Abstract
Psychological stress and physical activity contribute to blood pressure (BP) variability, which is a significant and independent risk factor for cardiovascular events. We examined the effects of physical activity level in the 5 min before each BP measurement and psychological stress on ambulatory BP and pulse rate variability in daily life. During a 24 h monitoring period, BP and pulse rate were measured by a multisensor ABPM device (TM-2441; A&D Co.) at 30 min intervals, and physical activity was continuously recorded by an actigraph built into the ABPM device. Psychological stress was assessed from negative emotions or worksite location in the participants’ situational information at each BP measurement, which was self-reported on a paper pad immediately (or as soon as possible) after the measurement. A total of 642 ABPM readings with corresponding situational information were obtained from 50 high-risk patients and showed that BP and pulse rate were significantly associated with actigraph-recorded physical activity (increase against the physical-activity-above-walking level: 4.2 ± 2.0 mmHg, p = 0.036 for SBP; 5.4 ± 1.1 bpm, p < 0.001 for pulse rate). When self-reported situational factors were additionally included in the analysis model as variables, negative emotions (7.4 ± 2.5 mmHg, p = 0.003 for SBP) and worksite location (5.8 ± 2.1 mmHg, p = 0.005 for SBP) were significantly associated with BP increase, while the association between BP and physical activity was weakened (p > 0.05). The pulse rate increased against the physical-activity-above-walking level but did not change for negative emotions. In conclusion, the effect of negative emotions on BP was greater than that of physical activity, whereas no similar effect on pulse rate was found. Simultaneous monitoring of BP, pulse rate, and actigraph-recorded physical activity could detect psychological stress-induced BP elevation.
Keywords: Ambulatory blood pressure, Physical activity, Emotional stress, Blood pressure variability
Introduction
Physical activity and psychological stress during daily life contribute to ambulatory blood pressure (BP) variability [1]. In 1987, Clark et al. reported that BP changes were associated with various daily activities [2]. Behaviors related to physical activity (e.g., walking) and activities under psychologically stressful situations (e.g., business meetings) were significantly associated with BP elevation. Schwartz et al. reported that body position and location at the time of measurement accounted for a substantial part of BP and pulse rate variability. As these results indicate, BP and pulse rate variability are related to daily activities [3]. Ambulatory BP, which can be measured by an ambulatory BP monitoring (ABPM) device and a recently developed wearable wrist watch-type oscillometric BP monitoring (WBPM) device, reflects physical and psychological stress during daily activity. Elevated daytime BP, monitored by ABPM with an upper arm cuff, has been reported to be a risk factor for cardiovascular disease [4, 5]. We recently demonstrated that ambulatory BP self-measured by WBPM significantly increased when measured under negative and stressful emotions [6, 7]. We also performed an analysis using a model including psychological stress and self-reported physical activity and found a tendency of BP elevation in response to self-reported physical activity (4.5 mmHg increase in SBP, p = 0.074) [6, 7]. In the same study, ABPM at 30 min intervals and continuous monitoring of physical activity by an actigraph built into the ABPM device were conducted simultaneously with WBPM. The present post hoc analysis using ABPM data and actigraph-recorded physical activity assessed the effects of fine-scale physical activity over 5 min before each BP measurement and psychological stress on ambulatory BP and pulse rate variability in daily life.
Subjects and methods
Study design
This study was conducted as a post hoc analysis of the data from a study comparing a recently developed WBPM device and a traditional ABPM device. Details of the comparison study design have been published previously [8]. Briefly, 50 adult outpatients who were scheduled to conduct ABPM in their clinical practice were consecutively recruited at Jichi Medical University Hospital and were asked to simultaneously wear the WBPM and an ABPM device on the same nondominant arm (Supplementary Fig. 1). The ABPM device was set to measure systolic BP (SBP), diastolic BP (DBP), and pulse rate every 30 min for 24 h. Fine-scale physical activity was continuously monitored by a high-sensitivity actigraph built into the ABPM device during the ambulatory BP monitoring period (i.e., 24 h in this study). Study participants were instructed to self-measure their BP with the WBPM device immediately after each automatic ABPM measurement ≥10 times while awake. In addition, participants were asked to keep a self-report diary describing their situation (i.e., location, emotion, body position, and self-reported intensity of physical activity) at the time of each WBPM measurement (Supplementary Fig. 2). The study protocol was approved by the institutional review board of Jichi Medical University School of Medicine (rin-B18-030) and was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000036689). All of the participants provided written informed consent to participate.
For the present analysis, we used ambulatory BP and fine-scale physical activity monitored by the ABPM device and investigated the influence of physical and psychological factors in daily life on ambulatory BP and pulse rate variability (Supplementary Fig. 1).
Multisensor ABPM
ABPM was performed at 30 min intervals for 24 h using a TM-2441 oscillometric device with a BP cuff worn on the upper arm (A&D Co., Tokyo) [9]. The main body of the device is worn around the waist and incorporates a high-sensitivity actigraph that can detect the fine-scale physical movements of the wearer in three directions, a thermometer, and a barometer. All BP readings, physical activity data, temperature data, and barometric pressure data are stored in the memory of the device.
Self-report diary at the time of BP measurement
The participants were provided a diary in which they were asked to answer questions about several situational conditions (the location, intensity of physical activity, behavior, emotional state, and body position) corresponding to each WBPM measurement at least 10 times while awake (Supplementary Fig. 2) [6]. The diary was a paper pad with the list of choices printed on it, as shown in Supplementary Fig. 2, and the participants self-reported the situation at the time of each BP measurement in the diary immediately after the measurement (or as soon as possible after the measurement, recalling the situation at the time of measurement).
Statistical analyses
All statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC). A mixed-effects model for repeated-measures analysis with a compound symmetry covariance structure was used to examine associations of stress-related factors with BP and pulse rate after adjusting for age, sex, and body mass index (BMI). Actigraph-recorded physical activity was calculated as the sum of the physical movement over the 5 min before each ABPM measurement (5 min of physical activity). Then, the 5 min of physical activity was divided into the following three categories based on the activity level: <200 G was considered a resting level; ≥200 and <1000 G was considered a light work level; and >1000 G was considered an above-walking level. Participants described their self-reported physical activity using a four-point scale (resting, mild [walking level], moderate [fast-walking level], or high [running level], Supplementary Fig. 2) at the time of each BP measurement. Since there was only one BP measurement corresponding to a high physical activity level, that measurement was combined with the moderate physical activity level into one category (moderate or high) and used in the analysis on a three-point scale. Values of p < 0.05 were considered significant.
Results
A total of 50 outpatients were included in this study. Their mean age was 66.1 ± 10.8 years, 60% were male, and the average BMI was 23.4 ± 4.8 kg/m2. The prevalence of regular alcohol use was 30%; current smoking, 4%; hypertension, 98%; antihypertensive medication use, 94%; history of atherosclerotic cardiovascular disease, 20%; and history of heart failure, 6%.
In total, 2217 readings from the ABPM device were obtained from the participants. Of those, 1580 readings were taken while awake (daytime measurements), and 642 of these readings had corresponding diary information. Details of the diary information of these 642 readings were described in the previous report (Supplementary Table 1) [6]. The self-reported emotional states corresponding to the 642 measures were happy (n = 92), calm (n = 510), anxious (n = 10), and nervous (n = 58) [some patients reported multiple items for single measurements]. We grouped these reports into two emotional states: positive (happy and/or calm; n = 575) and negative (anxious and/or tense; n = 67). The distribution of 5 min physical activity levels for the 642 measures with corresponding diary information was as follows: <200 G (n = 241), >200 G and <1000 G (n = 331), and >1000 G (n = 70) (Table 1). There was no significant difference between measurements corresponding to the diary reports (n = 642, used in the present study) and all daytime measurements (n = 1580) in the averages of ambulatory SBP, DBP, pulse rate, and physical activity (SBP: 129.6 mmHg vs. 129.1 mmHg, p = 0.597; DBP: 81.5 mmHg vs. 80.0 mmHg, p = 0.503; pulse rate: 73.4 bpm vs. 72.8 bpm, p = 0.355; physical activity 446.1 G vs. 423.0 G, p = 0.276). Of a total of 642 measurements, 94.4% were measured between 6:00 and 23:00. The number of measurements in each hour ranged from 24 to 44 times, with no time period having a particularly higher number of measurements than the others.
Table 1.
The effect of 5 min of physical activity and other situational factors on BP and pulse rate (n = 642 measurements)
| SBP, mmHg | DBP, mmHg | Pulse rate, bpm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient | 95% CI | P | Coefficient | 95% CI | P | Coefficient | 95% CI | P |
| Model I | |||||||||
| 5 min of physical activity, G | |||||||||
| −<200 G (rest), n = 241 | Ref. | Ref. | Ref. | ||||||
| −200-,<1000 G (light work level), n = 331 | 2.5 ± 1.2 | 0.1, 4.9 | 0.043 | 1.5 ± 0.8 | −0.1, 3.1 | 0.070 | 2.3 ± 0.7 | 0.9, 3.7 | 0.001 |
| −1000G- (above walking level), n = 70 | 4.2 ± 2.0 | 0.3, 8.1 | 0.036 | 1.3 ± 1.3 | −1.3, 3.9 | 0.329 | 5.4 ± 1.1 | 3.1, 7.6 | <0.001 |
| Model II (Model I + emotional state, location, and body position) | |||||||||
| Emotional state | |||||||||
| -Positive, n = 575 | Ref. | Ref. | Ref. | ||||||
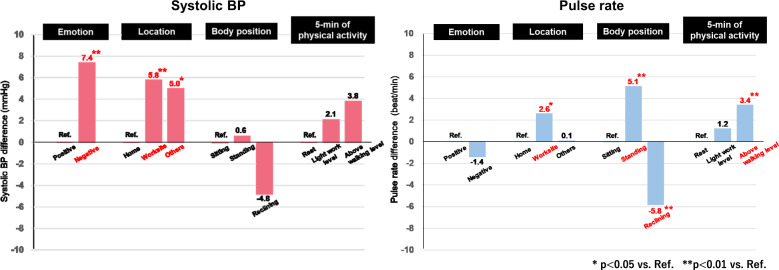
| -Negative, n = 67 | 7.4 ± 2.5 | 2.5, 12.3 | 0.003 | 5.1 ± 1.6 | 1.8, 8.3 | 0.002 | −1.4 ± 1.4 | −4.2, 1.4 | 0.324 |
| Location | |||||||||
| -Home, n = 473 | Ref. | Ref. | Ref. | ||||||
| -Worksite, n = 96 | 5.8 ± 2.1 | 1.7, 9.9 | 0.005 | 3.8 ± 1.4 | 1.1, 6.5 | 0.006 | 2.6 ± 1.2 | 0.3, 4.9 | 0.027 |
| -Others, n = 73 | 5.0 ± 2.0 | 1.1, 8.8 | 0.012 | 3.7 ± 1.3 | 1.2, 6.3 | 0.004 | 0.1 ± 1.1 | −2.1, 2.3 | 0.939 |
| Body position | |||||||||
| -Sitting, n = 500 | Ref. | Ref. | Ref. | ||||||
| -Standing, n = 115 | 0.6 ± 1.6 | −2.5, 3.7 | 0.704 | −0.02 ± 1.0 | −2.0, 2.0 | 0.982 | 5.1 ± 0.9 | 3.4, 6.8 | <0.001 |
| -Reclining, n = 26 | −4.8 ± 3.1 | −10.8, 1.2 | 0.114 | −2.2 ± 2.0 | −6.1, 1.8 | 0.283 | −5.8 ± 1.7 | −9.2, −2.4 | <0.001 |
| 5 min of physical activity, G | |||||||||
| -<200 G (rest), n = 241 | Ref. | Ref. | Ref. | ||||||
| −200-,<1000 G (light work level), n = 331 | 2.1 ± 1.2 | −0.3, 4.6 | 0.087 | 1.4 ± 0.8 | −0.2, 3.0 | 0.083 | 1.2 ± 0.7 | −0.2, 2.5 | 0.101 |
| −1000G- (above walking level), n = 70 | 3.8 ± 2.0 | −0.2, 7.7 | 0.064 | 1.2 ± 1.3 | −1.4, 3.9 | 0.352 | 3.4 ± 1.1 | 1.1, 5.6 | 0.004 |
Model I: Estimated changes were analyzed using a mixed-effect model adjusted for age, sex, BMI, and 5 min of physical activity
Model II: Estimated changes were analyzed using a mixed-effect model adjusted for age, sex, BMI, emotional state, location, body position, and 5 min of physical activity
BP indicates blood pressure, SBP systolic blood pressure, DBP diastolic blood pressure, BMI body mass index
In the analysis of the 642 measurements, the 5 min physical activity level was significantly associated with increases in BP and pulse rate (increase against the physical-activity-above-walking level: 4.2 ± 2.0 mmHg, p = 0.036 for SBP; 5.4 ± 1.1 bpm, p < 0.001 for pulse rate) (Table 1, Model I). When self-reported emotional state, location, and body position were additionally included in the analysis model as variables, negative emotions (7.4 ± 2.5 mmHg, p = 0.003 for SBP; 5.1 ± 1.6 mmHg, p = 0.002 for DBP) and worksite location (5.8 ± 2.1 mmHg, p = 0.005 for SBP; 3.8 ± 1.4 mmHg, p = 0.006 for DBP) were significantly associated with BP increase (Table 1, Model II). BP tended to increase in relation to the 5 min physical activity level, but the difference was not statistically significant. The pulse rate did not change for negative emotions but was significantly increased against the physical-activity-above-walking level. When self-reported behavior at the time of each BP measurement (18 behaviors selected in the diary, Supplementary Fig. 2) was additionally included in Model II, the results were similar to those from Model II.
In the same analysis using self-reported physical activity (three categories: rest, mild [walking level], and moderate or high [fast-walking and above level]) instead of 5 min of physical activity (n = 629 readings, after excluding 13 readings with missing self-reported physical activity), SBP was significantly associated with moderate or high self-reported physical activity level, while pulse rate was not (Supplementary Table 2). Similar results were observed when self-reported physical activity was divided into two categories (resting or active [including mild, moderate, and high physical activity level]) (Supplementary Table 3).
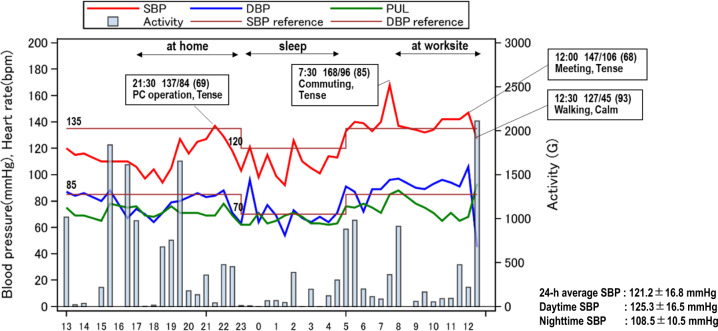
The Fig. 1 shows details for a representative case of a male in his 60 s with psychological stress-induced BP elevation detected by the multisensor ABPM device. BP and pulse rate variability essentially reflected physical activity in the 5 min before each measurement, but BP elevation not accompanied by higher physical activity was also observed at several time points when the patient felt tense (according to the diary information).
Fig. 1.
Time series of blood pressure, pulse rate, and 5 min physical activity: The case of a male in his 60s with psychological stress-induced blood pressure elevation detected by the multisensor ABPM. The reference values for SBP are 135 mmHg during a waking period and 120 mmHg during a sleeping period. The reference values for DBP are 85 mmHg during a waking period and 70 mmHg during a sleeping period. ABPM ambulatory blood pressure monitoring, SBP systolic blood pressure, DBP diastolic blood pressure
Discussion
This study demonstrated that psychological stress and physical activity over the 5 min prior to BP measurement contributed to ambulatory BP variability in daily life. The effect of psychological stress (such as negative emotions and worksite location) on BP was greater than that of physical activity. The pulse rate was influenced by 5 min of physical activity but not by negative emotions.
Effects of physical activity on BP and pulse rate
In an analysis on the effect of physical activity on BP and pulse rate, both self-reported and actigraph-recorded physical activity were significantly associated with BP elevation. Participants were asked to describe their self-reported physical activity using a four-point scale (resting, walking, fast-walking, or running level; Supplementary Fig. 2) “immediately before” each BP measurement. The time point of “immediately before” was somewhat subjective and varied slightly among participants. A previous study reported that the time period of activity that was most strongly related to the individual BP measurements was the 5 min period just before and 1 min during each BP measurement [10]. For pulse rate, the largest correlations were found with activity during the 1 min period before each measurement and during the 1 min measurement period itself. Therefore, quantification of physical activity over the 5 min before BP measurement is recommended to assess the effect of physical activity on BP.
BP reactivity in response to physical activity
An increase in BP in response to physical activity is a normal biological response, but the amplitude of BP elevation varies among individuals with different characteristics. Among healthy young individuals, a previous study showed that short-term BP variability immediately after exercise was suppressed in those who were physically trained (i.e., athletes) compared with untrained individuals without an exercise habit [11]. In a patient with a reduced left ventricular ejection fraction, BP reactivity was reported to be abnormally suppressed in response to physical activity [12]. In addition, even within the same individual, BP reactivity was found to be augmented by environmental factors [13]. Therefore, BP reactivity detected by simultaneous monitoring of BP and fine-scale physical activity (repeated monitoring in different seasons, if possible) might be useful to detect abnormal responses of BP to changes in physical activity.
Effects of psychological stress on BP and pulse rate
Consistent with the results of previous studies [1, 2, 10], a significant association between BP and physical activity was found in our study. However, when the effects of other situational factors were considered together, the amplitude of BP increase in response to physical activity was reduced, and increases during negative emotions and in out-of-home locations were greater than those against physical activity. Ambulatory BP during daytime daily activities was more impacted by psychological stress (as represented by negative emotions and worksite location) than by physical activity. Most of our study participants were hypertensive patients (98%) and were receiving antihypertensive treatment (94%). In our previous worksite study, in which BP at the worksite was measured using a conventional HBPM device, participants in the nonhypertension group had significantly higher worksite BP than morning BP, while this association was not observed in the participants in the hypertension group (including both treated and untreated patients). Given this result, it is possible that antihypertensive medications may have suppressed the increase in BP, but this hypothesis cannot be tested in the present study. Further studies should compare BP increase in response to psychological stress between healthy individuals or untreated hypertensive patients and treated hypertensive patients. A previous study of 477 healthy working adults found that weekly physical activity moderated the effect of stress on BP [14]. Although we examined the combined effect of psychological stress and physical activity on BP in the present study, the effect of regular physical activity (weekly average and exercise habits) on BP variability should be investigated in the future.
On the other hand, pulse rate exhibited no significant change during negative emotions. Consistent with previous results [3], pulse rate exhibited significant changes for worksite location and body position. “Negative emotions” and “worksite location” were considered psychological stress-related factors, but the response of the pulse rate to each was different. The physical activity level at the worksite might be relatively higher than that at home, and “worksite location” might reflect both physical activity and psychological stress. Although psychological stress-induced BP elevation could be detected by evaluating BP along with the patient’s situational information, collecting patients’ situational information using a paper pad questionnaire (employed in this study) is not convenient in clinical practice. Patients may find it troublesome to carry around the pad and record the information, and they may forget to fill them out. Using a smartphone app to collect information could reduce patient burden and missing information due to forgetfulness. Simultaneous monitoring of BP, pulse rate, and actigraph-recorded physical activity may help to obtain accurate and important information to identify pressor factors in individual patients without unduly burdening them.
Study limitations
There are several possible limitations to the present study. First, since this is a post hoc analysis of a study comparing ABPM and WBPM, less than half of the total set of daytime ABPM readings was available for analysis, including situational information. There was no significant difference in BP and physical activity levels between total daytime ABPM readings (n = 1580) and those used for the present analysis (n = 642). However, the present results using 642 data points do not reflect all the responses of daytime BP to psychological stress and physical activity. Second, we considered negative emotions (anxious and/or tense) and worksite location as psychological stressors. It would be desirable to quantitatively assess stress as well as physical activity, but no useful method has been established. However, assessing the degree of stress in addition to emotional state, as we have done in another study [7], could have allowed us to investigate the reactivity to various stresses. In the stress-filled era of the COVID-19 pandemic, a study of the impact of psychological stress, considering the intensity of stress on BP, is warranted.
Conclusions
BP and pulse rate variability were influenced by both physical activity in the 5 min before BP measurement and psychological stress. BP was more significantly increased by psychological stress, as represented by negative emotions and worksite location, than by physical activity, while pulse rate was not influenced by negative emotions. Our results suggest that simultaneous monitoring of BP, pulse rate, and actigraph-recorded physical activity can detect psychological stress-induced BP elevation and will facilitate personalized hypertension management that accounts for individual pressor factors in daily life.
Supplementary information
Compliance with ethical standards
Conflict of interest
KK has received research grants from Omron Healthcare, A&D, and Fukuda Denshi. NT was the recipient of a JSPS KAKENHI grant (no. 20K17127).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41440-022-01123-8.
References
- 1.Pickering TG, Schwartz JE, Stone A. Behavioral influences on diurnal blood pressure rhythms. Ann N. Y Acad Sci. 1996;783:132–40. doi: 10.1111/j.1749-6632.1996.tb26712.x. [DOI] [PubMed] [Google Scholar]
- 2.Clark LA, Denby L, Pregibon D, Harshfield GA, Pickering TG, Blank S, et al. A quantitative analysis of the effects of activity and time of day on the diurnal variations of blood pressure. J Chronic Dis. 1987;40:671–81. doi: 10.1016/0021-9681(87)90103-2. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz JE, Warren K, Pickering TG. Mood, location and physical position as predictors of ambulatory blood pressure and heart rate: application of a multi-level random effects model. Ann Behav Med. 1994;16:210–20. [Google Scholar]
- 4.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–83. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 5.Yano Y, Tanner RM, Sakhuja S, Jaeger BC, Booth JN, 3rd, Abdalla M, et al. Association of Daytime and Nighttime Blood Pressure With Cardiovascular Disease Events Among African American Individuals. JAMA Cardiol. 2019;4:910–7. doi: 10.1001/jamacardio.2019.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomitani N, Kanegae H, Suzuki Y, Kuwabara M, Kario K. Stress-Induced Blood Pressure Elevation Self-Measured by a Wearable Watch-Type Device. Am J Hypertens. 2021;34:377–82. doi: 10.1093/ajh/hpaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomitani N, Kanegae H, Kario K. Self-monitoring of psychological stress-induced blood pressure in daily life using a wearable watch-type oscillometric device in working individuals with hypertension. Hypertens Res. 2022;45:1531–37. [DOI] [PubMed]
- 8.Kario K, Shimbo D, Tomitani N, Kanegae H, Schwartz JE, Williams B. The first study comparing a wearable watch-type blood pressure monitor with a conventional ambulatory blood pressure monitor on in-office and out-of-office settings. J Clin Hypertens (Greenwich) 2020;22:135–41. doi: 10.1111/jch.13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kario K, Hoshide S, Saito K, Sato K, Hamasaki H, Suwa H, et al. Validation of the TM-2441 ambulatory blood pressure measurement device according to the ISO 81060-2: 2013 standard. Blood Press Monit. 2019;24:38–41. doi: 10.1097/MBP.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 10.Kario K, Schwartz JE, Pickering TG. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension. 1999;34:685–91. doi: 10.1161/01.HYP.34.4.685. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura K, Fujiwara T, Hoshide S, Ishiyama Y, Taki M, Ozawa S, et al. Differences in exercise-induced blood pressure changes between young trained and untrained individuals. J Clin Hypertens (Greenwich) 2021;23:843–8. doi: 10.1111/jch.14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narita K, Hoshide S, Kario K. Improvement of Actisensitivity After Ventricular Reverse Remodeling in Heart Failure: New ICT-Based Multisensor Ambulatory Blood Pressure Monitoring. Am J Hypertens. 2020;33:161–4. doi: 10.1093/ajh/hpz177. [DOI] [PubMed] [Google Scholar]
- 13.Kario K, Tomitani N, Kanegae H, Yasui N, Nishizawa M, Fujiwara T, et al. Development of a New ICT-Based Multisensor Blood Pressure Monitoring System for Use in Hemodynamic Biomarker-Initiated Anticipation Medicine for Cardiovascular Disease: The National IMPACT Program Project. Prog Cardiovasc Dis. 2017;60:435–49. doi: 10.1016/j.pcad.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Thomas MC, Kamarck TW, Li X, Erickson KI, Manuck SB. Physical activity moderates the effects of daily psychosocial stressors on ambulatory blood pressure. Health Psychol. 2019;38:925–35. doi: 10.1037/hea0000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.