Abstract
Background
Acute aortic dissection (AAD) is a rare condition but represents a time-sensitive disease for which a wrong and untimely identification in the triage phase could compromise the subsequent diagnostic, therapeutic path and patient's prognosis. The emergency nurse plays a crucial role in identifying and managing patients with possible AAD. The aim of this paper is to describe the emergency department nursing approach to critical patients with suspected hyperacute/acute AAD.
Purpose
It is crucial to examine the emergency departments nursing approach to patients with suspected AAD. It is fundamental to have a rapid and standardized approach related to life-saving procedures, practices, and management of critical patients during the triage phase, with the assessment of the most common presentation of clinical signs and symptoms and patient management during each step in the emergency department.
Conclusion
Early identification and diagnosis in ED allow prompt treatment that improves prognosis. The emergency nurse plays a crucial role in correctly identifying and managing patients with acute aortic dissection. High clinical suspicion from the triage stages, early diagnosis, monitoring, and initial clinical stabilization in the emergency department plays a key role while awaiting definitive treatment.
Keywords: triage, aorta, dissection, emergency nursing, emergency service, hospital
Introduction
Acute aortic syndromes (AASs) are life-threatening medical conditions sharing similar clinical characteristics and leading to a breakdown of the intima and media. AASs include all those diseases that involve an alteration in the vascular integrity of the aorta, associated with a risk of vascular wall rupture (Erbel et al., 2014). They represent surgical or medical emergencies in which chest pain is one of the most frequent symptom (Riambau et al., 2017). This condition may occur suddenly and is often described by the patient as a sharp, stabbing, sudden pain radiating anteriorly and/or posteriorly in the chest (Erbel et al., 2014; Riambau et al., 2017). Among AAS, the most common is acute aortic dissection (AAD) (85%–95%), followed by intramural hematoma (5%–25%), and a penetrating aortic ulcer (2%–7%) (Bossone et al., 2018; Erbel et al., 2014). Some authors include traumatic intimal tear or traumatic iatrogenic aortic injury as a subtype of AAS (Carpenter et al., 2014). When facing an AAD, early diagnosis and rapid identification are crucial to reducing the high mortality rate, which is reported to be up to 16.9% in a multicenter study (Conzelmann et al., 2016). Such complex diseases require a multidisciplinary approach, aiming to reduce the time between a patient's admission and possible definitive treatment. Early identification and initial supportive treatments should be considered a priority of emergency room physicians and nurses.
AAD is rare, with an incidence of 6 cases/100,000 person-years but it has a high mortality (Howard et al., 2013). It is more frequent in men but is associated with a worse prognosis in women because of the atypical manifestation often associated with delayed diagnosis (Howard et al., 2013; Landenhed et al., 2015). It is characterized by a split of the layers that compose the aortic wall, which results in the formation of a true lumen and a false lumen separated by a septum, sometimes called a flap (Mussa et al., 2016). It is believed that this process is initiated by a discontinuity in the intima that exposes the underlying tunica media to intraluminal pressure forces. In most cases, the dissection progresses anterogradely, thus dissecting the media from the intima and generating a false lumen. The intima may furtherly tear, opening a breach to re-enter into the true lumen (Nienaber & Clough, 2015; Riambau et al., 2017). The dissecting process may involve the origin of collateral vessels along its course: the most frequently affected vessels are the anonymous trunk, the left subclavian and the left renal artery (Erbel et al., 2014).
This paper aims to review the emergency department nursing approach to critical patients with suspected hyperacute/acute AAD, either during the triage phase, highlighting the most common signs and symptoms, and patient's management during the early step in the emergency room evaluation, where a quick and standardized approach can be crucial to reduce morbidity and mortality.
Brief Review
First Step: Nursing Triage
Correctly identifying signs and symptoms is crucial during the nursing triage phase. The Emergency Severity Index (ESI) is a simple-to-use, five-level triage algorithm that categorizes emergency department patients by evaluating both patient acuity and resource needs (Emergency Severity Index (ESI): A Triage Tool for Emergency Departments, 2020). An inaccurate evaluation of these patients can lead to a delay from symptom onset to diagnosis and treatment and increased mortality. Each patient with chest pain must be evaluated within the context of the ESI level-1 to determine whether the patient requires an immediate lifesaving intervention. The ESI level-1 patient always presents to the emergency department with an unstable condition. These patients are seen immediately because the timeliness of interventions can affect morbidity and mortality (Emergency Severity Index (ESI): A Triage Tool for Emergency Departments, 2020). Patients with chest pain who are physiologically unstable and require immediate interventions such as intubation or hemodynamic support should be triaged as ESI level-1. In contrast, the patients who meet ESI level-2 criteria should have their placement rapidly facilitated and should be evaluated as soon as possible (Emergency Severity Index (ESI): A Triage Tool for Emergency Departments, 2020). The triage nurse should keep a high level of suspicion in patients with chest pain localized anteriorly, between the base of the nose and the umbilicus and, posteriorly, between the occiput and the 12th vertebra (Stepinska et al., 2020). Chest pain is one of the most frequent symptoms, although AADs are only 0.2%–2% of chest pain presenting to the emergency department (Erbel et al., 2014; Riambau et al., 2017). The onset is sudden and abrupt; the pain is sharp and lacerating, stabbing-like.
Generally, two types of anatomical classification are used in AAD, the DeBakey and Stanford classification, which consider the anatomical location and the extension of the dissection (Erbel et al., 2014; Nienaber & Clough, 2015; Svensson et al., 1999).
A further non-anatomical classification has been proposed by the International Registry of Acute Aortic Dissection (IRAD), which recognizes four stages (Booher et al., 2013) concerning the time between the onset of symptoms and diagnosis:
- Hyperacute: <24 h
- Acute: 2–7 days
- Subacute: 8–30 days
- Chronic: ≥ 30 days
Anterior chest pain is most commonly associated with type A aortic dissection, whereas patients with type B dissection more frequently present with back or abdominal pain (Trimarchi et al., 2012). The clinical presentations of the two types of AAD may frequently overlap. Pain may migrate from its origin to other sites, following the dissection pathway as it extends through the aorta. The IRAD describes that hypotension, absence of chest/back pain, and branch vessel involvement are predictors of in-hospital mortality (Suzuki et al., 2003). Another potential sign of AAD, which is not uncommon, is pulse deficit. This occurs in 30% of patients with type A aortic dissection and 15% in those with type B, although evident ischemia of the lower limbs is rare (Bossone et al., 2002, 2018; Erbel et al., 2014). Assessment of the differential arterial pressure between the two arms, combined with an accurate evaluation of the radial and femoral pulses, may help the nurse to assign or confirm an acuity level for rapid access to treatment. AADs may be associated with symptoms of organ ischemia, such as stroke, mesenteric, renal, and lower limb ischemia, and acute coronary syndrome. Physical examination may be normal or may show signs of poor organ perfusion (neurological signs, pulse deficit, oliguria) or frank rupture (cardiac tamponade, aortic valve failure, hemothorax, hemoperitoneum) (Hagan et al., 2000; Pape et al., 2015). Ischemia or myocardial infarction may occur in 10%–15% of patients with AAD (Jánosi et al., 2009). If the patient does not require immediate lifesaving intervention (ESI level 1), with the visual assessment, the triage nurse should assess the main signs/symptoms, evaluate the presence of any suspicious clinical or risk factors and then checks vital signs, including blood pressure (BP). The presence of significantly different blood pressure values between the two arms (>15 mmHg) should raise suspicion of aortic dissection (Kuan et al., 2016). A pulse examination must follow this. Sometimes patients may have minimal symptoms, which could be a problem during the triage evaluation. Given the low specificity and sensitivity of individual signs and symptoms, carefully collecting the patient's history is crucial during the triage phase. There may be clinical conditions associated with typical symptoms that predispose to AADs (Pape et al., 2015; Sampson et al., 2014; Sidloff et al., 2014).
- Hypertension (most prevalent risk factor)
- Smoking
- Cocaine and amphetamine misuse
- Congenital connective tissue diseases (Marfan syndrome, Loeys-Dietz syndrome, etc.)
- Known aortic diseases (aneurysm, bicuspid aortic valve, aortic coarctation)
- Previous cardiac surgery or interventional cardiology with involvement of the heart and/or aorta
- Family history of aortic disease (Albornoz et al., 2006)
In the more recent European Society of Cardiology (ESC) guidelines (Erbel et al., 2014), a score was developed to identify patients at risk of developing an episode of AAD. Three main categories were identified: high-risk related diseases, high-risk related pain, and high-risk related clinical signs. Inside each main category, the various risk factors are listed. Therefore, each patient will be assigned a point and a risk class (low score 0–1 vs. high score 2–3) whenever they present at least one risk factor within each category (Table 1). It might be helpful to implement the application of a similar score already in the triage phase to identify patients with a potential risk of AAD earlier. Unlike ESI level-1 patients, the emergency nurse can initiate care through protocols without a physician immediately at the bedside. These patients may require a diagnostic electrocardiogram (EKG) within 10 min of arrival if has chest pain because the priority is always to exclude an eventual acute coronary syndrome (ACS). It is not uncommon to have coronary involvement during AAD with associated myocardial ischemia and ischemic EKG changes (ST-elevation or T-wave inversions). However, non-specific EKG changes are frequent, and only 1/3 of patients have a completely normal EKG (Bossone et al., 2018).
Table 1.
Aortic Dissection Detection Risk Score (1 Point/Category).
| High-risk conditions | High-risk pain features | High-risk examination features |
|---|---|---|
|
Chest, back, or abdominal pain described as any of the following:
|
|
Score 0: low risk; Score 1: medium risk; Score 2–3: high risk.
Second Step: Assessment and Initial Support
Once the correct acuity level is confirmed at triage, the emergency physician has to begin the medical examination of the critical patient immediately. While the emergency physician begins his clinical assessment, the nurse should first obtain two large-caliber (≥ 18 gauge) peripheral intravascular catheters (PIVC) and draw blood samples to send immediately to the emergency laboratory.
Even in an emergency situation, the order of draw should be respected (citrate, serum, heparin, EDTA, glycolysis inhibitors) (Giavarina & Lippi, 2017).
When facing a patient with difficult intravenous vascular access, if a vascular access team is available (Bell & Spencer, 2021), ultrasound is recommended to reduce pain and the number of venipunctures, therefore shortening the time required for correct PIVC placement field (Mahler et al., 2011; Privitera et al., 2022). Continuous vital signs monitoring is mandatory: respiratory rate, pulsation rate (with 5-lead electrocardiographic monitoring), oxygen saturation, and blood pressure monitoring at short intervals (3–5 min) (Wilcox, 2019). In the event of different blood pressures between the two arms, the arm with higher pressure will be monitored continuously. During each phase, it is essential to keep the patient informed to explain what will be done, involving the patient directly to reduce stress and anxiety. Once continuous monitoring of vital parameters has begun, the diagnostic phase has to start.
Third Step: Essential Examinations and Related Diagnostic Procedures
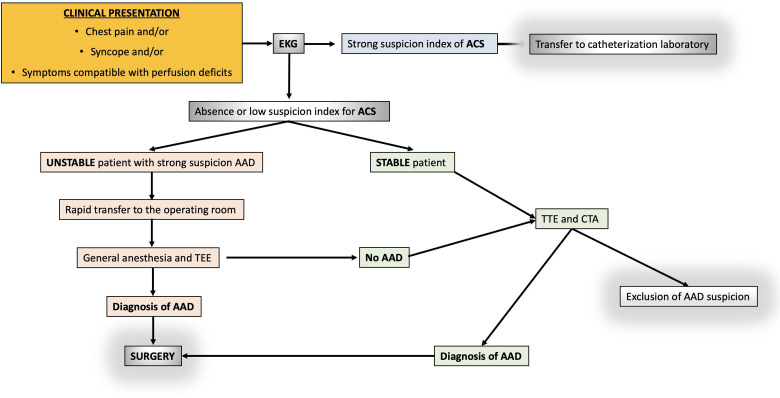
It is up to the emergency physician to decide which diagnostic tests to perform based on the pretest risk of AADs and the presenting clinical features (Table 1). The main diagnostic imaging modalities currently in use in a critically ill patient are: computed tomography angiography (CTA) of the chest, transthoracic echocardiogram (TTE), and transesophageal echocardiogram (TEE). The choice depends on the availability and expertise of the particular hospital as well as the patient's presenting clinical status. A diagnostic decision algorithm is suggested in Figure 1. Close supervision by medical/nursing staff will be required during each of these diagnostic procedures: continuous monitoring of vital signs by multi-parameter monitors and assessment of consciousness.
Figure 1.
A diagnostic decision algorithm for acute chest pain.
EKG = electrocardiogram; ACS = acute coronary syndromes; TEE = transesophageal echo; TTE = transthoracic echo.
Computed tomography angiography
Most emergency departments have a computed tomography scanner located nearby for rapid imaging of unstable patients. CTA of the chest is the first choice examination when an AAD is suspected (sensitivity 100%; specificity 98%) (Shiga et al., 2006). The advantages of this examination are the speed, availability, and the possibility of studying the entire aorta quickly. It is possible to identify the various types of AADs (aortic dissection, penetrating ulcer, or intramural hematoma). CT scan has disadvantages compared with echocardiography, which can be performed at the patient's bedside; it cannot easily be performed if the patient is hemodynamically unstable, and contrast medium can also cause allergy and or renal failure.
Transthoracic echocardiogram
It is an excellent tool for rapidly detecting life-threatening clinical conditions in AAD, such as aortic regurgitation, pericardial effusion, cardiac tamponade, or alterations in myocardial contractility. It is widely used in emergency situations, useful for rapid diagnosis at the patient's bedside (Neskovic et al., 2013). However, its low specificity and sensitivity in the diagnosis of AAD (sensitivity of 78%–100% for type A and 31%–55% for type B), in the event of a negative test, does not exclude potential AAD (Goldstein et al., 2015).
The main limitations are two: it is an “operator-dependent” examination and may require intra-procedural patient sedation unless the patient is unconscious. Simultaneously to bedside evaluations, management must focus on organization/transfer to CTA or directly to presurgical TEE in theatre.
Transesophageal echocardiogram
TEE has high diagnostic sensitivity and specificity (99%; 89%, respectively) (Bossone et al., 2007; Hilberath et al., 2010). Short execution times, feasibility at the patient's bedside, even in the case of an unstable patient, and the fact that it does not require the administration of contrast medium or ionizing radiation make it one of the most appropriate diagnostic tests as an alternative to or as a diagnostic addition to CT if the latter is doubtful, but pretest probability is high. The same limitations of TTE are also present in this exam.
Laboratory tests
Laboratory tests are useful for differential diagnosis and for detecting life-threatening complications. Among these, D-dimer is the most widely used diagnostic biomarker in the clinical practice (Erbel et al., 2014; Suzuki et al., 2013). Several studies have shown that a cut-off level of 500 ng/mL (currently used for pulmonary embolism) is highly sensitive for ruling out classical AAD within the first 6 h of symptom onset (Minegishi et al., 2016; Watanabe et al., 2016). However, D-dimer is a biomarker with high sensitivity but low specificity in AAD, as it may be elevated in many other disorders, including AAD, acute myocardial infarction, and acute pulmonary embolism (Erbel et al., 2014).
Fourth Step: Monitoring and Stabilization
Treatment should begin as soon as the diagnosis of aortic dissection is confirmed. Hemodynamically unstable patients require immediate stabilization. Immediate treatment aims to reduce the progression of the dissection while awaiting early surgical intervention (Aggarwal & Raymond, 2015). Continuous nursing supervision must always be guaranteed to the patient, together with adequate intensive monitoring of vital signs (Downey et al., 2018). Recommended a nurse-to-patient ratio of 2:1 (two nurses to one patient) (Falk & Wallin, 2016). In all cases of AADs, initial medical therapy is focused on reducing wall stress to limit the extent of the dissection.
Control of blood pressure (systolic pressure between 100 and 120 mmHg; mean pressure 60 mmHg) and heart rate (<60 bpm) is essential (Erbel et al., 2014). Intravenous beta-blockers represent the first-line drugs (Erbel et al., 2014; Morello et al., 2021). Either esmolol or labetalol represents an ideal drug providing both vasodilation and heart rate control. In patients who do not respond to beta-blockers or do not tolerate the drug, alternative options are calcium channel antagonists, urapidil, or clonidine. Nitroprusside is an alternative if target blood pressure is not obtained after titration of a beta-blocker and after assuring adequate heart rate control (Morello et al., 2021). It is necessary to have an accurate blood pressure value measured in both arms, with the higher value being taken as the baseline before therapy is administered. This requires placing the blood pressure cuff on the limb with the highest pressure. However, invasive blood pressure monitoring should be considered as soon as possible. Avoid the placement of femoral artery catheters, which is the preferred site for endovascular procedures. It is useful to monitor pulse oximetry and saturation by using an adhesive saturation meter also placed on the most perfused limb. This will not only provide a reliable SpO2 value but will also allow the volume changes in a certain district to be displayed graphically through plethysmography. Placing it on the hypotensive limb may not guarantee accurate monitoring of these parameters due to a reduced perfusion index.
The placement of a bladder catheter is considered mandatory. This allows monitoring of diuresis and if the catheter has the thermometer feature, hourly body temperature (Andrews & Nolan, 2006; Celotto et al., 2003)
It is essential to have adequate pain control (intravenous opioid analgesia). Pain management is an essential aspect of the patient management (Erbel et al., 2014). Reduction of pain leads, in turn to a reduction in the adrenergic component, which affects blood pressure, and a reduction in respiratory rate, which is likely to increase SpO2. It is useful to monitor pain using a validated score scale such as the Numerical Rating Scale (Karcioglu et al., 2018; Morello et al., 2021), considering a value of ≤ 4 as the optimal range. Pain control must avoid alteration of consciousness and breathing depression so that any increase in pain can still be detected as a symptom indicating possible dissection progression (Wilcox, 2019).
Continuous monitoring of signs or symptoms associated with cardiac tamponade (Type A dissection), which may occur if the dissection proceeds proximally, is crucial (Wilcox, 2019). Cardiac tamponade prevents the heart chambers from filling adequately, reducing cardiac output and causing obstructive shock if left untreated. The most common symptom is shortness of breath, while the three most common signs are hypotension, muffled heart sounds, and jugular venous distension (Beck's triad) (Alerhand et al., 2022). Any alteration or appearance of adverse signs and/or symptoms should be reported immediately.
Last Step: Surgical Treatment
If type A AAD has been diagnosed, surgery will be the first-choice treatment. Surgery reduces 1-month mortality from 90% to 30%. The advantage of surgery over conservative therapy is particularly obvious in the long-term follow-up (Perko et al., 1995). ED management should focus on patient support and preparation for surgery in these patients, including requests for compatible blood units (Morello et al., 2021). A previous study demonstrated that treating type A aortic dissection with a dedicated multi-disciplinary thoracic aortic team significantly improved peri-operative outcomes (Andersen et al., 2014).
In the case of type B AAD, the recommended treatment is medical (pain, pressure, and frequency management). Afterwards, endovascular or surgical interventions must be considered in patients presenting severe aortic dilatation, signs of impending rupture, aortic rupture, and organ malperfusion (Erbel et al., 2014; Morello et al., 2021)..
Importance to the Nursing Profession
AAD is a rare condition with a high mortality rate. Classic studies indicate a mortality rate of 1%–2% per hour in untreated patients. Early identification and diagnosis in the ED enable timely treatment that improves prognosis. The first person to see the patient is the emergency nurse, who plays a crucial role in correctly identifying and managing patients with AAD. Therefore, high clinical suspicion from the triage stages, early diagnosis, monitoring, and initial clinical stabilization in the emergency department plays a key role while waiting for definitive treatment.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article
ORCID iD: Daniele Privitera https://orcid.org/0000-0001-5804-5488
References
- Aggarwal B., Raymond C. E. (2015). Therapeutic goals in patients with acute aortic dissection: Management before surgery. Journal of the American College of Cardiology, 65(15), 1599–1600. 10.1016/j.jacc.2014.11.077 [DOI] [PubMed] [Google Scholar]
- Albornoz G., Coady M. A., Roberts M., Davies R. R., Tranquilli M., Rizzo J. A., Elefteriades J. A. (2006). Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. The Annals of Thoracic Surgery, 82(4), 1400–1405. 10.1016/j.athoracsur.2006.04.098 [DOI] [PubMed] [Google Scholar]
- Alerhand S., Adrian R. J., Long B., Avila J. (2022). Pericardial tamponade: A comprehensive emergency medicine and echocardiography review. The American Journal of Emergency Medicine, 58, 159–174. 10.1016/j.ajem.2022.05.001 [DOI] [PubMed] [Google Scholar]
- Andersen N. D., Ganapathi A. M., Hanna J. M., Williams J. B., Gaca J. G., Hughes G. C. (2014). Outcomes of acute type a dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. Journal of the American College of Cardiology, 63(17), 1796–1803. 10.1016/j.jacc.2013.10.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews F. J., Nolan J. P. (2006). Critical care in the emergency department: Monitoring the critically ill patient. Emergency Medicine Journal, 23(7), 561–564. 10.1136/emj.2005.029926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. A., Spencer T. R. (2021). Implementing an emergency department vascular access team: A quality review of training, competency, and outcomes. The Journal of Vascular Access, 22(1), 81–89. 10.1177/1129729820924554 [DOI] [PubMed] [Google Scholar]
- Booher A. M., Isselbacher E. M., Nienaber C. A., Trimarchi S., Evangelista A., Montgomery D. G., Froehlich J. B., Ehrlich M. P., Oh J. K., Januzzi J. L., O’Gara P., Sundt T. M., Harris K. M., Bossone E., Pyeritz R. E., Eagle K. A. (2013). The IRAD classification system for characterizing survival after aortic dissection. American Journal of Medicine, 126(8), 730.e19–730.e24. 10.1016/j.amjmed.2013.01.020 [DOI] [PubMed] [Google Scholar]
- Bossone E., Evangelista A., Isselbacher E., Trimarchi S., Hutchison S., Gilon D., Llovet A., O'Gara P., Cooper J. V., Fang J., Januzzi J. L., Mehta R. H., Distante A., Nienaber C. A., Eagle K., Armstrong W. F., & International Registry of Acute Aortic Dissection Investigators (2007). Prognostic role of transesophageal echocardiography in acute type A aortic dissection. American Heart Journal, 153(6), 1013–1020. 10.1016/j.ahj.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Bossone E., LaBounty T. M., Eagle K. A. (2018). Acute aortic syndromes: Diagnosis and management, an update. European Heart Journal, 39(9), 739–749d. 10.1093/eurheartj/ehx319 [DOI] [PubMed] [Google Scholar]
- Bossone E., Rampoldi V., Nienaber C. A., Trimarchi S., Ballotta A., Cooper J. V., Smith D. E., Eagle K. A., Mehta R. H. (2002). Usefulness of pulse deficit to predict in-hospital complications and mortality in patients with acute type A aortic dissection. American Journal of Cardiology, 89(7), 851–855. 10.1016/S0002-9149(02)02198-7 [DOI] [PubMed] [Google Scholar]
- Carpenter S. W., Kodolitsch Y. V., Debus E. S., Wipper S., Tsilimparis N., Larena-Avellaneda A., Diener H., Kölbel T. (2014). Acute aortic syndromes: Definition, prognosis and treatment options. The Journal of Cardiovascular Surgery, 55(2 Suppl 1), 133–144. PMid:24796906. [PubMed] [Google Scholar]
- Celotto S., Nesci M., Lucchini A., Bellani S., Bombino M. (2003). I parametri vitali del monitoraggio emodinamico [vital signs of hemodynamic monitoring]. Minerva Anestesiologica, 69(4), 289–296. [PubMed] [Google Scholar]
- Conzelmann L. O., Weigang E., Mehlhorn U., Abugameh A., Hoffmann I., Blettner M., Etz C. D., Czerny M., Vahl C. F. (2016). Mortality in patients with acute aortic dissection type A: Analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). European Journal of Cardio-Thoracic Surgery, 49(2), e44–e52. 10.1093/ejcts/ezv356 [DOI] [PubMed] [Google Scholar]
- Downey C. L., Chapman S., Randell R., Brown J. M., Jayne D. G. (2018). The impact of continuous versus intermittent vital signs monitoring in hospitals: A systematic review and narrative synthesis. International Journal of Nursing Studies, 84, 19–27. 10.1016/j.ijnurstu.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Emergency Severity Index (ESI): A Triage Tool for Emergency Departments. Content last reviewed May 2020. Agency for Healthcare Research and Quality, Rockville, MD.
- Erbel R., Aboyans V., Boileau C., Bossone E., Bartolomeo R. D., Eggebrecht H., Evangelista A., Falk V., Frank H., Gaemperli O., Grabenwöger M., Haverich A., Iung B., Manolis A. J., Meijboom F., Nienaber C. A., Roffi M., Rousseau H., Sechtem U., … ESC Committee for Practice Guidelines (2014). 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European society of cardiology (ESC). European Heart Journal, 35(41), 2873–2926. 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- Falk A. C., Wallin E. M. (2016). Quality of patient care in the critical care unit in relation to nurse patient ratio: A descriptive study. Intensive and Critical Care Nursing, 35, 74–79. 10.1016/j.iccn.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Giavarina D., Lippi G. (2017). Blood venous sample collection: Recommendations overview and a checklist to improve quality. Clinical Biochemistry, 50(10), 568–573. 10.1016/j.clinbiochem.2017.02.021 [DOI] [PubMed] [Google Scholar]
- Goldstein S. A., Evangelista A., Abbara S., Arai A., Asch F. M., Badano L. P., Bolen M. A., Connolly H. M., Cuéllar-Calàbria H., Czerny M., Devereux R. B., Erbel R. A., Fattori R., Isselbacher E. M., Lindsay J. M., McCulloch M., Michelena H. I., Nienaber C. A., Oh J. K., … Schepens M. (2015). Multimodality imaging of diseases of the thoracic aorta in adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging: Endorsed by the society of cardiovascular computed tomography and society for cardiovascular magnetic resonance. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography, 28(2), 119–182. 10.1016/j.echo.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Hagan P. G., Nienaber C. A., Isselbacher E. M., Bruckman D., Karavite D. J., Russman P. L., Evangelista A., Fattori R., Suzuki T., Oh J. K., Moore A. G., Malouf J. F., Pape L. A., Gaca C., Sechtem U., Lenferink S., Deutsch H. J., Diedrichs H., Marcos y Robles J., … Eagle K. A. (2000). The international registry of acute aortic dissection (IRAD): New insights into an old disease. JAMA, 283(7), 897–903. 10.1001/jama.283.7.897 [DOI] [PubMed] [Google Scholar]
- Hilberath J. N., Oakes D. A., Shernan S. K., Bulwer B. E., D’Ambra M. N., Eltzschig H. K. (2010). Safety of transesophageal echocardiography. Journal of the American Society of Echocardiography, 23(11), 1115–1127. 10.1016/j.echo.2010.08.013 [DOI] [PubMed] [Google Scholar]
- Howard D. P. J., Banerjee A., Fairhead J. F., Perkins J., Silver L. E., Rothwell P. M. (2013). Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford vascular study. Circulation, 127(20), 2031–2037. 10.1161/CIRCULATIONAHA.112.000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jánosi R. A., Buck T., Erbel R. (2009). Mechanism of coronary malperfusion due to type-A aortic dissection. Herz, 34(6), 478. 10.1007/s00059-009-3272-z [DOI] [PubMed] [Google Scholar]
- Karcioglu O., Topacoglu H., Dikme O., Dikme O. (2018). A systematic review of the pain scales in adults: Which to use? American Journal of Emergency Medicine, 36(4), 707–714. 10.1016/j.ajem.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Kuan P. X., Tan P. W., Jobli A. T., Razak N. A. (2016). Discrepancy in blood pressure between the left and right arms - importance of clinical diagnosis and role of radiological imaging. Medical Journal of Malaysia, 71(4), 206–208. PMID: 27770122. [PubMed] [Google Scholar]
- Landenhed M., Engström G., Gottsäter A., Caulfield M. P., Hedblad B., Newton-Cheh C., Melander O., Smith J. G. (2015). Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: A prospective cohort study. Journal of the American Heart Association, 4(1), e001513. 10.1161/JAHA.114.001513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S. A., Wang H., Lester C., Skinner J., Arnold T. C., Conrad S. A. (2011). Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: A prospective randomized study. American Journal of Emergency Medicine, 29(9), 1194–1197. 10.1016/j.ajem.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Minegishi S., Watanabe H., Horita N., Shibata Y., Kaneko T., Ishigami T. (2016). The current evidence on diagnosis and treatment of acute aortic syndrome. Journal of Thoracic Disease, 8(12), E1617–E1619. 10.21037/jtd.2016.12.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello F., Santoro M., Fargion A. T., Grifoni S., Nazerian P. (2021). Diagnosis and management of acute aortic syndromes in the emergency department. Internal and Emergency Medicine, 16(1), 171–181. 10.1007/s11739-020-02354-8 [DOI] [PubMed] [Google Scholar]
- Mussa F. F., Horton J. D., Moridzadeh R., Nicholson J., Trimarchi S., Eagle K. A. (2016). Acute aortic dissection and intramural hematoma: A systematic review. JAMA, 316(7), 754–763. 10.1001/jama.2016.10026 [DOI] [PubMed] [Google Scholar]
- Neskovic A. N., Hagendorff A., Lancellotti P., Guarracino F., Varga A., Cosyns B., Flachskampf F. A., Popescu B. A., Gargani L., Zamorano J. L., Badano L. P. (2013). Emergency echocardiography: The European Association of Cardiovascular Imaging recommendations. European Heart Journal Cardiovascular Imaging, 14(1), 1–11. 10.1093/ehjci/jes193 [DOI] [PubMed] [Google Scholar]
- Nienaber C. A., Clough R. E. (2015). Management of acute aortic dissection. The Lancet, 385(9970), 800–811. 10.1016/S0140-6736(14)61005-9 [DOI] [PubMed] [Google Scholar]
- Pape L. A., Awais M., Woznicki E. M., Suzuki T., Trimarchi S., Evangelista A., Myrmel T., Larsen M., Harris K. M., Greason K., Di Eusanio M., Bossone E., Montgomery D. G., Eagle K. A., Nienaber C. A., Isselbacher E. M., O’Gara P. (2015). Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. Journal of the American College of Cardiology, 66(4), 350–358. 10.1016/j.jacc.2015.05.029 [DOI] [PubMed] [Google Scholar]
- Perko M. J., Norgaard M., Herzog T. M., Olsen P. S., Schroeder T. V., Pettersson G. (1995). Unoperated aortic aneurysm: A survey of 170 patients. The Annals of Thoracic Surgery, 59, 1204–1209. 10.1016/0003-4975(95)00132-5 [DOI] [PubMed] [Google Scholar]
- Privitera D., Mazzone A., Pierotti F., Airoldi C., Galazzi A., Geraneo A., Cozzi M., Mora Garrido R., Vailati P., Scaglioni R., Capsoni N., Ganassin E. C., Salinaro G., Scala C., Dal Molin A. (2022). Ultrasound-guided peripheral intravenous catheters insertion in patient with difficult vascular access: Short axis/out-of-plane versus long axis/in-plane, a randomized controlled trial. The Journal of Vascular Access, 23(4), 589–597. 10.1177/11297298211006996 [DOI] [PubMed] [Google Scholar]
- Riambau V., Böckler D., Brunkwall J., Cao P., Chiesa R., Coppi G., Czerny M., Fraedrich G., Haulon S., Jacobs M. J., Lachat M. L., Moll F. L., Setacci C., Taylor P. R., Thompson M., Trimarchi S., Verhagen H. J., Verhoeven E. L., Committee E. G., … Schmidli J. (2017). Editor's choice - management of descending thoracic aorta diseases: Clinical practice guidelines of the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery: The Official Journal of the European Society for Vascular Surgery, 53(1), 4–52. 10.1016/j.ejvs.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Sampson U. K., Norman P. E., Fowkes F. G., Aboyans V., Song Y., Harrell F. E., Jr, Forouzanfar M. H., Naghavi M., Denenberg J. O., McDermott M. M., Criqui M. H., Mensah G. A., Ezzati M., Murray C. (2014). Global and regional burden of aortic dissection and aneurysms: Mortality trends in 21 world regions, 1990 to 2010. Global Heart, 9(1), 171–180.e10. 10.1016/j.gheart.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Shiga T., Wajima Z., Apfel C. C., Inoue T., Ohe Y. (2006). Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: Systematic review and meta-analysis. Archives of Internal Medicine, 166(13), 1350–1356. 10.1001/archinte.166.13.1350 [DOI] [PubMed] [Google Scholar]
- Sidloff D., Choke E., Stather P., Bown M., Thompson J., Sayers R. (2014). Mortality from thoracic aortic diseases and associations with cardiovascular risk factors. Circulation, 130(25), 2287–2294. 10.1161/CIRCULATIONAHA.114.010890 [DOI] [PubMed] [Google Scholar]
- Stepinska J., Lettino M., Ahrens I., Bueno H., Garcia-Castrillo L., Khoury A., Lancellotti P., Mueller C., Muenzel T., Oleksiak A., Petrino R., Guimenez M. R., Zahger D., Vrints C. J., Halvorsen S., de Maria E., Lip G. Y., Rossini R., Claeys M., Huber K. (2020). Diagnosis and risk stratification of chest pain patients in the emergency department: Focus on acute coronary syndromes. A position paper of the Acute Cardiovascular Care Association. European Heart Journal: Acute Cardiovascular Care, 9(1), 76–89. 10.1177/2048872619885346 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Bossone E., Sawaki D., Jánosi R. A., Erbel R., Eagle K., Nagai R. (2013). Biomarkers of aortic diseases. American Heart Journal, 165(1), 15–25. 10.1016/j.ahj.2012.10.006 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Mehta R. H., Ince H., Nagai R., Sakomura Y., Weber F., Sumiyoshi T., Bossone E., Trimarchi S., Cooper J. V., Smith D. E., Isselbacher E. M., Eagle K. A., Nienaber C. A., & International Registry of Aortic Dissection (2003). Clinical profiles and outcomes of acute type B aortic dissection in the current era: Lessons from the International Registry of Aortic Dissection (IRAD). Circulation, 108(Suppl 1), II312–II317. 10.1161/01.cir.0000087386.07204.09 [DOI] [PubMed] [Google Scholar]
- Svensson L. G., Labib S. B., Eisenhauer A. C., Butterly J. R. (1999). Intimal tear without hematoma. Circulation, 99(10), 1331–1336. 10.1161/01.cir.99.10.1331 [DOI] [PubMed] [Google Scholar]
- Trimarchi S., Tolenaar J. L., Tsai T. T., Froehlich J., Pegorer M., Upchurch G. R., Fattori R., Sundt T. M., III, Isselbacher E. M., Nienaber C. A., Rampoldi V., Eagle K. A. (2012). Influence of clinical presentation on the outcome of acute B aortic dissection: Evidences from IRAD. The Journal of Cardiovascular Surgery, 53(2), 161–168. PMID: 22456637. [PubMed] [Google Scholar]
- Watanabe H., Horita N., Shibata Y., Minegishi S., Ota E., Kaneko T. (2016). Diagnostic test accuracy of D-dimer for acute aortic syndrome: Systematic review and meta-analysis of 22 studies with 5000 subjects. Scientific Reports, 6, 26893. 10.1038/srep26893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox G. (2019). Nursing patients with acute aortic dissection in emergency departments. Emergency Nurse: The Journal of the RCN Accident and Emergency Nursing Association, 27(3), 32–41. 10.7748/en.2019.e1916 [DOI] [PubMed] [Google Scholar]