Abstract
The vertebrate main-body axis is laid down during embryonic stages in an anterior-to-posterior (head-to-tail) direction, driven and supplied by posteriorly located progenitors. Whilst posterior expansion and segmentation appears broadly uniform along the axis, there is developmental and evolutionary support for at least two discrete modules controlling processes within different axial regions: a trunk and a tail module. Here, we identify Nuclear receptor subfamily 6 group A member 1 (Nr6a1) as a master regulator of trunk development in the mouse. Specifically, Nr6a1 was found to control vertebral number and segmentation of the trunk region, autonomously from other axial regions. Moreover, Nr6a1 was essential for the timely progression of Hox signatures, and neural versus mesodermal cell fate choice, within axial progenitors. Collectively, Nr6a1 has an axially-restricted role in all major cellular and tissue-level events required for vertebral column formation, supporting the view that changes in Nr6a1 levels may underlie evolutionary changes in axial formulae.
Subject terms: Body patterning, Development
The authors identify Nuclear receptor subfamily 6 group A member 1 (Nr6a1) as a master regulator of elongation, segmentation, patterning and lineage allocation specifically within the trunk region of the mouse, acting downstream of the major signals known to control vertebral column formation.
Introduction
Vertebrate animals exhibit great diversity in their axial formulae, that is, the number and identity of elements that constitute the vertebral column. Axial formulae are established at early developmental stages when regional identity is superimposed on the continual process of somitogenesis1. During primary body formation, presacral vertebrae arise from dual-fated progenitor cells known as neuromesodermal progenitors (NMPs) that reside in the epiblast2,3, with the process of gastrulation providing a constant supply of descendant cells directly to the posterior presomitic mesoderm (PSM). The resulting PSM expansion is balanced by the periodic budding of tissue from the anterior PSM, generating vertebrae precursors known as somites. As gastrulation terminates, a subset of NMPs relocate internally to the chordoneural hinge of the tailbud, where they continue to supply descendant cells destined to form post-sacral vertebrae during secondary body formation4,5. This continues until progenitor exhaustion/PSM decline halts elongation and defines total vertebral number for that species6–8.
While each newly formed somite appears seemingly identical along the entire anterior-to-posterior (A-P) axis, the positional information that instructs regionally-appropriate vertebra morphology is already present prior to somite scission9,10. Central orchestrators of this positional information are the homeodomain-containing Hox proteins, a series of 39 transcription factors (in mouse/human) that work within a complex spatio-temporal hierarchy to pattern the vertebrae11. Specifically, it is the temporally-ordered activation of Hox gene expression within axial progenitors12, reinforced by their genomic clustering at 4 distinct loci13, that results in a spatially overlapping “Hox-code” along the A-P axis that governs regional identity11.
Evolutionary changes in vertebral number that selectively alter either the trunk or the tail/caudal regions have been repeatedly observed across the vertebrates14–17, supporting the view that axial elongation is driven by consecutive developmental modules that can be manipulated independently. At a cellular level, the trunk-to-tail (T-to-T) transition is marked by a switch in axial progenitor source from epiblast- to tailbud-derived8,18, and is coincident with a shift from an expanding to a depleting PSM size6. Transplantation experiments have shown that these T-to-T changes in cellular state and activity are not irreversible4, demonstrating that intrinsic exhaustion of axial progenitors does not follow a timer mechanism and that axial identity is able to be reset to a more anterior fate based on environmental cues4,19. These data, together with the observation of a temporally changing transcriptomic signature of axial progenitors20,21, as opposed to a stable signature, suggest an evolving developmental program as the main-body axis is laid down but one that can be broadly delineated into trunk and tail modules.
Gdf11 is one of the most prominent factors known to control the correct timing of the T-to-T transition. Genetic deletion of Gdf1122 or its receptors Acvr2a and Acvr2b23,24 in the mouse significantly expands the trunk region, while constitutive activation of this signalling pathway had the opposite effect25. However, Gdf11 signalling has pleiotropic effects across the vertebral column, with patterning changes as far anterior as the cervical region as well as tail truncation observed in Gdf11−/− mice22. It is currently unclear whether the cell-intrinsic mechanisms downstream of Gdf11 signalling are common across all phenotypes, though axially-restricted effectors such as Pou5f1/Oct4 in the trunk26, and the Lin28-let7axis in the tail27–29 have been identified.
Nuclear receptor subfamily 6 group A member 1 (Nr6a1; previously called GCNF) is an orphan-nuclear receptor whose genetic deletion in the mouse results in early embryonic lethality30. Nr6a1 is expressed widely within the early mouse embryo30,31, and is one of a select suite of marker genes that exhibits temporally-restricted expression within NMPs at embryonic day (E)8.520. Of particular note, an activating single nucleotide polymorphism (SNP) within Nr6a1 has been associated with increased trunk vertebral number in the domesticated pig32–35, a trait that has been selected for increased meat production36, with similar SNPs now found in domesticated sheep37. In mouse and other vertebrate species, the Nr6a1 3’-untranslated region harbours multiple binding sites for miR-196 and let-738–40, microRNA families known to constrain trunk38 and tail27 vertebral number respectively, suggesting the potential for Nr6a1 to function during vertebral column formation in an axially-restricted manner, though to date, this remains to be tested.
Here, we identify Nr6a1 as a master regulator of trunk elongation, segmentation, patterning and lineage allocation in the mouse. Nr6a1 expression within axial progenitors is dynamic, being positively reinforced by Wnt signalling at early stages and sharply terminated by the combined actions of Gdf11 and miR-196 at the T-to-T transition. Nr6a1 acts in a dose-dependent manner to control the number of thoraco-lumbar elements in a meristic not homeotic manner, with the subsequent termination of Nr6a1 expression essential for tail development. Furthermore, Nr6a1 controls the timely progression of Hox expression derived from all Hox clusters. Specifically, Nr6a1 activity enhances the expression of several trunk Hox genes whilst temporally constraining the expression of posterior Hox genes. Collectively, our data reinforce the view that axial elongation is controlled by at least two developmental modules, the first being Nr6a1-dependent and the second Nr6a1-independent, with Nr6a1 providing central cross-talk between elongation and patterning.
Results
Expression of Nr6a1 correlates with trunk formation in the mouse
The genomic locus encompassing Nr6a1 produces multiple transcripts in the mouse (Supplementary Fig. 1A). These include two largely overlapping Nr6a1 sense transcripts that produce proteins with identical DNA-binding and ligand-binding domains (Supplementary Fig. 1B), with a common 3’untranslated region harbouring multiple microRNA (miRNA) binding sites (Supplementary Fig. 1C). Within intron 3 of the long isoform, multiple antisense transcripts were identified including a long non-coding RNA (Nr6a1os) and a polycistronic transcript that encodes the miR-181a2 and miR-181b-2 microRNAs (miRNAs).
Whole mount in situ hybridisation utilising a riboprobe that bound both protein-coding sense transcripts revealed widespread Nr6a1 expression at embryonic day (E) 8.5 (Fig. 1A), including the posterior growth zone which houses various progenitor populations required for axial elongation8. Interrogation of a published single-cell RNA-seq dataset produced from E6.5 and E8.5 embryos41 confirmed robust expression of Nr6a1 at these early stages in NMPs, caudal mesoderm and the caudolateral epiblast (Fig. 1B), with lower expression in many other progenitor and mature populations where it has the potential to function. At E9.5, widespread Nr6a1expression was largely maintained consistent with previous studies42,43, though with notable absence of expression in the heart and a visible clearing of expression from the posterior growth zone during the period known as the T-to-T transition (Fig. 1A). This temporal decline in Nr6a1 within E9.5 NMPs is supported at the single-cell level20. At E10.5, restricted expression became apparent within the mid- and hind-brain, cranial neural crest cells, otic vesicle and dorsal root ganglia of the trunk, with a complete absence of expression caudal to the last-formed somite. By E12.5, Nr6a1 expression was barely detectable throughout the embryo, consistent with a “mid-gestation” pattern of expression39.
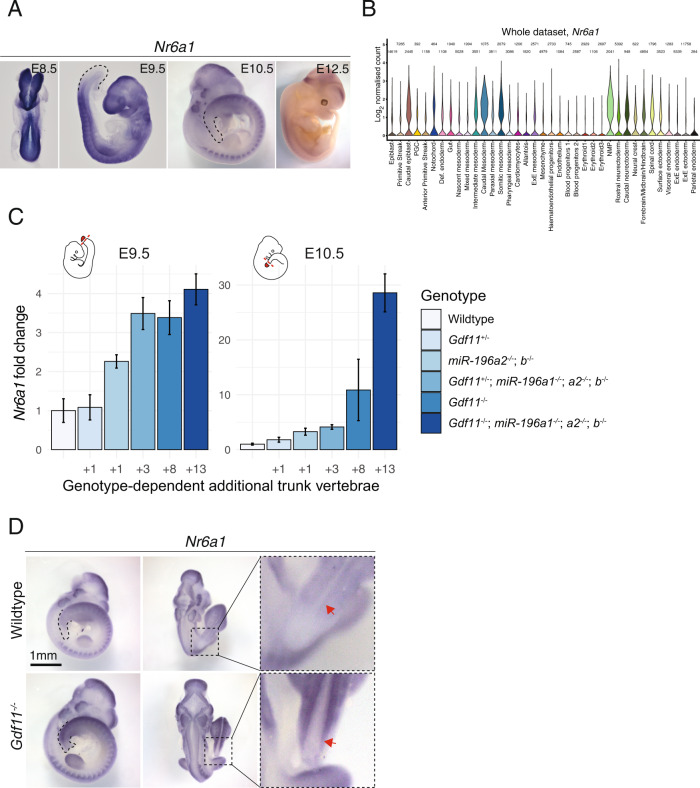
Fig. 1. Nr6a1 expression level positively correlates with the trunk vertebral number and is regulated by known mediators of the trunk-to-tail transition.
A Nr6a1 is expressed broadly at embryonic day (E)8.5, with expression becoming progressively excluded from the posterior growth zone starting E9.5. By E10.5 Nr6a1 exhibits tissue-specific expression, followed by complete tissue clearance by E12.5. B Violin plot illustrates the Log2 normalised count, which was derived from the raw counts from each cells divided by their size factors, of Nr6a1 in cells of the E6.5 to E8.5 mouse embryo, revealing robust expression within neuromesodermal progenitors (NMP), caudal mesoderm and caudolateral epiblast. Data derived from ref. [41]. C Expression level of Nr6a1 within the tailbud positively correlates with the number of trunk vertebrae that form later in development. Quantitative PCR analysis of Nr6a1 within tailbud tissue isolated from Gdf11 and miR-196 single and compound mutant embryos, represented as a fold change compared to wildtype (defined as 1). The number of biologically independent samples assessed: WT E9.5 n = 3, E10.5 n = 4; Gdf11+/− E9.5 n = 3, E10.5 n = 3; miR-196a2−/−;b−/− E9.5 n = 3, E10.5 n = 4; Gdf11+/−; miR-196a1−/−;a2−/−;b−/− E9.5 n = 3, E10.5 n = 4; Gdf11−/− E9.5 n = 3, E10.5 n = 2; Gdf11−/−; miR-196a1−/−;a2−/−;b−/− E9.5 n = 3, E10.5 n = 2; Error bar represents standard deviation. Source data are provided as a Source Data file. The number of additional thoraco-lumbar vertebrae observed across the allelic deletion series44 is indicated. D Whole mount in situ hybridisation reveals that the quantitative increase of Nr6a1 in E10.5 Gdf11−/− tailbud tissue relative to wildtype reflects a lack of timely clearance from the posterior growth zone. Consistent spatial changes were observed in 2 biologically independent samples/genotype. Red arrow indicates the neural tube.
The rapid clearance of Nr6a1 from the tailbud around E9.5 was striking and suggested the posterior limit of its expression may be controlled by factors that position the T-to-T transition. Indeed, Nr6a1 expression levels are known to be moderately repressed in vivo by miR-196, a factor that constrains thoraco-lumbar (T-L) number by 138. To dissect Nr6a1 regulatory mechanisms at this posterior boundary further, we took advantage of a series of recently generated mouse mutant lines which harbour an increasing number of thoraco-lumbar vertebrae dependent on the individual and combinatorial deletion of miR-196 and Gdf11 alleles44. Quantification of Nr6a1 expression within E9.5 tailbud tissue from across this allelic series revealed an overall positive correlation between early Nr6a1 expression level and the number of additional T-L vertebrae that form later in development (Fig. 1C). This increase in Nr6a1 expression relative to wildtype persisted at E10.5 and in extreme genotypes, where 8 or 13 additional T-L elements ultimately form, this differential expression escalated even further.
Spatial analysis of Nr6a1 in E10.5 Gdf11−/− embryos confirmed persistent expression in the caudal neural tube and mesoderm expanding toward the embryonic tip (Fig. 1D), reminiscent of ectopic Lin28a and Lin28b expression observed in embryos of the same genotype28. Collectively, these data revealed a dynamic pattern of Nr6a1 expression within tailbud tissue over time, with expression terminated by the synergistic action of miR-196 and Gdf11 signalling.
Nr6a1 supports trunk elongation and inhibits sacro-caudal identity
To provide insights into the mechanistic role of Nr6a1 in axial elongation, we first performed microarray transcriptomic analyses on E9.0-9.5 wildtype and Nr6a1−/− mutant embryos. The top 50 genes downregulated in Nr6a1−/− mutants compared to wildtype were analysed by ToppGene, which predicted that regionalisation, pattern specification, skeletal system development and morphogenesis, and anterior-posterior patterning were key biological processes disrupted in Nr6a1−/− mutants (Supplementary Data 1 and Supplementary Fig. 2A). ToppGene analysis also predicted multiple phenotypes associated with abnormal morphology of the axial skeleton as likely outcomes resulting from Nr6a1 loss-of-function (Supplementary Table 1). Lastly, DAVID analysis revealed that the protein families over-represented in the top 50 downregulated genes in Nr6a1−/− mutants included Homeobox proteins and Homeodomain-like proteins (Supplementary Table 1) suggesting a putative link between Nr6a1 and Hox-dependent patterning mechanisms.
The early lethality of Nr6a1−/− embryos complicates evaluation of its role during vertebral column formation30,45. To circumvent this, we next generated a ubiquitous but temporally-controlled conditional knockout model, crossing the Nr6a1flx/flx line46 with the CMV-CreERT2 deleter line47. Low dose Tamoxifen (Tam) was administered on a single day of development from E6.25-E9.25 and skeletal analysis performed at E18.5, revealing a reduction in the number of thoracic elements in CMV- CreERT2; Nr6a1flx/flx embryos when Tam was administered at or before E8.25 (Supplementary Fig. 2B, C). With the time-window of action defined, we next deleted Nr6a1 specifically within axial progenitors using the TCreERT2 deleter line48. Following administration of Tam at E7.5, a dose-dependent reduction in total vertebral number (TVN) and widespread vertebral patterning changes could be observed (Fig. 2A, B and Supplementary Fig. 3A). Compared to TCreERT2-positive control embryos, heterozygous conditional deletion of Nr6a1 resulted in 2 less T elements (Fig. 2A) and an overall reduction in TVN by 1 (Supplementary Fig. 3A) which we suggest stems from serial posteriorising homeotic transformations from the lower thoracic region onwards. For example, while the normal complement of 7 sternal rib attachments were unchanged in TCreERT2;Nr6a1+/flx embryos, the transitional vertebra (vertebra 17 (T10) in controls) was anteriorly displaced by one and all elements from the first lumbar vertebra onwards were anteriorly displaced by two. This phenotype was enhanced following homozygous conditional deletion of Nr6a1, with the number of sternal rib attachments reduced to 5, an overall loss of 4 T elements and a reduction in TVN by 3 (Fig. 2A and Supplementary Fig. 3A).
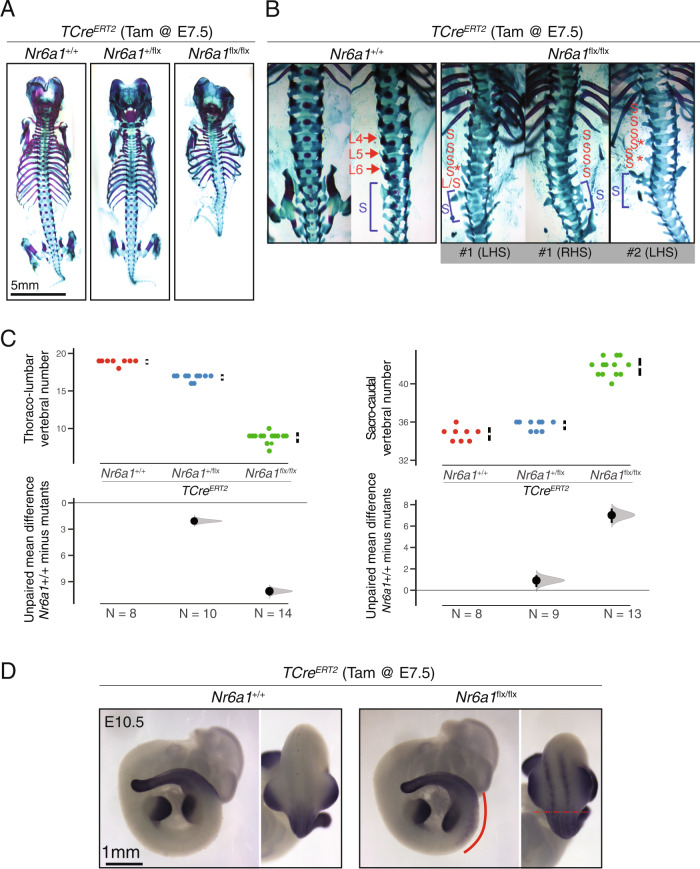
Fig. 2. Nr6a1 is required for trunk elongation and timely activation of sacro-caudal identity.
A Skeletal preparation of E18.5 embryos following conditional deletion of Nr6a1 revealed a dose-dependent reduction in the number of thoraco-lumbar elements. The number of biologically independent samples assessed: Nr6a1+/+ n = 8; Nr6a1+/− n = 10; Nr6a1−/− n = 14. B In WT skeletons, removal of pelvic bones allowed visualisation of transverse process morphology characteristic of lumbar (L) and sacral (blue S) elements. In two representative TCreERT2;Nr6a1flx/flx embryos, positioning of the normal sacral region is defined by the presence of rudimentary pelvic bones (blue S) and lumbar elements that have assumed sacral identity marked (red S). Red asterisk indicates lateral fusions between two adjacent elements. LHS = left-hand side; RHS = right-hand side. C Quantification of thoraco-lumbar and sacro-caudal vertebral number following conditional deletion of Nr6a1 alleles. Raw data is presented in the upper plots. Mean differences relative to wildtype (+/+) are presented in the lower plots as bootstrap sampling distributions. The mean difference for each genotype is depicted as a black dot and 95% confidence interval is indicated by the ends of the vertical error bar. D Whole mount in situ hybridisation for Hoxd11 in E10.5 embryos revealed a rostral shift in the anterior limit of expression in TCreERT2;Nr6a1flx/flx embryos compared to control. Ectopic Hoxd11 expression in TCreERT2;Nr6a1flx/flx embryos is marked with a red line in lateral view. In dorsal view, the normal anterior limit is marked with a red dotted line, with ectopic Hoxd11 expression restricted to the paraxial mesoderm. Consistent spatial changes were observed in 3 biologically independent samples/genotype.
Removal of the pelvic bones in a WT embryo allowed visualisation of the normal winged-shaped transverse process on sacral elements S1 and S2 which at this stage are becoming fused laterally, and the rostrally-pointing transverse process of lumbar elements L4, L5, L6 (Fig. 2B). In TCreERT2;Nr6a1flx/flx embryos, positioning of the normal (albeit malformed) sacral region was appropriately spaced relative to the last formed thoracic element, defined by the presence of adjacent rudimentary pelvic/hindlimb bones (blue S, Fig. 2B). Rostral to this, the transverse processes of all intervening post-thoracic vertebral elements assume a flattened morphology which, in numerous embryos, show lateral fusion between adjacent elements (red S, Fig. 2B; asterisk marks lateral fusions). These unique characteristics of sacral morphology indicated an almost complete transformation of lumbar to sacral identity in TCreERT2;Nr6a1flx/flx embryos.
To corroborate this morphological assessment, we performed whole-mount in situ hybridisation for Hoxd11, a known regulator of sacral identity49. At E10.5, we see a striking shift in the rostral boundary of Hoxd11 in TCreERT2;Nr6a1flx/flx embryos compared to somite-matched WT embryos (Fig. 2D). This shift was specific to the paraxial mesoderm, with ectopic Hoxd11 expression observed in at least 6-7 somites rostral to the normal boundary of expression, correlating well with the extent of skeletal transformations observed (Fig. 2B). We also assessed the expression of retinoic acid signalling components Aldh1a2 and Cyp26a1 at E9.5 and E10.5 but see no major spatio-temporal differences between genotypes (Supplementary Fig. 4) that prefigure the rostral shift in Hox expression or that may underlie body plan changes. Collectively, these results demonstrated an essential and dose-dependent role for Nr6a1 in the appropriate timing of patterning events during axial elongation, with the precocious activation of more posterior programs following Nr6a1 depletion predicted to temporally advance termination of elongation28,44,50, leading to the reduction in TVN observed.
In addition to these patterning and numerical changes, homozygous conditional deletion of Nr6a1 resulted in rib fusion defects and vertebral malformations throughout the entire thoracic and extended-sacral regions (Fig. 2A and Supplementary Fig. 5). Surprisingly, however, vertebral morphology immediately after the hindlimb reverted back to normal, with tail elements of TCreERT2;Nr6a1flx/flx embryos indistinguishable from controls (Fig. 3A). Consistent with this, characterisation of the early somite marker Uncx4.1 at E10.5 revealed highly dysmorphic somite formation in the interlimb and hindlimb regions of TCreERT2;Nr6a1flx/flx embryos compared to controls, with a sharp switch back to normal appearance immediately caudal to the hindlimb bud (Fig. 3B). Collectively, these results demonstrate an axially-restricted function for Nr6a1 in terms of vertebral number, patterning and morphology, reinforcing the view that distinct programs govern the trunk and the tail regions - the former being Nr6a1-dependent and the latter Nr6a1-independent.
Fig. 3. Nr6a1 function within somitic tissue is restricted to the trunk region.
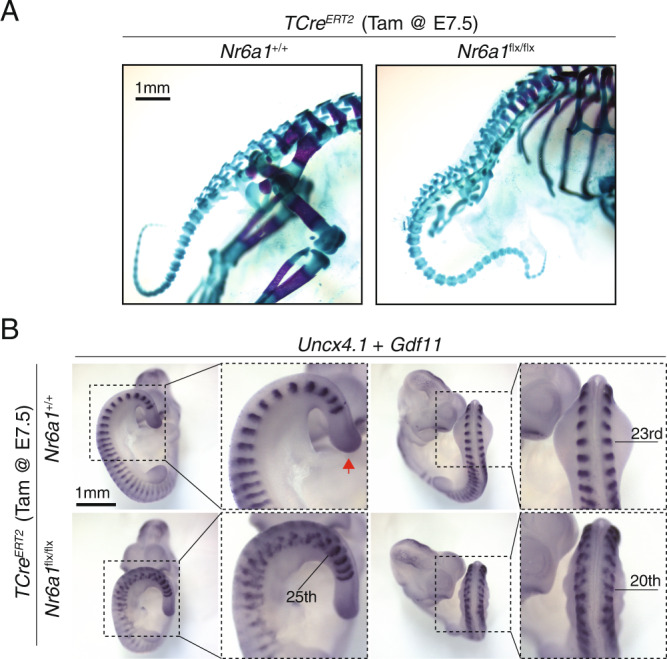
A Analysis of caudal elements in wildtype and TCreERT2;Nr6a1flx/flx and Nr6a1flx/flx embryos (n = 13 and n = 8 biologically independent samples, respectively) revealed no difference in vertebrae morphology or number. B Whole mount in situ hybridisation for the somite marker Uncx4.1 revealed a rostral shift in hindlimb positioning in TCreERT2;Nr6a1flx/flx embryos compared to control, somite number is marked in the right panel. Additionally, somite morphology was specifically disrupted in the future thoraco-lumbar region of TCreERT2;Nr6a1flx/flx embryos, returning to normal immediately after the hindlimb bud. Co-expression analysis of Gdf11 (red arrows) revealed no change in expression in the tailbud between genotypes. Consistent expression patterns were observed in 2 biologically independent samples/genotype.
Nr6a1 controls timely posterior Hox expression in the tailbud
The sequential activation of Hox genes in time, and subsequently in space, underpins body patterning along the vertebrate A-P axis12,49,51. Given the rostral shift in Hoxd11 expression within maturing somites following conditional deletion of Nr6a1 (Fig. 2D), we next wanted to address whether this was secondary to the precious temporal activation of this gene, and potentially other Hox10-13 paralog genes, within the E9.5 tailbud tissue. Tissue was collected from somite-matched (+/−1) embryos (Supplementary Fig. 6) and the expression of several posterior Hox genes found to be significantly upregulated in TCreERT2;Nr6a1flx/flx tailbuds compared to controls, including Hoxc12, Hoxc12, Hoxb13, Hoxc13 and Hoxd13, with others following a similar trend (Fig. 4A). For many of these genes, a dose-dependent control of Hox expression levels by Nr6a1 is suggested, although not significant with current sample numbers. The most highly upregulated Hox gene amongst this list was Hoxb13, increasing more than 200-fold in TCreERT2;Nr6a1flx/flx tailbuds (Fig. 4A).
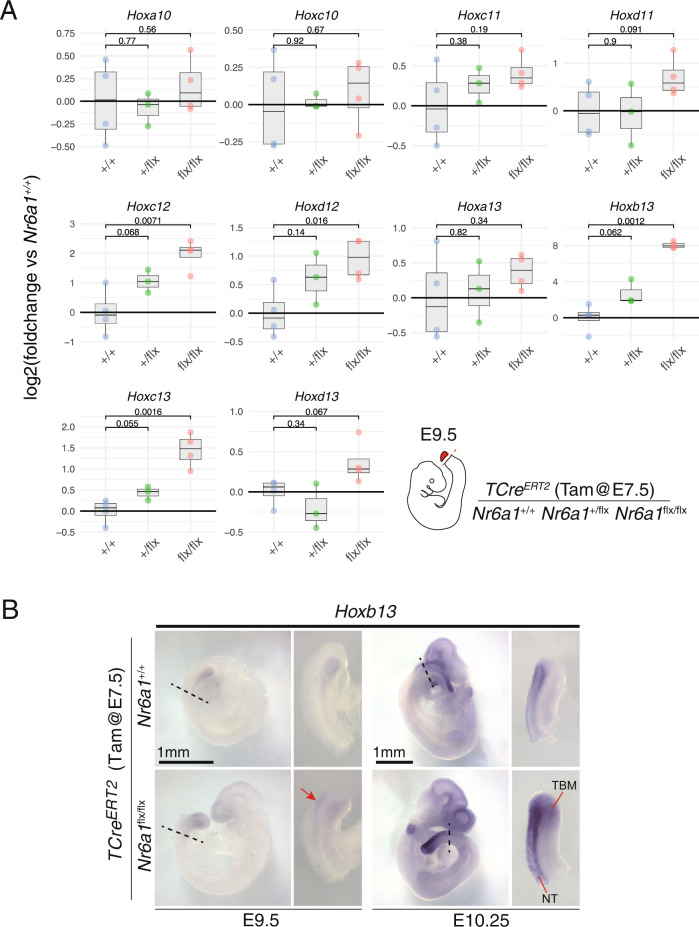
Fig. 4. Nr6a1 suppresses posterior Hox gene expression.
A Quantitative PCR analysis of posterior Hox gene expression within tailbud tissue isolated from TCreERT2 controls, TCreERT2;Nr6a1+/flx and TCreERT2;Nr6a1flx/flx embryos at E9.5, n = 3 biologically independent samples per genotype. Statistical analysis of differences between genotypes was performed using an unpaired two-sample Welch’s t test, p values are provided in the figure. Source data are provided as a Source Data file. B Whole mount in situ hybridisation revealed a spatial expansion of Hoxb13 expression in TCreERT2;Nr6a1+/flxembryos compared to wildtype, at both E9.5 and E10.5. Red arrow at E9.5 indicates ectopic Hoxb13 expression. TBM = tail bud mesoderm; NT = neural tube.
Spatial analysis of Hoxb13 in wildtype E9.5 embryos (Fig. 4B) confirmed expression was limited to endodermal cells of the hindgut at this stage52. In contrast, E9.5 TCreERT2;Nr6a1flx/flx embryos showed additional ectopic expression in the tailbud mesoderm (Fig. 4B). Ectopic Hoxb13 expression persisted in E10.5 TCreERT2;Nr6a1flx/flx embryos throughout the tailbud mesoderm, and increased Hoxb13 expression levels were observed in the neural tube relative to wildtype (Fig. 4B). These data reveal an essential role for Nr6a1 in ensuring correct spatio-temporal activation of a collective posterior Hox code within the tailbud and, specifically, in preventing their precocious expression at stages prior to the T-to-T transition.
Nr6a1 clearance is essential for the trunk-to-tail transition
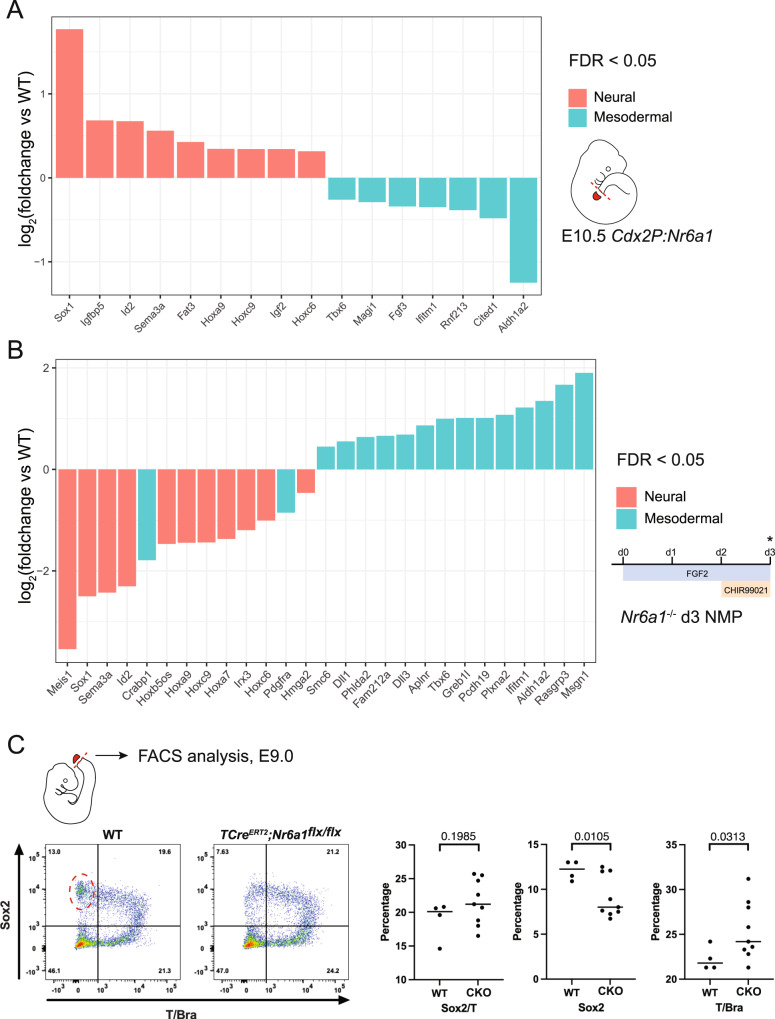
During development, the clearance of expression signatures can be instructive or passive. To test the morphological and molecular importance of timely Nr6a1 clearance at the T-to-T transition, we next turned to a gain-of-function approach where transgenic expression of Nr6a1 downstream of Cdx2 regulatory elements53 maintained gene expression in the tailbud throughout axial elongation (Cdx2P:Nr6a1). Skeletal analysis of Cdx2P:Nr6a1 embryos at E18.5 revealed thoraco-lumbar expansion, constituting an additional 2T and 2-4L vertebrae relative to wildtype (Fig. 5A). As the number of sternal rib attachments was unaltered in these transgenics (Supplementary Fig. 7A), the additional T elements are considered to be of caudal thoracic identity. Within the Cdx2P:Nr6a1 extended lumbar region, almost all of the elements harboured an accessory process known as the anapophysis, a feature usually restricted to the first 3 lumbar elements in wildtype (Fig. 5B). As such, we can pinpoint the presacral expansion in Cdx2P:Nr6a1 embryos to elements immediately surrounding the T-L junction, in terms of their identity.
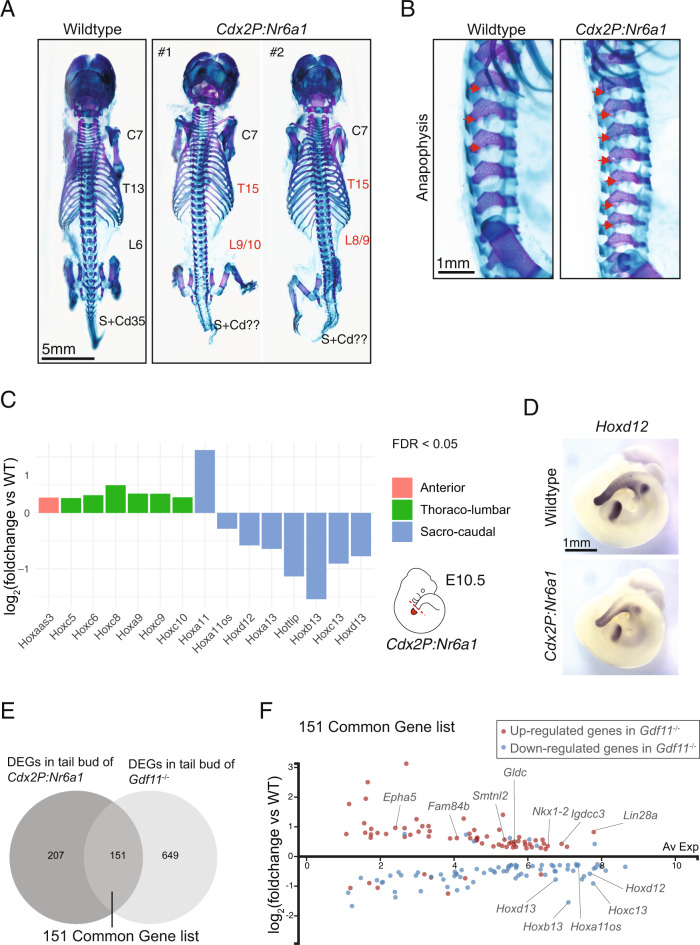
Fig. 5. Prolonged Nr6a1 activity is sufficient to increase thoraco-lumbar count and sustain trunk expression signatures.
A Skeletal preparation of E18.5 embryos, dorsal view, revealed an increase in the number of thoraco-lumbar elements in Cdx2P:Nr6a1 embryos (n = 2 independent samples) compared to wildtype (n = 8 independent samples). C = cervical, T = thoracic, L = lumbar, S = sacral and Cd = caudal vertebrae. B Higher magnification, lateral view, of the lumbar region in embryos from (A) revealed the anapophysis (red arrow) normally present on the first 3 lumbar elements in wildtype was now present on 7 of the 8 lumbar elements in Cdx2P:Nr6a1 embryos. Experimental numbers as per 5A. C RNAseq analysis of E10.5 wildtype and Cdx2P:Nr6a1 tailbuds revealed widespread changes in Hox expression downstream of Nr6a1. Results are presented as a log2-transformed fold change in Cdx2P:Nr6a1 samples relative to wildtype, n = 2 for Cdx2P:Nr6a1 and n = 4 for wildtype. Only those Hox genes with a false discovery rate (FDR) < 0.05 are displayed, and are colour-coded based on the axial region where the Hox protein functions. Source data are provided as a Source Data file. D Whole mount in situ hybridisation revealed reduced expression of Hoxd12 within the tail of E10.5 Cdx2P:Nr6a1embryos compared to wildtype. Consistent spatial changes were observed in 2 biologically independent samples/genotype. E Venn diagram overlay of differentially expressed genes (DEGs) in Cdx2P-Nr6a1 (this work) and Gdf11−/− 28 tailbud tissue reveals substantial overlap. Source data are provided as a Source Data file. F Analysis of the 151 co-regulated genes identified in (D), presented here as log2-transformed fold change in Cdx2P:Nr6a1 samples relative to wildtype, revealed that 136 genes (90%) displayed the same direction of regulation in both genetically altered backgrounds. Red and blue dots indicate upregulated and downregulated genes in Gdf11−/− tailbuds, respectively. Source data are provided as a Source Data file.
Increased Nr6a1 levels did not overtly affect vertebrae morphology within the T–L region up until the most caudal lumbar elements, after which, all sacro-caudal elements became small, fused and exhibited defects in dorsal closure (Fig. 5A and Supplementary Fig. 7B). These regionally-restricted phenotypic changes were complementary to those observed following conditional deletion of Nr6a1 (Fig. 2A), and demonstrate that the normal clearance of Nr6a1 at the T-to-T transition is not an inconsequential outcome of an altered developmental program but rather, Nr6a1 clearance is essential for correct sacral positioning and normal tail morphology.
To ascertain whether Hox expression dynamics were altered in Cdx2P:Nr6a1 embryos, we performed RNA-seq analysis of tailbud tissue at E10.5, a time when Nr6a1 should no longer be expressed at this site. Compared to wildtype controls, the prolonged maintenance of Nr6a1 activity resulted in a modest upregulation of several trunk Hox genes, including Hoxc6, Hoxc8, Hoxa9, Hoxc9 and Hoxc10, genes known to participate in conveying T-L identity49,51,54 (Fig. 5C). Conversely, genes of the Hox11-13 paralog groups and two 5’-Hox cluster antisense genes Hoxa11os and Hottip showed reduced expression in Cdx2P:Nr6a1 tailbuds (Fig. 5C), with Hoxb13 again having the largest differential response to altering Nr6a1 levels. One exception to this trend was Hoxa11, which was found to be upregulated in Cdx2P:Nr6a1 tailbuds. A similar inverse relationship between Hoxa11 and other posterior Hox cluster genes, including Hoxa11os, has also been observed in E10.5 Gdf11;miR-196single and compound mutant mice which harbour additional thoraco-lumbar elements44. An antagonistic relationship between Hoxa11os and Hoxa11 exists within the early mouse limb bud, resolving into mutually exclusive domains of expression as the limb bud elongates55. In this context, Hoxa11os transcription is dependent on Hoxa13/d13 and suppresses Hoxa11, with our data supporting the potential for a parallel regulatory network within the paraxial mesoderm.
We went on to assess the expression of Hoxd12 by whole mount in situ hybridisation in E10.5 Cdx2P:Nr6a1 embryos, revealing a general reduction in expression throughout the entire tail compared to WT somite-matched embryos, with clear reductions observed in tailbud mesoderm and somitic tissues (Fig. 5D). In summary, we show that the Nr6a1, likely acting with as-yet unknown protein partners, is sufficient to control the temporal progression of Hox activation of all 4 Hox clusters. This control over Hox cluster progression by Nr6a1 correlated well with altered patterning outcomes observed following manipulation of Nr6a1 levels. However, the increase in T-L vertebral number seen in Cdx2P:Nr6a1 embryos was harder to reconcile, since meristic changes (i.e. changes in vertebral number) are historically not thought to ensue downstream of changes to a single Hox gene or Hox paralog group. Growing evidence however has challenged this dogma44,50 supporting the view that the observed altered Hox code may indeed drive meristic changes.
Opposing effects of Nr6a1 and Gdf11 on a trunk regulatory module
The similarity of T-L expansion phenotypes seen in Nr6a1 gain-of-function (Fig. 5A) and Gdf11 loss-of-function22 mice prompted us to compare the global molecular changes identified in our E10.5 Cdx2P:Nr6a1 RNAseq dataset with those of a published E10.5 Gdf11−/− tailbud dataset28. This analysis revealed 151 conserved differentially expressed genes (Fig. 5D; Supplementary Data 2), 90% of which displayed the same direction of regulation in both mutants (Fig. 5F). 63 of the 136 co-regulated genes were upregulated, including genes enriched in NMPs (Nkx1-2, Epha5, Gpm6a and Gldc)20, as well as genes known to promote axial elongation such as Lin28a, Lin28b27–29. On the other hand, 73 of the 136 co-regulated genes were downregulated and included many posterior Hox genes, supporting an antagonistic arrangement whereby Nr6a1 promotes and Gdf11 terminates a core trunk gene regulatory network.
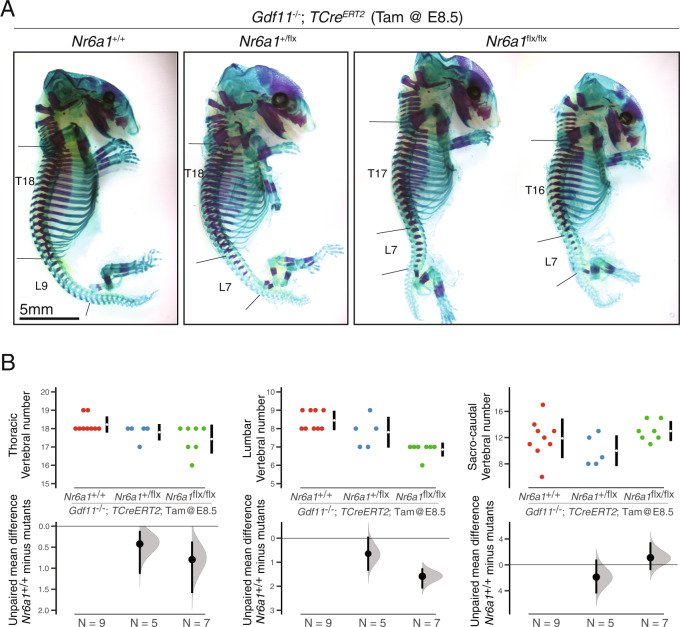
To test the in vivo epistatic interaction between Nr6a1 and Gdf11, we set out to assess whether Nr6a1 mediates Gdf11-dependent phenotypes through compound mutant analysis. Conditional deletion of Nr6a1 using the T-CreERT2 deleter line was repeated with Tam administration at E8.5, one day later than previous experiments and at a time when deletion was anticipated to have little to no phenotypic consequence that would confound compound mutant analysis. Indeed, no change in TVN was observed in TCreERT2;Nr6a1flx/flx embryos when Tam was administered at E8.5, on an otherwise wildtype (Gdf11+/+) or Gdf11+/− background (Supplementary Fig. 3A). However on a Gdf11−/− background which is known to display an expanded T-L count of 27 vertebrae (T18 L9; Fig. 5B, C and ref. [22]), conditional deletion of Nr6a1 at E8.5 significantly rescued T-L count by 3-4 elements (T16/17 L7), with Nr6a1 dose-dependent effects revealed (Fig. 6A, B). This temporal deletion of Nr6a1 did not rescue aberrant tail vertebrae morphology nor tail truncation known to occur in Gdf11−/− embryos, consistent with earlier interpretation that Nr6a1 function was not required at this site. This work identifies separable phenotypes, trunk elongation and tail morphology, that each depend on Gdf11 activity but utilise different downstream mechanisms. Collectively, this work establishes Nr6a1 as a master intrinsic regulator of T-L vertebral number and morphology, and reveals that termination of primary body axis formation by Gdf11 is mediated, likely to a large extent, through the clearance of Nr6a1 expression.
Fig. 6. Gdf11 signalling terminates Nr6a1-dependent trunk elongation.
A Skeletal preparation of E18.5 embryos, lateral view, revealed a dose-dependent rescue of Gdf11−/− thoraco-lumbar expansion following conditional deletion of Nr6a1 alleles. The number of biologically independent samples assessed: Nr6a1+/+;Gdf11−/− n = 9; Nr6a1+/−;Gdf11−/− n = 5; Nr6a1−/−;Gdf11−/− n = 7. T = thoracic, L = lumbar. B Quantification of thoracic, lumbar and sacro-caudal vertebral number across genotypes. Raw data is presented in the upper plots. Mean differences were calculated relative to Gdf11−/−;Nr6a1+/+ and presented in the lower plots as bootstrap sampling distributions. The mean difference for each genotype is depicted as a black dot and 95% confidence interval is indicated by the ends of the vertical error bar.
Nr6a1, along with the external factors, regulates Hox timing
To more precisely delineate the upstream signals influencing Nr6a1 expression, and the downstream molecular events requiring Nr6a1 activity, we turned to an in vitro embryonic stem cell (ESC) differentiation approach that models the developmental kinetics of a posterior growth zone by the sequential addition of FGF2 on day (d) 0, WNT pathway agonist CHIR99021 on d2, with or without the addition of Gdf11 on d3 (Supplementary Fig. 8A; based on refs. [20,56]. The loss of epiblast markers (Supplementary Fig. 8B), the induction of NMP identity (Supplementary Fig. 8B, C) and 3’ Hox cluster activation (Supplementary Fig. 8D) were all confirmed soon after Wnt activation. Collinear trunk Hox activation ensued quickly thereafter, with progression through to a posterior Hox code (Supplementary Fig. 8D) and a robust sacro-caudal producing E9.5-NMP molecular signature (Supplementary Fig. 8E) requiring Gdf11.
In wildtype cells, Nr6a1 was present in epiblast-like d2 cells and its expression was substantially enhanced on d2.5 and d3 following the induction of an NMP-like state (Supplementary Fig. 9A). While Nr6a1 expression was already decreasing after d3, it was sharply terminated following the addition of exogenous Gdf11 (Supplementary Fig. 9A), consistent with earlier in vivo results. Interestingly, an inverse temporal pattern of expression was observed for Nr6a1os, remaining very low until the addition of Gdf11 when levels increased 4-fold (Supplementary Fig. 9A). We find that the promoter of Nr6a1os is accessible in epiblast stem cells treated with or without Chiron for 48 h (Supplementary Fig. 9B)57, and harbours several consensus binding motifs for Smad2/3 (Supplementary Fig. 9C), ultimate transducers of Gdf11 signalling24 and Smad4, the common-mediator Smad, which forms heterotrimeric complex with Smad2/3 to control the target genes58. This raises the intriguing possibility that Gdf11 suppression of Nr6a1 sense transcripts is in fact mediated via a direct activation of Nr6a1os and subsequent genomic interactions between sense and antisense loci that establish mutually exclusive expression patterns, as has been observed for other developmental loci55.
With wildtype differentiation conditions established, we next generated Nr6a1−/− ESC clones by CRISPR/Cas959,60, deleting the DNA-binding domain from both alleles (Supplementary Fig. 10). These Nr6a1−/− ESCs were able to generate NMPs with equal kinetics to wildtype cells (Supplementary Fig. 11A–D), though whether the temporal progression of axial identity within these NMPs was altered, as may be predicted from the in vivo deletion of Nr6a1, remained unclear.
To this end, we performed RNAseq analysis as differentiation proceeded, and first assessed expression of the known Nr6a1 target43 and E8.5-NMP marker20, Pou5f1 (Oct4). Pou5f1 expression was slightly increased on d2 before the addition of Wnt, with a peak in enhanced differential expression observed on d3 (Supplementary Fig. 12B). This was accompanied by a statistical enrichment of many E8.5-NMP signature genes on d3, and concomitant reduction in E9.5-NMP signature genes (Supplementary Fig. 12C). This surprising result suggests that the deletion of Nr6a1 does not autonomously cause a speeding up of developmental timing within NMPs, and that additional factor(s) may be required for the earlier observations in vivo.
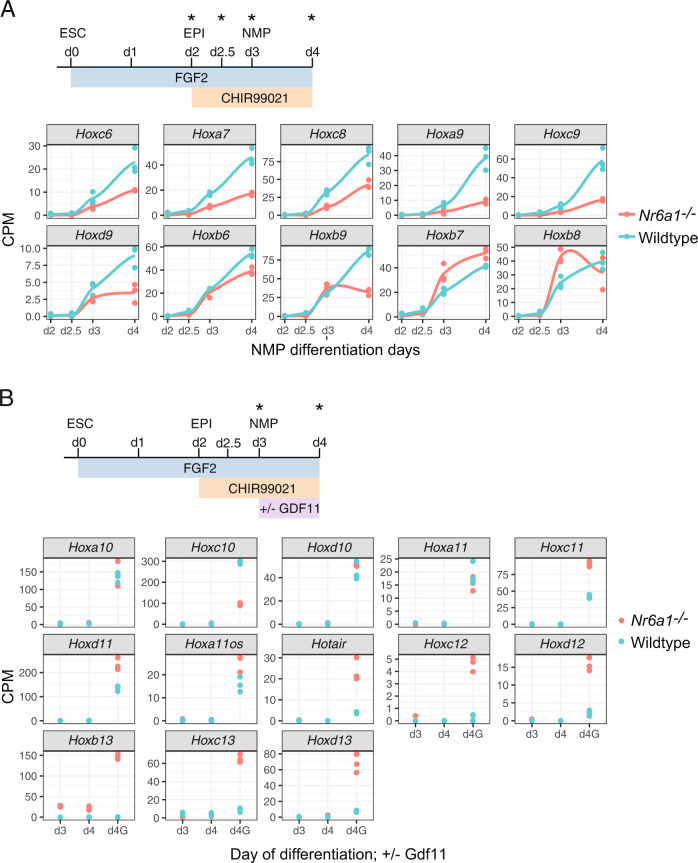
We next went on to characterise Hox expression dynamics within this RNAseq dataset. The robust initiation of a trunk Hox code normally seen 24 h after Wnt activation was reduced in Nr6a1−/− cells particularly for genes of the Hoxa, Hoxc and Hoxd clusters, with the expression of Hoxc6, Hoxa7, Hoxc8, Hoxa9, Hoxc9 and Hoxd9 all diminished relative to WT on d3 (Fig. 7A). This relative reduction was exacerbated even further when cells were cultured in Fgf/Wnt alone for a further 24 h. Genes of the Hoxb cluster showed mixed responses in Nr6a1−/− cells, however collectively, these observed expression signatures support the view that Nr6a1 does not serve a major role in the initiation of trunk Hox gene expression but is required to achieve their full expression potential.
Fig. 7. Nr6a1 and Gdf11 signalling cooperate in the control of Hox cluster transitions.
A, B Differential Hox expression kinetics observed during in vitro differentiation of Nr6a1 and wildtype ESCs. Source data are provided as a Source Data file. A RNAseq analysis of trunk Hox gene expression (Hox6-9) in Nr6a1−/− (red) and wildtype (blue) cells. In vitro differentiation protocol schematised, analysis timepoints marked with asterisk and data represented as counts per million (CPM). Only those genes showing a false discovery rate (FDR) < 0.05 are visualised. B RNAseq analysis of posterior Hox gene expression (Hox10-13) in Nr6a1−/− (red) and wildtype (blue) cells. In vitro differentiation protocol schematised, analysis timepoints marked with asterisk and data represented as counts per million (CPM). Only those genes showing a false discovery rate (FDR) < 0.05 are visualised. G = addition of Gdf11.
Under these standard Fgf/Wnt culture conditions, the activation of posterior Hox10-13 genes was almost universally absent in cells of both genotypes (Fig. 7B). For wildtype cells this is expected, since Gdf11 is required in these cultures for robust activation (Supplementary Fig. 8D)61,62. For Nr6a1−/− cells, however, these results allowed us to build a molecular hierarchy which was not able to be deciphered from in vivo experiments: the loss of Nr6a1 in general is not sufficient on its own to activate posterior Hox expression and requires positive input from additional signal(s). Once Gdf11 was exogenously applied to these cultures, Nr6a1−/− cells activated posterior Hox expression with far greater amplitude than wildtype cells, with this heightened response to Gdf11 more evident the further 5’ within a cluster the gene was located (Fig. 7B and Supplementary Fig. 12D).
One striking exception to this identified hierarchy was Hoxb13. 24 h after the activation of Wnt, and without any exogenous Gdf11, Hoxb13 expression was induced 25-fold relative to wildtype cells where a complete absence of expression was expected and observed (Fig. 7B). Expression persisted at this level without further signal input, and upon addition of Gdf11, Hoxb13 showed the largest differential increase in expression relative to wildtype of any posterior Hox gene (150-fold). The mechanism behind this unique regulation of Hoxb13 versus other posterior Hox genes remains to be defined, though is consistent with the heightened regulation of Hoxb13 in Nr6a1 in vivo gain-of-function experiments. With this exception noted, our in vitro results globally reinforce the dominant role of Gdf11 in activating posterior Hox expression, and additionally builds in an essential function for Nr6a1 in suppressing the untimely/precocious activation of posterior Hox expression and in defining their precise levels once expression has been activated.
Nr6a1 biases cell lineage choice in vitro and in vivo
The allocation of NMP descendants towards neural or mesodermal lineages is guided by both extrinsic63–65 and intrinsic factors66,67, with a disproportionate allocation to one lineage often resulting in termination of elongation68,69. Progenitor cells within the Gdf11−/− tailbud are biased toward a neural fate and an enlarged neural tube forms within caudal regions of these truncated mutant embryos28. In other developmental contexts, Nr6a1 activity has been linked to neural specification and differentiation in vitro70,71 and in vivo during mouse42 and Xenopus72,73 development, raising the possibility that Nr6a1 may promote neural cell fate within bipotent NMPs.
To address this, we first analysed our various RNAseq datasets for differential expression (FDR < 0.05) of genes previously found to delineate mesodermal progenitors (MP) or neural progenitors (NP) of the E8.5 tailbud20. Interrogation of in vitro differentiation samples 24 h after Wnt activation revealed heightened expression of MP genes and reduced expression of NP genes within Nr6a1−/− cells compared to wildtype (Fig. 7A; Note: Aldh1a2 in this context is a marker of MPs20). Conversely, interrogation of in vivo Cdx2P:Nr6a1 tail buds revealed the opposite, NP genes were found to be upregulated while MP genes were downregulated when Nr6a1 was ectopically maintained (Fig. 7B). While some of these lineage markers assessed are not uniquely restricted to these lineages, particularly at differentiation stages, the inclusion of key lineage markers within tailbud progenitor stages, such as Sox1 (NP) and Tbx6 or Msg1 (MP), supported further investigation of a potential bias in lineage choice in vivo.
To this end, we performed Flow cytometry analysis of E9.0 wildtype and TCreERT2;Nr6a1flx/flx tailbuds, to quantify any shift in the numbers of T/Bra+; Sox2+ (NMPs), T/Braneg; Sox2+ (NP) and T/Bra+; Sox2neg (MPs). The time point is important, since it is when cells that will go on to form the thoraco-lumbar region - the axial region that is strongly dependent on Nr6a1 function - are transiting through the PSM. While we observed no difference in the number of NMPs between WT and TCreERT2;Nr6a1flx/flx tailbuds we saw a significant decrease in NPs and increase in MPs following loss of Nr6a1 (Fig. 8C; WT n = 4 and CKO n = 9 individual tailbuds across two litters), consistent with the molecular biases observed (Fig. 8A, B). Together, these results identify Nr6a1 as an intrinsic factor that has a minor role in regulating the balance between neural and mesodermal fates within NMPs during axial elongation.
Fig. 8. Nr6a1 activity regulates neural vs mesodermal fate choice in vitro and in vivo.
A Heightened neural (red) and reduced mesodermal (blue) gene expression signatures were observed following prolonged maintenance of Nr6a1 within the mouse tailbud. RNAseq analysis was presented as a log2-transformed fold change in Cdx2P:Nr6a1 samples relative to wildtype, n = 2 for Cdx2P:Nr6a1 and n = 4 for wildtype. Neural and mesodermal gene lists were selected based on in vivo single-cell RNAseq analysis20, and only genes with a false discovery rate (FDR) < 0.05 are displayed. Source data are provided as a Source Data file. B Heightened mesodermal (blue) and reduced neural (red) gene expression signatures were observed in Nr6a1−/− in vitro-derived NMPs compared to wildtype. In vitro differentiation protocol schematised, analysis time-point (d3) marked with asterisk. RNAseq analysis is presented as a log2-transformed fold change in Nr6a1−/− samples relative to wildtype, n = 3/genotype. Neural and mesodermal gene lists as per above, and only genes with a false discovery rate (FDR) < 0.05 are displayed. Source data are provided as a Source Data file. C Flow cytometry analysis of Sox2 and T/Bra protein expression within cells of the E9.0 tailbud, collected from WT (n = 4 biologically independent samples) and TCreERT2;Nr6a1flx/flx (n = 9 biologically independent samples) embryos. Single Sox2-positive, single T/Bra-positive, or dual positive cells are presented as a percentage of all single, viable cells. Statistical comparison between genotypes was performed using an unpaired t-test with Welch’s correction, p-values are provided in the figure.
Discussion
The initial stages of vertebral column formation require many cellular decisions and tissue-level processes that, despite their required coordination, are often considered separately in terms of genetic regulation. Here, we have identified a single factor that coordinately controls vertebral number and identity, somite segmentation and NMP cell lineage choice in the mouse (summarised in Fig. 9). The axially-restricted manner in which Nr6a1 functions reinforces the existence of distinct developmental programs controlling trunk vs. tail formation, emphasising that this is not a developmental continuum that is delineated by a switch in patterning program and progenitor location alone. Rather, this switch involves additional changes in the genetic regulation of segmentation and regional control of vertebral number, all processes unified in their requirement for precise levels of Nr6a1 activity.
Fig. 9. Schematic summary highlighting the multiple roles of Nr6a1 within the tailbud.
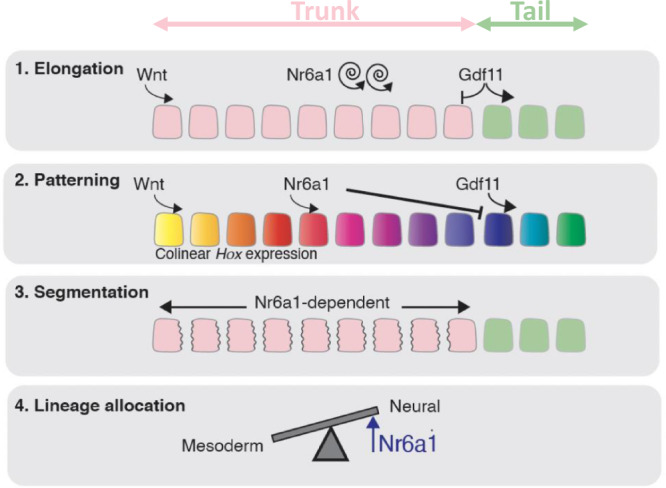
Blocks represent somites, pink = trunk-forming somites and green = tail-forming somites.
Complete loss of Nr6a1 in the mouse was found to slow the overall pace of development soon after the initiation of somitogenesis and, morphologically, these null embryos failed to progress through the period of embryonic turning (E8.75)30. Nonetheless, up until approximately E9.5 when Nr6a1−/− embryos died, the very anterior and posterior embryonic ends continued to expand and differentiate while the trunk region stalled, consistent with our collective analyses establishing Nr6a1 as a selective regulator of trunk development. Nr6a1 transcripts have been detected ubiquitously in the mouse embryo at E6.530,43, however, the first 7-13 somites still form in the complete absence of Nr6a1 activity30, broadly correlating with the future cervico-thoracic boundary. Here, Nr6a1 expression levels rise sharply in cells of the posterior embryo central to axial elongation41, a site high in Wnt activity74 which we show in vitro has the ability to rapidly enhance Nr6a1 transcript levels. As the T-L region is being laid down, Nr6a1 levels remain high, and a correlation between caudal Nr6a1 expression level and total T-L vertebral number was observed across our miR-196-TKO;Gdf11−/−deletion series. Indeed, Nr6a1 levels were shown to be quantitatively instructive based on the dose-dependent phenotypes observed in both Nr6a1 gain- and loss-of-function scenarios. As development proceeds and the primary body elongation program comes to its eventual completion, we show an essential requirement for the clearance of Nr6a1 expression within the tailbud. This clearance was achieved by multiple mechanisms, including the rising levels of Gdf11 signalling as well as microRNA repression by the posteriorly-expressed miR-196 paralogs.
The 3’UTR of Nr6a1 in fact houses binding sites for several microRNAs, including another Hox-embedded miRNA family, miR-10, as well as the developmental timing microRNA family, let-7 (Supplementary Fig. 1C). miR-10 paralogs are genomically positioned between Hox4/5 paralogs and thus are expressed from early stages of axial elongation75,76. This raises the intriguing possibility that miR-10 may act to buffer the functional domain of Nr6a1 activity at more anterior locations, while miR-196 reinforces its posterior boundary, collectively delimiting Nr6a1 output to the T-L region in synchrony with the temporal progression of Hox cluster activation. Finally, within the tail-forming region, the rising levels of Gdf11, and decreasing levels of Nr6a1 activates expression of progressively more posterior Hox genes, including the Hox13 paralogs which shift the Lin28-let-7 axis in favour of let-7 expression28, a potent repressor of Nr6a1 at least in vitro39 thus reinforcing termination of expression. Collectively, this work has revealed a highly integrated series of regulatory mechanisms defining axially-restricted expression of Nr6a1, and ultimately, defining vertebral number within the trunk region of the mouse.
The consequences of sustaining Nr6a1 activity in vivo for longer than normal bared resemblance to those observed following sustained activity of the Pou-domain transcription factor Oct4/Pou5f1, both in terms of expanded trunk vertebral number and in the delayed activation of posterior Hox expression26. This parallel is counterintuitive since Nr6a1 is a well-characterised direct repressor of Oct4 in ESCs71,77–79 and, consistent with this view, Oct4 expression is enhanced in the posterior growth zone of Nr6a1 null embryo at E8.543 and in our equivalent-stage Nr6a1−/− ESC-derived NMPs (Day2.5 and Day3 NMPs; Supplementary Fig. 12B). Why then does in vivo deletion of Nr6a1 reduce trunk vertebral number if Oct4 expression is heightened? We suggest the explanation for this result lies in the timing of gene regulatory mechanisms. While loss of Nr6a1 upregulates Oct4 within trunk-forming progenitors (E8.5 in vivo, Day2.5/3 in vitro), it is known that ectopic Oct4 activity has little to no phenotypic consequence within its normal (cervical-thoracic) domain of activity26. It is only at later stages, when endogenous Oct4 is normally extinguished, that persistent/exogenous Oct4 acts to drive trunk elongation26. Extending our in vivo analysis to these later stages showed that Oct4 transcripts were undetectable in both wildtype and TCreERT2;Nr6a1flx/flx tailbuds at E9.5 by qPCR. Similarly, in vitro, Oct4 expression dropped to negligible levels in both wildtype and Nr6a1−/− cells on d4 of differentiation (Supplementary Fig. 12D), indicating that the upregulation of Oct4 following loss of Nr6a1 is transient. Gdf11 and its signalling pathway components became robustly expressed with or without Nr6a1 (In vitro: d4 and d4G, Supplementary Fig. 12D; In vivo: TCreERT2;Nr6a1flx/flx, Fig. 3B), and thus would be expected to clear ectopic Oct4 expression even in Nr6a1 loss-of-function settings, while at the same time as amplifying precocious posterior Hox gene expression, collectively reinforcing the premature termination of trunk formation.
In parallel with, or likely integrated with, its role in controlling axial elongation and vertebral number, this work has identified Nr6a1 as a critical intrinsic regulator of Hox cluster temporal progression and body patterning. Both loss- and gain-of function functional analyses supported this conclusion, with the respective precocious or delayed posterior Hox activation being the primary phenotypic driver in our view. For example, in Cdx2P:Nr6a1 gain-of-function tailbuds (Fig. 5C), we do not believe the minor increase in trunk Hox expression seen in is the cause of the expanded thoraco-lumbar region, but rather, it is the delay in overall trunk-to-tail Hox code transition. Simply maintaining a trunk Hox code, in the absence of posterior Hox activation, would suffice. Moreover, it is possible that the minor increase in trunk Hox expression may be a secondary effect. In WT tailbuds, the timely activation of a posterior Hox code may feedback and reduce trunk Hox expression levels, a scenario that is not occurring at the same time in Cdx2P:Nr6a1 tailbuds.
At a mechanistic level, Nr6a1-dependent regulation of Hox cluster expression could be indirect via secondary signal(s), particularly as all 4 clusters are concurrently affected. However, our reanalysis of chromatin immunoprecipitation studies characterising mouse mesenchymal stem cells in vitro39 has identified putative Nr6a1 binding sites within each of the four Hox clusters, raising the possibility of a more direct mode of regulation. To date, Nr6a1 has been characterised as a transcriptional repressor80,81 which, in the context of the Oct4 promoter in ESCs, recruits the methyl transferases such as Dnmt3a, Mdb2 and Mdb3 to silence expression78,79. Additionally, Nr6a1 has been shown in vitro to interact with the histone deacetylase complex component Nuclear receptor corepressor 1 (Ncor1)80 and Ubiquitin interacting motif containing 1 (UIMC1)82 in the repression of downstream target genes. Regarding the latter, the particular SNP identified within Nr6a1 as associated with additional trunk vertebrae in pigs was shown to enhance binding between Nr6a1 and Ncor1/UIMC135, supporting the potential for a direct mode of Nr6a1 regulation in vivo. While mechanism remains to be elucidated, the global effect of Nr6a1 in regulating the temporal activation of expression from all Hox clusters supports an ancient role for this nuclear receptor, at least back to the common ancestor of vertebrates when a single Hox cluster existed.
An unexpected aspect of Nr6a1 function revealed in these studies was its axially-restricted role in segmentation. Thoraco-lumbar segmentation required the presence of Nr6a1, though was resistant to exaggerated Nr6a1 levels. Conversely, the active clearance of Nr6a1 function was essential in allowing normal post-sacral segmentation. This switch in the requirement for Nr6a1 activity in either scenario was incredibly sharp, visualised both in terms of somite and vertebrae morphology, supporting a very rapid transition in the genetic networks driving this developmental process.
Axially-restricted contribution to pre-sacral or post-sacral segmentation has been observed in a small number of studies, highlighting potential downstream effectors of Nr6a1 in this context. For example, some Hox genes have been shown to cycle in the PSM83 and, while altered Hox expression or Hox function has not generally not been linked to defects in segmentation, the in vivo ectopic expression of a linker region-deficient Hoxb6 protein was shown to cause severe defects in segmentation posterior to the hindlimb only84. In the case of Nr6a1 deficiency, Hox protein function is not expected to be altered, though it remains possible that aberrant levels of Hox activity may impact segmentation. A second, and more likely, mediator is Lunatic Fringe (Lfng), a core component/inhibitor of the Notch signalling pathway. Throughout segmentation, Lfng is expressed both in an oscillatory pattern in the posterior PSM and stably at the anterior PSM border85,86, controlled by independent enhancers87,88. The specific abolition of oscillatory Lfng expression via enhancer deletion resulted in highly malformed vertebrae of reduced number specifically within the presacral region, with the tail extending largely unaffected89–91. This regional phenotype was very similar to what was observed in TCre:Nr6a1−/− mutant embryos (Fig. 2F), and moreover, cyclic expression of Lfng has been found to be greatly reduced in Nr6a1−/− embryos while the stable band of Lfng in anterior PSM was unaffected30. Collectively, this supports the oscillatory expression of Lfng, downstream of Nr6a1, as essential for presacral somite segmentation.
While we have focused on the paraxial mesoderm and its major derivative, it is clear that the entire trunk region expands or contracts following manipulation of Nr6a1 level, suggesting that cell-autonomous Nr6a1 actions are likely to extend across multiple tissue/germ layers in addition to more global mechanisms of tissue-level coordination. For the lateral plate mesoderm, a change in hindlimb positioning that mirrors the anterior or posterior displacement of sacral vertebrae has been observed in Gdf11 signalling gain- and loss-of-function mouse mutants22,25 and also here in Cdx2P:Nr6a1 gain-of-function embryos. In TCre:Nr6a1−/− embryos however, the positioning of rudimentary pelvic bones maintained an almost “wildtype” distance from the last formed rib-bearing thoracic element, despite all intervening elements being transformed from lumbar to sacral identity. This suggests a likely dissociation in Nr6a1’s regulation of Hox signatures between tissue layers, and in the downstream events they instruct92. From an evolutionary perspective, our work provided experimental support for changes in Nr6a1 activity underlying the modest, albeit economically-beneficial, changes in axial formulae observed in domesticated pigs and sheep. Whether analogous changes are widespread across the vertebrates and whether more dramatic changes in Nr6a1 expression or function may have supported the evolution of extreme phenotypes such as the elongated vertebral column in snakes remain important areas for future investigation.
Methods
Experimental models and subject details
Animal models and ethical approval
All animal procedures in Monash University were performed in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (2013). These experiments were approved by the Monash Animal Ethics Committee under project number 21616. Animal experiments performed at the Stowers Institute for Medical Research were conducted in accordance with an Institutional Animal Care and Use Committee approved protocol (IACUC #2019-097).
Mice
Gdf11−/− 22, Nr6a1−/− 30, Nr6a1flx/flx 46, CMVCre47 and TCreERT248 mouse lines have been previously described, and were maintained on a C56BL/6 background. TCreERT2 and Nr6a1flx/flx lines were intercrossed to generate TCreERT2;Nr6a1+/flx males, which were subsequently bred with Nr6a1+/flx or Nr6a1flx/flx dams for time-mate collection of embryos. A similar breeding strategy was employed to incorporate the Gdf11+/− allele onto this TCre ERT2;Nr6a1+/flx compound line. Conditional deletion of Nr6a1 was performed by intraperitoneal administration of Tam (60 mg/kg) into the pregnant dam at the specified embryonic stages. Cdx2P:Nr6a1 transgenic embryos were generated by pronuclear injection by the Monash Gene Modification Platform. The full coding sequence of Nr6a1 long isoform was synthesised with Not1 restriction enzyme site addition at the 5’ and 3’ ends by Biomatik, USA, sequence verified, digested, and cloned downstream of the Cdx2P promotor53. Two adult transgene-positive chimeras were recovered from multiple rounds of injection, however, a stable line could not be produced as one founder was infertile and one did not transmit the transgene. Thus, transient transgenic embryos were collected at E10.5 for tailbud RNA extraction and/or in situ hybridisation, and at E18.5 for skeleton preparation. Transgene copy number for each embryo was confirmed by droplet digital PCR.
Genotyping
For routine genotyping, tail tissue was collected from postnatal pups and yolk sac collected from embryonic samples. Genotyping was performed by Transnetyx or in-house. For detection of Nr6a1 conditional deletion via TCreERT2 and Tam administration at E7.5 or E8.5, digits of the left hindlimb were collected based on known pattern of expression of the T promoter48,93. To isolate the DNA, digit tissue was treated with Proteinase K (20 µg/ml) in 500 µL of Tris-HCl buffer (Composition: 100 mM Tris-HCl (pH8.0); 5 mM EDTA; 200 mM NaCl; 0.2% SDS) overnight at 56°C. The DNA was precipitated by adding equal volume of isopropanol and pelleted at 13,000 rpm. Next, the pellet was washed twice with 70% ethanol and eluted in nuclease-free water. At this point, genotyping was performed by standard PCR method using primers described in46.
ESCs maintenance
A mouse Bruce-4 ESC line was used for all in vitro differentiation assays. ESCs were routinely maintained on an in-house generated mitotically inactive primary mouse embryo fibroblast feeder layer in ES medium (81.8% Knockout DMEM (Gibco, 10829-018); 15% gamma irradiated foetal bovine serum (Gibco, 10101-145); 1% Pen/Strep (Gibco, 15140-122); 1% GlutaMAX-I (Gibco, 35050-061); 1% MEM NEAA (Gibco, 11140-040), 0.2% 2-Mercaptoethanol (Gibco, 21985-023)) supplemented with LIF (1000x, made in-house).
Generation of Nr6a1−/− ESC line
Nr6a1 null allele generation was performed by the self-cloning CRISPR/Cas9 (scCRISPR) method59,60. Two protospacers in Nr6a1 exon4 were determined using the tool, CRISPOR (http://crispor.tefor.net/)94. The sgRNA oligonucleotides order for scCRISPR contains the protospacer (19-21 bp), flanked by sgPal7 (Addgene, 71484) homology (U6 promoter and sgRNA) sequence, total 60 bp. Three consecutive PCR steps were performed using three pairs of standard primer sets (sgRNA_HDRstep1-3) for preparing homology directed repair (HDR). ESCs used for electroporation were cultured and maintained as previously described without antibiotics. Once the ESCs were ready, they were pelleted and suspended in buffer R (ThermoFisher, MPK1025) at a density of 1*107 cells/ml. 100 µl of the ESCs were then mixed with the scCRISPR construct (Composition: 10 µl elute of HDR PCR product; 1 µl sgPal7 (Addgene, 71484); 1 µl spCas9-BlastR (Addgene, 71489). ESCs were electroporated at pulse voltage 1,400 and width (ms) 3 for three cycles, and then plated on a 10 mm feeder-covered plate. 96 single colonies were selected and expanded independently in a 96-well plate. Multiple independent knockout lines were produced by this method and clones selected based on normal morphology. Clone B1 used in these studies harboured independent genomic deletions within exon 4 of each Nr6a1 allele, one deleting 125 bp (Δ125) and the second 219 bp (Δ219) (Supplementary Fig. 8). Compared to the wildtype Nr6a1-long isoform protein product of 495 amino acids, Δ125 generates a truncated protein of 126 amino acids with no DNA binding and ligand-binding domain, while the in-frame deletion Δ219 allele encoded a protein of 422 amino acids with no DNA-binding domain, both supportive of a functional null45.
Gene cloning
Nr6a1 isoform sequences were confirmed using gene amplification from E10.5 embryonic tissue. RNA was extracted (Macherey-Nagel) and cDNA prepared (Roche, 04897030001) using both random hexamers and oligodT primers. Sequence-specific primers were designed based on the version mm10 genome (UCSC) as detailed in Key resources table. Amplified sequences were cloned into pGEM-T easy vector (Promega) and used as the template for synthesising RNA riboprobes.
Whole-mount in situ hybridisation
Whole-mount in situ hybridisation was performed as previously described95 with minor modifications. Mouse embryos were dissected and placed in iced-cold PBS (Gibco, 14190-144). For embryos E9.5 or older, the brain’s 4th ventricle was pierced to prevent probe trapping. Embryos were fixed in 4% paraformaldehyde (PFA) at 4°C, rocking overnight. Fixed embryos were washed twice in PBT (Composition: 137 mM NaCl; 2.7 mM KCl; 10 mM Na2HPO4; 2 mM KH2PO4 in DEPC-H2O (pH7.4); 0.1% Tween) for 5 min and dehydrated into methanol using a graded MeOH/PBT series (25%, 50%, 75%, 100% MeOH) for 5-20 mins each with gentle rocking at room temperature (RT). To start in situ hybridisation, embryos were rehydrated into PBT using a graded MeOH/PBT series (75%, 50%, 25% MeOH) for 5-20 mins each and washed in PBT twice for 5 min with gentle rocking at RT. The embryos were then treated with 10 µg/ml of proteinase K in PBT as follows: E8.5 for 3.5 min, E9.5 for 8 min, E10.5 for 15 min and E12.5 for 25 min at RT with gentle rocking. The reaction was quenched by washing embryos twice in PBT for 5 min and the following postfix in 4% PFA with 0.2% glutaraldehyde for 20 min at RT. Embryos were then washed twice in PBT for 5 min at RT and transferred to hybridisation solution (Composition: 50% formamide; 5x SSC (pH4.5), 1% SDS; 50 µg/ml heparin; 50 µg/ml yeast tRNA (Sigma, R6750)) at 70°C with constant rocking for at least 2 hr before 1 µg/ml DIG-labelled riboprobe was added and incubated overnight. On the second day, the embryos were washed 3 times at 70°C in pre-warmed solution I (Composition: 50% formamide; 5x SSC (pH4.5); 1% SDS) for 30 min, followed by 3 washes at 65°C in pre-warmed solution II (Composition: 50% formamide; 2x SSC (pH4.5); 0.1% Tween-20) with gentle rocking. The embryos were then washed 3 times in TBST (Composition: 137 mM NaCl; 2.7 mM KCl; 25 mM Tris-HCl (pH7.5); 0.1% Tween-20) for 5 min at RT and blocked in TBST containing 10% heat-inactivated sheep serum (HISS) for 2 hrs at RT with gentle rocking. The embryos were then transferred to TBST containing 10% HISS and anti-DIG-AP antibody (Roche, 11093274910) at a dilution of 1:2000 overnight at 4°C with constant rocking. On the third day, the embryos were washed at least 5 times in TBST for 1 hr each wash at RT with gentle rocking and then overnight in TBST at 4°C. On the fourth day, embryos were equilibrated into NTT (Composition: 100 mM NaCl, 100 mM Tris-HCl (pH9.5); 0.1% Tween-20) by washing 3 times for 10 min each at RT with gentle rocking. To develop the colour, the embryos were incubated in BM purple (Roche, 11442074001) at RT. To stop the colour reaction, embryos were washed 3 times in PBT for 5 min, and postfixed in 4% PFA for 20 min at RT. The embryos were then washed 3 times in PBT for 5 min and stored in PBT at 4°C until photographing.
Microarray
E9.0-E9.5 wildtype and Nr6a1−/−− embryos were isolated and yolk sac DNA genotyped in parallel. Total RNA was extracted from each whole embryo using TRIzol reagent and processed through an RNeasy column (QIAGEN) using the RNA clean-up protocol. Concentration and quality of RNA were determined by spectrophotometer and Agilent bioanalyzer analysis (Agilent Technologies, Inc., Palo Alto, CA). For array analysis, labelled mRNA targets were prepared from 150 ng total RNA using MessageAmp III RNA Amplification Kit (Applied Biosystems / Ambion, Austin, TX) according to manufacturer specifications. Array analysis was performed using Affymetrix GeneChip GeneChip Mouse Genome 430 2.0 Arrays processed with the GeneChip Fluidics Station 450 and scanned with a GeneChip Scanner 3000 7G using standard protocols.
Tailbud dissection for gene expression analysis
For E9.5 tailbud collection, all tissue at and caudal to the posterior neuropore was collected. For E10.5 tailbud collection, all tissue caudal to the last somite was collected. Dissected tissue was immediately placed into lysis buffer (Macherey-Nagel) on dry ice and stored at −80°C. RNA was extracted (Nucleospin RNA kit, Macherey-Nagel, 740955). The remaining part of each embryo was processed for whole-mount in situ hybridisation detection of Uncx4.1 and accurate somite count determination, with embryo comparisons restricted to those within ±1 somite.
Quantitative PCR using BioMark Fluidigm
100 ng RNA isolated from E9.5 and E10.5 tailbud tissue was used as template for cDNA synthesis performed using RT-Vilo (ThermoFisher). Quantitative PCR was performed using the 96*96 BioMark Fluidigm format. Raw Ct values were analysed using a modified version of the qPCR-Biomark script (https://github.com/jpouch/qPCR-Biomark) and normalised as previously described96. Only Ct values in the optimal range for the Biomark system of 6-25 were used for further analysis. All genes were first normalised against the mean raw Ct-values of five housekeeping gene probes yielding ΔCt values, then normalised against the wildtype condition yielding ΔΔCt values.
Quantitative PCR using Roche Lightcycler
RNA isolated from one E9.5 tailbud or 1 mg RNA isolated from the in vitro cells was used as template for cDNA synthesis (Roche, 04897030001) using random hexamers and eluted in 200 µl and 100 µl H2O respectively. Each 10 µl PCR reaction reaction (5 µl SYBR Green I Master Mix (Roche, 04887352001); 2.5 µl H2O; 2 µl cDNA; 0.25 µl 10 mM forward primer; 0.25 µl 10 mM reverse primer) was run on a Lightcycler 480 (Roche) using the program: 95°C for 10 s (1 cycle), 95°C for 10 s, 60°C for 15 s, 72°C for 10 s (45 cycles). Biological samples were processed in technical triplicate for each gene and the expression of housekeeping gene Pol2a used for normalising gene expression using the ΔΔCt method.
RNA-seq
For in vivo sample RNAseq, tissue was dissected and RNA extracted as described above. RNA quality and quantity assessed using the Agilent Technologies BioAnalyser. Multiplexed RNAseq libraries, generated from 50 ng total RNA per sample, were prepared using Illumina TruSeq Stranded mRNA Sample prep (Protocol 15031047 Rev Oct 2013). 75 bp single-end (SE) sequencing was performed on the NextSeq500 Illumina platform. On average, 69 million SE reads were obtained for each library. For in vitro cell sample RNAseq, libraries were prepared by an in-house method. The index is added during initial pA priming and pooled samples amplified using template switching oligos. P5 was added by PCR and P7 by Nextera transposase. The final library structure is as follows: P5-Rd1->8 bp index-10bpUMI-pA then cDNA < -Rd2 primer i7 index P7. Paired end sequencing was performed on the NextSeq550 Illumina platform for 19 bp forward reads of index and 72 bp reverse reads of cDNA. On average, 20 M raw reads were obtained for each library.
Skeleton preparation and imaging
Skeletal preparation was performed on E16.5 and E18.5 embryos as previously described97. Embryos were skinned, then incubated for 2 days at RT with constant rocking in each of the following solutions: 95% ethanol, 100% acetone, staining solution (Composition: 15 mg alcian blue and 5 mg alizarin red S in 70% ethanol with 0.5% glacial acetic acid). To clear the skeletons, stained embryos were washed in 1% KOH at RT with constant rocking for 2-5 days, then transferred into glycerol using a graded glycerol/1% KOH series (25%, 50%, 75%, 100% glycerol) for 24 hr in each solution with gentle rocking at RT. Finally, the embryos were transferred in 100% glycerol with 0.02% sodium azide for long-term storage. Images were acquired with a Vision Dynamic BK Lab System at the Monash University Paleontology Lab. Images were taken with a Canon 5d MkII with a 100 mm Macro lens (focus stop 1:3/1:1). Multiple images were taken to extend the focal depth, and stacked in ZereneStacker using the PMax algorithm.
NMP differentiation of ESCs
NMP differentiation was based on the published protocols20,56 with minor modifications. In preparation for differentiation, ESCs were feeder-depleted and plated in gelatin-coated (0.1% Sigma, G1890-100G) 6-well plates (Falcon, 353046) at a density of 8×103 cells/cm2 in ES medium. On D0 of differentiation media was changed to N2B27 media (Composition: 49.5% Advanced Dulbecco’s Modified Medium F-12 (Gibco, 12634028); 49% Neurobasal medium (Gibco, 21103049); 0.5% N2-supplement (Gibco, 17502001); 1% B27-supplement (Gibco, 17504044)) supplied with 1x Glutamax (Gibco, 17504044), 40 µg/ml BSA Fraction V (Gibco, 15260037) and 100 mM 2-Mercaptoethanol (Gibco, 21985-023)) supplemented with 10 ng/ml hFGF-2 (Miltenyi Biotec, 130-104-925). The media was further supplemented with 5 µM CHIR99021 (StemMACS, 130-103-926) on D2 and with or without 50 ng/ml hGdf11 (130-105-776, Miltenyi Biotec) on D3. Throughout differentiation, the medium was refreshed every 24 h. NMP identity at D3 was routinely confirmed by co-expression analysis of Sox2 and T/Bra using immunofluorescence.
Flow cytometry analysis
Samples for flow cytometry were isolated and analysed from individual embryos. All tissue posterior to the first visible somite condensate was dissected from E9.0 WT or T-CreERT2;Nr6a1flox/flox (Tamoxifen-treated @ E7.5) embryos in cold PBS and dissociated for 20’ in 120 µL Accutase at 37 °C. Single cell suspension was achieved by pipetting up and down with a P200 low bind pipette tip before adding 800 µL of PBS + 2% Foetal Bovine Serum (FBS) and pipetting up and down with a P1000. This single-cell suspension was then filtered through a 70 µm mesh cap and cells pelleted by centrifugation at 400 g. Cells were stained using the viability dye Zombie Aqua (BioLegend), fixed and permeabilised using the True Nuclear Transcription Factor Buffer Set (BioLegend), both following manufacturer’s instructions. Cells were incubated with primary antibodies (rat anti-Sox2, 1:200, eBiosciences; rabbit anti-T/Bra, 1:200, Abcam) for 30 minutes in PERM buffer in the dark at room temperature (RT), then washed once in PERM buffer. Secondary antibodies (goat anti-rat-AlexaFlour555, Invitrogen; goat anti-rabbit- AlexaFlour 647, Invitrogen), were incubated in PERM buffer for 30 minutes in the dark at RT, washed once in PERM buffer followed by a second wash in PBS + 2%FBS. Cells were pelleted by centrifugation at 400 g, resuspended in PBS + 2%FBS and 1 mg/ml Propidium iodide and analysed by flow cytometry on a LSRFortessa (BD). A minimum of 8,000 and a maximum of 16,000 events were recorded per sample. To set gates, all non-tailbud tissue was pooled and stained for 1) both secondary antibodies, 2) anti-Sox2 primary and secondary alone and 3) anti-T/Bra primary and secondary alone. Experimental cells were first gated to remove debris, then gated based on size to select for single cells, and finally, cells that had taken up Propidium iodide were excluded leaving single viable cells for analysis of Sox2 and T/Bra expression as depicted in Supplementary Fig. 13.
Quantification and statistical analysis
Microarray analysis
Microarray CEL files were analysed using RMA98 and limma99 in the R statistical package. Following alignment and annotation of genes, the Microarray data was filtered to export differentially expressed genes with an adjusted p-value or false discovery rate of 0.05 or lower and a fold change less than or equal to −2 and greater than or equal to 2 (log2FoldChange less than or equal to −1 and greater than or equal to 1). Genes were then sorted to extract the top 50 most downregulated genes, which were analysed via DAVID100,101 and ToppGene102 utilising standard settings provided in each program. The results were then exported into tables in Excel and the bar graph of biological processes affected by the top 50 downregulated genes was generated using -log10(p-value) parameters and plotted using PRISM.
Vertebral formulae—CMVCreERT2 analysis
For statistical analysis and data visualisation of vertebral numbers, Graph Pad Prism 9.3.1 (471) was used. A two-tailed T-test was employed. Error bars represent the mean with standard deviation.
Vertebral formulae—TCreERT2 analysis
For statistical analysis and data visualisation of vertebral numbers, R-package “Data Analysis using Bootstrap-Coupled ESTimation” (dabestr) was used, and the background of the methods were previously described103. To determine mean differences to the respective shared control, 5000 bootstrap samples were taken and the confidence interval was bias-corrected and accelerated. In the visualisation, 95% confidence interval is indicated by the ends of the vertical error bar and the sampling error distribution is diagrammed as a grey filled curve. The codes are available at https://github.com/ACCLAB/dabestr.
Quantitative PCR using Roche Lightcycler
All genes were first normalised against the mean raw Ct-values of housekeeping gene, Pol2a, yielding ΔCt values. Following normalisation of gene expression using the ΔΔCt method, statistical analysis was performed using the Wilcoxon test.
RNAseq analysis
All sequencing reads were aligned to the reference genome using STAR aligner104. Only the genes with counts, which are greater than 10, and with CPM, which are greater than 2 in two biological replicates were used for further analysis. Differential gene expression between control and mutants were performed using edgeR105,106. Here we used false discovery rate (FDR), the adjusted p-value, to display significance of the differential gene expression between the controls and the mutants. Genes with a FDR < 0.05 were considered to be significantly differentially expressed in the mutants.
Resource availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by Edwina McGlinn (edwina.mcglinn@monash.edu).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors acknowledge the excellent technical assistance provided by Monash Gene Modification Platform, Monash Animal Research Platform and the MHTP Medical Genomics Facility, Karin Zueckert-Gaudenz and Allison Peak for their microarray technical expertise and to Dr Chris Seidel for bioinformatic analysis support. We also greatly appreciate Melissa Childers and the Laboratory Animal Services Facility at the Stowers Institute for their care and maintenance of our mice. The Gdf11 mutant mouse line was kindly provided by Professor Se-Jin Lee and the Nr6a1flx line was kindly provided by Professor Austin Cooney. Y.-C.C. and G.M.H. were supported by an Australian Government Research Training Program Scholarship. A.A. was the recipient of an American Association for Anatomy Post-Doctoral Fellowship. J.M.P. is supported by an Australian Research Council Future Fellowship. This work was supported by Australian Research Council Discovery Project DP180102157 to E.M. and J.M.P. Research in the Trainor laboratory is supported by the Stowers Institute for Medical Research. The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government.
Source data
Author contributions
E.M. and Y.-C.C. conceived the study and wrote the manuscript. Y.-C.C., S.F.L.W., G.M.H., N.A.S., E.L.M., A.A., V.G., J.M. and P.T. performed experiments and analysed data. J.S. and J.M.P. provided advice on experimental design and analysed the RNA-seq data. All authors have commented on the manuscript and approve submission of the manuscript.
Peer review
Peer review information
Nature Communications thanks Valerie Wilson, and the other, anonymous, reviewer for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The data that support this study are available from the corresponding author upon request. The raw microarray data generated in this study have been deposited in the Gene Expression Omnibus (GEO) and are publicly available using the accession number GSE166458 (CEL datasets). Original data relating to the microarray experiment can also be accessed from the Stowers Original Data Repository LIBPB-1647. The raw in vitro and in vivo RNAseq data generated in this study have been deposited in the GEO and are publicly available using the accession number GSE180427. This includes raw data for graphs presented in this study. Source data are provided with this paper.
Competing interests
The authors declare no competing interests
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-35303-4.
References
- 1.Bénazéraf B, Pourquié O. Formation and segmentation of the vertebrate body axis. Annu. Rev. Cell Dev. Biol. 2013;29:1–26. doi: 10.1146/annurev-cellbio-101011-155703. [DOI] [PubMed] [Google Scholar]
- 2.Cambray N, Wilson V. Two distinct sources for a population of maturing axial progenitors. Development. 2007;134:2829–2840. doi: 10.1242/dev.02877. [DOI] [PubMed] [Google Scholar]
- 3.Forlani S, Lawson KA, Deschamps J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development. 2003;130:3807–3819. doi: 10.1242/dev.00573. [DOI] [PubMed] [Google Scholar]
- 4.Cambray N, Wilson V. Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development. 2002;129:4855–4866. doi: 10.1242/dev.129.20.4855. [DOI] [PubMed] [Google Scholar]
- 5.Dias, A. et al. A Tgfbr1/Snai1-dependent developmental module at the core of vertebrate axial elongation. Elife9, e56615 (2020). [DOI] [PMC free article] [PubMed]
- 6.Gomez C, et al. Control of segment number in vertebrate embryos. Nature. 2008;454:335–339. doi: 10.1038/nature07020. [DOI] [PubMed] [Google Scholar]
- 7.Denans, N., Iimura, T. & Pourquié, O. Hox genes control vertebrate body elongation by collinear Wnt repression. Elife4, e04379 (2015). [DOI] [PMC free article] [PubMed]
- 8.Wymeersch FJ, et al. Position-dependent plasticity of distinct progenitor types in the primitive streak. Elife. 2016;5:e10042. doi: 10.7554/eLife.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kieny M, Mauger A, Sengel P. Early regionalization of somitic mesoderm as studied by the development of axial skeleton of the chick embryo. Dev. Biol. 1972;28:142–161. doi: 10.1016/0012-1606(72)90133-9. [DOI] [PubMed] [Google Scholar]
- 10.Carapuço M, Nóvoa A, Bobola N, Mallo M. Hox genes specify vertebral types in the presomitic mesoderm. Genes Dev. 2005;19:2116–2121. doi: 10.1101/gad.338705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wellik DM. Hox patterning of the vertebrate axial skeleton. Dev. Dyn. 2007;236:2454–2463. doi: 10.1002/dvdy.21286. [DOI] [PubMed] [Google Scholar]
- 12.Burke AC, Nelson CE, Morgan BA, Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–346. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- 13.Soshnikova N, Duboule D. Epigenetic temporal control of mouse Hox genes in vivo. Science. 2009;324:1320–1323. doi: 10.1126/science.1171468. [DOI] [PubMed] [Google Scholar]
- 14.Polly, P. D., Head, J. J. & Cohn, M. J. in Beyond Heterochrony: The Evolution of Development (ed. Zelditch, M. L.) 307–335 (Wiley-Liss, Inc, 2001).
- 15.Narita Y, Kuratani S. Evolution of the vertebral formulae in mammals: a perspective on developmental constraints. J. Exp. Zool. B: Mol. Dev. Evol. 2005;304:91–106. doi: 10.1002/jez.b.21029. [DOI] [PubMed] [Google Scholar]
- 16.Ward AB, Brainerd EL. Evolution of axial patterning in elongate fishes. Biol. J. Linn. Soc. Lond. 2007;90:97–116. doi: 10.1111/j.1095-8312.2007.00714.x. [DOI] [Google Scholar]
- 17.Buchholtz EA. Modular evolution of the Cetacean vertebral column. Evol. Dev. 2007;9:278–289. doi: 10.1111/j.1525-142X.2007.00160.x. [DOI] [PubMed] [Google Scholar]
- 18.Guillot, C., Michaut, A., Rabe, B. & Pourquié, O. Dynamics of primitive streak regression controls the fate of neuro-mesodermal progenitors in the chicken embryo. Elife10, e64819 (2021). [DOI] [PMC free article] [PubMed]
- 19.McGrew MJ, et al. Localised axial progenitor cell populations in the avian tail bud are not committed to a posterior Hox identity. Development. 2008;135:2289–2299. doi: 10.1242/dev.022020. [DOI] [PubMed] [Google Scholar]
- 20.Gouti M, et al. A gene regulatory network balances neural and mesoderm specification during vertebrate trunk development. Dev. Cell. 2017;41:243–261.e7. doi: 10.1016/j.devcel.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wymeersch, F. J. et al. Transcriptionally dynamic progenitor populations organised around a stable niche drive axial patterning. Development146, dev168161 (2019). [DOI] [PMC free article] [PubMed]
- 22.McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat. Genet. 1999;22:260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- 23.Lee YJ, et al. Growth differentiation factor 11 signaling controls retinoic acid activity for axial vertebral development. Dev. Biol. 2010;347:195–203. doi: 10.1016/j.ydbio.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh SP, et al. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 2002;16:2749–2754. doi: 10.1101/gad.1021802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jurberg AD, Aires R, Varela-Lasheras I, Nóvoa A, Mallo M. Switching axial progenitors from producing trunk to tail tissues in vertebrate embryos. Dev. Cell. 2013;25:451–462. doi: 10.1016/j.devcel.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Aires R, et al. Oct4 is a key regulator of vertebrate trunk length diversity. Dev. Cell. 2016;38:262–274. doi: 10.1016/j.devcel.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Robinton DA, et al. The Lin28/let-7 pathway regulates the mammalian caudal body axis elongation program. Dev. Cell. 2019;48:396–405.e3. doi: 10.1016/j.devcel.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Aires R, et al. Tail bud progenitor activity relies on a network comprising Gdf11, Lin28, and Hox13 genes. Dev. Cell. 2019;48:383–395.e8. doi: 10.1016/j.devcel.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Miyazawa H, Muramatsu Y, Makino H, Yamaguchi Y, Miura M. Temporal regulation of Lin28a during mammalian neurulation contributes to neonatal body size control. Dev. Dyn. 2019;248:931–941. doi: 10.1002/dvdy.87. [DOI] [PubMed] [Google Scholar]
- 30.Chung AC, et al. Loss of orphan receptor germ cell nuclear factor function results in ectopic development of the tail bud and a novel posterior truncation. Mol. Cell. Biol. 2001;21:663–677. doi: 10.1128/MCB.21.2.663-677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Süsens U, Aguiluz JB, Evans RM, Borgmeyer U. The germ cell nuclear factor mGCNF is expressed in the developing nervous system. Dev. Neurosci. 1997;19:410–420. doi: 10.1159/000111238. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, Ren J, Zhang Z, Huang L. Genetic evidence for the introgression of Western NR6A1 haplotype into Chinese Licha breed associated with increased vertebral number. Anim. Genet. 2009;40:247–250. doi: 10.1111/j.1365-2052.2008.01820.x. [DOI] [PubMed] [Google Scholar]
- 33.Rubin C-J, et al. Strong signatures of selection in the domestic pig genome. Proc. Natl Acad. Sci. USA. 2012;109:19529–19536. doi: 10.1073/pnas.1217149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgos C, et al. Allelic frequencies of NR6A1 and VRTN, two genes that affect vertebrae number in diverse pig breeds: a study of the effects of the VRTN insertion on phenotypic traits of a Duroc×Landrace–Large White cross. Meat Sci. 2015;100:150–155. doi: 10.1016/j.meatsci.2014.09.143. [DOI] [PubMed] [Google Scholar]
- 35.Mikawa S, et al. Fine mapping of a swine quantitative trait locus for number of vertebrae and analysis of an orphan nuclear receptor, germ cell nuclear factor (NR6A1) Genome Res. 2007;17:586–593. doi: 10.1101/gr.6085507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King JWB, Roberts RC. Carcass length in the bacon pig; its association with vertebrae numbers and prediction from radiographs of the young pig. Anim. Sci. 1960;2:59–65. [Google Scholar]
- 37.Zhang X, et al. Association analysis of polymorphism in the NR6A1 gene with the lumbar vertebrae number traits in sheep. Genes Genomics. 2019;41:1165–1171. doi: 10.1007/s13258-019-00843-5. [DOI] [PubMed] [Google Scholar]
- 38.Wong SFL, et al. Independent regulation of vertebral number and vertebral identity by microRNA-196 paralogs. Proc. Natl Acad. Sci. USA. 2015;112:E4884–E4893. doi: 10.1073/pnas.1512655112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurtan AM, et al. Let-7 represses Nr6a1 and a mid-gestation developmental program in adult fibroblasts. Genes Dev. 2013;27:941–954. doi: 10.1101/gad.215376.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal, V., Bell, G. W., Nam, J.-W. & Bartel, D. P. Predicting effective microRNA target sites in mammalian mRNAs. Elife4, e05005 (2015). [DOI] [PMC free article] [PubMed]
- 41.Pijuan-Sala B, et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature. 2019;566:490–495. doi: 10.1038/s41586-019-0933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung AC-K, Xu X, Niederreither KA, Cooney AJ. Loss of orphan nuclear receptor GCNF function disrupts forebrain development and the establishment of the isthmic organizer. Dev. Biol. 2006;293:13–24. doi: 10.1016/j.ydbio.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Fuhrmann G, et al. Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Dev. Cell. 2001;1:377–387. doi: 10.1016/S1534-5807(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 44.Hauswirth GM, et al. Breaking constraint of mammalian axial formulae. Nat. Commun. 2022;13:243. doi: 10.1038/s41467-021-27335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lan Z-J, Chung AC-K, Xu X, DeMayo FJ, Cooney AJ. The embryonic function of germ cell nuclear factor is dependent on the DNA binding domain. J. Biol. Chem. 2002;277:50660–50667. doi: 10.1074/jbc.M209586200. [DOI] [PubMed] [Google Scholar]
- 46.Lan Z-J, Xu X, Cooney AJ. Generation of a germ cell nuclear factor conditional allele in mice. Genesis. 2003;37:172–179. doi: 10.1002/gene.10239. [DOI] [PubMed] [Google Scholar]
- 47.Sladitschek HL, Neveu PA. MXS-chaining: a highly efficient cloning platform for imaging and flow cytometry approaches in mammalian systems. PLoS ONE. 2015;10:e0124958. doi: 10.1371/journal.pone.0124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson MJ, et al. TCreERT2, a transgenic mouse line for temporal control of Cre-mediated recombination in lineages emerging from the primitive streak or tail bud. PLoS ONE. 2013;8:e62479. doi: 10.1371/journal.pone.0062479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wellik DM, Capecchi MR. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301:363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- 50.Young T, et al. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev. Cell. 2009;17:516–526. doi: 10.1016/j.devcel.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 51.McIntyre DC, et al. Hox patterning of the vertebrate rib cage. Development. 2007;134:2981–2989. doi: 10.1242/dev.007567. [DOI] [PubMed] [Google Scholar]
- 52.Zeltser L, Desplan C, Heintz N. Hoxb-13: a new Hox gene in a distant region of the HOXB cluster maintains colinearity. Development. 1996;122:2475–2484. doi: 10.1242/dev.122.8.2475. [DOI] [PubMed] [Google Scholar]
- 53.Benahmed F, et al. Multiple regulatory regions control the complex expression pattern of the mouse Cdx2 homeobox gene. Gastroenterology. 2008;135:1238–1247. doi: 10.1053/j.gastro.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 54.van den Akker E, et al. Axial skeletal patterning in mice lacking all paralogous group 8 Hox genes. Development. 2001;128:1911–1921. doi: 10.1242/dev.128.10.1911. [DOI] [PubMed] [Google Scholar]
- 55.Kherdjemil Y, et al. Evolution of Hoxa11 regulation in vertebrates is linked to the pentadactyl state. Nature. 2016;539:89–92. doi: 10.1038/nature19813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gouti M, et al. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 2014;12:e1001937. doi: 10.1371/journal.pbio.1001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neijts R, et al. Polarized regulatory landscape and Wnt responsiveness underlie Hox activation in embryos. Genes Dev. 2016;30:1937–1942. doi: 10.1101/gad.285767.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 59.Arbab, M. & Sherwood, R. I. Self-Cloning CRISPR. Curre. Protoc. Stem Cell Biol.38, 5B.5.1–5B.5.16 (2016). [DOI] [PMC free article] [PubMed]
- 60.Arbab, M., Srinivasan, S., Hashimoto, T., Geijsen, N. & Sherwood, R. I. Cloning-free CRISPR. Stem Cell Rep.5, 908–917 (2015). [DOI] [PMC free article] [PubMed]
- 61.Lippmann, E. S., Williams, C. E., Ruhl, D. A. & Estevez-Silva, M. C. Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Rep.4, 632–644 (2015). [DOI] [PMC free article] [PubMed]
- 62.Jansz N, et al. Smchd1 regulates long-range chromatin interactions on the inactive X chromosome and at Hox clusters. Nat. Struct. Mol. Biol. 2018;25:766–777. doi: 10.1038/s41594-018-0111-z. [DOI] [PubMed] [Google Scholar]
- 63.Olivera-Martinez I, Harada H, Halley PA, Storey KG. Loss of FGF-dependent mesoderm identity and rise of endogenous retinoid signalling determine cessation of body axis elongation. PLoS Biol. 2012;10:e1001415. doi: 10.1371/journal.pbio.1001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olivera-Martinez I, Storey KG. Wnt signals provide a timing mechanism for the FGF-retinoid differentiation switch during vertebrate body axis extension. Development. 2007;134:2125–2135. doi: 10.1242/dev.000216. [DOI] [PubMed] [Google Scholar]
- 65.Martin BL, Kimelman D. Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev. Cell. 2012;22:223–232. doi: 10.1016/j.devcel.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takemoto T, et al. Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature. 2011;470:394–398. doi: 10.1038/nature09729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koch F, et al. Antagonistic activities of Sox2 and Brachyury control the fate choice of neuro-mesodermal progenitors. Dev. Cell. 2017;42:514–526.e7. doi: 10.1016/j.devcel.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 68.Chapman DL, Papaioannou VE. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature. 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- 69.Nowotschin S, Ferrer-Vaquer A, Concepcion D, Papaioannou VE, Hadjantonakis A-K. Interaction of Wnt3a, Msgn1 and Tbx6 in neural versus paraxial mesoderm lineage commitment and paraxial mesoderm differentiation in the mouse embryo. Dev. Biol. 2012;367:1–14. doi: 10.1016/j.ydbio.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akamatsu W, DeVeale B, Okano H, Cooney AJ, van der Kooy D. Suppression of Oct4 by germ cell nuclear factor restricts pluripotency and promotes neural stem cell development in the early neural lineage. J. Neurosci. 2009;29:2113–2124. doi: 10.1523/JNEUROSCI.4527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gu P, et al. Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol. Cell. Biol. 2005;25:8507–8519. doi: 10.1128/MCB.25.19.8507-8519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 72.Barreto G, Borgmeyer U, Dreyer C. The germ cell nuclear factor is required for retinoic acid signaling during Xenopus development. Mech. Dev. 2003;120:415–428. doi: 10.1016/S0925-4773(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 73.Barreto G, Reintsch W, Kaufmann C, Dreyer C. The function of Xenopus germ cell nuclear factor (xGCNF) in morphogenetic movements during neurulation. Dev. Biol. 2003;257:329–342. doi: 10.1016/S0012-1606(03)00109-X. [DOI] [PubMed] [Google Scholar]
- 74.Mohamed OA, Clarke HJ, Dufort D. Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev. Dyn. 2004;231:416–424. doi: 10.1002/dvdy.20135. [DOI] [PubMed] [Google Scholar]
- 75.Kloosterman WP, Wienholds E, de Bruijn E, Kauppinen S, Plasterk RHA. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat. Methods. 2006;3:27–29. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- 76.Mansfield JH, et al. MicroRNA-responsive ‘sensor’ transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression. Nat. Genet. 2004;36:1079–1083. doi: 10.1038/ng1421. [DOI] [PubMed] [Google Scholar]
- 77.Mullen EM, Gu P, Cooney AJ. Nuclear receptors in regulation of mouse ES cell pluripotency and differentiation. PPAR Res. 2007;2007:61563. doi: 10.1155/2007/61563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu P, Xu X, Le Menuet D, Chung AC-K, Cooney AJ. Differential recruitment of methyl CpG-binding domain factors and DNA methyltransferases by the orphan receptor germ cell nuclear factor initiates the repression and silencing of Oct4. Stem Cells. 2011;29:1041–1051. doi: 10.1002/stem.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato N, Kondo M, Arai K-I. The orphan nuclear receptor GCNF recruits DNA methyltransferase for Oct-3/4 silencing. Biochem. Biophys. Res. Commun. 2006;344:845–851. doi: 10.1016/j.bbrc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Yan, Z. & Jetten, A. M. Characterization of the repressor function of the nuclear orphan receptor RTR/GCNF. J. Biol. Chem. 275, 35077–35085 (2000). [DOI] [PubMed]
- 81.Cooney AJ, Hummelke GC, Herman T, Chen F, Jackson KJ. Germ cell nuclear factor is a response element-specific repressor of transcription. Biochem. Biophys. Res. Commun. 1998;245:94–100. doi: 10.1006/bbrc.1998.8391. [DOI] [PubMed] [Google Scholar]
- 82.Yan Z, Kim Y-S, Jetten AM. RAP80, a novel nuclear protein that interacts with the retinoid-related testis-associated receptor. J. Biol. Chem. 2002;277:32379–32388. doi: 10.1074/jbc.M203475200. [DOI] [PubMed] [Google Scholar]
- 83.Zákány J, Kmita M, Alarcon P, de la Pompa JL, Duboule D. Localized and transient transcription of Hox genes suggests a link between patterning and the segmentation clock. Cell. 2001;106:207–217. doi: 10.1016/S0092-8674(01)00436-6. [DOI] [PubMed] [Google Scholar]
- 84.Casaca A, Nóvoa A, Mallo M. Hoxb6 can interfere with somitogenesis in the posterior embryo through a mechanism independent of its rib-promoting activity. Development. 2016;143:437–448. doi: 10.1242/dev.133074. [DOI] [PubMed] [Google Scholar]
- 85.Zhang N, Gridley T. Defects in somite formation in lunatic fringe-deficient mice. Nature. 1998;394:374–377. doi: 10.1038/28625. [DOI] [PubMed] [Google Scholar]
- 86.Evrard YA, Lun Y, Aulehla A, Gan L, Johnson R. L. lunatic fringe is an essential mediator of somite segmentation and patterning. Nature. 1998;394:377–381. doi: 10.1038/28632. [DOI] [PubMed] [Google Scholar]
- 87.Morales AV, Yasuda Y, Ish-Horowicz D. Periodic Lunatic fringe expression is controlled during segmentation by a cyclic transcriptional enhancer responsive to notch signaling. Dev. Cell. 2002;3:63–74. doi: 10.1016/S1534-5807(02)00211-3. [DOI] [PubMed] [Google Scholar]
- 88.Cole SE, Levorse JM, Tilghman SM, Vogt TF. Clock regulatory elements control cyclic expression of Lunatic fringe during somitogenesis. Dev. Cell. 2002;3:75–84. doi: 10.1016/S1534-5807(02)00212-5. [DOI] [PubMed] [Google Scholar]
- 89.Williams DR, Shifley ET, Lather JD, Cole SE. Posterior skeletal development and the segmentation clock period are sensitive to Lfng dosage during somitogenesis. Dev. Biol. 2014;388:159–169. doi: 10.1016/j.ydbio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Stauber M, Sachidanandan C, Morgenstern C, Ish-Horowicz D. Differential axial requirements for lunatic fringe and Hes7 transcription during mouse somitogenesis. PLoS One. 2009;4:e7996. doi: 10.1371/journal.pone.0007996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shifley ET, et al. Oscillatory lunatic fringe activity is crucial for segmentation of the anterior but not posterior skeleton. Development. 2008;135:899–908. doi: 10.1242/dev.006742. [DOI] [PubMed] [Google Scholar]
- 92.Moreau C, et al. Timed collinear activation of hox genes during gastrulation controls the avian forelimb position. Curr. Biol. 2019;29:35–50.e4. doi: 10.1016/j.cub.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perantoni AO, et al. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132:3859–3871. doi: 10.1242/dev.01945. [DOI] [PubMed] [Google Scholar]
- 94.Concordet J-P, Haeussler M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018;46:W242–W245. doi: 10.1093/nar/gky354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McGlinn E, Holzman MA, Mansfield JH. Detection of gene and protein expression in mouse embryos and tissue sections. Methods Mol. Biol. 2019;1920:183–218. doi: 10.1007/978-1-4939-9009-2_12. [DOI] [PubMed] [Google Scholar]
- 96.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 97.McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- 98.Irizarry RA, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 99.Smyth, G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article3 (2004). [DOI] [PubMed]
- 100.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 101.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–W311. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A. Moving beyond P values: data analysis with estimation graphics. Nat. Methods. 2019;16:565–566. doi: 10.1038/s41592-019-0470-3. [DOI] [PubMed] [Google Scholar]
- 104.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support this study are available from the corresponding author upon request. The raw microarray data generated in this study have been deposited in the Gene Expression Omnibus (GEO) and are publicly available using the accession number GSE166458 (CEL datasets). Original data relating to the microarray experiment can also be accessed from the Stowers Original Data Repository LIBPB-1647. The raw in vitro and in vivo RNAseq data generated in this study have been deposited in the GEO and are publicly available using the accession number GSE180427. This includes raw data for graphs presented in this study. Source data are provided with this paper.