Abstract
Objective
The objective of the study was to reveal the presence of cellular interplay through extracellular vesicle (EV) microRNAs (miRs), to dampen the vicious cycle to degenerate human corneal endothelium (HCE) tissues.
Design
Prospective, comparative, observational study.
Methods
The miR levels in neonate-derived corneal tissues, in the aqueous humor (AqH) of bullous keratoplasty and cataract patients, as well as in the culture supernatant (CS) and EV of cultured human corneal endothelial cells (hCECs), were determined using 3D-Gene human miR chips and then validated using the real-time polymerase chain reaction. The extracellularly released miRs were profiled after the forced downregulation of cellular miR-34a, either by an miR-34a inhibitor or exposure to H2O2. The senescence-associated secretory phenotypes and mitochondrial membrane potential (MMP) were assessed to determine the functional features of the released miRs.
Main Outcome Measures
Identification of functional miRs attenuating HCE degeneration.
Results
The miRs in AqH were classified into 2 groups: expression in 1 group was significantly reduced in neonate-derived tissues, whereas that in the other group remained almost constant, independent of aging. The miR-34a and -29 families were typical in the former group, whereas miR-184 and -24-3p were typical in the latter. Additionally, a larger amount of the latter miRs was detected in AqH compared with those of the former miRs. There was also a greater abundance of miR-184 and -24-3p in hCECs, EV, and CS in fully mature CD44−/dull hCEC, leading to sufficient clinical tissue regenerative capacity in cell injection therapy. The repression of cellular miR-34a, either due to miR-34a inhibitors or exposure to oxidative stress, unexpectedly resulted in the elevated release of miR-184 and -24-3p. Secretions of VEGF, interleukin 6, monocyte chemotactic protein-1, and MMP were all repressed in both mature CD44−/dull and degenerated CD44+++ hCEC, transfected with an miR-184 mimic.
Conclusions
The elevated release of miR-184 into AqH may constitute cellular interplay that prevents the aggravation of HCE degeneration induced by oxidative stress, thereby sustaining tissue homeostasis in HCE.
Keywords: Corneal endothelium degeneration, Extracellular vesicle, MiR-184, Mitochondria metabolic homeostasis, Oxidative stress
Abbreviations and Acronyms: AqH, aqueous humor; AQP-1, aquaporin 1; CS, culture supernatant; ECD, endothelial cell density; ER, endoplasmic reticulum; EV, extracellular vesicle; HCE, human corneal endothelium; hCEC, cultured human corneal endothelial cell; IL-6, interleukin 6; MCP-1, monocyte chemotactic protein-1; miR, microRNA; MMP, mitochondrial membrane potential; SASP, senescence-associated secretory phenotype; SLC4A11, solute carrier family 4 member 11; SP, subpopulation
In our recent publication, we revealed the mechanisms underlying the initiation of human corneal endothelium (HCE) failure, namely the functional disparity between degenerated and nondegenerated cultured human corneal endothelial cell (hCEC) at the early stage of cell-fate decision.1 We confirmed that p53-inducible cellular miR-34a downregulates CD44 expression, in a concerted manner with repressed c-Myc, and that the miR-34a/CD44 axis regulates downstream mitochondrial bioenergetics.1,2 Upon exposure to extracellular oxidative stress, the hCECs showed a reduction in cellular miR-34a expression and impaired mitochondrial homeostasis. However, how this cell degeneration in the HCE will be either aggravated to a larger proportion of HCE tissues or the disposition will be coped with the feedback loop to attenuate the further spreading of the degeneration to neighboring tissues has not been answered.
Because of the stability of miRNAs (miRs) in body fluids such as blood and aqueous humor (AqH), as well as in cells and tissues,3, 4, 5 miRs are potential biomarkers for the diagnosis of tissue disorders. We recently reported that hCECs sharing a CD44−/dull mature differentiated phenotype can be discriminated by measuring either the amounts of cellular miRs or those in extracellular vesicles (EVs) released from them.2,6 The analysis of heterotypic cell-to-cell communication via these EVs might open a novel avenue to better understand the cell-fate decision leading to functional HCE failure.7,8
Matthaei et al9 indicated a significant downregulation of the cellular miR-29 family in the HCE of patients with late-onset Fuchs’ endothelial corneal dystrophy (FECD). In addition, Iliff et al10 described a single-base-pair substitution in cellular miR-184 in regard to the disease phenotype of EDICT, a syndrome characterized by endothelial dystrophy, iris hypoplasia, congenital cataracts, and stromal thinning. However, the actual function involved in “the loss of the specified function” of miR-184 in these diseased tissues remains undetermined.
In this study, we show that large quantities of both miR-184 and miR-24-3p were detected in presurgical AqH of bullous keratopathy (BK) patients; additionally, extracellularly released miR-184 and miR-24-3p were more abundant in EVs and culture supernatants (CSs) derived from nondegenerated CD44−/dull hCECs. It is of interest that the repression of cellular miR-34a expression, either by transfection of an miR-34a inhibitor or exposure to oxidative stress, resulted in the elevated release of miR-184 as inclusion molecules in EVs. The reduced production of senescence-associated secretory phenotypes (SASPs)11, 12, 13, 14 by transfection of miR-184 mimics and the repression of mitochondrial membrane potential (MMP) by transfection of its inhibitor has led to a new hypothesis that the cellular interplay through EV miR-184 in AqH may alleviate the exacerbated degeneration in single-layer HCE tissue. Additionally, the cellular miR-34a/CD44 axis1,2 may orchestrate cell competition, potentially sustaining tissue homeostasis in the HCE.
Materials and Methods
Patients and Approval for AqH Acquisition
This study and the acquisition of AqH were approved by the Institutional Review Board of Kyoto Prefectural University of Medicine, Kyoto, Japan (Approval No.: ERB-C-245-8). The human tissue used in this study was handled in accordance with the tenets set forth by the Declaration of Helsinki. HCEC were obtained, including informed written consent for eye donation to research, from human donor corneas supplied by CorneaGen (Seattle, WA, USA) eye bank.
All procedures were conducted in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Human Materials in Ophthalmic and Vision Research, and all details of the current experimental study protocols were approved by the Institutional Ethical Committee of Kyoto Prefectural University of Medicine, Kyoto, Japan.
HCE Donors, Cultures of HCE Cells, and Reagents
Human tissues used in this study were handled and cultured as detailed in previous publications.15, 16, 17 The rho-associated protein kinase inhibitor Y-27632, epidermal growth factor, p38 mitogen-activated protein (MAP) kinase inhibitor SB203580, transforming growth factor-β receptor kinase inhibitor SB431543, Dulbecco’s modified Eagle’s medium, high glucose, and fetal bovine serum were also handled, cultured, and analyzed as previously described.15, 16, 17 The hCECs, passages 2 to 5, were used for all experiments.
Human AqH
AqH was obtained at the beginning of surgery, without blood contamination, using either a specially designed 30-gauge needle attached to a disposable pipette (Nipro), as described previously18, or a disposable 1-ml syringe with a 30-gauge needle. Approximately 150 μl of AqH was collected, immediately frozen, and stored at −80°C until analysis.
The patients’ demographics are presented in Table S1; they were undergoing cell injection therapy.
RNA Extraction and miRNA Profiling 3D-Gene Microarray Analysis
RNA extraction was performed as previously described.6 For miR expression profiling, 3D-Gene Human miRNA Oligo Chips (miRBase, version 17-19; Toray Industries) were used and analyzed as previously reported.6 All data were globally normalized per microarray such that the median of the signal intensity was adjusted to 25. Cellular miR expression profiles were distinct among hCEC subpopulations (SPs); they showed heterogeneous expression levels of CD44. Using these hCEC SPs, miRs exhibiting distinct intracellular expression levels were selected using volcano plots, in which the x- and y-axes indicated the log2 value of the expression fold change (abundance ratio) and the negative log10 value of P values, respectively.
Exosome Isolation Using the MagCapture Isolation Kit
Exosome belongs to EVs with a specified distinctive particulate size (50∼150 nm). CSs were harvested after incubation in the culture medium for 72 hours. Collected CSs were then centrifuged at 2000g for 10 minutes before being filtered through a 0.22-μm filter (Millex-GV, Merck). Additionally, to gain EVs, CSs were centrifuged at 10 000g for 30 minutes at 4°C to remove large EVs. The exosomes were concentrated via the phosphatidylserine affinity method using the MagCapture Exosome Isolation Kit PS2 (FUJIFILM Wako). The mixture of CS and exosome capture-immobilized beads was then rotated for 1 hour at 4°C. The beads were washed twice with an exosome capture immobilizing/washing buffer, and the bound exosomes were then eluted with elution buffer, according to the manufacturer’s instructions (Fig S1).
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from 200 μl of either CSs or EVs (or Exosomes) using the miRNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol with minor modifications. To improve the RNA yield, 1.6 μg of MS2 RNA (Roche Applied Science) was applied, and 5 μl of 0.5 nM Syn-cel-mir-39 miScript miRNA mimic (Qiagen) was added, as a spike-in control for purification efficiency, before the addition of chloroform. miRNA and gene expressions were normalized to RNU44, cel-mir-39, or 18S using the comparative cycle threshold method (2ˆ-ΔCT). Primers and probes were purchased from Thermo Fisher Scientific and are listed in Table S2.
Transfection of miR Mimics or Inhibitors
Transfection of either miR mimics or inhibitors at 30 nM was conducted using a DharmaFECT Duo reagent (GE Healthcare Dharmacon) in accordance with the manufacturer’s protocol. The mimics and inhibitors targeting miRNA-184, 24-3p, 23a-3p, and 34a-5p and the negative control mimics and inhibitors were purchased from Thermo Fisher Scientific.
Mitochondrial Membrane Potential
Changes in the MMP were detected using the JC-1 MitoMP Detection Kit (Dojindo Laboratories). After the treatment, cells were harvested via TrypLE Select (Thermo Fisher Scientific) treatment and suspended at a density of 106 cells/ml in the medium. Collected cells were incubated with 2 μM JC-1 for 30 minutes at 37°C. After washing with Hanks balanced salt solution, the cells were analyzed using a BD FACSCanto II Flow Cytometry System (BD Biosciences). For fluorescence imaging analysis, cells were incubated with 2 μM JC-1 for 30 minutes at 37°C and analyzed using a BZ X-700 Microscope System (Keyence Corporation).
Western Blotting
The procedures followed those previously described.1 The primary antibodies purchased were as follows: β−actin (#4967) and mitochondrial transcription factor A (#8076) from Cell Signaling Technology; TOMM20 (ab56783), COXIV (ab202554), and voltage-dependent anion channel (ab235143) from Abcam; and peroxisome proliferator-activated receptor-gamma coactivator 1-α (NBP1-04676) from Novus Biologicals. Goat anti-mouse IgG and goat anti-rabbit IgG (H + L; Southern Biotech) were used as secondary antibodies. MagicMark XP Western Protein Standard (Thermo Fisher Scientific) was used as the molecular weight marker. The protein bands were visualized using a Western BLoT HRP substrate series (TakaraBio).
Statistical Analysis
Data are presented as the mean ± standard deviation. Statistical analysis of differences was performed using Student's t test for comparisons between 2 groups; for analysis of variance, this was followed by either Tukey's or Dunnett's test. Values shown in the graphs represent the mean ± standard deviation.
Results
Distinct miRNA Profiles in AqH of BK Patients
Among the top 50 ranked miRs based on the amount detected in the individual AqH of cataract (109) and BK (97) patients, 73 were shared commonly (Fig 1A). Large amounts of MiR-184, -24-3p, and -92b-5p were detected in AqH of patients who experienced HCE failure; the amounts were greater in cataract patients (N = 19) than those in BK patients (N = 16) (P = 0.002 for both miR-184 and -24-3p and P = 0.027 for miR-92b-5p) (Fig 1B); this is consistent with the previous findings reported.19,20 Reportedly, the miR-184 and -29 families are eye-specific without background contamination from plasma.21 Mir-34a-5p, as a critical cellular constituent of the HCE, and the cellular miR-29 family were both at relatively lower levels in AqH (Fig 1B). MiR-34a is directly involved in hCEC differentiation and degeneration through its regulation of CD44 expression.1
Figure 1.
miRNA (miR) profiles in aqueous humor of bullous keratopathy (BK) patients. A, The profiles of miRs in aqueous humor (AqH) were analyzed using 3D-Gene human microRNA chips. miRs ranked in the top 50 in individual AqH were 109 among 19 cataract patients (control) and 97 among 16 BK patients. Among these miRs, 73 were detected in both cataract and BK patients. Almost 4000 miR species detected for each individual patient, selection of top 50 ranked miRs in each individual of cataract (19 patients × 50) and BK (16 patients × 50) resulted in the 109 and 97 species, respectively, after depletion of the redundant species present in 950 and 800. B, Variations in miR expression levels are illustrated for 12 typical miRs in AqH of cataract and BK patients. The selection is primarily based on levels of miRs expressed either only in AqH of cataract patients or in those of both cataract and BK patients.
Differential miR Expression in Corneal Endothelium Tissues
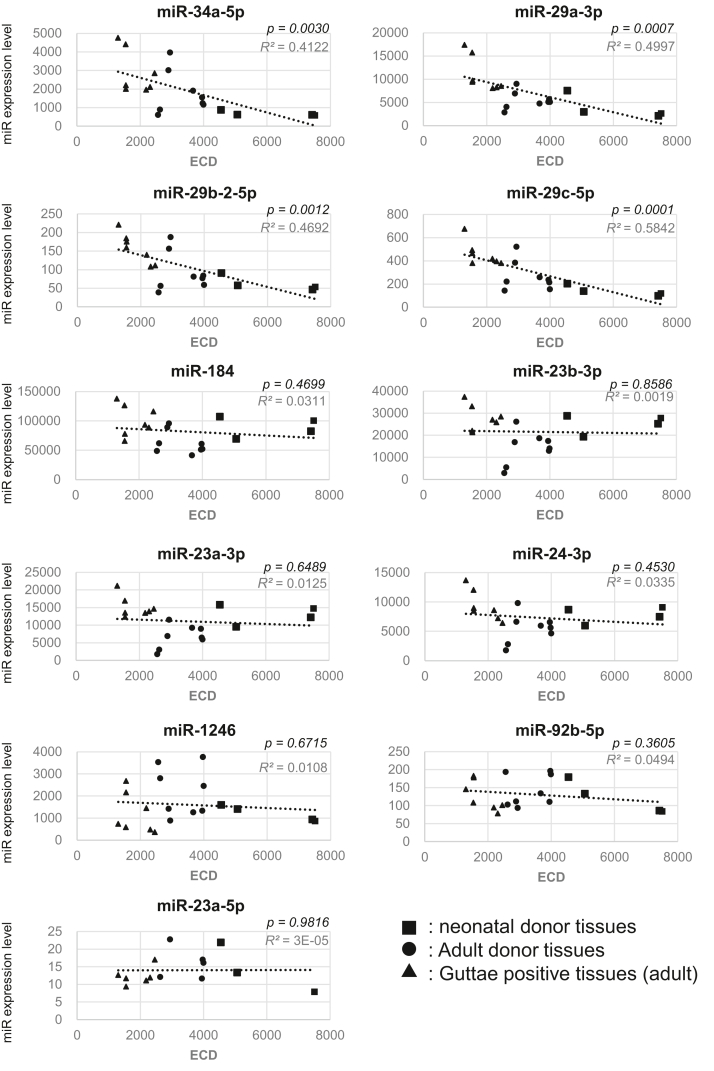
The distinct amounts of miRs in AqH of cataract and BK patients indicate the presence of secretion, excretion, or decomposition of those miRs in AqH. The absolute intracellular amounts that exist in HCE cells may be likely to influence the amounts of miRs in AqH. These miRs were classified into 2 groups according to their correlations with the endothelial cell density (ECD) (Table S3). One paralleled ECD values, whereas the other was almost constantly independent of ECD values (Fig 2). The former group was made up of miR-34a and -29 families, whereas the latter primarily contained miR-184, -24-3p, and -92b-5p. It is worth noting that the expression levels of miR-34a and -29 families in neonatal corneal tissues (Fig 2), with ECD over 4500 to 7500, were significantly downregulated, whereas those of miR-184, -24-3p, -23a-5p, -92b-5p, -23b-3p, and -1246 families remained almost constant among F5 to F20 HCE tissues (Table S3) without a significant decrease (Fig 2).
Figure 2.
Scatter plots of miRNA (miR) expression levels in 20 fresh corneal endothelial tissues regarding endothelial cell density (ECD). The tissues were a gift from CorneaGen, Inc. It should be noted that no diagnostic test was performed to determine whether donors of the endothelium tissues with Guttae were affected with Fuchs’ endothelial corneal dystrophy (FECD). Donor ages and ECDs are listed in Table S4. Among the 20 fresh tissues, 4 (F1∼F4) were from neonatal donors younger than 12 months old. The other 16 tissues were from adult donors ranging from 12 to 73 years of age; ECDs ranged from 3998 to 1301. Seven HCE specimens showed the presence of marginal, not severe, levels of Guttae. For miR expression profiling, 3D-Gene Human miRNA Oligo Chips (miRBase version 17-19, Toray Industries) were used.6 All data were globally normalized per microarray such that the median of the signal intensity was adjusted to 25. HCE = human corneal endothelium.
miR Profiles in CSs of hCECs
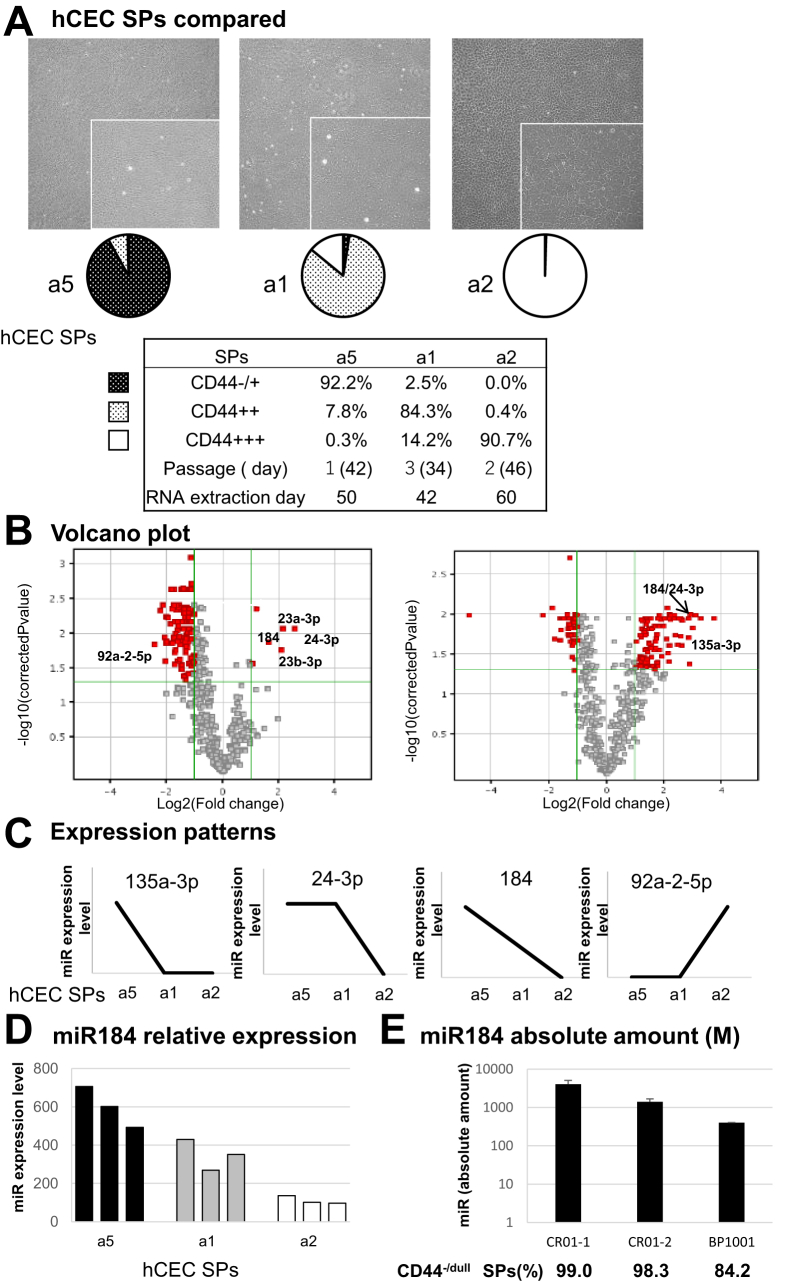
P53-inducible cellular miR-34a downregulates CD44 expression in a concerted manner with repressed c-Myc, whereas the miR-34a/CD44 axis regulates the downstream mitochondrial homeostasis.1,2 The miR profiles in CSs of the heterogeneous hCEC SPs were largely different. The volcano plots of cellular miR profiles between fully and intermediately matured SPs (Fig 3B, right) and those between intermediately matured and immature SPs (Fig 3B, left) revealed the significant and selective upregulations of cellular miR-184 and -24-3p in mature SPs. miR expression patterns were classified into 4 groups across these 3 hCEC SPs (Fig 3C). miR-184 and miR-24-3p in CSs were inversely correlated with the increase of CD44 (Fig 3C). This inverse correlation was also confirmed in terms of the absolute amount of miR-184 in CSs of hCECs produced in the cell-processing center in the clinical setting for cell infusion therapy (Fig 3E). The absolute quantification results for miR-184 in CSs were obtained using normalization by ratios of added spike-in cel-miR-39. Conversely, miR-92a-2-5p in CSs was positively correlated with CD44 expression (Fig 3C). miRs (miR-184, -24-3p, -92b-5p, and -23a-3p) that were abundant in AqH were also abundant in CSs and EVs released by mature hCECs (Fig 4A–C). Extracellular miR-184 and -24-3p were downregulated in CSs and EVs in CD44+++ hCEC SPs (Fig 4B, C vs. Fig 4D). MiR-24-3p was more abundant in CSs than in EVs, although the presence of miR-184 was comparable in both, suggesting that most extracellular miR-184 may be released as inclusion bodies in EVs (Fig 4C). The expressions of miR-23a-3p and -92b-5p in CSs and EVs were low; no significant difference between mature nondegenerated and immature degenerated cells was found.
Figure 3.
miRNA (miR) expression profiles of cultured human corneal endothelial cells (hCECs). A, hCECs were cultured according to the protocol described in the text (a5, seeding cell density was 800 cells/mm2, without the addition of SB431542; a1, the same conditions as a5, but with 1 mM SB431542; a2, seeding cell density was 380 cells/mm2 with 1 mM SB431542). B, Volcano plots of cellular miRs analyzed with 3D-Gene Human miRNA Oligo Chips, as in Figure 2, between fully matured subpopulations (SPs) (CD44−/dull 92.2%, passage 1, from the 19-year-old [y/o] male donor) and intermediately matured SPs (CD44++ 84.3%, passage 3, from the 10 y/o female donor) (B, right) and between intermediately matured and immature SPs (CD44+++ 90.7%, passage 2, from the 55 y/o male donor) (B, left). C, Distinctions of miR expression levels among 3 hCEC SPs (a5, a1, and a2) were schematically classified into 4. miRs belonging to each class are shown. D, miR-184 expression levels are inversely dependent on the expression levels of CD44 among the 3 hCEC SPs (a5, a1, and a2). E, The quantification of absolute amounts of mir-184 in the culture supernatants (CSs) of hCECs produced in the cell-processing center in a clinical setting. Absolute amounts were obtained using normalization by ratios of added spike-in cel-miR-39.
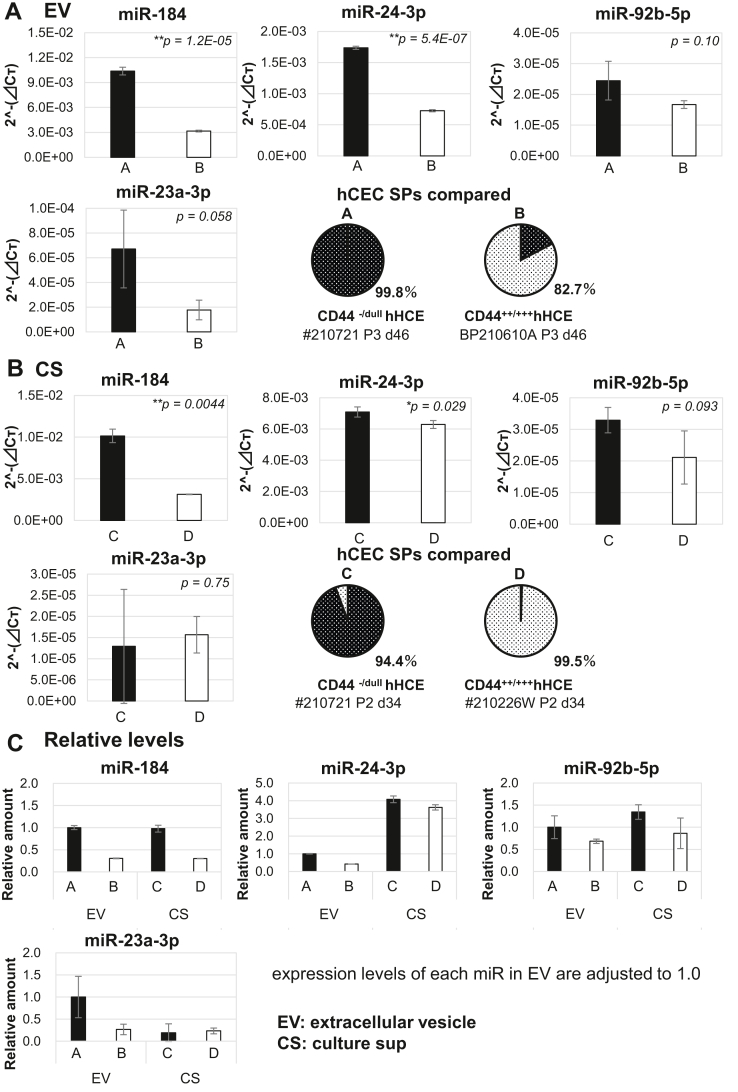
Figure 4.
miRNA (miR)-184 prevails in culture supernatants (CSs) of mature human corneal endothelial cell (hCEC) subpopulations (SPs). A, Released extracellular vesicles (EVs) were concentrated by repeated centrifugation of the cultured hCEC SPs from the CS #210721 (CD44−/dull SPs 99.8%, a culture additive, with 10 μM Y27632, without SB203580, SB431542, or EGF, passage 3) and BP210610A (CD44−/dull SPs 17.3%, culture additives, without Y27632, and with 10 μM SB203580, 1 μM SB431542, and 5 ng/ml EGF, passage 3) according to the described method. B, CSs of hCEC SPs, #210721 (CD44−/dull SPs 94.4%, passage 2) and #210226W (CD44−/dull SP 0.5%, culture additives, without Y27632, and with 10 μM SB203580, 1 μM SB431542, and 5 ng/ml EGF, passage 2) were harvested after 72 hours of incubation. The levels of miR-184, miR-24-3p, miR-92b-5p, and miR-23a-3p were evaluated via quantitative real-time polymerase chain reaction. C, Relative levels of miR-184, miR-24-3p, miR-92b-5p, and miR-23a-3p in EVs from BP210610A, CS from #210721, and CS from #210226W were compared with those in EVs from #210721.
MiR-34a-5p Regulates Release of EV miR-184
An miR-34a-5p inhibitor was transfected into CD44−/dull hCEC SPs to determine the level of participation of cellular miR-34a-5p in the release of miRs. Results indicated that the cellular miR-184 expression level in miR-34a-5p–transfected cells was only slightly elevated. Notably, the extracellular miR-184 and -24-3p levels in CSs and EVs were markedly upregulated (Fig 5A–C). Extracellular miR-23-3p and -92b-5p levels, whether in CSs or EVs, showed no significant difference as a result of the transfection.
Figure 5.
Cellular miRNA (miR)-34a-5p regulates release of extracellular vesicle (EV) miR184 and -24-3p. Mature CD44−/dull cultured human corneal endothelial cell (hCEC) subpopulations (SPs) were transiently transfected with either the miR-34a-5p inhibitor or control inhibitor. A, Forty-eight hours after transfection, cellular levels of miR-34a-5p and miR-184 were evaluated via quantitative real-time polymerase chain reaction (qRT-PCR). The relative expression of miR was analyzed using RNU44 for normalization. B, C, Extracellular levels of miR-184, miR-24-3p, and miR-92b-5p in the EVs (B) and culture supernatants (CS) (C) were also evaluated via qRT-PCR. The relative expression of miR was analyzed using cel-miR-39 for normalization. The hCECs analyzed were #210512 for (A) and (B) and #210721 for (C).
Oxidative Stress Enhances Release of miR-184 and -24-3p
Recently, we found that the exposure of hCECs to extracellular oxidative stress depressed cellular miR-34a-5p expression.1 Mature CD44−/dull hCEC SPs were exposed to 1 mM H2O2 for 24 hours. Cellular expression levels of miR-184 and -24-3p, as well as that of miR-34a-5p, decreased (Fig 6A) as expected, while miR-92b-5p levels did not change. Intriguingly, the exposure significantly upregulated the release of miR-184 and -24-3p in EVs and CSs (Fig 6B, C).
Figure 6.
Oxidative stress induces the release of miRNA (miR)-184 and -24-3p. Mature CD44−/dull cultured human corneal endothelial cell (hCEC) subpopulations (SPs) at passage 3 were washed with exosome-depleted Nancy medium, which was replaced with fresh medium; they were then immediately exposed, or not, to 1 mM H2O2 for 24 hours before RNA was extracted. A, The cellular levels of miR-34a-5p, miR-184, miR24-3p, and miR-92b-5p were evaluated via quantitative real-time polymerase chain reaction (qRT-PCR). The relative expression of miR was analyzed using RNU44 for normalization. B, C, The extracellular levels of miR-184, miR24-3p, and miR-92b-5p in the culture supernatants (CSs) (B) and extracellular vesicles (EVs) (C) were also evaluated via qRT-PCR. The relative expression of miR was analyzed using cel-miR-39 for normalization.
miR-184 Regulates Expression of SASP and Mitochondrial Function
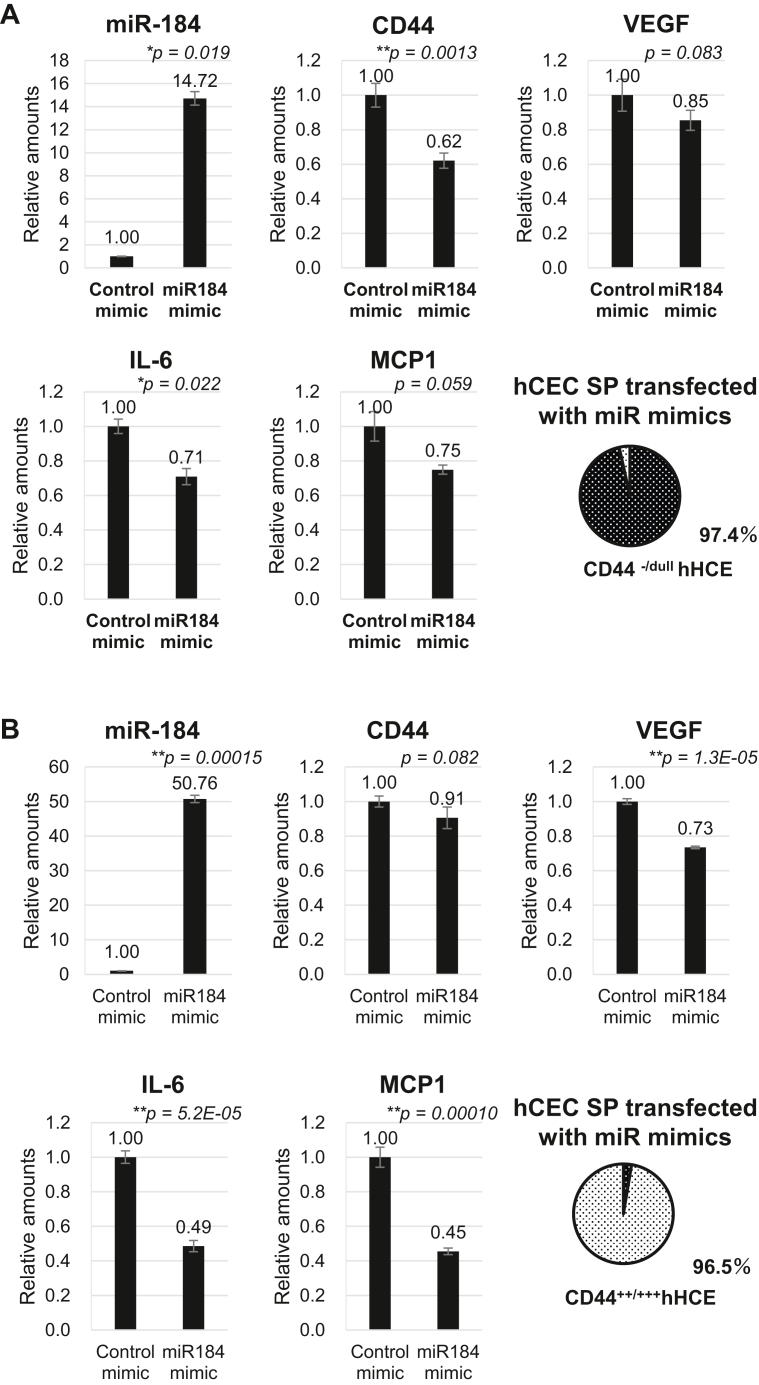
Finally, we explored the roles of extracellular miR-184 and -24-3p in regulating SASP expression and mitochondrial functions. The upregulation of cellular miR-184 via the transfection of its mimic into mature CD44−/dull hCEC SPs clearly showed the downregulated gene activation of CD44 and SASP genes, namely VEGF, IL-6, and MCP-1 (Fig 7A). Gene activation was similarly observed in degenerated immature CD44+++ hCEC SPs, and the negative regulation of these SASP gene activations was more prominent in the latter SPs as compared with mature CD44−/dull hCEC SPs (Fig 7B), indicating an improvement in hCEC cellular senescence due to the upregulated miR-184 in the degenerated CD44+++ hCEC SPs. The transfection of either the miR-184 or miR-24-3p inhibitor into mature CD44−/dull hCEC SPs induced the reduction of mitochondrial homeostasis as compared with the control; MMP, as shown through JC-1 staining (Fig 7C), and mitochondrial biogenesis monitored by the expression of a peroxisome proliferator-activated receptor-gamma coactivator 1-α, voltage-dependent anion channel, and mitochondrial transcription factor A (Fig 7D, Western blot).
Figure 7.
Regulation of senescence-associated secretory phenotype and mitochondrial homeostasis by miR-184. A, B, The changes in mRNA expression levels of CD44, interleukin 6 (IL-6), VEGF, and monocyte chemotactic protein-1 (MCP-1) in mature CD44−/dull cultured human corneal endothelial cell (hCEC) subpopulations (SPs) (A) and immature CD44+++ hCEC SPs (B) by transfection of an miRNA (miR)-184 mimic. The relative expression of mRNA was analyzed using 18S mRNA for normalization. Transfection of miR mimics at 30 nM was conducted using a DharmaFECT Duo reagent (GE Healthcare Dharmacon) in accordance with the manufacturer’s protocol. C, Changes in mitochondrial membrane potentials of mature CD44−/dull hCECs following transfection with either an miR-184 or miR-24-3p inhibitor. Seventy-two hours after transfection, the cells were incubated with JC-1 dye, and relative mitochondrial membrane potentials were observed using fluorescence microscopy. D, Qualitative analyses of JC-1 and Western blotting (WB). The changes in expression levels of PGC1α, VDAC1, TFAM, TOMM20, COXIV, and β-actin in mature CD44−/dull hCECs due to transfection using the miR-184 or miR-24-3p inhibitor. Forty-eight hours after transfection, expression levels were assessed using WB. Proteins were loaded at 25 μg protein/lane.
Discussion
In this study, we generated a novel concept whereby the elevated release of EV miR-184 into AqH may constitute cellular interplay that alleviates aggravated HCE degeneration. Cell competition is an important mechanism whereby cell society robustly orchestrates tissue homeostasis, through cell death, cellular senescence, or cell extrusion due to physical pressures.22, 23, 24 Therefore, HCEC SPs with an identical lineage but distinct functional phenotypes, such as CD44−/dull and CD44+++ hCEC SPs, compete with each other for space in single-layer tissues.22, 23, 24
The downregulation of the cellular miR-29 family in the HCE of late-onset FECD patients9 and the disease phenotype EDICT have been shown to be associated with single-base-pair substitution in miR-184.10 Zhao et al25 identified different expressions of miRs in the mouse CE during aging. More recently, Buono et al26 analyzed the effects of mesenchymal stem cell–derived EVs in an in vitro endoplasmic reticulum (ER) stress model of corneal dystrophy and found that its effects were correlated with the transfer of ER stress targeting miRs to CEC. There are few additional reports describing the function of miRs in AqH upon the HCE.19,27,28
In this study, we first observed the skewed presence of a cluster of miRs in AqH of BK patients (Fig 1). Relatively large amounts of miR-184, -24-3p, -92b-5p, and -23b-3p were detected in AqH of 19 cataract and 16 BK patients undergoing cell injection therapy (Fig 1 and Table S1).
When analyzing the multiomic landscape of AqH, Yamaguchi et al noted a rapid postoperative ECD decrease in patients with severe pre-existing iris atrophy after corneal transplant.20,29 Jurkunas et al also demonstrated that abnormal AqH-induced mitochondrial dysfunctions resulted in the decrease of ECD20,29,30; however, neither study provided any information on either the presence or role of miRs in AqH.
It is worthy of note that the expression levels of cellular miR-34a and -29 families in the neonatal corneal tissues, with the ECD of 4500 to 7500, were significantly downregulated (Fig 2). To our surprise, no downregulation of expression levels of cellular miR-184, -24-3p, -23b-3p, -23a-3p, or -92b-5p was observed in HCE tissues (Fig 2 and Table S2). All adult-derived tissues here handled either lacked Guttae entirely or were found to have marginal levels (Table S4). The regulatory mechanisms underlying the senescence of the HCE that leads to the decrease in the ECD are largely unknown.25,31 The expression level of cellular miR-184 varied during development, cell differentiation, and wound healing.32, 33, 34, 35
Cultured HCEC are composed of a dysregulated expression of a hierarchy of miR clusters, probably due to cell degeneration in combination with the elevated expression of CD44 and senescence.2 We recently found that functional phenotypes directly link to trans-endothelial fluid movement in mature CD44−/dull hCEC SPs,1,34,36 expressing AQP1, SLC4A11, and ZO-1.37,38
Increases in cellular miR-184 and -24-3p in hCECs were inversely proportional to the decrease of CD44 expression (Fig 3). Similarly biased amounts of miR-184, -24-3p, -23a-3p, and -92b-5p were also confirmed in both EVs and CSs (Fig 4A, B). It is of note that most extracellular miR-184 may be released as inclusion bodies in EVs, whereas the others are not (Fig 4C). Considering the elevated release of miR-184, -24-3p, and -92b-5p in CSs and EVs (Fig 5) due to the forced downregulation of cellular miR-34a, this might function as autocrine feedback to recover the dysfunctional intracellular events initiated by the repression of intracellular miR-34a. The activation of p53 by stress signals results in enhanced EV production by hCEC SPs.2,39,40 Members of the miR-34 family were identified as the most prevalent p53-induced miRs41 and were involved in senescence targeting the p53 pathway. Oxidative stress also plays a major role in the chronic degenerative process of HCE in FECD.42, 43, 44 The oxidative cellular stress that caused hCEC degeneration was accompanied by reduced miR-34a expression1 (Fig 6) and dysfunctional mitochondria biogenesis1 as well as the increased release of miR-184 and -24-3p (Fig 6).
All previous observations, together with those described here, validate the key role of EV miR-184 in coping with the ER stress responsible for HCE degeneration through reduced secretions of SASP, VEGF, IL-6, and MCP-1 and upregulations of mitochondrial MMP and biogenesis (Figs 7 and 8).
Figure 8.
Cellular interplay through exosome miRNA (miR)-184, 24-3p alleviates corneal endothelium degeneration: hypothetical schema. Here, newly presented findings, together with our previous observations, support the protective role of extracellular vesicle (EV) miR-184 against endoplasmic reticulum stress responsible for human corneal endothelium (HCE) degeneration through the reduced secretion of senescence-associated secretory phenotypes (SASPs), VEGF, interleukin 6 (IL-6), and monocyte chemotactic protein-1 (MCP-1) and upregulations of the mitochondrial membrane potential and mitochondrial biogenesis. Our findings generate the novel concept that nondegenerated CD44−/dull HCE cells compete with degenerated CD44+++ HCE cells for space in single-layer HCE tissues through the alleviation of HCE degeneration caused by EV miR-184, being released by the former winner cells (thick blue arrow), in addition to autocrine positive feedback (gray arrow) that suppresses the cycle. The failure in this cell competition among HCE SPs with identical lineages but a distinct functional phenotype may be at least partially responsible for the common pathogeneses of bullous keratopathy and Fuchs’ endothelial corneal dystrophy.
Our findings generate a novel concept that nondegenerated CD44−/dull HCE cells compete with degenerated CD44+++ HCE cells for space in single-layer HCE tissues through EV miR184 released by the former winner cells (Fig 8) in addition to the autocrine positive feedback that suppresses the vicious cycle. The failure of this cell competition among HCE SPs with an identical lineage but distinct functional phenotypes may be at least partially responsible for the common pathogeneses of BK and FECD. However, this interpretation might be too simplistic, as EVs released into AqH would act as cargo for diverse molecules like protein, nucleic acid, DNA, and mRNA. In fact, our preliminary results indicate that the presence of miRs actively degenerates mature, functional CD44−/dull HCE cells. Nonetheless, considering the association of miR-184 with BK, FECD,9 EDICT,10 and corneal dystrophy,26 loss of the function of EV miR-184 to alleviate the oxidative stress–induced degeneration, as noted in this study, heralds a potential new way to understand the molecular pathogeneses of these diseases.
SLC4A11 has also been shown to be mutated in late-onset FECD and in congenital hereditary endothelial dystrophy of CE.45, 46, 47, 48, 49 SLC4A11 is an electrogenic H+ transporter that facilitates glutamine catabolism and suppresses the production of mitochondrial superoxide by providing ammonia-sensitive H+ uncoupling.50 It may be of interest to define the role of the SLC4A11 transporter protein in the release of EVs discussed here, especially considering the null expression of SLC4A11 in hCEC SPs with an insufficient regenerative capacity.37,38
Acknowledgments
The authors wish to thank Eiko Ito, Asako Hiraga, and Michio Hagiya for their technical assistance and Keiko Takada and Yoko Hamuro for their secretarial assistance throughout the study. The authors are also grateful to CorneaGen Inc for the generous gift of corneal tissues.
Manuscript no. XOPS-D-22-00125.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The authors have made the following disclosures: S.K.: All support for the present manuscript (e.g., funding, provision of study materials, medical writing, article processing charges, etc) – Japan Agency for Medical Research and Development (AMED) JSPS, Japan Society for the Promotion of Science (JSPS), CorneaGen; Grant titled “Research Center Network for Realization of Regenerative Medicine”, Grant titled “KAKENHI Grant”, Research material of corneal tissues.; Grants or contracts from any entity – Santen Pharmaceutical Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Senju Pharmaceutical Co, Ltd, Kowa Co, Ltd, HOYA Corporation, Oncolys Biopharma Inc, LION Corporation, CorneaGen; Patent license fee – CorneaGen, Aurion Biotech; Honorarium for consulting – Santen Pharmaceutical Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Alcon Japan, Ltd, Novartis, Astellas Pharma; Honorarium for lecture – Santen Pharmaceutical Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Senju Pharmaceutical Co, Ltd, Kowa Co, Ltd, Alcon Japan, Ltd, Abbott Medical Optics, Inc.
C.S.: Grant-in-Aid for Scientific Research – Japanese Ministry of Health, Labor and Welfare; Research Grant – Japanese Ministry of Education, Culture, Sports, Science and Technology, Japan Agency for Medical Research and Development, Santen Pharmaceutical Co, Ltd, Senju Pharmacoitical Co, Ltd, Otsuka Pharmacoitical Co, Ltd, Nitto Medic Co, Ltd, SEED Co, Ltd, Nitten Pharmaceutical Co, Ltd, Kowa Company, Ltd, CorneaGen, Hirosaki LI; Honorarium for lecture – Otsuka Pharmaceutical Co, Ltd, Santen Pharmaceutical Co, Ltd, Senju Pharmaceutical Co, Ltd, Toa Pharmaceutical Co, Ltd.
M.U.: Grant titled “Research Center Network for Realization of Regenerative Medicine” – Japan Agency for Medical Research and Development (AMED) JSPS, Grant titled “KAKENHI Grant” – Japan Society for the Promotion of Science (JSPS); Patent license fee - CorneaGen, Aurion Biotech.
Supported by Projects for Technological Development from the Japan Agency for Medical Research and Development, AMED (Tokyo, Japan) 19bm0404033h0002, and JSPS KAKENHI grant number JP26293376. The sponsor or funding organization had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. The human tissue used in this study was handled in accordance with the tenets set forth by the Declaration of Helsinki. HCECs were obtained, including informed written consent for eye donation to research, from human donor corneas supplied by CorneaGen (Seattle, WA) eye bank. All procedures were conducted in accordance with the ARVO Statement for the Use of Human Materials in Ophthalmic and Vision Research, and all the details of the current experimental study protocols were approved by the Institutional Ethical Committee of the Kyoto Prefectural University of Medicine, Kyoto, Japan.
No animal subjects were included in this study.
Author Contributions:
Research design: Kazuko Asada, Shigeru Kinoshita, Junji Hamuro
Data acquisition and/or research execution: Tomoko Yamashita, Kazuko Asada, Nao Hiramoto, Munetoyo Toda, Junji Hamuro
Data analysis and/or interpretation: Tomoko Yamashita, Kazuko Asada, Morio Ueno, Nao Hiramoto, Tomoko Fujita, Chie Sotozono, Shigeru Kinoshita, Junji Hamuro.
Obtained funding: Shigeru Kinoshita, Junji Hamuro, Morio Ueno
Manuscript preparation: Tomoko Yamashita, Morio Ueno, Tomoko Fujita, Chie Sotozono, Shigeru Kinoshita, Junji Hamuro
Supplementary Data
Exosome Concentration by MagCapture
Demographics of Patients
Primer and Assay Lists
Distinct Expression of miRs in AqH of Cataract and BK Patients
Human Corneal Tissues Used
References
- 1.Hamuro J., Asada K., Ueno M., et al. Repressed miR-34a expression dictates the cell fate to corneal endothelium failure. Invest Ophthalmol Vis Sci. 2022;63:22. doi: 10.1167/iovs.63.4.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueno M., Asada K., Toda M., et al. Concomitant evaluation of a panel of exosome proteins and MiRs for qualification of cultured human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2016;57:4393–4402. doi: 10.1167/iovs.16-19805. [DOI] [PubMed] [Google Scholar]
- 3.Kosaka N., Iguchi H., Yoshioka Y., et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindoso R.S., Collino F., Bruno S., et al. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev. 2014;23:1809–1819. doi: 10.1089/scd.2013.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Zhang X., Li X. Intraocular exosomes in eye diseases. Curr Mol Med. 2022;22:540–548. doi: 10.2174/1566524021666210901122948. [DOI] [PubMed] [Google Scholar]
- 6.Ueno M., Asada K., Toda M., et al. MicroRNA profiles qualify phenotypic features of cultured human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2016;57:5509–5517. doi: 10.1167/iovs.16-19804. [DOI] [PubMed] [Google Scholar]
- 7.Jadli A.S., Ballasy N., Edalat P., Patel V.B. Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem. 2020;467:77–94. doi: 10.1007/s11010-020-03703-z. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto S., Fujita Y., Kadota T., et al. Intercellular communication by vascular endothelial cell-derived extracellular vesicles and their MicroRNAs in respiratory diseases. Front Mol Biosci. 2021;7 doi: 10.3389/fmolb.2020.619697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthaei M., Hu J., Kallay L., et al. Endothelial cell microRNA expression in human late-onset Fuchs' dystrophy. Invest Ophthalmol Vis Sci. 2014;55:216–225. doi: 10.1167/iovs.13-12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iliff B.W., Riazuddin S.A., Gottsch J.D. A single-base substitution in the seed region of miR-184 causes EDICT syndrome. Invest Ophthalmol Vis Sci. 2012;53:348–353. doi: 10.1167/iovs.11-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safwan-Zaiter H., Wagner N., Michiels J.F., Wagner K.D. Dynamic spatiotemporal expression pattern of the senescence-associated factor p16Ink4a in development and aging. Cells. 2022;11:541. doi: 10.3390/cells11030541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manakanatas C., Ghadge S.K., Agic A., et al. Endothelial and systemic upregulation of miR-34a-5p fine-tunes senescence in progeria. Aging (Albany NY) 2022;14:195–224. doi: 10.18632/aging.203820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prattichizzo F., Giuliani A., Recchioni R., et al. Anti-TNF-α treatment modulates SASP and SASP-related microRNAs in endothelial cells and in circulating angiogenic cells. Oncotarget. 2016;7:11945–11958. doi: 10.18632/oncotarget.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman P.R., Chang G., Hutas G., et al. Age-associated stresses induce an anti-inflammatory senescent phenotype in endothelial cells. Aging (Albany NY) 2013;5:913–924. doi: 10.18632/aging.100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamuro J., Numa K., Fujita T., et al. Metabolites interrogation in cell fate decision of cultured human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2020;61:10. doi: 10.1167/iovs.61.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Numa K., Ueno M., Fujita T., et al. Mitochondria as a platform for dictating the cell fate of cultured human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2020;61:10. doi: 10.1167/iovs.61.14.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toda M., Ueno M., Hiraga A., et al. Production of homogeneous cultured human corneal endothelial cells indispensable for innovative cell therapy. Invest Ophthalmol Vis Sci. 2017;58:2011–2020. doi: 10.1167/iovs.16-20703. [DOI] [PubMed] [Google Scholar]
- 18.Terao N., Koizumi H., Kojima K., et al. Distinct aqueous humour cytokine profiles of patients with pachychoroid neovasculopathy and neovascular age-related macular degeneration. Sci Rep. 2018;8 doi: 10.1038/s41598-018-28484-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunmire J.J., Lagouros E., Bouhenni R.A., et al. MicroRNA in aqueous humor from patients with cataract. Exp Eye Res. 2013;108:68–71. doi: 10.1016/j.exer.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Chen S., Yuan M., Liu Y., et al. Landscape of microRNA in the aqueous humour of proliferative diabetic retinopathy as assessed by next-generation sequencing. Clin Exp Ophthalmol. 2019;47:925–936. doi: 10.1111/ceo.13554. [DOI] [PubMed] [Google Scholar]
- 21.Wecker T., Hoffmeier K., Plötner A., et al. MicroRNA profiling in aqueous humor of individual human eyes by next-generation sequencing. Invest Ophthalmol Vis Sci. 2016;57:1706–1713. doi: 10.1167/iovs.15-17828. [DOI] [PubMed] [Google Scholar]
- 22.Maruyama T., Fujita Y. Cell competition in vertebrates - a key machinery for tissue homeostasis. Curr Opin Genet Dev. 2022;72:15–21. doi: 10.1016/j.gde.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Clavería C., Torres M. Cell competition: mechanisms and physiological roles. Annu Rev Cell Dev Biol. 2016;32:411–439. doi: 10.1146/annurev-cellbio-111315-125142. [DOI] [PubMed] [Google Scholar]
- 24.Bowling S., Lawlor K., Rodríguez T.A. Cell competition: the winners and losers of fitness selection. Development. 2019;146:dev167486. doi: 10.1242/dev.167486. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X., Huang Y., Wang Y., et al. MicroRNA profile comparison of the corneal endothelia of young and old mice: implications for senescence of the corneal endothelium. Mol Vis. 2013;19:1815–1825. [PMC free article] [PubMed] [Google Scholar]
- 26.Buono L., Scalabrin S., De Iuliis M., et al. Mesenchymal stem cell-derived extracellular vesicles protect human corneal endothelial cells from endoplasmic reticulum stress-mediated apoptosis. Int J Mol Sci. 2021;22:4930. doi: 10.3390/ijms22094930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dismuke W.M., Challa P., Navarro I., et al. Human aqueous humor exosomes. Exp Eye Res. 2015;132:73–77. doi: 10.1016/j.exer.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragusa M., Caltabiano R., Russo A., et al. MicroRNAs in vitreus humor from patients with ocular diseases. Mol Vis. 2013;19:430–440. [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii N., Yamaguchi T., Yazu H., et al. Factors associated with graft survival and endothelial cell density after Descemet's stripping automated endothelial keratoplasty. Sci Rep. 2016;6:25276. doi: 10.1038/srep25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benischke A.S., Vasanth S., Miyai T., et al. Activation of mitophagy leads to decline in Mfn2 and loss of mitochondrial mass in Fuchs endothelial corneal dystrophy. Sci Rep. 2017;7:6656. doi: 10.1038/s41598-017-06523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laule A., Cable M.K., Hoffman C.E., Hanna C. Endothelial cell population changes of human cornea during life. Arch Ophthalmol. 1978;96:2031–2035. doi: 10.1001/archopht.1978.03910060419003. [DOI] [PubMed] [Google Scholar]
- 32.Liu X., Yang Y., Wang X., et al. MiR-184 directly targets Wnt3 in cardiac mesoderm differentiation of embryonic stem cells. Stem Cells. 2020 doi: 10.1002/stem.3282. [DOI] [PubMed] [Google Scholar]
- 33.Shalom-Feuerstein R., Serror L., De La Forest Divonne S., et al. Pluripotent stem cell model reveals essential roles for miR-450b-5p and miR-184 in embryonic corneal lineage specification. Stem Cells. 2012;30:898–909. doi: 10.1002/stem.1068. [DOI] [PubMed] [Google Scholar]
- 34.Cao Q., Xu W., Chen W., et al. MicroRNA-184 negatively regulates corneal epithelial wound healing via targeting CDC25A, CARM1, and LASP1. Eye Vis (Lond) 2020;7:35. doi: 10.1186/s40662-020-00202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagosa S., Leesch F., Putin D., et al. microRNA-184 induces a commitment switch to epidermal differentiation. Stem Cell Rep. 2017;9:1991–2004. doi: 10.1016/j.stemcr.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamuro J., Deguchi H., Fujita T., et al. Polarized expression of ion channels and solute carrier family transporters on heterogeneous cultured human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2020;61:47. doi: 10.1167/iovs.61.5.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deguchi H., Yamashita T., Hiramoto N., et al. Intracellular pH affects mitochondrial homeostasis in cultured human corneal endothelial cells prepared for cell injection therapy. Sci Rep. 2022;12:6263. doi: 10.1038/s41598-022-10176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueno M., Toda M., Numa K., et al. Superiority of mature differentiated cultured human corneal endothelial cell injection therapy for corneal endothelial failure. Am J Ophthalmol. 2022;237:267–277. doi: 10.1016/j.ajo.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Pigati L., Yaddanapudi S.C., Iyengar R., et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X., Harris S.L., Levine A.J. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 41.Cairns R.A., Harris I.S., Mak T.W. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 42.Azizi B., Ziaei A., Fuchsluger T., et al. p53-regulated increase in oxidative-stress--induced apoptosis in Fuchs endothelial corneal dystrophy: a native tissue model. Invest Ophthalmol Vis Sci. 2011;52:9291–9297. doi: 10.1167/iovs.11-8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z., Handa J.T., Green W.R., et al. Advanced glycation end products and receptors in Fuchs' dystrophy corneas undergoing Descemet's stripping with endothelial keratoplasty. Ophthalmology. 2007;114:1453–1460. doi: 10.1016/j.ophtha.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 44.Kumar V., Jurkunas U.V. Mitochondrial dysfunction and mitophagy in Fuchs endothelial corneal dystrophy. Cells. 2021;10:1888. doi: 10.3390/cells10081888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vilas G.L., Loganathan S.K., Liu J., et al. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet. 2013;22:4579–4590. doi: 10.1093/hmg/ddt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eghrari A.O., Riazuddin S.A., Gottsch J.D. Fuchs corneal dystrophy. Prog Mol Biol Transl Sci. 2015;134:79–97. doi: 10.1016/bs.pmbts.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Alka K., Casey J.R. Molecular phenotype of SLC4A11 missense mutants: setting the stage for personalized medicine in corneal dystrophies. Hum Mutat. 2018;39:676–690. doi: 10.1002/humu.23401. [DOI] [PubMed] [Google Scholar]
- 48.Patel S.P., Parker M.D. SLC4A11 and the pathophysiology of congenital hereditary endothelial dystrophy. Biomed Res Int. 2015;2015 doi: 10.1155/2015/475392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vallabh N.A., Romano V., Willoughby C.E. Mitochondrial dysfunction and oxidative stress in corneal disease. Mitochondrion. 2017;36:103–113. doi: 10.1016/j.mito.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Bonanno J.A., Shyam R., Choi M., Ogando D.G. The H+ transporter SLC4A11: roles in metabolism, oxidative stress and mitochondrial uncoupling. Cells. 2022;11:197. doi: 10.3390/cells11020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exosome Concentration by MagCapture
Demographics of Patients
Primer and Assay Lists
Distinct Expression of miRs in AqH of Cataract and BK Patients
Human Corneal Tissues Used