B, Individual OCT angiography (OCTA) 10 scans, taken sequentially 1 minute apart, formatted into video, showing a normal foveal avascular zone (FAZ) and parafoveal microvasculature. Red A labels the associated arteriole, and blue V’s label the associated venules. Box localizes the region of interest (ROI) imaged with adaptive optics scanning light ophthalmoscopy (AOSLO). C1, Confocal AOSLO imaging showed normal microvascular structure. C2, Nonconfocal AOSLO imaging showed more detail of vessel wall and blood cell structures compared with confocal AOSLO imaging. The AOSLO videos are composed of 30 frames and played at 16 frames per second.
VidClip of left eye of patient 1. B, OCTA 10 scans, formatted into video, revealed intermittent nonperfusion of a perivenular capillary segment at the temporal border of the FAZ. Red A’s label the associated arterioles, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. C1, Confocal AOSLO imaging showed poor blood flow in this capillary segment, followed by restoration of perfusion. C2, Nonconfocal AOSLO imaging revealed a torpedo-shaped single sickled red blood cell (RBC) and an RBC rouleau temporarily stuck in place within the capillary segment. With careful observation, individual stacked RBCs can be seen within the rouleau. The AOSLO videos are composed of 27 frames and are played at 16 frames per second.
VidClip of right eye of patient 2. B, OCTA 10 scans, formatted into video, revealed a poorly perfused barely visible perivenular capillary segment at the inferior border of the FAZ. Red A’s label the associated arterioles, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. C1, Confocal AOSLO imaging revealed poor blood flow in this capillary segment (yellow and red arrows). C2, Nonconfocal AOSLO imaging showed blood cell stasis (yellow arrow) and sluggish blood cell flow (red arrow) within the capillary segment. Blue V’s label the venule from (B). The AOSLO videos are composed of 30 frames and are played at 16 frames per second.
VidClip of left eye of patient 3. B, OCTA 10 scans, formatted into video, raised suspicion for the presence of a nonperfused capillary segment at the irregular superior FAZ border. Red A’s label the associated arterioles, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. C1, Confocal AOSLO imaging revealed a dilated capillary segment with multiple immobile reflective foci within the capillary. These were suggestive of blood cell aggregation and stasis (yellow arrow). C2, Nonconfocal AOSLO imaging confirmed the presence of compromised blood flow, revealing details of the thrombus containing static, sludged blood cells (yellow arrow). Also visible was a plasma-filled capillary segment with occasional tumbling RBCs spilling over from the nearby venule (red arrow); and, a sclerotic capillary segment (blue arrow). The AOSLO videos are composed of 20 frames and are played at 16 frames per second.
VidClip of right eye of patient 4, imaged 2 months apart without changes in disease management. C2, Nonconfocal AOSLO imaging at baseline revealed an RBC (yellow arrow) caught against the capillary wall, which partially blocked the entrance into the capillary segment at its bifurcation from its feeder arteriole. The partial blockage caused downstream blood cell flow to be sparse (red arrow). C4, Nonconfocal AOSLO imaging 2 months after initial presentation revealed a resolution of the partial blockage (yellow arrow), and restoration of perfusion (red arrow). Blue V labels the associated venule. Insets in C2 and C4 represent OCTA 10 scans, formatted into video, of the ROI. AOSLO videos are composed of 19 frames and are played at 16 frames per second.
VidClip of right eye of patient 5. B, OCTA 10 scans, formatted into video, suggested the presence of nonperfused capillaries near the nasal FAZ border. Red A labels the associated arteriole, and blue V labels the associated venule. Box represents ROI imaged with AOSLO. C1, Confocal AOSLO imaging revealed the presence of a capillary segment not visible on OCTA (black arrow). A reflective immobile intravascular mass was visible at the bifurcation of the upstream feeder capillary (yellow arrow). C2, Nonconfocal AOSLO vascular imaging confirmed the presence of a capillary segment and revealed that there were no blood cells flowing through it (black arrow). Blood cells with associated material forming a thrombus stuck to the capillary wall were visible at the bifurcation of the upstream feeder capillary (yellow arrow). Spillover and peristaltic backflow of blood cells into the nonperfused capillary segment from a downstream perfused capillary segment was visible (red arrow). In the parallel perfused capillary segment, a transient RBC rouleau was visible (blue arrow). Blue V’s label the venule from (B). The AOSLO videos are composed of 40 frames and played at 16 frames per second.
VidClip of right eye of patient 6 at baseline and 2 months after initiation of oral hydroxyurea therapy. B, OCTA 10 scans, formatted into video, revealed an intermittently nonperfused capillary segment near the superior-temporal border of the FAZ. Red A labels the associated arteriole, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. C, Nonconfocal AOSLO vascular imaging. C4, C5, Imaged approximately 5 minutes apart at baseline, documenting the formation of a thrombus of blood cells at a capillary bifurcation (yellow arrows), along with its growth and extension into the downstream capillary lumen. The other downstream capillary segment was the poorly perfused capillary seen on OCTA (red arrow). C6, After 2 months of oral hydroxyurea therapy, the thrombus previously seen at the capillary bifurcation was now fully resolved (yellow arrow), and blood cell flow through the downstream capillary segments now appeared restored (red arrow). The AOSLO videos are composed of 20 frames and are played at 16 frames per second.
VidClip of left eye of patient 7. B, OCTA 10 scans, formatted into video, showed the presence of intermittently nonperfused perivenular capillary segments near the inferior-temporal border of the FAZ. Red A’s label the associated arterioles, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. C1, Confocal AOSLO imaging revealed the formation of a cellular thrombus blocking perfusion through the capillary segment (yellow arrow). C2, Nonconfocal AOSLO imaging revealed the formation of the cellular thrombus (yellow arrow) in greater detail. An adjacent capillary segment also showed poor blood cell flow (red arrow). The AOSLO videos are composed of 40 frames and are played at 16 frames per second.
VidClip of right eye of patient 8. B, OCTA 10 scans show the presence of an intermittently nonperfused capillary segment near the superior temporal border of the FAZ. Red A labels the associated arteriole, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. Confocal (C1) and nonconfocal (C2) AOSLO imaging showed a thrombus (blue arrow) containing blood cells partially obstructing the capillary lumen and causing sparse blood cell flow through the downstream capillary segment. During this time period, blood cells bypassing the partial obstruction and flowing through the poorly perfused downstream capillary segment were seen (red and yellow arrows). The AOSLO videos are composed of 30 frames and are played at 16 frames per second.
References
- 1.Kato G.J., Piel F.B., Reid C.D., et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4 doi: 10.1038/nrdp.2018.10. [DOI] [PubMed] [Google Scholar]
- 2.Hassell K.L. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Piel F.B., Hay S.I., Gupta S., et al. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne A.B., Mehal J.M., Chapman C., et al. Trends in sickle cell disease-related mortality in the United States, 1979 to 2017. Ann Emerg Med. 2020;76(3S):S28–S36. doi: 10.1016/j.annemergmed.2020.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dina S. Cost of illness of sickle cell disease in the US, payers’ perspective: (CRESCENT). Poster presentation at Virtual ISPOR 2021. May 17-20, 2021.
- 6.Farrell A.T., Panepinto J., Carroll C.P., et al. End points for sickle cell disease clinical trials: patient-reported outcomes, pain, and the brain. Blood Adv. 2019;3:3982–4001. doi: 10.1182/bloodadvances.2019000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner S.K., Fu D.J., Faes L., et al. Insights into systemic disease through retinal imaging-based oculomics. Transl Vis Sci Technol. 2020;9:6. doi: 10.1167/tvst.9.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spaide R.F., Fujimoto J.G., Waheed N.K., et al. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia Y., Tan O., Tokayer J., et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitao Guerra R.L., Leitao Guerra C.L., Bastos M.G., et al. Sickle cell retinopathy: What we now understand using optical coherence tomography angiography. A systematic review. Blood Rev. 2019;35:32–42. doi: 10.1016/j.blre.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Alam M., Thapa D., Lim J.I., et al. Quantitative characteristics of sickle cell retinopathy in optical coherence tomography angiography. Biomed Opt Express. 2017;8:1741–1753. doi: 10.1364/BOE.8.001741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch G., Scott A.W., Linz M.O., et al. Foveal avascular zone morphology and parafoveal capillary perfusion in sickle cell retinopathy. Br J Ophthalmol. 2020;104:473–479. doi: 10.1136/bjophthalmol-2019-314567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alam M., Thapa D., Lim J.I., et al. Computer-aided classification of sickle cell retinopathy using quantitative features in optical coherence tomography angiography. Biomed Opt Express. 2017;8:4206–4216. doi: 10.1364/BOE.8.004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fares S., Hajjar S., Romana M., et al. Sickle cell maculopathy: microstructural analysis using OCTA and identification of genetic, systemic and biological risk factors. Am J Ophthalmol. 2021;224:7–17. doi: 10.1016/j.ajo.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Zhou D.B., Castanos M.V., Pinhas A., et al. Quantification of intermittent retinal capillary perfusion in sickle cell disease. Biomed Opt Express. 2021;12:2825–2840. doi: 10.1364/BOE.418874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan J.I. The fundus photo has met its match: optical coherence tomography and adaptive optics ophthalmoscopy are here to stay. Ophthalmic Physiol Opt. 2016;36:218–239. doi: 10.1111/opo.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roorda A., Williams D.R. The arrangement of the three cone classes in the living human eye. Nature. 1999;397:520–522. doi: 10.1038/17383. [DOI] [PubMed] [Google Scholar]
- 18.Dubra A., Sulai Y., Norris J.L., et al. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed Opt Express. 2011;2:1864–1876. doi: 10.1364/BOE.2.001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takayama K., Ooto S., Hangai M., et al. High-resolution imaging of the retinal nerve fiber layer in normal eyes using adaptive optics scanning laser ophthalmoscopy. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scoles D., Sulai Y.N., Dubra A. In vivo dark-field imaging of the retinal pigment epithelium cell mosaic. Biomed Opt Express. 2013;4:1710–1723. doi: 10.1364/BOE.4.001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chui T.Y., Gast T.J., Burns S.A. Imaging of vascular wall fine structure in the human retina using adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2013;54:7115–7124. doi: 10.1167/iovs.13-13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi E.A., Granger C.E., Sharma R., et al. Imaging individual neurons in the retinal ganglion cell layer of the living eye. Proc Natl Acad Sci U S A. 2017;114:586–591. doi: 10.1073/pnas.1613445114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bower A.J., Liu T., Aguilera N., et al. Integrating adaptive optics-SLO and OCT for multimodal visualization of the human retinal pigment epithelial mosaic. Biomed Opt Express. 2021;12:1449–1466. doi: 10.1364/BOE.413438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Migacz J.V., Otero-Marquez O., Zhou R., et al. Imaging of vitreous cortex hyalocyte dynamics using non-confocal quadrant-detection adaptive optics scanning light ophthalmoscopy in human subjects. Biomed Opt Express. 2022;13:1755–1773. doi: 10.1364/BOE.449417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sredar N., Razeen M., Kowalski B., et al. Comparison of confocal and non-confocal split-detection cone photoreceptor imaging. Biomed Opt Express. 2021;12:737–755. doi: 10.1364/BOE.403907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chui T.Y.P., Mo S., Krawitz B., et al. Human retinal microvascular imaging using adaptive optics scanning light ophthalmoscopy. Int J Retina Vitreous. 2016;2:11. doi: 10.1186/s40942-016-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chui T.Y., Vannasdale D.A., Burns S.A. The use of forward scatter to improve retinal vascular imaging with an adaptive optics scanning laser ophthalmoscope. Biomed Opt Express. 2012;3:2537–2549. doi: 10.1364/BOE.3.002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scoles D., Sulai Y.N., Langlo C.S., et al. In vivo imaging of human cone photoreceptor inner segments. Invest Ophthalmol Vis Sci. 2014;55:4244–4251. doi: 10.1167/iovs.14-14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozaffari S., Jaedicke V., LaRocca F., et al. Versatile multi-detector scheme for adaptive optics scanning laser ophthalmoscopy. Biomed Opt Express. 2018;9:5477–5488. doi: 10.1364/BOE.9.005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mece P., Gofas-Salas E., Rui Y., et al. Spatial-frequency-based image reconstruction to improve image contrast in multi-offset adaptive optics ophthalmoscopy. Opt Lett. 2021;46:1085–1088. doi: 10.1364/OL.417903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gofas-Salas E., Rui Y., Mece P., et al. Design of a radial multi-offset detection pattern for in vivo phase contrast imaging of the inner retina in humans. Biomed Opt Express. 2022;13:117–132. doi: 10.1364/BOE.441808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sapoznik K.A., Luo T., de Castro A., et al. Enhanced retinal vasculature imaging with a rapidly configurable aperture. Biomed Opt Express. 2018;9:1323–1333. doi: 10.1364/BOE.9.001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moerdler S., Manwani D. New insights into the pathophysiology and development of novel therapies for sickle cell disease. Hematology Am Soc Hematol Educ Program. 2018;2018:493–506. doi: 10.1182/asheducation-2018.1.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chylack L.T., Jr., Wolfe J.K., Singer D.M., et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111:831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 35.Lynch G., Romo J.S.A., Linderman R., et al. Within-subject assessment of foveal avascular zone enlargement in different stages of diabetic retinopathy using en face OCT reflectance and OCT angiography. Biomed Opt Express. 2018;9:5982–5996. doi: 10.1364/BOE.9.005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett A.G., Rudnicka A.R., Edgar D.F. Improvements on Littmann's method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994;232:361–367. doi: 10.1007/BF00175988. [DOI] [PubMed] [Google Scholar]
- 37.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubra A., Harvey Z. In: Biomedical Image Registration. Fischer B., Dawant B.M., Lorenz C., editors. Springer-Verlag; Berlin, Heidelberg: 2010. Registration of 2D images from fast scanning ophthalmic instruments; pp. 60–71. [Google Scholar]
- 39.Sulai Y.N., Scoles D., Harvey Z., Dubra A. Visualization of retinal vascular structure and perfusion with a nonconfocal adaptive optics scanning light ophthalmoscope. J Opt Soc Am A Opt Image Sci Vis. 2014;31:569–579. doi: 10.1364/JOSAA.31.000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obiefuna P.C., Photiades D.P. Sickle discocytes form more rouleaux in vitro than normal erythrocytes. J Trop Med Hyg. 1990;93:210–214. [PubMed] [Google Scholar]
- 41.Kviatkovsky I., Zeidan A., Yeheskely-Hayon D., et al. Measuring sickle cell morphology during blood flow. Biomed Opt Express. 2017;8:1996–2003. doi: 10.1364/BOE.8.001996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobuchi Y., Ito T., Ogiwara A. A model for rouleaux pattern formation of red blood cells. J Theor Biol. 1988;130:129–145. doi: 10.1016/s0022-5193(88)80089-4. [DOI] [PubMed] [Google Scholar]
- 43.Stuart M.J., Nagel R.L. Sickle-cell disease. Lancet. 2004;364:1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 44.Sundd P., Gladwin M.T., Novelli E.M. Pathophysiology of sickle cell disease. Annu Rev Pathol. 2019;14:263–292. doi: 10.1146/annurev-pathmechdis-012418-012838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novelli E.M., Gladwin M.T. Crises in sickle cell disease. Chest. 2016;149:1082–1093. doi: 10.1016/j.chest.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elagouz M., Jyothi S., Gupta B., Sivaprasad S. Sickle cell disease and the eye: old and new concepts. Surv Ophthalmol. 2010;55:359–377. doi: 10.1016/j.survophthal.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Lemaire C., Lamarre Y., Lemonne N., et al. Severe proliferative retinopathy is associated with blood hyperviscosity in sickle cell hemoglobin-C disease but not in sickle cell anemia. Clin Hemorheol Microcirc. 2013;55:205–212. doi: 10.3233/CH-2012-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grover S., Sambhav K., Chalam K.V. Capillary nonperfusion by novel technology of OCT angiography in a patient with sickle cell disease with normal fluorescein angiogram. Eur J Ophthalmol. 2016;26:e121–123. doi: 10.5301/ejo.5000765. [DOI] [PubMed] [Google Scholar]
- 49.Ong S.S., Linz M.O., Li X., et al. Retinal thickness and microvascular changes in children with sickle cell disease evaluated by optical coherence tomography (OCT) and OCT angiography. Am J Ophthalmol. 2020;209:88–98. doi: 10.1016/j.ajo.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 50.Otero-Marquez O, Rosen RB, Castanos Toral MV, et al. Preferential vulnerability of capillary occlusion in sickle cell retinopathy. Poster Presentation at the Imaging in the Eye Annual Conference, May 1–7, 2021.
- 51.Kaul D.K., Fabry M.E. In vivo studies of sickle red blood cells. Microcirculation. 2004;11:153–165. [PubMed] [Google Scholar]
- 52.Pauling L., Itano H.A., Singer S.J., Wells I.C. Sickle cell anemia a molecular disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 53.Marotta C.A., Wilson J.T., Forget B.G., Weissman S.M. Human beta-globin messenger RNA. III. Nucleotide sequences derived from complementary DNA. J Biol Chem. 1977;252:5040–5053. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VidClip of left eye of a 34-year-old unaffected control. B, Individual OCT angiography (OCTA) 10 scans, taken sequentially 1 minute apart, formatted into video, showing a normal foveal avascular zone (FAZ) and parafoveal microvasculature. Red A labels the associated arteriole, and blue V’s label the associated venules. Box localizes the region of interest (ROI) imaged with adaptive optics scanning light ophthalmoscopy (AOSLO). C1, Confocal AOSLO imaging showed normal microvascular structure. C2, Nonconfocal AOSLO imaging showed more detail of vessel wall and blood cell structures compared with confocal AOSLO imaging. The AOSLO videos are composed of 30 frames and played at 16 frames per second.
VidClip of left eye of patient 1. B, OCTA 10 scans, formatted into video, revealed intermittent nonperfusion of a perivenular capillary segment at the temporal border of the FAZ. Red A’s label the associated arterioles, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. C1, Confocal AOSLO imaging showed poor blood flow in this capillary segment, followed by restoration of perfusion. C2, Nonconfocal AOSLO imaging revealed a torpedo-shaped single sickled red blood cell (RBC) and an RBC rouleau temporarily stuck in place within the capillary segment. With careful observation, individual stacked RBCs can be seen within the rouleau. The AOSLO videos are composed of 27 frames and are played at 16 frames per second.
VidClip of right eye of patient 2. B, OCTA 10 scans, formatted into video, revealed a poorly perfused barely visible perivenular capillary segment at the inferior border of the FAZ. Red A’s label the associated arterioles, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. C1, Confocal AOSLO imaging revealed poor blood flow in this capillary segment (yellow and red arrows). C2, Nonconfocal AOSLO imaging showed blood cell stasis (yellow arrow) and sluggish blood cell flow (red arrow) within the capillary segment. Blue V’s label the venule from (B). The AOSLO videos are composed of 30 frames and are played at 16 frames per second.
VidClip of left eye of patient 3. B, OCTA 10 scans, formatted into video, raised suspicion for the presence of a nonperfused capillary segment at the irregular superior FAZ border. Red A’s label the associated arterioles, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. C1, Confocal AOSLO imaging revealed a dilated capillary segment with multiple immobile reflective foci within the capillary. These were suggestive of blood cell aggregation and stasis (yellow arrow). C2, Nonconfocal AOSLO imaging confirmed the presence of compromised blood flow, revealing details of the thrombus containing static, sludged blood cells (yellow arrow). Also visible was a plasma-filled capillary segment with occasional tumbling RBCs spilling over from the nearby venule (red arrow); and, a sclerotic capillary segment (blue arrow). The AOSLO videos are composed of 20 frames and are played at 16 frames per second.
VidClip of right eye of patient 4, imaged 2 months apart without changes in disease management. C2, Nonconfocal AOSLO imaging at baseline revealed an RBC (yellow arrow) caught against the capillary wall, which partially blocked the entrance into the capillary segment at its bifurcation from its feeder arteriole. The partial blockage caused downstream blood cell flow to be sparse (red arrow). C4, Nonconfocal AOSLO imaging 2 months after initial presentation revealed a resolution of the partial blockage (yellow arrow), and restoration of perfusion (red arrow). Blue V labels the associated venule. Insets in C2 and C4 represent OCTA 10 scans, formatted into video, of the ROI. AOSLO videos are composed of 19 frames and are played at 16 frames per second.
VidClip of right eye of patient 5. B, OCTA 10 scans, formatted into video, suggested the presence of nonperfused capillaries near the nasal FAZ border. Red A labels the associated arteriole, and blue V labels the associated venule. Box represents ROI imaged with AOSLO. C1, Confocal AOSLO imaging revealed the presence of a capillary segment not visible on OCTA (black arrow). A reflective immobile intravascular mass was visible at the bifurcation of the upstream feeder capillary (yellow arrow). C2, Nonconfocal AOSLO vascular imaging confirmed the presence of a capillary segment and revealed that there were no blood cells flowing through it (black arrow). Blood cells with associated material forming a thrombus stuck to the capillary wall were visible at the bifurcation of the upstream feeder capillary (yellow arrow). Spillover and peristaltic backflow of blood cells into the nonperfused capillary segment from a downstream perfused capillary segment was visible (red arrow). In the parallel perfused capillary segment, a transient RBC rouleau was visible (blue arrow). Blue V’s label the venule from (B). The AOSLO videos are composed of 40 frames and played at 16 frames per second.
VidClip of right eye of patient 6 at baseline and 2 months after initiation of oral hydroxyurea therapy. B, OCTA 10 scans, formatted into video, revealed an intermittently nonperfused capillary segment near the superior-temporal border of the FAZ. Red A labels the associated arteriole, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. C, Nonconfocal AOSLO vascular imaging. C4, C5, Imaged approximately 5 minutes apart at baseline, documenting the formation of a thrombus of blood cells at a capillary bifurcation (yellow arrows), along with its growth and extension into the downstream capillary lumen. The other downstream capillary segment was the poorly perfused capillary seen on OCTA (red arrow). C6, After 2 months of oral hydroxyurea therapy, the thrombus previously seen at the capillary bifurcation was now fully resolved (yellow arrow), and blood cell flow through the downstream capillary segments now appeared restored (red arrow). The AOSLO videos are composed of 20 frames and are played at 16 frames per second.
VidClip of left eye of patient 7. B, OCTA 10 scans, formatted into video, showed the presence of intermittently nonperfused perivenular capillary segments near the inferior-temporal border of the FAZ. Red A’s label the associated arterioles, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. C1, Confocal AOSLO imaging revealed the formation of a cellular thrombus blocking perfusion through the capillary segment (yellow arrow). C2, Nonconfocal AOSLO imaging revealed the formation of the cellular thrombus (yellow arrow) in greater detail. An adjacent capillary segment also showed poor blood cell flow (red arrow). The AOSLO videos are composed of 40 frames and are played at 16 frames per second.
VidClip of right eye of patient 8. B, OCTA 10 scans show the presence of an intermittently nonperfused capillary segment near the superior temporal border of the FAZ. Red A labels the associated arteriole, and blue V labels the associated venule. Box localizes ROI imaged with AOSLO. Confocal (C1) and nonconfocal (C2) AOSLO imaging showed a thrombus (blue arrow) containing blood cells partially obstructing the capillary lumen and causing sparse blood cell flow through the downstream capillary segment. During this time period, blood cells bypassing the partial obstruction and flowing through the poorly perfused downstream capillary segment were seen (red and yellow arrows). The AOSLO videos are composed of 30 frames and are played at 16 frames per second.