Abstract
Salmonella pathogenicity island 1 (SPI-1) encodes virulence determinants, which are important for enteropathogenicity in calves. To determine whether the Salmonella enterica serovar Typhimurium SPI-1 effector proteins SspA and SptP are important for enteropathogenicity, strains lacking these proteins were tested during oral infection of calves. Calves infected with a sptP mutant or its isogenic parent developed diarrhea and lethal morbidity. In contrast, calves infected with an sspA mutant developed diarrhea, which resolved within 10 days but did not result in mortality. The sspA mutant was recovered from bovine intestinal tissues at numbers similar to those obtained for its isogenic parent and caused marked intestinal lesions. Thus, the severity of pathological changes caused by serovar Typhimurium strains or their ability to cause diarrhea were not predictive of their ability to cause lethal morbidity in calves. We conclude that factors other than or in addition to bacterial colonization, intestinal lesions, or electrolyte loss contribute to lethal morbidity in calves infected with serovar Typhimurium.
The incidence of human salmonellosis is estimated to range between 800,000 and 3,700,000 cases per year in the United States (7). Each year, approximately 40,000 cases are serotyped and reported to the Centers for Disease Control and Prevention (6). The incidence of reported Salmonella infections in the United States is highest among children aged less than 1 year, with 111 cases per 100,000 population (5). Between 21 and 38% of human salmonellosis cases in North America and Europe are due to infection with a single serovar, Salmonella enterica serovar Typhimurium (4, 20, 37). Serovar Typhimurium infections in humans commonly remain localized to the intestine and draining lymph nodes and manifest within 48 h after ingestion of contaminated food or water with nausea, vomiting, and acute diarrhea which progresses toward dysentery (30). Since serovar Typhimurium usually causes a self-limited infection with diarrhea resolving within 10 days, replacement of fluids and electrolytes is the primary approach to treatment (23). Serovar Typhimurium produces bacteremia in 1.1% of patients (37), and in these cases antibiotic therapy can be lifesaving. The testing of S. typhimurium isolates for antibiotic sensitivity has shown that in recent years, an increasing proportion is resistant to multiple antimicrobial agents (14). The continuing epidemic of multidrug-resistant serovar Typhimurium will likely reduce the efficacy of antibiotic therapy in the future. In order to devise alternate strategies for the control of salmonellosis, it will be necessary to develop a better understanding of the fundamental factors that serovar Typhimurium uses to cause morbidity and mortality.
In addition to its association with human disease, serovar Typhimurium is a major cause of calf morbidity and mortality in the United States and in Europe (29, 32). A survey performed in Britain revealed that Salmonella serovars are associated with 12% of diarrhea outbreaks among calves (28). Two serotypes, Typhimurium and Dublin, are associated with more than 95% of the salmonellosis cases reported from cattle (13, 34, 35). Since bovine enteritis closely resembles the illness produced by serovar Typhimurium in humans, recent studies have focused on experimental oral infection of calves to establish an animal model for diarrheal disease in humans (38, 40). Approximately 75% of serovar Typhimurium infections occur in calves less than 2 months of age, before the animals are weaned (34). Calves inoculated orally with serovar Typhimurium develop diarrhea within 48 h (27), while the infection remains primarily enteric, with only occasional bacteremia (43). Oral inoculation of calves at a dose of 105 to 107 organisms usually results in a transient diarrhea which resolves within 10 days, but oral inoculation at higher doses may lead to mortality (33, 43). Lethal signs of disease in calves include anorexia and central nervous system depression (33). At necropsy, calves infected with a lethal dose of serovar Typhimurium present with marked intestinal lesions. These include acute fibrinopurulent necrotizing enteritis with pseudomembrane deposition at the luminal surface of the terminal 5 m of the ileum and the cranial 1 to 2 m of the colon. Furthermore, serovar Typhimurium infection results in lymphoid depletion in germinal centers of intestinal lymphoid follicles and in the mesenteric lymph node (38, 43). Although severe intestinal lesions, acute diarrhea, and the resulting dehydration are striking features of bovine enteritis, it is not clear whether these signs of disease contribute to mortality in calves.
Several studies have implicated the invasion-associated type III secretion system encoded by Salmonella pathogenicity island 1 (SPI-1) in causing diarrhea and mortality in cattle. For instance, mutations in prgH, hilA, and invH, three genes located on SPI-1, result in strongly reduced diarrhea and marked attenuation of serovar Typhimurium during oral infection of calves (38, 40). Furthermore, SPI-1 mutants of serovars Dublin and Typhimurium elicit reduced fluid accumulation and neutrophil influx in bovine ligated ileal loops (1, 12). However, the mechanisms by which SPI-1 promotes enteropathogenicity are unknown.
The main function of the type III secretion system encoded by SPI-1 is to translocate bacterial effector proteins into the cytosol of the host cell. Some of the effector proteins translocated by the invasion associated type III secretion system are encoded by genes that are not located on SPI-1. For example SopB (also known as SigD), a protein with inositol phosphate phosphatase activity, is translocated into epithelial cells by an SPI-1-dependent pathway (12, 24). The sopB gene is located on SPI-5, and mutational inactivation results in a modest reduction of fluid secretion elicited by serovar Dublin in bovine ligated ileal loops (12, 41). However, unlike strains carrying a mutation in hilA or prgH, a serovar Typhimurium sopB mutant causes severe diarrhea and mortality at wild-type levels during oral infection of calves (38). These data suggest that in addition to SopB, other SPI-1-secreted effector proteins play a role in enteropathogenicity.
Genes located on SPI-1 encode eight secreted targets of the invasion-associated type III export apparatus, including InvJ (SpaN), SpaO, AvrA, SptP, SspA (SipA), SspB (SipB), SspC (SipC), and SspD (SipD) (9–11, 15, 17–19). Five of these targets, AvrA, SptP, SspA, SspB, and SspC, are translocated into epithelial cells and may thus be considered secreted effector proteins (8, 9, 15, 44). The SspB protein has two functions. It is an effector protein which, after injection into macrophages, causes apoptosis by binding to caspase-1 (16). In addition, SspB is part of a translocation apparatus (formed by SspB, SspC, and SspD) which is required for the delivery of secreted effector proteins (e.g., SptP, AvrA, SopB, SopE, SspB, and SspC) into the eukaryotic cytoplasm (8, 11, 12, 15, 42). Inactivation of sspB results in a dramatic reduction in fluid secretion and polymorphonuclear cell influx elicited by serovar Dublin in bovine ligated ileal loops (12). However, since a mutation in sspB prevents translocation of a number of targets of the invasion-associated type III secretion system, it is not clear which secreted effector proteins are responsible for this phenotype. Furthermore, it is not apparent from results obtained using the ligated ileal loop assay how a mutation may effect other aspects of disease, including the severity of intestinal lesions or lethality (38). To determine the role of individual SPI-1-secreted effector proteins in producing virulence-associated phenotypes, such as diarrhea, intestinal lesions, or lethality, we have investigated the effect of mutations in sspA and sptP on enteropathogenicity during oral infection of serovar Typhimurium in calves.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Derivatives of ATCC strain 14028, a bovine serovar Typhimurium isolate, are listed in Table 1. Strain IR715 is a spontaneous nalidixic acid-resistant derivative of 14028 (36). Strain CS401 is a spontaneous streptomycin-resistant (Strr) derivative of 14028 which carries a phoN::Tn10d(Cm) insertion (26). Bacteria were cultured aerobically at 37°C in Luria-Bertani (LB) broth (per liter, 10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl) or on LB agar (16 g/liter) plates. If appropriate, antibiotics were added at the following concentrations: kanamycin, 100 mg/liter; chloramphenicol, 30 mg/liter; ampicillin, 100 mg/liter; streptomycin, 100 mg/liter; nalidixic acid, 50 mg/liter.
TABLE 1.
Derivatives of serovar Typhimurium strain ATCC 14028 used in this study
| Strain | Genotype | Reference |
|---|---|---|
| IR715 | 14028 wild type, nalidixic acid resistant | 36 |
| CS401 | 14028 phoN::Tn10d(Cm) Strr | 26 |
| MJH2362 | CS401 ΔsptP | This study |
| EE633 | CS401 sspA::Tn5lacZY | 3 |
| CAS152 | CS401 ΔsspB | This study |
| CAS108 | CS401 ΔsspC | C. A. Scherer, E. Cooper, and S. I. Miller, submitted for publication |
| CAS153 | CS401 ΔsspD | This study |
| TK091 | CS401 ΔprgH–K | This study |
Construction of mutants.
A nonpolar deletion of the prgHIJK operon was constructed by allelic exchange. DNA fragments of approximately 1 kb originating upstream and downstream of prgH and prgK, respectively, were amplified by PCR. The fragment upstream of the deletion contained the first 15 nucleotides of prgH, while the downstream fragment contained the last 192 nucleotides of prgK. The upper and lower flanking regions were ligated together in plasmid pKAS32, a λpir-dependent suicide vector encoding resistance to ampicillin and an rpsL allele conferring streptomycin sensitivity (32), to generate pTK62. Plasmid pTK62 was introduced into Escherichia coli strain Sm10λpir and transferred into Salmonella serovar Typhimurium strain CS401 by conjugation. Exconjugants containing pTK62 integrated into the serovar Typhimurium chromosome were selected on LB agar plates supplemented with ampicillin and chloramphenicol. Deletion of the rpsL allele of plasmid pTK62 by a second recombination event was selected for by plating exconjugants on LB agar plates containing streptomycin. An Strr isolate (TK091) containing the prgHIJK deletion was identified by Southern blot analysis using a 4,307-bp DNA probe containing the prgHIJK operon. Using the above strategy, a nonpolar deletion of sptP was constructed by digesting a cloned 5.5-kb BamHI DNA fragment containing sptP with EcoRV and AflII. Religation of the plasmid resulted in a 1,737-bp deletion which removed sequences 247 bp upstream of the sptP start codon to 50 bp downstream of the codon for the conserved active-site cysteine of SptP. Following integration of a suicide vector containing the 1,736-bp deletion and streptomycin treatment, an ampicillin-sensitive Strr isolate (MJH2362) was analyzed by Southern hybridization using the 5.5-kb BamHI fragment as a probe. Nonpolar deletions of sspB and sspD were constructed using the same strategy. Regions approximately 1 kb directly upstream and downstream of the coding sequences of each gene were PCR amplified. The upper and lower flanking regions were ligated together in pKAS32 to generate pMM02 (sspB deletion vector) and pMM04 (sspD deletion vector). These plasmid were introduced into CS401 by conjugative transfer, and exconjugants were selected for by growth on LB agar plates containing ampicillin and chloramphenicol. Deletion of plasmid sequences was induced by growth on LB agar plates supplemented with streptomycin, and clones containing the deletions were identified by Southern blot analysis using a DNA probe specific to sspC. The resultant clones contained a complete deletion of the sspB coding sequence (CAS152) and a deletion of the sspD coding sequence through the HindIII site at nucleotide 1013 (CAS153). The lack of SspB and SspD expression was confirmed by Western blot analysis (data not shown) with trichloroacetic acid precipitates of culture supernatants and whole cell lysates of bacterial cultures (grown to an optical density at 600 nm of 1.2 to 1.5), using antibodies generated to secreted proteins as described previously (17).
Animal experiments.
Milk-fed male Friesian/Holstein calves, aged 3 to 4 weeks, were obtained from a commercial dairy calf rearing operation. The weight of the calves ranged between 45 and 52 kg. Animals were cared for according to Association for Assessment and Accreditation of Laboratory Animal Care guidelines. Calves were fed 2 liters of milk replacer twice daily and were given water ad libitum. Before being used for experiments, calves were screened for elevated white blood cell counts, fever, and infection with Salmonella serovars. Salmonella serovars were detected in fecal swabs by enrichment in tetrathionate broth (Difco) and plating on brilliant green agar (BBL).
Oral infection of bull calves was performed as described previously (22). In brief, the optical density of overnight cultures at 600 nm was determined to estimate the number of bacteria per milliliter. A volume containing the desired numbers of bacteria was added to 50 ml of a suspension of 5% magnesium trisilicate, 5% sodium bicarbonate, and 5% magnesium carbonate buffer. The inoculum was added to 950 ml of milk replacer and fed orally to calves. Serial 10-fold dilutions of the inoculum were spread on LB plates to determine the exact number of CFU fed to each animal.
A previous report indicates that in calves which survive a serovar Typhimurium infection, diarrhea does not persist beyond the day 10 postinfection (27). Calves were hence monitored for 10 days postinfection and then euthanized. When calves developed anorexia or were unable to stand, they were euthanized for humane reasons as described previously (22). Weights and fecal scores (assessment of diarrhea) of calves were recorded daily. Shedding of serovar Typhimurium was monitored by taking daily rectal swabs, subsequent enrichment in tetrathionate broth (Difco), and plating on brilliant green agar (BBL). Blood samples were taken before (two samples taken on different days) and at 1 and 2 days after infection. Determination of concentrations of sodium, chloride, glucose, blood urea nitrogen, creatinine, aspartate transaminase, alkaline phosphatase, creatinine kinase, phosphorus, albumen, anion gap, total calcium, total protein, and total bilirubin in plasma samples was performed by Clinical Pathology Ektachem (Texas Veterinary Medical Center, Texas A&M University). The normal range of blood values was determined from 56 blood samples taken prior to infection from 28 calves (two blood samples were taken from each calf on two separate days).
During competitive infection experiments, groups of four calves were inoculated at a total dose of approximately 109 CFU/animal with a 1:1 mixture of wild-type and mutant bacteria. At 4 days postinfection, animals were euthanized. Tissues were collected, homogenized in phosphate-buffered saline, and plated in the presence of the appropriate antibiotics for enumeration of mutant and wild-type bacteria to determine the output ratio. Data were normalized by dividing the wild type/mutant output ratio of by the wild type/mutant input ratio. All data were converted logarithmically prior to the calculation of averages and statistical analysis. Student's t test was used to determine whether the wild type/mutant ratio recovered from infected organs was significantly different from the wild type/mutant ratio present in the inoculum.
To evaluate the effect of fluid and electrolyte replacement, four calves were each infected with approximately 1010 CFU of serovar Typhimurium strain IR715. Two calves were given 1 liter of rehydration solution (90 mM sodium, 20 mM potassium, 80 mM chloride, 30 mM bicarbonate, 111 mM glucose) intragastrically through a feeding tube three times daily. Blood values were taken prior to infection and on days 1 and 2 postinfection. The experiment was repeated with four calves, two of which received oral rehydration therapy.
Histopathology.
Calves infected with approximately 1010 CFU of mutant or wild-type bacteria were euthanized at identical time points postinfection to allow direct comparison of the histopathological lesions. Euthanasia and preparation of tissue samples for histopathology were performed as described previously (22). At necropsy, tissue samples from Peyer's patches, ileum, and mesenteric lymph node were collected; a portion was homogenized in phosphate-buffered saline and plated in the presence of the appropriate antibiotics for enumeration of bacteria. The remaining tissue samples were coded for blind examination. Tissue samples were fixed in 10% formalin, embedded in paraffin, thin sectioned, and stained with hematoxylin and eosin for histopathological examination.
RESULTS
Role of SPI-1 effector genes in calf virulence.
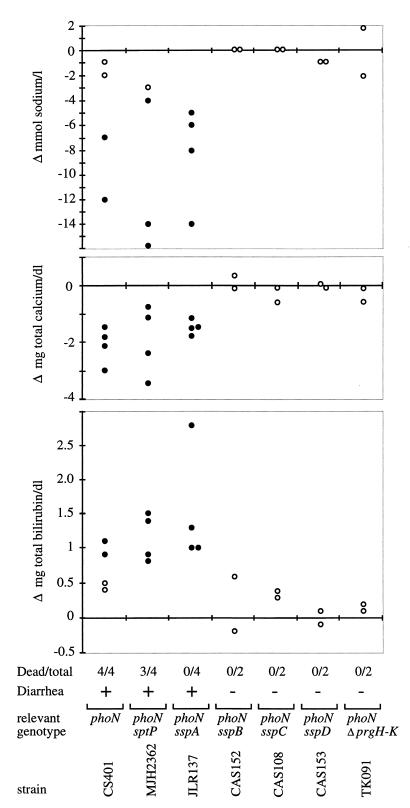
Calves infected with 1010 CFU of strain CS401, a wild-type derivative marked by insertion of an antibiotic resistance cassette into the phoN gene, had aqueous diarrhea with feces containing various combinations of blood, fibrin, and mucus. All calves developed signs of terminal illness (anorexia or inability to stand) between days 2 and 5 postinfection and were euthanized (Fig. 1). In contrast, mutations which are known to prevent type III secretion (phoN ΔprgHIJK) or translocation of effector proteins into host cells (ΔsspB, ΔsspC, or ΔsspD) resulted in marked attenuation. Calves infected with 1010 CFU of strain CAS152 (phoN ΔsspB), CAS108 (phoN ΔsspC), CAS153 (phoN ΔsspD), or TK091 (phoN ΔprgHIJK) either had only soft feces for 3 to 4 days or normal feces. After 10 days, all calves were healthy. These data confirm previous reports that mutations in prgH or hilA, which inactivate the SPI-1-encoded type III secretion system, reduce the severity of diarrhea and cause marked attenuation during serovar Typhimurium infection in calves (38). To assess the role in calf virulence of SPI-1 effector proteins, which are not required for protein translocation, groups of four animals were infected orally with 1010 CFU of strain MJH2362 (phoN sptP) or EE633 (phoN sspA). In both groups, all calves developed aqueous diarrhea comparable to that caused by their isogenic parent (CS401). The sptP mutant (MJH2362) caused lethal morbidity in three of four infected calves. In calves infected with the sspA mutant (EE633), the diarrhea resolved within 10 days and all animals recovered from the infection.
FIG. 1.
Enteropathogenicity of serovar Typhimurium SPI-1 mutants. Graphs show the difference in the plasma concentrations of sodium (top), total calcium (middle), and total bilirubin (bottom) determined for samples collected 2 days postinfection relative to a preinfection sample. Each circle represents data from an individual animal. Open circles indicate plasma values which were within the normal range of 133 to 143 mM (sodium), 9.1 to 10.6 mg/dl (total calcium), or 0.2 to 1.0 mg/dl (total bilirubin); closed circles indicate plasma values outside the normal range. The ability of serovar Typhimurium strains to cause lethal infection and diarrhea is shown below.
To assess the severity of clinical signs of disease quantitatively, we determined how the composition of blood changes during infection. Blood was collected from calves before infection and at day 2 after infection. This time point was chosen because calves infected with strain CS401 (phoN) developed signs of terminal illness starting at day 2 postinfection and were euthanized. The sodium concentration in plasma of animals that developed diarrhea was decreased at day 2 postinfection and was below the normal range of 133 to 141 mM in 9 out of 12 animals with diarrhea (Fig. 1). The plasma concentration of sodium did not decrease notably in calves, which did not develop diarrhea. The decrease in the plasma sodium concentration in calves infected with strain CS401 (phoN), MJH2362 (phoN sptP), or EE633 (phoN sspA) was likely caused by electrolyte loss due to intestinal fluid secretion. These data thus confirmed that calves infected with CS401 (phoN), MJH2362 (phoN sptP), or EE633 (phoN sspA) developed diarrhea of similar severity.
The amount of total calcium decreased in calves with diarrhea and was below the normal range of 9.1 to 10.5 mg/dl at day 2 postinfection in all animals infected with strain CS401 (phoN), MJH2362 (phoN sptP), or EE633 (phoN sspA) (Fig. 1). In contrast, the plasma concentration of calcium stayed within the normal range in all calves that did not develop diarrhea. A decrease in the total calcium concentrations may be the result of a diarrhea-induced loss of plasma proteins, which bind calcium. A decrease in the plasma protein content in serovar Typhimurium-infected calves with severe diarrhea has been reported previously (33). Similarly, we found that the total protein content of plasma tended to decrease in calves with severe diarrhea but stayed within the normal range of 5.0 to 7.1 g/dl in all calves.
The amount of total bilirubin increased in all calves infected with strain CS401 (phoN), MJH2362 (phoN sptP), or EE633 (phoN sspA) and was above the normal range of 0.3 to 1.0 mg/dl in 10 out of 12 animals with diarrhea (Fig. 1). The amount of total bilirubin remained within the normal range in animals infected with strain CAS152 (phoN ΔsspB), CAS108 (phoN ΔsspC), CAS153 (phoN ΔsspD), or TK091 (phoN ΔprgHIJK). The reason for the increase in the concentration of total bilirubin in animals infected with strain CS401 (phoN), MJH2362 (phoN sptP), or EE633 (phoN sspA) is unclear.
Ability of serovar Typhimurium mutants to colonize bovine intestinal tissue and cause lesions.
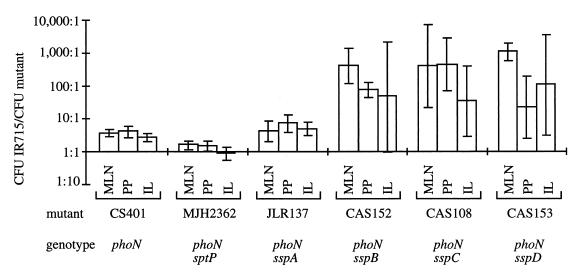
To assess the effect of mutations in sptP and sspA on invasion of the intestinal mucosa in calves, each strain was tested for its ability to compete with serovar Typhimurium strain IR715 (nalidixic acid-resistant wild type) for colonization of bovine tissues. Groups of four calves were infected orally with a 1:1 mixture of wild type (IR715) and one mutant. Four days postinfection, calves were euthanized and organs (ileum, Peyer's patch, and mesenteric lymph node) were collected. The resistance marker of the phoN::Tn10d(Cm) allele was used to distinguish the CS401 derivatives from the competing wild-type strain (IR715). A competitive infection of strain CS401(phoN) and strain IR715 revealed that strain CS401 displayed a modest defect in competitive colonization (IR715 was recovered in approximately fivefold-higher numbers than CS401) (Fig. 2). The colonization defect of CS401 may be due either to its phoN mutation or to the mutation leading to streptomycin resistance, but this was not further investigated. Mutations in sptP (MJH2362) or sspA (EE633) did not further reduce the ability of a phoN mutant to colonize bovine intestinal tissues (Fig. 2). To compare these results with a colonization defect caused by inactivation of the type III secretion/translocation machinery, competitive infections with IR715 were performed with strain CAS152 (phoN ΔsspB), CAS108 (phoN ΔsspC), or CAS153 (phoN ΔsspD). The wild type (IR715) was recovered in approximately 500- to 1,000-fold-higher numbers from bovine Peyer's patches than CAS152, CAS108, or CAS153. This observation is consistent with a previous report that during competitive infection experiments, the serovar Typhimurium wild type is recovered in 500- to 5,000-fold-higher numbers from bovine Peyer's patches than strains carrying mutations in hilA or prgH, two genes required for SPI-1 dependent type III secretion (39). Thus, sspB, sspC, or sspD, but not sptP or sspA, is required for colonization of the bovine intestine by serovar Typhimurium. Furthermore, there was a good correlation between the ability of a strain to invade the intestinal mucosa (Fig. 2) and its ability to cause diarrhea in calves (Fig. 1).
FIG. 2.
Ability of serovar Typhimurium mutants to compete with a nalidixic acid-resistant wild-type strain (IR715) for colonization of bovine tissues. For each mutant, competitive infection was studied with groups of four animals infected with a 1:1 mixture of wild-type (IR715) and mutant bacteria. The wild type/mutant ratio recovered from organs of infected calves 4 days postinfection is shown as mean (bars) ± standard deviation. MLN, mesenteric lymph node; PP, ileal Peyer's patch proximal to cecum; IL, terminal ileum.
To determine whether attenuation of the sspA mutant is related to the severity of intestinal lesions caused by this strain, two calves were infected either with CAS152 (phoN sspB), EE633 (phoN sspA), or their isogenic parent (CS401). One calf in each group was euthanized at days 1 and 2 postinfection. At necropsy, gross pathologic examination was performed and tissue samples from Peyer's patches, ileum, and mesenteric lymph node were collected for histopathologic examination. Strains EE633 (phoN sspA) and its isogenic parent (CS401) produced macroscopically severe acute fibrinopurulent necrotizing enteritis with segmental or continuous pseudomembrane deposition. No gross pathological lesions were detected in calves infected with CAS152 (phoN ΔsspB). Histopathologic analysis revealed severe lymphoid depletion and confirmed the severe acute fibrinopurulent necrotizing enteritis in calves infected with EE633 (phoN sspA) or CS401 (phoN) (data not shown). Intestinal lesions were either negligible or absent in calves infected with CAS152 (phoN ΔsspB). Thus, the severity of intestinal lesions correlated with the ability of a mutant to colonize bovine intestinal tissues during a competitive infection experiment.
Effect of oral rehydration therapy on mortality in calves infected with serovar Typhimurium.
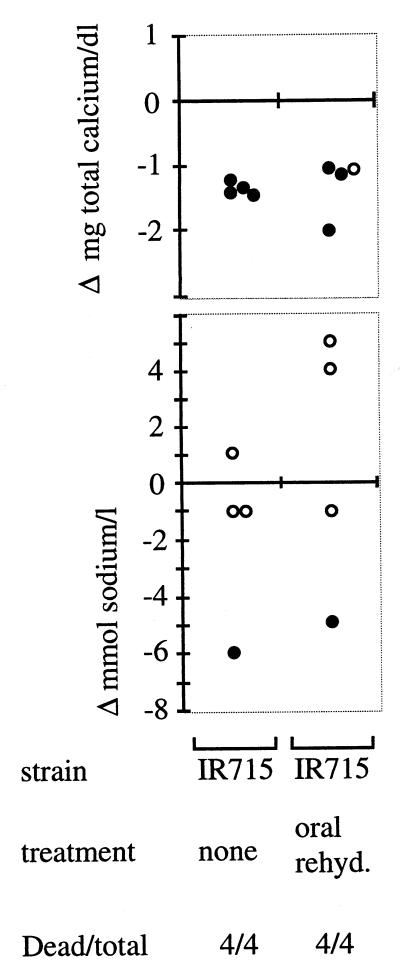
To determine whether mortality in calves could be prevented by oral rehydration, eight calves, each infected with 1010 CFU of strain IR715 (wild type), were treated using oral rehydration therapy (four calves) or were not treated (four calves). The sodium concentration in blood samples collected before and 1 day after infection were determined. All calves infected with IR715 developed lethal signs of disease and were euthanized, regardless of whether oral rehydration therapy was performed. The observed changes in sodium concentrations of treated or untreated calves overlapped over a wide range (Fig. 3). Despite an increase (relative to preinfection values) in sodium concentrations observed in two of the calves rehydrated orally, these animals developed lethal signs of disease and were euthanized 1 day postinfection. Thus, oral rehydration therapy practiced as described above did not reduce mortality during experimental infection.
FIG. 3.
Effect of oral rehydration therapy on plasma concentrations of total calcium (top) and sodium (bottom). Graphs show the difference in the plasma concentrations determined for samples collected 1 day postinfection relative to a preinfection sample. Each circle represents data from an individual animal. Open circles indicate plasma values which were within the normal range of 133 to 143 mM (sodium) or 9.1 to 10.6 mg/dl (total calcium); closed circles indicate plasma values outside the normal range.
DISCUSSION
Serovar Typhimurium requires a functional invasion-associated type III secretion apparatus to cause diarrhea and mortality in calves. For instance, mutations in hilA, encoding an activator of SPI-1 genes (2), or prgH, encoding a component of the type III secretion complex (21, 25), result in marked attenuation and strongly reduced diarrhea during oral infection of calves (38). Furthermore, a mutation in sspB (sipB) causes a dramatic reduction in fluid secretion and polymorphonuclear cell influx elicited by serovar Dublin in bovine ligated ileal loops (12). SspB is part of a translocation complex (formed by SspB, SspC, and SspD) which is required for the delivery of effector proteins into the eukaryotic cytoplasm (8, 11, 12, 15, 42). Consistent with a role of SPI-1 in enteropathogenicity, we found that mutations which prevent type III secretion (prgHIJK) or translocation of effector proteins into host cells (sspB, sspC, or sspD) reduced the severity of diarrhea and resulted in marked attenuation of serovar Typhimurium during oral infection of calves (Fig. 1). These data confirmed that delivery of one or several translocated effector proteins by the invasion-associated type III secretion system is required for enteropathogenicity in calves. To assess the contribution of individual effector proteins encoded by SPI-1 to enteropathogenicity, we determined the calf virulence of serovar Typhimurium sspA and sptP mutants.
Three of four calves infected with the serovar Typhimurium sptP mutant and all calves inoculated with its isogenic parent developed terminal signs of disease and were euthanized. Thus, mutational inactivation of sptP did not result in overt attenuation. In contrast, all calves inoculated with the serovar Typhimurium sspA mutant (EE633) survived an infection (Fig. 1). Mutational inactivation of sspA does not result in an obvious invasion defect in tissue culture cells (17, 18), although entry is delayed at early time points (44). Similarly, a mutation in sspA did not result in a competitive colonization defect for bovine Peyer's patches, ileum, or mesenteric lymph node (Fig. 2). Furthermore, colonization of bovine tissues by the sspA mutant resulted in intestinal lesions similar to those observed in calves infected with its isogenic parent. Despite its inability to produce lethal morbidity, calves infected with the sspA mutant developed severe diarrhea. Measurement of the plasma sodium concentration of infected calves suggested that the diarrhea-induced electrolyte loss produced by a serovar Typhimurium sspA mutant was similar to that observed in animals infected with the isogenic parent (Fig. 1). Significantly, these results suggest that the loss of fluids and electrolytes may not be the sole cause of death during bovine enteritis. It has been speculated that mortality in calves may be caused by massive bacterial growth leading to septicemia (33), and this possibility cannot be ruled out. We were, however, unable to detect evidence for endotoxic shock by determining concentrations of circulating endotoxin in blood samples taken from moribund calves infected with serovar Typhimurium (data not shown).
SspA is an effector protein which binds and stabilizes actin filaments and modulates the actin-bundling activity of T-plastin, resulting in a more pronounced outward extension of serovar Typhimurium-induced membrane ruffles in epithelial cells in vitro (44, 45). There is currently no obvious connection between this in vitro observation and the role of SspA in causing lethal morbidity in calves. Mutational inactivation of sspA does not result in attenuation of serovar Typhimurium in the mouse typhoid model, in which diarrhea does not develop (18). Thus, the virulence defect in calves caused by a mutation in sspA was not predicted by results obtained using either the mouse or tissue culture model of serovar Typhimurium infection. These findings illustrate the importance of using an animal model, which resembles the natural course of infection and the typical signs of disease, to elucidate serovar Typhimurium virulence mechanisms important for morbidity and mortality during diarrheal disease.
ACKNOWLEDGMENTS
We thank C. Tanksley and T. Parsons for care of animals, R. Barthel and J.-A. Gutiérrez-Pabello for assistance with necropsies, and R. deLima Santos for critical comments on the manuscript.
Support was provided by USDA/NRICGP grant 9702568 to R.M.T., NRSA AI09312 to C.A.S., a Poncin fellowship to T.K., PHS grant AI30479 to S.I.M., USDA Formula Animal Health Funding to T.A.F. and A.J.B., USDA/NRICGP grant 9802610 to A.J.B., T.A.F., and L.G.A., and PHS grants AI40124 and AI44170 to A.J.B.
REFERENCES
- 1.Ahmer B M, van Reeuwijk J, Watson P R, Wallis T S, Heffron F. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol Microbiol. 1999;31:971–982. doi: 10.1046/j.1365-2958.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj V, Hwang C, Lee C A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj V, Lucas R L, Hwang C, Lee C A. Co-odinate regulation of Salmonella typhimurium invasion genes by environmental factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. Epidemiologic notes and reports update: Salmonella enteritidis infections and shell eggs—United States, 1990. Morbid Mortal Weekly Rep. 1990;39:909–912. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Incidence of foodborne illnesses–FoodNet, 1997. Morbid Mortal Weekly Rep. 1998;47:782–786. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Summary of notifiable diseases, United States 1997. Morbid Mortal Weekly Rep. 1998;46:1–107. [PubMed] [Google Scholar]
- 7.Chalker R B, Blaser M J. A review of human salmonellosis. III. Magnitude of Salmonella infection in the United States. Rev Infect Dis. 1988;10:111–124. doi: 10.1093/clinids/10.1.111. [DOI] [PubMed] [Google Scholar]
- 8.Collazo C M, Galan J E. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 9.Collazo C M, Galan J E. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun. 1996;64:3524–3531. doi: 10.1128/iai.64.9.3524-3531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collazo C M, Zierler M K, Galán J E. Functional analysis of the Salmonella typhimurium invasion genes invI and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995;15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y, Galan J E. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol Microbiol. 1998;27:359–368. doi: 10.1046/j.1365-2958.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 12.Galyov E E, Wood M W, Rosqvist R, Mullan P B, Watson P R, Hedges S, Wallis T S. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol. 1997;25:903–912. doi: 10.1111/j.1365-2958.1997.mmi525.x. [DOI] [PubMed] [Google Scholar]
- 13.Gibson E A. Salmonellosis in calves. Vet Rec. 1961;73:1284–1296. [PubMed] [Google Scholar]
- 14.Glynn M K, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo F J. Emergence of multidrug-resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 15.Hardt W D, Galan J E. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc Natl Acad Sci USA. 1997;94:9887–9892. doi: 10.1073/pnas.94.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinsky A. The salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueck C J, Hantman M J, Bajaj V, Johnston C, Lee C A, Miller S I. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaniga K, Trollinger D, Galan J E. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol. 1995;177:7078–7085. doi: 10.1128/jb.177.24.7078-7085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaniga K, Tucker S, Trollinger D, Galán J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khakhria R, Woodward D, Johnson W M, Poppe C. Salmonella isolated from humans, animals and other sources in Canada, 1983–92. Epidemiol Infect. 1997;119:15–23. doi: 10.1017/s0950268897007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galan J E, Aizawa S I. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 22.Libby S J, Adams L G, Ficht T A, Allen C, Whitford H A, Buchmeier N A, Bossie S, Guiney D G. The spv genes of the Salmonella dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect Immun. 1997;65:1786–1792. doi: 10.1128/iai.65.5.1786-1792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller S I, Hohmann E L, Pegues D A. Salmonella (including Salmonella typhi) In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. Vol. 2. New York, N.Y: Churchill Livingstone; 1995. pp. 2013–2033. [Google Scholar]
- 24.Norris F A, Wilson M P, Wallis T S, Galyov E E, Majerus P W. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sci USA. 1998;95:14057–14059. doi: 10.1073/pnas.95.24.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pegues D A, Hantman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 26.Rakeman J L, Bonifield H R, Miller S I. A HilA-independent pathway to Salmonella typhimurium invasion gene transcription. J Bacteriol. 1999;181:3096–3104. doi: 10.1128/jb.181.10.3096-3104.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankin J D, Taylor R J. The estimation of the doses of Salmonella typhimurium for experimental production of disease in calves. Vet Rec. 1966;78:706–707. doi: 10.1136/vr.78.21.706. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds D J, Morgan J H, Chanter N, Jones P W, Bridger J C, Debney T G, Bunch K J. Microbiology of calf diarrhoea in southern Britain. Vet Rec. 1986;119:34–39. doi: 10.1136/vr.119.2.34. [DOI] [PubMed] [Google Scholar]
- 29.Rothenbacher H. Mortality and morbidity in calves with salmonellosis. J Am Vet Med Assoc. 1965;147:1211–1214. [PubMed] [Google Scholar]
- 30.Saphra I, Winter J W. Clinical manifestations of salmonellosis in man: an evaluation of 7779 human infections identified at the New York Salmonella Center. N Engl J Med. 1957;256:1128. doi: 10.1056/NEJM195706132562402. [DOI] [PubMed] [Google Scholar]
- 31.Skorupski K, Taylor R K. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 32.Smith B P, Da Roden L, Thurmond M C, Dilling G W, Konrad H, Pelton J A, Picanso J P. Prevalence of salmonellae in cattle and in the environment on California dairies. J Am Vet Med Assoc. 1994;205:467–471. [PubMed] [Google Scholar]
- 33.Smith B P, Habasha F, Reina-Guerra M, Hardy A J. Bovine salmonellosis: experimental production and characterization of the disease in calves, using oral challenge with Salmonella typhimurium. Am J Vet Res. 1979;40:1510–1513. [PubMed] [Google Scholar]
- 34.Sojka W J, Field H I. Salmonellosis in England and Wales 1958–1967. Vet Bull. 1970;40:515–531. [Google Scholar]
- 35.Sojka W J, Wray C, Hudson E B, Benson J A. Incidence of salmonella infection in animals in England and Wales, 1968–73. Vet Rec. 1975;96:280–284. [Google Scholar]
- 36.Stojiljkovic I, Bäumler A J, Heffron F. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutH gene cluster. J Bacteriol. 1995;177:1357–1366. doi: 10.1128/jb.177.5.1357-1366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Threlfall E J, Hall M L, Rowe B. Salmonella bacteraemia in England and Wales, 1981–1990. J Clin Pathol. 1992;45:34–36. doi: 10.1136/jcp.45.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsolis R M, Adams L G, Ficht T A, Bäumler A J. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect Immun. 1999;67:4879–4885. doi: 10.1128/iai.67.9.4879-4885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsolis R M, Townsend S M, Miao E A, Miller S I, Ficht T A, Adams L G, Bäumler A J. Identification of a putative Salmonella enterica serotype Typhimurium host range factor with homology to IpaH and YopM by signature-tagged mutagenesis. Infect Immun. 1999;67:6385–6393. doi: 10.1128/iai.67.12.6385-6393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson P R, Galyov E E, Paulin S M, Jones P W, Wallis T S. Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect Immun. 1998;66:1432–1438. doi: 10.1128/iai.66.4.1432-1438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood M W, Jones M A, Watson P R, Hedges S, Wallis T S, Galyov E E. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol Microbiol. 1998;29:883–891. doi: 10.1046/j.1365-2958.1998.00984.x. [DOI] [PubMed] [Google Scholar]
- 42.Wood M W, Rosqvist R, Mullan P B, Edwards M H, Galyov E E. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol Microbiol. 1996;22:327–338. doi: 10.1046/j.1365-2958.1996.00116.x. [DOI] [PubMed] [Google Scholar]
- 43.Wray C, Sojka W J. Experimental Salmonella typhimurium infection in calves. Res Vet Sci. 1978;25:139–143. [PubMed] [Google Scholar]
- 44.Zhou D, Mooseker M S, Galán J E. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science. 1999;283:2092–2095. doi: 10.1126/science.283.5410.2092. [DOI] [PubMed] [Google Scholar]
- 45.Zhou D, Mooseker M S, Galan J E. An invasion-associated Salmonella protein modulates the actin-bundling activity of plastin. Proc Natl Acad Sci USA. 1999;96:10176–10181. doi: 10.1073/pnas.96.18.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]