Abstract
Malaria merozoites require the presence of specific surface receptors on the red blood cell for invasion. Plasmodium vivax, requires the Duffy blood group antigen as an obligate receptor for invasion. The parasite Duffy binding protein (DBP) is the ligand involved in this process, making the DBP a potential vaccine candidate. A preliminary objective was to study whether people exposed to vivax malaria acquire antibodies that have the ability to block erythrocyte cytoadherence to the PvDBP. In comparison, we studied the immunogenicity of various recombinant DBP vaccines and investigated their potential to induct antifunctional antibodies. In order to do so, recombinant proteins to different regions of the putative ectodomain of the DBP and a DNA vaccine were used to immunize laboratory animals. An in vitro cytoadherence assay was used to investigate the presence of antifunctional antibodies in plasmas from people naturally exposed to vivax malaria, as well as in antisera obtained by animal vaccination. Our results showed that human plasma from populations naturally exposed to vivax malaria, as well as antisera obtained by vaccination using recombinant proteins, a DNA vaccine, and a synthetic peptide to DBP, inhibited in vitro binding of human erythrocytes to the DBP ligand domain (DBPII) in correlation to their previously measured antibody titer. Our results provide further evidence for the vaccine potential of this essential parasite adhesion molecule.
Invasion of an erythrocyte by the merozoite of Plasmodium vivax requires specific interactions between its surface ligands and reticulocyte receptors (10). This cascade of events represents potential targets for reducing or eliminating the blood stages of malaria parasites and their pathological outcomes through the use of drugs or vaccine blockade strategies (16).
The Duffy binding protein (DBP) of P. vivax is required for junction formation during merozoite invasion. Junction formation is a critical event of the invasion process, committing the parasite to invade a particular host cell (2). This role makes DBP, as well as other micronemal proteins, important determinants in host cell specificity. The DBP is a type I membrane protein belonging to a family of erythrocyte binding proteins having a conserved molecular structure characterized by two cysteine-rich domains. The amino cysteine-rich domain, also known as region II, was directly identified as the domain binding to the Duffy blood group antigen (or Duffy antigen receptor for chemokines) present on human erythrocytes (3). This study used an in vitro erythrocyte-binding assay in which the putative ligand domain of the DBP was expressed on the surface of cultivated mammalian cells. This assay proved a valuable tool for studying the specific interaction between other parasite ligands and erythrocyte receptors (12, 17, 18). The ability to study the specific receptor-ligand interaction in vitro is particularly valuable for P. vivax adhesion molecules since it obviates the use of primate infections otherwise necessary for this type of analysis. A hypervariable portion of DBP region II was identified and foreseen as a potential target for immune selection (20). Previous studies demonstrated that a recombinant protein corresponding to the P. vivax PvDBP regions II to IV (PvDBPII–IV) was immunogenic in laboratory animals and that antibodies against this recombinant protein reacted with P. vivax and P. knowlesi in an immunofluorescence assay (IFA) (9). Serologic assays with this recombinant protein revealed the presence of naturally occurring antibodies in populations exposed to vivax malaria in areas of Papua New Guinea (PNG) where the disease is highly endemic (9), as well as in regions of Colombia where the organism is less common (14). In these studies, the enzyme-linked immunosorbent assay (ELISA) antibody titer showed that the average immunological response increased with age, demonstrating a boosting effect which was likely due to repeated exposures to infection. Nevertheless, a significant proportion of these populations also exhibited either a low or an absent response to DBP, grouping individuals in the population into three categories as high, low, or nonresponders.
The purpose of the present study was to determine the inhibitory efficacy of anti-DBP antibodies using the in vitro binding assay. We initially investigated the potential for naturally acquired antibodies against PvDBP to inhibit the binding activity of the PvDBPII to erythrocytes. Different vaccine constructs (recombinant proteins, DNA, and a peptide) were then evaluated for their ability to elicit antibodies that could block binding of erythrocytes to PvDBPII expressed in vitro.
In this study, we provide evidence that naturally acquired and vaccine-elicited antibodies can block in vitro the specific interaction between the PvDBP ligand domain and its receptor present on the erythrocyte surface.
MATERIALS AND METHODS
Recombinant proteins of the PvDBP.
Antisera to PvDBP were produced by vaccination with three different recombinant proteins, a DNA vaccine and a synthetic peptide (Fig. 1).
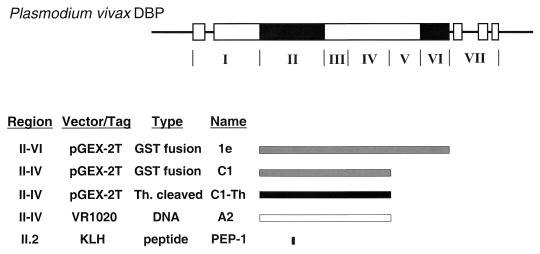
FIG. 1.
Vaccine constructs and their corresponding regions on the P. vivax DBP Sal-1 (8). The exons of the PvDBP are boxed, and the black areas identify the cysteine-rich regions. The structurally distinct regions of the EBP family products are shown under the diagram in roman numerals (1). Region II is the principal ligand domain or erythrocyte binding domain (3). The putative ectodomain of the PvDBP, regions II to VI, was expressed as a GST fusion protein designated 1e (9). A truncated version (C1) was derived from the full-length construct by excision of an EcoRI fragment encoding regions V and VI (9). The C1 product, regions II to IV, was purified as a soluble GST fusion protein and was used as such for vaccination or cleaved with thrombin (Th) and purified separate from the GST component (C1-Th). The same portion of PvDBP, regions II to IV, was inserted in the DNA vector VR1020. This construct (A2) was used for DNA vaccination. Peptide PEP-1 corresponding to a hypervariable region of the PvDBP domain II.2 was synthesized and conjugated to maleimide-activated KLH for use as a vaccine.
(i) Recombinant protein rPvDBPII–VI (1e).
The putative full-length ectodomain of the PvDBP Sal-1 (M37514) (8), from amino acids 132 to 1005 (regions II to VI), was inserted in frame with glutathione S-transferase (GST) in the plasmid expression vector pGEX-2T (19), as described earlier (9). This fragment is thought to correspond to the major portion of the soluble form of the parasite-derived erythrocyte binding protein (21). This full-length construct expressed a GST-PvDBPII–VI recombinant protein designated 1e (Fig. 1).
(ii) Recombinant protein rPvDBPII–IV (C1).
A truncated recombinant protein, from amino acids 132 to 771 (regions II to IV), was derived directly from the expression plasmid containing the full-length ectodomain (9). This truncated expression construct expressed a GST-PvDBPII–IV recombinant protein designated C1 (Fig. 1).
(iii) Expression and purification.
The GST-PvDBP recombinant proteins were expressed using only minor modifications of the recommended protocol (19) as described earlier (9). The standard method to isolate GST fusion proteins was followed. The thrombin-cleaved recombinant protein DBPII–IV was purified separate from the GST component and is referred to as C1-Th (Fig. 1).
DNA vaccine rDBPII–IV (A2).
Regions II to IV of PvDBP corresponding to C1 (Fig. 1) were cloned into a DNA VR1020 vector (Vical, Inc.) obtained from W. Rogers and R. Hedstrom (Naval Medical Research Center, Rockville, Md.). The plasmid contains a polyadenylation termination sequence downstream of the multiple-cloning site (MCS), a prokaryotic origin of replication, and the kanamycin resistance gene as a selectable marker. A strong eukaryotic cytomegalovirus (CMV) promoter drives expression of the gene of choice. Plasmid VR1020 contains a tissue plasminogen activator signal sequence upstream of the MCS. Plasmid pGEX-1e was cut with EcoRI, and free ends were filled in using the Klenow fragment (Gibco-BRL). The C1-equivalent PvDBPII–IV was removed from this linearized construct using restriction enzyme BamHI and ligated into VR1020 previously linearized with BglII, treated with the Klenow fragment and cut with BamHI to ensure directional cloning. This DNA vector construct was referred to as A2 (Fig. 1).
Synthetic peptide (PEP-1).
Synthetic peptide (FGTDEKAQQRRKQWC*) corresponding to a hypervariable region of the PvDBP domain II.2 (SalI: amino acids 336 to 349) was prepared at the Bioscience Core Facility at the University of Notre Dame. A cysteine residue (indicated by an asterisk) was added at the carboxyl terminus of the peptide hapten to allow conjugation to maleimide-activated keyhole limpet hemocyanin (KLH) (Pierce, Inc). Conjugation was performed according to the manufacturer's recommendations. Briefly, 2 mg of peptide was dissolved in 500 μl of phosphate-buffered saline (PBS; pH 7.4) and incubated for 2 h with solution containing 2 mg of maleimide-activated KLH. The conjugate was dialyzed overnight in pH 7.4 to remove EDTA and stored frozen at −20°C until use. This synthetic peptide is referred to as PEP-1 (Fig. 1). A second unrelated synthetic peptide, PfERD2 (C-YFALAKWYGKKLVLPFNGEV), conjugated to KLH (7) was prepared as described earlier (15) and referred to as PEPc (peptide control).
Animals.
New Zealand White rabbits were bred and maintained at the Freiman Animal Facility of the University of Notre Dame. A group of six congenic strains of mice (six individuals per strain) on a B10 background with different H-2 phenotypes were used: B10, B10-BR, B10-D2, B10-M, B10-PL, and B10-S. R. Schwartz (National Institutes of Health) provided these mice. Another group of four congenic mice strains (five or six individuals per strain), one of which (indicated by an asterisk) lacks the ability to respond to endotoxin or lipopolysaccharide (LPS), were also used: C3H/He/OuJ, C3He/B/FeJ, C3H/HeSnJ, and C3H/HeJ*. Mice of both sexes were purchased from the Jackson Laboratory (Bar Harbor, Maine).
Immunizations and antisera.
Rabbits were immunized with purified recombinant proteins, DNA vaccine or synthetic peptide according to approved protocols. For each GST fusion protein, 100 μg in solution were emulsified with Freund's complete adjuvant for the first injection administered subcutaneously. To boost the antibody response, the antigen was emulsified in incomplete Freund's adjuvant, and rabbits were vaccinated subcutaneously at 2-week intervals: five times for the C1 fusion protein and six times for the 1e fusion protein. Because the full-length ectodomain 1e recombinant protein remained adsorbed to the glutathione beads, the immunization protocol was modified so that the animal was injected with an emulsion of a suspension containing antigen-adsorbed beads. The thrombin-cleaved C1 recombinant protein (C1-Th) was injected as mentioned above at 125 μg per injection. Ten days after the last immunization, sera were collected from clotted blood, decomplemented, aliquoted, and stored at −20 or −80°C. Anti-GST serum was prepared against a nonfusion GST protein produced by expression of nonrecombinant pGEX-2T.
DNA vaccine VR1020-A2 was injected five times at 4-week intervals. Each time a total of 100 μg was injected at four intradermal and two intramuscular sites. Antisera were collected and stored as described above.
Each synthetic peptide-KLH conjugate (2.5 mg) was emulsified with Freund's complete adjuvant for the first injection administered subcutaneously. To boost the antibody response, the antigen was emulsified in incomplete Freund's adjuvant, and rabbits were vaccinated subcutaneously at 2-week intervals. Antisera were collected and stored as described above.
All congenic strains of mice were immunized with the purified C1-Th. The antigen was emulsified with Freund's complete adjuvant for the first injection and injected subcutaneously. To boost the antibody response, the antigen was emulsified in incomplete Freund's adjuvant, and mice were vaccinated subcutaneously at 2-week intervals. Sera were collected (100 μl, retro-orbitally) for each mouse prior to the first injection (a prebleed sample was used as control) and 1 day prior subsequent injections. Mice were bled out 14 days after last injection.
Human plasma.
Human plasma samples were collected in microhematocrit tubes from P. vivax-exposed residents of the Madang or East Sepik provinces, PNG, as described earlier (9, 11). The total volume of these plasma samples was generally 50 to 200 μl; therefore, the anti-DBP cytoadherence inhibition had to rely on pooled samples to obtain sufficient volumes for the analysis method. Their specific PvDBP antibody reactivities were determined by ELISA against the purified C1-Th, as described below. Human plasma samples were pooled in three different groups according to their antibody titer and designated as high, low, and nonresponders (PNG+, PNG+/−, and PNG−, respectively). Immune sera, obtained from North American residents with P. vivax infections, were pooled and designated NA-Pv. Negative control plasma samples were collected from North American volunteers with no previous vivax malaria exposure (nonexposed North American [NENA]).
Serologic assays (ELISA).
ELISAs were performed, with a few modifications, as previously described (9). Purified C1-Th diluted to 2 μg/ml in PBS was adsorbed overnight at 4°C onto microtiter ELISA plates (Immunolon I; Dynatech), rinsed in wash buffer (0.2% Tween 20 in PBS), incubated 15 min with block buffer (1% bovine serum albumin [BSA] in PBS), rinsed again in wash buffer, and used immediately or else stored sealed at 4°C until use. The antigen-coated wells were incubated for 60 min with triplicate serum samples diluted 1:400 in block buffer. Plates were rinsed in wash buffer, incubated 60 min with the appropriate species-specific secondary antibody (e.g., goat anti-human immunoglobulin G [IgG; H+L]-alkaline phosphatase conjugate) diluted to 0.5 μg/ml in block buffer, and rinsed in wash buffer, and p-nitrophenolphosphate was added to each well. The anti-PvDBP antibody activity was detected by recording the absorbance at 405 nm, the absorbance of blank wells (with substrate alone) was subtracted from all values. The average absorbance was calculated for each serum sample. Significantly different A405 values at each time point were assessed by multivariate analysis of variance and Tukey's high-significance-degree analysis using Systat 5.2 for Macintosh software package.
COS-7 cell surface expression of DBPII and erythrocyte binding-inhibition assay. (i) COS-7 cells transfected with pRE4-DBPII.
The pRE4 construct containing the P. vivax DBP region II was described earlier (3). The vector contains the herpes simplex virus glycoprotein D1 (HSVgD1), a type I integral membrane protein open reading frame (ORF). A Rous sarcoma virus long terminal repeat drives expression of gD1. The HSVgD1 ORF contains unique ApaI and PvuII restriction sites where PvDBPII was cloned as an HSVgD1 chimera.
Recombinant plasmids were purified using an endotoxin-free plasmid purification kit (Qiagen). COS-7 cells were plated into six-well culture plates and then transfected with the pRE4-DBPII DNA (1 μg per well) using Lipofectamine (Gibco-BRL) in Dulbecco modified Eagle medium (DMEM; Sigma) without serum. After 24 h, transfection medium was replaced by DMEM with 10% fetal bovine serum, and cells were incubated for 24 h. Transfected COS-7 cells were incubated at 37°C for 1 h with 1 ml of the different antisera diluted in DMEM without serum. After this treatment the transfected cells were incubated for 2 h at room temperature with human erythrocytes (1% final suspension, previously washed in RPMI 1640). Cells were washed three times with PBS to remove nonadherent erythrocytes. Counting rosettes in 30 fields at a magnification of ×200 was used to score binding. Rosettes were counted as positive when adherent erythrocyte covered more than 50% of the cell surface. Experiments for each serum were done in three replicates and were repeated three times unless otherwise stated. All antisera were first tested at a 1:10 dilution to assess their inhibition activity. Serial dilutions were then tested for each antiserum when positive for inhibition at the 1:10 dilution level. The binding for each antiserum dilution was compared to the binding of transfected COS-7 cells incubated with a 1:10 dilution preimmune serum. Results were expressed as the relative percent binding (preimmune serum = 100% binding). A 1:10 dilution anti-PEPc rabbit serum was used as a negative control for antisera to the DBP peptide PEP-1. A 1:10 dilution anti-GST serum was also used as a negative control for antisera to recombinant GST fusion proteins and as a common reference for all assays.
Synthetic peptide PEP-1 (stock solution, 100 μM concentration in PBS) was also tested for its ability to inhibit erythrocyte adhesion to DBP. Transfected COS-7 cells were incubated at 37°C for 1 h with 1 ml of a PEP-1 solution at a final concentration of 1 μM in DMEM without serum.
(ii) COS-7 cells transfected with pEGFP-DBPII.
Plasmid pEGFP-N1 (Clontech) encodes a red-shifted variant of the wild-type green fluorescent protein (GFP) transcribed by the immediate-early promoter of human CMV. This plasmid allows expression of a recombinant fusion protein to the N terminus of the EGFP used as a transfection marker. The HSVgD1-DBPII chimera described above was PCR amplified from the pRE4-DBPII plasmid construct (5′ primer [HindIII, underscored], ATAAAGCTTCAGCGCGAACGACC; 3′ primer [BamHI, underscored], ATAGGATCCTTGTAAAAGAAGGGCTGGTGCGA). The PCR-amplified product was cleaved with the appropriate restriction enzymes and cloned into the HindIII site at the 5′ end and into the BamHI site at the 3′ end of the pEGFP-N1 polylinker. This construct was designated pEGFP-DBPII and was purified using an endotoxin-free plasmid purification kit (Qiagen). Transfections of COS-7 cells were performed as described above. Transfected COS-7 cells were incubated at 37°C for 1 h with various dilutions of the different pooled human plasma in DMEM without serum at room temperature and under agitation (the medium volume was minimized to 750 μl). Transfected cells were then incubated for 2 h at room temperature with human O+ erythrocytes to prevent agglutination (1% final suspension, previously washed in DMEM without serum). Cells were washed three times with PBS to remove nonadherent erythrocytes. Binding was scored as described above. All pooled human plasmas were first tested (three replicates) at a 1:10 dilution to assess their inhibitory activity. Serial dilutions were then tested for each plasma pool when positive for inhibition at the 1:10 dilution level. Binding for each plasma dilution was compared to binding of transfected COS-7 cells incubated with a 1:10 dilution of NENA plasma.Results were expressed as the relative percent binding (NENA = 100% binding).
Fluorescence microscopy.
Mammalian COS-7 cells transfected with the VR1020 construct A2 were visualized by an IFA to assess expression of DBPII–IV. Briefly, COS-7 cells were transfected with 2 μg of the plasmid DNA using Lipofectin (Gibco-BRL) in DMEM without serum. Surface expression was detected using a 1:20 dilution antiserum against A2, obtained as described above, and affinity-purified goat IgG anti-rabbit IgG (FC) conjugated to fluorescein isothiocyanate (FITC) (Kirkegaard & Perry) was used as secondary antibody. Cells were observed unfixed on an MRC-1024 Laser Scanning Confocal Imaging System (Bio-Rad) fitted with a krypton-argon laser.
Surface expression of COS-7 cells transfected with pRE4-DBPII was detected by using monoclonal antibodies 1D3 and DL6 against HSVgD1 epitopes remaining in the expression construct (4).
COS-7 cells transfected with pEGFP-DBPII were observed using an MRC-1024 Laser Scanning Confocal Imaging System (Bio-Rad) fitted with a krypton-argon laser. Pictures were analyzed and manipulated using Adobe Photoshop 5.0 for Macintosh. The original pictures were split into gray and green color channels corresponding to bright-field and fluorescence views, respectively.
RESULTS AND DISCUSSION
Acquired antibodies responses to PvDBP: inhibition properties.
The objective in developing the PvDBP as a vaccine against blood-stage malaria is to elicit an antibody response that blocks the adhesion of this parasite molecule to human erythrocytes. Residents living in regions where malaria is endemic do acquire an antibody response to the PvDBP from exposure to vivax malaria (9, 14). The profiles of serological reactivities to PvDBP observed in these individuals, even in regions where vivax malaria is highly endemic, varied considerably from a few with high titers to mostly poor and negligible anti-PvDBP reactivity. The consistently significant percentage of apparent nonresponders in all groups suggests that the PvDBP is a poor immunogen. However, the serologic responses of residents in vivax malaria-endemic regions did demonstrate a positive correlation with age, both in average and maximum response levels (9, 14). In the development of the PvDBP as a potential vaccine, it is important to know whether the antibody response elicited by natural exposure to P. vivax can block the PvDBP erythrocyte binding activity. If so, vaccination could potentially enhance a naturally acquired immunity in low responders, and reinfection would serve to boost the immune response.
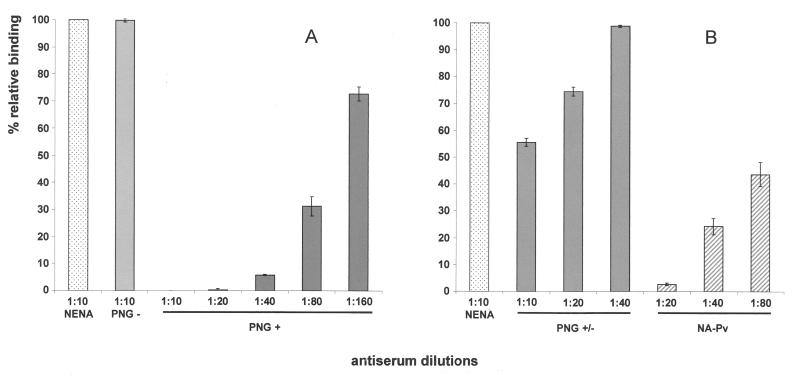
We sought to determine whether the level of serologic response correlated with a protective antifunctional inhibitory response against PvDBP cytoadherence activity. An in vitro binding assay was used to study the antifunctional inhibitory response of human plasmas against PvDBP cytoadherence activity. In this assay COS-7 cells were transfected with a plasmid expressing DBPII as a fusion protein to the N terminus of the GFP. This in vitro cytoadherence assay permits analysis only of the specific interaction of the cysteine-rich DBL ligand domain (DBPII) expressed on the surface of transfected COS-7 cells with the erythrocyte receptor. Binding of erythrocytes to the transfected cells was measured by counting the number of rosettes (Fig. 2A and C ), and transfection was assessed concomitantly by detecting the expression of the GFP in fluorescence microscopy (Fig. 2B and D). We used plasma from residents living in different regions where malaria is endemic in Papua New Guinea who were chronically exposed to P. vivax. The antibody responses of these individuals were previously characterized as high, low, and nonresponders by ELISA (9). Pooled immune plasmas from individuals of PNG blocked binding of the DBPII to human erythrocytes. The efficacy of inhibition positively correlated with the strength of their antibody responses. Pooled plasmas from high responders (PNG+) showed 100% inhibition at the 1:10 dilution level (Fig. 2C) and down to the 1:20 dilution level (Fig. 3A). Significant inhibition (30%) was still observed at 1:160 dilution (Fig. 3A). In contrast, a 1:10 dilution of pooled plasma from low responders (PNG+/−) showed only 50% inhibition (Fig. 3B). No inhibition was observed when using pooled plasma from nonresponders (PNG−) at the same dilution (Fig. 2A and 3A).
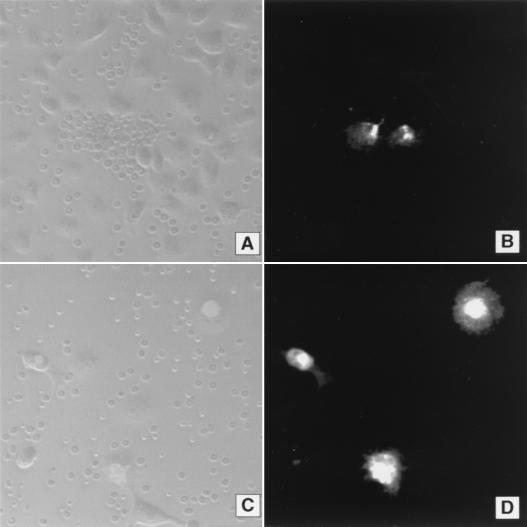
FIG. 2.
Inhibition of erythrocyte binding to P. vivax DBPII expressed in COS-7 cells as an N terminus GFP fusion protein. (A) Rosetting of erythrocytes on COS-7 cells transfected with pEGFP-DBPII construct. Cells were incubated with a 1:10 plasma dilution of PNG residents characterized as anti-PvDBP nonresponders (PNG−). No inhibition of erythrocyte binding to transfected COS-7 cells was observed compared to a 1:10 dilution of pooled plasma from North Americans never exposed to malaria (NENA) used as a negative control (not shown). (B) Cell-associated fluorescence using FITC filter settings on the same field as in panel A and showing expression of the GFP-DBPII fusion protein in the COS-7 cells associated with erythrocyte binding. (C) COS-7 cells were transfected with the pEGFP-DBPII construct and incubated with 1:10 dilution of pooled plasma from PNG residents characterized as anti-PvDBP high responders (PNG+). Complete inhibition of erythrocyte binding to transfected COS-7 cells was observed. (D) Cell-associated fluorescence using FITC filter settings on the same field as in panel C and showing expression of the GFP-DBPII fusion protein.
FIG. 3.
Inhibition of erythrocyte binding to P. vivax DBP region II expressed on the surface of COS-7 cells by naturally acquired antibodies to the PvDBP. COS-7 cells were transfected with pEGFP-DBPII plasmid DNA expressing a GFP-DBPII fusion protein. Cells were subsequently incubated with different human plasma samples at various dilutions prior to the addition of human erythrocytes. Binding was scored after counting rosettes in 30 fields at a magnification of ×200. The percent binding was determined relative to a 1:10 dilution of pooled plasma from NENA samples used as a negative control. (A) Percent relative binding after incubation of COS-7 cells with plasma from PNG residents characterized as nonresponders (PNG−) and high responders (PNG+) to the PvDBP as previously determined by ELISA (9). (B) Percent relative binding after incubation of COS-7 cells with pooled plasma from PNG residents characterized as low responders (PNG+/−) and with pooled plasma from North American residents with P. vivax infections (i.e., NA-Pv). Error bars correspond to ± the standard error (the standard deviation/the square root of the sample size).
Acquisition of a high-level antibody response in PNG residents exposed to vivax malaria was previously found associated with the presence of antibodies reactive to linear epitopes present in reduced and denatured recombinant PvDBP (9). Therefore, for comparison we tested a sample of pooled immune human serum that did not react with linear epitopes of PvDBPII–IV, but still had a positive anti-DBP ELISA activity level comparable to PNG high responders (9). This pooled serum (NA-Pv), prepared from North American residents with P. vivax infections, effectively inhibited the erythrocyte binding activity of PvDBP (Fig. 3B). Serum samples from North American residents without infection with P. vivax or a history of exposure to the parasite (i.e., NENA samples) had no inhibitory effect on erythrocyte adherence to PvDBP. These results indicated that antibodies against the neutralizing epitopes of the PvDBP DBL domain could be acquired from natural exposure to the parasite. These inhibitory antibodies were elicited even without repeated exposure, but the increased antibody response associated with chronic exposure in regions where malaria is endemic resulted in a more effective antibody inhibition of PvDBP cytoadherence.
Inhibition of DBP cytoadherence by antibodies elicited both in limited infections and by chronic exposure suggests that effective inhibition can be manifested in various ways. Observed polymorphism in the DBP ligand domain (20) may play a role in permitting reinfection, even in persons with an inhibitory response against one allelic form of DBP. Our limited plasma sample volumes did not permit adequate analysis of a random screening of individual samples of high responders; however, it would not be surprising if some lacked inhibitory antibodies against the Sal-1 variant of DBP used in our cytoadherence assays. Protective immunity against P. vivax merozoites will likely depend not only on inhibitory responses against multiple variants of the DBP but also against other invasion-related molecules such as AMA-1 variants (5).
Blocking antibodies induced by vaccination with rPvDBPII–IV.
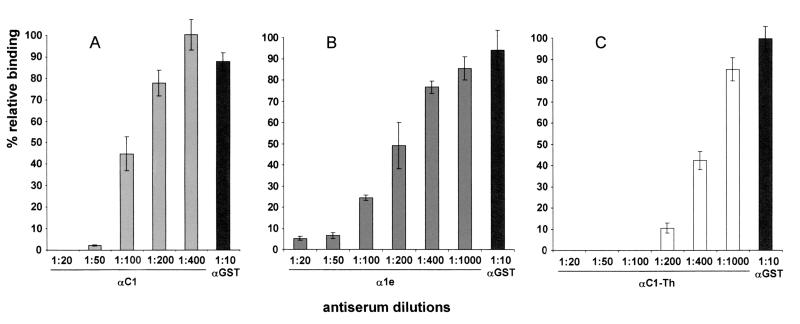
Previous studies demonstrated that PvDBPII–IV expressed as a recombinant GST fusion protein in bacteria induced an antibody response in rabbits that reacted with the P. vivax DBP (9). An in vitro cytoadherence assay described previously (3) was used to test whether these antibodies induced against rPvDBPII–IV could block the erythrocyte binding to the PvDBP ligand domain. Antibodies prepared against recombinant PvDBP GST fusion proteins (C1 and 1e) effectively blocked the DBP-erythrocyte receptor interaction at dilutions of up to 1:50 and 1:20, respectively (Fig. 4A and B). The difference in the inhibitory efficacies of these antisera is reflective of the difference in their antibody titers. All values are given as the percent binding relative to the average cytoadherence counted in the presence of a prevaccination serum from the corresponding animal. A 1:10 dilution of anti-GST immune serum was used in each assay as an additional negative control and a standard reference among assays. To confirm that the inhibitory effect was directed against the recombinant DBL domain, a separate antiserum was prepared against the purified rPvDBPII–IV released from the GST fusion by thrombin cleavage (C1-Th). This antiserum had a very high inhibitory efficacy in blocking the in vitro binding of human erythrocytes to the transfected COS-7 cells expressing the PvDBP DBL domain (Fig. 4C).
FIG. 4.
Inhibition of erythrocyte binding to P. vivax DBP region II expressed on the surface of COS-7 cell antibodies to the PvDBP elicited by vaccination with recombinant proteins. COS-7 cells were transfected with pRE4-DBPII plasmid DNA expressing the DBPII as a HSVgD1 chimera (3). COS-7 cells were subsequently incubated with different rabbit antisera at various dilutions prior to the addition of human erythrocytes. Binding was scored after counting rosettes in 30 fields at a magnification of ×200. The percent binding was determined relative to a 1:10 dilution of preimmune rabbit sera used as a negative control. A 1:10 dilution of anti-GST antiserum (αGST) was also used as a negative control for recombinant GST fusion proteins and as a common reference for all assays. (A) Percent relative binding after incubation of COS-7 cells with different dilutions of rabbit antiserum to the GST-PvDBPII–IV recombinant protein (αC1). (B) Percent relative binding after incubation of COS-7 cells with different dilutions of rabbit antiserum to the GST-PvDBPII–IV recombinant protein (α1e). (C) Percent relative binding after incubation of COS-7 cells with different dilutions of rabbit antiserum to thrombin-cleaved recombinant protein PvDBPII–IV (αC1-Th). Error bars correspond to ± the standard error (the standard deviation/the square root of the sample size).
Immunogenicity of rPvDBPII–IV vaccine.
The significant number of nonresponder PNG residents suggested major histocompatibility complex (MHC) restriction of the immune response. Therefore, we tested the immunogenicity of a recombinant PvDBPII–IV for evidence of MHC-related differences in antibody responses, using mouse strains congenic at the MHC locus with different H-2 phenotypes. Such mouse strains previously showed significant differences in their responses to recombinant PvMSP-119 in ELISAs (13).
Six mouse strains congenic at the MHC locus on a B10 background were vaccinated intraperitoneally with the recombinant protein emulsified in Freund's complete adjuvant. All mouse strains had a positive antibody response to PvDBPII–IV after the first vaccination, but responses by the B10.PL mice were significantly lower than by the other strains (P < 0.05). Boosting with additional vaccinations overcame the low responsiveness of the B10.PL mice and elicited an equivalent antibody response in all strains (Fig. 5). There was no evidence that trace LPS contamination possibly present in the purified rPvDBPII–IV played a role for the antibody response to the recombinant protein when using a congenic mouse strain lacking the ability to respond to LPS (data not shown).
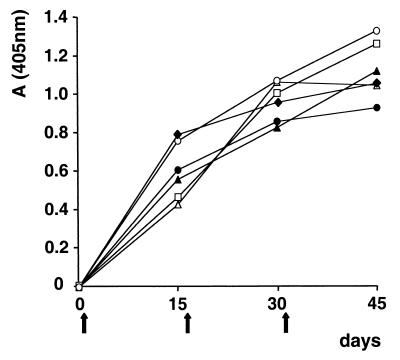
FIG. 5.
Antibody response to thrombin-cleaved recombinant protein PvDBPII–IV in congenic mice on the B10 background as determined by ELISA. Serum samples were taken 1 day before each injection (vertical arrows), and mice were bled out 14 days after the last injection. The plates were coated with PvDBPII–IV at a 2-μg/ml concentration. A405 values were averaged from six mice per strain. Mouse strains: ●, B10(b); ⧫, B10.BR(k); □, B10.D2(d); ▴, B10.M(f); ▵, B10.PL(u); ○, B10.S(s); letters in parentheses indicate H-2 alleles.
The boosting of the anti-DBP responses observed in residents of different regions where malaria is endemic, responses which correlated with age, is consistent with the results of the mouse vaccinations. Low-responder individuals may require repeated or active exposure to acquire or maintain a positive response, and many infections may be insufficient to elicit a sustained high-level response. Therefore, vaccination should be an effective way to elicit and boost existing immune responses directed against the PvDBP in both naive and chronically exposed individuals. Obviously, further detailed analysis of DBP immunogenicity is necessary to determine the complex nature of the immune response against DBP and the most effective methods to elicit a protective immune response by vaccination. As a step toward developing a DBP vaccine, we tested two additional common vaccination methods, DNA delivery and a synthetic peptide, to determine their abilities to induce blocking antibody relative to the recombinant protein immunogen.
DNA vaccine.
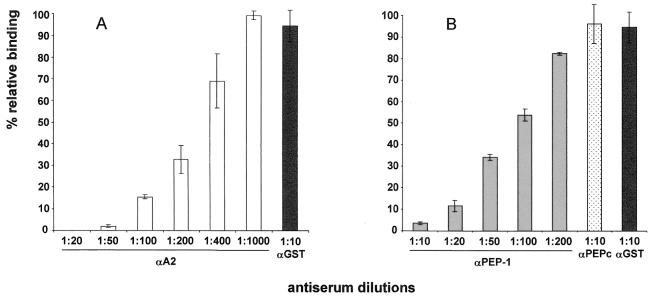
DNA vaccines offer a promising alternative to the recombinant protein vaccines (6). A PvDBP DNA vaccine was designed to express the region equivalent to the rPvDBPII–IV in the expression vector VR1020. This plasmid vector was selected to target the PvDBP through the secretory pathway in order to promote formation of its native secondary structure. Expression of DBPII–IV by this vector in a transfected eukaryotic cell monolayer was confirmed by IFA (Fig. 6). The DNA vaccine was first tested in mice that effectively induced a positive antibody response with only two injections; however, there was a significant delay in the onset of the anti-PvDBP response (4 months), and the level of the antibody response was significantly lower compared with two vaccinations of the protein vaccine (data not shown). Five injections of the DNA vaccine were required to induce a relatively strong antibody response, as determined by ELISA. Antibodies developed against this DBP immunogen were produced in rabbits and effectively inhibited the erythrocyte binding activity of the PvDBP ligand domain in our in vitro cytoadherence assay (Fig. 7A).
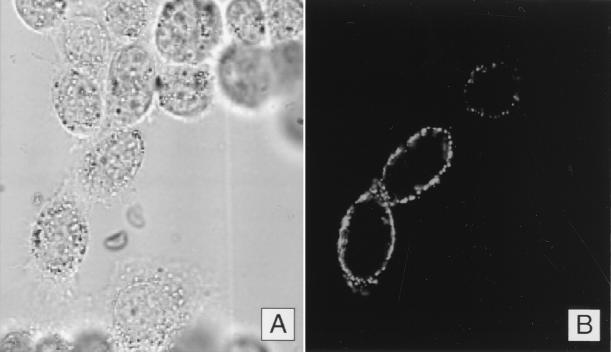
FIG. 6.
Expression of PvDBPII in COS-7 cells transfected with plasmid used as DNA vaccine. COS-7 cells were transfected with 2 μg of plasmid VR1020 containing PvDBPII. Cells were observed unfixed on an MRC-1024 Laser Scanning Confocal Imaging System (Bio-Rad) fitted with a krypton-argon laser. (A) COS-7 cells monolayer observed in a bright field. (B) Immunofluorescent localization of PvDBPII–IV on the surface of COS-7 cells corresponding to field shown in panel A.
FIG. 7.
Inhibition of erythrocyte binding to P. vivax DBP region II expressed on the surface of COS-7 cells by antibodies to the PvDBP elicited by vaccination using a vaccine DNA and a synthetic peptide. COS-7 cells were transfected with pRE4-DBPII plasmid DNA expressing the DBPII as a HSVgD1 chimera (3). COS-7 cells were subsequently incubated with different rabbit antisera at various dilutions prior to the addition of human erythrocytes. Binding was scored after counting the rosettes in 30 fields at a magnification of ×200. The percent binding was determined relative to a 1:10 dilution of preimmune rabbit sera used as a negative control. (A) Percent relative binding after incubation of COS-7 cells with different dilutions of rabbit antiserum to the PvDBPII expressed in plasmid vector VR1020 (αA2). (B) Percent relative binding after incubation of COS-7 cells with different dilutions of rabbit antiserum to the KLH-conjugated synthetic peptide corresponding to PvDBP hypervariable region II.2 (αPEP-1). Rabbit serum raised against an irrelevant synthetic peptide conjugated to KLH was used as control (αPEPc). Error bars correspond to ± the standard error (the standard deviation/the square root of the sample size).
Subunit peptide vaccine.
Synthetic peptides using defined neutralizing epitopes offer another alternative to recombinant protein vaccines. A potentially important target of antibody inhibition was identified in the central portion of the PvDBP erythrocyte binding domain. This predicted alpha-helical surface epitope is a hypervariable region between the cysteine-rich portions of the DBL domain (20). The biased pattern of amino acid substitutions presented evidence that the variability of this motif was the result of immune selective pressure, presumably as the target of inhibitory antibody against the DBL domain. In spite of its high degree of variability, the extent of variation was limited to certain types of amino acids, suggesting there are also important functional constraints on this region. A synthetic peptide (PEP-1) spanning this motif was conjugated to KLH and used to elicit a high-titer antibody response reactive with the peptide (conjugated to BSA). Nevertheless, this serum was not reactive with the parasite in IFA (data not shown) and correspondingly showed significantly less inhibition of erythrocyte binding activity as measured by the in vitro cytoadherence assay (Fig. 7B) compared to the other means of vaccinations. No significant inhibition of binding was observed with a negative control rabbit antiserum raised against an irrelevant peptide (PEPc) (Fig. 7B). Although the antiserum to PEP-1 had demonstrable inhibitory specificity against the DBP ligand, its failure to completely inhibit erythrocyte adhesion even at a high concentration was suggestive of low affinity for the native conformation of the ligand domain. In addition, the soluble PEP-1 did not hinder erythrocyte binding activity to PvDBP DBL domain in the cytoadherence assay even at high concentrations (1 μM), thus confirming the lack of inherent erythrocyte binding activity of the peptide alone (data not shown). These results with the anti-peptide antibody suggested that the hypervariable motif was not readily accessible to antibodies or, more likely, that the peptide immunogen could not assume its native conformation outside the context of the DBL domain. Restrained synthetic peptides or ones considerably longer may be more effective in eliciting an effective inhibitory response.
Conclusion.
These studies of acquired and vaccine-elicited antibody responses to the PvDBP provide positive evidence for the vaccine potential of this essential parasite adhesion molecule. Inhibitory antibodies against the DBL ligand domain developed in humans even after limited exposure. Elevated antibody responses to the PvDBP correlated to increased blocking capacity of the immune sera. Importantly, immunization of laboratory animals with PvDBP recombinant proteins, a DNA vaccine and, to a lesser extent, a synthetic peptide elicited inhibitory antibodies. Evidence for secondary boosting of an initially low-responder immune response was demonstrated in congenic mice. These data provide hope that vaccination against the PvDBP will boost the inherent low responsiveness in residents of malaria-endemic regions to this protein and help confer protection against the severe morbidity caused by the blood-stage parasite. Knowing the significance of the antibody response to the DBP, as studied through the in vitro cytoadherence assay, requires a better understanding of what constitutes immunity against blood-stage malaria. Identifying and analyzing the individual components of protective immunity through other in vitro assays or parasite neutralization studies will facilitate this understanding. The hope of developing vaccines against blood-stage malaria will depend on knowing what needs to be targeted and how the immune response needs to be directed.
ACKNOWLEDGMENTS
We appreciate the cooperation of the Madang and Wosera residents in supporting this research. We thank Bill Collins, Blaise Genton, David Kaslow, Sanjai Kumar, Betsy Majane, and Bill Rogers for their advice.
This work was supported by a Public Health Service grant (R29 AI-33656) from the National Institute of Allergy and Infectious Diseases and the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases. J.H.A. is a Burroughs Wellcome Fund New Investigator in Molecular Parasitology.
REFERENCES
- 1.Adams J H, Sim B K L, Dolan S A, Fang X, Kaslow D C, Miller L H. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1992;89:7085–7089. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aikawa M, Miller L H, Johnson J, Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol. 1978;77:72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chitnis C, Miller L H. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med. 1994;180:497–506. doi: 10.1084/jem.180.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen G H, Wilcox W C, Sodora D L, Long D, Levin J Z, Eisenberg R J. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J Virol. 1988;62:1932–1940. doi: 10.1128/jvi.62.6.1932-1940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crewther P E, Matthew M L, Flegg R H, Anders R F. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect Immun. 1996;64:3310–7. doi: 10.1128/iai.64.8.3310-3317.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly J J, Ulmer J B, Liu M A. DNA vaccines. Dev Biol Stand. 1998;95:43–53. [PubMed] [Google Scholar]
- 7.Elmendorf H G, Haldar K. Identification and localization of ERD2 in the malaria parasite Plasmodium falciparum: separation from sites of sphingomyelin synthesis and implications for organization of the Golgi. EMBO J. 1993;12:4763–4773. doi: 10.1002/j.1460-2075.1993.tb06165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang X D, Kaslow D C, Adams J H, Miller L H. Cloning of the Plasmodium vivax Duffy receptor. Mol Biochem Parasitol. 1991;44:125–32. doi: 10.1016/0166-6851(91)90228-x. [DOI] [PubMed] [Google Scholar]
- 9.Fraser T, Michon P, Barnwell J W, Noe A R, AlYaman F, Kaslow D C, Adams J H. Expression and serologic activity of a soluble recombinant Plasmodium vivax Duffy binding protein. Infect Immun. 1997;65:2772–2777. doi: 10.1128/iai.65.7.2772-2777.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galinski M R, Barnwell J W. Plasmodium vivax: merozoites, invasion of reticulocytes and considerations for malaria vaccine development. Parasitol Today. 1996;12:20–29. doi: 10.1016/0169-4758(96)80641-7. [DOI] [PubMed] [Google Scholar]
- 11.Genton B, al-Yaman F, Beck H P, Hii J, Mellor S, Narra A, Gibson N, Smith T, Alpers M P. The epidemiology of malaria in the Wosera area, East Sepik Province, Papua New Guinea, in preparation for vaccine trials. I. Malariometric indicies and immunity. Ann Trop Med Parasitol. 1995;89:359–376. doi: 10.1080/00034983.1995.11812965. [DOI] [PubMed] [Google Scholar]
- 12.Kappe S H I, Noe A R, Fraser T S, Blair P L, Adams J H. A family of chimeric erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1998;95:1230–1235. doi: 10.1073/pnas.95.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaslow D C, Kumar S. Expression and immunogenicity of the C-terminus of a major blood-stage surface protein of Plasmodium vivax, Pv200-19, secreted from Saccharomyces cervisiae. Immunol Lett. 1996;51:187–189. doi: 10.1016/0165-2478(96)02570-9. [DOI] [PubMed] [Google Scholar]
- 14.Michon P, Arevalo-Herrera M, Fraser T, Herrera S, Adams J H. Serologic responses to recombinant Plasmodium vivax Duffy binding protein in a Colombian village. Am J Trop Med Hyg. 1998;59:597–599. doi: 10.4269/ajtmh.1998.59.597. [DOI] [PubMed] [Google Scholar]
- 15.Noe, A. R., D. J. Fishkind, and J. H. Adams. Spatial and temporal dynamics of the secretory pathway during differentiation of the Plasmodium yoelii schizont. Mol. Biochem. Parasitol., in press. [DOI] [PubMed]
- 16.Oaks, J., S. C., V. S. Mitchell, G. W. Pearson, and C. J. Carpenter. 1991. Malaria: obstacles and opportunities. National Academy Press, Washington, D.C. [PubMed]
- 17.Rowe J A, Moulds J M, Newbold C I, Miller L H. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 18.Sim B K L, Chitnis C E, Wasniowska T J, Hadley T J, Miller L H. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 19.Smith D B, Corcoran L M. Expression and purification of glutathione-S-transferase fusion proteins. In: Ausubel F M, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons; 1991. pp. 16.7.1–16.7.8. [DOI] [PubMed] [Google Scholar]
- 20.Tsuboi T, Kappe S H I, Al-Yaman F, Prickett M D, Alpers M, Adams J H. Natural variation within the principal adhesion domain of the Plasmodium vivax duffy binding protein. Infect Immun. 1994;62:5581–5586. doi: 10.1128/iai.62.12.5581-5586.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wertheimer S P, Barnwell J W. Plasmodium vivax interaction with the human Duffy blood group glycoprotein: identification of a parasite receptor-like protein. Exp Parasitol. 1989;69:340–350. doi: 10.1016/0014-4894(89)90083-0. [DOI] [PubMed] [Google Scholar]