Abstract
DNA methylation is one of the most important epigenetic mechanisms that governing regulation of gene expression, aberrant DNA methylation patterns are strongly associated with human malignancies. Long non-coding RNAs (lncRNAs) have being discovered as a significant regulator on gene expression at the epigenetic level. Emerging evidences have indicated the intricate regulatory effects between lncRNAs and DNA methylation. On one hand, transcription of lncRNAs are controlled by the promoter methylation, which is similar to protein coding genes, on the other hand, lncRNA could interact with enzymes involved in DNA methylation to affect the methylation pattern of downstream genes, thus regulating their expression. In addition, circular RNAs (circRNAs) being an important class of noncoding RNA are also found to participate in this complex regulatory network. In this review, we summarize recent research progress on this crosstalk between lncRNA, circRNA, and DNA methylation as well as their potential functions in complex diseases including cancer. This work reveals a hidden layer for gene transcriptional regulation and enhances our understanding for epigenetics regarding detailed mechanisms on lncRNA regulatory function in human cancers.
Keywords: lncRNA, circRNA, DNA methylation, histone modification, transcriptional regulation, regulatory network
Introduction
DNA methylation is an epigenetic modification involving the transfer of the methyl group onto the C5 position of the cytosine at CpG dinucleotide sites to form the 5-methylcytosine (5mC). It has been widely recognized for DNA methylation as a major epigenetic mechanism in regulating gene expression, genome stability and cell fate (Deaton and Bird, 2011; Moore et al., 2013). DNA methylation at promoter region could determine the regulatory activity of the target genes by regulating chromatin accessibility and blocking recruitment of transcription factors (Blattler and Farnham, 2013; Hu et al., 2013). CpG islands within promoter regions are usually unmethylated and associated with a transcriptionally permissive state in normal physiology, whereas methylated CpG islands, which are often observed in cancer, generally associated with the closed chromatin configuration and lead to gene repression (Feinberg et al., 2006). DNA methylation status alterations are well known to influence transcript abundance of many cancer-related genes, thus may define different types of “driver” events, such as cell growth, proliferation, differentiation, and apoptosis processes (Borgel et al., 2010; Jones, 2012; Kulis et al., 2015; Fialkova et al., 2017).
DNA methylation is highly spatio-temporal specific across different cell types and developmental stages, and its emergence and maintenance are complex processes under precise regulation (Lister et al., 2009; Ziller et al., 2013). In mammalian cells, transfer of the methyl group to cytosine is catalyzed by three DNA methyltransferases (DNMTs): DNMT3A, DNMT3B, and DNMT1. It is recognized that DNMT3A and DNMT3B are de novo methyltransferases that establish DNA methylation patterns early in development, whereas DNMT1 functions to preserve DNA methylation patterns from parental to daughter strand during every DNA replication cycle (Lyko, 2018). DNA demethylation is mainly mediated by the Ten-eleven translocation (TET) family members (TET1, TET2, and TET3). These enzymes are responsible for the hydroxylation of 5mC and its further oxidation, which finally get replaced by cytosine following base excision repair (Melamed et al., 2018). The DNA methylation status at particular site is not only determined by activity of DNMTs, which present limited sequence specificity (Furuta et al., 2014), but is also affected by coordinated function of other complexes, particularly chromatin-remodeling complexes and histone modification enzymes (Hervouet et al., 2018). For instance, it has been found that the maintenance of DNA methylation in heterochromatin requires the DNMT1/HDAC1 interaction and deacetylation state of histones, and the presence of 5mC is often correlated with histone deacetylation (Fuks et al., 2000). The Ubiquitin-like containing PHD Ring Finger 1 (UHRF1), which constitutes a complex with HDAC1, could interact with DNMT1 to promote DNA methylation inheritance during mid to late S phase (Liu et al., 2013; Nishiyama et al., 2020). Another example is the Polycomb Repressive Complex 2 (PRC2) protein EZH2, which has been shown to interact with DNMTs and is crucial for recruitment of DNMTs to specific loci (Vire et al., 2006; Wu et al., 2008). DNA hypermethylation observed in colon cancer could be partially regulated by interactions between DNMT3B and PRC1 or PRC2 (Jin et al., 2009). In recent years, accumulating evidence points towards long non-coding RNAs (lncRNAs) being an important piece in this jigsaw puzzle, representing a distinct class of epigenetic regulators that influence genome-wide DNA methylation patterns.
LncRNAs are defined as non-coding transcripts whose length ranges from 200 nt to more than 10 kb, and have been implicated in many physiological and pathological processes, including cancer (Cabili et al., 2011; Fatica and Bozzoni, 2014). A vast majority of lncRNAs are characterized as tissue and developmental stage specific with important functions in gene expression regulation, often act as competing endogenous RNA (ceRNA) to regulate the expression of downstream genes by binding to their common microRNA (miRNA) regulators (Ponting et al., 2009; Tay et al., 2014). In fact, lncRNAs could regulate gene expression via multiple mechanisms, including modulation of transcription, mRNA stability, translation and protein subcellular location by interacting with DNA, RNA or protein to form large complexes (Statello et al., 2021). Many lncRNAs act as scaffold or decoy to recruit or sequester other proteins or RNAs. They could affect chromatin architecture and genome organization to regulate gene expression by different mechanisms of action (Yao et al., 2019). Meanwhile, circular RNAs (circRNAs) being a new subtype of non-coding RNA formed by covalently closed loops through back splicing, now exhibit great potential with different cellular functions (Liu and Chen, 2022). They are involved in gene expression regulation by acting as sponge for miRNAs, or with other aspects of mechanisms. LncRNAs and circRNAs are widely implicated in the epigenetic regulatory mechanisms, such as DNA methylation and histone modification, and involved in the development and progression of many human malignancies (Hanly et al., 2018; Morselli and Dieci, 2022).
Evidence has indicated that transcriptional control of lncRNAs and circRNAs are similar to that of protein-coding genes (PCGs), with their expression regulated by promoter methylation status (Wu et al., 2010; Li et al., 2015; Xu et al., 2018). On the other hand, studies also indicate that they are pivotal regulators modulating the epigenome by interacting with different epigenetic factors (Ferreira and Esteller, 2018). LncRNAs and circRNAs could regulate DNA methylation via interaction with DNMTs or other genes involved in chromatin organization, thereby regulating target gene expression in diverse biological processes (Mercer and Mattick, 2013). The dynamic nature of their repertoire and plasticity for lncRNAs and circRNAs in interacting with different molecules made this crosstalk between lncRNAs and DNA methylation a complex regulatory network to be elucidated at the system level (Figure 1). Therefore, a comprehensive review for achievements of the experimentally verified regulatory relationships among lncRNA, circRNA and DNA methylation is critically needed. Here we lay emphasis on those lncRNAs and circRNAs that have been identified to regulate DNA methylation with various mechanisms, as well as their roles in cancer development. Indeed, the broad phylogenies of lncRNAs and circRNAs and their important biological roles lead to the hypothesis that they could constitute another regulatory layer that shapes the epigenetic landscape, with great potential for diagnosis, prognosis, and personalized treatment of cancer.
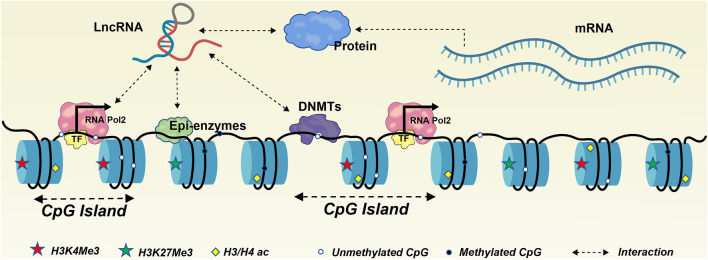
FIGURE 1.
Complex regulatory network involving lncRNAs and DNA methylation. On one hand, DNA methylation change targeting promoters of lncRNA genes may affect its expression as observed for PCGs. On another hand, lncRNAs can modulate DNA methylation and transcription of proximal and distant genes by interacting with enzymes or proteins involved in epigenetic regulation.
DNA methylation contributes to long non-coding RNA expression regulation
Beneath the aberrant cell proliferation of tumor formation is the complex interactions between a striking diversity of genetic and epigenetic factors, and the mechanisms of cancer development can be largely attributed to epimutations, which include the aberrant histone modifications and DNA hyper- and hypomethylation events across the genome (Banno et al., 2012). CpG hypermethylation is associated with specific chromatin conformation in blocking the recruitment of transcription factors, and generally promotes the transcription inhibition of tumor suppressor genes in cancer, whereas hypomethylation may lead to upregulation of oncogenes (Domcke et al., 2015). LncRNAs resemble mRNAs in length and biological characteristics but lack extended open reading frames (ORFs). Most of them are transcribed by RNA polymerase II, capped, polyadenylated, and often spliced, thus it is not surprise lncRNAs share similar epigenetic regulatory mechanisms with PCGs (Okazaki et al., 2002; Sati et al., 2012; Hangauer et al., 2013). This was confirmed by the observation of the lncRNA promoter methylation alterations in cancers (Yan et al., 2015), and also by the altered expression of numerous lncRNAs in response to the treatment with DNA methylation inhibitor 5-aza-2′-deoxycytidine (5-AZA-CdR) (Cao et al., 2016). Many lncRNAs that undergo cancer-associated methylation changes are found at the crossroads of key oncogenic pathways (Table 1). For example, a p53-induced lncRNA TP53TG1 present promoter hypermethylation in gastric and colon cancers. This lncRNA was found to interact with the DNA/RNA binding protein YBX1, impede its nuclear localization and prevent YBX1-mediated activation of other oncogenes (Diaz-Lagares et al., 2016). Another example is the tumor suppressor lncRNA GAS5 (Growth Arrest-Specific transcript 5), which was found downregulated in gastric cancer via promoter hypermethylation. This lncRNA plays a key role in adriamycin sensitivity, and represents a novel marker of prognosis and potential therapeutic target for gastric cancer (Sun et al., 2014; Zhang et al., 2016). LncRNA CRNDE presents promoter hypermethylation and downregulated expression in B lymphocytes of chronic lymphocytic leukemia (CLL) patients. It acts as a competing endogenous RNA (ceRNA) to repress miR-28, thereby regulating NDRG2 expression. Overexpression of CRNDE by DNA methylation inhibitor 5-AZA-CdR promotes NDRG2 expression, thereby inhibit cell proliferation and promote apoptosis in CLL (Ni et al., 2021).
TABLE 1.
Representative lncRNAs whose expression regulated by promoter methylation.
| LncRNA name | Methylation pattern | Tissue/disease | Target | Function | References |
|---|---|---|---|---|---|
| TP53TG1 | Hypermethylation | Gastric cancer; colon cancer | YBX1 | Cellular death resistance | Diaz-Lagares et al. (2016) |
| GAS5 | Hypermethylation | Gastric cancer | Cell proliferation promotion | Sun et al. (2014); Zhang et al. (2016) | |
| CRNDE | Hypermethylation | Chronic lymphocytic leukemia | miR-28 | Competing endogenous RNA, cell proliferation promotion | Ni et al. (2021) |
| H19 | Hypomethylation | Bladder cancer | Takai et al. (2001) | ||
| H19 | Hypomethylation | Colorectal cancer | Tian et al. (2012) | ||
| H19 | Hypomethylation | Oral squamous cell carcinoma | Lee et al. (2021) | ||
| H19 | Hypermethylation | Peripheral blood of gastric cancer patients | Hu et al. (2021) | ||
| PlncRNA-1 | Hypomethylation | Breast cancer | miR-136 | Competing endogenous RNA, epithelial–mesenchymal transition (EMT) | Kang et al. (2020) |
| Esrp2-as | Hypomethylation | Breast cancer | Cell motility and proliferation promotion | Heilmann et al. (2017) | |
| HNF1A-AS1 | Hypermethylation | Laryngeal squamous cell carcinoma | Shi et al. (2020) | ||
| LINC00299 | Hypermethylation | Breast cancer (TNBC) | Manoochehri et al. (2020) | ||
| LINC00472 | Hypermethylation | Gastric cancer | Tsai et al. (2019) | ||
| RP11-713P17.4 | Hypermethylation | Breast cancer | Pangeni et al. (2022) | ||
| SNHG12 | Hypermethylation | Glioblastoma | miR-129-5p | Competing endogenous RNA | Lu et al. (2020) |
| SNHG11 | Hypermethylation | Colorectal cancer | Promote CRC cell migration and metastasis under hypoxia | Xu et al. (2020) | |
| CCND2 AS1 | Hypomethylation | Cervical cancer | Inhibited the proliferation and cell cycle progression | Zhao et al. (2020a) | |
| SOX21-AS1 | Hypomethylation | Cervical cancer | Regulation of the Wnt signaling pathway | Du et al. (2021a) | |
| H19 | Hypomethylation | Nasopharyngeal carcinoma | Ng et al. (2003) | ||
| H19 | Hypomethylation | Colorectal cancer | Cui et al. (2002) | ||
| H19 | Hypermethylation | Cervical cancer | Roychowdhury et al. (2020) | ||
| MEG3 | Hypermethylation | Esophageal squamous cell carcinoma | miR-9 | Competing endogenous RNA, promote cell proliferation and invasion | Dong et al. (2017) |
| PLUT | Hypermethylation | Lung adenocarcinoma | Kim-Wanner et al. (2020) | ||
| LINC00473 | Hypermethylation | Colorectal cancer | Ruiz-Banobre et al. (2022) | ||
| MEG3 | Hypermethylation | Breast cancer | Pan et al. (2022) | ||
| LINC00261 | Hypermethylation | Pancreatic cancer | C-myc | Repressing c-Myc expression | Liu et al. (2020c) |
| BLAT1 | Hypomethylation | Breast cancer | Increased apoptosis, accumulation of DNA damage | Han et al. (2018) | |
| LINC00886 | Hypermethylation | Laryngeal squamous cell carcinoma | Mitigated cell proliferation, migration and invasion, VEGFA/PI3K/AKT signaling pathways and epithelial-mesenchymal transition | Lan et al. (2020) | |
| SSTR5-AS1 | Hypermethylation | Laryngeal squamous cell carcinoma | E-cadherin | Inhibits laryngeal carcinoma cells proliferation, migration and invasion | Wang et al. (2019a) |
| GAS5 | Hypermethylation | Cervical cancer | Inhibited proliferation, cell cycle progression, invasion, migration while inducing apoptosis | Yang et al. (2019b) | |
| MALAT1 | Hypomethylation | Non-small cell lung cancer | CXCL5 | Decrease cell migration and invasion | Guo et al. (2015) |
| TRPM2-AS1 | Hypomethylation | Colorectal cancer | Promote proliferation and drug resistance of colorectal cancer cell | Ghasemi et al. (2021) |
In addition to promoter hypermethylation, hypomethylation is also widely observed for many lncRNA genes. For instance, the well-known lncRNA H19 displays aberrant promoter hypomethylation in many different cancer-types, including bladder cancer (Takai et al., 2001), colorectal cancer (Tian et al., 2012), and oral squamous cell carcinoma (Lee et al., 2021). One exception was found in the peripheral blood of gastric cancer patients, where hypermethylation of H19 was observed that associated with poor prognosis (Hu et al., 2021). Another lncRNA PlncRNA-1 was found hypomethylated in breast cancer tissue and accompanied by overexpression. It also functions as a ceRNA in the regulatory axis of miR-136—Smad3, regulating epithelial–mesenchymal transition (EMT) (Kang et al., 2020). Besides proximal promoter regions, aberrant DNA methylation at enhancer region has also been observed for lncRNA genes. For example, hypomethylation of the enhancer mapping to Esrp2-as is associated with its overexpression in breast cancer. This lncRNA locates in proximity to Esrp2 (epithelial splicing regulatory protein 2), coordinated overexpression of Esrp2 and Esrp2-as inversely correlates with hypomethylation in the enhancer and promotes cell motility and proliferation (Heilmann et al., 2017). Some other representative examples of aberrant methylation of lncRNA promoter in different cancers are summarized in Table 1.
In recent years, circRNA as another important class of non-coding RNAs has gained much attention due to its promising regulatory roles in cellular systems. CircRNAs are generated from precursor mRNA and are derived from non-canonical back-splice junction by linking 3′ splice site to a downstream 5′ splice site (Ashwal-Fluss et al., 2014). In this case, circRNA are thought to share the same transcription regulatory mechanism with their host genes. A previous study found a group of six circRNAs with their host genes undergo cancer-specific hypermethylation-associated transcriptional silencing, this phenomenon is suggested to be wide spread among different types of human malignancies (Ferreira et al., 2018). Another example was from multiple myeloma (MM), circRNA ciRS-7 is downregulated in MM cells with immunomodulatory drug resistance. The decrease of its expression is associated with promoter hypermethylation of its host gene LINC00632 (Jakobsen et al., 2021). However, evidence also suggests that many circRNAs may be transcriptionally regulated independently from their linear isoforms, resulting in different levels between their expression and that of their cognate linear mRNAs (Salzman et al., 2013; Rybak-Wolf et al., 2015). But the detailed mechanism of epigenetic regulation on circRNA biogenesis is largely unknown and remains further investigation.
It is worth noting that improvements in high-throughput sequencing technologies have led to the development of DNA methylome approaches, such as Whole Genome Bisulfite Sequencing (WGBS), Reduced Representation Bisulfite Sequencing (RRBS), DNA Immunoprecipitation Sequencing (MeDIP-seq), Methylation-sensitive restriction enzyme digestion sequencing (MRE-seq) and Human Methylation BeadChip Array (450K, EPIC). These technologies allow comprehensive characterization of human cancers via integrative analyses of genome, epigenome, and transcriptome data, and enable identification of global aberrant epigenetic patterns implicating deregulated lncRNAs and circRNAs. For example, by applying a combined strategy of MeDIP-seq and MRE-seq, Zhang et al. (2014) investigated the genome-wide DNA methylome profile in endometrial cancer, with hundreds of differentially methylated regions (DMRs) identified that co-localized with the promoters of lncRNA genes, including the well-known Xist which is critical for establishing inactivation of the X chromosome. Another study based on integrative analysis of MeDIP-seq and RNA-seq data identified differentially methylated lncRNAs in bladder cancer, with 26 lncRNAs presenting reverse correlation between methylation and expression (Zhang et al., 2019). Another integrative analysis of RRBS and RNA-seq, now in lung cancer, identified eight lncRNAs whose expression are associated with methylation in promoter regions (Sun et al., 2021). Due to the complex processing procedures and high cost of high-throughput sequencing based methylome technology, studies that identify global DNA methylation patterns for lncRNAs are still limited. For this reason, the Illumina Infinium Human Methylation450 BeadChip Array and its successor, the MethylationEPIC Array, are now commonly used to investigate DNA methylation profiles for different scenarios. Many studies have developed re-annotation strategies to identify array probes located in genome loci that associated with lncRNAs and to obtain lncRNA methylation profiles for a large number of samples (Zhi et al., 2014; Zhi et al., 2018). For example, one study performed in-depth characterization of DNA methylation landscape of lncRNA genes in 20 cancer types from The Cancer Genome Atlas (TCGA), discovering that the expression of lncRNAs is recurrently activated in tumors by hypomethylation. Overexpression of lncRNA EPIC1 was identified to enhance tumor growth in vitro and in vivo for breast cancer, and is associated with poor prognosis of the patients (Wang et al., 2018b). Many other studies utilized bioinformatics and systems biology approaches to investigate differential methylation patterns of lncRNAs and their associated functions at pan-cancer wide (Ma et al., 2017; Xiao et al., 2018; Li et al., 2020; Ji et al., 2020; Xu et al., 2021; Zhong et al., 2021; Zhao et al., 2022). Although most of these DNA methylation related lncRNA dysregulation remains further confirmation and mechanism investigation, these current progresses indicate that many lncRNA genes are recurrently targeted by DNA methylation alterations in tumors, and could play an important role in tumor initiation and progression, and are worth being further evaluated for usage as cancer biomarkers.
Long non-coding RNAs as DNA methylation regulator
One of the major advances for functional study of lncRNAs over the past decade has been their participation in epigenetic control. The regulation by lncRNAs on DNA methylation has been proved to be an important mechanism that controls gene expression during cancer development (Ferreira and Esteller, 2018). For instance, we have previously shown that the well-known lncRNA HOTAIR is associated with methylation profile enriched for polycomb group target (PCGT) genes in ovarian cancer, this HOTAIR-associated DNA methylation signature could serve as biomarkers for mesenchymal differentiation and also as for carboplatin resistance of the tumor cell (Teschendorff et al., 2015). LncRNA associated DNA methylome deviation is achieved through direct or indirect interactions with DNMT or TET members to recruit or sequester these enzymes from specific genome loci, resulting in promotion or repression of the DNA methylation in cis or in trans. HOTAIR and some other lncRNAs, such as particle, are found to recruit epigenetic modifiers to RNA binding loci in the genome by formation of triple helix, which functions to modulate global methylation in cancer cells (Kalwa et al., 2016; O'Leary et al., 2017). The effect of lncRNAs on DNA methylation dysregulation of their target genes affects multiple cellular regulatory networks, revealing their importance for tumorigenesis and progression.
Long non-coding RNAs interact with DNA methyltransferases
As the core enzyme involved in DNA methylation, interfering with DNMTs could be the most effective way for its function disturbance. Many lncRNAs were identified that physically interact with DNMTs to regulate methylation on target genes (Figure 2A). Merry et al. (2015) discovered 148 lncRNAs that interact with DNMT1 in colon cancer by using the RNA immunoprecipitation sequencing (RIP-seq) method. Among these, one named DACOR1 (DNMT1-associated colon cancer repressed lncRNA 1), which presents downregulated expression in colon cancer, was identified to interact with DNMT1 and recruit this macromolecular complex at specific genomic sites to influence DNA methylation and gene expression. Induction of DACOR1 in colon cancer cells results in global hypermethylation at multiple loci without changing the DNMT1 expression level, many of the hypermethylated regions are associated with genes that participate in cancer related pathways, such as TGF-β/BMP signaling (Somasundaram et al., 2018). Similarly, another lncRNA SAMD12-AS1 was found highly up-regulated in gastric cancer. SAMD12-AS1 may facilitate the repression of p53 by recruiting DNMT1, thus promoting the progression of gastric cancer (Lu et al., 2021). In chronic myelocytic leukemia (CML), the lncRNA HOTAIR was found to enhance the methylation of PTEN promoter by recruiting DNMT1. Overexpression of HOTAIR could facilitate the proliferation, invasion, and migration of CML cells (Song et al., 2021). Besides PCGs, lncRNAs associated DNA methylation dysregulation are also widely found in promoters of other types of ncRNAs, such as miRNA. In hepatocellular carcinoma (HCC), miR-122 was identified as the methylation target of HOTAIR, the downregulated expression of miR-122 by HOTAIR leads to the activation of oncogene Cyclin G1 and promotion of tumorigenesis in HCC (Cheng et al., 2018). Another example is TINCR, this lncRNA can recruit DNMT1 to the promoter of miR-503 gene in breast cancer. Overexpression of TINCR could increase methylation and suppress the transcription of miR-503-5p. Of note, TINCR can also act as a ceRNA for miR-503-5p to regulate EGFR and interfere with JAK2–STAT3 signaling (Wang et al., 2021).
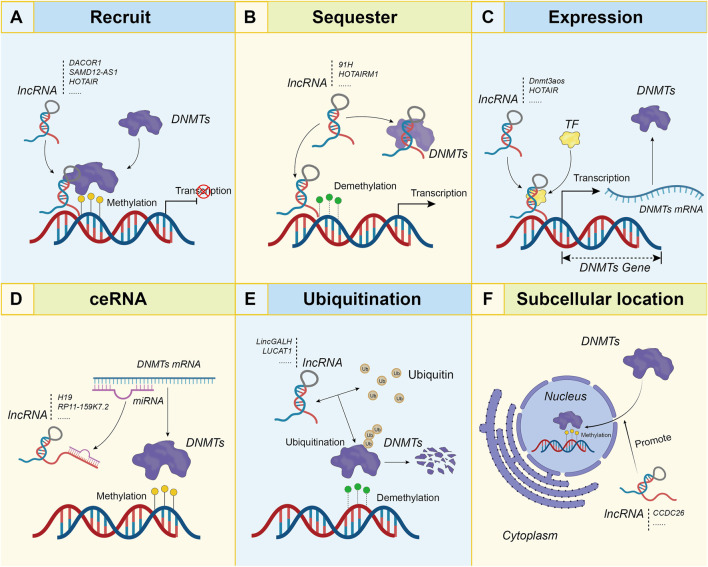
FIGURE 2.
Detailed mechanism for DNA methylation regulation by lncRNAs in direct mode. (A). LncRNAs recruit DNMTs to genome loci; (B). LncRNAs sequester DNMTs from genome loci; (C). LncRNAs regulate expression level of DNMTs; (D). LncRNAs function as ceRNA to regulate DNMT expression level; (E). LncRNAs influence the ubiquitination of DNMT proteins to affect the degradation. (F). LncRNAs promote subcellular location of DNMT proteins. It is worth noting that similar mechanisms also applies to TET family members.
Besides the recruitment mechanism, lncRNA also sequester DNMTs from particular genome loci by a competitive interaction mode (Figure 2B). A lncRNA arising from the CEBPA gene locus termed ecCEBPA could compete with DNMT1, thus inhibit methylation of CEBPA gene and facilitate CEBPA expression in leukemic cells. (Di Ruscio et al., 2013). This lncRNA was later identified to interact with DNA strand by forming a DNA:RNA triple helices and protect regions near its binding site from methylation (Ogunleye et al., 2021). Another lncRNA, named 91H which located at the H19/IGF2 locus and transcribed in H19 antisense orientation, is overexpressed in breast cancer and prevent the maternal allele at the H19/IGF2 locus from DNA methylation, by this mechanism to induce overexpression of oncogenic H19 (Vennin et al., 2017). LncRNA HOTAIRM1 (HOX antisense intergenic RNA myeloid 1), which is located between the HOXA1 and HOXA2 genes, could interact with DNMTs and other epigenetic factors to sequester them away from HOXA1 promoter in glioblastoma multiforme (GBM). Upregulation of HOTAIRM1 could lead to reduced methylation levels of HOXA1 and finally to its upregulation of expression (Li et al., 2018). A similar observation was found in dental follicle stem cells (hDFSCs), in which HOTAIRM1 binding to the CpG islands of the HOXA2 promoter and reduce the binding of DNMT1 at the HOXA2 promoter, resulting in HOXA2 hypomethylation and deviant induction (Chen et al., 2020). These examples indicate that this regulatory mechanism by HOTARIM1 within the HOXA cluster could be universal across tissues and diseases.
LncRNAs are also found to interact with other DNA methyltransferases in addition to DNMT1 to influence the methylation pattern of target genes. For instance, lncRNA HOTAIR was shown to recruit DNMT3B to increase HOXA5 promoter methylation and silence its expression in acute myeloid leukemia (AML). HOTAIR silence and HOXA5 activation were found to induce apoptosis and reduce proliferation of AML cells (Wang et al., 2019d). Another lncRNA MROS-1 was found to modulate tumor suppressor PRUNE2 expression by interacting with DNMT3A in oral squamous cell carcinoma (OSCA). Higher methylation levels of PRUNE2 promoter induced by MROS-1 were associated with cell migration and metastases (Su et al., 2021). The lncRNA TTTY15 could interact with DNMT3A and prevent its binding to TBX4 promoter in non-small cell lung cancer (NSCLC), the lower expression level of TTTY15 and the associated downregulation of TBX4 is connected with metastasis and worse prognosis of NSCLC patients (Lai et al., 2019).
Besides interacting with DNMT proteins, lncRNAs could also regulate their expression level with different mechanisms (Figure 2C). For instance, one lncRNA named Dnmt3aos (DNA methyltransferase 3A, opposite strand) located on the antisense strand of DNMT3A was found to participate in the regulation of DNMT3A expression. Dnmt3aos is highly expressed in M(IL-4) macrophages, which leads to the highly coordinated expression of this sense-antisense pair of DNMT3A and Dnmt3aos. Elevated expression of Dnmt3aos and DNMT3A results in global DNA methylation changes in M(IL-4) macrophages (Li et al., 2020). In small cell lung cancer (SCLC), HOTAIR was found to inhibit expression of DNMT1 and DNMT3B, thus regulating the methylation of HOXA1 to mediate chemoresistance of SCLC (Fang et al., 2016). Whereas in AML patients, HOTAIR present up-regulated expression, which leads to downregulation of PTEN via DNMT3B-dependent pathway, and lead to doxorubicin resistance (Zhou et al., 2021).
LncRNAs have long been recognized to regulate gene expression via the ceRNA mechanism, by which lncRNAs act as a “sponge” to combine with miRNAs and sequester their interactions with mRNAs to de-repress the expression of targets. Many examples have been found for lncRNAs that regulate the expression of DNMTs as ceRNA (Figure 2D). In laryngeal squamous cell carcinoma (LSCC), H19 was found to be the sponge for miR-148a-3p, through which to regulate DNMT1 expression. Overexpression of H19 in LSCC leads to elevated expression of DNMT1 and genome wide change of DNA methylation, including MGMT (Wu et al., 2016). Similar observation was also found for the RP11-159K7.2—miR-206 – DNMT3A axis in LSCC. Overexpressed RP11-159K7.2 could interact with miR-206, which binds with DNMT3A 3′-UTR. Interestingly, DNMT3A was also found to inhibit the expression of miR-206 via a DNA methylation-dependent manner, thus a feedback loop is maintained between DNMT3A and miR-206 to keep its internal balance (Wang et al., 2020). In hepatocytes, HOTAIR was found as sponge of miR-29b, which also regulates the expression of DNMT3B to regulate the methylation level of PTEN (Yu et al., 2020). Besides interactions with miRNAs, lncRNAs are also found to regulate the mRNA level of DNMTs by interacting with other proteins. For instance, the RMST, a lncRNA capable of upregulating DNMT3B expression by interaction with the RNA binding protein HuR, leads to alterations in global methylation in cancers (Peng et al., 2020).
LncRNAs could also function to regulate protein expression for DNMTs, such as by mechanism of ubiquitination (Figure 2E). In HCC, lncRNA linc-GALH overexpression could enhance the ubiquitination of DNMT1 to accelerate its degradation. In this way, linc-GALH reduces the methylation level of Gankyrin to promote its expression (Xu et al., 2019). In another example, lncRNA LUCAT1 was found to interact with DNMT1 but now to inhibit the ubiquitination in esophageal squamous cell carcinoma (ESCC). Upregulated LUCAT1 thus stabilizes DNMT1 to enhance the methylation and inhibit the expression of tumor suppressors (Yoon et al., 2018). In addition, lncRNAs could also regulate local concentration of DNMTs by interfering with its subcellular location (Figure 2F). For example, the lncRNA CCDC26 could promote DNMT1 localization from cytoplasm to nucleus. In absence of CCDC26, DNMT1 is found mis-located in the cytoplasm, resulting in global hypomethylation (Jones et al., 2021). Examples of lncRNAs that interact with DNMTs to regulate methylation of downstream genes and their functions in cancers are summarized in Table 2.
TABLE 2.
Representative lncRNAs that regulate DNA methylation of other genes in cancers and other disease.
| LncRNA name | Cofactor | Interaction mode | Target | Tissue/cancer | Function | References |
|---|---|---|---|---|---|---|
| DACOR1 | DNMT1 | Recruit | Genome wide | Colon cancer | TGF-β/BMP signaling | Merry et al. (2015); Somasundaram et al. (2018) |
| SAMD12-AS1 | DNMT1 | Recruit | p53 | Gastric cancer | P53 signaling pathway | Lu et al. (2021) |
| HOTAIR | DNMT1 | Recruit | PTEN | Chronic myelocytic leukemia | Song et al. (2021) | |
| HOTAIR | DNMT1 | Recruit | miR-122 | Hepatocellular carcinoma | Cyclin G1 repression | Cheng et al. (2018) |
| TINCR | DNMT1 | Recruit | miR-503-5p | Breast cancer | EGFR and JAK2–STAT3 signaling | Wang et al. (2021b) |
| ecCEBPA | DNMT1 | Sequester | CEBPA; genome wide | Di Ruscio et al. (2013); Ogunleye et al. (2021) | ||
| 91H | DNMT1 | Sequester | H19; IGF2 | Breast cancer | Vennin et al. (2017) | |
| HOTAIRM1 | DNMTs; G9a; EZH2 | Sequester | HOXA1 | Glioblastoma multiforme | Li et al. (2018) | |
| HOTAIRM1 | DNMT1 | Sequester | HOXA2 | Dental follicle stem cell | Osteogenesis | Chen et al. (2020b) |
| HOTAIR | DNMT3B | Recruit | HOXA5 | Acute myeloid leukemia | Apoptosis | Wang et al. (2019d) |
| MROS-1 | DNMT3A | Recruit | PRUNE2 | Su et al. (2021) | ||
| TTTY15 | DNMT3A | Sequester | TBX4 | Non-small cell lung cancer | Metastasis | Lai et al. (2019) |
| Dnmt3aos | DNMT3A | Expression | Genome wide | M(IL-4) macrophage | Macrophage polarization | Li et al. (2020a) |
| HOTAIR | DNMT1; DNMT3B | Expression | HOXA1 | Small cell lung cancer | Chemoresistance | Fang et al. (2016) |
| HOTAIR | DNMT3B | Expression | PTEN | Acute myeloid leukemia | Adriacin doxorubicin resistance | Zhou et al. (2021b) |
| H19 | miR-148a-3p—DNMT1 | ceRNA | MGMT; Genome wide | Laryngeal squamous cell carcinoma | Cell proliferation | Wu et al. (2016) |
| RP11-159K7.2 | miR-206—DNMT3B | ceRNA | miR-206 | Laryngeal squamous cell carcinoma | Wang et al. (2020) | |
| HOTAIR | miR-29b—DNMT3B | ceRNA | PTEN | Hepatocytes | Liver fibrosis | Yu et al. (2020a) |
| RMST | HuR—DNMT3B | RNA stability | Genome wide | Peng et al. (2020) | ||
| Linc-GALH | Ubiquitin—DNMT1 | Ubiquitination | Gankyrin | Hepatocellular carcinoma | AKT signaling | Xu et al. (2019c) |
| LUCAT1 | Ubiquitin—DNMT1 | Ubiquitination | Esophageal squamous cell carcinoma | Cell proliferation, apoptosis | Yoon et al. (2018) | |
| CCDC26 | DNMT1 | Subcellular location | Genome wide | Apoptosis | Jones et al. (2021) | |
| MAGI2-AS3 | TET1 | Recruit | MAGI2 | Breast cancer | Cell proliferation and migration | Xu et al. (2021b) |
| MAGI2-AS3 | TET2 | Recruit | LRIG1 | Acute myeloid leukaemia | Leukaemic stem cell self-renewal suppression | Chen et al. (2020a) |
| TARID | GADD45A—TET1 | Recruit | TCF21 | Arab et al. (2014); Arab et al. (2019) | ||
| HOTAIR | TET1 | Expression | SOX17; MAGI2 | Cervical cancer (Hela cell) | Wnt/β-catenin signaling | Salmeron-Barcenas et al. (2019) |
| H19 | let-7—TET1 | ceRNA | TGFBR2; TSP1 | Atherosclerotic coronary arteries | TGF-β signaling | Cao et al. (2020) |
| H19 | let-7—TET3 | ceRNA | HMGA2 | Uterine leiomyomas | Proliferation | Cao et al. (2019) |
| TETILA | TET2 | Ubiquitination; subcellular location; recruit | MMP-9 | Diabetic skin | Wound healing | Zhou et al. (2019a) |
| PYCARD-AS1 | G9a; DNMT1 | Recruit | PYCARD | Breast cancer | Miao et al. (2019) | |
| KCNQ1OT1 | HP1α | Recruit | Genome wide | Lung fibroblast | Heterochromatin reorganization | Zhang et al. (2022b) |
| LINC01133 | EZH2 | Recruit | DKK1 | Pancreatic cancer | Wnt signaling | Weng et al. (2019) |
| HOXB13-AS1 | EZH2; DNMT3B | Recruit | HOXB13 | Glioma | Xiong et al. (2018) | |
| Lnc-LALC | EZH2; DNMTs | Recruit | LZTS1 | Colorectal cancer | Liver metastasis | Zhang et al. (2021a) |
| LUCAT1 | EZH2; DNMTs | Recruit | CXXC4; SFRP2 | Gastric cancer | Wnt/β-catenin signaling | Byun et al. (2020) |
| SNHG22 | EZH2; DNMT1 | Recruit | miR-16-5p | Hepatocellular carcinoma | Cell proliferation | Zhang et al. (2021c) |
| GIHCG | EZH2; DNMT1 | Recruit | miR-200b/a/429 | Hepatocellular carcinoma | Cell proliferation and migration | Sui et al. (2016) |
| SChLAP1 | EZH2; DNMT3A; miR-340-5p—DNMT3A | Recruit; expression | miR-340-5p; miR-143-3p; miR-145-5p | Prostate cancer | Cell proliferation and migration | Huang and Tang, (2021) |
| HOXA11-AS | EZH2; LSD1; DNMT1; miR-1297—EZH2 | Recruit; ceRNA | PRSS8; KLF2 | Gastric cancer | Cell proliferation, migration and apoptosis | Sun et al. (2016) |
| LINC00470 | miR-101—EZH2; miR-101—EED | ceRNA | ELFN2 | Glioblastoma | Cell autophagy | Liu et al. (2018) |
| H19 | SAHH | Interaction | Nctc1; genome wide | Zhou et al. (2015) | ||
| H19 | SAHH | Interaction | HNF4α | Liver of metformin-exposed fetuses | Liver development and function | Deng et al. (2017) |
| H19 | SAHH | Interaction | Beclin1 | Breast cancer | Autophagy | Wang et al. (2019c) |
| H19 | SAHH | Interaction | LINE-1 | Lung | Fu et al. (2018) | |
| SNHG6 | miR-1297—MAT2A; MAT1A | ceRNA; subcellular location | Genome wide | Hepatocellular carcinoma | Guo et al. (2018) | |
| LINC00662 | MAT1A; SAHH | Interaction | Genome wide | Hepatocellular carcinoma | Guo et al. (2020) | |
| PARTICLE | G9a; SUZ12 | Recruit | MAT2A | Breast cancer cell line | Response to irradiation | O'Leary et al. (2015) |
| LINC00261 | DNMTs | Recruit | DYPD | Esophageal cancer | 5-fluorouracil resistance | Lin et al. (2019) |
| LINC01419 | DNMTs | Recruit | GSTP1 | Esophageal cancer | 5-fluorouracil resistance | Chen et al. (2019b) |
| LINC00673 | DNMTs | Recruit | KLF4 | Prostate cancer | Paclitaxel resistance | Jiang et al. (2020) |
| LINC00628 | DNMTs | Recruit | LAMA3 | Lung adenocarcinoma | Vincristine resistance | Xu et al. (2019b) |
| LINC00607 | DNMTs | Recruit | CASP9 | Thyroid cancer | Doxorubicin resistance | Li et al. (2021a) |
| 91H | DNMTs | Recruit | CDK4 | Osteosarcoma | Tumor migration and invasion | Cheng et al. (2021) |
| H19 | DNMT3B | Expression | Genome wide | Endometrial cancer; breast cancer | Cell proliferation | Zhong et al. (2017) |
| HOTAIR | EZH2; DNMTs | Interaction | ALDH1A1 | Ovarian cancer | Spheroid formation and colony-forming | Wang et al. (2021c) |
| HOTAIR | miR-126—DNMT1 | ceRNA | CDKN2A | Osteosarcoma | Cell viability and apoptosis | Li et al. (2017) |
| LINC00240 | miR-124-3p—DNMT3B | ceRNA | miR-124-3p | Gastric cancer | Cell proliferation, invasion and migration | Li et al. (2020c) |
| XIST | miR-149-5p—DNMT3A | ceRNA | miR-149-5p | Cartilage | Cell proliferation, apoptotic and ENC degradation | Liu et al. (2020d) |
| HOTTIP | miR-101—DNMT3B | ceRNA | HoxA13 | Cartilage | Cartilage development and destruction | Kim et al. (2013) |
| IRAIN | DNMT1; DNMT3A; DNMT3B | Recruit | VEGFA | Renal carcinoma | Cell proliferation, migration and apoptosis | Li et al. (2020b) |
| AS1DHRS4 | G9a; EZH2 | Recruit | DHRS4L1; DHRS4L2 | Li et al. (2012) | ||
| PRKCA-AS1 | DNMT1 | Recruit | PRKCA | Heart | p38/MAPK pathway | Xie et al. (2021) |
| LINC00518 | DNMT1; DNMT3A; DNMT3B | Recruit | CDX2 | Breast cancer | Cell proliferation, invasion, migration and EMT | Wang et al. (2019b) |
| RCPCD | DNMT1; DNMT2; DNMT3 | Recruit | HCN4 | Embryonic stem cells | Differentiation of ESCs into pacemakelike cells | Zhu et al. (2021) |
| LINC00313 | DNMT1; DNMT3B | Recruit | ALX4 | Thyroid cancer | AKT/mTOR signaling, cell proliferative, migratory, invasive abilities as well as EMT | Zhao and Hu, (2019) |
| LINC00152 | DNMTs | Recruit | BRCA1/PTEN | Breast cancer | Tumorigenesis and metastasis | Wu et al. (2018) |
| LINC00470 | DNMT3A | Recruit | PTEN | Endometrial cancer | Cell invasiveness, migration and angiogenesis, facilitate tumorigenesis and metastasis | Yi et al. (2021) |
| LINC00922 | DNMT1; DNMT3A; DNMT3B | Recruit | NKD2 | Breast cancer | Wnt signaling pathway | Wang et al. (2021d) |
| LINC01419 | DNMT1; DNMT3A; DNMT3B | Recruit | ZIC1 | Hepatocellular carcinoma | PI3K/Akt signaling pathway, tumor formation and metastasis | Hou et al. (2021) |
| MIR210HG | DNMT1 | Recruit | CACNA2D2 | Non-small cell lung cancer | Cell proliferation and migration | Kang et al. (2019) |
| SNHG1 | DNMT1 | Recruit | Bcl-2 | Sepsis | Cell inflammation and apoptotic | Zhang et al. (2022a) |
| ADAMTS9-AS2 | DNMT1; DNMT3A; DNMT3B | Recruit | CDH3 | Esophageal cancer | Cell proliferation, invasion and migration | Liu et al. (2020a) |
| ELFN1-AS1 | DNMT1; DNMT3A; DNMT3B | Recruit | ZBTB16 | Gastric cancer | PI3K/AKT signaling pathway | Zhuang et al. (2022) |
| IGF2-AS | DNMT1 | Recruit | IGF2 | Breast cancer | PI3K/AKT/mTOR signaling pathway | Zhang et al. (2021d) |
| NEAT1 | G9a; DNMT1; Snail | Recruit | CDH1 | Osteosarcoma | Metastasis in vitro and in vivo, EMT | Li and Cheng, (2018) |
| PCAT-14 | DNMT1; DNMT3A; DNMT3B | Recruit | miR-372 | Hepatocellular carcinoma | Cell proliferation, invasion, cell cycle arrest | Wang et al. (2017) |
| HAGLR | DNMT1 | Recruit | E2F1 | Lung adenocarcinoma | Cell growth | Guo et al. (2019) |
| XIST | DNMT1; DNMT3A; DNMT3B | Recruit | TIMP-3 | Cartilage | Collagen degradation | Chen et al. (2019a) |
| RAMP2-AS1 | DNMT1; DNMT3B | Recruit | CXCL11 | Breast cancer | Tumor growth | Li et al. (2022a) |
| TNRC6C-AS1 | DNMT1; DNMT3A; DNMT3B | Recruit | STK4 | Thyroid cancer | Hippo signaling pathway | Yang et al. (2019a) |
| yylncT | DNMT3B | Recruit | Embryo | Embryonic cell fate transition | Frank et al. (2019) | |
| SNHG1 | DNMT1 | Expression | PTBP1 | Bone marrow | Adipogenic differentiation and contributed to osteoporosis | Yu et al. (2022a) |
| FAS-AS1 | DNMT3B | Expression | SIRT1; FAS | Leukemia | Yuan et al. (2020) | |
| Linc-POU3F3 | EZH2; DNMT1; DNMT3A; DNMT3B | Recruit | POU3F3 | Esophageal squamous cell carcinoma | Cell proliferation and ability to form colonies | Li et al. (2014) |
| PVT1 | EZH2; DNMT1 | Recruit | miR-18b-5p; HIF1A | Gallbladder cancer | Cell proliferation | Jin et al. (2020) |
| ROIT | DNMT3A | Ubiquitination | Nkx6.1 | Pancreas islet | Glucose homeostasis and insulin transcription | Zhang et al. (2020a) |
| Platr10 | TET1 | Recruit | Oct4 | Modulating chromatin architecture | Du et al. (2021b) | |
| WT1-AS | TET2; TET3; DNMTs | Recruit | WT1 | Leukemia | McCarty and Loeb, (2015) | |
| NEAT1 | DNMTs | Recruit | miR-129-5p; WNT4 | Breast cancer | WNT signaling | Lo et al. (2016) |
| Evf2 | MECP2 | Recruit | DLX1/2 | Forebrain | Berghoff et al. (2013) | |
| NKILA | NF-κB; DNMT3A | Recruit | KLF4 | Vascular endothelium | Endothelium inflammation | Zhu et al. (2019) |
| HOTAIR | DNMT1; DNMT3B; EZH2 | Expression | HOXA1 | Small cell lung cancer | Multidrug resistance | Fang et al. (2018) |
| ANRIL | EZH2 | Recruit | ERRFI1 | Cholangiocarcinoma | Cell proliferation and migration | Yu et al. (2020b) |
| LINC00858 | DNMTs | Recruit | WNK2 | Colon cancer | Cell apoptosis, autophagy and senescence | Wu et al. (2020) |
| UCA1 | EZH2 | Recruit | p21 | Breast cancer | PI3K/AKT signaling pathway | Li et al. (2019) |
| H19 | EZH2 | Recruit | BIK | Breast cancer | Paclitaxel (PTX) resistance | Si et al. (2016) |
| AC092723.1 | TET1 | Recruit | IRF8 | Zhou et al. (2022) | ||
| HOTAIR | EZH2 | Recruit | E-cadherin | Oral squamous cell carcinoma | Cell invasion, migration and apoptosis | Wu et al. (2015) |
| LINC00887 | DNMT1 | Recruit | CA9 | Tongue squamous carcinoma | Suppress oncogenic CA9 | Shen et al. (2021b) |
| LINC00472 | DNMTs | Recruit | MCM6 | Breast cancer | Inhibite tumor growth and metastasis | Shao et al. (2021) |
| LINC01270 | DNMTs | Recruit | GSTP1 | Esophageal cancer | Cell proliferation, migration, invasion and drug resistance | Li et al. (2021b) |
| HOTAIR | DNMTs | Recruit | MTHFR | Esophageal cancer | Cell apoptosis and proliferation | Zhang et al. (2020b) |
| BZRAP1-AS1 | DNMT3B | Recruit | THBS1 | Hepatocellular carcinoma | Angiogenesis and tumor growth | Wang et al. (2019e) |
| PVT1 | DNMT1 | Recruit | BNIP3 | Gastric cancer | Cell proliferation | Xin et al. (2021) |
| SNHG3 | EZH2 | Recruit | MED18 | Gastric cancer | Cell migration and invasion | Xuan and Wang, (2019) |
| HOTAIR | DNMT1; EZH2 | Recruit | miR-454-3p | Gastric cancer | Cell apoptosis and autophagy | Bao et al. (2017) |
| LINC00630 | DNMT3B; EZH2 | Recruit | BEX1 | Colorectal cancer | Cell apoptosis and radio-resistance | Liu et al. (2020b) |
| Lnc34a | DNMT3A; PHB2 | Recruit | miR-34a | Colorectal cancer | Cell proliferation | Wang et al. (2016) |
| SATB2-AS1 | TETs; GADD45A | Recruit | SATB2 | Colorectal cancer | Cell metastasis and immune response | Xu et al. (2019a) |
| Dali | DNMT1 | Recruit | Pou3f3; genome wide | Central nervous system | Cell differentiation | Chalei et al. (2014) |
| Dum | DNMT1; DNMT3A; DNMT3B | Recruit | Dppa2 | Skeletal myoblast cell | Myogenesis | Wang et al. (2015) |
| lincRNA-p21 | HNRNPK – DNMT1; SETDB1 | Recruit | Nanog | Pluripotent stem cell | Cell differentiation | Bao et al. (2015) |
| Kcnq1ot1 | DNMT1; EZH2; G9a | Recruit | Kcnq1 | Placenta | Gene imprinting | Mohammad et al. (2010) |
| THAP9-AS1 | DNMTs | Recruit | SOCS3 | Osteosarcoma | JAK2/STAT3 signaling | Yang et al. (2021b) |
| H19 | PRC2 | Recruit | genome wide | Neuroendocrine prostate cancer | Metastatic | Singh et al. (2021) |
| KCNQ1OT1 | DNMT1 | Recruit | PTEN | Triple negative breast cancer | Cell proliferation, invasion, and migration | Shen et al. (2021a) |
| KCNQ1OT1 | DNMT1; DNMT3A; DNMT3B | Recruit | EIF2B5 | Ovarian cancer | Metastasis | He et al. (2022) |
| KAT7 | DNMTs | Recruit | miR-10a | Non-small cell lung cancer | Gao et al. (2021) | |
| SNHG3 | miR-448—DNMT1 | ceRNA | SEPT9 | Gastric cancer | Cell growth, metastasis | Li et al. (2022d) |
| KIF9-AS1 | DNMT1 | Recruit | RAI2 | Hepatocellular carcinoma | Cell proliferation, migration, apoptosis | Yu et al. (2022b) |
| PVT1 | EZH2; DNMT1 | Recruit | ZBP1 | Liver cell | Response to nonylphenol | Qiannan et al. (2022) |
| ZFAS1 | DNMT3B | Recruit | Notch1 | Myocardial ischemia-reperfusion injury | Apoptosis | Li et al. (2022b) |
| UCA1 | EZH2; DNMT1 | Recruit | APAF1 | Myocardial ischemia-reperfusion injury | Jin et al. (2022) | |
| LINC01270 | DNMT1; DNMT3A; DNMT3B | Recruit | LAMA2 | Breast cancer | MAPK signaling pathway | Li et al. (2022c) |
| UCA1 | DNMT1; DNMT3A; DNMT3B | Recruit | METTL14 | Breast cancer | Cell proliferation, invasion, metastasis | Zhao et al. (2022a) |
Long non-coding RNAs interact with ten-eleven translocation enzymes
DNMTs are responsible for catalyzing the conversion of cytosine to 5-mC whereas TET enzymes catalyze the successive conversion of 5mC to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) to promote locus-specific removal of methylation. DNA demethylation can be achieved either as a process in the absence of functional DNA methylation maintenance mechanism during DNA replication or through TET-mediated 5mC oxidation. In this case, regulation to TET family affects the methylation level of downstream genes as well. Studies have discovered many lncRNAs interact with TETs to regulate methylation process (Table 2). For example, lncRNA MAGI2-AS3 (MAGI2 antisense RNA 3) which is transcribed from the antisense strand near the MAGI2, acts as cis-acting factor to downregulate the DNA methylation level of the MAGI2 promoter by interaction with TET1 and promotes apoptosis by activating the Fas/FasL signaling pathway in breast cancer (Xu et al., 2021). In AML, MAGI2-AS3 recruits TET2 to the LRIG1 promoter region in trans and causes DNA demethylation of LRIG1. Downregulation of MAGI2-AS3 suppresses the self-renewal capacity of leukemic stem cell by promoting LRIG1 expression (Chen et al., 2020). LncRNAs are also found to recruit TET enzymes in an indirect mode. The lncRNA TARID (TCF21 antisense RNA inducing demethylation) could interact with both the TCF21 promoter and GADD45A protein, whereas GADD45A in turn recruits TET1 to activate the expression of TCF21 (Arab et al., 2014). The authors further show that TARID combine to TCF21 promoter to form an R-loop of DNA–RNA hybrids, which is recognized by GADD45A and then triggers TET1-dependent DNA demethylation (Arab et al., 2019).
TETs are also found to be regulated by lncRNAs at the transcriptional, posttranscriptional, and protein expression levels. In cervical cancer, the HOTAIR could regulate TET1 expression, which leads to promoter hypermethylation of Wnt/β-catenin signaling related genes. In Hela cells, upregulated HOTAIR leads to the decreased TET1 expression, which is associated with the transcriptional activity of Wnt/β-catenin pathway genes, such as PCDH10, SOX17, AJAP1, and MAGI2 (Salmeron-Barcenas et al., 2019). At the posttranscriptional level, TET1 is found to be regulated by lncRNA H19 via miRNA let-7 with ceRNA mode, TET1 expression alteration due to upregulation of H19 promotes TGF-β signaling related endothelial–mesenchymal transition in endothelial cells of atherosclerotic coronary arteries (Cao et al., 2020). A similar observation was found for TET3 in uterine leiomyomas, a H19—let-7—TET3 axis was identified for methylation regulation of fibroid-promoting gene and to drive proliferation of leiomyoma cells (Cao et al., 2019). At the protein expression level, a multifunctional lncRNA TETILA was found in diabetic skin that play a key role in wound healing. Zhou et al. (2019a) indicated this lncRNA could regulate TET2 stability through the ubiquitin-proteasome pathway and also promote TET2 nuclear translocation. In addition, TETILA also acts as a scaffold to recruit thymine-DNA glycosylase (TDG), which simultaneously interacts with TET2 at the promoter of MMP-9 for its demethylation and transcriptional activation.
Long non-coding RNAs interact with other epigenetic factors
One of the most intriguing observations have recently emerged in epigenetics is the subtle crosstalk between DNA methylation and other epigenetic modifications. Accumulating literature has revealed complex mechanisms underlying the interplay between DNA methylation and histone modification. Many partners of DNMTs have been found that involved in both of the DNA methylation and histone modification. In addition, DNA methylation status within genome present concomitant presence with other repressive marks, such as histone deacetylation. For example, HDAC1 has the ability to bind DNMT1, the histone deacetylase activity is required for DNMT1 related DNA methylation maintenance in heterochromatin (Fuks et al., 2000). DNMTs have also been identified to interact with G9a, which is responsible for mono-, di-and slowly trimethylation of histone H3 lysine 9 (H3K9). This interaction has been shown to play a role in the establishment of DNA methylation pattern for key genes in ES cells (Xin et al., 2003; Esteve et al., 2006). In addition, the PRC2 system, which has histone methyltransferase activity for H3K27me3, is connected to DNA methylation related gene silencing at specific loci. The PRC2 core component EZH2-dependent recruitment of DNMT3A was found to be associated with H3K27me3 and DNA methylation (Jin et al., 2009; Rush et al., 2009; Li et al., 2021c). This explains how lncRNAs interact with epigenetic factors to regulate DNA methylation at particular loci (Figure 3A). For instance, the PYCARD-AS1, which is antisense to the pro-apoptotic gene PYCARD, functions to induce DNA methylation and H3K9me2 modification of PYCARD promoter by recruiting the chromatin-suppressor proteins G9a and DNMT1 in breast cancer (Miao et al., 2019). Another example is the lncRNA KCNQ1OT1, which binds and recruits the heterochromatin protein HP1α, and finally lead to DNA methylation and H3K9me3 modification in the genome. One repeat-rich region within KCNQ1OT1 is identified mainly responsible for Hoogsteen base pairing with double-stranded DNA, by which to fulfill the function of protein recruitment. This observation demonstrates an example for lncRNA to induce and maintain epigenetic silencing at repetitive DNA elements, in order to safeguard against genome instability (Zhang et al., 2022). In pancreatic cancer, the upregulated LINC01133 was found to recruit EZH2 to for histone methylation and also to promote the promoter methylation of DKK1, thus activate Wnt signaling (Weng et al., 2019). LncRNA HOXB13-AS1 is found upregulated in glioma and negatively correlated with its surrounding gene HOXB13, this lncRNA could increase DNMT3B-mediated methylation of HOXB13 promoter by binding with EZH2 (Xiong et al., 2018). Similar examples include the regulation of LZTS1 by lnc-LALC during liver metastasis of colorectal cancer (Zhang et al., 2021), regulation of CXXC4 and SFRP2 by LUCAT1 in gastric cancer (Byun et al., 2020). In addition, lncRNA could also regulate promoter methylation of miRNA genes by interacting EZH2. For instance, lncRNA SNHG22 was found to recruit DNMT1 to miR-16-5p DNA promoter through EZH2 and inhibited miR-16-5p transcription via DNA methylation (Zhang et al., 2021c). LncRNA GIHCG physically associates with EZH2 and recruits EZH2 and DNMT1 to promoter regions of the miR-200b/a/429, which lead to changes of H3K27me3 and DNA methylation levels in the miR-200b/a/429 promoter, and dramatically silences their expression (Sui et al., 2016).
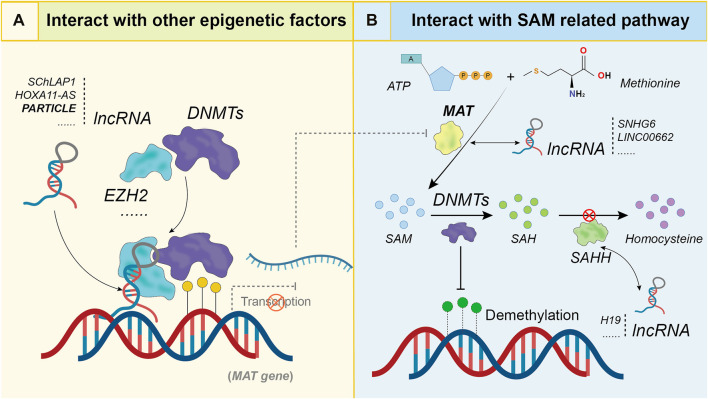
FIGURE 3.
Detailed mechanism for DNA methylation regulation by lncRNAs in indirect mode. (A). LncRNAs interact with other epigenetic factors, such as EZH2, to affect methylation level of downstream genes; (B). LncRNAs interfere with DNMT functions by interacting with S-adenosylmethionine related pathway.
It is worth noting that many miRNAs regulated by lncRNA through promoter DNA methylation are also found to regulate the upstream lncRNAs or other epigenetic factors, by which a feedback loop formed to control the internal gene expression. For example, lncRNA SChLAP1 was found to recruit EZH2 and DNMT3A to repress multiple miRNA expression in prostate cancer, including the miR-340-5p/miR-143-3p/miR-145-5p, these miRNAs in turn regulate DNMT3A expression (Huang and Tang, 2021). In gastric cancer, EZH2 along with the histone demethylase LSD1 and DNMT1 were recruit by the lncRNA HOXA11-AS, this lncRNA also acts as sponge for miR-1297, antagonizing its ability to repress EZH2 protein translation (Sun et al., 2016). In glioblastoma, LINC00470 could enhance the expression of ELFN2 through adsorption of miR-101, and also affect the methylation level of ELFN2 by decreasing H3K27me3 occupancy (Liu et al., 2018). The above examples indicate that lncRNAs are able to control genes at the transcriptional level or post-transcriptional level through a variety of different mechanisms to achieve accurate regulation of expression levels for downstream target genes.
Long non-coding RNAs interact with S-adenosylmethionine related pathway
All DNA methyltransferases are known to use S-adenosylmethionine (SAM) as the methyl donor and generate S-adenosylhomocysteine (SAH) as by-product. The methyl donor SAM is synthesized from ATP and methionine by the methionine adenosyltransferase (MAT) (Lu and Mato, 2012), whereas SAH could be eliminated by S-adenosylhomocysteine hydrolase (SAHH), SAH also acts as feedback inhibitor of DNMTs (Lyko, 2018). Regulation on the genes involved SAM synthesis or SAH degradation by lncRNAs may lead to malfunction of DNMTs to interference DNA methylation (Figure 3B). The H19 for instance, could bind to SAHH and inhibits its function of SAH hydrolyzing, then give rise to genome-wide methylation alteration (Zhou et al., 2015). This mechanism was further observed in liver of metformin-exposed fetuses to induce hypomethylation and increased expression of HNF4α (Deng et al., 2017), and also in tamoxifen-resistant breast cancer to induce the upregulation of Beclin1 (Wang et al., 2019c), as well as in human lung tissue to regulate the LINE-1 methylation (Fu et al., 2018).
Interference to MAT may result in the alteration of the SAM concentration and disturbance of DNA methylation process. This has been confirmed by the interaction between lncRNA SNHG6 and MAT family members of MAT1A and MAT2A. On one hand, SNHG6 was found to upregulate MAT2A expression by act as sponge for miR-1297, on another hand, this lncRNA also downregulate MAT1A translation by suppressing the nucleus-cytoplasmic shuttling of MAT1A mRNA, thereby regulate genome wide methylation in hepatoma cells of HCC (Guo et al., 2018). Another lncRNA LINC00662 was identified to induce decay of MAT1A mRNA and also the degradation of SAHH protein by ubiquitination mechanism, in this way to reduce SAM and enhance SAH levels, which finally leads to global hypomethylation (Guo et al., 2020). It is worth mentioning a dual functional lncRNA PARTICLE in response to low-dose irradiation. Over expressed PARTICLE upon irradiation recruits the PRC2 to the promoter region of MAT2A in a DNA-RNA triplex form, in this way to regulate MAT2A expression via methylation. The altered expression level of MAT2A lead to changed concentration of SAM, which further influence the methylation level of downstream genes (O'Leary et al., 2015) (Figures 3A,B). This triplex-mediated expression regulation based on interaction between lncRNA PARTICLE and DNA strand was further proved to be widespread in the human genome (O'Leary et al., 2017). In summary, these studies indicate that lncRNAs could regulate methylation level of downstream genes by regulating the SAM related pathway genes.
Implications of long non-coding RNA mediated DNA methylation in drug treatment of cancer
Studies have indicated that lncRNAs could modulate gene for degradation and/or elimination of endogenous and exogenous toxins or medicines, by which they are able to exert their effects on drug metabolism and response to treatment (Table 2). For example, LINC00261 was found to recruit DNMTs to the promoter of the dihydropyrimidine dehydrogenase (DYPD), which is mainly responsible for 5-fluorouracil (5-FU) degradation. Increased LINC00261 promotes the methylation level within the DPYD promoter region and leads to its downregulation in esophageal cancer. As a result, 5-FU degradation is inhibited, finally results in an elevated sensitivity to 5-FU of the cancer cell (Lin et al., 2019). Similar observations were also found for the effect of LINC01419-GSTP1 regulation in esophageal cancer (Chen et al., 2019). In prostate cancer, regulation of KLF4 promoter methylation by LINC00673 is associated with paclitaxel resistance (Jiang et al., 2020). In lung adenocarcinoma, vincristine resistance is meditated by promoter methylation of LAMA3 induced by LINC00628 (Xu et al., 2019). In thyroid cancer, LINC00607 mediates doxorubicin resistance through the regulation of CASP9 methylation (Li et al., 2021). These observations lead to the thought that the chemical drug effectiveness can be improved for better treatment by regulating the expression level of these lncRNAs.
Another possible direction for cancer treatment is to interfere with lncRNAs involved in DNA methylome regulation by using gene editing methods. One example is the lncRNA 91H which is reasonable for inducing methylation of CDK4 promoter, knockdown of this lncRNA could suppress the tumorigenesis of osteosarcoma (Cheng et al., 2021). Some small molecules directly interfering lncRNAs responsible for methylation regulation could also be efficient treatment targets. For instance, metformin was found to induce H19 repression and the genome-wide DNA methylation alterations by modulating the activity of H19—SAHH axis, this observation provides a novel explanation for the mechanism and function of the metformin for the epigenetic regulation effect in cancer (Zhong et al., 2017). In addition, some chemical compound that interrupts the HOTAIR—EZH2 interaction are found to inhibit cancer cell invasion and migration, which was thought to be a potential approach for targeted therapy of cancers (Ren et al., 2019; Wang et al., 2021). In summary, lncRNAs involved in DNA methylation regulation are promising targets for applications in cancer therapy. Representative lncRNAs currently identified that are involved in DNA methylation regulation, and the associated cofactors, interaction mode, as well as target genes are listed in Table 2. This comprehensive summary revealed us a complex interaction network based on epigenetic regulatory mechanisms that remains to be further explored. In-depth analysis of non-coding RNA and other epigenetic regulatory elements including DNA methylation at the systemic level will help us to reveal the underlying mechanisms of tumor development and development, thus providing a new perspective for personalized tumor therapy.
Role of circular RNAs in DNA methylation regulation
In recent years, circRNAs have been revealed for their crucial role during the onset and progression of human disease by their important regulatory effect. The capacity of circRNAs interact with proteins involved in epigenetic modification manifests itself the ability for the transcriptional regulation on target genes (Table 3). Examples include a circRNA termed ACR (autophagy related circular RNA), which directly binds to DNMT3B and block DNMT3B-mediated DNA methylation of Pink1 promoter. Pink1 further brings about phosphorylation of the downstream target FAM65B, and finally inhibits autophagy and cell death in the heart (Zhou et al., 2019). An exosome derived circRNA circ_6790 from bone marrow mesenchymal stem cell was found to increase the nuclear translocation of CBX7, by this indirect interaction mode to recruit DNMTs and induce the methylation of S100A11 in pancreatic ductal adenocarcinoma (Gao et al., 2022). Many circRNAs are found to regulate the expression level of DNMT genes and finally influence the downstream target methylation. For example, hsa_circ_0012919 is downregulated in CD4+ T cells of systemic lupus erythematous (SLE) and results in the increased the expression of DNMT1 and finally leads to the hypermethylation of CD70 and CD11a (Zhang et al., 2018). A similar example is the circ-Amotl1, which interacts with STAT3 and facilitate its nuclear translocation and the binding to the promoter of DNMT3A gene, the activated DNMT3A further induce miR-17 promoter methylation and decrease its expression (Yang et al., 2017). In addition, a multi-functional circRNA was found that regulate downstream methylation by different mechanisms. The circRNA derived from FLI1 termed FECR1 is able to recruit TET1 to the promoter of the host gene and lead to the hypomethylation in cis, in addition, this circRNA could also bind to the DNMT1 promoter, where it downregulates DNMT1 transcription in trans. In this manner, this circRNA regulator controls tumor growth and metastasis of breast cancer (Chen et al., 2018).
TABLE 3.
Representative circRNAs that regulate DNA methylation of other genes in cancers and other disease.
| CircRNA name | Cofactor | Interaction mode | Target | Tissue/disease | Function | References |
|---|---|---|---|---|---|---|
| ACR | DNMT3B | Recruit | Pink1 | Myocardial ischemia/infarction | Autophagy | Zhou et al. (2019b) |
| Circ_6790 | CBX7—DNMTs | Recruit | S100A11 | Pancreatic ductal adenocarcinoma | Cell proliferation, apoptosis, metastasis, immune escape | Gao et al. (2022) |
| Hsa_circ_001291 | DNMT1 | Expression | CD11a; CD70 | Systemic lupus erythematosus | Zhang et al. (2018) | |
| Circ-Amotl1 | STAT3—DNMT3A | Expression | miR-17-5p | Wound healing | Cell adhesion, migration, proliferation, wound repair | Yang et al. (2017) |
| FECR1 | TET1; DNMT1 | Recruit; expression | FLI1; SERTED2 | Breast cancer | Tumor invasion, metastasis | Chen et al. (2018) |
| Circ_0040809 | miR-515-5p—DNMT1 | ceRNA | Colorectal cancer | Cell proliferation, migration, apoptosis | Mao et al. (2021) | |
| CircSOD2 | miR-502-5p—DNMT3A | ceRNA | SOCS3 | Hepatocellular carcinoma | JAK2/STAT3 signaling | Zhao et al. (2020b) |
| CircMEMO1 | miR-106b-5p—TET1 | ceRNA | TCF21 | Hepatocellular carcinoma | Cell proliferation, invasion, metastasis, EMT, sorafenib sensitivity | Dong et al. (2021) |
| CircTRIM33–12 | miR-191—TET1 | ceRNA | WWC3; TP53INP1; ULBP1; JHDM1D | Hepatocellular carcinoma | Cell proliferation, migration, invasion, immune evasion | Zhang et al. (2019a) |
| CircIBTK | miR-29b | — | Genome wide | Systemic lupus erythematosus | AKT signaling | Zhang et al. (2019a) |
| Circ-ATAD1 | — | — | miR-34b | Acute myeloid leukemia | Cell proliferation | Wu et al. (2021b) |
| Circ-ATAD1 | — | — | miR-10a | Endometrial cancer | Cell invasion, migration | Yang et al. (2021a) |
| CircFAT1 | — | — | miR-21 | Endometrial cancer | Cell stemness increase | Wu et al. (2021a) |
| CircSEPT9 | — | — | miR-186 | Endometrial cancer | Cell invasion, migration | Guo et al. (2022) |
| CircRIMS | — | — | miR-613 | Esophageal squamous cell carcinoma | Cell proliferation | Wan et al. (2021) |
| CircSKA3 | — | — | miR-1 | Glioblastoma | Cell proliferation | Zhou et al. (2021a) |
| CircFADS2 | — | — | miR-195-5p | Osteoarthritis | Apoptosis | Zhang et al. (2021b) |
The ceRNA mechanism is also widely involved in the processes of methylation regulation by circRNAs. For example, hsa_circ_0040809 regulates cell proliferation of colorectal cancer by upregulating DNMT1 via targeting miR-515-5p (Mao et al., 2021). Another example is from HCC, the circSOD2 was activated by promoter modification of H3K27ac and H3K4me3, the activated circSOD2 inhibits miR-502-5p expression and rescues miR-502-5p target gene DNMT3A expression (Zhao et al., 2020). Similar observations include the circMEMO1—miR-106b-5p—TET1 axis (Dong et al., 2021) and circTRIM33–12—miR-191—TET1 axis (Zhang et al., 2019), which play key roles for controlling cell proliferation, migration and immune evasion. This ceRNA mechanism for downstream target methylation regulation was also found during SLE development (Wang et al., 2018). Interestingly, miRNA genes are also found to be the methylation targets of circRNA regulators. For instance, the circ-ATAD1 leads to miR-34b gene methylation in AML to increase the cell proliferation (Wu et al., 2021). This very circRNA was found to regulate miR-10a gene methylation in endometrial cancer (Yang et al., 2021). Other similar examples are also identified in many types of diseases (Table 3) (Wu et al., 2021; Zhou et al., 2021; Zhang et al., 2021; Wan et al., 2021; Guo et al., 2022). However, the detailed mechanism on how circRNA influence the methylation of miRNA gene promoters are largely unknown and remains to be further investigation.
Concluding remarks
One of the major findings in cancer epigenetics is that genes encoding lncRNAs and circRNAs are widely connected with DNA methylome regulation in tumorigenesis. First of all, lncRNAs as well as circRNAs could be targets of DNA methylation regulation bases on the canonical epigenetic regulatory mechanism. Aberrant methylation changes at lncRNA and circRNA promoters are widely observed in a variety of physiological and pathological circumstances. Studies have identified the lncRNAs and circRNAs whose transcriptional deviation are associated with aberrant promoter methylation (Lujambio et al., 2010; Morenos et al., 2014; Boque-Sastre et al., 2015; Lu et al., 2020; Pangeni et al., 2022). On the other hand, lncRNAs and circRNAs could also regulate DNA methylation level of target genes by interaction with DNMTs or other genes involved in this process, either directly or indirectly. The study of the lncRNA-DNAm interactions has shifted our understanding of gene expression and regulation. LncRNAs usually do not function alone, but by interaction with proteins or other biomolecules to play a regulatory role in different biological processes (Teng et al., 2020; Wang et al., 2021). As a rapid way for gene expression regulation, impact on target genes by lncRNAs by re-shaping the epigenome is an effective approach to adjust cell function, through which cells can respond to diverse stimuli rapidly. Given the diversity and tissue specificity of their expression pattern, lncRNAs and circRNAs taking part in multiple cellular regulatory networks have revealed their importance in various physiological processes, and also the implications in cancer. Indeed, by using a systems biology approach, we have revealed lncRNAs that constitute master regulators of the DNA methylome in pan-cancer wide, which implicated in regulating the DNA methylation and expression levels of key genes involved in cancer development as targets (Yang et al., 2021). It is likely that lncRNAs and circRNAs establish an additional layer for transcriptional and posttranscriptional regulation defined by epigenetic landscape, which leads to reconsideration of our concept about epigenetics. As summarized in this review, evidences of the regulatory networks among lncRNAs and DNA methylation in human diseases are increasing rapidly, although many important questions regarding detailed mechanism on lncRNA regulatory complexity remain to be solved. In this context, lncRNAs could be exploited not only as specific biomarkers for early diagnosis and prognosis, but also for combined epigenetic targeting of personalized treatment of cancer.
Author contributions
ZY designed the study and wrote the main manuscript text. FX prepared figures. AT edited the manuscript. YZ, LY, JL, and YH collected data. All authors read and approved the final manuscript.
Funding
This work is supported by National Natural Science Foundation of China (91959106, 31871255), Shanghai Municipal Science and Technology (2017SHZDZX01) and the Project of Science and Technology Department of Sichuan Provincial of China (2019JDJQ0035).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Arab K., Karaulanov E., Musheev M., Trnka P., Schafer A., Grummt I., et al. (2019). GADD45A binds R-loops and recruits TET1 to CpG island promoters. Nat. Genet. 51 (2), 217–223. 10.1038/s41588-018-0306-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab K., Park Y. J., Lindroth A. M., Schafer A., Oakes C., Weichenhan D., et al. (2014). Long noncoding RNA TARID directs demethylation and activation of the tumor suppressor TCF21 via GADD45A. Mol. Cell 55 (4), 604–614. 10.1016/j.molcel.2014.06.031 [DOI] [PubMed] [Google Scholar]
- Ashwal-Fluss R., Meyer M., Pamudurti N. R., Ivanov A., Bartok O., Hanan M., et al. (2014). circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 56 (1), 55–66. 10.1016/j.molcel.2014.08.019 [DOI] [PubMed] [Google Scholar]
- Banno K., Kisu I., Yanokura M., Tsuji K., Masuda K., Ueki A., et al. (2012). Epimutation and cancer: A new carcinogenic mechanism of lynch syndrome (review). Int. J. Oncol. 41 (3), 793–797. 10.3892/ijo.2012.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X., Ren T., Huang Y., Sun K., Wang S., Liu K., et al. (2017). Knockdown of long non-coding RNA HOTAIR increases miR-454-3p by targeting Stat3 and Atg12 to inhibit chondrosarcoma growth. Cell Death Dis. 8 (2), e2605. 10.1038/cddis.2017.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X., Wu H., Zhu X., Guo X., Hutchins A. P., Luo Z., et al. (2015). The p53-induced lincRNA-p21 derails somatic cell reprogramming by sustaining H3K9me3 and CpG methylation at pluripotency gene promoters. Cell Res. 25 (1), 80–92. 10.1038/cr.2014.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff E. G., Clark M. F., Chen S., Cajigas I., Leib D. E., Kohtz J. D. (2013). Evf2 (Dlx6as) lncRNA regulates ultraconserved enhancer methylation and the differential transcriptional control of adjacent genes. Development 140 (21), 4407–4416. 10.1242/dev.099390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattler A., Farnham P. J. (2013). Cross-talk between site-specific transcription factors and DNA methylation states. J. Biol. Chem. 288 (48), 34287–34294. 10.1074/jbc.R113.512517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boque-Sastre R., Soler M., Oliveira-Mateos C., Portela A., Moutinho C., Sayols S., et al. (2015). Head-to-head antisense transcription and R-loop formation promotes transcriptional activation. Proc. Natl. Acad. Sci. U. S. A. 112 (18), 5785–5790. 10.1073/pnas.1421197112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgel J., Guibert S., Li Y., Chiba H., Schubeler D., Sasaki H., et al. (2010). Targets and dynamics of promoter DNA methylation during early mouse development. Nat. Genet. 42 (12), 1093–1100. 10.1038/ng.708 [DOI] [PubMed] [Google Scholar]
- Byun H. J., Yoon J. H., Lee S. K. (2020). LUCAT1 epigenetically downregulates the tumor suppressor genes CXXC4 and SFRP2 in gastric cancer. Yonsei Med. J. 61 (11), 923–934. 10.3349/ymj.2020.61.11.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabili M. N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A., et al. (2011). Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25 (18), 1915–1927. 10.1101/gad.17446611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Song N., Zhang M., Di C., Yang Y., Lu Y., et al. (2016). Systematic study of novel lncRNAs in different gastrointestinal cancer cells. Discov. Med. 21 (115), 159–171. [PubMed] [Google Scholar]
- Cao T., Jiang Y., Li D., Sun X., Zhang Y., Qin L., et al. (2020). H19/TET1 axis promotes TGF-beta signaling linked to endothelial-to-mesenchymal transition. FASEB J. 34 (6), 8625–8640. 10.1096/fj.202000073RRRRR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T., Jiang Y., Wang Z., Zhang N., Al-Hendy A., Mamillapalli R., et al. (2019). H19 lncRNA identified as a master regulator of genes that drive uterine leiomyomas. Oncogene 38 (27), 5356–5366. 10.1038/s41388-019-0808-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalei V., Sansom S. N., Kong L., Lee S., Montiel J. F., Vance K. W., et al. (2014). The long non-coding RNA Dali is an epigenetic regulator of neural differentiation. Elife 3, e04530. 10.7554/eLife.04530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Yang S., Shao R. (2019a). Long non-coding XIST raises methylation of TIMP-3 promoter to regulate collagen degradation in osteoarthritic chondrocytes after tibial plateau fracture. Arthritis Res. Ther. 21 (1), 271. 10.1186/s13075-019-2033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Lin Z. X., Qin Y. S., She Y. Q., Chen Y., Chen C., et al. (2019b). Overexpression of long noncoding RNA LINC01419 in esophageal squamous cell carcinoma and its relation to the sensitivity to 5-fluorouracil by mediating GSTP1 methylation. Ther. Adv. Med. Oncol. 11, 1758835919838958. 10.1177/1758835919838958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Fan X., Zhu J., Chen X., Liu Y., Zhou H. (2020a). LncRNA MAGI2-AS3 inhibits the self-renewal of leukaemic stem cells by promoting TET2-dependent DNA demethylation of the LRIG1 promoter in acute myeloid leukaemia. RNA Biol. 17 (6), 784–793. 10.1080/15476286.2020.1726637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhao G., Yan X., Lv Z., Yin H., Zhang S., et al. (2018). A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 19 (1), 218. 10.1186/s13059-018-1594-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Zheng J., Hong H., Chen D., Deng L., Zhang X., et al. (2020b). lncRNA HOTAIRM1 promotes osteogenesis of hDFSCs by epigenetically regulating HOXA2 via DNMT1 in vitro . J. Cell. Physiol. 235 (11), 8507–8519. 10.1002/jcp.29695 [DOI] [PubMed] [Google Scholar]
- Cheng D., Deng J., Zhang B., He X., Meng Z., Li G., et al. (2018). LncRNA HOTAIR epigenetically suppresses miR-122 expression in hepatocellular carcinoma via DNA methylation. EBioMedicine 36, 159–170. 10.1016/j.ebiom.2018.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Zheng J., Liu X., Shi J., Gong F., Zhang X., et al. (2021). Knockdown of 91 H suppresses the tumorigenesis of osteosarcoma via inducing methylation of CDK4 promoter. Technol. Cancer Res. Treat. 20, 1533033821990006. 10.1177/1533033821990006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H., Onyango P., Brandenburg S., Wu Y., Hsieh C. L., Feinberg A. P. (2002). Loss of imprinting in colorectal cancer linked to hypomethylation of H19 and IGF2. Cancer Res. 62 (22), 6442–6446. [PubMed] [Google Scholar]
- Deaton A. M., Bird A. (2011). CpG islands and the regulation of transcription. Genes Dev. 25 (10), 1010–1022. 10.1101/gad.2037511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Mueller M., Geng T., Shen Y., Liu Y., Hou P., et al. (2017). H19 lncRNA alters methylation and expression of Hnf4α in the liver of metformin-exposed fetuses. Cell Death Dis. 8 (12), e3175. 10.1038/cddis.2017.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ruscio A., Ebralidze A. K., Benoukraf T., Amabile G., Goff L. A., Terragni J., et al. (2013). DNMT1-interacting RNAs block gene-specific DNA methylation. Nature 503 (7476), 371–376. 10.1038/nature12598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Lagares A., Crujeiras A. B., Lopez-Serra P., Soler M., Setien F., Goyal A., et al. (2016). Epigenetic inactivation of the p53-induced long noncoding RNA TP53 target 1 in human cancer. Proc. Natl. Acad. Sci. U. S. A. 113 (47), E7535–E7544. 10.1073/pnas.1608585113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domcke S., Bardet A. F., Adrian Ginno P., Hartl D., Burger L., Schubeler D. (2015). Competition between DNA methylation and transcription factors determines binding of NRF1. Nature 528 (7583), 575–579. 10.1038/nature16462 [DOI] [PubMed] [Google Scholar]
- Dong Z. R., Ke A. W., Li T., Cai J. B., Yang Y. F., Zhou W., et al. (2021). CircMEMO1 modulates the promoter methylation and expression of TCF21 to regulate hepatocellular carcinoma progression and sorafenib treatment sensitivity. Mol. Cancer 20 (1), 75. 10.1186/s12943-021-01361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., Zhang A., Liu S., Lu F., Guo Y., Zhang G., et al. (2017). Aberrant methylation-mediated silencing of lncRNA MEG3 functions as a ceRNA in esophageal cancer. Mol. Cancer Res. 15 (7), 800–810. 10.1158/1541-7786.MCR-16-0385 [DOI] [PubMed] [Google Scholar]
- Du P., Zhi Y., Wang R., Li Y., Li H., Zhang X., et al. (2021a). Aberrant methylation of the SOX21-AS1 promoter region promotes gene expression and its clinical value in cervical cancer. Reprod. Sci. 28 (2), 532–540. 10.1007/s43032-020-00335-y [DOI] [PubMed] [Google Scholar]
- Du Z., Wen X., Wang Y., Jia L., Zhang S., Liu Y., et al. (2021b). Chromatin lncRNA Platr10 controls stem cell pluripotency by coordinating an intrachromosomal regulatory network. Genome Biol. 22 (1), 233. 10.1186/s13059-021-02444-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve P. O., Chin H. G., Smallwood A., Feehery G. R., Gangisetty O., Karpf A. R., et al. (2006). Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes Dev. 20 (22), 3089–3103. 10.1101/gad.1463706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S., Gao H., Tong Y., Yang J., Tang R., Niu Y., et al. (2016). Long noncoding RNA-HOTAIR affects chemoresistance by regulating HOXA1 methylation in small cell lung cancer cells. Lab. Invest. 96 (1), 60–68. 10.1038/labinvest.2015.123 [DOI] [PubMed] [Google Scholar]
- Fang S., Shen Y., Chen B., Wu Y., Jia L., Li Y., et al. (2018). H3K27me3 induces multidrug resistance in small cell lung cancer by affecting HOXA1 DNA methylation via regulation of the lncRNA HOTAIR. Ann. Transl. Med. 6 (22), 440. 10.21037/atm.2018.10.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatica A., Bozzoni I. (2014). Long non-coding RNAs: New players in cell differentiation and development. Nat. Rev. Genet. 15 (1), 7–21. 10.1038/nrg3606 [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Ohlsson R., Henikoff S. (2006). The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 7 (1), 21–33. 10.1038/nrg1748 [DOI] [PubMed] [Google Scholar]
- Ferreira H. J., Davalos V., de Moura M. C., Soler M., Perez-Salvia M., Bueno-Costa A., et al. (2018). Circular RNA CpG island hypermethylation-associated silencing in human cancer. Oncotarget 9 (49), 29208–29219. 10.18632/oncotarget.25673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira H. J., Esteller M. (2018). Non-coding RNAs, epigenetics, and cancer: Tying it all together. Cancer Metastasis Rev. 37 (1), 55–73. 10.1007/s10555-017-9715-8 [DOI] [PubMed] [Google Scholar]
- Fialkova V., Vidomanova E., Balharek T., Marcinek J., Kudela E., Hanysova S., et al. (2017). DNA methylation as mechanism of apoptotic resistance development in endometrial cancer patients. Gen. Physiol. Biophys. 36 (5), 521–529. 10.4149/gpb_2017032 [DOI] [PubMed] [Google Scholar]
- Frank S., Ahuja G., Bartsch D., Russ N., Yao W., Kuo J. C., et al. (2019). yylncT defines a class of divergently transcribed lncRNAs and safeguards the T-mediated mesodermal commitment of human PSCs. Cell Stem Cell 24 (2), 318–327. 10.1016/j.stem.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Fu Y., Wang W., Li X., Liu Y., Niu Y., Zhang B., et al. (2018). LncRNA H19 interacts with S-adenosylhomocysteine hydrolase to regulate LINE-1 Methylation in human lung-derived cells exposed to Benzo[a]pyrene. Chemosphere 207, 84–90. 10.1016/j.chemosphere.2018.05.048 [DOI] [PubMed] [Google Scholar]
- Fuks F., Burgers W. A., Brehm A., Hughes-Davies L., Kouzarides T. (2000). DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat. Genet. 24 (1), 88–91. 10.1038/71750 [DOI] [PubMed] [Google Scholar]
- Furuta Y., Namba-Fukuyo H., Shibata T. F., Nishiyama T., Shigenobu S., Suzuki Y., et al. (2014). Methylome diversification through changes in DNA methyltransferase sequence specificity. PLoS Genet. 10 (4), e1004272. 10.1371/journal.pgen.1004272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Wang L., Li C. (2022). Circ_0006790 carried by bone marrow mesenchymal stem cell-derived exosomes regulates S100A11 DNA methylation through binding to CBX7 in pancreatic ductal adenocarcinoma. Am. J. Cancer Res. 12 (5), 1934–1959. [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Zhao H., Mu L. (2021). LncRNA-KAT7 negatively regulates miR-10a through an epigenetic pathway to participate in nonsmall cell lung cancer. Cancer biother. Radiopharm. 36 (5), 441–445. 10.1089/cbr.2019.3228 [DOI] [PubMed] [Google Scholar]
- Ghasemi T., Khalaj-Kondori M., Hosseinpour Feizi M. A., Asadi P. (2021). Aberrant expression of lncRNAs SNHG6, TRPM2-AS1, MIR4435-2HG, and hypomethylation of TRPM2-AS1 promoter in colorectal cancer. Cell Biol. Int. 45 (12), 2464–2478. 10.1002/cbin.11692 [DOI] [PubMed] [Google Scholar]
- Guo F., Guo L., Li Y., Zhou Q., Li Z. (2015). MALAT1 is an oncogenic long non-coding RNA associated with tumor invasion in non-small cell lung cancer regulated by DNA methylation. Int. J. Clin. Exp. Pathol. 8 (12), 15903–15910. [PMC free article] [PubMed] [Google Scholar]
- Guo T., Gong C., Wu P., Battaglia-Hsu S. F., Feng J., Liu P., et al. (2020). LINC00662 promotes hepatocellular carcinoma progression via altering genomic methylation profiles. Cell Death Differ. 27 (7), 2191–2205. 10.1038/s41418-020-0494-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Wang H., Liu P., Xiao Y., Wu P., Wang Y., et al. (2018). SNHG6 acts as a genome-wide hypomethylation trigger via coupling of miR-1297-mediated S-Adenosylmethionine-Dependent positive feedback loops. Cancer Res. 78 (14), 3849–3864. 10.1158/0008-5472.CAN-17-3833 [DOI] [PubMed] [Google Scholar]
- Guo X., Chen Z., Zhao L., Cheng D., Song W., Zhang X. (2019). Long non-coding RNA-HAGLR suppressed tumor growth of lung adenocarcinoma through epigenetically silencing E2F1. Exp. Cell Res. 382 (1), 111461. 10.1016/j.yexcr.2019.06.006 [DOI] [PubMed] [Google Scholar]
- Guo X., Zeng Y., Ye G., Yang J., Wu Q., Shi Y., et al. (2022). Circular RNA circSEPT9 is upregulated in endometrial cancer and promotes cell invasion and migration by downregulating miR-186 through methylation. Ann. Clin. Lab. Sci. 52 (3), 399–405. [PubMed] [Google Scholar]
- Han Y. J., Boatman S. M., Zhang J., Du X. C., Yeh A. C., Zheng Y., et al. (2018). LncRNA BLAT1 is upregulated in basal-like breast cancer through epigenetic modifications. Sci. Rep. 8 (1), 15572. 10.1038/s41598-018-33629-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangauer M. J., Vaughn I. W., McManus M. T. (2013). Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 9 (6), e1003569. 10.1371/journal.pgen.1003569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanly D. J., Esteller M., Berdasco M. (2018). Interplay between long non-coding RNAs and epigenetic machinery: Emerging targets in cancer? Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20170074. 10.1098/rstb.2017.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S. L., Chen Y. L., Chen Q. H., Tian Q., Yi S. J. (2022). LncRNA KCNQ1OT1 promotes the metastasis of ovarian cancer by increasing the methylation of EIF2B5 promoter. Mol. Med. 28 (1), 112. 10.1186/s10020-022-00521-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann K., Toth R., Bossmann C., Klimo K., Plass C., Gerhauser C. (2017). Genome-wide screen for differentially methylated long noncoding RNAs identifies Esrp2 and lncRNA Esrp2-as regulated by enhancer DNA methylation with prognostic relevance for human breast cancer. Oncogene 36 (46), 6446–6461. 10.1038/onc.2017.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervouet E., Peixoto P., Delage-Mourroux R., Boyer-Guittaut M., Cartron P. F. (2018). Specific or not specific recruitment of DNMTs for DNA methylation, an epigenetic dilemma. Clin. Epigenetics 10, 17. 10.1186/s13148-018-0450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Chen K., Liao R., Li Y., Yang H., Gong J. (2021). LINC01419-mediated epigenetic silencing of ZIC1 promotes metastasis in hepatocellular carcinoma through the PI3K/Akt signaling pathway. Lab. Invest. 101 (5), 570–587. 10.1038/s41374-021-00539-z [DOI] [PubMed] [Google Scholar]
- Hu D., Lou X., Meng N., Li Z., Teng Y., Zou Y., et al. (2021). Peripheral blood-based DNA methylation of long non-coding RNA H19 and metastasis-associated lung adenocarcinoma transcript 1 promoters are potential non-invasive biomarkers for gastric cancer detection. Cancer Control. 28, 10732748211043667. 10.1177/10732748211043667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Wan J., Su Y., Song Q., Zeng Y., Nguyen H. N., et al. (2013). DNA methylation presents distinct binding sites for human transcription factors. Elife 2, e00726. 10.7554/eLife.00726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., Tang Y. (2021). SChLAP1 promotes prostate cancer development through interacting with EZH2 to mediate promoter methylation modification of multiple miRNAs of chromosome 5 with a DNMT3A-feedback loop. Cell Death Dis. 12 (2), 188. 10.1038/s41419-021-03455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen T., Dahl M., Dimopoulos K., Gronbaek K., Kjems J., Kristensen L. S. (2021). Genome-wide circular RNA expression patterns reflect resistance to immunomodulatory drugs in multiple myeloma cells. Cancers (Basel) 13 (3), 365. 10.3390/cancers13030365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J., Zhao L., Zhao X., Li Q., An Y., Li L., et al. (2020). Genomewide DNA methylation regulation analysis of long noncoding RNAs in glioblastoma. Int. J. Mol. Med. 46 (1), 224–238. 10.3892/ijmm.2020.4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Zhang Y., Chen X., Wu P., Chen D. (2020). Long non-coding RNA LINC00673 silencing inhibits proliferation and drug resistance of prostate cancer cells via decreasing KLF4 promoter methylation. J. Cell. Mol. Med. 24 (2), 1878–1892. 10.1111/jcmm.14883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin B., Yao B., Li J. L., Fields C. R., Delmas A. L., Liu C., et al. (2009). DNMT1 and DNMT3B modulate distinct polycomb-mediated histone modifications in colon cancer. Cancer Res. 69 (18), 7412–7421. 10.1158/0008-5472.CAN-09-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G., Zheng J., Zhang Y., Yang Z., Chen Y., Huang C. (2022). LncRNA UCA1 epigenetically suppresses APAF1 expression to mediate the protective effect of sevoflurane against myocardial ischemia-reperfusion injury. Funct. Integr. Genomics 22 (5), 965–975. 10.1007/s10142-022-00874-4 [DOI] [PubMed] [Google Scholar]
- Jin L., Cai Q., Wang S., Wang S., Wang J., Quan Z. (2020). Long noncoding RNA PVT1 promoted gallbladder cancer proliferation by epigenetically suppressing miR-18b-5p via DNA methylation. Cell Death Dis. 11 (10), 871. 10.1038/s41419-020-03080-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A. (2012). Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13 (7), 484–492. 10.1038/nrg3230 [DOI] [PubMed] [Google Scholar]
- Jones R., Wijesinghe S., Wilson C., Halsall J., Liloglou T., Kanhere A. (2021). A long intergenic non-coding RNA regulates nuclear localization of DNA methyl transferase-1. iScience 24 (4), 102273. 10.1016/j.isci.2021.102273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalwa M., Hanzelmann S., Otto S., Kuo C. C., Franzen J., Joussen S., et al. (2016). The lncRNA HOTAIR impacts on mesenchymal stem cells via triple helix formation. Nucleic Acids Res. 44 (22), 10631–10643. 10.1093/nar/gkw802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W., Wang Q., Dai Y., Wang H., Wang M., Wang J., et al. (2020). Hypomethylation of PlncRNA-1 promoter enhances bladder cancer progression through the miR-136-5p/Smad3 axis. Cell Death Dis. 11 (12), 1038. 10.1038/s41419-020-03240-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X., Kong F., Huang K., Li L., Li Z., Wang X., et al. (2019). LncRNA MIR210HG promotes proliferation and invasion of non-small cell lung cancer by upregulating methylation of CACNA2D2 promoter via binding to DNMT1. Onco. Targets. Ther. 12, 3779–3790. 10.2147/OTT.S189468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Song J., Han J., Kim Y., Chun C. H., Jin E. J. (2013). Two non-coding RNAs, MicroRNA-101 and HOTTIP contribute cartilage integrity by epigenetic and homeotic regulation of integrin-α1. Cell. Signal. 25 (12), 2878–2887. 10.1016/j.cellsig.2013.08.034 [DOI] [PubMed] [Google Scholar]
- Kim-Wanner S. Z., Assenov Y., Nair M. B., Weichenhan D., Benner A., Becker N., et al. (2020). Genome-wide DNA methylation profiling in early stage I lung adenocarcinoma reveals predictive aberrant methylation in the promoter region of the long noncoding RNA PLUT: An exploratory study. J. Thorac. Oncol. 15 (8), 1338–1350. 10.1016/j.jtho.2020.03.023 [DOI] [PubMed] [Google Scholar]
- Kulis M., Merkel A., Heath S., Queiros A. C., Schuyler R. P., Castellano G., et al. (2015). Whole-genome fingerprint of the DNA methylome during human B cell differentiation. Nat. Genet. 47 (7), 746–756. 10.1038/ng.3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai I. L., Chang Y. S., Chan W. L., Lee Y. T., Yen J. C., Yang C. A., et al. (2019). Male-specific long noncoding RNA TTTY15 inhibits non-small cell lung cancer proliferation and metastasis via TBX4. Int. J. Mol. Sci. 20 (14), E3473. 10.3390/ijms20143473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L., Cao H., Chi W., Meng W., Zhao L., Cui W., et al. (2020). Aberrant DNA hypermethylation-silenced LINC00886 gene accelerates malignant progression of laryngeal carcinoma. Pathol. Res. Pract. 216 (4), 152877. 10.1016/j.prp.2020.152877 [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Song J. M., Kim H. J., Park H. R. (2021). Hypomethylation of lncRNA H19 as a potential prognostic biomarker for oral squamous cell carcinoma. Arch. Oral Biol. 129, 105214. 10.1016/j.archoralbio.2021.105214 [DOI] [PubMed] [Google Scholar]
- Li L., Gan Y. P., Peng H. (2022a). RAMP2-AS1 inhibits CXCL11 expression to suppress malignant phenotype of breast cancer by recruiting DNMT1 and DNMT3B. Exp. Cell Res. 416 (2), 113139. 10.1016/j.yexcr.2022.113139 [DOI] [PubMed] [Google Scholar]
- Li L., Gao Z., Zhao L., Ren P., Shen H. (2021a). Long non-coding RNA LINC00607 silencing exerts antioncogenic effects on thyroid cancer through the CASP9 Promoter methylation. J. Cell. Mol. Med. 25 (16), 7608–7620. 10.1111/jcmm.16265 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li M., Jiao L., Shao Y., Li H., Sun L., Yu Q., et al. (2022b). LncRNA-ZFAS1 promotes myocardial ischemia-reperfusion injury through DNA methylation-mediated Notch1 down-regulation in mice. JACC. Basic Transl. Sci. 7 (9), 880–895. 10.1016/j.jacbts.2022.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Zhao Z., Miao F., Cai S., Liu P., Yu Y., et al. (2021b). Silencing of long non-coding RNA LINC01270 inhibits esophageal cancer progression and enhances chemosensitivity to 5-fluorouracil by mediating GSTP1methylation. Cancer Gene Ther. 28 (5), 471–485. 10.1038/s41417-020-00232-1 [DOI] [PubMed] [Google Scholar]
- Li Q., Dong C., Cui J., Wang Y., Hong X. (2018). Over-expressed lncRNA HOTAIRM1 promotes tumor growth and invasion through up-regulating HOXA1 and sequestering G9a/EZH2/Dnmts away from the HOXA1 gene in glioblastoma multiforme. J. Exp. Clin. Cancer Res. 37 (1), 265. 10.1186/s13046-018-0941-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Su Z., Xu X., Liu G., Song X., Wang R., et al. (2012). AS1DHRS4, a head-to-head natural antisense transcript, silences the DHRS4 gene cluster in cis and trans. Proc. Natl. Acad. Sci. U. S. A. 109 (35), 14110–14115. 10.1073/pnas.1116597109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Hu J., Li G., Mai H., Gao Y., Liang B., et al. (2022c). Epigenetic regulation of LINC01270 in breast cancer progression by mediating LAMA2 promoter methylation and MAPK signaling pathway. Cell Biol. Toxicol. 10.1007/s10565-022-09763-9 [DOI] [PubMed] [Google Scholar]
- Li W., Ma X., Wang F., Chen S., Guo Q., Sun F., et al. (2022d). SNHG3 affects gastric cancer development by regulating SEPT9 methylation. J. Oncol. 2022, 3433406. 10.1155/2022/3433406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zheng J., Deng J., You Y., Wu H., Li N., et al. (2014). Increased levels of the long intergenic non-protein coding RNA POU3F3 promote DNA methylation in esophageal squamous cell carcinoma cells. Gastroenterology 146 (7), 1714–1726. 10.1053/j.gastro.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Li X., Lu H., Fan G., He M., Sun Y., Xu K., et al. (2017). A novel interplay between HOTAIR and DNA methylation in osteosarcoma cells indicates a new therapeutic strategy. J. Cancer Res. Clin. Oncol. 143 (11), 2189–2200. 10.1007/s00432-017-2478-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen X., Lu C. (2021c). The interplay between DNA and histone methylation: Molecular mechanisms and disease implications. EMBO Rep. 22 (5), e51803. 10.15252/embr.202051803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang Y., Pei W., Zhang M., Yang H., Zhong M., et al. (2020a). LncRNA Dnmt3aos regulates Dnmt3a expression leading to aberrant DNA methylation in macrophage polarization. FASEB J. 34 (4), 5077–5091. 10.1096/fj.201902379R [DOI] [PubMed] [Google Scholar]
- Li Y., Cheng C. (2018). Long noncoding RNA NEAT1 promotes the metastasis of osteosarcoma via interaction with the G9a-DNMT1-Snail complex. Am. J. Cancer Res. 8 (1), 81–90. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li Y., Luo Q., Li Z., Wang Y., Zhu C., Li T., et al. (2020b). Long non-coding rna irain inhibits vegfa expression via enhancing its dna methylation leading to tumor suppression in renal carcinoma. Front. Oncol. 10, 1082. 10.3389/fonc.2020.01082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Yan J., Wang Y., Wang C., Zhang C., Li G. (2020c). LINC00240 promotes gastric cancer cell proliferation, migration and EMT via the miR-124-3p/DNMT3B axis. Cell biochem. Funct. 38 (8), 1079–1088. 10.1002/cbf.3551 [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang Y., Li S., Lu J., Chen J., Wang Y., et al. (2015). Genome-wide DNA methylome analysis reveals epigenetically dysregulated non-coding RNAs in human breast cancer. Sci. Rep. 5, 8790. 10.1038/srep08790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Tan H., Yu H., Deng Z., Zhou X., Wang M. (2020d). DNA methylation and gene expression profiles characterize epigenetic regulation of lncRNAs in colon adenocarcinoma. J. Cell. Biochem. 121 (3), 2406–2415. 10.1002/jcb.29463 [DOI] [PubMed] [Google Scholar]
- Li Z., Yu D., Li H., Lv Y., Li S. (2019). Long noncoding RNA UCA1 confers tamoxifen resistance in breast cancer endocrinotherapy through regulation of the EZH2/p21 axis and the PI3K/AKT signaling pathway. Int. J. Oncol. 54 (3), 1033–1042. 10.3892/ijo.2019.4679 [DOI] [PubMed] [Google Scholar]
- Lin K., Jiang H., Zhuang S. S., Qin Y. S., Qiu G. D., She Y. Q., et al. (2019). Long noncoding RNA LINC00261 induces chemosensitization to 5-fluorouracil by mediating methylation-dependent repression of DPYD in human esophageal cancer. FASEB J. 33 (2), 1972–1988. 10.1096/fj.201800759R [DOI] [PubMed] [Google Scholar]
- Lister R., Pelizzola M., Dowen R. H., Hawkins R. D., Hon G., Tonti-Filippini J., et al. (2009). Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462 (7271), 315–322. 10.1038/nature08514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Fu H., Liu X., Lei Q., Zhang Y., She X., et al. (2018). LINC00470 coordinates the epigenetic regulation of ELFN2 to distract GBM cell autophagy. Mol. Ther. 26 (9), 2267–2281. 10.1016/j.ymthe.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. X., Chen L. L. (2022). Circular RNAs: Characterization, cellular roles, and applications. Cell 185 (12), 2016–2034. 10.1016/j.cell.2022.04.021 [DOI] [PubMed] [Google Scholar]
- Liu D., Wu K., Yang Y., Zhu D., Zhang C., Zhao S. (2020a). Long noncoding RNA ADAMTS9-AS2 suppresses the progression of esophageal cancer by mediating CDH3 promoter methylation. Mol. Carcinog. 59 (1), 32–44. 10.1002/mc.23126 [DOI] [PubMed] [Google Scholar]
- Liu F., Huang W., Hong J., Cai C., Zhang W., Zhang J., et al. (2020b). Long noncoding RNA LINC00630 promotes radio-resistance by regulating BEX1 gene methylation in colorectal cancer cells. IUBMB Life 72 (7), 1404–1414. 10.1002/iub.2263 [DOI] [PubMed] [Google Scholar]
- Liu S., Zheng Y., Zhang Y., Zhang J., Xie F., Guo S., et al. (2020c). Methylation-mediated LINC00261 suppresses pancreatic cancer progression by epigenetically inhibiting c-Myc transcription. Theranostics 10 (23), 10634–10651. 10.7150/thno.44278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Gao Q., Li P., Zhao Q., Zhang J., Li J., et al. (2013). UHRF1 targets DNMT1 for DNA methylation through cooperative binding of hemi-methylated DNA and methylated H3K9. Nat. Commun. 4, 1563. 10.1038/ncomms2562 [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu K., Tang C., Shi Z., Jing K., Zheng J. (2020d). Long non-coding RNA XIST contributes to osteoarthritis progression via miR-149-5p/DNMT3A axis. Biomed. Pharmacother. 128, 110349. 10.1016/j.biopha.2020.110349 [DOI] [PubMed] [Google Scholar]
- Lo P. K., Zhang Y., Wolfson B., Gernapudi R., Yao Y., Duru N., et al. (2016). Dysregulation of the BRCA1/long non-coding RNA NEAT1 signaling axis contributes to breast tumorigenesis. Oncotarget 7 (40), 65067–65089. 10.18632/oncotarget.11364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Wei Y., Wang X., Zhang Z., Yin J., Li W., et al. (2020). DNA-methylation-mediated activating of lncRNA SNHG12 promotes temozolomide resistance in glioblastoma. Mol. Cancer 19 (1), 28. 10.1186/s12943-020-1137-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G. H., Zhao H. M., Liu Z. Y., Cao Q., Shao R. D., Sun G. (2021). LncRNA SAMD12-AS1 promotes the progression of gastric cancer via DNMT1/p53 Axis. Arch. Med. Res. 52 (7), 683–691. 10.1016/j.arcmed.2021.04.004 [DOI] [PubMed] [Google Scholar]
- Lu S. C., Mato J. M. (2012). S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 92 (4), 1515–1542. 10.1152/physrev.00047.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujambio A., Portela A., Liz J., Melo S. A., Rossi S., Spizzo R., et al. (2010). CpG island hypermethylation-associated silencing of non-coding RNAs transcribed from ultraconserved regions in human cancer. Oncogene 29 (48), 6390–6401. 10.1038/onc.2010.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyko F. (2018). The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 19 (2), 81–92. 10.1038/nrg.2017.80 [DOI] [PubMed] [Google Scholar]
- Ma X., Yu L., Wang P., Yang X. (2017). Discovering DNA methylation patterns for long non-coding RNAs associated with cancer subtypes. Comput. Biol. Chem. 69, 164–170. 10.1016/j.compbiolchem.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Manoochehri M., Jones M., Tomczyk K., Fletcher O., Schoemaker M. J., Swerdlow A. J., et al. (2020). DNA methylation of the long intergenic noncoding RNA 299 gene in triple-negative breast cancer: Results from a prospective study. Sci. Rep. 10 (1), 11762. 10.1038/s41598-020-68506-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G., Zhou B., Xu W., Jiao N., Wu Z., Li J., et al. (2021). Hsa_circ_0040809 regulates colorectal cancer development by upregulating methyltransferase DNMT1 via targeting miR-515-5p. J. Gene Med. 23 (12), e3388. 10.1002/jgm.3388 [DOI] [PubMed] [Google Scholar]
- McCarty G., Loeb D. M. (2015). Hypoxia-sensitive epigenetic regulation of an antisense-oriented lncRNA controls WT1 expression in myeloid leukemia cells. PLoS One 10 (3), e0119837. 10.1371/journal.pone.0119837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed P., Yosefzon Y., David C., Tsukerman A., Pnueli L. (2018). Tet enzymes, variants, and differential effects on function. Front. Cell Dev. Biol. 6, 22. 10.3389/fcell.2018.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer T. R., Mattick J. S. (2013). Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 20 (3), 300–307. 10.1038/nsmb.2480 [DOI] [PubMed] [Google Scholar]
- Merry C. R., Forrest M. E., Sabers J. N., Beard L., Gao X. H., Hatzoglou M., et al. (2015). DNMT1-associated long non-coding RNAs regulate global gene expression and DNA methylation in colon cancer. Hum. Mol. Genet. 24 (21), 6240–6253. 10.1093/hmg/ddv343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Wang L., Zhan H., Dai J., Chang Y., Wu F., et al. (2019). A long noncoding RNA distributed in both nucleus and cytoplasm operates in the PYCARD-regulated apoptosis by coordinating the epigenetic and translational regulation. PLoS Genet. 15 (5), e1008144. 10.1371/journal.pgen.1008144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad F., Mondal T., Guseva N., Pandey G. K., Kanduri C. (2010). Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development 137 (15), 2493–2499. 10.1242/dev.048181 [DOI] [PubMed] [Google Scholar]
- Moore L. D., Le T., Fan G. (2013). DNA methylation and its basic function. Neuropsychopharmacology 38 (1), 23–38. 10.1038/npp.2012.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morenos L., Chatterton Z., Ng J. L., Halemba M. S., Parkinson-Bates M., Mechinaud F., et al. (2014). Hypermethylation and down-regulation of DLEU2 in paediatric acute myeloid leukaemia independent of embedded tumour suppressor miR-15a/16-1. Mol. Cancer 13, 123. 10.1186/1476-4598-13-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli M., Dieci G. (2022). Epigenetic regulation of human non-coding RNA gene transcription. Biochem. Soc. Trans. 50 (2), 723–736. 10.1042/BST20210860 [DOI] [PubMed] [Google Scholar]
- Ng A., Tang J. P., Goh C. H., Hui K. M. (2003). Regulation of the H19 imprinting gene expression in human nasopharyngeal carcinoma by methylation. Int. J. Cancer 104 (2), 179–187. 10.1002/ijc.10926 [DOI] [PubMed] [Google Scholar]
- Ni J., Hong J., Li Q., Zeng Q., Xia R. (2021). Long non-coding RNA CRNDE suppressing cell proliferation is regulated by DNA methylation in chronic lymphocytic leukemia. Leuk. Res. 105, 106564. 10.1016/j.leukres.2021.106564 [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Mulholland C. B., Bultmann S., Kori S., Endo A., Saeki Y., et al. (2020). Two distinct modes of DNMT1 recruitment ensure stable maintenance DNA methylation. Nat. Commun. 11 (1), 1222. 10.1038/s41467-020-15006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary V. B., Ovsepian S. V., Carrascosa L. G., Buske F. A., Radulovic V., Niyazi M., et al. (2015). PARTICLE, a triplex-forming long ncRNA, regulates locus-specific methylation in response to low-dose irradiation. Cell Rep. 11 (3), 474–485. 10.1016/j.celrep.2015.03.043 [DOI] [PubMed] [Google Scholar]
- O'Leary V. B., Smida J., Buske F. A., Carrascosa L. G., Azimzadeh O., Maugg D., et al. (2017). PARTICLE triplexes cluster in the tumor suppressor WWOX and may extend throughout the human genome. Sci. Rep. 7 (1), 7163. 10.1038/s41598-017-07295-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunleye A. J., Romanova E., Medvedeva Y. A. (2021). Genome-wide regulation of CpG methylation by ecCEBPα in acute myeloid leukemia. F1000Res. 10, 204. 10.12688/f1000research.28146.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y., Furuno M., Kasukawa T., Adachi J., Bono H., Kondo S., et al. (2002). Analysis of the mouse transcriptome based on functional annotation of 60, 770 full-length cDNAs. Nature 420 (6915), 563–573. 10.1038/nature01266 [DOI] [PubMed] [Google Scholar]
- Pan T., Ding H., Jin L., Zhang S., Wu D., Pan W., et al. (2022). DNMT1-mediated demethylation of lncRNA MEG3 promoter suppressed breast cancer progression by repressing Notch1 signaling pathway. Cell Cycle 21, 2323–2337. 10.1080/15384101.2022.2094662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangeni R. P., Olivaries I., Huen D., Buzatto V. C., Dawson T. P., Ashton K. M., et al. (2022). Genome-wide methylation analyses identifies Non-coding RNA genes dysregulated in breast tumours that metastasise to the brain. Sci. Rep. 12 (1), 1102. 10.1038/s41598-022-05050-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W. X., Koirala P., Zhang W., Ni C., Wang Z., Yang L., et al. (2020). lncRNA RMST enhances DNMT3 expression through interaction with HuR. Mol. Ther. 28 (1), 9–18. 10.1016/j.ymthe.2019.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ponting C. P., Oliver P. L., Reik W. (2009). Evolution and functions of long noncoding RNAs. Cell 136 (4), 629–641. 10.1016/j.cell.2009.02.006 [DOI] [PubMed] [Google Scholar]
- Qiannan D., Qianqian J., Jiahui S., Haowei F., Qian X. (2022). LncRNA PVT1 mediates the progression of liver necroptosis via ZBP1 promoter methylation under nonylphenol exposure. Sci. Total Environ. 844, 157185. 10.1016/j.scitotenv.2022.157185 [DOI] [PubMed] [Google Scholar]
- Ren Y., Wang Y. F., Zhang J., Wang Q. X., Han L., Mei M., et al. (2019). Targeted design and identification of AC1NOD4Q to block activity of HOTAIR by abrogating the scaffold interaction with EZH2. Clin. Epigenetics 11 (1), 29. 10.1186/s13148-019-0624-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury A., Samadder S., Das P., Mazumder D. I., Chatterjee A., Addya S., et al. (2020). Deregulation of H19 is associated with cervical carcinoma. Genomics 112 (1), 961–970. 10.1016/j.ygeno.2019.06.012 [DOI] [PubMed] [Google Scholar]
- Ruiz-Banobre J., Rodriguez-Casanova A., Costa-Fraga N., Bao-Caamano A., Alvarez-Castro A., Carreras-Presas M., et al. (2022). Noninvasive early detection of colorectal cancer by hypermethylation of the LINC00473 promoter in plasma cell-free DNA. Clin. Epigenetics 14 (1), 86. 10.1186/s13148-022-01302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M., Appanah R., Lee S., Lam L. L., Goyal P., Lorincz M. C. (2009). Targeting of EZH2 to a defined genomic site is sufficient for recruitment of Dnmt3a but not de novo DNA methylation. Epigenetics 4 (6), 404–414. 10.4161/epi.4.6.9392 [DOI] [PubMed] [Google Scholar]
- Rybak-Wolf A., Stottmeister C., Glazar P., Jens M., Pino N., Giusti S., et al. (2015). Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell 58 (5), 870–885. 10.1016/j.molcel.2015.03.027 [DOI] [PubMed] [Google Scholar]
- Salmeron-Barcenas E. G., Illades-Aguiar B., Del Moral-Hernandez O., Ortega-Soto A., Hernandez-Sotelo D. (2019). HOTAIR knockdown decreased the activity wnt/β-catenin signaling pathway and increased the mRNA levels of its negative regulators in hela cells. Cell. Physiol. biochem. 53 (6), 948–960. 10.33594/000000188 [DOI] [PubMed] [Google Scholar]
- Salzman J., Chen R. E., Olsen M. N., Wang P. L., Brown P. O. (2013). Cell-type specific features of circular RNA expression. PLoS Genet. 9 (9), e1003777. 10.1371/journal.pgen.1003777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sati S., Ghosh S., Jain V., Scaria V., Sengupta S. (2012). Genome-wide analysis reveals distinct patterns of epigenetic features in long non-coding RNA loci. Nucleic Acids Res. 40 (20), 10018–10031. 10.1093/nar/gks776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao G., Fan X., Zhang P., Liu X., Huang L., Ji S. (2021). Methylation-dependent MCM6 repression induced by LINC00472 inhibits triple-negative breast cancer metastasis by disturbing the MEK/ERK signaling pathway. Aging (Albany NY) 13 (4), 4962–4975. 10.18632/aging.103568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Li Y., Ye Q., Qin Y. (2021a). YY1-mediated long non-coding RNA Kcnq1ot1 promotes the tumor progression by regulating PTEN via DNMT1 in triple negative breast cancer. Cancer Gene Ther. 28 (10-11), 1099–1112. 10.1038/s41417-020-00254-9 [DOI] [PubMed] [Google Scholar]
- Shen T., Xia W., Min S., Yang Z., Cheng L., Wang W., et al. (2021b). A pair of long intergenic non-coding RNA LINC00887 variants act antagonistically to control Carbonic Anhydrase IX transcription upon hypoxia in tongue squamous carcinoma progression. BMC Biol. 19 (1), 192. 10.1186/s12915-021-01112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Zhang Q., Xie M., Feng Y., Ma S., Yi C., et al. (2020). Aberrant methylationmediated decrease of lncRNA HNF1AAS1 contributes to malignant progression of laryngeal squamous cell carcinoma via EMT. Oncol. Rep. 44 (6), 2503–2516. 10.3892/or.2020.7823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X., Zang R., Zhang E., Liu Y., Shi X., Zhang E., et al. (2016). LncRNA H19 confers chemoresistance in ERα-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget 7 (49), 81452–81462. 10.18632/oncotarget.13263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Ramnarine V. R., Song J. H., Pandey R., Padi S. K. R., Nouri M., et al. (2021). The long noncoding RNA H19 regulates tumor plasticity in neuroendocrine prostate cancer. Nat. Commun. 12 (1), 7349. 10.1038/s41467-021-26901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram S., Forrest M. E., Moinova H., Cohen A., Varadan V., LaFramboise T., et al. (2018). The DNMT1-associated lincRNA DACOR1 reprograms genome-wide DNA methylation in colon cancer. Clin. Epigenetics 10 (1), 127. 10.1186/s13148-018-0555-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Chen L., Liu W., Xu X., Zhou Y., Zhu J., et al. (2021). Depleting long noncoding RNA HOTAIR attenuates chronic myelocytic leukemia progression by binding to DNA methyltransferase 1 and inhibiting PTEN gene promoter methylation. Cell Death Dis. 12 (5), 440. 10.1038/s41419-021-03637-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statello L., Guo C. J., Chen L. L., Huarte M. (2021). Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22 (2), 96–118. 10.1038/s41580-020-00315-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S. C., Yeh C. M., Lin C. W., Hsieh Y. H., Chuang C. Y., Tang C. H., et al. (2021). A novel melatonin-regulated lncRNA suppresses TPA-induced oral cancer cell motility through replenishing PRUNE2 expression. J. Pineal Res. 71 (3), e12760. 10.1111/jpi.12760 [DOI] [PubMed] [Google Scholar]
- Sui C. J., Zhou Y. M., Shen W. F., Dai B. H., Lu J. J., Zhang M. F., et al. (2016). Long noncoding RNA GIHCG promotes hepatocellular carcinoma progression through epigenetically regulating miR-200b/a/429. J. Mol. Med. 94 (11), 1281–1296. 10.1007/s00109-016-1442-z [DOI] [PubMed] [Google Scholar]
- Sun M., Jin F. Y., Xia R., Kong R., Li J. H., Xu T. P., et al. (2014). Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer 14, 319. 10.1186/1471-2407-14-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Nie F., Wang Y., Zhang Z., Hou J., He D., et al. (2016). LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 76 (21), 6299–6310. 10.1158/0008-5472.CAN-16-0356 [DOI] [PubMed] [Google Scholar]
- Sun X., Yi J., Yang J., Han Y., Qian X., Liu Y., et al. (2021). An integrated epigenomic-transcriptomic landscape of lung cancer reveals novel methylation driver genes of diagnostic and therapeutic relevance. Theranostics 11 (11), 5346–5364. 10.7150/thno.58385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai D., Gonzales F. A., Tsai Y. C., Thayer M. J., Jones P. A. (2001). Large scale mapping of methylcytosines in CTCF-binding sites in the human H19 promoter and aberrant hypomethylation in human bladder cancer. Hum. Mol. Genet. 10 (23), 2619–2626. 10.1093/hmg/10.23.2619 [DOI] [PubMed] [Google Scholar]
- Tay Y., Rinn J., Pandolfi P. P. (2014). The multilayered complexity of ceRNA crosstalk and competition. Nature 505 (7483), 344–352. 10.1038/nature12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng X., Chen X., Xue H., Tang Y., Zhang P., Kang Q., et al. (2020). NPInter v4.0: An integrated database of ncRNA interactions. Nucleic Acids Res. 48 (D1), D160–D165. 10.1093/nar/gkz969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff A. E., Lee S. H., Jones A., Fiegl H., Kalwa M., Wagner W., et al. (2015). HOTAIR and its surrogate DNA methylation signature indicate carboplatin resistance in ovarian cancer. Genome Med. 7, 108. 10.1186/s13073-015-0233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F., Tang Z., Song G., Pan Y., He B., Bao Q., et al. (2012). Loss of imprinting of IGF2 correlates with hypomethylation of the H19 differentially methylated region in the tumor tissue of colorectal cancer patients. Mol. Med. Rep. 5 (6), 1536–1540. 10.3892/mmr.2012.833 [DOI] [PubMed] [Google Scholar]
- Tsai K. W., Tsai C. Y., Chou N. H., Wang K. C., Kang C. H., Li S. C., et al. (2019). Aberrant DNA hypermethylation silenced LncRNA expression in gastric cancer. Anticancer Res. 39 (10), 5381–5391. 10.21873/anticanres.13732 [DOI] [PubMed] [Google Scholar]
- Vennin C., Spruyt N., Robin Y. M., Chassat T., Le Bourhis X., Adriaenssens E. (2017). The long non-coding RNA 91H increases aggressive phenotype of breast cancer cells and up-regulates H19/IGF2 expression through epigenetic modifications. Cancer Lett. 385, 198–206. 10.1016/j.canlet.2016.10.023 [DOI] [PubMed] [Google Scholar]
- Vire E., Brenner C., Deplus R., Blanchon L., Fraga M., Didelot C., et al. (2006). The Polycomb group protein EZH2 directly controls DNA methylation. Nature 439 (7078), 871–874. 10.1038/nature04431 [DOI] [PubMed] [Google Scholar]
- Wan H., Yuan B., Jiang K., Wei J., Feng X., Sun B., et al. (2021). CircRNA CircRIMS is overexpressed in esophageal squamous cell carcinoma and downregulate miR-613 through methylation to increase cell proliferation. Cancer Manag. Res. 13, 4587–4595. 10.2147/CMAR.S282983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Zhao L., Chi W., Cao H., Cui W., Meng W. (2019a). Aberrant methylation-mediated downregulation of lncRNA SSTR5-AS1 promotes progression and metastasis of laryngeal squamous cell carcinoma. Epigenetics Chromatin 12 (1), 35. 10.1186/s13072-019-0283-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. B., Wei H., Wang J. S., Li L., Chen A. Y., Li Z. G. (2019b). Down-regulated expression of LINC00518 prevents epithelial cell growth and metastasis in breast cancer through the inhibition of CDX2 methylation and the Wnt signaling pathway. Biochim. Biophys. Acta Mol. Basis Dis. 1865 (3), 708–723. 10.1016/j.bbadis.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Wang J., Xie S., Yang J., Xiong H., Jia Y., Zhou Y., et al. (2019c). The long noncoding RNA H19 promotes tamoxifen resistance in breast cancer via autophagy. J. Hematol. Oncol. 12 (1), 81. 10.1186/s13045-019-0747-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhao Y., Gong W., Liu Y., Wang M., Huang X., et al. (2021a). Edlmfc: An ensemble deep learning framework with multi-scale features combination for ncRNA-protein interaction prediction. BMC Bioinforma. 22 (1), 133. 10.1186/s12859-021-04069-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Bu P., Ai Y., Srinivasan T., Chen H. J., Xiang K., et al. (2016). A long non-coding RNA targets microRNA miR-34a to regulate colon cancer stem cell asymmetric division. Elife 5, e14620. 10.7554/eLife.14620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhao Y., Bao X., Zhu X., Kwok Y. K., Sun K., et al. (2015). LncRNA Dum interacts with Dnmts to regulate Dppa2 expression during myogenic differentiation and muscle regeneration. Cell Res. 25 (3), 335–350. 10.1038/cr.2015.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Liu J., You Z., Yin Y., Liu L., Kang Y., et al. (2021b). LncRNA TINCR favors tumorigenesis via STAT3-TINCR-EGFR-feedback loop by recruiting DNMT1 and acting as a competing endogenous RNA in human breast cancer. Cell Death Dis. 12 (1), 83. 10.1038/s41419-020-03188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. L., Huang Y., Su R., Yu Y. Y. (2019d). Silencing long non-coding RNA HOTAIR exerts anti-oncogenic effect on human acute myeloid leukemia via demethylation of HOXA5 by inhibiting Dnmt3b. Cancer Cell Int. 19, 114. 10.1186/s12935-019-0808-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Chen G., Wang B., Yuan Z., Liu G., Niu B., et al. (2019e). Long non-coding RNA BZRAP1-AS1 silencing suppresses tumor angiogenesis in hepatocellular carcinoma by mediating THBS1 methylation. J. Transl. Med. 17 (1), 421. 10.1186/s12967-019-02145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Fang F., Ozes A., Nephew K. P. (2021c). Targeting ovarian cancer stem cells by dual inhibition of HOTAIR and DNA methylation. Mol. Cancer Ther. 20 (6), 1092–1101. 10.1158/1535-7163.MCT-20-0826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Yu B., Jin Q., Zhang J., Yan B., Yang L., et al. (2020). Regulation of laryngeal squamous cell cancer progression by the lncRNA RP11-159K7.2/miR-206/DNMT3A axis. J. Cell. Mol. Med. 24 (12), 6781–6795. 10.1111/jcmm.15331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang C., Wu Z., Chen Y., Shi W. (2018a). CircIBTK inhibits DNA demethylation and activation of AKT signaling pathway via miR-29b in peripheral blood mononuclear cells in systemic lupus erythematosus. Arthritis Res. Ther. 20 (1), 118. 10.1186/s13075-018-1618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Dong T., Wang P., Li S., Wu G., Zhou J., et al. (2021d). LINC00922 regulates epithelial-mesenchymal transition, invasive and migratory capacities in breast cancer through promoting NKD2 methylation. Cell. Signal. 77, 109808. 10.1016/j.cellsig.2020.109808 [DOI] [PubMed] [Google Scholar]
- Wang Y., Hu Y., Wu G., Yang Y., Tang Y., Zhang W., et al. (2017). Long noncoding RNA PCAT-14 induces proliferation and invasion by hepatocellular carcinoma cells by inducing methylation of miR-372. Oncotarget 8 (21), 34429–34441. 10.18632/oncotarget.16260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang B., Zhang M., Guo W., Wu Z., Wang Y., et al. (2018b). lncRNA epigenetic landscape analysis identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell-cycle progression in cancer. Cancer Cell 33 (4), 706–720. 10.1016/j.ccell.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y. C., Ma J., Zhang J., Wang J. C. (2019). Long non-coding RNA LINC01133 silencing exerts antioncogenic effect in pancreatic cancer through the methylation of DKK1 promoter and the activation of Wnt signaling pathway. Cancer Biol. Ther. 20 (3), 368–380. 10.1080/15384047.2018.1529110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Meng X., Gao R., Jia Y., Chai J., Zhou Y., et al. (2020). Long non-coding RNA LINC00858 inhibits colon cancer cell apoptosis, autophagy, and senescence by activating WNK2 promoter methylation. Exp. Cell Res. 396 (1), 112214. 10.1016/j.yexcr.2020.112214 [DOI] [PubMed] [Google Scholar]
- Wu J., Shuang Z., Zhao J., Tang H., Liu P., Zhang L., et al. (2018). Linc00152 promotes tumorigenesis by regulating DNMTs in triple-negative breast cancer. Biomed. Pharmacother. 97, 1275–1281. 10.1016/j.biopha.2017.11.055 [DOI] [PubMed] [Google Scholar]
- Wu S. C., Kallin E. M., Zhang Y. (2010). Role of H3K27 methylation in the regulation of lncRNA expression. Cell Res. 20 (10), 1109–1116. 10.1038/cr.2010.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Qu L., He G., Tian L., Li L., Zhou H., et al. (2016). Regulation of laryngeal squamous cell cancer progression by the lncRNA H19/miR-148a-3p/DNMT1 axis. Oncotarget 7 (10), 11553–11566. 10.18632/oncotarget.7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Zhou J., Wu Y., Tang X., Zhu W. (2021a). Overexpression of circRNA circFAT1 in endometrial cancer cells increases their stemness by upregulating miR-21 through methylation. Cancer biother. Radiopharm. 37, 843–849. 10.1089/cbr.2020.4506 [DOI] [PubMed] [Google Scholar]
- Wu X., Gong Y., Yue J., Qiang B., Yuan J., Peng X. (2008). Cooperation between EZH2, NSPc1-mediated histone H2A ubiquitination and Dnmt1 in HOX gene silencing. Nucleic Acids Res. 36 (11), 3590–3599. 10.1093/nar/gkn243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Gao B., Qi X., Bai L., Li B., Bao H., et al. (2021b). Circular RNA ATAD1 is upregulated in acute myeloid leukemia and promotes cancer cell proliferation by downregulating miR-34b via promoter methylation. Oncol. Lett. 22 (5), 799. 10.3892/ol.2021.13060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhang L., Zhang L., Wang Y., Li H., Ren X., et al. (2015). Long non-coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E-cadherin in oral squamous cell carcinoma. Int. J. Oncol. 46 (6), 2586–2594. 10.3892/ijo.2015.2976 [DOI] [PubMed] [Google Scholar]
- Xiao W., Cao Y., Long H., Luo Z., Li S., Deng N., et al. (2018). Genome-wide DNA methylation patterns analysis of noncoding RNAs in temporal lobe epilepsy patients. Mol. Neurobiol. 55 (1), 793–803. 10.1007/s12035-016-0353-x [DOI] [PubMed] [Google Scholar]
- Xie Z., Wang Q., Hu S. (2021). Coordination of PRKCA/PRKCA-AS1 interplay facilitates DNA methyltransferase 1 recruitment on DNA methylation to affect protein kinase C alpha transcription in mitral valve of rheumatic heart disease. Bioengineered 12 (1), 5904–5915. 10.1080/21655979.2021.1971482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L., Lu H., Liu C., Zeng F., Yuan Y. W., Wu Y., et al. (2021). Methionine deficiency promoted mitophagy via lncRNA PVT1-mediated promoter demethylation of BNIP3 in gastric cancer. Int. J. Biochem. Cell Biol. 141, 106100. 10.1016/j.biocel.2021.106100 [DOI] [PubMed] [Google Scholar]
- Xin Z., Tachibana M., Guggiari M., Heard E., Shinkai Y., Wagstaff J. (2003). Role of histone methyltransferase G9a in CpG methylation of the Prader-Willi syndrome imprinting center. J. Biol. Chem. 278 (17), 14996–15000. 10.1074/jbc.M211753200 [DOI] [PubMed] [Google Scholar]
- Xiong Y., Kuang W., Lu S., Guo H., Wu M., Ye M., et al. (2018). Long noncoding RNA HOXB13-AS1 regulates HOXB13 gene methylation by interacting with EZH2 in glioma. Cancer Med. 7 (9), 4718–4728. 10.1002/cam4.1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Wang L., Pang S., Cao M., Wang W., Yu X., et al. (2021a). The functional characterization of epigenetically related lncRNAs involved in dysregulated CeRNA-CeRNA networks across eight cancer types. Front. Cell Dev. Biol. 9, 649755. 10.3389/fcell.2021.649755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wang Z., Li S., Chen J., Zhang J., Jiang C., et al. (2018). Combinatorial epigenetic regulation of non-coding RNAs has profound effects on oncogenic pathways in breast cancer subtypes. Brief. Bioinform. 19 (1), 52–64. 10.1093/bib/bbw099 [DOI] [PubMed] [Google Scholar]
- Xu L., Huan L., Guo T., Wu Y., Liu Y., Wang Q., et al. (2020). LncRNA SNHG11 facilitates tumor metastasis by interacting with and stabilizing HIF-1α. Oncogene 39 (46), 7005–7018. 10.1038/s41388-020-01512-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Xu X., Pan B., Chen X., Lin K., Zeng K., et al. (2019a). LncRNA SATB2-AS1 inhibits tumor metastasis and affects the tumor immune cell microenvironment in colorectal cancer by regulating SATB2. Mol. Cancer 18 (1), 135. 10.1186/s12943-019-1063-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S. F., Zheng Y., Zhang L., Wang P., Niu C. M., Wu T., et al. (2019b). Long non-coding RNA LINC00628 interacts epigenetically with the LAMA3 promoter and contributes to lung adenocarcinoma. Mol. Ther. Nucleic Acids 18, 166–182. 10.1016/j.omtn.2019.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Lou Y., Tang J., Teng Y., Zhang Z., Yin Y., et al. (2019c). The long non-coding RNA Linc-GALH promotes hepatocellular carcinoma metastasis via epigenetically regulating Gankyrin. Cell Death Dis. 10 (2), 86. 10.1038/s41419-019-1348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Yuan X., Ni J., Guo J., Gao Y., Yin W., et al. (2021b). MAGI2-AS3 inhibits breast cancer by downregulating DNA methylation of MAGI2. J. Cell. Physiol. 236 (2), 1116–1130. 10.1002/jcp.29922 [DOI] [PubMed] [Google Scholar]
- Xuan Y., Wang Y. (2019). Long non-coding RNA SNHG3 promotes progression of gastric cancer by regulating neighboring MED18 gene methylation. Cell Death Dis. 10 (10), 694. 10.1038/s41419-019-1940-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Hu Z., Feng Y., Hu X., Yuan J., Zhao S. D., et al. (2015). Comprehensive genomic characterization of long non-coding RNAs across human cancers. Cancer Cell 28 (4), 529–540. 10.1016/j.ccell.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. X., Wu J., Guo M. L., Zhang Y., Ma S. G. (2019a). Suppression of long non-coding RNA TNRC6C-AS1 protects against thyroid carcinoma through DNA demethylation of STK4 via the Hippo signalling pathway. Cell Prolif. 52 (3), e12564. 10.1111/cpr.12564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Yun K., Zhang R. (2021a). CircRNA circ-ATAD1 is downregulated in endometrial cancer and suppresses cell invasion and migration by downregulating miR-10a through methylation. Mamm. Genome 32 (6), 488–494. 10.1007/s00335-021-09899-9 [DOI] [PubMed] [Google Scholar]
- Yang S., Wang B., Liu C., Wang Q., Wang R., Su W., et al. (2021b). THAP9-AS1 promotes tumorigenesis and reduces ROS generation through the JAK2/STAT3 signaling pathway by increasing SOCS3 promoter methylation in osteosarcoma. Oxid. Med. Cell. Longev. 2021, 5620475. 10.1155/2021/5620475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Xu X., Hong L., Wang Q., Huang J., Jiang L. (2019b). Upregulation of lncRNA GAS5 inhibits the growth and metastasis of cervical cancer cells. J. Cell. Physiol. 234 (12), 23571–23580. 10.1002/jcp.28926 [DOI] [PubMed] [Google Scholar]
- Yang Z. G., Awan F. M., Du W. W., Zeng Y., Lyu J., Wu D., et al. (2017). The circular RNA interacts with STAT3, increasing its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Mol. Ther. 25 (9), 2062–2074. 10.1016/j.ymthe.2017.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Xu F., Wang H., Teschendorff A. E., Xie F., He Y. (2021c). Pan-cancer characterization of long non-coding RNA and DNA methylation mediated transcriptional dysregulation. EBioMedicine 68, 103399. 10.1016/j.ebiom.2021.103399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R. W., Wang Y., Chen L. L. (2019). Cellular functions of long noncoding RNAs. Nat. Cell Biol. 21 (5), 542–551. 10.1038/s41556-019-0311-8 [DOI] [PubMed] [Google Scholar]
- Yi T., Song Y., Zuo L., Wang S., Miao J. (2021). LINC00470 stimulates methylation of PTEN to facilitate the progression of endometrial cancer by recruiting DNMT3A through MYC. Front. Oncol. 11, 646217. 10.3389/fonc.2021.646217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. H., You B. H., Park C. H., Kim Y. J., Nam J. W., Lee S. K. (2018). The long noncoding RNA LUCAT1 promotes tumorigenesis by controlling ubiquitination and stability of DNA methyltransferase 1 in esophageal squamous cell carcinoma. Cancer Lett. 417, 47–57. 10.1016/j.canlet.2017.12.016 [DOI] [PubMed] [Google Scholar]
- Yu F., Chen B., Dong P., Zheng J. (2020a). HOTAIR epigenetically modulates PTEN expression via MicroRNA-29b: A novel mechanism in regulation of liver fibrosis. Mol. Ther. 28 (12), 2703. 10.1016/j.ymthe.2020.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Song M. S., Rong P. Z., Chen X. J., Shi L., Wang C. H., et al. (2022a). LncRNA SNHG1 modulates adipogenic differentiation of BMSCs by promoting DNMT1 mediated Opg hypermethylation via interacting with PTBP1. J. Cell. Mol. Med. 26 (1), 60–74. 10.1111/jcmm.16982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Chen Q., Zhang X., Yang J., Lin K., Ji C., et al. (2020b). Long noncoding RNA ANRIL promotes the malignant progression of cholangiocarcinoma by epigenetically repressing ERRFI1 expression. Cancer Sci. 111 (7), 2297–2309. 10.1111/cas.14447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Lu X., Yan Y., Wang Y., Meng J., Tian S., et al. (2022b). The lncRNA KIF9-AS1 accelerates hepatocellular carcinoma growth by recruiting DNMT1 to promote RAI2 DNA methylation. J. Oncol. 2022, 3888798. 10.1155/2022/3888798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q., Zhang H., Pan Z., Ling X., Wu M., Gui Z., et al. (2020). Regulatory loop between lncRNA FAS-AS1 and DNMT3b controls FAS expression in hydroquinone-treated TK6 cells and benzene-exposed workers. Environ. Pollut. 261, 114147. 10.1016/j.envpol.2020.114147 [DOI] [PubMed] [Google Scholar]
- Zhang B., Xing X., Li J., Lowdon R. F., Zhou Y., Lin N., et al. (2014). Comparative DNA methylome analysis of endometrial carcinoma reveals complex and distinct deregulation of cancer promoters and enhancers. BMC Genomics 15, 868. 10.1186/1471-2164-15-868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wang L., Jin C., Zhou J., Peng C., Wang Y., et al. (2021a). Long non-coding RNA Lnc-LALC facilitates colorectal cancer liver metastasis via epigenetically silencing LZTS1. Cell Death Dis. 12 (2), 224. 10.1038/s41419-021-03461-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wang X., Chen Y., Wu Z., Zhang C., Shi W. (2018). The down-regulation of hsa_circ_0012919, the sponge for miR-125a-3p, contributes to DNA methylation of CD11a and CD70 in CD4(+) T cells of systemic lupus erythematous. Clin. Sci. 132 (21), 2285–2298. 10.1042/CS20180403 [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang X., Li X., Zhao N., Wang Y., Han X., et al. (2017). The landscape of DNA methylation-mediated regulation of long non-coding RNAs in breast cancer. Oncotarget 8 (31), 51134–51150. 10.18632/oncotarget.17705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. F., Liu Y. H., Wang D. W., Liu T. S., Yang Y., Guo J. M., et al. (2020a). Obesity-induced reduced expression of the lncRNA ROIT impairs insulin transcription by downregulation of Nkx6.1 methylation. Diabetologia 63 (4), 811–824. 10.1007/s00125-020-05090-y [DOI] [PubMed] [Google Scholar]
- Zhang H., Ge J., Lu X. (2021b). CircFADS2 is downregulated in osteoarthritis and suppresses LPS-induced apoptosis of chondrocytes by regulating miR-195-5p methylation. Arch. Gerontol. Geriatr. 96, 104477. 10.1016/j.archger.2021.104477 [DOI] [PubMed] [Google Scholar]
- Zhang N., Wang A. Y., Wang X. K., Sun X. M., Xue H. Z. (2016). GAS5 is downregulated in gastric cancer cells by promoter hypermethylation and regulates adriamycin sensitivity. Eur. Rev. Med. Pharmacol. Sci. 20 (15), 3199–3205. [PubMed] [Google Scholar]
- Zhang P. F., Wei C. Y., Huang X. Y., Peng R., Yang X., Lu J. C., et al. (2019a). Circular RNA circTRIM33-12 acts as the sponge of MicroRNA-191 to suppress hepatocellular carcinoma progression. Mol. Cancer 18 (1), 105. 10.1186/s12943-019-1031-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Niu Z., Liu J., Dang X., Feng H., Sun J., et al. (2022a). LncRNA SNHG1 promotes sepsis-induced myocardial injury by inhibiting Bcl-2 expression via DNMT1. J. Cell. Mol. Med. 26 (13), 3648–3658. 10.1111/jcmm.17358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Cao H., Ye L., Wen X., Wang S., Zheng W., et al. (2019b). Cancer-associated methylated lncRNAs in patients with bladder cancer. Am. J. Transl. Res. 11 (6), 3790–3800. [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zheng F., Zhang L., Huang Z., Huang X., Pan Z., et al. (2020b). LncRNA HOTAIR-mediated MTHFR methylation inhibits 5-fluorouracil sensitivity in esophageal cancer cells. J. Exp. Clin. Cancer Res. 39 (1), 131. 10.1186/s13046-020-01610-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Jiang Q., Li J., Zhang S., Cao Y., Xia X., et al. (2022b). KCNQ1OT1 promotes genome-wide transposon repression by guiding RNA-DNA triplexes and HP1 binding. Nat. Cell Biol. 24, 1617–1629. 10.1038/s41556-022-01008-5 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lu C., Cui H. (2021c). Long non-coding RNA SNHG22 facilitates hepatocellular carcinoma tumorigenesis and angiogenesis via DNA methylation of microRNA miR-16-5p. Bioengineered 12 (1), 7446–7458. 10.1080/21655979.2021.1975969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yan H., Jiang Y., Chen T., Ma Z., Li F., et al. (2021d). Long non-coding RNA IGF2-AS represses breast cancer tumorigenesis by epigenetically regulating IGF2. Exp. Biol. Med. 246 (4), 371–379. 10.1177/1535370220966253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Ling X., Xia Y., Yan B., Guan Q. (2022a). LncRNA UCA1 promotes SOX12 expression in breast cancer by regulating m(6)A modification of miR-375 by METTL14 through DNA methylation. Cancer Gene Ther. 29 (7), 1043–1055. 10.1038/s41417-021-00390-w [DOI] [PubMed] [Google Scholar]
- Zhao C., Liu J., Wu H., Hu J., Chen J., Chen J., et al. (2020a). Aberrant methylation-mediated downregulation of lncRNA CCND2 AS1 promotes cell proliferation in cervical cancer. J. Biol. Res. 27, 11. 10.1186/s40709-020-00122-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Guo M., Zhang C., Wang C., Wang K. (2022b). Pan-cancer methylated dysregulation of long non-coding RNAs reveals epigenetic biomarkers. Front. Cell Dev. Biol. 10, 882698. 10.3389/fcell.2022.882698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Hu X. (2019). Downregulated long noncoding RNA LINC00313 inhibits the epithelial-mesenchymal transition, invasion, and migration of thyroid cancer cells through inhibiting the methylation of ALX4. J. Cell. Physiol. 234 (11), 20992–21004. 10.1002/jcp.28703 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Song J., Tang B., Fang S., Zhang D., Zheng L., et al. (2020b). CircSOD2 induced epigenetic alteration drives hepatocellular carcinoma progression through activating JAK2/STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 39 (1), 259. 10.1186/s13046-020-01769-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi H., Li X., Wang P., Gao Y., Gao B., Zhou D., et al. (2018). Lnc2Meth: A manually curated database of regulatory relationships between long non-coding RNAs and DNA methylation associated with human disease. Nucleic Acids Res. 46 (D1), D133–D138. 10.1093/nar/gkx985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi H., Ning S., Li X., Li Y., Wu W., Li X. (2014). A novel reannotation strategy for dissecting DNA methylation patterns of human long intergenic non-coding RNAs in cancers. Nucleic Acids Res. 42 (13), 8258–8270. 10.1093/nar/gku575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q., Lu M., Yuan W., Cui Y., Ouyang H., Fan Y., et al. (2021). Eight-lncRNA signature of cervical cancer were identified by integrating DNA methylation, copy number variation and transcriptome data. J. Transl. Med. 19 (1), 58. 10.1186/s12967-021-02705-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong T., Men Y., Lu L., Geng T., Zhou J., Mitsuhashi A., et al. (2017). Metformin alters DNA methylation genome-wide via the H19/SAHH axis. Oncogene 36 (17), 2345–2354. 10.1038/onc.2016.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yang L., Zhong T., Mueller M., Men Y., Zhang N., et al. (2015). H19 lncRNA alters DNA methylation genome wide by regulating S-adenosylhomocysteine hydrolase. Nat. Commun. 6, 10221. 10.1038/ncomms10221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Ren M., Zeng T., Wang W., Wang X., Hu M., et al. (2019a). TET2-interacting long noncoding RNA promotes active DNA demethylation of the MMP-9 promoter in diabetic wound healing. Cell Death Dis. 10 (11), 813. 10.1038/s41419-019-2047-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. Y., Zhai M., Huang Y., Xu S., An T., Wang Y. H., et al. (2019b). The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/FAM65B pathway. Cell Death Differ. 26 (7), 1299–1315. 10.1038/s41418-018-0206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Li H., Chen K., Ding W., Yang C., Wang X. (2021a). CircSKA3 downregulates miR-1 through methylation in glioblastoma to promote cancer cell proliferation. Cancer Manag. Res. 13, 509–514. 10.2147/CMAR.S279097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Zhu X., Ye Z., Wang Y. F., Yao C., Xu N., et al. (2022). Lupus enhancer risk variant causes dysregulation of IRF8 through cooperative lncRNA and DNA methylation machinery. Nat. Commun. 13 (1), 1855. 10.1038/s41467-022-29514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Xu S., Chen X., Wang C. (2021b). HOTAIR suppresses PTEN via DNMT3b and confers drug resistance in acute myeloid leukemia. Hematology 26 (1), 170–178. 10.1080/16078454.2021.1880733 [DOI] [PubMed] [Google Scholar]
- Zhu X., Du J., Yu J., Guo R., Feng Y., Qiao L., et al. (2019). LncRNA NKILA regulates endothelium inflammation by controlling a NF-κB/KLF4 positive feedback loop. J. Mol. Cell. Cardiol. 126, 60–69. 10.1016/j.yjmcc.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Zhu Y., You J., Wei W., Gu J., Xu C., Gu X. (2021). Downregulated lncRNA RCPCD promotes differentiation of embryonic stem cells into cardiac pacemaker-like cells by suppressing HCN4 promoter methylation. Cell Death Dis. 12 (7), 667. 10.1038/s41419-021-03949-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S. H., Meng C. C., Fu J. J., Huang J. (2022). Long non-coding RNA ELFN1-AS1-mediated ZBTB16 inhibition augments the progression of gastric cancer by activating the PI3K/AKT axis. Kaohsiung J. Med. Sci. 38 (7), 621–632. 10.1002/kjm2.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziller M. J., Gu H., Muller F., Donaghey J., Tsai L. T., Kohlbacher O., et al. (2013). Charting a dynamic DNA methylation landscape of the human genome. Nature 500 (7463), 477–481. 10.1038/nature12433 [DOI] [PMC free article] [PubMed] [Google Scholar]