Abstract
The polysaccharide fucoidin is a selectin blocker that inhibits leukocyte recruitment into the cerebrospinal fluid (CSF) during experimental pneumococcal meningitis. In the present study, the effect of fucoidin treatment on the release of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-8 into the CSF was investigated. Rabbits (n = 7) were treated intravenously with 10 mg of fucoidin/kg of body weight every second hour starting 4 h after intracisternal inoculation of ∼106 CFU of Streptococcus pneumoniae type 3 (untreated control group, n = 7). CSF samples were obtained every second hour during a 16-h study period. Treatment with fucoidin caused a consistent and significant decrease in CSF IL-1 levels (in picograms per milliliter) between 12 and 16 h (0 versus 170, 0 versus 526, and 60 versus 1,467, respectively; P < 0.02). A less consistent decrease in CSF TNF-α levels was observed in the fucoidin-treated group, but with no significant difference between the two groups (P > 0.05). In contrast, there was no attenuation in CSF IL-8 levels. Indeed, there was a significant increase in CSF IL-8 levels (in picograms per milliliter) in the fucoidin-treated group at 10 and 12 h (921 versus 574 and 1,397 versus 569, respectively; P < 0.09). In conclusion, our results suggest that blood-derived leukocytes mainly are responsible for the release of IL-1 and to some degree TNF-α into the CSF during pneumococcal meningitis, whereas IL-8 may be produced by local cells within the brain.
Bacterial meningitis is characterized by neutrophil accumulation in the meninges and the cerebrospinal fluid (CSF). The role of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) in bacterial meningitis has been studied extensively through the last decade (24). Intracisternal injection of endotoxins from gram-negative bacteria or cell wall components from gram-positive bacteria has previously been shown to induce a sequential release of TNF-α prior to the appearance of neutrophils in the CSF, though the timing of the IL-1 release is less clear (3, 13, 25). Moreover, intracisternal injection of these cytokines induces neutrophil pleocytosis (18, 19, 21, 23), whereas monoclonal antibodies to TNF-α and IL-1 introduced intracisternally inhibit neutrophil pleocytosis in experimental meningitis (19, 21), indicating an essential role of these cytokines in the generation of neutrophil pleocytosis. On the other hand, recent studies show that blood-derived leukocytes are an important source of TNF-α and IL-1 production in pneumococcal meningitis and therefore, to some degree, suggest that these cytokines more likely are the result rather than the cause of the pleocytosis (2, 26). Thus, several aspects of the role of TNF-α and IL-1 in bacterial meningitis remain to be defined.
IL-8 is a CXC chemokine and one of the most potent inducers of neutrophil chemotaxis and activation in vitro and in vivo (9) and, therefore, a possible candidate in the generation of neutrophil pleocytosis. Patients with bacterial meningitis have highly elevated CSF IL-8 levels, and this may be useful in the differential diagnosis between bacterial and viral meningitis (16). Only one study has previously been published addressing the role of IL-8 in experimental meningitis (15). In that study, low IL-8 levels in the CSF were associated with diminished neutrophil pleocytosis.
The polysaccharide fucoidin is a selectin blocker that inhibits leukocyte rolling and subsequent leukocyte transendothelial migration. Treatment with fucoidin intravenously has previously been found to attenuate the pleocytosis in experimental pneumococcal meningitis (1, 8). Thus, it would be likely that a diminished pleocytosis would cause an attenuation in levels of cytokines primarily produced by blood-derived leukocytes in the CSF, whereas this should not be the case for cytokines primarily produced by cells within the central nervous system.
The aim of the present study was to investigate whether a blockage of leukocyte recruitment into the CSF would influence the release of the proinflammatory cytokines TNF-α, IL-1, and IL-8 into the CSF during experimental pneumococcal meningitis.
MATERIALS AND METHODS
Bacterial strain.
The bacterial strain used in the present study was a Streptococcus pneumoniae type 3 strain (68034; Statens Serum Institut [SSI], Copenhagen, Denmark). After several passages in rabbit CSF, the organism was cultured on 5% blood agar plates (SSI), suspended in sterile beef broth, and kept at −80°C. For experiments, the frozen organism was thawed and grown on 5% blood agar plates for 24 h, and the colonies were suspended in sterile beef broth to an optical density of 0.30 at 540 nm and incubated for 1 h. The test organism was diluted in sterile beef broth to a final concentration of ∼5 × 106 CFU/ml, as confirmed by quantitative cultures, and 0.2 ml was used for intracisternal inoculation.
Experimental meningitis model.
The experimental protocols were approved by the Danish animal experiment inspectorate. A well-established meningitis model (15, 17) originally described by Dacey and Sande (4) was used. New Zealand White rabbits weighing approximately 2.5 kg were anesthetized subcutaneously with 0.5 mg of midazolam (Dormicum; F. Hoffmann-La Roche AG, Basel, Switzerland)/kg of body weight and intramuscularly with 0.35 ml of Hypnorm/kg, and a dental acrylic helmet containing a half turnbuckle was attached to the skull. The next day the rabbits were reanesthetized subcutaneously with 1.75 g of ethyl carbamate (Urethane; Fluka Kemi AG, Buchs, Switzerland)/kg and 10 mg of pentobarbital (Nykomed, Roskilde, Denmark)/kg and immobilized in a stereotaxic frame. A 25-gauge spinal needle was introduced into the cisterna magna for the inoculation of 0.2 ml of bacterial suspension and repetitive CSF sampling every second hour during a 16-h study period. The rate of removal of CSF did not exceed the rate of CSF formation, which was approximately 0.4 ml/h (22). Blood samples were taken from a central ear artery. After testing for bacterial concentration and white blood cell (WBC) count, CSF and blood samples were centrifuged and supernatants were immediately stored at −80°C for subsequent analysis.
Fucoidin treatment.
Fucoidin (F-5631, lot 56 H 3799; Sigma Chemical Co., St. Louis, Mo.) was dissolved in pyrogen-free phosphate-buffered saline (SSI) to a final concentration of 10 mg/ml and was passed through a 0.2-μm-pore-size sterile filter (Millipore, Bedford, Mass.). Because unexpected lethargic septic shock developed after five or fewer bolus injections of ∼2.5 ml of fucoidin/2 h in initial experiments, the endotoxin content in the fucoidin solution was measured by a Limulus assay and found to be as high as ∼200 ng/ml. Therefore, subsequent removal of endotoxin by adsorption to a polymyxin B-Sepharose column was performed, as previously described (12). The final content of endotoxin in the fucoidin solution after column passage was ∼16 ng/ml, which caused no symptoms of septic shock in the fucoidin-treated rabbits.
Animals (n = 7) were treated with 10 mg of fucoidin/kg, prepared as described above, every second hour starting 4 h after bacterial inoculation. This dose has previously been shown to attenuate pleocytosis in experimental pneumococcal meningitis (8). Two fucoidin-treated rabbits were injected intracisternally with 0.2 ml of pyrogen-free NaCl and served as uninfected controls. The number of infected rabbits in the untreated control group was 7.
CSF analysis. (i) Bacterial concentration.
Bacterial titers in CSF and blood were determined by plating undiluted samples and 10-fold serial dilutions on 5% blood agar plates (SSI). The lowest detectable bacterial concentration was 50 CFU/ml.
(ii) WBC, glucose, lactate, and protein levels.
WBC and differential counts were determined on an automatic cell counter (Swelab, Årsta, Sweden). The lowest detectable WBC count was 100 × 103 cells/ml. Lactate and glucose contents were measured with commercial kits (Sigma). Protein content was determined as described by Lowry et al. (10).
(iii) TNF-α.
CSF TNF-α levels were measured by bioassay as previously described (15). Briefly, 5 × 104 cells of WEHI 164, subclone 13 (kindly provided by T. Espevik), per well in RPMI 1640 (SSI) with 0.25 μg of actinomycin D (Sigma)/ml were incubated with a standard (recombinant human TNF-α; Genzyme Diagnostics, Cambridge, Mass.) or 10-μl samples in triplicate on microtiter plates (Nunc, Roskilde, Denmark) for 18 to 24 h in ambient air at 37°C. Thiazolyl blue (1 mg/ml; MTT; Sigma) was used to indicate cell kill. Twenty percent (wt/vol) sodium dodecyl sulfate (Sigma) was dissolved in 50% (vol/vol) dimethylformamide (Sigma) and in water and used as a lysing buffer, and the optical density was measured on an enzyme-linked immunosorbent assay (ELISA) reader (Bio-Tec, Vinooski, Vt.) at 570 nm. The coefficient of variation was less than 20%. The lowest detection level was 50 pg/ml. The addition of goat anti-rabbit TNF-α (Research Diagnostics Inc., Flanders, N.J.) completely neutralized the cytotoxic activity in CSF samples.
(iv) IL-1.
CSF IL-1 levels were measured in a two-step assay, as previously described (15). In the first step, cells of the murine T-cell line EL-4, subclone NOB 1 (2 × 105/well; kindly provided by M. Svendsen, Copenhagen, Denmark), which produces IL-2 upon exposure to IL-1 in RPMI 1640 medium, were incubated with a standard (recombinant human IL-1β; Genzyme) or 10-μl CSF samples in triplicate on microtiter plates (Nunc) for 18 to 24 h in ambient air at 37°C. In the second step, 100 μl of each of the supernatants was carefully collected for subsequent analysis of IL-2 content with a commercial mouse IL-2 ELISA kit (Genzyme), essentially as described by the manufacturer. The coefficient of variation was less than 20%. The lowest detection level was 31 pg/ml. The addition of goat anti-rabbit IL-1β (Research Diagnostics Inc.) inhibited, although not completely, the IL-1 activity in CSF samples.
(v) IL-8.
CSF IL-8 levels were measured with a rabbit-specific ELISA as previously described (15). Briefly, microtiter plates (Nunc) were coated overnight with WS-4 (kindly provided by K. Matsushima, Tokyo, Japan), a mouse monoclonal antibody to human and rabbit IL-8, at 1.5 μg/ml in 0.05 M carbonate buffer (pH 9.6) at 4°C. After the unbound sites were blocked, the standard (rabbit IL-8, kindly provided by K. Matsushima) or samples diluted in the assay buffer (1% [wt/vol] bovine serum albumin in phosphate-buffered saline containing 0.05% Tween 20 [Sigma]) were added in duplicate and incubated overnight at 4°C. Goat anti-human IL-8 immunoglobulin G (R&D Systems Europe Ltd., Abingdon, United Kingdom) at 1 μg/ml was added as the detection antibody and incubated at 37°C for 2 h. The specificity of goat anti-human IL-8 for rabbit IL-8 has previously been tested by Western blot analysis and found to be similar to that of guinea pig anti-rabbit IL-8 (unpublished observations). In the next step, horseradish peroxidase-conjugated rabbit and anti-goat immunoglobulin G (R&D Systems) diluted 1:3,000 was added and incubated for an additional 1 h at 37°C. o-Phenylenediamine (Kem-En-Tec, Copenhagen, Denmark) was added and allowed to react for 30 min at room temperature. The enzyme reaction was stopped by the addition of 100 μl of 4.5 N H2SO4, and the optical density at 490 nm was measured with the ELISA reader. The lowest detection level was 20 pg/ml. The coefficient of variation was less than 10%.
Statistical analysis.
All results are provided as medians and 25/75 percentiles. Comparison of groups was performed by the nonparametric Mann-Whitney U test. If a significant difference was detected at only one time point, the result was corrected for multiple comparison with the Bonferroni coefficient. A P value of <0.05 was considered significant.
RESULTS
Effect of fucoidin treatment on CSF WBCs.
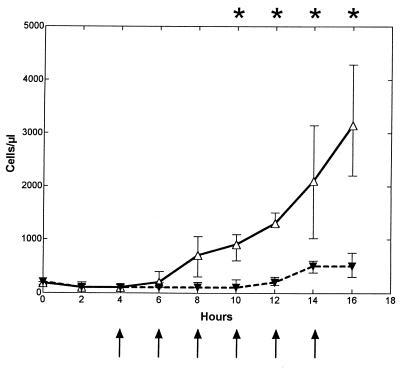
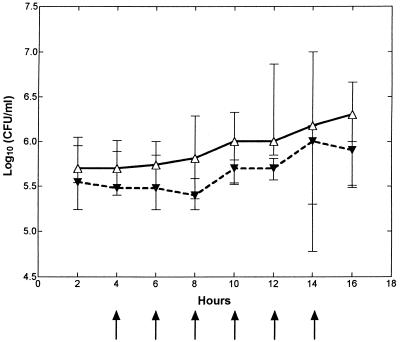
CSF WBCs (per microliter) started to increase at ∼8 h (6 to 10 h) after the bacterial inoculation and continued to increase throughout the study period in the untreated control group (n = 7) (Fig. 1), whereas this increase was inhibited in the fucoidin-treated group (n = 7), with a significant difference between the two groups between 10 and 16 h (900 [600 to 1,089]) versus 100 [100 to 250], 1,300 [1,250 to 1,500] versus 200 [150 to 300], 2,100 [1,020 to 3,150] versus 500 [382 to 600], and 3,150 [2,200 to 4,289] versus 500 [300 to 750], respectively; P < 0.005). At the end of the study period (at 14 and 16 h), there was a minor elevation in CSF WBCs in the fucoidin-treated group. No difference in the differential count was observed between the two groups, with a predominance of neutrophils throughout the 16-h study period (data not shown). Fucoidin treatment (n = 2) caused no pleocytosis in uninfected rabbits (data not shown).
FIG. 1.
Median (25/75 percentiles) CSF WBC levels in fucoidin-treated and untreated rabbits during experimental pneumococcal meningitis. Fucoidin treatment (10 mg/kg) was administered every second hour (arrows) starting 4 h after intracisternal bacterial challenge (at 0 h). The CSF WBC level was significantly attenuated in the fucoidin-treated group (closed symbols and dashed line; n = 7) compared to the untreated control group (open symbols and solid line; n = 7) between 10 and 16 h. ∗, P < 0.05 (Mann-Whitney U test).
Effect of fucoidin treatment on CSF cytokine levels.
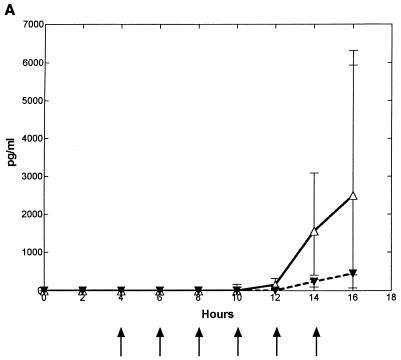
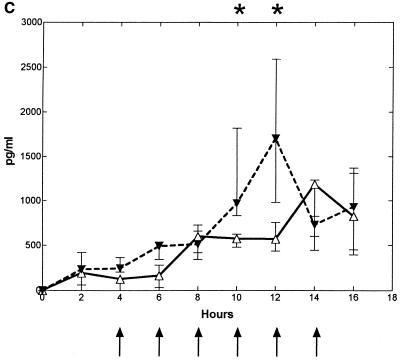
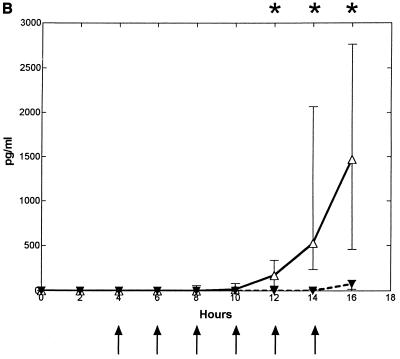
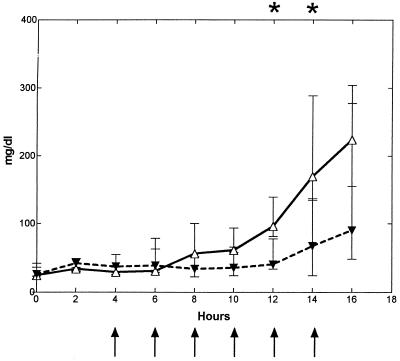
Intracisternal challenge with pneumococci resulted in elevated levels of TNF-α, IL-1, and IL-8 in the CSF in the untreated control group (Fig. 2). The CSF IL-8 levels started to increase ∼7 h (6 to 8 h) after the bacterial inoculation, which was ∼0 to 2 h before the initial increase in CSF WBCs. This was in contrast to the elevation of TNF-α and IL-1, which started to increase ∼12 (10 to 14 h) and ∼10 h (8 to 12 h) after inoculation, respectively, which was ∼2 to 4 h after the initial increase in CSF WBCs.
FIG. 2.
Median (25/75 percentiles) CSF cytokine kinetics in fucoidin-treated and untreated rabbits during experimental pneumococcal meningitis. Symbols are as described for Fig. 1. Fucoidin treatment (10 mg/kg; n = 7) was administered every second hour starting 4 h after intracisternal bacterial challenge (at 0 h). There was no significant difference in CSF TNF-α (A) levels between the two groups. CSF IL-1 (B) levels were significantly attenuated between 12 and 16 h, whereas CSF IL-8 (C) levels were significantly enhanced in the fucoidin-treated group at 10 and 12 h compared to those in the untreated control group (n = 7).
Treatment with fucoidin caused a consistent and significant decrease in CSF IL-1 levels (in picograms per milliliter) at 12 to 16 h after the bacterial inoculation compared to the untreated control group (0 [0] versus 170 [53 to 337], 0 [0 to 18] versus 526 [233 to 2,065], and 60 [17 to 114] versus 1,467 [456 to 2,765], respectively; P < 0.02). Less consistent was the decrease in CSF TNF-α levels (in picograms per milliliter) in the fucoidin-treated group compared to the untreated control group, with a significant difference between the two groups only at the 14-h point (1,556 [404 to 3,092] versus 229 [97 to 290]; P = 0.035, but corrected with the Bonferroni coefficient of 3, P > 0.05). In contrast to this, no attenuation was observed in CSF IL-8 levels. Indeed, a significant increase in CSF IL-8 levels (in picograms per milliliter) was observed in the fucoidin-treated group compared to the untreated control group after 10 and 12 h (970 [833 to 1,820] versus 574 [479 to 625] and 1,703 [984 to 2,591] versus 569 [438 to 757], respectively; P < 0.009). Fucoidin treatment caused no detectable CSF cytokine production in uninfected rabbits (data not shown).
Bacterial concentration in the CSF and blood.
No significant difference in the bacterial concentrations in the CSF (Fig. 3) and in the blood (data not shown) was observed between the two groups during the 16-h study period.
FIG. 3.
Median (25/75 percentiles) CSF bacterial concentration in fucoidin-treated and untreated rabbits during experimental pneumococcal meningitis. Symbols are as described for Fig. 1. Fucoidin treatment (10 mg/kg; n = 7) was administered every second hour starting 4 h after intracisternal bacterial challenge (at 0 h). There was no significant difference in bacterial concentration between the fucoidin-treated group and the untreated control group (n = 7).
Indices of blood-brain barrier damage.
The CSF protein concentration gradually increased from ∼8 h after the bacterial inoculation and throughout the study period in the untreated control group (Fig. 4). No increase in CSF protein concentration (in milligrams per deciliter) was detected in the fucoidin-treated group, and there was a significant difference at 12 and 14 h between the two groups (40.0 [33.6 to 77.6] versus 96.0 [80.8 to 139.2] and 66.6 [minimum, 24.0; maximum, 137.6] versus 169.6 [134.4 to 288.8], respectively; P < 0.05). No difference in levels of glucose and lactate in the CSF was observed between the two groups during the study period (data not shown).
FIG. 4.
Median (25/75 percentiles) CSF protein levels in fucoidin-treated and untreated rabbits during experimental pneumococcal meningitis. Symbols are as described for Fig. 1. Fucoidin treatment (10 mg/kg) was administered every second hour starting 4 h after intracisternal bacterial challenge (at 0 h). The CSF protein levels were significantly reduced in the fucoidin-treated group (n = 7) compared to the untreated control group (n = 7) at 12 and 14 h.
DISCUSSION
In the present study, we found that the release of IL-8 into CSF starts to increase prior to the initiation of pleocytosis, indicating that local cells within the brain are able to produce IL-8, whereas the release of TNF-α and IL-1 starts to increase after the pleocytosis has already started. Several other studies have measured CSF TNF-α and IL-1 levels in pneumococcal meningitis (2, 3, 5, 6, 14, 26). To our knowledge, only two of these have measured CSF cytokine kinetics in samples taken every second to fourth hour, as in our study. In a study by Friedland et al. in which live pneumococci (inoculum size, ∼3 × 103 CFU) were intracisternally injected, TNF-α started to increase at the same time as the start of the pleocytosis (6), whereas in a study by Burroughs et al. using heat-killed pneumococci (inoculum size, ∼107 CFU) as the pathogen, TNF-α but not IL-1 preceded the pleocytosis (3). We have further explored the discrepancy between these two studies and found that intracisternal injection of high concentrations of living or heat-killed pneumococci (∼1 × 108 CFU) resulted in elevated TNF-α (but not IL-1) levels prior to the pleocytosis, whereas intracisternal injections of live pneumococci at lower concentrations (≤1 × 106 CFU) resulted in no elevation in TNF-α levels prior to the pleocytosis (unpublished data).
In further support of the proposition that IL-8 may be produced by local cells within the brain during pneumococcal meningitis, we found that blocking of leukocyte entry into the brain by fucoidin—most importantly—caused no attenuation in CSF IL-8 levels. This is in accordance with findings about lipopolysaccharide-induced arthritis, which showed that synovial IL-8 levels were not affected by a depletion of neutrophils (11). Besides this, and maybe of minor importance, there was even a small increase in CSF IL-8 levels in fucoidin-treated rabbits, but whether such an increase is part of a higher local chemotactic signal trying to induce pleocytosis remains to be clarified. However, we have excluded three other theoretical explanations.
(i) We found no difference in bacterial concentration between the fucoidin-treated group and the untreated control group. Thus, a reduced pleocytosis causes no increase in the bacterial load and therefore no higher antigenic stimulation of CSF IL-8 production.
(ii) Fucoidin treatment itself caused no release of IL-8 into the CSF: intravenous injections of fucoidin were not able to induce IL-8 production in the CSF of uninfected rabbits.
(iii) Fucoidin treatment caused no increase in blood-brain barrier breakdown and therefore no higher diffusion of systematically produced IL-8 into the CSF, since CSF protein levels, indeed, were lower in the fucoidin-treated group.
In this study, we found a consistent decrease in CSF IL-1 levels, but no significant decrease in TNF-α levels, when leukocyte appearance in the CSF was inhibited by fucoidin, suggesting that IL-1 is produced by blood-derived leukocytes. A recent study by Granert et al. also found decreased IL-1 levels in the CSF during fucoidin treatment at 6 h but not at 12 h after inoculation of heat-killed pneumococci (7). Moreover, CSF TNF-α levels were depressed at 12 h but not at 6 h. Furthermore, no significant difference in TNF-α and IL-1 levels between fucoidin-treated rabbits and untreated rabbits was detected late in the course of antibiotic-treated pneumococcal meningitis. We do not believe that the two studies are comparable, because of differences in the study design. (i) We used live pneumococci, whereas Granert et al. used heat-killed pneumococci, which may cause different cytokine kinetics (please see above). (ii) Granert et al. gave ampicillin together with fucoidin late in the course of pneumococcal meningitis. (iii) Very few time points were included in that study compared to ours. (iv) The measured TNF-α and IL-1 levels in the study by Granert et al. were extremely low: 184 to 1,010 and 2 to 1,000 pg/liter, respectively, which was much lower than our results and even lower than the lower detection limits for the assays in this study (TNF-α, 15,000 pg/liter; IL-1, 8,000 pg/liter). In accordance with our study, Zysk et al. found decreased levels of IL-1, but no decrease in TNF-α levels, in CSF when blood monocytes were depleted from the circulation and no pleocytosis was observed (26). Moreover, Sáez-Llorens et al. found no decrease in CSF TNF-α levels when the recruitment of leukocytes was inhibited by treatment with a monoclonal antibody to CD11 (20). Thus, blood-derived leukocytes are likely an important source of IL-1 production, whereas the cellular source of TNF-α is less clear.
In conclusion, fucoidin treatment inhibits the release of IL-1 and to some degree TNF-α into the CSF, whereas it enhances the release of IL-8. This indicates that IL-1 is produced by blood-derived leukocytes, whereas IL-8 is produced by local cells within the central nervous system. Further studies are required to investigate the cellular source of the proinflammatory cytokine production in bacterial meningitis, as well as the functions of the cytokines.
ACKNOWLEDGMENTS
This work was supported in part by the læge Sofus Carl Emil Friis'-, Ebbe Celinder's, Ydes', and købmand Svend Hansen's Foundations.
We thank K. Matsushima for providing rabbit IL-8 and WS-4 for use in the IL-8 ELISA. We also thank Bo Sønder Hansen and Jacob Vang for skillful technical assistance.
REFERENCES
- 1.Angstwurm K, Weber J R, Segert A, Burger W, Weih M, Freyer D, Einhaupl K M, Dirnagl U. Fucoidin, a polysaccharide inhibiting leukocyte rolling, attenuates inflammatory responses in experimental pneumococcal meningitis in rats. Neurosci Lett. 1995;191:1–4. doi: 10.1016/0304-3940(95)11541-4. [DOI] [PubMed] [Google Scholar]
- 2.Bitsch A, Trostdorf F, Bruck W, Schmidt H, Fischer F R, Nau R. Central nervous system TNF alpha-mRNA expression during rabbit experimental pneumococcal meningitis. Neurosci Lett. 1997;237:105–108. doi: 10.1016/s0304-3940(97)00830-6. [DOI] [PubMed] [Google Scholar]
- 3.Burroughs M H, Tsenova-Berkova L, Sokol K, Ossig J, Tuomanen E, Kaplan G. Effect of thalidomide on the inflammatory response in cerebrospinal fluid in experimental bacterial meningitis. Microb Pathog. 1995;19:245–255. doi: 10.1016/s0882-4010(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 4.Dacey R G, Sande M A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974;6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelhard D, Pomeranz S, Gallily R, Strauss N, Tuomanen E. Serotype-related differences in inflammatory response to Streptococcus pneumoniae in experimental meningitis. J Infect Dis. 1997;175:979–982. doi: 10.1086/514005. [DOI] [PubMed] [Google Scholar]
- 6.Friedland I R, Paris M M, Hickey S, Shelton S, Olsen K, Paton J C, McCracken G H. The limited role of pneumolysin in the pathogenesis of pneumococcal meningitis. J Infect Dis. 1995;172:805–809. doi: 10.1093/infdis/172.3.805. [DOI] [PubMed] [Google Scholar]
- 7.Granert C, Raud J, Waage A, Lindquist L. Effects of polysaccharide fucoidin on cerebrospinal fluid interleukin-1 and tumor necrosis factor alpha in pneumococcal meningitis in the rabbit. Infect Immun. 1999;67:2071–2074. doi: 10.1128/iai.67.5.2071-2074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granert C, Raud J, Xie X, Lindquist L, Lindbom L. Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J Clin Investig. 1994;93:929–936. doi: 10.1172/JCI117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 10.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 11.Matsukawa A, Yoshinaga M. Sequential generation of cytokines during the initiative phase of inflammation, with reference to neutrophils. Inflamm Res. 1998;47:S137–S144. doi: 10.1007/s000110050304. [DOI] [PubMed] [Google Scholar]
- 12.Mølvig J, Baek L. Removal of endotoxin from culture media by a polymyxin B sepharose column. The activity of contaminating endotoxin in culture media measured by the interleukin 1 inducing effect on human monocyte cultures and by the Limulus test. Scand J Immunol. 1987;26:611–619. doi: 10.1111/j.1365-3083.1987.tb02296.x. [DOI] [PubMed] [Google Scholar]
- 13.Mustafa M M, Ramilo O, Olsen K D, Franklin P S, Hansen E J, Beutler B, McCracken G H. Tumor necrosis factor in mediating experimental Haemophilus influenzae type B meningitis. J Clin Investig. 1989;84:1253–1259. doi: 10.1172/JCI114292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nau R, Zysk G, Schmidt H, Fischer F R, Stringaris A K, Stuertz K, Bruck W. Trovafloxacin delays the antibiotic-induced inflammatory response in experimental pneumococcal meningitis. J Antimicrob Chemother. 1997;39:781–788. doi: 10.1093/jac/39.6.781. [DOI] [PubMed] [Google Scholar]
- 15.Østergaard C, Benfield T, Gesser B, Kharazmi A, Frimodt-Møller N, Espersen F, Lundgren J D. Pretreatment with granulocyte colony-stimulating factor attenuates the inflammatory response but not the bacterial load in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect Immun. 1999;67:3430–3436. doi: 10.1128/iai.67.7.3430-3436.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Østergaard C, Benfield T L, Sellebjerg F, Kronborg G, Lohse N, Lundgren J D. Interleukin-8 in cerebrospinal fluid from patients with septic and aseptic meningitis. Eur J Clin Microbiol Infect Dis. 1996;15:166–169. doi: 10.1007/BF01591492. [DOI] [PubMed] [Google Scholar]
- 17.Østergaard C, Sørensen T K, Knudsen J D, Frimodt-Møller N. Evaluation of moxifloxacin, a new 8-methoxyquinolone, for treatment of meningitis caused by a penicillin-resistant pneumococcus in rabbits. Antimicrob Agents Chemother. 1998;42:1706–1712. doi: 10.1128/aac.42.7.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quagliarello V J, Wispelwey B, Long W J, Jr, Scheld W M. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat. Characterization and comparison with tumor necrosis factor. J Clin Investig. 1991;87:1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramilo O, Saez-Llorens X, Mertsola J, Jafari H, Olsen K D, Hansen E J, Yoshinaga M, Ohkawara S, Nariuchi H, McCracken G H., Jr Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J Exp Med. 1990;172:497–507. doi: 10.1084/jem.172.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sáez-Llorens X, Jafari H S, Severien C, Parras F, Olsen K D, Hansen E J, Singer I I, McCracken G H. Enhanced attenuation of meningeal inflammation and brain edema by concomitant administration of anti-CD18 monoclonal antibodies and dexamethasone in experimental Haemophilus meningitis. J Clin Investig. 1991;88:2003–2011. doi: 10.1172/JCI115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector R, Lorenzo A V. Inhibition of penicillin transport from the cerebrospinal fluid after intracisternal inoculation of bacteria. J Clin Investig. 1974;54:316–325. doi: 10.1172/JCI107767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang T, Frenette P S, Hynes R O, Wagner D D, Mayadas T N. Cytokine-induced meningitis is dramatically attenuated in mice deficient in endothelial selectins. J Clin Investig. 1996;97:2485–2490. doi: 10.1172/JCI118695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tauber M G, Moser B. Cytokines and chemokines in meningeal inflammation: biology and clinical implications. Clin Infect Dis. 1999;28:1–12. doi: 10.1086/515079. [DOI] [PubMed] [Google Scholar]
- 25.Waage A, Halstensen A, Shalby R, Brandtzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor-alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zysk G, Brück W, Huitinga I, Fischer F R, Flachsbarth F, van Rooijen N, Nau R. Elimination of blood-derived macrophages inhibits the release of interleukin-1 and the entry of leukocytes into the cerebrospinal fluid in experimental pneumococcal meningitis. J Neuroimmunol. 1997;73:77–80. doi: 10.1016/s0165-5728(96)00173-7. [DOI] [PubMed] [Google Scholar]