Highlights
-
•
The review summarizes major findings and recent advances in magnetic resonance spectroscopy (MRS) of migraine.
-
•
The most reproducible results were decreased N-acetyl-aspartate (NAA) in cerebellum in patients with hemiplegic migraine and in the thalamus of chronic migraine patients.
-
•
Increased lactate in the occipital cortex was found for migraine with aura patients but not for migraine without aura.
-
•
Multinuclear imaging studies predominantly investigated phosphorous. The most consistent finding was changes in PCr in various brain regions.
Keywords: Migraine, MRS, 1H-MRS, 31P-MRS, Metabolic, Headache
Abstract
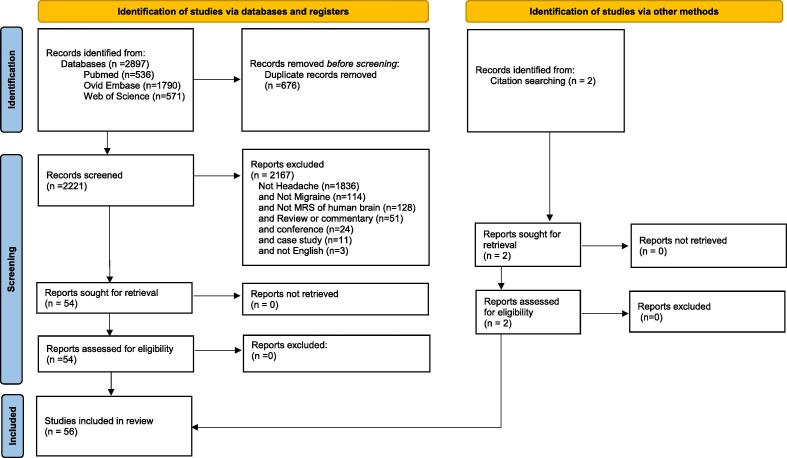
This review summarizes major findings and recent advances in magnetic resonance spectroscopy (MRS) of migraine. A multi database search of PubMed, EMBASE, and Web of Science was performed with variations of magnetic resonance spectroscopy and headache until 20th September 2021. The search generated 2897 studies, 676 which were duplicates and 1836 were not related to headache. Of the remaining 385 studies examined, further exclusions for not migraine (n = 114), and not MRS of human brain (n = 128), and non-original contributions (n = 51) or conferences (n = 24) or case studies (n = 11) or non-English (n = 3), were applied. The manuscripts of all resulting reports were reviewed for their possible inclusion in this manuscript (n = 54). The reference lists of all included reports were carefully reviewed and articles relevant to this review were added (n = 2).
Included are 56 studies of migraine with and without aura that involve magnetic resonance spectroscopy of the human brain. The topics are presented in the form of a narrative review. This review aims to provide a summary of the metabolic changes measured by MRS in patients with migraine. Despite the variability reported between studies, common findings focused on regions functionally relevant to migraine such as occipital cortices, thalamic nuclei, cerebellum and cingulate. The most reproducible results were decreased N-acetyl-aspartate (NAA) in cerebellum in patients with hemiplegic migraine and in the thalamus of chronic migraine patients. Increased lactate (Lac) in the occipital cortex was found for migraine with aura but not in subjects without aura. MRS studies support the hypothesis of impaired energetics and mitochondrial dysfunction in migraine. Although results regarding GABA and Glu were less consistent, studies suggest there might be an imbalance of these important inhibitory and excitatory neurotransmitters in the migraine brain. Multinuclear imaging studies in migraine with and without aura, predominantly investigating phosphorous, report alterations of PCr in occipital, parietal, and posterior brain regions. There have been too few studies to assess the diagnostic relevance of sodium imaging in migraine.
Introduction
Migraine is a neurological disease affecting over a billion individuals worldwide (Stovner et al., 2018). Migraine attacks, which last between 4 and 72 h, typically include moderate to severe throbbing headache with a combination of nausea, vomiting, sensitivity to light and sound, and worsening of symptoms with routine physical activity (Cephalalgia, 2018). Research neuroimaging studies have identified abnormalities in brain structure and function associated with migraine, often in pain processing regions (the ‘pain matrix’), regions responsible for multisensory integration (Rocca et al., 2006, Valfre et al., 2008, Ellingson et al., 2019), and in regions responsible for processing of other sensory stimuli (e.g. visual regions). Studies performed during migraine attacks have identified early involvement of hypothalamic and brainstem regions, suggesting that changes in their activity and functional connectivity are associated with generation of the migraine attack (Schulz et al., 2007, Stankewitz et al., 2011, Stankewitz and May, 2011, Chong et al., 2017, Hougaard et al., 2017, Schulte and May, 2017, May and Burstein, 2019, Mehnert and May, 2019, Schulte et al., 2020).
Metabolic information from specific brain regions of interest can be obtained via magnetic resonance spectroscopy (MRS), a non-invasive imaging technique. Conventional MRS imaging measures signal from protons, which are abundant due to the high water and fat content in tissue. Proton 1H MRS spectra can be acquired with conventional hardware on a clinical MRI system. The measurable metabolites with 1H MRS are N- Acetyl Aspartate (NAA, neuronal marker), creatine (Cr, cell energy marker), choline (Cho, cell membrane turnover marker), myoinositol (mI, astrocyte marker), glutamate-glutamine complex (Glx, excitatory neurotransmitters), γ-aminobutyric acid (GABA, inhibitory neurotransmitter), and lactate (Lac, marker of hypoxia). Most clinical systems have 1H MRS protocols which can measure each metabolite except for GABA. The latter requires a specialized developmental sequence, thereby limiting the prevalence of acquired data in the clinical setting. There are nuclei that are detectable with specialized hardware, such as sodium (23Na) and phosphorus (31P). 23Na imaging can detect sodium concentration and pH due to chemical shift. 31P can provide information on metabolites such as phosphocreatine (PCr), adenosine diphosphate (ADP) and adenosine triphosphate (ATP). These non 1H MRS studies are not as widely used but are very useful for identification of cellular energetics and ionic imbalances.
There are numerous spectroscopy imaging studies of migraine, but the results are highly variable, making interpretation difficult. The voxels selected for spectroscopic imaging range widely and include cortical and non-cortical regions. Data is acquired using different coils and different magnetic field strengths,processed by different software and presented in different units (absolute vs ratios), all of which makes results hard to compare. As such, the interpretation of published MRS results is not straightforward.
The majority of the published results in migraine are collected using 1H MRS, but there are also multinuclear studies using 31P or 23Na. Numerous reports demonstrate metabolic aberrations in the migraine brain, such as increased glutamate levels (signifying excitotoxicity), decreased levels of N-Acetyl Aspartate (suggestive of neuronal damage), as well as abnormal levels of GABA in pain processing areas. It is possible that migraine metabolic biomarkers exist, but improved and consistent MRS techniques are needed to achieve their identification.
The goal of this review is to provide a summary of the metabolic changes measured by all types of MRS in people with migraine, focusing on the most prevalent findings and those that correlate with clinical characteristics.
Methods
This review followed the Preferred Reporting Items for Systematic Reviews and meta-Analysis statement (PRISMA 2020), (Page et al., 2021). Studies were included in this review if they contained ((migraine OR migraineurs OR headache) AND (in-vivo NMR spectroscopy OR magnetic resonance spectroscopy OR magnetic resonance spectroscopies OR in-vivo NMR spectroscopy OR MRS)) in their text, title, or abstract. Studies that did not involve headache were excluded. The remaining studies were examined and excluded if they were not specific to migraine, not related to MRS of the human brain, unoriginal contributions (review or letters), a conference proceeding, case study, or written in a language other than English. Three databases were used to search for studies: OVID EMBASE, WEB of SCIENCE and PubMed. Studies were included in this review if they were published before Sept 20, 2021. In addition to identifying articles using the search strategy described above, the reference lists of all included articles were inspected to make sure that studies were not missed.
The abstracts of all articles were screened for inclusion. Reasons for inclusion and exclusion were documented. All duplicate studies were removed. Results created from the same subjects were excluded. Each database was reviewed independently prior to duplication removals. Many studies were reviewed up to 3 times. The Ovid (EMBASE) search provided article type (article, conference, review) that was leveraged during the screening process. No automated tools were used.
Data collected from each study included the type of nuclei studied (1H, 23Na or 31P), the migraine subtype (e.g. migraine with aura, migraine without aura, hemiplegic migraine), the imaging sequence parameters such as repetition time (TR) and echo time (TE), the field strength the study was performed at, the number of patients (and controls when available), the voxel location, the type of imaging sequence used (PRESS, GRI, CSI etc.), the software used for data analysis, the main findings, and bibliometric data including authors and year of publication. All data items are presented in Table 1 and the inclusion process is outlined in Fig. 1. The visual presentation of results by metabolite and brain region is shown in Fig. 2. The results synthesis examines each metabolite individually as it relates to various brain regions in migraine. Conventional 1H-MRS is considered separately from multinuclear, functional MRS, and multidimensional studies.
Table 1.
Summary of MRS in migraine including migraine phenotype, methodology, and main findings.
| Nuclei | Migraine Phenotype* | TR [ms]/TE[ms] | Field Strength [T] | Number of Controls | Number of Patients | Voxel Location | Sequence | Software | Main Findings | Year | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1H | M | 1800/68 | 3 | 19 | 19 | Posterior Cigulate Cortex (PCC) | MEGA-PRESS | Gannet | Increased GABA+ (p = 0.002) in migraine patients (median, 1.41 institutional units (IU); interquartile range, 1.31–1.50 IU) relative to controls (median, 1.18 IU; interquartile range, 1.12–1.35 IU). Results give optimal GABA + cut-off value for migraine of 1.30 IU, with a sensitivity of 84.2 %, specificity of 68.4 % and positive likelihood ratio of + 2.67. The study demonstrates thatGABA + concentration has good diagnostic accuracy for migraine. | 2015 | Aguila, M.E., et al. |
| 1H | M | 1800/68 | not reported | 20 | 20 | PCC | MEGA-PRESS | Gannet | Positive association between GABA levels in the posterior cingulate of individuals with migraine and pain scores, specifically total short form McGill Pain Questionaire-2 (SF-MPQ-2) scores (ρ = 0.47; P = 0.04) and SF-MPQ-2 scores on intermittent (τ = 0.33; P = 0.04), neuropathic (τ = 0.37; P = 0.03), and affective (ρ = 0.49; P = 0.03) pain subscales. Positive association between GABA levels and Central Sensitization Inventory (CSI) scores (ρ = 0.48; P = 0.03). CSI was shown to have good diagnostic accuracy for migraine prediction. | 2016 | Aguila, M.R., et al. |
| 1H | MwA, MwoA | 6000/30 | 3 | 17 | 17 | WMH, contralateral | PRESS | LCModel | Decrease of NAA and Cr in the WMH + migraine voxels compared to WMH − migraine (NAA, P = 0.031, Cr, P = 0.001) and healthy controls (NAA P = 0.016, Cr P = 0 0.002). No statistical difference among the 3 voxel groups for Glx, Cho, Cr, mI. No Lac was present. | 2013 | Aradi, M., et al., |
| 1H | MwA | 5000/36.5 | 3 | 14 | 15 | visual cortex | PRESS | LCModel | In patients, increased Lac was observed during hypoxia of 61 % (CI 13–108 %), and during sham of 7 % (CI–21–35 %), p = 0.028. No changes in glutamate concentration and other metabolites (NAA, tCr) were reported in migraine patients realative to sham. There were no changes in any of the metabolites between controls and patients at 180 min (p > 0.05) or at 240 min (p > 0.05). | 2016 | Arngrim, N., et al. |
| 31P | M | 5000/(N/A) | 1.5 | 15 | 8 | occipital | DRESS | Gencap | Reduced phosphocreatine to inorganic phosphate ratio (PCr/P) in all patients, ranging from 1.75 to 2.48 with 3.57(0.27) in healthy controls. PCr/ATP ratio was reduced in five patients and borderline in other three wrt control average of 1.25(0.12). | 1990 | Barbiroli, B., et al. |
| 31P | MwA, MwoA | 5000/(N/A) | 1.5 | N/A | MwA 12 MwoA 4 |
occipital | DRESS | Gencap | Lower PCr (3.73 (0.30)) in patients vs 4.47 (0.27) in controls, and increased ADP in all patients (37 (6.5)) vs 28.9(2.51) for controls. | 1992 | Barbiroli, B., et al. |
| 1H | MwoA | 2000/30 | 3 | 15 | 15 | occipital/thalamus | MEGA PRESS | LCModel | Increased GLX levels in both the primary occipital cortex (z = 2.08, p = 0.038) and thalamus (z = 2.54, p = 0.011). No group differences were observed in GABA/tCr, NAA/tCr and Cr/H20 for these two regions. No significant correlations between pain intensity and levels of GLX or GABA in either of the two brain regions. | 2018 | Bathel A, et al. |
| 1H | EM | 2000/(31–229) | 3 | 33 | 32 | anterior cingulate cortex (ACC) | 2D MRS modified j-resolved PRESS | ProFit | No significant differences for Cr normalized metabolite levels. Reduction in Asp/Cr, NAA/Cr, and Gln/Cr reported in ACC in the migraine cohort (not statistically significant). | 2016 | Becerra, L., et al. |
| 1H | MwA, MwoA | 1800/80 | 3 | 31 | 25 (pediatric) MwA = 9, MwoA = 15 | thalamus, visual cortex, sensorimotor cortex | MEGA PRESS | Gannet | No significant differences reported in Glx (F(1, 51) = 0.241, P = 0.626), Glu (F(1, 51) = 0.001, P = 0.974), or GABA (F(1, 49) = 0.431, P = 0.515) levels in the thalamus between migraine and control groups. No significant differences in any of the metabolites in the sensorimotor cortex (Glx: F(1, 50) = 0.870, P = 0.356; Glu: F(1, 50) = 1.047, P = 0.311; GABA: F(1, 49) = 0.927, P = 0.340; GABA/Glx: F(1, 47) = 1.258, P = 0.268. There were no significant differences in Glx (F(1, 48) = 1.989, P = 0.165), Glu (F(1, 48) = 2.812, P = 0.100), GABA F(1, 48) = 0.264 in the visual cortex between migraine patients and controls. Levels of Glu were significantly lower in migraine with aura (P = 0.022) compared to controls. |
2021 | Bell, T., et al |
| 1H | MwA, MwoA | 2000/72 | 4 | 9 | 19, 9 MwA and 10 MwA | occipital | 3D LASER | 3D FT custom | GABA levels are not significantly different in migraineurs and controls, or in migraine with or without aura. There were no significant differences in GABA (F(2, 46) = 0.458, P = 0.635), Glx (F(2, 46) = 2.798, P = 0.071), or GABA/Glx ratios (F(2, 45) = 1.024, P = 0.367) in the visual cortex between the 3 groups (MwA, MwoA and controls) | 2008 | Bigal, M.E., et al. |
| 31P | MwA, MwoA, HM | 1000/(N/A) | 3 | 40 | 46 (19MwA, 19MwoA, 8 HM) | posterior lower slice (calcarine cortex, temporal gyri, occipital gyri); anterior center slice (frontal gyri, frontal forceps, genu of corpus callosum); posterior center slice (occipital cortex association regions with some of the calcarine cortex); and occipital cortex | 3D CSI | SPARC 20 | Pronounced changes observed in hemiplegic migraine patients. Significantly decreased [Mg2+] in patients relative to control subjects in posterior but not anterior brain regions. A trend toward decreased [PCr] reported in the occipital cortex. No significant changes found in [PME], [PDE], or [Pi] in these regions. No significant metabolite changes were found in MwoA and MwA patients. | 2002 | Boska, M.D., et al. |
| 1H | MwA | 4000/8.5 | 3 | 13 | 13 | occipital | SPECIAL | LCModel | Reduced occipital GABA levels (by 10 %) in migraine patients relative to controls (t = 1.8, p = 0.042). No changes in glutamate levels between patients and controls (t = 0.85, p = -0.41). Glutamate levels in migraine patients correlated with the blood-oxygenation-level-dependent (BOLD) signal in the primary visual cortex during visual stimulation. | 2015 | Bridge H., rt al. |
| 1H | MwA, MwoA | 1500/68 | 3 | 16 | 16 (9 MwA, 7 MwoA) | occipital | MEGA PRESS | Gannet | Decreased Glx in migraineurs relative to controls (Controls: 1.90 ± 0.27iu, Migraineurs:1.61 ± 0.12iu; Welch’s t (20.94) = 4.04, p < 0.001, Cohen’s d = 1.42, , resulting in a significantly higher GABA/Glx ratio in the migraine cohort (Controls: 1.78 ± 0.36iu, Migraineurs: 2.14 ± 0.19iu; Welch’s t(22.78) = 3.54, p = 0.002; Cohen’s d = 1.25, . No change in occipital GABA levels between groups (mean ± sd: Controls: 3.31 ± 0.32iu, Migraineurs: 3.42 ± 0.24iu; t-test: t(30) = 1.12, p = 0.27; Cohen’s d = 0.39, . Neither GABA levels, nor Glx levels correlated with rivalry percept duration data. | 2019 | Chan YM, et al. |
| 1H | MwoA | 2000/144 | 1.5 | 16 | 15 | occipital lobe | PRESS | JMRUI | Significant reduction of NAA/Cr ratio in patient group relative to control group (p = 0.0001). Increased Cho/Cr (0.671 controls, 1.159 patients, p = 0.000*) and mI/NAA in the patient group (p = 0.004). The Cho/Cr ratio significantly correlated with disease duration (r = 0.686, p < 0.001) and number of disease attacks (r = 0.758, p < 0.001). No significant relationship between Increased duration of illness and frequency of attack with mI/NAA ratio (r = 0.125, p = 0.569). | 2020 | Dehghan, A., et al. |
| 1H | HM | 2000/35 | 1.5 | 17 | 15 | SCV, visual, parietal | PRESS | LCModel | Significant disease effect for metabolite concentrations (reduction of NAA, Glu and increase in mI) in the superior cerebeller vermis (Wilks λ = 0.395, F = 7.036, df = 5.000, p < 0.001). No significant effects in the visual cortex (Wilks λ = 0.858, F = 0.759, df = 5.000, p = 0.588) and in the parietal cortex (Wilks λ = 0.816, F = 0.991, df = 5.000, p = 0.446). | 2005 | Dichgans, M., et al. |
| 1H | VM | TR not provided, TE = 135 | 1.5 | 20 | 25 | occipital lobe | long echo time MRS | not specified | Lactate peaks detected in occipital lobes of patients. Lactate correlates with presence of non paroxysmal positional nystagmus. | 2020 | ElSherif, M., et al. |
| 1H | MwA, MwoA | 6000/30 | 3 | N/A | 17 (10 MwA, 7 MwoA) | WMH, contralateral | PRESS | LCModel | Significantly decreased NAA (median values 8.133 vs 7.153 mmol/L, P = 0.009) and creatine/phosphocreatine (median values 4.970 vs 4.641 mmol/L, P = 0.015) concentrations were reported compared to baseline, indicative of axonal loss and glial hypocellularity with decreased intracellular energy production. |
2015 | Erdelyi-Botor, S., et al. |
| 1H | M, Pain and cognitive impairment | 2000/35 | 1.5 | 193 | 207 (33 with Migraine) | PCC | PRESS | LCModel | Significantly lower NAA (P = 0.003) and NAA/Cr (P = 0.015) in migraine group relative to controls. Significantly greater Glx (glutamate + glutamine) (P < 0.001) and Glx/Cr (P < 0.000) in patients relative to controls. Glu, Glx, NAA, and their Cr ratios exhibited a negative correlation with age, whereas ml and Cho exhibited a positive correlation with age. | 2014 | Fayed, N., et al. |
| 1H | MwA, MwoA | 2500/66 | 3 | 19 | 27 | paracingulate and occipital cortices | PRESS | LCModel | Higher Glu/Gln ratio was reported in the occipital cortex of migraine patients compared with healthy control subjects (4.87 for migraineurs -standard deviation (SD) = 2.74 and 3.42 for controls (SD = 1.52, P = 0.042). The authors observed elevated Glu levels (6.98 for migraineurs (SD = 0.85) and 6.22 for controls (SD = 0.97, P = 0.007), and higher Glu/Cr + PCr ratio (1.18 for migraineurs (SD = 0.18) and 1.00 for controls (SD = 0.16, P = 0.001) in anterior paracingulate cortex in migraine patients. | 2013 | Gonzalez de la Aleja, J., et al. |
| 1H | M and trigeminal neuralgia | 1000/144 | 3 | 14 | 36 | multi voxel | CSI | Functool software provided by the Advanted windows of General Electric |
Significant difference in NAA/Cho between migraine and control groups (p = 0.05). In trigeminal neuralgia group, NAA/Cho of both sides was lower than those of control group (p = 0.05). For the affected side, the difference of NAA/Cho in migraine group was observed in the left side, not in the right side (p = 0.05). Bilateral differences were observed in NAA/Cr and Cho/Cr in trigeminal neuralgia group. Comparing the metabolite concentration ratios of affected and contralateral thalami in migraine and trigeminal neuralgia groups, only NAA/Cr showed a significantly difference (p = 0.05). | 2008 | Gu, T., et al. |
| 1H | MwoA | 2000/144 | 3 | 14 | 15 MwoA, 14 cervicogenic headache (CH) | thalamus, ACC, paracentral gyrus (posterior) | 2D multivoxel PRESS | manufacturer supplied | Increased NAA/Cr in bilateral thalamus in MwoA patients after acupuncture threatment (left: 1.90 ± 0.22 vs 2.11 ± 0.35, T = 3.43, ES = 0.68, p = 0.006), (right: 1.83 ± 0.18 vs 1.96 ± 0.14, T = 3.38, ES = 0.81, p = 0.006). In the ACC there was no change in NAA/Cr in MwoA, decrease of NAA/Cr and Cho/NAA in CH group, and increase in NAA/Cr in HC group. No changes were observed in Cho/NAA. | 2018 | Gu, T., et al. |
| 1H | EM, CM | 1000/144 | 1.5 | 16 | 19 EM, 53 CM | brainstem | PRESS | GE scanner | Increased NAA/Cr ratios in dorsal pons in patients with episodic migraine (right, P = 0.014; left, P = 0.034) relative to chronic migraine and controls. NAA/Cr ratios in the dorsal pons inversely correlated with headache frequency (right, r = − 0.350, P = 0.004; left, r = − 0.284, P = 0.019) and intensity (right, r = − 0.286, P = 0.019; left, r = − 0.244, P = 0.045), but not disease duration. | 2012 | Lai, T.H., et al. |
| 1H | MwA, MwoA | 2000/68 | 3 | N/A | 1 MwA, 13 MwoA | anterior cortex, posterior cingulate cortex and medial prefrontal cortex | MEGA-PRESS | not specified | Significant decrease of GABA + in PCC (p = 0.015) for migraine patients responding to levetiracetam treatment. | 2018 | Li, Q. et al. |
| 1H | M | 1500/35 | 1.5 | 30 (15 with and 15 without major depressive disorder) | dorsolateral prefrontal cortex | PRESS | GE SAGE | No differences between NAA/tCr and Cho/tCr ratios between migraine patients with and without depressive disorder. Relative to patients without major depressive disorder (MDD), migraine patients with MDD had higher mI/tCr ratios in the bilateral dorsolateral prefrontal cortex (p = 0.02, left; p = 0.02, right, Mann-Whitney U test). The mI/tCr ratios in the right dorsolateral prefrontal cortex were positively correlated with BDI scores (r = 0.52, p = 0.003). | 2015 | Lirng J. et al. | |
| 31P | MS, MwoA, MwA, and cluster headache | 4500/(N/A) | 1.5 | 36 | 78 (7 M stroke, 21 MwoA, 50 MwA, 13 cluster headache) | occipital | DRESS | not specified | Statistically significant differences in Mg2+ among different groups of subjects in the occipital lobes of migraine patients with migraine stroke (152 microM +/- 6) was significantly (p < 0.05) lower than in controls (184 microM +/- 5) and in patients with prolonged aura (156 microM =/- 7; p < 0.05), with typical aura or basilar migraine (159 micorM =/- 4; p < 0.05) and without aura (169 microM +/- 6; p < 0.05). In patients with cluster headache, brain free Mg2+ (166 microM +/- 5) was similar to findings in patients with migraine without aura and significantly (p < 0.05) lower than controls. |
2001 | Lodi, R., et al. |
| 31P | juvenile M | 5000/(N/A) | 1.5 | 12 | 15 | occipital | DRESS | Gencap | Reduced brain intracellular free Mg2+ concentration (by 25 %) in patients relative to controls. | 1997 | Lodi, R., et al., |
| 1H | MwA | 1500/30 | 1.5 | 7 | 8 | cerebellum | PRESS | JMRUI | Reduced Cho/NAA (p = 0.005) and Cho/Cr (p = 0.003) in the patient group with respect to the age-matched control group. No significant differences in Cr/NAA and mI/NAA or mI/Cr. | 2003 | Macrı, M. et al |
| 23Na | M, tension type | 120/0.2 | 3 | 12 | 24 (12 migraine, 12 migraine with tension headache) | CSF | 3D GRE | Osirix | Significantly higher 23Na concentration was observed in patients compared to controls (p < 0.001). Increased Na concentrations in CSF of migraine and mixed migraine/TTH group relative to control group (p = 0.007 and p < 0.001). A positive correlation was noted between pain level and TSC in the CSF (r = 0.62) in patients. | 2019 | Meyer, M.M., et al |
| 1H | MwoA | 1000/144 | 1.5 | 10 | 22 | bilateral thalamus | PRESS | Functool software provided by the Advanted windows of General Electric |
NAA/Cho and NAA/Cr ratios were significantly decreased in patients relative to controls (P = 0.003 and 0.027, respectively). The mean Cho/Cr, MI/NAA and Lac/NAA ratios showed no significant differences between patients and controls (P = 0.83, 0.10, and 0.09, respectively). | 2013 | Mohamed, R.,et al. |
| 31P | MwoA | 5000/(N/A) | 1.5 | 18 | 22 | occipital cortex | DRESS | in house software | Significantly low PCr content in MwoA compared to healthy controls (P < 0.001). Mean P was increased in migraine group (not significant). ADP was increased and PP was decreased in the migraine group (p < 0.001) | 1994 | Montagna, P., et al. |
| 1H | CM, EM | 1500/30 | 3 | 25 | 25 CM, 24 EM | bilateral medial walls of the brain | EPI | LCModel | In the right hemisphere of chronic migraine patients, NAA reduction was found for the ACC (F = 5.08, P = 0.009) and the thalamus (F = 7.39, P = 0.001) but not for the occipital cortex (F = 1.91, P = 0.155). Significant group effects were found for tCr in the left ACC (F = 3.406, P = 0.039) and the left thalamus (F = 5.144; P = 0.008) and for myo-inositol in the right ACC (F = 3.144, P = 0.050). | 2018 | Niddam DM, et al. |
| 1H | CM | 2530/3 | 3 | 16 | 16 | thalamus, ACC, PCC and mid cingulate cortex | MRSI (EPI) | LCModel | Reduced thalamic tNAA (left p = 0.005, right p = 0.0008) and Cr (left p = 0.003), reduced mI in the anterior cingulate cortex (left p = 0.004, right p = 0.001). Elevated choline (p = 0.048) and Glx (p = 0.006) in the right mid cingulate cortex in both patient groups. A negative association between mI laterality index in the anterior cingulate cortices and number of days per month with acute medication use was found across all patients. | 2020 | Niddam DM, et al. |
| 1H | M, whiplash headache, low back pain | 2000/68 | 3 | 22 | 56 (20 migraine) | PCG | MEGA-PRESS | Gannet | Increased GABA + levels in the posterior cingulate gyrus migraine and low back pain participants relative to controls (migraine 4.89 IU +/-0.62 vs controls 4.62 IU +/-0.38, p = 0.02). | 2021 | Peek, A.L., et al. |
| 1H | M | 2000/(30–260) | 4 | 8 | 10 | ACC, left insula | PRESS | LCModel | No significant metabolite differences between the two subject cohorts in the ACC and the insula using ANOVA. Differences in NAAG and GLn between subject cohorts were observed using Linear discriminant analysis (LDA) in the ACC and insula. | 2009 | Prescot, A., et al. |
| 31P | MwA, MwoA | 1512/(N/A) | 1.89 | 25 | 19 | frontal, frontotemporal, parieto-occipital or occipital cortex | PRESS | in house software | Statistically significant decrease in brain Mg between migraine patients and controls (p < 0.02). | 1989 | Ramadan, N.,et al. |
| 1H | MwoA | 4000/(30–288) | 3 | 25 | 22 | visual cortex | PRESS | JMRUI | No changes found in any metabolites in patients without aura. | 2010 | Reyngoudt, H., et al. |
| 1H | MwoA | 2000/288 | 3 | 20 | 20 | visual cortex | PRESS | jMRUI | No significant differences in metabolate ratios and absolute metabolite concentrations, including Lac, between MwoA patients and controls before photic stimulation | 2011(b) | Reyngoudt, H., et al. |
| 31P | MwoA | 1550/2.37 | 3 | 26 | 19 | visual cortex | 2D CSI | syngo | Decreased PCr in MwoA patients (p = 0.001). Significantly lower ATP in MwoA (p = 023). | 2011(a) | Reyngoudt, H., et al. |
| 1H | MwA | 1500/288 | 1.5 | 11 | 10 | visual cortex | PRESS, functional MRSI | not specified | Functional MRS. Higher visual cortex Lac/NAA in migraine with aura (MwA) compared with healthy volunteers (HV) or migraine with aura with at least one of the following comorbidities: paraesthesia, paresis or dysphasia (MwAplus) (p < 0.05 for MwA vs HVs, P < 0.001 for MwA vs MwAplus). Lower Lac/NAA in the MwAplus group compared with healthy volunteers. No differences in Lac/NAA before stimulation between groups in non-visual cortices. During visual stimulation, the Lac/NAA in visual cortices remained high in MwA compared with the other groups also in the resting state after stimulation (P < 0.01 for MwA vs MwAplus, P < 0.05 for MwA vs HVs). In MwA plus lactate increased only during stimulation, only in visual cortex; in MwA resting lactate was high in visual cortex, without further increase during stimulation. | 2005 | Sandor, P.S., et al. |
| 1H | MwA, MwoA | 2000/144 | 1.5 | 10 | 44 (22 MwA, 22MwoA) | visual cortex | PRESS, functional MRSI | not specified | Decrease of NAA (-14.61 %) following photic stimulation in patients with migraine with aura realative to migraine patients without aura and control subjects accompanied by a slight increase in lactate. No differences observed in Cr and Cho signals between groups. | 2005 | Sarchielli, P., et al |
| 31P, 1H | MwA | 31P: 2500/(N/A) 1H 1500/135 |
2 | 16 | 21 | cortical | 1H: PRESS 31P: FID |
JMRUI | Decreased PCr/Pi (p < 0.003) in patients with hemiplegic migraine. No differences noted in metabolites measured by 1H. | 2007 | Schulz, U.G., et al. |
| 31P | MS, MwoA | 1500/135 | 2 | 14 | 14 | lentiform nucleus | PRESS | JMRUI | Patients with persistent aura without infarction had lower PCr/Pi ratios (mean = 1.61, SD = 0.10) compared with controls (1.94,0.35, P = 0.011) and with patients with migrainous stroke (1.96,0.16, P = 0.0001). The differences were present in cortical tissue only. In migrainous stroke patients, no significant differences were found in the metabolite ratios from those of controls without migraine. | 2009 | Schulz, U.G., et al. |
| 1H | MwA | 2000/37 | 3 | 10 | 10 | visual cortex | PRESS | Philips curve fitting software | Significantly higher (Glx/Cr) reported in migraineurs relative to healthy controls (p = 0.036).The baseline values for the NAA/Cr ratio and of Cr did not differ between groups for both sessions and for both baseline recordings. | 2012 | Siniatchkin M., et al. |
| 1H | MwA | 2000/44 | 3 | 16 | 14 | occipital and somatosensory cortices | SPECIAL | LCModel | No differences GABA/Cr in the occipital lobes of 14 migraine with aura patients relative to 16 matched healthy controls (p = 0.744). Similarly, no difference for the same two groups in the somatosensory cortex (p = 0.305). | 2019 | Staermose, T.G., et al. |
| 31P | HM | 5000/3.3 | 1.5 | 2 | 3 | occipital | DRESS | Gencap | Reduced PCr and high ADP in occipital regions. Also reportred was a decreased phosphorylation potential in HM. | 1995 | Uncini, A., et al. |
| 1H | CM and Cluster Headache | 1500/144 | 1.5 | 21 | 47 cluster headache, 16 CM | bilateral hypothalami | PRESS | Functool software provided by the Advanted windows of General Electric |
Significant reduction in 1H‐MRS metabolite ratios (NAA/Cr and Cho/Cr) in the hypothalamus in patients with episodic cluster headache compared to controls and CM (NAA/Cr p < 0.001, Cho/Cr p = 0.006). No change in metabolite ratios in CM compared to HC. | 2006 | Wang, S.J., et al. |
| 1H | M, MwA | 1500/270 | 1.5 | 6 | 6 | occipital | SE sequence | not specified | High lactate levels in 5 patients with migraine attacks within 2 months. The patient with no migraine attack history for previous 4 years did not have a Lac peak. | 1996 | Watanabe, H., et al., |
| 31P | M | 1512/(N/A) | 1.89 | 12 | 27 | right or left frontal, fronto temporal,parieto occipital and occipital | FID | not specified | No changes in pHi were measured during a migraine attack. | 1988 | Welch, K.M., et al. |
| 31P | M | 1512/(N/A) | 1.89 | 12 | 27 | right or left frontal, fronto temporal,parieto occipital and occipital | not specified | not specified | Compared with normal subjects, a lower mean PCr/Pi ratio was observed in patients studied during a migraine attack. Reduction in mean PCr/TP ratio and increase in mean Pi/TP ratio (all p values < 0.05). No change in brain pHi was reported during or between attacks. Disordered nergy phosphate metabolism was reported during a migraine attack. | 1989 | Welch, K.M., et al. |
| 1H | Migraine Like Headache | 3000/38 | 3 | N/A | 17 | brainstem, thalamus | PRESS | LCModel | In migraine subjects, increase in Glu in brainstem was reported after sildenafil administration (5.6 %, P = 0.039) compared to placebo injection. No changes in GLU, LAC or NAA in thalamus. | 2018 | Younis, S., et al. |
| 1H | MwoA | 3000/38.3 | 3 | N/A | 26 | pons | PRESS | LCModel | Increased pontine tCr (3.5 %, p = 0.041) and tNAA levels (3.5 %, p = 0.014). No change in pontine GLU (p = 0.873) or Lac (p = 0.099). | 2021a | Younis, S., et al. |
| 1H | MwoA | 3000/38.3 | 3 | 16 | 33 | pons | PRESS | LCModel | No altered interictal pontine NAA, Glx or lactate levels were found in migraine. Increases of tCr (9 %,p = 0.009) were reported suggestive of altered pontine energy metabolism. | 2021b | Younis, S., et al. |
| 1H | MwoA | 2000/35 | 3 | 13 | 19 MwoA (6 woA during attack, 13 during interictal period) | occipital cortex | PRESS | LCModel | Reduced GSH/tCr in patients without aura during attack was found relative to patients with aura during interictal period (MWoA-DI) and HC (p = 0.008 and p = 0.011). MWoA-DI patients showed lower tCho/tCr than those in the other two groups (p = 0.031 and p = 0.022). The GSH/tCr ratio was positively correlated with attack frequency in the MWoA-DI group. The tCho/tCr ratio was positively correlated with attack frequency and Migraine Disability Assessment Scale (MIDAS) scores in the MWoA-DA group. There were no statistical differences in tCho/tCr between the MWoA-DA and HC groups. There were no statistical differences in NAA/tCr, mI/tCr, and Glu/tCr among the three groups (all p > 0.05). | 2021 | Zhang,L., et al. |
| 1H | HM | 2000/21 | 7 | 19 | 18 | pons, hypothalamus, cerebellum and visual cortex | STEAM | LCModel | Decreased NAA/Cr in cerebellum (median 0.73, range 0.59–1.03) compared to healthy controls (median 0.79, range (0.67–0.95); p = 0.02) | 2014 | Zielman, R., et al. |
| 1H | MwA, MwoA, HM | 4000/10 | 7 | 43 | 38 MwA, 27 MwoA, 18 HM | CSF | NOESY | in house software | 2-Hydroxybutyrate was significantly lower for hemiplegic migraine (23 mM+/-9.2) compared to controls (29 mM+/-12, p = 0.003). 2-Hydroxyisovalerate levels were significantly lower for hemiplegic migraine (5 mM +/- 1.4) compared to controls (7 mM +/- 2.1, p = 0.003). Cho was significantly lower in both migraine with and without aura. | 2016 | Zielman, R., et al. |
| 1H | MwA, MwoA | 5000/30 | 7 | 24 | 50 (23 MwA, 27 MwoA) | visual cortex | semi-LASER, diffusion weigted PRESS | LCModel | Increased Glu levels reported in visual cortices of migrainers without aura (7.02 ± 0.50 mM) compared to healthy controls (6.40 ± 0.78 mM, P = 0.042) | 2017 | Zielman, R., et al. |
Point Resolved Spectroscopy (PRESS), Depth Resolved Surface Coil Spectroscopy (DRESS), Free induction Decay (FID), Stimulated Echo Acquisition Mode (STEAM),Localization by adiabatic selective refocussing (LASER), Nuclear Overhauser Effect Spectroscopy(NOESY) Grey Matter (GM), White Matter(WM) Cerebrospinal fluid (CSF).
Not applicable (N/A).
Fig. 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and other sources.
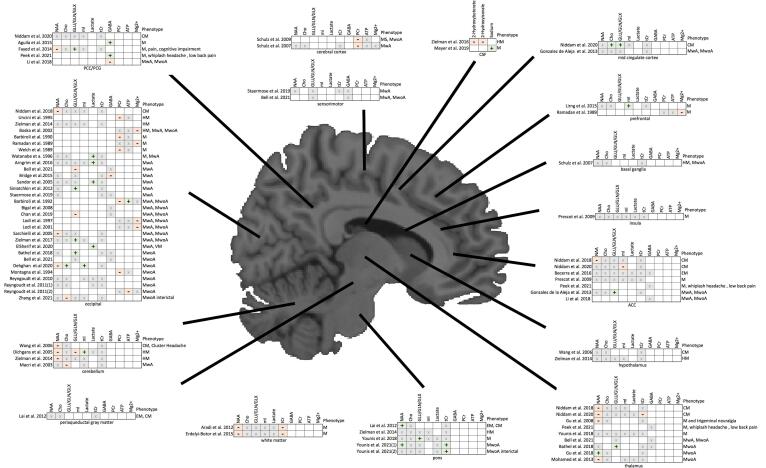
Fig. 2.
Summary of main findings from all studies. Abbreviations are as follows: M = Migraine, CM = chronic migraine, EM = episodic migraine, MS = migrainous stroke, MwA = Migraine with aura, MwoA = Migraine without aura, VM = visual migraine, HM = hemiplegic migraine, PCC = posterior cingulate cortex, PCG = posterior cingulate gyrus, ACC = anterior cingulate cortex, NAA = N-Acetyl Aspartate, Glx = Glutamate and Glutamine complex, Lac = Lactate, Cr = Creatine, Pcr = Phosphocreatine, tCr = total Creatine, Cho = Choline, tCho = Choline + Phosphocholine, mI = Myo-inositol. Plus signs (+) indicate increases were reported, Negative signs (–) represent reported decreases, and × means no change was reported. Blank squares indicate that metabolite was not investigated in the study.
Results
Study selection
The multiple database search of PubMed, EMBASE, and Web of Science for variations of search terms for MRS and headache until 20th September 2021 resulted in identifying 2897 studies, 676 which were duplicates and 1836 which were not related to headache. Of the remaining 385 studies examined, articles were excluded for not studying migraine (n = 114), not being MRS studies of the human brain (n = 128), for not being original contributions (n = 52), and for being conference papers (n = 24) or case studies (n = 11), or for not being written in English (n = 3). The manuscripts of all resulting reports were included in this review (n = 54). The reference lists of all included reports were carefully read, and relevant references were added (n = 2). One article had overlapping patient groups. The migraine patients included in Aradi et al., (Aradi et al., 2013) were re-used for a longitudinal study by Erdelyi-Botor et al., (Erdelyi-Botor et al., 2015). Included in this review are 56 studies of migraine with and without aura that involve MRS of the human brain.
Study characteristics
The field strengths of the included studies consisted of eighteen at 1.5 T, five at 1.9 to 2 T, twenty-seven at 3 T, two at 4 T, three at 7 T and one which was undisclosed. In regard to the pulse sequences used, twenty-seven studies used PRESS MRS pulse sequence, six studies used MEGA-Press, six used DRESS, three -CSI, two -SPECIAL, two -fid, one used LASER, one -semi-LASER, one −3D GRE, two -EPI, one -NOESY, one -long echo time MRS, one -STEAM and one was unspecified. In terms of analysis software, eighteen used LCModel, eight -MRI manufacturer’s software, six -JMRUI, five -Gannet, four -Gencap, one -OSIRIX, one -ProFit, one -Sparc20, four used custom in-house software, and eight were not specified. In terms of voxel selection, the majority of the studies (28) examined the occipital lobe, nine studied the thalamus, seven used the anterior cingulate cortex (ACC), five used the posterior cingulate cortex/posterior cingulate gyrus (PCC/PCG), five studied the pons, four used the cerebellum, and other brain regions were investigated in two or fewer studies.
Results of individual studies
Summaries of study parameters and results as described by the authors are presented in Table 1. The metabolic results from each study, organized by brain region and migraine phenotype are shown in Fig. 2.
Discussion
Summary of results from migraine studies
MRS studies reported varying levels of NAA in migraine. In this review, compared to controls, 24 studies reported no difference, 13 reported lower NAA, and 3 reported higher NAA levels. NAA is one of the most abundant metabolites in the brain. It has contributions of N-Acetyl aspartyl glutamate (NAAG), glycoproteins, and amino acids. NAA is primarily of neuronal origin and is considered a maker of neuronal density and integrity. Its reduction signifies neuronal loss or dysfunction. Decreased NAA was reported in the occipital lobes of MwoA patients relative to healthy controls (Sarchielli et al., 2005, Dehghan et al., 2020) and in the cerebellum in patients with hemiplegic migraine (Dichgans et al., 2005, Zielman et al., 2014), perhaps indicating reduced neuronal viability in these migraine populations. Reduction of NAA/Cho was observed in the left thalamus of a migraine cohort, suggestive of neuronal damage of left thalamic neurons. Reduced tNAA has been found in the anterior cingulate cortex and thalamus of chronic migraine patients compared to healthy controls and those with episodic migraine (Mohamed, 2013, Niddam et al., 2018, Niddam et al., 2020). These regions in which reduced NAA has been identified have also been implicated as part of migraine pathophysiology in other imaging studies. The anterior cingulate cortex is commonly implicated in migraine and pain pathophysiology and is thought to participate in the affective components of pain processing (Fuchs et al., 2014). Most studies demonstrating abnormal structure of the anterior cingulate cortex in migraine demonstrate less volume or thickness of this region, sometimes the extent to which correlates with headache frequency. MRS studies demonstrating reduced NAA in the anterior cingulate cortex could suggest that the reduced volume and cortical thickness are at least partially due to neuronal loss. Abnormal structure and function of the cerebellum is another common finding in migraine studies, including being strongly implicated in hemiplegic migraine and related genetic diseases (Vincent and Hadjikhani, 2007, Kim et al., 2008, Schmidt-Wilcke et al., 2008, Valfre et al., 2008, Barros et al., 2013, Jin et al., 2013, Hougaard et al., 2016). The thalamus and altered thalamocortical connectivity have been suggested to play roles in multiple aspects of migraine, including pain processing, pain modulation, allodynia, photosensitivity, and multisensory integration (Tu et al., 2019, Younis et al., 2019). Several studies have demonstrated abnormal thalamic structure and function in those with migraine (Xue et al., 2013, Magon et al., 2015, Coppola et al., 2016, Wang et al., 2016, Wei et al., 2019). Combined MRS and structural imaging studies might be useful for understanding the pathology underlying structural changes associated with migraine. For example, reduced NAA measures in brain regions that also demonstrate loss of cortical thickness or volume could suggest that the structural changes are at least partially due to neuronal loss or dysfunction.
In this review, 12 studies reported no changes in Lac, while four showed an increase in Lac levels in the visual cortices of migraine patients. Lac is an end-product of anaerobic glycolysis. As a marker of anaerobic metabolism, it is not present in high enough quantities to be detected in spectra derived from healthy brain tissue. Increases of Lac are observed in necrotic areas, in the presence of various infections, hypoxia, stroke, and with some mitochondrial diseases. Increased Lac levels have been demonstrated in those with vestibular migraine (ElSherif et al., 2020) and in the visual cortices of migraine subjects as a result of visual stimulation (Watanabe et al., 1996, Montagna et al., 2000, Sandor et al., 2005, Arngrim et al., 2016). Increased Lac was shown to accompany hypoxia induced migraine in the visual cortical areas of migraine subjects (Arngrim et al., 2016). Increased Lac in those with migraine could relate to migraine-associated mitochondrial dysfunction, the cerebral hypoperfusion associated with cortical spreading depolarization and the resulting migraine aura.
Another metabolite difficult to quantify with conventional MRS is glutamate (Glu). Glu and Glutamine (Gln) are neurotransmitters compartmentalized in neurons and glia. Glutamate is the primary excitatory neurotransmitter in the brain, with a vital role in normal brain function, memory and learning, and mood and anxiety disorders (Hasler et al., 2019). Glu and Gln have similar metabolic structures and have similar, hard to resolve spectra. Isolating Glu from Gln is difficult with standard MRS techniques. Combined Glu and Gln values referred to as Glx are typically reported but the combined results are much more difficult to interpret. The majority of migraine studies (18), studying different ROIs, reported no change in Glu/Glx levels in migraine. Overall, migraine studies reported conflicting information including increases in occipital, thalamic, PCC, MCC and ACC Glx levels (Siniatchkin et al., 2012, Gonzalez de la Aleja et al., 2013, Fayed et al., 2014, Bridge et al., 2015, Zielman et al., 2017, Bathel et al., 2018, Younis et al., 2018, Niddam et al., 2020, Bell et al., 2021), occipital and cerebellar decreases in Glx (Dichgans et al., 2005, Chan et al., 2019, Bell et al., 2021), or no changes in occipital or anterior cingulate Glx (Watanabe et al., 1996, Sandor et al., 2005, Sarchielli et al., 2005, Bigal et al., 2008, Prescot et al., 2009, Reyngoudt et al., 2010, Reyngoudt et al., 2011b, Aradi et al., 2013, Zielman et al., 2014, Bridge et al., 2015, Erdelyi-Botor et al., 2015, Arngrim et al., 2016, Becerra et al., 2016, Zielman et al., 2017, Bathel et al., 2018, Niddam et al., 2018, Dehghan et al., 2020, ElSherif et al., 2020, Niddam et al., 2020, Bell et al., 2021, Zhang et al., 2021). Results are variable probably due to the difficulty of resolving the Glu-Gln peak at low magnetic fields.
GABA is the main inhibitory neurotransmitter in the brain. GABA can be measured by using specialized 1H spectral editing techniques using sequences such as MEGA-PRESS that filter the GABA signal based on J-coupling of the molecule. Altered GABA concentrations and GABA-Glu ratios could lead to an imbalance of inhibitory-excitatory process in the migraine brain.
There are discrepancies in the reports of GABA levels in the reported migraine studies. Increases in GABA were found in two studies in the PCC and PCG (Aguila et al., 2015), (Peek et al., 2021). Such increases have also been reported in non-migraine chronic pain conditions; therefore, they might not be attributable specifically to migraine (Enna and McCarson 2006). Decreased occipital and ACC levels of GABA were reported in two studies, indicative of reduction of inhibitory mechanisms (Bigal et al., 2008, Bridge et al., 2015, Li et al., 2018). No changes in GABA levels of migraine subjects in occipital, thalamic, ACC and sensorimotor regions were reported by eight studies (Bigal et al., 2008, Becerra et al., 2016, Bathel et al., 2018, Li et al., 2018, Chan et al., 2019, Staermose et al., 2019, Bell et al., 2021, Peek et al., 2021). Similar to other MRS-measured metabolites, GABA levels might be highly dependent on the timing of measurement in relation to the most recent migraine attack, overall headache frequency, and timing of the most recent aura event.
In MRS studies, there is insufficient signal-to-noise to resolve the Cr and PCr peaks, hence, combined values of Cr and PCr are most often reported. The combined Cr + PCr, referred to as total creatine or tCr in 1H-MRS studies, provides information about intracellular energetics. In this review, 24 migraine studies showed no changes in tCr levels, suggesting intact brain energy metabolism. Of the ones that showed changes, 2 reported increases in the pons (Younis et al., 2021a, Younis et al., 2021b), 2 reported decreases in white matter (Aradi et al., 2013, Erdelyi-Botor et al., 2015), and one reported decrease in thalamic regions in migraine (Niddam et al., 2020). Although most of the studies demonstrated no differences in tCr in those with migraine, discrepancies that were reported are likely due to the difficulty in resolving the Cr and PCr peaks with conventional MRS. Discrepancies for Cr are particularly concerning since Cr is typically used as an internal reference standard for reporting metabolite ratios. If its measurement is not precise, there is uncertainty about results that use Cr as a reference standard.
Multinuclear imaging studies predominantly investigated phosphorous. Phosphorus MRS allows for in-vivo measurements of energy metabolism. Commonly reported metabolites in phosphorous MRS studies are levels of phosphorus (P), phosphocreatine (PCr), inorganic phosphate (Pi), adenosine diphosphate (ADP), adenosine triphosphate (ATP), and phosphorylation potential (PP). The most consistent results of the 31P studies in migraine were decreased levels of occipital PCr (Welch et al., 1988, Barbiroli et al., 1990, Barbiroli et al., 1992, Montagna et al., 1994, Uncini et al., 1995), as well as increased ADP in migraine patients with and without aura with respect to controls (Barbiroli et al., 1990, Barbiroli et al., 1992) and decreased PCr/P in migraine patients with persistent aura and in patients with hemiplegic migraine (Schulz et al., 2007, Schulz et al., 2009). Low concentrations of PCr/P could signify low availability of free energy in the cells. Increased ADP is indicative of higher metabolic function or low energy reserves. Studies demonstrating disrupted and unstable energy metabolism between migraine attacks (i.e. during the interictal phase) could suggest that an altered brain metabolic state contributes to migraine susceptibility and the initiation of migraine attacks (Montagna et al., 1994, Uncini et al., 1995, Lodi et al., 2001). In addition, studies showed a reduction of phosphorylation potential (Barbiroli et al., 1990, Montagna et al., 1994, Lodi et al., 1997, Reyngoudt et al., 2011a). These abnormalities could indicate enhanced brain excitability or the presence of mitochondrial dysfunction. In addition to MRS findings, there are several lines of evidence that suggest the presence of mitochondrial dysfunction in migraine: a) higher migraine frequency in females; b) maternal inheritance of migraine; c) high prevalence of migraine amongst those with mitochondrial diseases; d) triggering of migraine attacks by conditions that impact energetics, such as stress, missed meals, and physical exercise; and e) migraine preventive effect of riboflavin and coenzyme Q10 (Vollono et al., 2018, Fila et al., 2019, Tiehuis et al., 2020, Terrin et al., 2022). An additional factor that can contribute to this dysfunction is reduced levels of Mg. Decreased levels of magnesium have been linked to several components of the migraine attack including the induction of cortical spreading depression and inappropriate modulation of nociceptive signals (Domitrz and Cegielska 2022). Several studies have reported low brain Mg2+ in migraine populations with and without aura (Lodi et al., 1997, Lodi et al., 2001, Ramadan et al., 1989, Boska et al., 2002), and low magnesium serum levels have been associated with increased risk of developing migraine attacks (Assarzadegan et al., 2016).
Functional MRS studies examine metabolic changes nearly in real-time (i.e. time scale on the order of seconds). The mechanisms and temporal dynamics of the regulation of these metabolites are not well understood within the context of migraine. Functional phosphorus MRS studies have shown reductions in PCr and ATP in the occipital lobes of interictal migraine without aura subjects reflective of altered cortical energy metabolism (Reyngoudt et al., 2011a). No abnormalities were found in Cho, Glu, Lac or NAA in the visual cortex after visual stimulation in migraine without aura subjects, providing evidence that there is not a switch to non-aerobic glucose metabolism in response to photic stimulation (Reyngoudt et al., 2011b).
The reviewed MRS studies of migraine reported different and often conflicting information. The varied results might be attributable to the use of different MRS methods, as well as the heterogeneous migraine populations studied, including patient use of medications, the migraine subtypes of studied patients (e.g. migraine with aura, migraine without aura, hemiplegic migraine), frequency of headaches and migraine attacks (e.g. episodic migraine, chronic migraine), whether acquisition occurred during migraine attacks or interictally, and the amount of time that had passed since the end of the prior migraine attack and until the next attack (if studied interictally). Other considerations when interpreting MRS results include field strength, MRS sequences used, quality of data, region of interest (ROI) selection and variability, post processing methods, and how the metabolite concentrations are presented. All of these factors can impact the reported results and their interpretation.
Field strength
The studies reported in this review were performed at different field strengths. The signal to noise ratio and spectral resolution are dependent on the magnetic field strength and effect metabolite detectability and estimation. Higher magnetic fields provide better resolved spectra and improved detection of individual peaks. For example, at 1.5 T there is an overlap between Glu, Gln, and GABA. At higher magnetic fields, Glx can be resolved and Glu and Gln can be studied independently.
MRS sequences used
The choice and availability of MRS sequences are dependent on the scanner and support at each imaging site. The list of sequences used in each report is included in Table 1. Additional factors such as echo time and repetition time are also of interest for the quality of detectable metabolites.
New sequences are frequently developed and work in progress sequences are available to be used for investigational purposes. Availability of specialized imaging sequences such as MEGA-PRESS is of particular importance for measuring GABA. Changes of GABA have not been widely reproduced, possibly due to the limited availability of the specialized sequences and post-processing expertise.
Quality of data
The quality of the data in MRS studies is either presented as the linewidth of the water peak, or as Cramer-Rao Lower bounds (CRLB) of the metabolite estimations. Inferring quality from the standard deviation of group averages is not recommended. To confound matters, different post-processing techniques often use different terminology to report quality of estimated fits, which makes them difficult to compare. The exclusion criteria varied between studies for a threshold of CRLB and many studies were unable to detect various metabolites. For example, Lac was not reported in most studies. Of the 43 1H MRS studies, only 16 reported Lac.
Presentation of metabolic concentrations
The two standard formats for reporting of results are as ratios or concentrations. Reporting results as ratios requires the selection of a reference metabolite to be used as the denominator (commonly tCr) and is the simplest since it requires no additional measurements. However, this approach is inherently unable to independently measure a single metabolite. Absolute concentration measurements require an additional measurement to be used as a reference. The most common approach is to re-acquire the signal from unsuppressed water in the voxels in-vivo. This approach is further refined by decomposing the tissue in the voxel (using an anatomical image) into grey matter, white matter, and CSF to improve correction factors. A less common practice involves replacing the patient with a container of a known mixture to create an ex-vivo reference.
Region of interest location and voxel placement
The voxel size and location are limiting factors in many single voxel MRS studies. With the most commonly used imaging equipment, regions closer to the surface (e.g. cortical regions) have greater spectral quality than deep brain structures (pons, midbrain, thalamus. Deeper structures require more acquisition time which limits the number of ROI measurements that can be fit into an imaging session. Further challenges with single voxel-MRS can be encountered when encompassing the ROI in a single voxel while avoiding CSF. This presents two types of possible errors where either 1) the voxel is too large and additional regions are introduced into the measurement, or 2) the voxel is too small, and portions of the ROI are excluded from the measurement. ROI placement may present issues in reproducibility. A recommended practice for single voxel spectroscopy is to report the percent of the target ROI measured and the percent of the ROI that is the intended target as assessed by a volumetric atlas for reference.
Most of the studies in this review were single voxel spectroscopy studies. The use of single voxels allows for investigation of metabolites within specific rectangular ROIs. This approach does not typically reflect actual underlying brain anatomy and it omits the rest of the brain. Advances in 2D chemical shift imaging and 3D MRS imaging may avoid these limitations. 3D MRS is still in development and may prove to be useful in examining the whole brain instead of only targeted ROIs.
Post processing methods
There are many different post processing procedures that utilize various software in different stages of validation. There are numerous algorithms used to estimate metabolite concentrations (e.g. Gannet, LCModel, Tarquin, Midas, FSL-MRS, MRI scanner), which complicates inter-study comparisons (Table 1).
There are considerable technical variations in the acquisition and processing of data amongst MRS studies of migraine. Future research would benefit from more consistent and robust approaches to reporting negative results, absolute spectral quantification instead of presenting results as ratios, whole brain MRS instead of single voxel, and a shared database for retrospective studies.
There are considerable technical variations in the acquisition and processing of data amongst MRS studies of migraine. Despite these and other sources of variability, there were a few brain regions that were commonly demonstrated to have atypical metabolite concentrations, including the occipital lobes, thalamic nuclei, cerebellum and cingulate. Numerous MRS studies support the hypothesis that there is impaired energetics and mitochondrial dysfunction in migraine. Although results regarding GABA and Glu are less consistent, MRS studies suggest there might be an imbalance of these important inhibitory and excitatory neurotransmitters in the migraine brain. To better understand migraine pathophysiology, future research would benefit from more consistent and robust approaches and optimized MRS techniques.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Simona Nikolova: Conceptualization, Methodology, Writing – original draft. Todd J. Schwedt: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Simona Nikolova, Email: nikolova.simona@mayo.edu.
Todd J. Schwedt, Email: Schwedt.Todd@mayo.edu.
References
- Aguila M.E., Lagopoulos J., Leaver A.M., Rebbeck T., Hubscher M., Brennan P.C., Refshauge K.M. Elevated levels of GABA+ in migraine detected using (1) H-MRS. NMR Biomed. 2015;28(7):890–897. doi: 10.1002/nbm.3321. [DOI] [PubMed] [Google Scholar]
- Aradi M., Schwarcz A., Perlaki G., Orsi G., Kovacs N., Trauninger A., Kamson D.O., Erdelyi-Botor S., Nagy F., Nagy S.A., Doczi T., Komoly S., Pfund Z. Quantitative MRI studies of chronic brain white matter hyperintensities in migraine patients. Headache. 2013;53(5):752–763. doi: 10.1111/head.12013. [DOI] [PubMed] [Google Scholar]
- Arngrim N., Schytz H.W., Britze J., Amin F.M., Vestergaard M.B., Hougaard A., Wolfram F., de Koning P.J., Olsen K.S., Secher N.H., Larsson H.B., Olesen J., Ashina M. Migraine induced by hypoxia: an MRI spectroscopy and angiography study. Brain. 2016;139(Pt 3):723–737. doi: 10.1093/brain/awv359. [DOI] [PubMed] [Google Scholar]
- Assarzadegan F., Asgarzadeh S., Hatamabadi H.R., Shahrami A., Tabatabaey A., Asgarzadeh M. Serum concentration of magnesium as an independent risk factor in migraine attacks: a matched case-control study and review of the literature. Int. Clin. Psychopharmacol. 2016;31(5):287–292. doi: 10.1097/YIC.0000000000000130. [DOI] [PubMed] [Google Scholar]
- Barbiroli B., Montagna P., Cortelli P., Martinelli P., Sacquegna T., Zaniol P., Lugaresi E. Complicated migraine studied by phosphorus magnetic resonance spectroscopy. Cephalalgia. 1990;10(5):263–272. doi: 10.1046/j.1468-2982.1990.1005263.x. [DOI] [PubMed] [Google Scholar]
- Barbiroli B., Montagna P., Cortelli P., Funicello R., Iotti S., Monari L., Pierangeli G., Zaniol P., Lugaresi E. Abnormal brain and muscle energy metabolism shown by 31P magnetic resonance spectroscopy in patients affected by migraine with aura. Neurology. 1992;42(6):1209–1214. doi: 10.1212/wnl.42.6.1209. [DOI] [PubMed] [Google Scholar]
- Barros J., Damasio J., Tuna A., Alves I., Silveira I., Pereira-Monteiro J., Sequeiros J., Alonso I., Sousa A., Coutinho P. Cerebellar ataxia, hemiplegic migraine, and related phenotypes due to a CACNA1A missense mutation: 12-year follow-up of a large Portuguese family. JAMA Neurol. 2013;70(2):235–240. doi: 10.1001/jamaneurol.2013.591. [DOI] [PubMed] [Google Scholar]
- Bathel A., Schweizer L., Stude P., Glaubitz B., Wulms N., Delice S., Schmidt-Wilcke T. Increased thalamic glutamate/glutamine levels in migraineurs. J. Headache Pain. 2018;19(1):55. doi: 10.1186/s10194-018-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L., Veggeberg R., Prescot A., Jensen J.E., Renshaw P., Scrivani S., Spierings E.L.H., Burstein R., Borsook D. A 'complex' of brain metabolites distinguish altered chemistry in the cingulate cortex of episodic migraine patients. Neuroimage Clin. 2016;11:588–594. doi: 10.1016/j.nicl.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell T., Stokoe M., Khaira A., Webb M., Noel M., Amoozegar F., Harris A.D. GABA and glutamate in pediatric migraine. Pain. 2021;162(1):300–308. doi: 10.1097/j.pain.0000000000002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal M.E., Hetherington H., Pan J., Tsang A., Grosberg B., Avdievich N., Friedman B., Lipton R.B. Occipital levels of GABA are related to severe headaches in migraine. Neurology. 2008;70(22):2078–2080. doi: 10.1212/01.wnl.0000313376.07248.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boska M.D., Welch K.M., Barker P.B., Nelson J.A., Schultz L. Contrasts in cortical magnesium, phospholipid and energy metabolism between migraine syndromes. Neurology. 2002;58(8):1227–1233. doi: 10.1212/wnl.58.8.1227. [DOI] [PubMed] [Google Scholar]
- Bridge H., Stagg C.J., Near J., Lau C.I., Zisner A., Cader M.Z. Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia. 2015;35(11):1025–1030. doi: 10.1177/0333102414566860. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (2018). Cephalalgia. 38(1):1-211. [DOI] [PubMed]
- Chan Y.M., Pitchaimuthu K., Wu Q.Z., Carter O.L., Egan G.F., Badcock D.R., McKendrick A.M. Relating excitatory and inhibitory neurochemicals to visual perception: a magnetic resonance study of occipital cortex between migraine events. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0208666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C.D., Plasencia J.D., Frakes D.H., Schwedt T.J. Structural alterations of the brainstem in migraine. Neuroimage Clin. 2017;13:223–227. doi: 10.1016/j.nicl.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G., Di Renzo A., Tinelli E., Lepre C., Di Lorenzo C., Di Lorenzo G., Scapeccia M., Parisi V., Serrao M., Colonnese C., Schoenen J., Pierelli F. Thalamo-cortical network activity between migraine attacks: Insights from MRI-based microstructural and functional resting-state network correlation analysis. J. Headache Pain. 2016;17(1) doi: 10.1186/s10194-016-0693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan A., Saatchian E., Sobhani M., Montazerabadi A. Neurochemical metabolite alterations of the occipital lobe in migraine without aura by proton magnetic resonance spectroscopy. Neuroradiol. J. 2020;33(5):410–415. doi: 10.1177/1971400920932793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichgans M., Herzog J., Freilinger T., Wilke M., Auer D.P. 1H-MRS alterations in the cerebellum of patients with familial hemiplegic migraine type 1. Neurology. 2005;64(4):608–613. doi: 10.1212/01.WNL.0000151855.98318.50. [DOI] [PubMed] [Google Scholar]
- Domitrz I., Cegielska J. Magnesium as an important factor in the pathogenesis and treatment of migraine-from theory to practice. Nutrients. 2022;14(5):1089. doi: 10.3390/nu14051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson B.M., Hesterman C., Johnston M., Dudeck N.R., Charles A.C., Villablanca J.P. Advanced imaging in the evaluation of migraine headaches. Neuroimaging Clin. N. Am. 2019;29(2):301–324. doi: 10.1016/j.nic.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSherif M., Reda M.I., Saadallah H., Mourad M. Eye movements and imaging in vestibular migraine. Acta Otorrinolaringol. Esp. (Engl. Ed.) 2020;71(1):3–8. doi: 10.1016/j.otorri.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Enna S.J., McCarson K.E. The role of GABA in the mediation and perception of pain. Adv. Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- Erdelyi-Botor S., Aradi M., Kamson D.O., Kovacs N., Perlaki G., Orsi G., Nagy S.A., Schwarcz A., Doczi T., Komoly S., Deli G., Trauninger A., Pfund Z. Changes of migraine-related white matter hyperintensities after 3 years: a longitudinal MRI study. Headache. 2015;55(1):55–70. doi: 10.1111/head.12459. [DOI] [PubMed] [Google Scholar]
- Fayed N., Andres E., Viguera L., Modrego P.J., Garcia-Campayo J. Higher glutamate+glutamine and reduction of N-acetylaspartate in posterior cingulate according to age range in patients with cognitive impairment and/or pain. Acad. Radiol. 2014;21(9):1211–1217. doi: 10.1016/j.acra.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Fila M., Pawlowska E., Blasiak J. Mitochondria in migraine pathophysiology - does epigenetics play a role? Arch. Med. Sci. 2019;15(4):944–956. doi: 10.5114/aoms.2019.86061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P.N., Peng Y.B., Boyette-Davis J.A., Uhelski M.L. The anterior cingulate cortex and pain processing. Front. Integr. Neurosci. 2014;8:35. doi: 10.3389/fnint.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez de la Aleja J., Ramos A., Mato-Abad V., Martinez-Salio A., Hernandez-Tamames J.A., Molina J.A., Hernandez-Gallego J., Alvarez-Linera J. Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache. 2013;53(2):365–375. doi: 10.1111/head.12030. [DOI] [PubMed] [Google Scholar]
- Hasler G., Buchmann A., Haynes M., Muller S.T., Ghisleni C., Brechbuhl S., Tuura R. Association between prefrontal glutamine levels and neuroticism determined using proton magnetic resonance spectroscopy. Transl. Psychiatry. 2019;9(1):170. doi: 10.1038/s41398-019-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard A., Amin F.M., Arngrim N., Vlachou M., Larsen V.A., Larsson H.B.W., Ashina M. Sensory migraine aura is not associated with structural grey matter abnormalities. Neuroimage Clin. 2016;11:322–327. doi: 10.1016/j.nicl.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard A., Amin F.M., Christensen C.E., Younis S., Wolfram F., Cramer S.P., Larsson H.B.W., Ashina M. Increased brainstem perfusion, but no blood-brain barrier disruption, during attacks of migraine with aura. Brain. 2017;140(6):1633–1642. doi: 10.1093/brain/awx089. [DOI] [PubMed] [Google Scholar]
- Jin C., Yuan K., Zhao L., Zhao L., Yu D., von Deneen K.M., Zhang M., Qin W., Sun W., Tian J. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013;26(1):58–64. doi: 10.1002/nbm.2819. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Suh S.I., Seol H.Y., Oh K., Seo W.K., Yu S.W., Park K.W., Koh S.B. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28(6):598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- Li Q., Chen C., Gong T. High-field MRS study of GABA+ in patients with migraine: response to levetiracetam treatment. NeuroReport. 2018;29(12):1007–1010. doi: 10.1097/WNR.0000000000001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi R., Montagna P., Soriani S., Iotti S., Arnaldi C., Cortelli P., Pierangeli G., Patuelli A., Zaniol P., Barbiroli B. Deficit of brain and skeletal muscle bioenergetics and low brain magnesium in juvenile migraine: an in vivo 31P magnetic resonance spectroscopy interictal study. Pediatr. Res. 1997;42(6):866–871. doi: 10.1203/00006450-199712000-00024. [DOI] [PubMed] [Google Scholar]
- Lodi R., Iotti S., Cortelli P., Pierangeli G., Cevoli S., Clementi V., Soriani S., Montagna P., Barbiroli B. Deficient energy metabolism is associated with low free magnesium in the brains of patients with migraine and cluster headache. Brain Res. Bull. 2001;54(4):437–441. doi: 10.1016/s0361-9230(01)00440-3. [DOI] [PubMed] [Google Scholar]
- Magon S., May A., Stankewitz A., Goadsby P.J., Tso A.R., Ashina M., Amin F.M., Seifert C.L., Chakravarty M.M., Muller J., Sprenger T. Morphological abnormalities of thalamic subnuclei in migraine: a multicenter MRI study at 3 tesla. J. Neurosci. 2015;35(40):13800–13806. doi: 10.1523/JNEUROSCI.2154-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A., Burstein R. Hypothalamic regulation of headache and migraine. Cephalalgia. 2019;39(13):1710–1719. doi: 10.1177/0333102419867280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehnert J., May A. Functional and structural alterations in the migraine cerebellum. J. Cereb. Blood Flow Metab. 2019;39(4):730–739. doi: 10.1177/0271678X17722109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed R.A.A., Al-Malt M. Interictal alterations of thalamic metabolic concentration ratios in migraine without aura detected by proton magnetic resonance spectroscopy. Egypt. J. Radiol. Nucl. Med. 2013;44(4):859–870. [Google Scholar]
- Montagna P., Cortelli P., Lodi R., Barbiroli B. Magnetic resonance spectroscopy of episodic ataxia type 2 and migraine. Ann. Neurol. 2000;47(6):838–839. doi: 10.1002/1531-8249(200006)47:6<838::aid-ana25>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Montagna P., Cortelli P., Monari L., Pierangeli G., Parchi P., Lodi R., Iotti S., Frassineti C., Zaniol P., Lugaresi E. 31P-magnetic resonance spectroscopy in migraine without aura. Neurology. 1994;44(4):666–669. doi: 10.1212/wnl.44.4.666. [DOI] [PubMed] [Google Scholar]
- Niddam D.M., Lai K.L., Tsai S.Y., Lin Y.R., Chen W.T., Fuh J.L., Wang S.J. Neurochemical changes in the medial wall of the brain in chronic migraine. Brain. 2018;141(2):377–390. doi: 10.1093/brain/awx331. [DOI] [PubMed] [Google Scholar]
- Niddam D.M., Lai K.L., Tsai S.Y., Lin Y.R., Chen W.T., Fuh J.L., Wang S.J. Brain metabolites in chronic migraine patients with medication overuse headache. Cephalalgia. 2020;40(8):851–862. doi: 10.1177/0333102420908579. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hrobjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek A.L., Leaver A.M., Foster S., Oeltzschner G., Puts N.A., Galloway G., Sterling M., Ng K., Refshauge K., Aguila M.-E., Rebbeck T. Increased GABA+ in people with migraine, headache and pain conditions- a potential marker of pain. J. Pain. 2021;22(12):1631–1645. doi: 10.1016/j.jpain.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot A., Becerra L., Pendse G., Tully S., Jensen E., Hargreaves R., Renshaw P., Burstein R., Borsook D. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol. Pain. 2009;5:34. doi: 10.1186/1744-8069-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan N.M., Halvorson H., Vande-Linde A., Levine S.R., Helpern J.A., Welch K.M. Low brain magnesium in migraine. Headache. 1989;29(9):590–593. doi: 10.1111/j.1526-4610.1989.hed2909590.x. [DOI] [PubMed] [Google Scholar]
- Reyngoudt H., De Deene Y., Descamps B., Paemeleire K., Achten E. (1)H-MRS of brain metabolites in migraine without aura: absolute quantification using the phantom replacement technique. MAGMA. 2010;23(4):227–241. doi: 10.1007/s10334-010-0221-z. [DOI] [PubMed] [Google Scholar]
- Reyngoudt H., Paemeleire K., Descamps B., De Deene Y., Achten E. 31P-MRS demonstrates a reduction in high-energy phosphates in the occipital lobe of migraine without aura patients. Cephalalgia. 2011;31(12):1243–1253. doi: 10.1177/0333102410394675. [DOI] [PubMed] [Google Scholar]
- Reyngoudt H., Paemeleire K., Dierickx A., Descamps B., Vandemaele P., De Deene Y., Achten E. Does visual cortex lactate increase following photic stimulation in migraine without aura patients? A functional (1)H-MRS study. J. Headache Pain. 2011;12(3):295–302. doi: 10.1007/s10194-011-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Ceccarelli A., Falini A., Colombo B., Tortorella P., Bernasconi L., Comi G., Scotti G., Filippi M. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37(7):1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- Sandor P.S., Dydak U., Schoenen J., Kollias S.S., Hess K., Boesiger P., Agosti R.M. MR-spectroscopic imaging during visual stimulation in subgroups of migraine with aura. Cephalalgia. 2005;25(7):507–518. doi: 10.1111/j.1468-2982.2005.00900.x. [DOI] [PubMed] [Google Scholar]
- Sarchielli P., Tarducci R., Presciutti O., Gobbi G., Pelliccioli G.P., Stipa G., Alberti A., Capocchi G. Functional 1H-MRS findings in migraine patients with and without aura assessed interictally. Neuroimage. 2005;24(4):1025–1031. doi: 10.1016/j.neuroimage.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Ganssbauer S., Neuner T., Bogdahn U., May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28(1):1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- Schulte L., May A. Pearls and pitfalls in migraine neuroimaging. Headache. 2017;57(6):850–851. doi: 10.1111/head.13140. [DOI] [PubMed] [Google Scholar]
- Schulte L.H., Mehnert J., May A. Longitudinal neuroimaging over 30 days: temporal characteristics of migraine. Ann. Neurol. 2020;87(4):646–651. doi: 10.1002/ana.25697. [DOI] [PubMed] [Google Scholar]
- Schulz U.G., Blamire A.M., Corkill R.G., Davies P., Styles P., Rothwell P.M. Association between cortical metabolite levels and clinical manifestations of migrainous aura: an MR-spectroscopy study. Brain. 2007;130(Pt 12):3102–3110. doi: 10.1093/brain/awm165. [DOI] [PubMed] [Google Scholar]
- Schulz U.G., Blamire A.M., Davies P., Styles P., Rothwell P.M. Normal cortical energy metabolism in migrainous stroke: a 31P-MR spectroscopy study. Stroke. 2009;40(12):3740–3744. doi: 10.1161/STROKEAHA.109.558163. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M., Sendacki M., Moeller F., Wolff S., Jansen O., Siebner H., Stephani U. Abnormal changes of synaptic excitability in migraine with aura. Cereb. Cortex. 2012;22(10):2207–2216. doi: 10.1093/cercor/bhr248. [DOI] [PubMed] [Google Scholar]
- Staermose T.G., Knudsen M.K., Kasch H., Blicher J.U. Cortical GABA in migraine with aura -an ultrashort echo magnetic resonance spectroscopy study. J. Headache Pain. 2019;20(1):110. doi: 10.1186/s10194-019-1059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewitz A., Aderjan D., Eippert F., May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J. Neurosci. 2011;31(6):1937–1943. doi: 10.1523/JNEUROSCI.4496-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewitz A., May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011;77(5):476–482. doi: 10.1212/WNL.0b013e318227e4a8. [DOI] [PubMed] [Google Scholar]
- Stovner L.J., Nichols E., Steiner T.J., Abd-Allah F., Abdelalim A., Al-Raddadi R.M., Ansha M.G., Barac A., Bensenor I.M., Doan L.P., Edessa D., Endres M., Foreman K.J., Gankpe F.G., Gopalkrishna G., Goulart A.C., Gupta R., Hankey G.J., Hay S.I., Hegazy M.I., Hilawe E.H., Kasaeian A., Kassa D.H., Khalil I., Khang Y.-H., Khubchandan J., Kim Y.J., Kokubo Y., Mohammed M.A., Mokdad A.H., Moradi-Lakeh M., Nguyen H.L.T., Nirayo Y.L., Qorbani M., Ranta A., Roba K.T., Safiri S., Santos I.S., Satpathy M., Sawhney M., Shiferaw M.S., Shiue I., Smith M., Szoeke C.E.I., Truong N.T., Venketasubramanian N., weldegwergs K.G., Westerman R., Wijeratne T., Tran B.X., Yonemoto N., Feigin V.L., Vos T., Murray C.J.L. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–976. doi: 10.1016/S1474-4422(18)30322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrin A., Bello L., Valentino M.L., Caporali L., Soraru G., Carelli V., Maggioni F., Zeviani M., Pegoraro E. The relevance of migraine in the clinical spectrum of mitochondrial disorders. Sci. Rep. 2022;12(1):4222. doi: 10.1038/s41598-022-08206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiehuis L.H., Koene S., Saris C.G.J., Janssen M.C.H. Mitochondrial migraine; a prevalence, impact and treatment efficacy cohort study. Mitochondrion. 2020;53:128–132. doi: 10.1016/j.mito.2020.05.004. [DOI] [PubMed] [Google Scholar]
- Tu Y., Fu Z., Zeng F., Maleki N., Lan L., Li Z., Park J., Wilson G., Gao Y., Liu M., Calhoun V., Liang F., Kong J. Abnormal thalamocortical network dynamics in migraine. Neurology. 2019;92(23):e2706–e2716. doi: 10.1212/WNL.0000000000007607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncini A., Lodi R., Di Muzio A., Silvestri G., Servidei S., Lugaresi A., Iotti S., Zaniol P., Barbiroli B. Abnormal brain and muscle energy metabolism shown by 31P-MRS in familial hemiplegic migraine. J. Neurol. Sci. 1995;129(2):214–222. doi: 10.1016/0022-510x(94)00283-t. [DOI] [PubMed] [Google Scholar]
- Valfre W., Rainero I., Bergui M., Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48(1):109–117. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- Vincent M., Hadjikhani N. The cerebellum and migraine. Headache. 2007;47(6):820–833. doi: 10.1111/j.1526-4610.2006.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollono C., Primiano G., Della Marca G., Losurdo A., Servidei S. Migraine in mitochondrial disorders: prevalence and characteristics. Cephalalgia. 2018;38(6):1093–1106. doi: 10.1177/0333102417723568. [DOI] [PubMed] [Google Scholar]
- Wang T., Zhan W., Chen Q., Chen N., Zhang J.P., Liu Q., He L., Zhang J.R., Huang H., Gong Q.Y. Altered resting-state ascending/descending pathways associated with the posterior thalamus in migraine without aura. NeuroReport. 2016;27(4):257–263. doi: 10.1097/WNR.0000000000000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Kuwabara T., Ohkubo M., Tsuji S., Yuasa T. Elevation of cerebral lactate detected by localized 1H-magnetic resonance spectroscopy in migraine during the interictal period. Neurology. 1996;47(4):1093–1095. doi: 10.1212/wnl.47.4.1093. [DOI] [PubMed] [Google Scholar]
- Wei H.L., Zhou X., Chen Y.C., Yu Y.S., Guo X., Zhou G.P., Zhou Q.Q., Qu L.J., Yin X.D., Li J.R., Zhang H. Impaired intrinsic functional connectivity between the thalamus and visual cortex in migraine without aura. J. Headache Pain. 2019;20(1) doi: 10.1186/s10194-019-1065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K.M., Levine S.R., D'Andrea G., Helpern J.A. Brain pH in migraine: an in vivo phosphorus-31 magnetic resonance spectroscopy study. Cephalalgia. 1988;8(4):273–277. doi: 10.1046/j.1468-2982.1988.0804273.x. [DOI] [PubMed] [Google Scholar]
- Xue T., Yuan K., Cheng P., Zhao L., Zhao L.M., Yu D.H., Dong T., von Deneen K.M., Gong Q.Y., Qin W., Tian J. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed. 2013;26(9):1051–1058. doi: 10.1002/nbm.2917. [DOI] [PubMed] [Google Scholar]
- Younis S., Hougaard A., Christensen C.E., Vestergaard M.B., Petersen E.T., Paulson O.B., Larsson H.B.W., Ashina M. Effects of sildenafil and calcitonin gene-related peptide on brainstem glutamate levels: a pharmacological proton magnetic resonance spectroscopy study at 3.0 T. J. Headache Pain. 2018;19(1):44. doi: 10.1186/s10194-018-0870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis S., Hougaard A., Noseda R., Ashina M. Current understanding of thalamic structure and function in migraine. Cephalalgia. 2019;39(13):1675–1682. doi: 10.1177/0333102418791595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis S., Christensen C.E., Vestergaard M.B., Lindberg U., Tolnai D., Paulson O.B., Larsson H.B., Hougaard A., Ashina M. Glutamate levels and perfusion in pons during migraine attacks: a 3T MRI study using proton spectroscopy and arterial spin labeling. J. Cereb. Blood Flow Metab. 2021;41(3):604–616. doi: 10.1177/0271678X20906902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis S., Hougaard A., Christensen C.E., Vestergaard M.B., Paulson O.B., Larsson H.B.W., Ashina M. Interictal pontine metabolism in migraine without aura patients: a 3 Tesla proton magnetic resonance spectroscopy study. Neuroimage Clin. 2021;32 doi: 10.1016/j.nicl.2021.102824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Huang J., Zhang Z., Cao Z. Altered metabolites in the occipital lobe in migraine without aura during the attack and the interictal period. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.656349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielman R., Teeuwisse W.M., Bakels F., Van der Grond J., Webb A., van Buchem M.A., Ferrari M.D., Kruit M.C., Terwindt G.M. Biochemical changes in the brain of hemiplegic migraine patients measured with 7 tesla 1H-MRS. Cephalalgia. 2014;34(12):959–967. doi: 10.1177/0333102414527016. [DOI] [PubMed] [Google Scholar]
- Zielman R., Wijnen J.P., Webb A., Onderwater G.L.J., Ronen I., Ferrari M.D., Kan H.E., Terwindt G.M., Kruit M.C. Cortical glutamate in migraine. Brain. 2017;140(7):1859–1871. doi: 10.1093/brain/awx130. [DOI] [PubMed] [Google Scholar]