Abstract
Torpor, and its differential expression, is essential to the survival of many mammals and birds. Physiological characteristics of torpor appear to vary between those species that express strict daily heterothermy and those capable of multiday hibernation, but comparisons are complicated by the temperature-dependence of variables. Previous reviews have compared these different torpor strategies by measuring the depth and duration of torpor in multiple species. However, direct comparison of multiple physiological parameters under similar thermal conditions are lacking. Here, we quantified three physiological variables; body temperature, metabolic rate (MR) and heart rate (HR) of two small heterothermic bats (daily heterotherm Syconycteris australis, and hibernator Nyctophilus gouldi) under comparable thermal conditions and torpor bout durations. When normothermic and resting both MR and HR were similar for the two species. However, during torpor the minimum HR was more than fivefold higher, and minimum MR was 6.5-fold higher for the daily heterotherm than for the hibernator at the same subcutaneous Tb (16 ± 0.5 °C). The data show that the degree of heterothermy defined using Tb is not necessarily a precise proxy for physiological capacity during torpor in these bats and is unlikely to reveal accurate energy budgets. Our study provides evidence supporting a distinction between daily torpor in a daily heterotherm and short term torpor in a hibernator, at least within the Chiroptera with regard to these physiological variables. This exists even when individuals display the same degree of Tb reduction, which has clear implications for the modelling of their energy expenditure.
Subject terms: Ecology, Physiology, Zoology
Introduction
Torpor expression has commonly been classified into two major strategies; daily torpor seen in daily heterotherms and multiday torpor seen in hibernators1. The comparison between these groups is complicated, however, by the strong and often direct temperature-dependence of variables of torpor, leading some to conclude that heterothermy falls on a body temperature (Tb) dependent continuum e.g.2. Yet there is a lack of consistent data that scrutinize the difference between these strategies under similar thermal conditions3. A proper understanding of the differences between daily heterotherms and hibernators in their physiological capacity for torpor use is essential for understanding the flexibility of their physiology and behaviour, particularly in our rapidly changing world.
Traditional interpretations of physiological capacity in heterotherms are derived from data on species that exhibit the extremes in torpor pattern and have become ‘model’ species of thermal biology, such as obligate seasonal hibernators4,5 or strict daily heterotherms6,7. Yet evidence is amassing from non-Holarctic species, and the wild, that indicates flexibility in torpor use across species and throughout the year8,9. It is evident that multiday torpor is not restricted to winter10, and species capable of multiday torpor can also enter short bouts of torpor throughout the year11. For example, the little brown bat Myotis lucifugus is capable of torpor bouts up to 45 days during winter hibernation, but in summer regularly expresses torpor bouts lasting less than 1 day12. Yet these short bouts are often referred to as ‘daily torpor’13 due to their temporal resemblance to torpor in daily heterotherms. Furthermore these bouts often occur at high ambient temperatures (Ta), where the degree of Tb reduction may also be similar among species. For example, the mouse lemur Microcebus griseorufus, is capable of multiday torpor bouts at a skin temperature of around 20 °C, but also can exhibit short torpor bouts of only a few hours in the same season14, making it difficult to distinguish from a daily heterotherm using either Tb or torpor bout duration alone.
The maximum degree of metabolic rate reduction has been used to distinguish daily heterotherms from hibernators in > 200 species15. This distinction is thought to be driven by the fact that in daily heterotherms metabolism during torpor is, to a large extent, a function of body temperature16, whereas hibernators possess the ability to further inhibit metabolism physiologically17. If daily heterotherms and hibernators differ in their degree of metabolic rate reduction, even at the same body temperature, then the short bouts of torpor in hibernators will provide greater energy savings than daily torpor in daily heterotherms. Altogether, this highlights the importance of considering ‘non-model’ species, conducting experiments under a range of ambient conditions, and including more proximal physiological traits in investigations9.
Bats are an ideal group in which to study torpor as they have an almost global distribution and the vast majority of species studied have been shown to express torpor18; even in the tropics, subtropics and arid zones19–22. Torpor strategies in bats are highly flexible. Unlike other Holarctic hibernating species, many bats are able to enter short bouts of deep torpor throughout the year and to enter multiday torpor, at high ambient temperatures23,24. While most research on torpor in bats has focussed on species that enter multiday torpor, detailed knowledge about daily heterothermy is scant. This is particularly true for measures of heart rate, with only a single record from a pteropodid in torpor25.
Here we compared heart rate (HR), oxygen consumption (; a proxy for metabolism) and subcutaneous temperature (Tsub) throughout torpor and at rest for two bat species that express either daily heterothermy (the 18 g blossom bat, Syconycteris australis, Pteropodidae), or hibernation (the 10 g long-eared bat, Nyctophilus gouldi, Vespertilionidae) to assess whether short-term torpor in a hibernator is comparable to a daily heterotherm. Syconycteris australis exhibit torpor patterns indicative of a daily heterotherm with torpor bouts generally < 12 h and minimum Tb in torpor of 18 °C26,27. In addition, this species has one of the highest recorded field metabolic rates for endotherms, suggesting that while torpor is essential for energy balance, the savings are minimal in this species as they only enter short, shallow torpor bouts in the wild28. Nyctophilus gouldi, while capable of hibernation, also enters short bouts of torpor throughout the year29. Previous investigations have shown that there is no effect of season on the electrocardiogram in this species during torpor30, which suggests that short torpor bouts may not differ physiologically from multiday torpor bouts at the same temperature. These two species overlap in their geographic distributions, providing a unique opportunity to compare hibernator and daily heterotherm species exposed to similar environment conditions in the wild and therefore experiencing similar selection pressures for torpor use. We hypothesized that the degree of energy savings from short torpor bouts in the hibernator would differ from the daily heterotherm, and that this would be evident even when species were exposed to the same ambient conditions. Furthermore, we predicted that cardiac function and metabolism would be lower in the hibernator than the daily heterotherm.
Results
Oxygen consumption and heart rate at rest
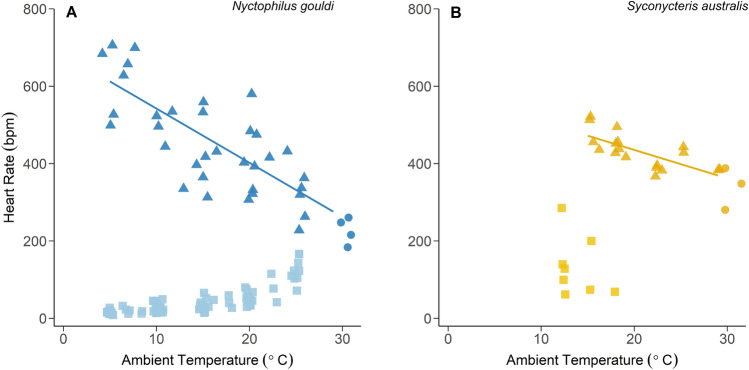
When bats were normothermic (S. australis, n = 4, Tsub = 33.8 ± 0.9 °C; N. gouldi, n = 13, Tsub = 34.4 ± 1.2 °C) increased linearly with decreasing Ta below the thermoneutral zone (Supplementary Figure 1A & B). Although appeared to increase more steeply in N. gouldi than S. australis, reflecting the difference in size and hence thermal conductance of the species, this difference in slope was not found to be statistically significant (lme, df = 25.09, t = 1.99, p = 0.06). This was also the case for resting HR (lme, df = 37.02, t = 1.70, p = 0.10). For N. gouldi, resting HR increased by 182 bpm between Ta 25 °C and 12 °C (Fig. 1A). In contrast, for S. australis the change in resting HR (95 bpm) was just over half that of N. gouldi over the same temperature range (Fig. 1B).
Figure 1.
Heart rate as a function of ambient temperature (Ta) for Nyctophilus gouldi and Syconycteris australis. Basal HR (filled circles) was recorded in the thermoneutral zone (TNZ; 29.5–34 °C). Below the TNZ, resting HR (triangles) increased linearly for both species; (A) HR (bpm) = 682.57 -14.03 (Ta), (B) HR (bpm) = 582.32 -7.34 (Ta). Bats entered torpor (filled squares) when exposed to Ta below 25 °C (Ng) and 20 °C (Sa).
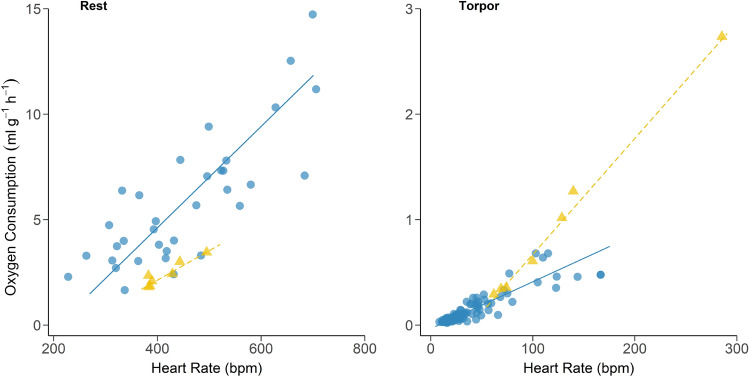
When we compare the relationship between resting and resting HR, however, the differences between the species became more pronounced (Fig. 2; sma; df = 1, χ = 5.32, p < 0.05). For every 10 bpm increase in HR the rate of increase in was almost twice as high for N. gouldi than S. australis; increasing by 0.24 ml O2 g−1 h−1 versus 0.14 ml O2 g−1 h−1 respectively.
Figure 2.
The relationship between HR and for N. gouldi (blue circles-solid line) and S. australis (yellow triangles-dashed line) during rest and torpor. N. gouldi; resting = 0.024(HR) − 5.104, r2 = 0.65, p < 0.001, torpid = 0.004(HR) − 0.034, r2 = 0.78, p < 0.001. S. australis; resting = 0.014(HR) − 3.467309, r2 = 0.88, p < 0.01, torpid = 0.011(HR) − 0.429, r2 = 0.99, p < 0.001.
Consequently, in N. gouldi oxygen pulse increased from an average of 1.01 × 10−4 ml O2 g−1 beat−1 when metabolic rate was basal in the thermoneutral zone, to an estimated 2.35 × 10−4 ml O2 g−1 beat−1 at Ta 12 °C (data taken from the curve fitted to the data). This represents a 48.7% increase in HR to account for increased , whereas for S. australis oxygen pulse increased from 0.89 × 10−4 to 1.57 × 10−4 ml O2 g−1 beat−1, which equated to a contribution of HR of only 38.3%.
Heart rate and metabolism during torpor
When bats were resting at similar Ta (15.0 ± 0.7 °C) both resting HR and were comparable between the species, with only a 51 bpm difference in resting HR and less than 0.5 ml g−1 h−1 difference in (Table 1). However, when both species entered torpor minimum HR and were more than fourfold higher on average in S. australis than N. gouldi (Table 1). When we compared individuals at their thermoconforming minimum, when Tsub was within 1 °C of one another, the difference in was even more pronounced at ninefold higher in S. australis than N. gouldi (0.36 versus 0.04 ml O2 g−1 h−1 respectively). In these same individuals heart rate was more than fivefold higher in S. australis than N. gouldi (74 and 14 bpm respectively). This difference was also found for the distributions of torpid HR and over the same Ta range (Fig. 2; ks-test for HR, D = 0.86, p < 0.001; ks-test for , D = 0.93, p < 0.001).
Table 1.
Average physiological parameters for Syconycteris australis and Nyctophilus gouldi at rest (normothermia) and in torpor where ambient temperature was 15 ± 0.7 °C (range 13–16.5 °C).
| Syconycteris australis | Nyctophilus gouldi | |
|---|---|---|
| HR (bpm; rest) | 482 ± 42 (n = 3, N = 4) | 431 ± 88 (n = 7, N = 7) |
| HR (bpm; torpor) | 137 ± 89 (n = 2, N = 2) | 32 ± 12 (n = 17, N = 19) |
| (ml g−1 h−1; rest) | 4.38 ± 0.28 (n = 4. N = 4) | 4.79 ± 1.91 (n = 7, N = 7) |
| (ml g−1 h−1; torpor) | 0.7 ± 0.55 (n = 2, N = 5) | 0.1 ± 0.04 (n = 17, N = 19) |
| Tsub (°C; rest) | 34.0 ± 0.9 (n=4, N = 8) | 34.4 ± 1.5 (n = 6, N = 6) |
| Tsub (°C; torpor) | 19.9 ± 3.5 (n = 4, N = 6) | 16.0 ± 0.8 (n = 17, N = 20) |
The proportional reduction of during torpor was greater in N. gouldi than S. australis at the same Ta (15.1 ± 0.1 °C). Average during torpor was 7.4 ± 0.7% of basal metabolic rate in N. gouldi compared to 31.7 ± 9.5% for S. australis. This was also true of the reduction in HR proportionate to basal heart rate, which was only 14.0 ± 1.2% in N. gouldi individuals compared to 60.7 ± 11.8% for S. australis. If we consider this in relation to resting metabolic rate at the same temperature, N. gouldi showed a 97.9 ± 0.2% reduction in during torpor compared to an average 88.4 ± 3.5% reduction for S. australis. Again, the proportional reduction in heart rate was also greater in N. gouldi than S. australis when compared to resting heart rate at the same Ta (92.6 ± 0.6% versus 71.6 ± 8.3%).
The absolute minimum HR in torpor for S. australis was 69 bpm recorded at Ta 12.6 °C with Tsub of 15.0 °C. The corresponding minimum was 0.29 ml g−1 h−1. The minimum HR in torpid N. gouldii was less than one quarter of S. australis at a similar temperature (Ta = 10.7 °C, Tsub = 11.2 °C) at 16 bpm and was one tenth that of S. australis at 0.02 ml g−1 h−1. Yet the absolute minimum HR we recorded in N. gouldi fell as low as 9 bpm (Tsub = 5.8 °C) with corresponding of 0.03 ml g−1 h−1 as these individuals were exposed to lower Ta than S. australis (in this case 5 °C).
During steady-state torpor bats of both species showed strong linear relationships between HR and (Sa r2 = 0.99, Ng r2 = 0.78, p < 0.01) (Fig. 2). However, there was a significant difference between the relationships for each species (χ = 23.38, df = 1, p < 0.001) as increased more steeply with increasing HR in torpid S. australis than for N. gouldi.
Subcutaneous temperature during torpor
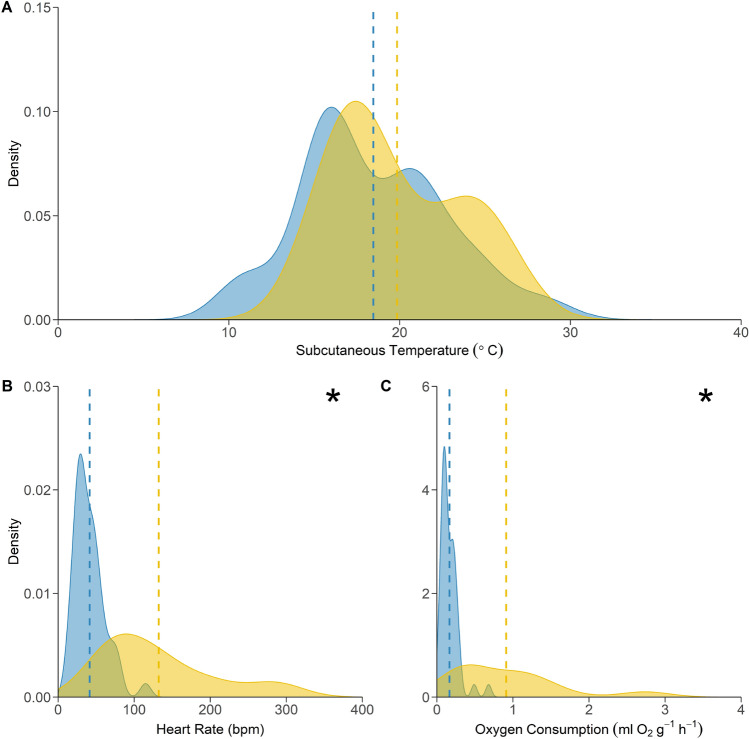
Both bat species maintained a mean Tsub around 34 °C (Sa 33.8 ± 0.9 °C; Ng 34.4 ± 1.2 °C) when normothermic and resting (Supplementary Figure 2). When animals entered torpor the relationship between Tsub and Ta differed between the two species, even though the distribution of Tsub of torpid bats over the same Ta range did not (Fig. 3; ks-test, D = 0.30, p = 0.55). Nyctophilus gouldi entered torpor at Ta ≤ 25 °C and Tsub remained within 2 °C of Ta in thermoconforming torpid individuals. Only eight individuals maintained Tsub > 2 °C (5.1 ± 0.7 °C) above Ta and these bats did so at mild temperatures between 19 and 22 °C on only one occasion each. In contrast, S. australis maintained a greater differential between Tsub and Ta when in torpor below 20 °C (5.4 ± 3.9 °C on average) and Tsub during torpor was highly variable at any given Ta. For example, Tsub ranged from 16.3 to 24.1 °C at Ta 14.9 ± 0.9 °C. Yet, the minimum Tsub for S. australis was 15.0 °C recorded at Ta of 12.6 °C.
Figure 3.
Kernel-density distributions of physiological parameters in torpor for N. gouldi (blue shading) and S. australis (yellow shading) at ambient temperatures between 10.5 and 24 °C. Mean values represented by dashed vertical lines. The distributions of both heart rate and oxygen consumption in torpor differed significantly between the two species (as determined via Kolmogorov–Smirnov tests and shown with asterix) even though subcutaneous temperature in torpor was not different.
Torpor entry and arousal
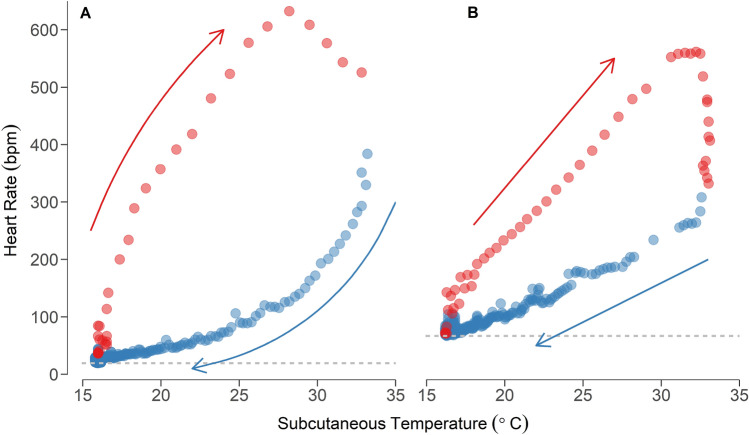
HR showed a pronounced hysteresis with Tsub during entry and arousal from torpor in both species, although absolute values of HR differed substantially between the two species (Fig. 4). During entry into torpor HR was slower at each Tsub than at the corresponding Tsub during rewarming. Figure 4 also illustrates the pronounced drop in HR during torpor entry with little change in Tsub in N. gouldi compared to a shallower linear decline in HR in S. australis. Yet the rapid increase in HR during rewarming over a small Tsub change and overshoot of HR at the end of arousal from torpor was similar in both species. For example, at the initiation of arousal in N. gouldi HR increased ~ eightfold over a rise in Tsub of only 1.6 °C, from 24 to 200 bpm. Similarly, HR of S. australis more than doubled when rewarming over a Tsub range of only 1.4 °C from 70 to 173 bpm. The overall time taken to rewarm showed a negative curvilinear response to increasing Ta (Supplementary Figure 3). The log transformed data showed a significant relationship between time taken to rewarm and Ta for N. gouldi (lme, df = 46.1, t = − 11.715, p < 0.01). Yet post-hoc analysis revealed no effect of Ta on arousal time for S. australis (lme, df = 35.15, t = − 0.741, p = 0.464) and no overall difference between the species (lme, df = 35.73, t = 0.631, p = 0.532).
Figure 4.
Heart rate as a function of subcutaneous temperature during entry (blue circles and arrow) and arousal (red circles and arrow) from a single torpor bout for N. gouldi (A) and S. australis (B) at an ambient temperature of 15 °C. Arrows indicated the progression of the torpor bout in time and dashed lines indicate minimum heart rate during torpor.
Discussion
Although HR, and Tsub were similar in resting normothermic bats at mild Ta, when bats entered torpor the mechanisms reducing HR, and Tsub were decidedly different between the daily heterotherm and hibernator. At all Ta measured, the hibernator N. gouldi, had lower and HR during torpor than corresponding measurements for the daily heterotherm, S. australis. This was true even when there was no significant difference in the distribution of Tsub between the two groups.
Our results are consistent when compared across several bat species whose geographic ranges overlap with N. gouldi and/or S. australis. During short term torpor at 15 °C, metabolic rate in two species capable of hibernation (10 g Nyctophilus bifax and 15 g Chalinolobus gouldii), was less than one third of S. australis at the same Ta; 0.11 and 0.06 versus 0.36 ml O2 g−1 h−1 respectively31,32. Heart rate was also substantially lower in torpid C. gouldii at only 20 bpm compared to our findings of 74 bpm for S. australis when Tb was ~ 16 °C32. This is interesting considering that these data were taken from N. bifax captured at the same location as S. australis in this study31, and that the range of body mass in C. gouldii converges with S. australis33. When we compare this to data from the only other daily heterotherm within this geographic area where torpor has been reported (16 g Macroglossus minimus) minimum metabolic rate in torpor was similar to that of S. australis at the same Ta; 0.58 versus 0.60 ml O2 g−1 h−1 at 18 °C26,34. These minima are more than fivefold the recorded metabolic rate of C. gouldii and threefold our findings for N. gouldi at the same Ta (0.10 ± 0.04 and 0.17 ± 0.06 ml O2 g−1 h−1, respectively)32. Taken together this suggests that the degree of physiological reduction in daily heterotherms and hibernators may be consistently different across bat species, even at the same ambient temperatures. It is important to recognise that these comparisons are between bats from two different families (Vespertilionidae and Pteropodidae), which complicates our comparison as the distinctions between torpor patterns are also divided across phylogenetic lines15. Further investigation of more species at the same ambient conditions is necessary for us to clarify these distinctions.
While our sample size for S. australis may be low, our findings for metabolic rate during torpor are supported by those of previous studies on this species which report mean minima between 0.47 and 0.75 ml O2 g−1 h−127,34,35.The only other pteropodid bat for which HR has been measured during torpor is the daily heterotherm Nyctimene albiventer (28 g) and even then, only two recordings were made25. During torpor at Ta 25 °C HR of N. albiventer was relatively low at 88 and 96 bpm with torpid of 0.67 ± 0.17 ml O2 g−1 h−1. Minimum average HR recorded for S. australis in our study was only slightly lower, 62 bpm at Ta 12.6 °C which corresponded to a of 0.29 ml O2 g−1 h−1, less than half that of N. albiventer. While we are confident that our data accurately reflect the cardiac function of S. australis in torpor and at rest, our small sample size should be taken into consideration. Therefore, we advocate that more investigation into cardiac function in daily heterotherm species, including S. australis, be undertaken to improve our understanding of this important physiological variable.
Short term torpor in bat species capable of hibernation is often referred to as daily torpor suggesting that it may differ from seasonal multiday torpor36. This is partially due to the fact that short bouts often occur at mild Ta, but may also reflect the assumption that hibernation requires seasonal preparation; such as tissue remodelling and differential gene expression37. Data reported for hibernating bat species after many days in torpor show that HR falls to around 10 bpm at Ta 5 °C38,39. Our data for N. gouldi are comparable, if not lower than those presented during longer torpor bouts at the same Ta, confirming that short term torpor in these bats is physiologically indistinguishable from multiday hibernation. This also supports previous findings for this species that showed no seasonal difference in electrocardiogram parameters during torpor30. The capacity for energy savings in hibernators even during short torpor bouts is important to recognise and investigate as there is a rise in the number of studies modelling energy expenditure in bats under varied ecological and climate change scenarios40,41.
Entry into torpor progressed more slowly in the daily heterotherm than the hibernator, reflected by a slower decline in HR during cooling, as illustrated by a more gradual slope of the hysteresis curve in S. australis compared to N. gouldi at the same Ta. During entry into torpor the hypothalamic set point for thermoregulation gradually falls to the new low set point for steady-state torpor42. Our results suggest that the fall in Tset occurs more slowly in daily heterotherms as animals defend Tb throughout, or for part of, cooling. The progression of torpor entry in the hibernator was more rapid, likely as a function of metabolic inhibition and active suppression of HR at the onset of cooling, and the much lower Tset in torpor.
The ability to rewarm from torpor is a defining feature of heterothermic animals and the arousal process is extremely costly. Arousal costs are reduced when rewarming rate is maximised43 and the importance of this was evident in both species of bats investigated here as there was no significant effect of torpor pattern on the time taken to rewarm. Our data for HR during rewarming also showed that both species were capable of increasing HR at the onset of arousal to ~ 200 bpm before Tsub had increased by more than 2 °C. This indicates that selection for swift rewarming may be consistent across species regardless of the depth or length of torpor bouts, as has been previously suggested44.
During periods of normothermy both bat species showed a qualitatively similar response of HR and to changes in Ta following the general endothermic pattern. Our results demonstrate that the relationship between HR and at rest vary between the two species reflected in both regression analyses and calculations of oxygen pulse. Although the oxygen pulse of both species increased with decreasing Ta, the percentage contribution of HR to oxygen transport differed between the two species. The proportional increase in HR for increased oxygen transport at low Ta was substantially lower in S. australis (38.3%) than N. gouldi (48.7%) across an equivalent Ta gradient. This suggests that for S. australis other aspects of the cardiovascular system, such as stroke volume, play a greater role in maintaining cardiac output at high metabolic rate. It is possible that these differences relate to energy acquisition and partitioning associated with flight and may not necessarily be a function of the species’ pattern of torpor use. Nectar feeding bats and birds are known to have the highest maximum rate of oxygen consumption amongst animals and consequently nectarivorous bats have comparatively high field metabolic rates28,45. Furthermore, flight dynamics have been shown to be highly correlated with heart mass46. It is possible that the differences reported here for resting HR and the relationship with in N. gouldi and S. australis relate to some extent to the relative size of the heart46 and a greater cardiac reserve which might be necessary for longer foraging times in the nectar feeding bats. However, this relationship should be reversed during torpor, as the larger mass and heart size in Syconycteris should result in a lower HR than in the smaller Nyctophilus, emphasising the physiological differences between these groups in relation to torpor.
From an ecological perspective, an understanding of the differences in physiological capacity between daily heterotherms and hibernators gives us a stronger tool to model and predict how environmental conditions effect energy budgets for these species. Here we show that a similar reduction in body temperature does not equate to the same level of energy savings for two species of heterothermic bat, and that these differences persist across a number of bat species at the same Ta. Ideally, with more data, phylogenetically controlled analyses could be incorporated to better address whether these differences reflect physiologically distinct groups (daily heterotherms vs hibernators), or are simply a function of phylogenetic effects (vespertilionids vs pteropodids). If short-term torpor of the same duration exhibited by N. gouldi was mistaken for daily torpor and ascribed a similar proportional reduction in metabolism to S. australis (8% of resting metabolism in S. australis compared to 0.8% for N. gouldi at the same Tsub) we would substantially overestimate the daily energy needs of these individuals. Using a novel approach of investigating different torpor patterns under similar experimental conditions, we provide evidence that short term torpor in vespertilionid hibernators provides proportionately greater energy savings than daily torpor in pteropodid daily heterotherms. Moreover, we provide additional evidence for the use of heart rate as a mechanism to investigate physiological capacity in heterothermic species.
Methods
Study species
Syconycteris australis are nectarivorous bats from tropical and subtropical eastern Australia and in captivity have been shown to use daily torpor throughout the year. Torpor bouts are generally restricted to < 12 h and minimum regulated Tb reported during torpor is ~ 18 °C27,35. The propensity of these bats to enter torpor has been suggested to have enabled S. australis to extend its range into higher latitudes where it overlaps with temperate species such as N. gouldi (Supplementary Figure 4)47,48. Nyctophilus gouldi are insectivorous bats that hibernate in winter, expressing torpor bouts of around 2 weeks and often express short-term torpor bouts of < 24 h throughout the year29. These bats maintain a low Tb during both short and long torpor bouts with reported minimum regulated Tb of ~ 1–2 °C49.
Adult male S. australis (n = 4, body mass at capture = 18.7 ± 0.5 g) were caught in mist nets at Iluka Nature Reserve in a subtropical habitat on the north coast of NSW, Australia (29°24’S, 153°22’E). Bats were transported to the University of New England (UNE) and housed in a large indoor flight cage (2 × 2 × 2 m), which was equipped with branches and large stands of foliage for individuals to roost in. Temperature of the room was maintained at 20–22 °C, with relative humidity > 55% and animals were exposed to natural light. Food, a blended mixture of fruit, juice and protein supplements27 was available ad libitum in modified plastic syringes that acted as feeders which were placed among branches. Feeders were refilled daily and washed/soaked overnight in Milton antibacterial solution to minimize microbial growth. Water was available in birdfeeders.
Adult N. gouldi (n = 21, body mass at capture = 10.5 ± 0.3 g) were caught at Imbota Nature Reserve and Newholme Field Station in a temperate habitat near Armidale, NSW (30°35’S, 151°44’E). At UNE, bats were kept in large outdoor flight cages (3 × 1.5 × 2 m) with a maximum of eight animals per cage and provided mealworms and water ad libitum. Mealworms were dusted with a supplement of Wombaroo™ Insectivore Rearing Mix twice a week. Additional food was supplied in the form of moths and other flying insects, and these were attracted into cages by a UV light. All bats were kept in captivity for a maximum period of seven months and each individual remained within 1 g of their body mass at the time of capture while in captivity (average BM; S. australis = 19.6 ± 0.7 g, N. gouldi = 11.0 ± 0.3 g).
Animal ethics declaration
This study was conducted under a scientific license provided by the NSW Parks and Wildlife Authority (SL100084) under the guidelines of the Environment Protection and Biodiversity Conservation Act 1999. Animal Ethics approval was granted by the animal ethics committee of the University of New England (AEC11-016 and AEC12-043). This study was carried out in compliance with the ARRIVE guidelines on animal research and the Australian Code for the Care and Use of Animals for Scientific Purposes.
Subcutaneous body temperature
Temperature-sensitive transponders (IPTT-300 Bio Medic Data Systems, Delaware, USA) were used to measure subcutaneous body temperature (Tsub). Transponders were calibrated over a range of 5 to 40 °C to the nearest 0.1 °C against a precision reference thermometer in a water bath prior to use. Bats were given a minimum of 3 days to acclimate to captivity and ensure stable body mass before transponder implantation. Transponders were implanted interscapularly under general Isoflurane/oxygen anaesthesia. The skin was cleaned with 70% alcohol before a small (~ 3 mm) incision was made in the skin just below the shoulder blades for insertion. The insertion site was closed with a single suture (chromic gut, Ethicon, Somerville, MA, USA). Bats were given 24 h to recover in small individual cages in a warm room before being returned to their flight cages.
Respirometry
Individuals were placed in respirometry chambers in the late afternoon/early evening and oxygen consumption () was measured as a proxy for MR over the course of the following day (at least ~ 20 h). Bats were weighed (to 0.1 g) before the start of experimentation and immediately after being removed from respirometry chambers. We calculated mass-specific assuming a linear rate of mass loss. Bats were exposed to Ta within the typical range of their natural habitat, as such the N. gouldi were measured from Ta 33 °C down to 4 °C and S. australis were only measured from Ta 33 °C down to 12 °C. During measurements bats were exposed to a simulated natural photoperiod.
Oxygen concentration of excurrent air was measured on a FC-1B Oxygen Analyser (Sable Systems, Nevada, USA). Measurements were taken from airflow through the chamber every minute for 15 min and then switched to outside air for reference readings (3 min). Chamber Ta was measured to the nearest 0.1 °C using a calibrated thermocouple placed ~ 5 mm within the chamber. Outputs of the digital thermocouple thermometer, flowmeter and oxygen analyser were recorded onto a personal computer using custom written data acquisition software (Gerhard Körtner) and was calculated using equation 3A of Withers50. Tsub was read from each animal with a DAS-7006/7R/S Handheld Reader (Bio Medic Data Systems, Delaware, USA) which was connected to a personal computer and programmed to take readings every minute, concurrent with respirometry measurements.
As body mass and roosting posture differed between the two species, respirometry chambers were of different sizes and roosting material was also different. Nyctophilus gouldi were placed in rectangular polycarbonate chambers (0.26, 0.40, or 0.53 l) where they roosted flush with the back wall of the chamber and clung to a small patch of hessian cloth. Syconycteris australis preferred to hang in the centre of the chamber (glass jar 0.75 l) where they were free hanging and not touching the glass. Individuals roosted from plastic mesh supported horizontally near the roof of the chamber by a wire frame. Flow rate was adjusted (180–290 ml min−1) to ensure that 99% equilibrium was reached in < 15 min. measurements were time adjusted for lag of the system, but not washout characteristics of the chambers, to correspond with measurements of HR and Tsub.
Electrocardiogram (ECG) measurements
For both species of bat ECGs could only be measured during the day as the animals did not tolerate electrode wires during their active phase (overnight). Following lights on in the morning, most bats were either already torpid or had begun to enter torpor (depending on Ta and species) and at that point ECG electrode wires were attached to the bats’ forearms (lead I arrangement). This disturbance resulted in a partial arousal and bats either remained normothermic and resting for the remainder of recording or returned to torpor, either with or without ECG electrodes attached. S. australis individuals were less likely to return to torpor, hence our smaller sample size for this species. However, metabolic rate in torpor was unchanged, or even slightly lower, in both species following ECG lead attachment (paired t-test; p = 0.07 Nyctophilus, p = 0.20 Syconycteris), therefore the data we were able to collect reflect undisturbed torpor.
For N. gouldi ECG’s were recorded following the methods of Currie30 using adhesive electrodes of appropriate length and width to fit the forearm. Adhesive electrodes were not suitable for the forearms of S. australis however, and thus we constructed ECG electrodes from modified metal ear tags for mice that fit around the forearm of the bats in a similar fashion to bat ID bands. Bands were kept on the animals throughout time spent in captivity and only one bat experienced irritation associated with the bands. In this case the band was removed and the individual excluded from experimentation until completed healed. Electrode leads were made from modified Kittycat™ Paediatric Monitoring Electrodes (Tyco Healthcare Group, Mansfield, USA) with stainless steel clips at one end. ECG electrode gel was applied to the metal clips to improve signal conduction only for S. australis.
Statistical analysis
To objectively assess whether the physiology of torpor differs between the two species, analyses were restricted to short torpor bouts of less than 24 h. Minimum values of Tsub, HR, and were averaged over 30 min from animals in steady-state torpor. On occasion transponders temporarily stopped working when the Tsub of N. gouldi in torpor fell below 7 °C. In these cases Tsub was estimated to be 0.5 °C above Ta, as this was the average differential for animals with similar whose transponders continued to work at low Ta.
Resting VO2 and HR for both species were either taken from animals that did not enter torpor during the day, or from the period following arousal. Additional resting VO2 values for S. australis were taken from the period in the afternoon when animals were first placed in the respirometry chamber. Basal metabolic rate (BMR) and basal heart rate (BHR) were measured following arousal from torpor while animals were resting and within the thermoneutral zone previously determined for each species27,49. Duration of arousal was calculated from the point at which Tsub began a steady continuous increase until maximum Tsub was reached for both species (average 34.9 ± 1.5 °C, range 32.1–37.9 °C).
The percentage contribution of resting HR to increased metabolism in normothermic individuals at low Ta, was calculated using the equation of Bartholomew and Tucker51;
using HR and oxygen pulse (OP; /HR) at Ta 1 (TNZ; 30.4 ± 0.8 °C) and Ta 2 (12.0 °C).
Statistical analyses were performed using R v4.0.3. Linear mixed effects models were used to interpret and compare the relationship between physiological variables and Ta between the species using the lme4 package with species as a fixed factor and individual as a random effect52. We used the package lmerTest to provide p-values for linear mixed effects models53. Within this package degrees of freedom and t-values are calculated using the Welch-Satterthwaite equation. Data was relevelled and tests were repeated to specifically assess the effect of Ta for each species, as such p-values were corrected using the Holm-Bonferroni method. Standardized major axis regressions were performed to assess the relationship between HR and using the smatr package54 as this enables us to account for measurement errors on both the x and y axes55. Pseudo-replication was accounted for by using the degrees of freedom as for mixed effects modelling in lmerTest. To determine whether the distributions of HR, MR and Tsub of torpid bats form a single continuous distribution for both species under the same ambient conditions, Kolmogorov–Smirnov tests were performed for all data collected at Ta between 10.5 and 24 °C.
Supplementary Information
Acknowledgements
We would like to thank Daniella Rojas and Liam Bailey for their helpful comments on the manuscript. Funding for this research was provided by the Australian Research Council and the University of New England.
Author contributions
S.E.C. conceptualized the project, conducted the research and data analysis, and lead the writing of the manuscript. G.K. assisted with data analysis and revisions of the manuscript. F.G. assisted with conceptualizing the project and revisions of the manuscript.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-25590-8.
References
- 1.Lyman CP, Willis JS, Malan A, Wang LCH. Hibernation and Torpor in Mammals and Birds. Academic Press; 1982. [Google Scholar]
- 2.Boyles JG, et al. A global heterothermic continuum in mammals. Glob. Ecol. Biogeogr. 2013;22:1029–1039. doi: 10.1111/geb.12077. [DOI] [Google Scholar]
- 3.Geiser F. Ecological Physiology of Daily Torpor and Hibernation. Springer; 2021. [Google Scholar]
- 4.Buck CL, Barnes BM. Effects of ambient temperature on metabolic rate, respiratory quotient and torpor in an arctic hibernator. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2000;279:R255–R262. doi: 10.1152/ajpregu.2000.279.1.R255. [DOI] [PubMed] [Google Scholar]
- 5.Ortmann S, Heldmaier G. Regulation of body temperature and energy requirements of hibernating Alpine marmots (Marmota marmota) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R698–R704. doi: 10.1152/ajpregu.2000.278.3.R698. [DOI] [PubMed] [Google Scholar]
- 6.Swoap SJ, Gutilla MJ. Cardiovascular changes during daily torpor in the laboratory mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R769–R774. doi: 10.1152/ajpregu.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirsch R, Ouarour A, Pévet P. Daily torpor in the Djungarian hamster (Phodopus sungorus): photoperiodic regulation, characteristics and circadian organization. J. Comp. Physiol. A. 1991;168:121–128. doi: 10.1007/BF00217110. [DOI] [PubMed] [Google Scholar]
- 8.Nowack J, Stawski C, Geiser F. More functions of torpor and their roles in a changing world. J. Comp. Physiol. (B) 2017;187:889–897. doi: 10.1007/s00360-017-1100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowack J, Levesque DL, Reher S, Dausmann KH. Variable climates lead to varying phenotypes: “Weird” mammalian torpor and lessons from non-holarctic species. Front. Ecol. Evol. 2020 doi: 10.3389/fevo.2020.00060. [DOI] [Google Scholar]
- 10.Hoelzl F, et al. How to spend the summer? Free-living dormice (Glis glis) can hibernate for 11 months in non-reproductive years. J. Comp. Physiol. B. 2015;185:931–939. doi: 10.1007/s00360-015-0929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiser F. Seasonal expression of avian and mammalian daily torpor and hibernation: not a simple summer-winter affair. F. Phys. 2020;11:436. doi: 10.3389/fphys.2020.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonasson KA, Willis CKR. Hibernation energetics of free-ranging little brown bats. J. Exp. Biol. 2012;215:2141–2149. doi: 10.1242/jeb.066514. [DOI] [PubMed] [Google Scholar]
- 13.Dietz M, Kalko EKV. Seasonal changes in daily torpor patterns of free-ranging female and male Daubenton’s bats (Myotis daubentonii) J. Comp. Physiol. B. 2006;176(3):223–231. doi: 10.1007/s00360-005-0043-x. [DOI] [PubMed] [Google Scholar]
- 14.Kobbe S, Ganzhorn JU, Dausmann KH. Extreme individual flexibility of heterothermy in free-ranging Malagasy mouse lemurs (Microcebus griseorufus) J. Comp. Physiol. B. 2011;181:165–173. doi: 10.1007/s00360-010-0507-5. [DOI] [PubMed] [Google Scholar]
- 15.Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol. Rev. 2015;90:891–926. doi: 10.1111/brv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- 17.Storey KB, Storey JM. Metabolic rate depression: the biochemistry of mammalian hibernation. Adv. Clin. Chem. 2010;52:77–108. doi: 10.1016/S0065-2423(10)52003-1. [DOI] [PubMed] [Google Scholar]
- 18.Stawski C, Willis CKR, Geiser F. The importance of temporal heterothermy in bats. J. Zool. 2014;292:86–100. doi: 10.1111/jzo.12105. [DOI] [Google Scholar]
- 19.Bondarenco A, Körtner G, Geiser F. Some like it cold: summer torpor by freetail bats in the Australian arid zone. J. Comp. Physiol. (B) 2013;183:1113–1122. doi: 10.1007/s00360-013-0779-7. [DOI] [PubMed] [Google Scholar]
- 20.O'Mara MT, et al. Heart rate reveals torpor at high body temperatures in lowland tropical free-tailed bats. R. Soc. Open Sci. 2017;4:171359. doi: 10.1098/rsos.171359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reher S, Ehlers J, Rabarison H, Dausmann KH. Short and hyperthermic torpor responses in the Malagasy bat Macronycteris commersoni reveal a broader hypometabolic scope in heterotherms. J. Comp. Physiol. B. 2018 doi: 10.1007/s00360-018-1171-4. [DOI] [PubMed] [Google Scholar]
- 22.Geiser F, et al. Hibernation and daily torpor in Australian and New Zealand bats: Does the climate zone matter? Aust. J. Zool. 2020 doi: 10.1071/ZO20025. [DOI] [Google Scholar]
- 23.Stawski C, Turbill C, Geiser F. Hibernation by a free-ranging subtropical bat (Nyctophilus bifax ) J. Comp. Physiol. (B) 2009;179:284–292. doi: 10.1007/s00360-008-0328-y. [DOI] [PubMed] [Google Scholar]
- 24.Levin E, et al. Subtropical mouse-tailed bats use geothermally heated caves for winter hibernation. Proc. R. Soc. Lond. B Biol. Sci. 2015;282:20142781. doi: 10.1098/rspb.2014.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartholomew GA, Dawson WR, Lasiewski RC. Thermoregulation and heterothermy in some of the smaller flying foxes (Megachiroptera) of New Guinea. Z. Vergl. Physiol. 1970;70:196–209. doi: 10.1007/BF00297716. [DOI] [Google Scholar]
- 26.Bartels W, Law BS, Geiser F. Daily torpor and energetics in a tropical mammal, the northern blossom-bat Macroglossus minimus (Megachiroptera) J. Comp. Physiol. (B) 1998;168:233–239. doi: 10.1007/s003600050141. [DOI] [PubMed] [Google Scholar]
- 27.Geiser F, Coburn DK, Körtner G, Law BS. Thermoregulation, energy metabolism, and torpor in blossom-bats, Syconycteris australis (Megachiroptera) J. Zool. 1996;239:538–590. doi: 10.1111/j.1469-7998.1996.tb05944.x. [DOI] [Google Scholar]
- 28.Geiser F, Coburn DK. Field metabolic rates and water uptake in the blossom-bat Syconycteris australis (Megachiroptera) J. Comp. Physiol. (B) 1999;169:133–138. doi: 10.1007/s003600050203. [DOI] [PubMed] [Google Scholar]
- 29.Turbill C. Roosting and thermoregulatory behaviour of male Gould’s long-eared bats, Nyctophilus gouldi: energetic benefits of thermally unstable tree roosts. Aust. J. Zool. 2006;54:57–60. doi: 10.1071/ZO05068. [DOI] [Google Scholar]
- 30.Currie SE. No effect of season on the electrocardiogram of long-eared bats (Nyctophilus gouldi) during torpor. J. Comp. Physiol. B. 2018;188:695–705. doi: 10.1007/s00360-018-1158-1. [DOI] [PubMed] [Google Scholar]
- 31.Stawski C, Geiser F. Do season and distribution affect thermal energetics of a hibernating bat endemic to the tropics and subtropics? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R542–R547. doi: 10.1152/ajpregu.00792.2010. [DOI] [PubMed] [Google Scholar]
- 32.Currie SE, Stawski C, Geiser F. Cold-hearted bats: uncoupling of heart rate and metabolism during torpor at subzero temperatures. J. Exp. Biol. 2018 doi: 10.1242/jeb.170894. [DOI] [PubMed] [Google Scholar]
- 33.Churchill S. Australian Bats. 2. Allen and Unwin; 2008. [Google Scholar]
- 34.Geiser F, Law BS, Körtner G. Daily torpor in relation to photoperiod in a subtropical blossom-bat, Syconycteris australis (Megachiroptera) J. Therm. Biol. 2005;30:574–579. doi: 10.1016/j.jtherbio.2005.08.002. [DOI] [Google Scholar]
- 35.Coburn DK, Geiser F. Seasonal changes in energetics and torpor patterns in the subtropical blossom-bat Syconycteris australis (Megachiroptera) Oecologia. 1998;113:467–473. doi: 10.1007/s004420050399. [DOI] [PubMed] [Google Scholar]
- 36.Dietz M, Kalko EKV. Seasonal changes in daily torpor patterns of free-ranging female and male Daubenton’s bats (Myotis daubentonii) J. Comp. Physiol. (B) 2006;176:223–231. doi: 10.1007/s00360-005-0043-x. [DOI] [PubMed] [Google Scholar]
- 37.Andrews MT. Advances in molecular biology of hibernation in mammals. BioEssays. 2007;29:431–440. doi: 10.1002/bies.20560. [DOI] [PubMed] [Google Scholar]
- 38.Twente JW, Twente J. Autonomic regulation of hibernation by Citellus and Eptesicus. In: Wang LCH, Hudson JW, editors. Strategies in Cold: Natural Torpidity and Thermogenesis. Academic Press; 1978. pp. 327–373. [Google Scholar]
- 39.Davis WH, Reite OB. Responses of bats from temperate regions to changes in ambient temperature. Biol. Bull. 1967;132:320–328. doi: 10.2307/1539637. [DOI] [PubMed] [Google Scholar]
- 40.Alston JM, Dillon ME, Keinath DA, Abernethy IM, Goheen JR. Daily torpor reduces the energetic consequences of microhabitat selection for a widespread bat. Ecology. 2022;103:e3677. doi: 10.1002/ecy.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphries MM, Thomas DW, Speakman JR. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature. 2002;418:313–316. doi: 10.1038/nature00828. [DOI] [PubMed] [Google Scholar]
- 42.Heller HC. Hibernation: neural aspects. Annu. Rev. Physiol. 1979;41:305–321. doi: 10.1038/nature00828. [DOI] [PubMed] [Google Scholar]
- 43.McKechnie AE, Wolf BO. The energetics of the rewarming phase of avian torpor. In: Barnes BM, Carey HV, editors. Life in the Cold: Evolution, Mechanisms, Adaptation and Application. University of Alaska; 2004. pp. 265–267. [Google Scholar]
- 44.Geiser F, Baudinette RV. The relationship between body mass and rate of rewarming from hibernation and daily torpor in mammals. J. Exp. Biol. 1990;151:349–359. doi: 10.1242/jeb.151.1.349. [DOI] [PubMed] [Google Scholar]
- 45.Voigt CC, Kelm DH, Visser GH. Field metabolic rates of phytophagous bats: do pollination strategies of plants make life of nectar-feeders spin faster? J. Comp. Physiol. (B) 2006;176:213–222. doi: 10.1007/s00360-005-0042-y. [DOI] [PubMed] [Google Scholar]
- 46.Bullen RD, McKenzie NL, Bullen KE, Williams MR. Bat heart mass: correlation with foraging niche and roost preference. Aust. J. Zool. 2009;57:399–408. doi: 10.1071/ZO09053. [DOI] [Google Scholar]
- 47.Law BS. Climatic limitation of the southern distribution of the common blossom bat Syconycteris australis in New South Wales. Aust. J. Ecol. 1994;19:366–374. doi: 10.1111/j.1442-9993.1994.tb00502.x. [DOI] [Google Scholar]
- 48.Bonaccorso FJ, McNab BK. Plasticity of energetics in blossom bats (Pteropodidae): impact on distribution. J. Mammal. 1997;78:1073–1088. doi: 10.2307/1383050. [DOI] [Google Scholar]
- 49.Geiser F, Brigham RM. Torpor, thermal biology and energetics in Australian long-eared bats (Nyctophilus) J. Comp. Physiol. (B) 2000;170:153–162. doi: 10.1007/s003600050270. [DOI] [PubMed] [Google Scholar]
- 50.Withers PC. Metabolic, respiratory and haematological adjustments of the little pocket mouse to circadian torpor cycles. Respir. Physiol. 1977;31:295–307. doi: 10.1016/0034-5687(77)90073-1. [DOI] [PubMed] [Google Scholar]
- 51.Bartholomew GA, Tucker VA. Control of changes in body temperature, metabolism and circulation by the Agamid lizard, Amphibolurus barbatus. Physiol. Zool. 1963;36:199–218. doi: 10.1086/physzool.36.3.30152307. [DOI] [Google Scholar]
- 52.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 53.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017;82:1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 54.Warton DI, Duursma RA, Falster DS, Taskinen S. smatr 3- an R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012;3:257–259. doi: 10.1111/j.2041-210X.2011.00153.x. [DOI] [Google Scholar]
- 55.Halsey LG, et al. Flexibility, variability and constraint in energy management patterns across vertebrate taxa revealed by long-term heart rate measurements. Funct. Ecol. 2019;33:260–272. doi: 10.1111/1365-2435.13264. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.