Abstract
The study rapidly reviewed and meta-analyzed the worldwide prevalence of depression and anxiety among pregnant women during the COVID-19 pandemic. A systematic search of the literature and meta-analyses were conducted from December 2019 – February 2021 with a total of 46 studies meeting inclusion criteria. Depression was assessed in 37 studies (N = 47,677), with a pooled prevalence of 25.6%. Anxiety was assessed in 34 studies (N = 42,773), with a pooled prevalence of 30.5%; moderation by time showed that prevalence of anxiety was higher in studies conducted later in the pandemic.
Keywords: Pregnancy, COVID-19, Mental health, Depression, Anxiety, Meta-analysis
Exposure to natural disasters and disease outbreaks increase the prevalence of mental health problems during pregnancy, which is already a period of increased risk for mental illness (Dennis et al., 2017; Gavin et al., 2005). Untreated mental illness in pregnancy is concerning because of the negative influence on pregnancy outcomes and postpartum mental health (Stein et al., 2014). Children of mothers who had untreated mental health problems in pregnancy are themselves more likely to have cognitive and behavioral problems and are at higher risk for later mental health problems (Stein et al., 2014; Van den Bergh et al., 2018).
Early reports from pregnant cohorts around the world suggest elevated symptoms of depression and anxiety among pregnant individuals during the COVID-19 pandemic. However, the exact prevalence is currently unknown, making the development of policy and practice recommendations difficult. Previous research suggests that certain socioeconomic and racial/ethnic groups are at heightened risk for mental illness in pregnancy, as are individuals experiencing chronic life and interpersonal stress but the extent to which is true during the pandemic is also unknown (Lancaster et al., 2010).
Rapid reviews of the literature have emerged as an efficient way to support health policy-making by providing timely and high-quality evidence about the state of the problem allowing for optimal policy level resource allocation (Langlois et al., 2019). The aim of the current study was therefore to conduct a rapid review of the prevalence of depression and anxiety experienced in pregnancy during the COVID-19 pandemic.
1. Study design
This rapid review was registered with PROSPERO [CRD42020205186]. PRISMA guidelines were followed for search strategy, article screening, and data extraction (Moher et al., 2015). A health sciences librarian conducted electronic searches in PsycINFO, Cochrane Central Register of Controlled Trails (CENTAL), EMBASE, and MEDLINE from inception up to February 10th, 2021. The patient population in question was pregnant women. The outcome was symptoms and/or diagnosis of depression and anxiety and the timeframe in question was studies conducted during the COVID-19 pandemic; the search strategy was crafted to include these three themes. Terms were searched as subject headings and keywords. Adjacency operators and truncation symbols were used to capture variations in key terms. A database of prints pre-publication for studies that matched the key terms “pregnan*” and “COVID-19” was also searched.
Inclusion criteria for the current study were: 1) study participants were pregnant; 2) a proportion of individuals in the study met clinical cut-offs for anxiety or depressive symptoms via a validated self-report measure or healthcare professional diagnosis; 3) data was obtained after the onset of COVID-19, 4) participants were ≥ 18 years; 5) study was empirical; and 6) written in English. Qualitative or case study reports were excluded. Using Covidence software, two authors reviewed titles and abstracts emerging from the search strategy to determine inclusion eligibility. Disagreements were resolved via consensus. Subsequently, full text articles were reviewed by a team of coders and reliability for the full text review. Disagreements were resolved by discussion.
2. Data extraction
Prevalence data of clinically elevated anxiety and depressive symptoms were extracted by one coder and 100% data check was conducted by a second coder. All studies were examined to ensure those included represented independent samples. Moderators extracted were: 1) study quality; 2) participant age (mean); 3) geographic location, 4) gestation (weeks pregnant), 5)% minority group members in the sample, and 6) month that data collection began for the study.
3. Study quality
A short 6-item study quality measure was used based on modified versions of the National Institute of Health Quality Assessment Tool for Observation Cohort and Cross-Sectional Studies; (Wells et al., 2013; National Heart L, and Blood Institute 2014) scores ranged from 0 to 6 (eTable 2).
4. Data analysis
All data was entered into Comprehensive Meta-Analysis (CMA version 3.0) (Comprehensive Meta-Analysis Software (CMA) 2013) where pooled prevalence and 95% confidence intervals (CIs) were computed. Random-effects models were used. Pooled prevalences were weighted by the inverse of their variance, giving greater weight to studies with larger samples. Tests of heterogeneity were examined with and without outliers to determine if outliers influenced between-study heterogeneity, which was examined using Q and I2 statistics. A Q statistic or I2 statistic greater than 75% suggests moderator analyses should be explored. Categorical moderators were only conducted when k ≥ 10 and with a minimum cell size of k > 3 were available. Random-effect meta-regressions were calculated for continuous moderators. The Egger test and funnel plots were used to examine publication bias.
5. Results
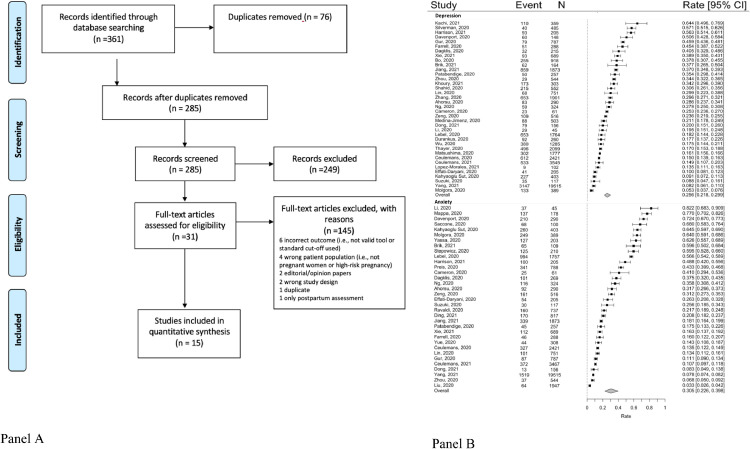
The search yielded 776 non-duplicate records (Fig. 1 a). 124 full text articles were reviewed and 46 met full inclusion criteria.
Fig. 1.
a PRISMA diagram and b. forest plots.
5.1. Study characteristics
Across all 46 studies, mean participant age was 30.63 years (range, 27.4–34.4) and mean gestational age was 23.78 weeks (range 7.04–31.63) (eTable 3). There were 22 countries represented, across North America (n = 9, 19.57%), East Asia (n = 17; 36.96%), Europe (n = 10, 21.74%), West Asia (n = 5, 10.87%), and South Asia (n = 4, 8.70%) and Europe/West Asia combined (n = 1, 2.17%) Mean study quality was 3.7 out of 6 (range: 2–5) see eTable 4.
5.2. Pooled prevalence of clinically elevated prenatal depressive symptoms during COVID-19
A random-effects meta-analysis of 37 studies revealed a pooled event rate of 0.256 (95% CI: 0.218, 0.299; Fig 1b), indicating a prevalence of clinically significant prenatal depression across studies of 25.6%. The funnel plot was symmetrical and the Egger test was not significant (eFigure 1; p < .06). There was significant between-study heterogeneity (Q = 2616.09, p < .001, I 2 = 98.62). Maternal age, gestational age, percent minority, and study quality were explored as potential moderators, but none emerged as significant (maternal age Z = −0.15, p < .89; gestational age Z = −0.25, p < .81; percent minority Z = −0.04, p < .98; study quality Z = −0.97, p < .34). No significant differences (Q = 0.54; p < .92) were observed in prevalence's across geographical regions (East Asia: k = 14, event rate = 0.25 [95% CI: 0.19, 0.33]; Europe: k = 8, event rate = 0.27 [95% CI: 0.19, 0.37]; North America: k = 6, event rate = 0.26 [95% CI: 0.17, 0.37]; West Asia: k = 5, event rate = 0.30 [95% CI: 0.18, 0.46]. There was not an effect of time on the prevalence rate of depression (Z = 1.61 p < .11).
5.3. Pooled prevalence of clinically elevated prenatal anxiety symptoms during COVID-19
A random-effects meta-analysis of 34 studies revealed a pooled event rate of 0.305 (95% CI: 0.226, 0.398); Fig 1b), indicating a prevalence of clinically significant prenatal anxiety across studies of 30.5%. The funnel plot was symmetrical; however, the Egger test was significant (eFigure 2; p = .01). There was significant between-study heterogeneity (Q = 5780.54, p < .001, I 2 = 98.43). Maternal age, gestational age, percent minority, and study quality were explored as potential moderators, but none emerged as significant (maternal age Z = 0.85, p < .40; gestational age Z = −0.43, p < .67; percent minority Z = −1.61, p < .11; study quality Z = 1.00, p < .32). However, significant differences (Q = 14.93; p < .01) were observed in prevalence across geographical regions, with prevalence in East Asia (k = 12, event rate = 0.16, 95% CI: 0.11, 0.23) being significantly lower than those in Europe (k = 10, event rate = 0.44, 95% CI: 0.27, 0.62), North America (k = 5, event rate = 0.43, 95% CI: 0.24, 0.63), but not significantly lower than those in West Asia (k = 4, event rate = 0.33 95% CI: 0.15, 0.58). There was a significantly effect of time on the prevalence rate of anxiety, such that studies with data collection later in the pandemic reported higher prevalence rate (Z = 2.12, p < .04).
6. Comment
In this rapid review and meta-analysis, we observed significantly elevated rates of antenatal depression and anxiety during the COVID-19 pandemic compared to historical norms that used similar methodology (Dennis et al., 2017; Gavin et al., 2005). We also observed that studies with data collected later in the pandemic reported higher anxiety prevalence, potentially linked to exposure to pandemic chronic stressors and ongoing uncertainty. Finally, rates of anxiety were lower in East Asia, compared to Europe, and North America, but not West Asia.
Recommendations have been rapidly developed to support women's mental health in pregnancy during the COVID-19 pandemic (Cohen et al., 2020). In line with pre-pandemic practice guidelines, the findings from this study suggest the continued need for screening and evidence based treatments for depression and anxiety in pregnancy (ACOG Committee Opinion 2018; Arch, 2014).
7. Future directions
There is moderate to high stability in symptoms of depression and anxiety from pregnancy to the postpartum period – understanding if chronicity of mental health symptoms is true of pandemic related mental health symptoms is an urgent research priority (Bayrampour et al., 2016). Findings from this study have implications for maternal-infant bonding and the cognitive, social and emotional development of children (Rogers et al., 2020). Continued surveillance of maternal, family and child outcomes that are associated with these concerning elevations in depression and anxiety is necessary as are health care system wide monitoring of birth outcomes and parental postpartum mood.
CRediT authorship contribution statement
Lianne M. Tomfohr-Madsen: Conceptualization, Methodology, Writing-Original draft, Writing - Review & Editing. Nicole Racine: Methodology, Data curation, Writing-Original draft, Writing - Review & Editing. Gerald F. Giesbrecht: Conceptualization, Writing - Review & Editing. Catherine Lebel: Conceptualization, Writing- Review & Editing. Sheri Madigan: Writing-Original draft, Data Visualization and Analysis, Writing- Review & Editing.
Declaration of Competing Interest
The authors have no conflicts of interest to report.
Acknowledgments
Acknowledgement(s)
We would like thank Nicole Dunnewold, MLIS, from the University of Calgary, for her assistance with the search strategy and Roshni Sohail and Jasleen Kaur for their assistance with data extraction. Funding was provided by the Canadian Child Health Clinician Scientist Program (LTM), the Canada Research Chairs Program (SM, CL) and Alberta Innovates (NR).
Financial Support
Salary support for this project was provided by the Canadian Child Health Clinician Scientist Program (CCHCSP; LTM).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psychres.2021.113912.
Appendix. Supplementary materials
References
- Dennis C.L., Falah-Hassani K., Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br. J. Psychiatry. 2017;210(5):315–323. doi: 10.1192/bjp.bp.116.187179. [DOI] [PubMed] [Google Scholar]
- Gavin N.I., Gaynes B.N., Lohr K.N., Meltzer-Brody S., Gartlehner G., Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet. Gynecol. 2005;106(5):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Stein A., Pearson R.M., Goodman S.H., et al. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B.R., Dahnke R., Mennes M. Prenatal stress and the developing brain: risks for neurodevelopmental disorders. Dev. Psychopathol. 2018;30(3):743–762. doi: 10.1017/S0954579418000342. [DOI] [PubMed] [Google Scholar]
- Lancaster C.A., Gold K.J., Flynn H.A., Yoo H., Marcus S.M., Davis M.M. Risk factors for depressive symptoms during pregnancy: a systematic review. Am. J. Obstet. Gynecol. 2010;202(1):5–14. doi: 10.1016/j.ajog.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois E.V., Straus S.E., Antony J., King V.J., Tricco A.C. Using rapid reviews to strengthen health policy and systems and progress towards universal health coverage. BMJ Glob. Health. 2019;4(1) doi: 10.1136/bmjgh-2018-001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G.A., Shea B., Higgins J.P., Sterne J., Tugwell P., Reeves B.C. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Res. Synth. Methods. 2013;4(1):63–77. doi: 10.1002/jrsm.1077. [DOI] [PubMed] [Google Scholar]
- National Heart L, and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Published 2014. Accessed. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- Compehensive Meta-Analysis Software (CMA) Version 3. Biostat; 2013. ([computer Program]). [Google Scholar]
- Cohen M.A., Powell A.M., Coleman J.S., Keller J.M., Livingston A., Anderson J.R. Special ambulatory gynecologic considerations in the era of coronavirus disease 2019 (COVID-19) and implications for future practice. Am. J. Obstet. Gynecol. 2020;223(3):372–378. doi: 10.1016/j.ajog.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACOG Committee Opinion No. 757: screening for perinatal depression. Obstet. Gynecol. 2018;132(5):e208–e212. doi: 10.1097/AOG.0000000000002927. [DOI] [PubMed] [Google Scholar]
- Arch J.J. Cognitive behavioral therapy and pharmacotherapy for anxiety: treatment preferences and credibility among pregnant and non-pregnant women. Behav. Res. Ther. 2014;52:53–60. doi: 10.1016/j.brat.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Bayrampour H., Tomfohr-Madsen L.M., Suzanne T. Trajectories of perinatal depressive and anxiety symptoms in a community cohort. J. Clin. Psychiatry. 2016;77(11):e1467–e1473. doi: 10.4088/JCP.15m10176. [DOI] [PubMed] [Google Scholar]
- Rogers A., Obst S., Teague S.J., et al. Association between maternal perinatal depression and anxiety and child and adolescent development. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.