Summary
Background
Patients with peripheral arterial disease (PAD) often remain undiagnosed and therefore suboptimally managed. Here, we investigated the diagnostic and prognostic potential of fatty acid binding protein 3 (FABP3) in patients with PAD.
Methods
In the discovery phase, 374 PAD and 184 non-PAD patients were recruited from vascular surgery ambulatory clinics at St. Michael's Hospital (Toronto, Ontario, Canada) between October 4, 2017 to October 29, 2018. The diagnostic ability of baseline FABP3 level was investigated through receiver operator characteristic (ROC) curves to determine two cutoff points: 1) an exclusionary “rule out” cutoff point, and 2) a confirmatory “rule in” cutoff point. Next, these cutoff points were confirmed in the external validation phase using a separate cohort of 312 patients (180 PAD and 132 non-PAD) recruited from ambulatory vascular surgery clinics at St. Michael's Hospital (Canada) between November 6, 2018–July 30, 2019. Cox regression analyses were used to explore the independent association between FABP3 and major adverse limb events (MALE – defined as need for arterial revascularization or major amputation) and decrease in ankle-brachial index (ABI -defined as drop ≥0.15) during 3 years of follow-up.
Findings
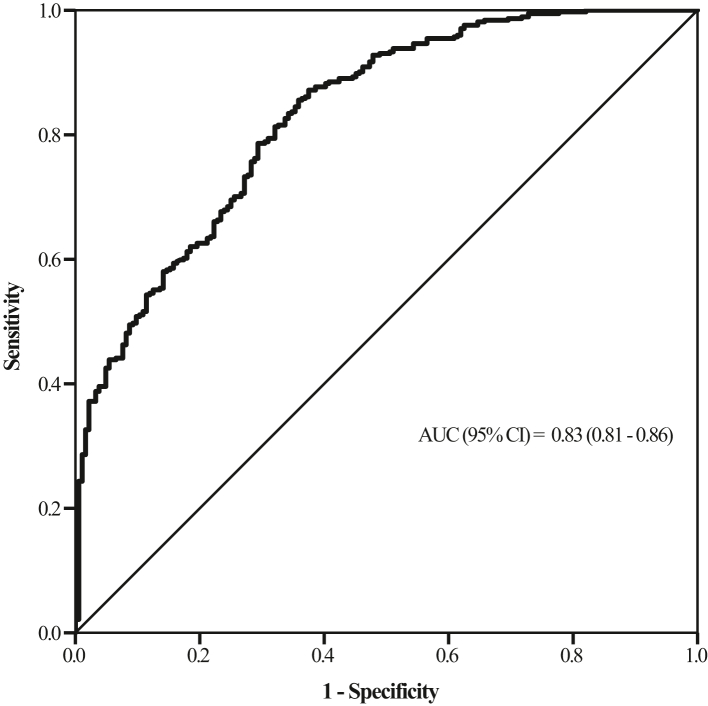
In the discovery phase, FABP3 levels were significantly elevated in patients with PAD compared to non-PAD patients. ROC analysis demonstrated that FABP3 had an AUC of 0.83 (95% CI: 0.81–0.86, p-value < 0.001). FABP3 exclusionary cutoff was <1.55 ng/ml (sensitivity = 96%; specificity = 40%), whereas FABP3 confirmatory cutoff was >3.55 ng/ml (sensitivity = 43%; specificity = 95%) – values that were confirmed in the external validation phase. Cox regression analysis demonstrated FABP3 to be an independent predictor of increase in MALE [HR = 1.14 (1.03–1.29); p-value = 0.010] and worsening PAD status (drop in ABI >0.15 [HR = 1.11 (1.02–1.19); p-value = 0.009]).
Interpretation
Our findings suggested that FABP3 levels can be used as both a diagnostic and prognostic biomarker for PAD, and may facilitate risk stratification in select individuals for purposes of vascular evaluation or intensive medical management.
Funding
Funding for this study was provided by the Bill and Vicky Blair Foundation.
Keywords: Peripheral arterial disease, Fatty acid binding protein 3, FABP3, Biomarker, Diagnostic, Prognostic
Research in context.
Evidence before this study
We searched PubMed and Embase with several key terms (e.g. “Peripheral Arterial Disease”, “Lower Extremity Occlusion”, “Lower Extremity Artery Disease”, “Lower Extremity Revascularization”) AND (e.g. “proteins”, “biomarkers”, “diagnosis”, “prognosis”, “risk stratification”, “outcome”) on Jan 10, 2022, for all English articles without date restrictions. While >20 published relevant manuscripts were yielded by the search, only one study looked at PAD-specific prognostic biomarkers as it relates to major adverse limb events (MALE). Collectively, the literature makes clear the critical limitations associated with the current diagnostic and prognostic tools for PAD and the urgent need for improved tools in this regard.
Added value of this study
The present study suggests that FABP3 has strong diagnostic potential for PAD as validated through an independent cohort of patients. In addition, our results suggested that FABP3 is a good predictor of PAD-related adverse outcomes: (i) MALE and, (ii) decrease in ABI which indicates disease progression. Furthermore, patients with elevated FABP3 levels had significantly increased risk for the individual components of MALE, as well as MI (in the case of MACE). Further analyses revealed elevated FABP3 levels in patients with PAD regardless of smoking history or diabetes status.
Implications of all the available evidence
Lack of a simple, readily available, and reliable test is often suggested as common barrier to performing PAD-related diagnostic and prognostic tests. FABP3, a protein expressed skeletal/myocardium muscles, has potential to be a novel diagnostic, prognostic, and risk-stratification test for adverse events in patients with PAD. Future multicentre studies are warranted to validate this biomarker in diverse populations as well as its integration into clinical guidelines and practice.
Introduction
Peripheral arterial disease (PAD) is a highly prevalent atherosclerotic disease that affects over 200 million people globally.1, 2, 3 Inadequate management of PAD can lead to catastrophic consequences, including nonfatal coronary or cerebral vascular events, lower-limb amputations, and death.
Patients managed early on in their PAD disease see improvements in their ambulatory and exercise tolerance; however, a substantial number of patients with PAD are either undiagnosed, misdiagnosed, or receive their diagnosis late in the disease process, putting them at an increased risk of morbidity and mortality.4, 5, 6
One reason for the delayed diagnosis (and subsequent poor prognosis) of patients with PAD is the lack of a simple, readily available, reliable test. The ankle-brachial index (ABI) is the current standard of practice for diagnosing PAD but has limitations including the need for extensive training of healthcare personnel, the time required to conduct the test, difficulty with interpreting results, physician knowledge gaps, and equipment unavailability.7, 8, 9, 10, 11 Consequently, the ABI is under-utilised and often incorrectly performed and interpreted in primary care settings.12 Furthermore, the ABI is not always reliable in all patient populations, as 25% of patients with diabetes have falsely elevated ABI values due to incompressible calcified vessels.13,14 In addition to diagnostic limitations, the predictive ability of ABI has also previously come into question.15,16
Improved approaches to diagnosis and risk stratification have the potential to improve limb-salvage rates and outcomes in this high risk population.17 Previous studies have highlighted the potential for a simple blood or urine test to achieve this goal in two completely different patient populations.18, 19, 20
In a previous pilot study, we found that patients with PAD had elevated blood levels of FABP3 compared to non-PAD patients.21 In this study, we further explored the use of FABP3 in the diagnosis of PAD, and also investigated FABP3's potential in predicting major adverse limb events (MALE) and disease progression (i.e. “decrease in ABI”) in patients with PAD.
Methods
Study design and participants
The study was approved by the Unity Health Toronto Research Ethics Board at St. Michael's Hospital—University of Toronto, Ontario, Canada. Informed written consent was obtained from all participants.
Discovery phase–study population
Consecutive patients presenting to ambulatory vascular surgery clinics at St. Michael's Hospital between October 4, 2017 and October 29, 2018 were invited to participate in this study. All patients underwent clinical examination of peripheral pulses and measurement of ABI. PAD was defined as ABI <0.9 in combination with evidence of atherosclerotic disease on duplex ultrasound, reduced peripheral pulses in at least one leg (with or without claudication). Non-PAD was defined as ABI ≥0.9 with palpable distal pulses, and no history of claudication. In situations where ABI values were erroneous due to non-compressible tibial vessels (ABI>1.4), the toe brachial index (TBI) measurements were utilised instead. Patients with TBI <0.7 were defined as having PAD.
Patients were ineligible for this study if they met one or more of the following criteria: 1) chronic kidney disease stages 3–5, 2) acute coronary syndrome (ACS), stroke, or transient ischemic attack (TIA) all within the past 30 days or, 3) elevated troponin levels. These patient populations were excluded due to potential confounding effect on plasma levels of FABP3.
Baseline measurements
The medical history of all patients was obtained, including history of cardiovascular disease and risk factors. Hypercholesteraemia was defined as total cholesterol >5.2 mmol/L, triglyceride >1.7 mmol/L, or using antihyperlipidemic medication.22 Hypertension was defined as systolic blood pressure of ≥130 mm Hg, diastolic pressure ≥80 mm Hg, or using antihypertensive medication. Diabetes mellitus was defined as glycosylated hemoglobin A1c of ≥6.5% or using antidiabetic medication.23
Sample collection and processing
Blood samples were drawn into EDTA-containing vacutainer tubes. After centrifugation at 3000 rpm for 10 min (4 °C), plasma was aliquoted and stored at −80 °C. Plasma samples were analyzed using MILLIPLEX MAP Human Cardiovascular Disease (CVD) Magnetic Bead Panel 1 (EMD-Millipore; Billerica, Mass) to determine FABP3 concentrations. Plasma sample analyses were performed on the same day to avert inter-assay variability. Sample intra-assay Coefficients of Variability (CV) was <10%, whereas inter-assay CV was 15%. The MagPix analyzer (Luminex Corp; Austin, TX, USA) was calibrated prior to analysis using Fluidics Verification and Calibration bead kits (Luminex Corp). A minimum of 50 beads for FABP3 were acquired using Luminex xPonent software and analyzed using Milliplex Analyst software (v.5.1; EMD-Millipore, Darmstadt, Germany).
External validation phase–confirmation of FABP3 diagnostic cutoff point within an external patient cohort
The ability of FABP3 to diagnose PAD based on our calculated cutoff points were validated using an independent cohort of consecutive patients presenting to ambulatory vascular surgery clinics at St. Michael's Hospital between November 4, 2018 - July 30, 2019. Similar to the discovery cohort, all patients underwent clinical and radiological examination of peripheral pulses and measurement of ABI. PAD was defined as ABI <0.9 in combination with evidence of atherosclerotic disease on duplex ultrasound, reduced peripheral pulses in at least one leg (with or without claudication). Non-PAD was defined as ABI ≥0.9 with palpable distal pulses, and no history of claudication. In situations where ABI values were erroneous due to non-compressible tibial vessels (ABI>1.4), the toe brachial index (TBI) measurements were utilised instead. Patients with TBI <0.7 were defined as having PAD.
Patients were ineligible if they met one or more of the following criteria: 1) chronic kidney disease stages 3–5, 2) acute coronary syndrome (ACS), stroke, or transient ischemic attack (TIA) all within the past 30 days or, 3) elevated troponin levels. Levels of FABP3 were quantified as aforementioned.
Surveillance protocol and measured outcomes
Outpatient clinic visits were scheduled at 12, 24 and 36 months upon recruitment for patients recruited to the discovery phase of this study. During these follow-up visits, ABI measurements, PAD-related management, surgical interventions (revascularisation or amputation), and incidence of cardiovascular related mortality, stroke, or myocardial infarction (MI) were assessed and recorded. Information pertaining to hospitalizations or emergency room visits were also recorded. The primary outcome was incidence of major adverse limb events (MALE) - defined as composite of PAD-specific severe limb ischemia requiring an arterial revascularization (open or endovascular intervention) or major limb amputation (i.e. above ankle-level limb loss). Secondary outcome was decrease in ABI, defined as drop in ABI ≥0.15 from baseline. The tertiary outcome was the incidence of major adverse cardiovascular events (MACE) - defined as composite incidence of cardiovascular related mortality, stroke, or myocardial infarction (MI).24,25 The primary, secondary, and tertiary outcomes were directly collected from patients/medical records and recorded on a standardised data collection form.
Sample size calculation
Sample size was calculated using MedCalc statistical software version 20.115 (MedCalc Software Ltd, Ostend, Belgium). Based on our pilot study, a total sample of 548 is required to achieve 90% power to detect an area under the curve (AUC) of 0.90 with a margin of error of 0.0521. Power calculations were done using a two-sided z-test at a significance level of 0.05.
Statistical analysis
Demographics and clinical characteristics were reported as medians with interquartile ranges (IQR), or frequencies with percentages (%). Comparative analyses were conducted using student's t-test for continuous data (if normally distributed), or the Mann–Whitney U test. Chi-square test or Fisher's exact test were used to compare categorical variables.
To determine the ability of FABP3 to diagnose PAD, a receiver operating characteristic (ROC) curve analysis was conducted. Two cutoff values were determined. First, an overall exclusionary “rule out” cutoff point was determined using a sensitivity above 95%. This high sensitivity was selected to ensure a low percentage of false negatives. Second, a confirmatory “rule in” cutoff point was determined using a specificity above 95% to ensure a low rate of false positives. We calculated the diagnostic performance (i.e., sensitivity, specificity, positive predictive value and negative predictive value, likelihood ratios, and area under curves (AUC)) for the two determined FABP3 cutoff points. We also calculated age-adjusted FABP3 “rule out” values for age groups <50, 50–75 and <75 years. The diagnostic accuracy of these cutoff points was then validated in an external and independent patient cohort. While the external validation cohort was comprised of a completely different set of patients, they still underwent the same research protocol as those within the discovery cohort.
Next, to ascertain FABP3's predictive potential with regards to the primary (MALE outcomes), secondary (decrease in ABI ≥0.15) and tertiary (MACE) outcomes, Cox proportional hazard analysis was performed. For the survival analyses, the starting timepoint for all eligible patients was between October 2017 and October 2018 (baseline). Patients were followed up with scheduled visits at 12-, 24- and 36-months post-recruitment. The last follow-up was scheduled in October 2021. Type and time of censoring was reported for the study cohort. Patients that dropped out (right censored) were assumed to have the same probability of experiencing an event as patients who remained in the study (i.e., non-informative censoring). Variables that were identified to be potential confounders (age (in years), sex (male vs female), hypertension (yes vs no), hypercholesteremia (yes vs no), smoking (non-smoker vs past smoker vs current smoker), diabetes (yes vs no), history of CAD (yes vs no), ASA (yes vs no), statins (yes vs no), and ACEi/ARB (yes vs no)) were entered into the multivariate analysis. Cox proportional hazards model assumptions were assessed with no violations detected. These included testing the proportional hazards assumption using Schoenfeld residuals, examining influential observations using deviance residual, and detecting nonlinearity between log hazard and covariates using martingale residual. For the Kaplan–Meier curves, FABP3 “rule in” cutoff point was used to categorise the study cohort into two groups - those above the cutoff value, and those below. Event-free curves were computed for decrease in ABI, composite MALE outcomes (need for arterial revascularization or major limb amputation), as well as individual components of MALE and MACE. Comparisons of event-free survival curves were performed using the log-rank test. Statistical significance was established at P-value < 0.05 (2-sided).
Role of the funding source
The funding source had no role or influence with regards to study design, data collection, data analysis, data interpretation, manuscript writing, or manuscript publication. All authors had access to the full data and the decision to submit this manuscript for publication was unanimously shared between all authors.
Results
Discovery phase- baseline characteristics
A total of 558 patients participated in this study, comprising 374 patients with PAD and 184 without PAD (controls). All patients with non-compressible ABI (n = 39) had TBI values < 0.7 (0.34 median, IQR 0.28–0.47), and were therefore classified as having PAD. Median age for the overall cohort was 66 years and 61% were male and 39% were female. Furthermore, 70% of study population were smokers, 61% had hypercholesteremia while 62% had hypertension (Table 1). Significant differences were noted between PAD and non-PAD groups on all measured baseline demographics and clinical characteristics (Table 1). Plasma FABP3 (ng/ml) levels were significantly higher in patients with PAD (median = 3.27; IQR 2.35–4.46) than those without PAD (median = 1.74; IQR 1.25–2.55, p-value < 0.001) (Table 1).
Table 1.
Baseline demographic and clinical characteristics of patients with and without peripheral arterial disease.
| Demographics and clinical characteristics | Overall (n = 558) | non-PAD (n = 184) | PAD (n = 374) | P-value a |
|---|---|---|---|---|
| Median (IQR)b | ||||
| ABI | 0.79 (0.58–1.00) | 1.01 (1.00–1.09) | 0.63 (0.50–0.79) | <0.001 |
| Age, years | 66 (55–74) | 49 (35–64) | 69 (63–79) | <0.001 |
| FABP3 (ng/ml) | 2.59 (1.83–3.70) | 1.74 (1.25–2.55) | 3.27 (2.35–4.46) | <0.001 |
| Frequency (%)c | ||||
| Sex, male | 343 (61) | 86 (47) | 257 (69) | <0.001 |
| Sex, female | 215 (39) | 98 (53) | 117 (31) | |
| Hypertension | 344 (62) | 60 (33) | 284 (76) | <0.001 |
| Hypercholesteremia | 343 (61) | 55 (30) | 288 (77) | <0.001 |
| Diabetes | 207 (37) | 22 (12) | 185 (49) | <0.001 |
| Renal insufficiency | 16 (3) | 0 (0) | 16 (4) | 0.004 |
| Past smoker | 256 (46) | 49 (27) | 207 (55) | <0.001 |
| Current smoker | 133 (24) | 34 (19) | 99 (27) | 0.037 |
| History of congestive heart failure | 14 (3) | 0 (0) | 14 (4) | 0.008 |
| History of coronary artery disease | 167 (30) | 25 (14) | 142 (38) | <0.001 |
| History of stroke | 59 (11) | 4 (2) | 55 (15) | <0.001 |
| Medications Frequency (%) | ||||
| Statins | 331 (59) | 45 (25) | 286 (76) | <0.001 |
| Aspirin | 232 (42) | 25 (14) | 207 (55) | <0.001 |
| ACEi/ARB | 248 (44) | 33 (18) | 215 (57) | <0.001 |
| Βeta Blockers | 136 (24) | 17 (9) | 119 (32) | <0.001 |
| Insulin | 41 (7) | 2 (1) | 39 (10) | <0.001 |
| Oral hypoglycemic | 127 (23) | 11 (6) | 116 (31) | <0.001 |
ABI, Ankle Brachial index.
ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Medians and interquartile ranges (IQR) were calculated for continuous variables.
Frequencies and percentages were calculated for categorical variables; all numbers were rounded up with zero decimal place.
All p-values were rounded to three decimal places.
The significance of the difference between PAD and non-PAD groups.
Differences between groups were compared using Mann–Whitney test.
Differences between groups were compared using chi-square test.
Diagnosis of PAD based on FABP3 levels
ROC analysis, conducted to determine the diagnostic potential of FABP3 for PAD, demonstrated an AUC of 0.83 (95% CI: 0.81–0.86, p-value < 0.001) (Fig. 1). Exclusionary and confirmatory cutoff points were calculated using a highly sensitive value (for ruling out non-PAD patients) and a highly specific value (for ruling in patients with PAD), respectively. The FABP3 exclusionary (“rule out”) cutoff point was 1.55 ng/ml, with a sensitivity of 96% and a specificity of 40% (negative likelihood ratio = 0.11). The FABP3 confirmatory (“rule in”) cutoff point was 3.55 ng/ml, with a sensitivity of 43% and a specificity of 95% (positive likelihood ratio = 10.1) (Table 2).
Fig. 1.
Receiver-operating characteristiccurve for FABP3-based diagnosis of PAD.
Table 2.
Optimal FABP3 cutoff points for confirmation or exclusion of peripheral arterial disease.
| FABP3 (ng/ml) | Sensitivity (%) | Specificity (%) | LR (positive) | LR (negative) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|
| Exclusionary (rule out) cutoff point | 1.55 | 96 | 40 | 1.57 | 0.11 | 76 | 81 |
| Confirmatory (rule in) cutoff point | 3.55 | 43 | 95 | 10.1 | 0.60 | 94 | 45 |
LR, Likelihood ratio.
PPV, positive predictive value; NPV, negative predictive value.
Since we previously demonstrated that age is a strong determinant of FABP3 levels, we calculated age-adjusted FABP3 “rule out” cutoff point. The rule out cutoff points were calculated to be 1.84 ng/ml (sensitivity = 92.0%, specificity = 66%), 2.12 ng/ml (sensitivity = 91%, specificity = 56%), and 2.98 ng/ml (sensitivity = 93%, specificity = 79%) for age groups <50, 50–75 and >75, respectively (Table 3).
Table 3.
Age-adjusted FABP3 cutoff points for the exclusion of peripheral arterial disease.
| Age (years) | FABP3 (ng/ml) “LR 0.1” |
Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| Exclusionary (rule out) cutoff point | <50 | 1.55 | 91 | 52 |
| 50–75 | 90 | 26 | ||
| >75 | 95 | 36 | ||
| Age adjusted rule out cutoff points | <50 | 1.84 | 92 | 66 |
| 50–75 | 2.12 | 91 | 56 | |
| >75 | 2.98 | 93 | 79 |
LR, Likelihood ratio.
External validation phase–confirmation of FABP3 diagnostic cutoff point within an external cohort
Next, we validated the FABP3 cutoff points in a separate patient cohort comprising of patients with and without PAD (n = 312). Overall, the external validation cohort comprised of 180 patients with PAD and 132 without PAD (controls). Median age for the overall cohort was 67 years, and was primarily composed of males (64%) (Supplementary Table S1).
ROC curve analysis using the patient data from the external validation cohort yielded a sensitivity of 97% and a specificity of 58% for the exclusionary cutoff point, and a sensitivity of 73% and specificity of 96% for the confirmatory cutoff point. In terms of the age-adjusted FABP3 exclusionary cutoff points, the validation ROC analysis yielded a sensitivity of 100% and specificity of 87% for age groups <50, a sensitivity of 95% and specificity of 82% for age groups 50–75, and a sensitivity of 90% and specificity of 92% for age groups >75 (Table 4).
Table 4.
Validation of FABP3 (ng/ml) cutoff values in the external validation cohort (n = 312).
| Category | FABP3 (ng/ml) Cutoff Point | Sensitivity (%) | Specificity (%) | LR (positive) | LR (negative) | PPV (%) | NPV (%) | Diagnostic Accuracy of Cutoff Point |
|---|---|---|---|---|---|---|---|---|
| Exclusionary (rule out) cutoff point | ||||||||
| Rule out, overall (n = 312) | 1.55 | 97 | 58 | 2.35 | 0.04 | 76 | 95 | 81 |
| <50 year (n = 39) | 1.84 | 100 | 87 | 7.60 | 0.01 | 31 | 100 | 87 |
| 50–75 years (n = 220) | 2.12 | 95 | 82 | 4.75 | 0.06 | 89 | 90 | 89 |
| >75 (n = 53) | 2.98 | 90 | 92 | 7.83 | 0.11 | 97 | 73 | 90 |
| Confirmatory (rule in) cutoff point | ||||||||
| All patients (n = 312) | 3.55 | 73 | 96 | 10.2 | 0.28 | 96 | 72 | 83 |
Clinical outcomes and survival analysis
Patients recruited to the discovery phase were prospectively followed. During the three-year follow-up period, 14 patients (3%) were truly lost to follow-up (right censoring that was accounted for in the calculation of patients at risk). All 14 patients were censored before their first follow-up at 12 months. An additional 20 patients assessed virtually for their final follow-up due to COVID-19 related lockdown measures. Patients were followed for a mean of 33.6 months [±0.32]. The primary outcome of composite MALE outcomes occurred in 69 (13%) patients. Individual components of MALE, namely need for arterial revascularization and need for major limb amputation, occurred in 63 (12%) patients and 18 (3%) patients, respectively. The secondary outcome, decrease in ABI (≥0.15), occurred in 59 (12%) patients. A significant difference was noted in median FABP3 levels (ng/ml) between patients with and without MALE (2.58 (1.79–3.67) vs. 3.52 (2.51–4.81), p-value = 0.001) and between patients with and without change in ABI ≥0.15 event (2.51 (1.67–3.48) vs. 3.69 (2.79–4.45), p-value = 0.005).
Event rates of MALE, need for arterial revascularization, need for major limb amputation and decrease in ABI were significantly higher in PAD group as compared to non-PAD group. MALE, need for arterial revascularization, and need for major limb amputation occurred in 19% (6.67/100PYs), 18% (6.08/100PYs), and 5% (1.76/100PYs) of patients with PAD, respectively. Decrease in ABI occurred in 18% (6.53/100PYs) of patients with PAD (Supplementary Table S3).
To investigate FABP3's potential for predicting PAD-related events, univariate Cox proportional hazard analysis were performed. For each unit increase in FABP3, significant hazard ratios (HR) were observed for MALE (HR 1.15 [95% CI: 1.05–1.21]; p-value < 0.001), need for arterial revascularization (HR 1.14 [95% CI: 1.06–1.15]; p-value < 0.001), need for major limb amputation (HR 1.11 [95% CI: 1.05–1.16]; p-value < 0.001), and decrease in ABI (HR 1.15 [95% CI: 1.07–1.16]; p-value = 0.001). For all adverse PAD-related events, significance was retained even after adjusting for age, sex, hypertension, hyperlipidemia, smoking, diabetes, history of CAD, ASA, statins, and ACEi/ARB (Table 5). Furthermore, we observed that for each one unit increase in FABP3 levels, significant hazard ratios were observed for myocardial infarction (MI) (HR 1.06 [95% CI: 1.02–1.18]; p-value = 0.016). However, no significant association was observed between an increase in FABP3 levels and MACE (HR 1.03 [95% CI: 0.85–1.28]; p-value = 0.290) (refer to Supplementary Table S4).
Table 5.
Univariate and multivariate cox hazard analyses for the observed study events.
| Event | Unadjusted HR (95% CI) | P-value | Adjusted HR (95% CI)a | P-value |
|---|---|---|---|---|
| MALE, n = 69 | 1.15 (1.05–1.21) | <0.001 | 1.14 (1.03–1.29) | 0.010 |
| Arterial revascularization, n = 63 | 1.14 (1.06–1.15) | <0.001 | 1.08 (1.05–1.14) | 0.005 |
| Major limb amputation, n = 18 | 1.11 (1.05–1.16) | <0.001 | 1.10 (1.04–1.18) | 0.002 |
| Change in ABI (>0.15) n = 59 | 1.15 (1.07–1.16) | 0.001 | 1.11 (1.02–1.19) | 0.009 |
Hazard ratio for study events for one unit increase of FABP3.
MALE, Major Adverse Limb Events (need for arterial revascularization or major amputation).
Adjusted for age, sex, hypertension, hypercholesteremia, smoking, diabetes, history of CAD, ASA, statins, and ACEi/ARB.
Risk stratification using FABP3 established cutoff points
To aid with clinical risk-stratification of patients with PAD, patients were divided into two groups based on the established FABP3 “rule out” cutoff point of 1.55 ng/ml. Clinical characteristics and risk factors were noted to be different amongst the two groups, with more risk factors identified in patients above “rule out” cutoff point (Supplementary Table S5). Moreover, patients with higher levels of FABP3 were more likely to experience MALE, need for an arterial revascularization, and decrease in ABI at 3 years (p-values: <0.001, <0.001, and 0.002 respectively) (Supplementary Table S2).
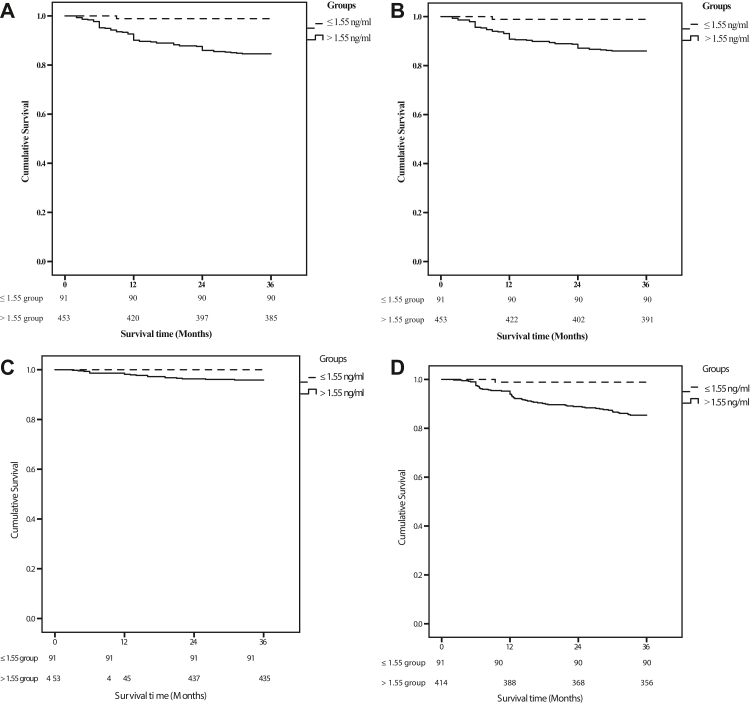
Kaplan–Meier analysis demonstrated that elevated levels of FABP3 could reliably risk stratify patients with PAD for MALE, need for arterial revascularization, and decrease in ABI (P = 0.001 for all) (Fig. 2A, B, and D), but not for need of major limb amputation (Fig. 2C). Regarding our primary outcome, MALE -free survival rates at 1 year, 2 years, and 3 years were 98.9%, 98.9% and 98.9% for the FABP3 ≤ 1.55 ng/ml group and 92.6%, 85.9% and 84.6% for the FABP3 > 1.55 ng/ml group (log-rank = 12.8, p-value < 0.001). With respect to need for arterial revascularization, event-free survival rates at 1 year, 2 years, and 3 years were 98.9%, 98.9% and 98.9% for the FABP3 ≤ 1.55 ng/ml cohort and 93.1%, 88.7% and 85.9% for FABP3 > 1.55 ng/ml cohort (log-rank = 11.4, p-value < 0.001). For our secondary outcome, event-free survival rates for change in ABI at 1 year, 2 years, and 3 years were 98.9%, 98.9% and 98.9% for the FABP3 ≤ 1.55 ng/ml cohort, and 93.9%, 88.9% and 85.4% for the FABP3 > 1.55 ng/ml cohort (log-rank = 11.7, p-value <0.001). Lastly, MACE-free survival rates at 1 year, 2 years, 3 years were 100%, 98.9%, 98.9% respectively for the FABP3 ≤ 1.55 ng/ml group and 98.2%, 91.0% and 85.7% respectively for the FABP3 > 1.55 ng/ml group (log-rank = 11.7, p-value < 0.001) (refer to Supplementary Fig. S1).
Fig. 2.
Kaplan–Meier curves (A: freedom from MALE, B and C: individual MALE outcomes and D: >0.15 decrease in ABI) in patients during the 36 month following presentation, expressed as a function of FABP3 concentration (log-rank test, P < 0.05). Patients were stratified into two groups according to FABP3 cutoff point at baseline. Dotted line, FABP3≤ 1.55 ng/mL; Solid line, FABP3>1.55 ng/mL. A) Major adverse limb events (MALE) (n = 544). B) Need for arterial revascularisation (n = 544). C) Need for Major Limb Amputation (n = 544). D) decrease in ABI (>0.15) event (n = 505).
Discussion
The results of the present study suggest that FABP3 has strong diagnostic potential for PAD as validated through an independent cohort of patients. In addition, our results show that FABP3 is a good predictor of PAD-related outcomes (i.e. MALE and decrease in ABI but not MACE). Furthermore, patients with elevated FABP3 levels had significantly increased risk for the individual components of MALE, as well as MI (in the case of MACE). Subgroup analyses revealed elevated FABP3 levels in patients with PAD regardless of smoking history or diabetes status (Supplementary Tables S6 and S7). Further analysis demonstrated that individuals who experienced either a primary or secondary outcome generally had elevated levels of FABP3, reduced ABI, and were more likely to be males with several cardiovascular risk factors (hypertension, hypercholesterolemia, diabetes, and prior history of coronary artery disease) (Supplementary Table S8). These data confirm FABP3 levels as a PAD-specific predictor of PAD progression and adverse limb-events.
The pathophysiological basis for the association between FABP3 and adverse PAD events is yet to be fully understood. FABP3 is an intracellular protein that is mainly expressed in skeletal and myocardial cells. While this protein has been extensively investigated in patients with myocardial conditions, it has been studied minimally within the PAD-context. In a previous pilot study, we demonstrated the association between elevated FABP3 levels and increased PAD disease severity.21 We also observed an upregulation in FABP3 expression within skeletal muscle samples obtained from patients with PAD when compared to non-PAD controls.21 Thus, elevated plasma levels of FABP3, a cytosolic marker, in patients with PAD reflect ongoing skeletal muscular injury. While the exact mechanisms behind skeletal injury and subsequent FABP3 release are incompletely understood, several mechanisms may account for the observed phenomenon: necrosis due to ischemia, increase plasma membrane permeability, and possibly chronic inflammation. Thus, while the pathophysiological pathways are yet to be fully understood, the associations between FABP3 and PAD/increased disease severity are plausible based on our current understanding of human anatomy and physiology.
In the present study, FABP3 diagnostic cutoff values and their associated sensitivities and specificities were reported. Using a cutoff point of <1.55ug/mL, PAD can be ruled out with 96% sensitivity. Thus, low FABP3 levels provide a high negative-predictive value (96%) with a negative likelihood ratio of 0.11, enabling the ruling out of PAD. Further confirmatory arterial imaging would not be required in this patient subgroup, and other causes behind the lower leg symptoms would need to be explored by the physician. Alternatively, PAD can be ruled in with a confirmatory cutoff point of 3.55ug/mL (95% specificity). Thus, elevated FABP3 levels provide a high positive-predictive value (83%) and positive likelihood ratio of 10.1 for the diagnosis of PAD. The exclusionary cutoff point for FABP3 is a low value, which increases the tests’ sensitivity but decreases its specificity. As such, this results in a decreased false negative rate but increased false positive rate. Contrarily, the confirmatory cutoff point, which included the majority of the PAD patients, had a higher FABP3 value. This lowers the false positive rate but increases the false negative rate. Patients with FABP3 levels in between these two cutoffs (1.55–3.5 ng/ml) are in the “grey zone”, where their PAD status remains uncertain. These patients would require further clinical and radiographical assessment to confirm their PAD status. These findings were consistent in an external validation cohort. Thus, this collective body of work represents a novel, FABP3-based, screening methodology to assess the PAD status of patients.
With regards to prognostication, we demonstrated FABP3's ability to predict adverse events in patients with PAD. Our findings showed that elevated levels of FABP3 at baseline were associated with an increased incidence of decrease in ABI as well as MALE outcomes, even after adjusting for known confounding factors. Based on the data presented herein, baseline FABP3 levels of >1.55ug/ml could reliably be used to facilitate risk stratification in patients with PAD for disease progression, MALE outcomes and need for arterial revascularization. Practically speaking, since patients with PAD are often underdiagnosed and sub-optimally managed, FABP3 levels may be used to assess the risk of PAD-related adverse events for each patient.4 Patients deemed high-risk may then benefit from early and aggressive medical management.26
Furthermore, to reduce underlying confounders, we stratified our patient cohort based on ABI quartiles, history of revascularization, and smoking status [Supplementary Tables S9–S11). In terms of ABI quartiles, we noted an increase in FABP3 levels as ABI values decreased (i.e. with increasing PAD severity) (Supplementary Table S9). Patients with advanced stages of PAD (Q3 and Q4) had a higher incidence of MALE, whereas early-stage patients with PAD (Q1 and Q2) had higher incidences of change in ABI (i.e., worsening PAD status). MACE incidence was highest among advanced-stage patients with PAD (Q3 and Q4), which reflects the increased atherosclerotic burden within these patients. Regarding revascularization history, a previous revascularization was noted to be a predictor of MALE (Supplementary Table S10). Finally, no significant differences in the primary and secondary outcomes were observed among patients with PAD after stratifying them by smoking status (non-smokers vs past smokers vs current smokers) (Supplementary Table S11). Based on these data, levels of FABP3 are elevated in patients with PAD, irrespective of revascularization history or smoking status.
Multiple biomarkers have been reported to be elevated in patients with PAD, including inflammatory cytokines, markers of endothelial dysfunction, mediators of angiogenesis, lipoproteins, and coagulation factors, among others.23,27, 28, 29, 30, 31, 32, 33, 34 However, none have been approved for clinical use, most likely due to their limited sensitivity and/or specificity for PAD,27,35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 and incomplete evaluation (e.g. only some have tested only in patients with diabetes).46,47 In contrast to other PAD-related biomarker investigations,23,27, 28, 29, 30, 31, 32, 33, 34 our study included a large cohort of diverse patients (in independent discovery and validation cohorts), proposed age-adjusted FABP3 cutoff points that can be used to diagnose PAD, and demonstrated FABP3's potential for risk stratification/prognostication as it relates to MALE and change in ABI. These findings contribute to the growing literature regarding improved PAD screening and risk stratification using FABP3 Fatty acid binding protein 4 (FABP4) in particular has been proposed as diagnostic marker and as a prognostic marker of MACE within patients with PAD, but not MALE.23,32,33 The authors are currently running research studies investigating the feasibility of utilizing both proteins for the diagnosis/prognosis of patients with PAD. Levels of FABP3 within urine can also serve as a non-invasive diagnostic or prognostic biomarker for PAD, but requires normalization for urinary creatinine.19,20 Factors such as equipment availability, level of training, blood laboratory accessibility, and geographic location, among others, could dictate the choice between using either urine or blood FABP3 levels for PAD-related diagnosis or prognosis.
This study has limitations. First, our findings would have been more generalizable had we recruited patients from multiple hospital-sites (as opposed to a single centre). Second, we did not perform serial measurement of FABP3 or evaluate response to therapy. Third, we excluded patients with advanced kidney disease or recent acute cardiovascular ischemic events. However, renal disease patients were excluded as FABP3 is cleared by the kidneys, which obfuscates the diagnostic potential of this protein within this specific patient population. The incidence of renal failure stages 4 and 5 is less than 0.23% within the general population, and less than 3% in the PAD population, thus it is not too large of a subset of patients.48,49 Fourth, linking to health data registries may have proved more reliable for capturing diagnosis and the study outcomes. Fifth, utilizing bootstrapping methods to conduct an internal validation analysis would have strengthened the analysis of this manuscript. Lastly, the authors may not have accounted for all possible confounding factors modulating FABP3 release. Additional multi-centre studies are needed to better understand the diagnostic and prognostic value of FABP3 within the context of PAD.
Evidence regarding the urgent and critical need for diagnostic and prognostic biomarkers for patients with PAD is growing within the literature.18 We studied the potential of a blood-based biomarker, FABP3, in diagnosing patients for PAD, as well as investigated its potential in identifying individuals at high risk of PAD-related adverse events. Our data demonstrates the potential for FABP3 levels in the diagnosis and risk stratification of patients with PAD who may benefit from vascular evaluation and intensive medical management. Economic evaluations of incorporating FABP3 within clinical practice are needed.
Contributors
MQ and RA conceptualised the study. The methodology was designed by AZ, MHS, RA, and MQ. The formal data analysis was conducted by AZ, but all authors (AZ, MHS, ODR, JE, DJK, KKS, RA and MQ) were collectively responsible for data interpretation and investigation. The original writing of the draft was prepared by AZ, MHS, and MQ. All authors (AZ, MHS, ODR, JE, DJK, KKS, RA and MQ) reviewed, edited, and approved the manuscript. MQ, AZ, and MHS had access to the full data, verified it, and accept responsibility to submit this body of work for publication purposes. All authors have read and agreed to the published version of the manuscript.
Data sharing statement
All relevant data are within the manuscript.
Declaration of interests
Dr. John Eikelboom reports consulting fees/honoraria and/or grant support from AstraZeneca, Bayer Boehringer-Ingelheim, Bristol-Myer-Squibb/Pfizer, Daiichi-Sankyo, Eli Lilly & Co, GlaxoSmithKline, Pfizer, Janssen, sanofi-aventis, Servier. The remaining authors have nothing to disclose.
Acknowledgments
Dr. Mohammad Qadura is supported by a Blair Early Career Professorship in Vascular Surgery. Dr. Ori Rotstein is supported by a Keenan Chair in Research Leadership.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101766.
Appendix A. Supplementary data
References
- 1.Fowkes F.G.R., Rudan D., Rudan I., et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Dhaliwal G., Mukherjee D. Peripheral arterial disease: epidemiology, natural history, diagnosis and treatment. Int J Angiol. 2007;16:36–44. doi: 10.1055/s-0031-1278244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weitz J.I., Byrne J., Clagett G.P., et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch A.T., Criqui M.H., Treat-Jacobson D., et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 5.Walsh D.B., Gilbertson J.J., Zwolak R.M., et al. The natural history of superficial femoral artery stenoses. J Vasc Surg. 1991;14:299–304. doi: 10.1067/mva.1991.30618. [DOI] [PubMed] [Google Scholar]
- 6.Whyman M., Ruckley C., Fowkes F. A prospective study of the natural history of femoropopliteal artery stenosis using duplex ultrasound. Eur J Vasc Surg. 1993;7:444–447. doi: 10.1016/s0950-821x(05)80264-6. [DOI] [PubMed] [Google Scholar]
- 7.Davies J.H., Kenkre J., Williams E.M. Current utility of the ankle-brachial index (ABI) in general practice: implications for its use in cardiovascular disease screening. BMC Fam Pract. 2014;15:1–11. doi: 10.1186/1471-2296-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford F., Welch K., Andras A., Chappell F.M. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst Rev. 2016;9(9) doi: 10.1002/14651858.CD010680.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolaï S.P., Kruidenier L.M., Rouwet E.V., Bartelink M.-L.E., Prins M.H., Teijink J.A. Ankle brachial index measurement in primary care: are we doing it right? Br J Gen Pract. 2009;59:422–427. doi: 10.3399/bjgp09X420932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hageman D., Pesser N., Gommans L.N., et al. Limited adherence to peripheral arterial disease guidelines and suboptimal ankle brachial index reliability in Dutch primary care. Eur J Vasc Endovasc Surg. 2018;55:867–873. doi: 10.1016/j.ejvs.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Chiu L.Y., Syed M.H., Zamzam A., et al. Perceived challenges to routine uptake of the ankle brachial index within primary care practice. J Clin Med. 2021;10:4371. doi: 10.3390/jcm10194371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schröder F., Diehm N., Kareem S., et al. A modified calculation of ankle-brachial pressure index is far more sensitive in the detection of peripheral arterial disease. J Vasc Surg. 2006;44:531–536. doi: 10.1016/j.jvs.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Association A.D. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 14.Conte M.S., Pomposelli F.B., Clair D.G., et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015;61:2S–41S.e1. doi: 10.1016/j.jvs.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Wolosker N., Rosoky R.A., Nakano L., Basyches M., Puech-Leão P. Predictive value of the ankle-brachial index in the evaluation of intermittent claudication. Rev Hosp Clin. 2000;55:61–64. doi: 10.1590/s0041-87812000000200005. [DOI] [PubMed] [Google Scholar]
- 16.Doobay A.V., Anand S.S. Sensitivity and specificity of the ankle–brachial index to predict future cardiovascular outcomes: a systematic review. Arterioscler Thromb Vasc Biol. 2005;25:1463–1469. doi: 10.1161/01.ATV.0000168911.78624.b7. [DOI] [PubMed] [Google Scholar]
- 17.Rudofker E.W., Hogan S.E., Armstrong E.J. Preventing major amputations in patients with critical limb ischemia. Curr Cardiol Rep. 2018;20:1–7. doi: 10.1007/s11886-018-1019-2. [DOI] [PubMed] [Google Scholar]
- 18.Cooke J.P., Wilson A.M. Biomarkers of peripheral arterial disease. J Am Coll Cardiol. 2010;55:2017–2023. doi: 10.1016/j.jacc.2009.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B., Zamzam A., Syed M.H., et al. Urinary fatty acid binding protein 3 has prognostic value in peripheral artery disease. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.875244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamzam A., Syed M.H., Harlock J., et al. Urinary fatty acid binding protein 3 (uFABP3) is a potential biomarker for peripheral arterial disease. Sci Rep. 2021;11:1–6. doi: 10.1038/s41598-021-90395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syed M.H., Zamzam A., Khan H., et al. Fatty acid binding protein 3 is associated with peripheral arterial disease. JVS. 2020;1:168–175. doi: 10.1016/j.jvssci.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy S.M. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 23.Zamzam A., Syed M.H., Greco E., et al. Fatty acid binding protein 4—a circulating protein associated with peripheral arterial disease in diabetic patients. J Clin Med. 2020;9:2843. doi: 10.3390/jcm9092843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicoloff A.D., Taylor L.M., Jr., Sexton G.J., et al. Relationship between site of initial symptoms and subsequent progression of disease in a prospective study of atherosclerosis progression in patients receiving long-term treatment for symptomatic peripheral arterial disease. J Vasc Surg. 2002;35:38–47. [PubMed] [Google Scholar]
- 25.Criqui M.H., Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 26.Gornik H.L., Creager M.A. Contemporary management of peripheral arterial disease: I. Cardiovascular risk-factor modification. Cleve Clin J Med. 2006;73:S30–S37. doi: 10.3949/ccjm.73.suppl_4.s30. [DOI] [PubMed] [Google Scholar]
- 27.Wilson A.M., Kimura E., Harada R.K., et al. β2-Microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation. 2007;116:1396–1403. doi: 10.1161/CIRCULATIONAHA.106.683722. [DOI] [PubMed] [Google Scholar]
- 28.Vu J.D., Vu J.B., Pio J.R., et al. Impact of C-reactive protein on the likelihood of peripheral arterial disease in United States adults with the metabolic syndrome, diabetes mellitus, and preexisting cardiovascular disease. Am J Cardiol. 2005;96:655–658. doi: 10.1016/j.amjcard.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 29.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 30.Dieplinger B., Lingenhel A., Baumgartner N., et al. Increased serum lipoprotein (a) concentrations and low molecular weight phenotypes of apolipoprotein (a) are associated with symptomatic peripheral arterial disease. Clin Chem. 2007;53:1298–1305. doi: 10.1373/clinchem.2007.088013. [DOI] [PubMed] [Google Scholar]
- 31.Tseng C.-H. Lipoprotein (a) is an independent risk factor for peripheral arterial disease in Chinese type 2 diabetic patients in Taiwan. Diabetes Care. 2004;27:517–521. doi: 10.2337/diacare.27.2.517. [DOI] [PubMed] [Google Scholar]
- 32.Hoebaus C., Herz C.T., Pesau G., Wrba T., Koppensteiner R., Schernthaner G.-H. FABP4 and cardiovascular events in peripheral arterial disease. Angiology. 2018;69:424–430. doi: 10.1177/0003319717728226. [DOI] [PubMed] [Google Scholar]
- 33.Dakhel A., Memon A.A., Zarrouk M., et al. Novel cardiovascular biomarkers associated with peripheral arterial disease in men screened for abdominal aortic aneurysm. Vasa. 2022;51:167–173. doi: 10.1024/0301-1526/a000999. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler L., Hedin U., Gottsäter A. Circulating biomarkers in lower extremity artery disease. Eur Cardiol. 2022;17 doi: 10.15420/ecr.2021.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiatt W.R., Zakharyan A., Fung E.T., et al. A validated biomarker panel to identify peripheral artery disease. Vasc Med. 2012;17:386–393. doi: 10.1177/1358863X12463491. [DOI] [PubMed] [Google Scholar]
- 36.Ceasovschih A., Sorodoc V., Tesloianu D., et al. Biomarker utility for peripheral artery disease diagnosis in real clinical practice: a prospective study. Diagnostics. 2020;10:723. doi: 10.3390/diagnostics10090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridker P.M., Cushman M., Stampfer M.J., Tracy R.P., Hennekens C.H. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 38.Tzoulaki I., Murray G.D., Lee A.J., Rumley A., Lowe G.D., Fowkes F.G.R. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: edinburgh Artery Study. Circulation. 2005;112:976–983. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 39.Murabito J.M., Keyes M.J., Guo C.-Y., et al. Cross-sectional relations of multiple inflammatory biomarkers to peripheral arterial disease: the Framingham Offspring Study. Atherosclerosis. 2009;203:509–514. doi: 10.1016/j.atherosclerosis.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittermayer F., Krzyzanowska K., Exner M., et al. Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler Thromb Vasc Biol. 2006;26:2536–2540. doi: 10.1161/01.ATV.0000242801.38419.48. [DOI] [PubMed] [Google Scholar]
- 41.Kappelmayer J., Nagy B., Miszti-Blasius K., Hevessy Z., Setiadi H. The emerging value of P-selectin as a disease marker. Clin Chem Lab Med. 2004;42:475–486. doi: 10.1515/CCLM.2004.082. [DOI] [PubMed] [Google Scholar]
- 42.Tsimikas S. Oxidized low-density lipoprotein biomarkers in atherosclerosis. Curr Atherosclerosis Rep. 2006;8:55–61. doi: 10.1007/s11883-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 43.Mueller T., Dieplinger B., Gegenhuber A., et al. Serum total 8-iso-prostaglandin F2α: a new and independent predictor of peripheral arterial disease. J Vasc Surg. 2004;40:768–773. doi: 10.1016/j.jvs.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 44.Seigneur M., Dufourcq P., Conri C., et al. Levels of plasma thrombomodulin are increased in atheromatous arterial disease. Thromb Res. 1993;71:423–431. doi: 10.1016/0049-3848(93)90116-6. [DOI] [PubMed] [Google Scholar]
- 45.Vidula H., Tian L., Liu K., et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med. 2008;148:85–93. doi: 10.7326/0003-4819-148-2-200801150-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J-y, Yang X-y, Wang X-f, et al. Siglec-5 is a novel marker of critical limb ischemia in patients with diabetes. Sci Rep. 2017;7:1–9. doi: 10.1038/s41598-017-11820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hung P.-H., Chen Y.-W., Cheng K.-C., et al. Plasma proteomic analysis of the critical limb ischemia markers in diabetic patients with hemodialysis. Mol Biosyst. 2011;7:1990–1998. doi: 10.1039/c1mb05055a. [DOI] [PubMed] [Google Scholar]
- 48.Bikbov B., Purcell C.A., Levey A.S., et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hopley C.W., Kavanagh S., Patel M.R., et al. Chronic kidney disease and risk for cardiovascular and limb outcomes in patients with symptomatic peripheral artery disease: the EUCLID trial. Vasc Med. 2019;24:422–430. doi: 10.1177/1358863X19864172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.