Abstract
Background
Donation after circulatory death (DCD) is becoming increasingly common, yet little is known about the way potential donors receive end-of-life care.
Purpose
The aims of this systematic review are to describe the current practice in end-of-life care for potential donors and identify metrics that are being used to assess discomfort among these patients.
Research design and Study Sample
This review encompasses published literature between June 1, 2000 and June 31, 2020 of end-of-life care received by potential DCD patients. The population of interest was defined as patients eligible for Maastracht classification III donation after circulatory death for a solid organ transplantation. Outcomes examined included: analgesic or palliative protocols, and surrogates of discomfort (eg dyspnea, agitation).
Results
Among 141 unique articles, 27 studies were included for full review. The primary reason for exclusion was lack of protocol description, or lack of reporting on analgesic medications. No primary research studies specifically examined distress in the DCD eligible population. Numerous professional guidelines were identified. Surveys of critical care practitioners identified concerns regarding the impact of symptom management on hastening the dying process in the DCD population as a potential barrier to end-of-life palliative treatment.
Conclusions
There is a paucity of empirical evidence for end-of-life symptom assessment and management for DCD patients. Key evidence gaps identified for DCD include the need for: i) a multidisciplinary structure of treatment teams and preferred environment for DCD, ii) objective tools for monitoring of distress in this patient population, and iii) evidence guiding the administration of analgesic medications following withdrawal of life sustaining therapy.
Keywords: donation after circulatory death, palliative care, organ donation, neurocritical care
In the last 2 decades, there has been a revival of donation after circulatory death, particularly arising from the limits of the existing donor pool from donation after brain death.1,2 Solid organ transplants, as of 2017, only met about 10% of the total donor need according to World Health Organization estimates. 3 Donation after circulatory death (DCD) generally occurs in patients with severe and irreversible brain injury in whom the decision has been made to withdraw life sustaining treatments. Patients are declared dead based on cardiopulmonary criteria as opposed to donation after brain death, which is defined as the irreversible loss of all functions of the entire brain determined by complete loss of consciousness, brainstem reflexes, ventilatory drive and absence of reversible features. 4
Despite unanimous support from the United Network for Organ Sharing, the Joint Commission on Accreditation of Healthcare organizations, and the Institute of Medicine 3 mandate that hospitals have comprehensive DCD protocols in place, wide and persistent variation in the final phase just prior to withdrawal of life sustaining treatments and before cardiopulmonary arrest persists.1,2 Largely driven by concerns regarding ethical ambiguity many experts in the field, as well as professional societies, have since ventured to address these issues by drafting guidelines. 5
In developing guidelines for DCD, it is essential that the decision to withdraw life sustaining therapy is decoupled from organ donation, and as such the procuring transplant team is never to be involved in conversations regarding prognosis or ventilator withdrawal.2,6 As a prerequisite, all eligible DCD cases comprise patients in whom the decision to transition to comfort care has been decided. If these patients expire quickly during this phase, organs may be recovered for transplant depending on organ viability.
Typical DCD subjects are from a heterogenous population often with numerous medical comorbidities, including those that have catastrophic brain injury, terminal neuromuscular disorders such as amyotrophic lateral sclerosis (ALS), and high spinal cord injuries. Generally, these are patients whose death would be considered imminent once life sustaining treatment is withdrawn. Since these patients do not meet brain death criteria, it is critically important that best practices are utilized when it comes to amelioration of discomfort at end-of-life.
There remain numerous situations in which patients are minimally conscious 7 following withdrawal of life support measurers, further emphasizing a need for appropriate comfort-based therapy. In this way, neurologists are uniquely poised to assess and identify responsiveness, and discomfort, in these individuals. As it stands a broad group of non-neurologists are more often involved in the end-of-life care of these neurologically complex patients. Given the heterogeneity in this population, neurohospitalists are positioned to identify patients at higher risk for retained awareness, and thus discomfort, during the withdrawl-of-life sustaining therapy (WLST), that may not be captured by imperfect measures of distress such as vital sign variation. Neurologists are often consulting on the care of these patients prior to WLST, or managing them as neurointensivists, thus there exists an opportunity for care of neurologically vulnerable patients to continue until death and potential organ procurement.”
The primary objective of this review is to describe variation in palliative and comfort focused care provided for the potential DCD donor prior to death, and to examine the metrics currently being utilized to assess discomfort during the final phase prior to donation.
Methods
An initial search strategy was modeled after recommendations of the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) statement 8 and the Assessment of Multiple Systematic Reviews (AMSTAR) tool. 9 The population of interest was defined as patients eligible for Maastricht classification III donation after circulatory death for a solid organ transplantation. Outcomes examined included the inclusion of analgesic or palliative protocols, as well as the recording of surrogates of discomfort such as pain, anxiety, tachycardia, dyspnea, or agitation.
An initial search strategy involved searching multiple databases including PubMed, Embase, the Cochrane Database and Web of Science between June 21, 2000 and July 1, 2020 for original English-language research published in peer-reviewed journals, which included reviews, editorials, cross sectional surveys, and professional society guidelines, between June 1, 2000 and June 31, 2020. Search terms were devised and included terms such as “palliative”, “comfort”, “pain”, “analgesia”, “sedation”, “opioids”, “dyspnea”, “symptoms”, “ethics”, “end-of-life care”, “NHBD”, “DCD”, and “organ donation”. Terms such as “Autism”, “developmental coordination disorder,” dynamic cooling device” were excluded to avoid search terms with similar acronyms utilized in other clinical contexts.
We reviewed titles and abstracts from the initial database searches to identify articles eligible for full text review. Prior to selecting an article for full review, text search functions were utilized to identify if end-of-life care was discussed in the body of the work. This search was supplemented by hand-searching the reference lists of the full-text articles to identify potentially relevant studies.
Studies that met the following criteria were included for full text review: reported the use of analgesic or sedative medications either in methods or as covariates/outcome, included patients eligible or undergoing Maastricht III organ transplants after cardiopulmonary arrest or represented institutional/societal guidelines and detailed recommendations for end-of-life care.
Results
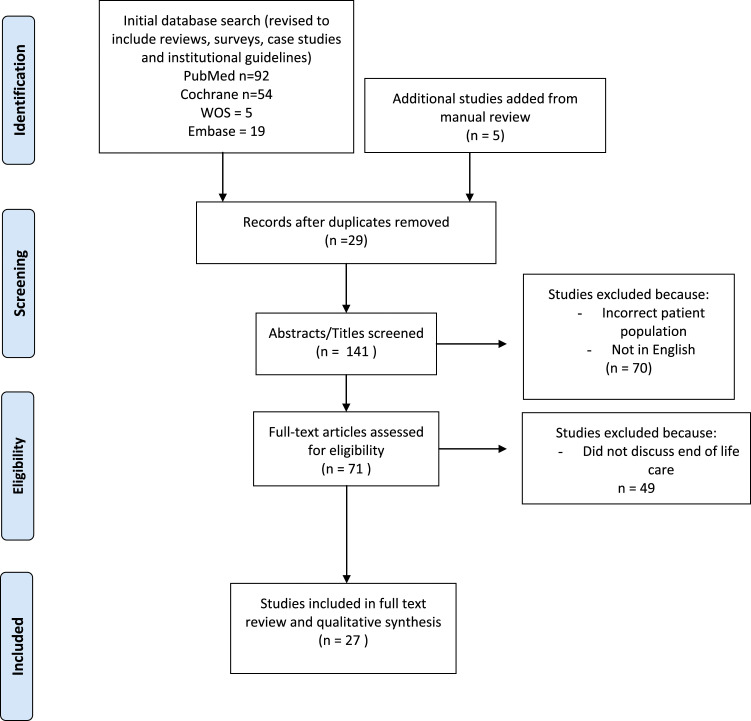
The search strategy (Figure 1) yielded 141 unique articles; after excluding studies from the database search that did not include the population of interest, 71 studies underwent brief review, and additional studies were further excluded if they did not discuss end-of-life care of potential donor patients in the body of the work. After including manually reviewed studies, 27 studies were selected for full text review. Of these, 2 articles were retrospective cohort studies,10,11 3 were cross sectional surveys of intensive care unit (ICU) practitioners,12–14 5 were case studies,15–19 6 were professional society or institutional guidelines,5,20–23 and the remainder (n = 11) were reviews or position papers. Primary sources of bias originate from the lack of primary research identified, with the only original empirical evidence stemming from retrospective or cross-sectional survey data.
Figure 1.
Search strategy and study screening protocol.
Comfort Focused Medications and Time to Death
Among these articles, 2 retrospective cohort studies examined the role of sedative analgesics in the potential DCD population, both examining the question of comfort-focused care expediting time to death in this population. Among 128 patients being considered for controlled DCD, LeDoux et al compared time to death after withdrawal of life sustaining therapy between DCD and non-DCD patients. 10 They found that a DCD protocol with comfort focused measures did not reduce time to cardiopulmonary arrest compared to non-DCD patients receiving standard comfort focused care. The study was limited by a lack of reporting for validated measures of discomfort, or surrogate markers, such as vital sign abnormalities, secretions, facial grimacing or perceived discomfort by bedside clinicians. Further, specific medications and treatment protocols utilized during withdrawal of life sustaining therapy were not described.
A separate retrospective study of 505 DCD patients examined the association of the use of comfort focused medications (opiates, benzodiazepines) at end-of-life and time to death. 11 Among 72% of patients receiving at least 1 comfort medication, there was a lower risk of death in the first 60 minutes after withdrawal of life sustaining therapy (aOR .35 CI [.21-.59]) in those treated with comfort focused medications.
Evaluation of Discomfort and Distress
No studies specifically examined discomfort or distress, or reported a specific protocol for use of comfort focused medications during DCD. The use of sedatives and analgesics, 5 and importance of involving trained palliative care practitioners 24 are mentioned, but protocols for evaluating DCD patients, including indications for palliative care specialist involvement are not suggested.
A survey of pediatric critical care nurses (n= 93) involved in DCD cases found 95% of respondents felt analgesics should be used for discomfort, and 11% expressed concern for undertreating pain. 12 In a cross sectional survey of critical care practitioners across Australia and New Zealand, the vast majority (92%) of physician respondents either agreed or strongly agreed with prescribing palliative medications during palliative ventilatory withdrawal, though 1 in 4 respondents reported they avoid higher doses given concern for perceived hastening of death. A similar percentage (23%) of physicians reported altering their end-of-life care in DCD eligible patients, with 60% of physicians reporting they were concerned that DCD eligible patients would receive inappropriate doses of palliative medication (either excess or inadequate). 13 In a large cross sectional survey in the United States (n = 3204), the majority of intensive care physicians and nurses felt they should “help manage potential donors”, but differences in end of life care among DCD were not reported. 14
The most specific model for caring for DCD patients was described by Kelso et al, who described co-management of DCD patients with palliative care providers alongside the intensive care team, and suggested monitoring for signs of discomfort, while treating episodes of tachypnea, moaning, grimacing, restlessness or myoclonus with opioids or sedatives accordingly. In this paradigm, the intensive care nurse administered medications prescribed by a palliative care provider at bedside, and the intensive care team pronounced death and facilitated transfer to the organ-recovery team. 25 Others suggest establishing vital sign parameters by which to administer medications such as titration of benzodiazepines and opiates to heart rate less than 100 beats per minute or respiratory rate less than 20.26,27 While oral secretions are unlikely to be distressing to the patient themselves, 28 anticholinergic medications such as scopolamine (ie 0.4mg IV or IM) or glycopyrrolate have been proposed for secretion management,25,28 especially if a patient’s family is at bedside.
Sedative and Analgesic Regimens
While there is emerging consensus emphasizing DCD patient’s equal right to comfort-focused care1,2,6,26,27 prior to circulatory arrest, there are few specific protocols or rigorous evaluations of sedative and analgesic regimens utilized during DCD. Many institutions may view DCD as the same as WLST, however different care processes and physical location within the hospital where DCD unfolds (ie operating room vs intensive care unit) warrants rigorous appraisal. While 1 study examined variation among adult donation center protocols (serviced by the New England Organ Bank), investigators found that 60% (54/90) of the participating hospitals specifically indicated that analgesics were accepted in the DCD protocol. 29
Many suggest that DCD organ donors prior to donation should be treated in concordance with institution specific standard of care in regard to end of life care.2,3,30–32 As such, assuming a protocol similar to that of the palliative ventilatory withdrawal of any other critically or neurologically ill patient could be proposed,26,33 the mainstay of which involves treatment with benzodiazepines and opioid pain medications. 34 Frontera26,30 suggests titration of benzodiazepines and opiate boluses to vital sign parameters, such as heart rate less than 100 beats per minute or respiratory rate less than 20. While Kelso et al 25 utilize a regimen by which 5-10mg IV morphine or 2-4mg IV midazolam are used for respiratory distress every 5 minutes titrated to distress, is cited as the standard of care at their institution.
Mounting evidence suggests palliative benefit in the administration of anticipatory doses of opioids prior to WLST to avoid respiratory distress, 35 and avoidance of sedative monotherapy given concerns around paradoxically worsening dyspnea There were no publications recommending premedication prior to palliative ventilatory withdrawal (anticipatory dosing) among DCD patients. However premedication with analgesic medications for dyspnea was mentioned in several case reports.17,36,37
Outcome data measuring donor patient comfort, and specific types of sedative analgesics utilized are extremely limited. The largest published single center cohort found 382 DCD cases between 1984 and 2000, 38 but there was no mention of sedative and analgesic regimens used among DCD patients prior to death. Only 1 study of pediatric DCD patients mentioned specific sedatives and analgesics used (fentanyl mean dose of 4 µg per kilogram and lorazepam mean dose of .1 mg per kilogram). 39 Many articles made no mention of palliative analgesics,38,40–43 while others described adhering to “institutional care and comfort measures”44,45
Consciousness and DCD
Undetected or covert consciousness is a major concern among DCD patients as places them at risk for distress, 46 yet few studies evaluated potential DCD patients for covert consciousness. A small case series of DCD eligible patients (n=3) demonstrated changes to bispectral index on processed electroencephalogram (EEG) during the period following withdrawal of life sustaining treatment (WLST). 15 Only 1 other small case series attempted to replicate these results, 16 however, given very few patients were included in these studies, no conclusions can be drawn. Task-based EEG monitoring 47 has not been evaluated in this population.
Patients known to have preserved awareness and arousal such as in amyotrophic lateral sclerosis (ALS), or high spinal cord injury, highlights the need for well-developed protocolization of treatment of peri-mortem distress in the DCD population. These patients are distinct from other DCD donors who often have devastating brain injury but fail to meet brain death criteria. While uncommon, multiple cases of DCD after progressive respiratory failure secondary to ALS have been reported.17,18 In all cases, organ donation per DCD protocol was desired by the donor and discussed in anticipation of an inevitable progression of their neuromuscular disease. As an example, one such patient was admitted electively in the setting of progressive disease; after a period of opioid dose titration, pre-medication with fentanyl and midazolam were utilized prior to withdrawal of ventilator support, and DCD. 17 Similarly, patients with high spinal cord injury, or brain stem injury that spare the cortical arousal networks, as in the locked-in patient, 19 emphasize the need for detailed multidisciplinary planning for DCD in this heterogenous population.
Institutional and Professional Society Guidelines
Many professional societies have published guidelines, with the goal of standardizing the DCD process and supporting best practices. The guidelines are comprehensive, offering frequent and broad assurances that the comfort of the donor is fundamental and not to be violated. The report from the national conference on donation after cardiac death, for example, states that “quality end of life care...is the absolute priority” and “sedatives and opioids should be administered in the customary manner”. 48 Similarly the Society of Critical Care Medicine asserts that the donor has “equivalent right” 5 to comfort care as other patients and, in concert with the American Thoracic Society and the United Network of Organ Sharing, released a statement that includes the mandate to “manage symptoms of pain, anxiety, and breathlessness”. 21 The American Society of Transplant Surgeons, 20 American Thoracic Society, 21 and the Canadian 22 position statements have similar declarations, mentioning specifically that procurement team members are not to be involved in managing potential discomfort of the patient. A review of 72 unique institutional DCD protocols across children’s hospitals found that 89% [CI 80-95%] of protocols highlighted the importance of palliative care, while only 7% [CI 2-15%] recommended or required palliative care specialist involvement. Specific palliative medications or measures of peri-mortem discomfort were not discussed. 23
Discussion
The DCD process is a unique clinical situation requiring specific evidence to reduce the potential for distress and discomfort. While there is consensus that comfort focused sedatives and analgesics have a role in end-of-life care for DCD patients, standards for monitoring and treating these patients are not clear. There is very little systematic empirical research examining palliative care during DCD. Professional society guidelines and position statements assert the importance of comfort-focused therapy without proposing a standard of care for such regimens. The evidence gap may be due to an assumption that existing end of life care practices are generalizable to DCD patients. The atypical setting, neurophysiological state, and heightened potential for clinician moral distress 49 are unique to DCD, and call for specific evidence to support best practices in the care of DCD patients.
The principle of double effect supports the use of sedative analgesics with the intent of alleviating distress even if there is a risk of hastening death. 50 Moreover, analgesic medications and benzodiazepines seem to prolong the time before cardiopulmonary arrest in DCD eligible populations,10,11 and previous experiences in critically ill patients suggest that sedatives do not hasten death.51,52 The DCD population includes patients with severe neuromuscular disease with preserved awareness, as well as those with brain injury that still maintain some cortical function, such as in patients with malignant middle cerebral artery infarctions or large brainstem hemorrhages. These patients may not be able to communicate discomfort but neurologically have intact pain and arousal networks, leaving them at risk for limited recognition and undertreatment of distress.
Most of the hallmark studies in the DCD literature make no mention of the use of sedation or analgesia in the donor prior to transplantation. This is likely due to the requirements of the donation process, which necessitates separation of the transplant recovery team and the treating physicians of the donor prior to death. Thus, by design, the transplant teams rarely have access to the data regarding how the donor patient was treated prior to arrest. However, minimal data suggest significant variation in donor environment, as preferred by organ procurement organizations. 53 There is likely additional variation as it relates to background or level of training (intern to attending staff or advanced practice providers), and area of specialization (anesthesiology, critical care, and palliative care), but this has not been extensively studied. 29
Potential DCD donors represent a heterogenous population of critically ill patients, with similarly varied degrees of awareness. Both the bedside evaluation and noninvasive neuromonitoring of consciousness among these patients are imperfect.7,46,47,54 This population of patients is at high risk for under-treatment of end-of-life distress and discomfort due to concerns surrounding expediting patient death, and uncertainty regarding the ability of patients with severe neurologic injury to experience distress. As such, neurologists and neurointensivists caring for these patients are uniquely positioned to develop evidence based guidelines for the DCD process in collaboration with other specialties providing end of life care. At present no major neurological professional society has developed or endorsed specific guidelines for the care of DCD patients.
There are multiple limitations to this review that are worth highlighting. Most strikingly, the lack of empirical evidence studying end-of-life care in the DCD population creates a space for conjecture in the synthesis of lower quality research. The authors attempted to mitigate this by using a systematic approach and openly calling attention to times when conclusions or future avenues of research were not supported by high quality empirical evidence. Additionally, while the search strategy followed PRISMA guidelines, the transplantation research is multi-discplinary, and it is possible our search strategy may have missed small empiric studies of end-of-life care among the broad array of sub-categories of patients.
Overall improved systems of support for clinicians caring for these patients may be beneficial, and foster an appreciation for unrecognized symptoms at end-of-life among DCD patients. Some institutions identified in this review designate specific palliative care providers to be responsible for administration of sedation and analgesia during the DCD process, while the critical care team is responsible for pronouncing death of the patient and facilitating transition to the transplant recovery team.25,31 Future research could support an empirical approach to optimal team composition and dynamics for reducing potential for distress, and to better characterize the experiences of those involved with potential DCD patients. Some evidence suggests that concerns regarding sedative and analgesia dosing decisions persist. 13 One potential strategy to address this gap could be a mixed methods study of neurologist and non-neurologists, to gain a detailed understanding of approaches to analgesic and sedative regimens. To extend the ongoing work of researchers in palliative and end-of-life care, 55 there is a role for neurologists to refine and validate objective measures of distress among patients with severe brain injury.
This review has identified significant evidence gaps in the practice of comfort focused care among patients at end-of-life who are being considered for DCD. Specifically, there is a need for the development of evidence-based interdisciplinary guidelines detailing: i) the multidisciplinary structure of treatment teams and the preferred environment for DCD, ii) objective tools for monitoring of distress, and iii) the administration of sedative and analgesic medications following withdrawal of life sustaining therapy among the DCD population.
Appendix.
Abbreviations
ALS (amyotrophic lateral sclerosis) DCD (donation after circulatory death) EEG (electroencephalogram)ICU (intensive care unit)
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SPC has nothing to disclose, WSS reports Ownership interest in MindRhythm, Inc, RS has nothing to disclose, DBW reports grants as below during the conduct of the study; personal fees from American Thoracic Society, personal fees from UpToDate, outside the submitted work; SLM has nothing to disclose, CRF has nothing to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Stefanie P Cappucci https://orcid.org/0000-0003-0061-8495
Corey R Fehnel https://orcid.org/0000-0003-1726-5809
References
- 1.Neyrinck A, Van Raemdonck D, Monbaliu D. Donation after circulatory death: current status. Curr Opin Anaesthesiol. 2013;1:382-390. [DOI] [PubMed] [Google Scholar]
- 2.Smith M, Dominguez-Gil B, Greer DM, Manara AR, Souter MJ. Organ donation after circulatory death: current status and future potential. Intensive Care Med. 2019;45:310-321. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine (US) . Committee on Non-Heart-Beating Transplantation II: The Scientific and Ethical Basis for Practice and Protocols. Non-Heart-Beating Organ Transplantation: Practice and Protocols. Washington (DC): National Academies Press (US); 2000. http://www.ncbi.nlm.nih.gov/books/NBK225025/ [PubMed] [Google Scholar]
- 4.Russell JA, Epstein LG, Greer DM, Kirschen M, Rubin MA, Lewis A. Brain death, the determination of brain death, and member guidance for brain death accommodation requests: AAN position statement. Neurology. Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology. 2019;92:228-232. [DOI] [PubMed] [Google Scholar]
- 5.A Position Paper by the Ethics Committee, American College of Critical Care Medicine, Society of Critical Care Medicine . Recommendations for nonheartbeating organ donation: Crit Care Med. 2001;29:1826–1831. [DOI] [PubMed] [Google Scholar]
- 6.Manara AR, Murphy PG, O’Callaghan G. Donation after circulatory death. Br J Anaesth. 2012;108:i108-i121. [DOI] [PubMed] [Google Scholar]
- 7.Giacino JT, Edlow BL. Covert Consciousness in the Intensive Care Unit. Trends Neurosci. 2019;42:844-847. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. British Medical Journal Publishing Group; 2009. https://www.bmj.com/content/339/bmj.b2535 [PMC free article] [PubMed] [Google Scholar]
- 9.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledoux D, Delbouille M-H, Deroover A, et al. Does comfort therapy during controlled donation after circulatory death shorten the life of potential donors? Clin Transplant. 2014;28:47-51. [DOI] [PubMed] [Google Scholar]
- 11.DeVita MA, Brooks MM, Zawistowski C, Rudich S, Daly B, Chaitin E. Donors after cardiac death: Validation of Identification Criteria (DVIC) Study for Predictors of Rapid Death. Am J Transplant. 2008;8:432-441. [DOI] [PubMed] [Google Scholar]
- 12.Mathur M, Taylor S, Tiras K, Wilson M, Abd-Allah S. Pediatric critical care nurses’ perceptions, knowledge, and attitudes regarding organ donation after cardiac death. Pediatr Crit Care Med. 2008;9:261-269. [DOI] [PubMed] [Google Scholar]
- 13.Lee YY, Ranse K, Silvester W, Mehta A, Van Haren FMP. Attitudes and Self-Reported End-Of-Life Care of Australian and New Zealand Intensive Care Doctors in the Context of Organ Donation after Circulatory Death. Anaesth Intensive Care. 2018;46:488-497. [DOI] [PubMed] [Google Scholar]
- 14.Hart JL, Kohn R, Halpern SD. Perceptions of organ donation after circulatory determination of death among critical care physicians and nurses: A national survey. Crit Care Med. 2012;40:2595-2600. [DOI] [PubMed] [Google Scholar]
- 15.Auyong DB, Klein SM, Gan TJ, Roche AM, Olson D, Habib AS. Processed Electroencephalogram During Donation After Cardiac Death. Anesth Analg. 2010;110:1428-1432. [DOI] [PubMed] [Google Scholar]
- 16.Norton L, Gibson RM, Gofton T, et al. Electroencephalographic recordings during withdrawal of life-sustaining therapy until 30 minutes after declaration of death. Can J Neurol Sci J Can Sci Neurol. 2017;44:139-145. [DOI] [PubMed] [Google Scholar]
- 17.Toossi S, Lomen‐Hoerth C, Josephson SA, et al. Organ donation after cardiac death in amyotrophic lateral sclerosis. Ann Neurol. 2012;71:154-156. [DOI] [PubMed] [Google Scholar]
- 18.Smith TJ, Vota S, Patel S, et al. Organ donation after cardiac death from withdrawal of life support in patients with amyotrophic lateral sclerosis. J Palliat Med. Mary Ann Liebert. 2011;15:16-19. [DOI] [PubMed] [Google Scholar]
- 19.Bruno B, Eves M. Discomfort as a catalyst: An ethical analysis of donation after cardiac death in a patient with locked-in syndrome. J Clin Ethics. 2018;29:313-318. [PubMed] [Google Scholar]
- 20.Reich DJ, Mulligan DC, Abt PL, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004-2011. [DOI] [PubMed] [Google Scholar]
- 21.Gries CJ, White DB, Truog RD, et al. An official american thoracic society/international society for heart and lung transplantation/society of critical care medicine/association of organ and procurement organizations/united network of organ sharing statement: ethical and policy considerations in organ donation after circulatory determination of death. Am J Respir Crit Care Med. American Thoracic Society - AJRCCM. 2013;188:103-109. [DOI] [PubMed] [Google Scholar]
- 22.Shemie SD, Baker AJ, Knoll G, et al. Donation after cardiocirculatory death in Canada. CMAJ Can Med Assoc J. 2006;175:S1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antommaria AHM, Trotochaud K, Kinlaw K, Hopkins PN, Frader J. Policies on Donation After Cardiac Death at Children’s Hospitals: A Mixed-Methods Analysis of Variation. JAMA. American Medical Association. 2009;301:1902-1908. [DOI] [PubMed] [Google Scholar]
- 24.Joris J, Kaba A, Lauwick S, et al. End of life care in the operating room for non–heart-beating donors: organization at the university hospital of Liège. Transplant Proc. 2011;43:3441-3444. [DOI] [PubMed] [Google Scholar]
- 25.Kelso CM, Lyckholm LJ, Coyne PJ, Smith TJ. Palliative care consultation in the process of organ donation after cardiac death. J Palliat Med. 2007;10:118-126. [DOI] [PubMed] [Google Scholar]
- 26.Frontera JA, Curtis JR, Nelson JE, et al. Integrating palliative care into the care of neurocritically Ill Patients: A report from the improving palliative care in the icu project advisory board and the center to advance palliative care. Crit Care Med. 2015;43:1964-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson TA, Bekker P, Vagefi PA. Anesthetic considerations in organ procurement surgery: A narrative review. Can J Anesth Can Anesth. 2015;62:529-539. [DOI] [PubMed] [Google Scholar]
- 28.Kompanje EJO. The death rattle” in the intensive care unit after withdrawal of mechanical ventilation in neurological patients. Neurocrit Care. 2005;3:107-110. [DOI] [PubMed] [Google Scholar]
- 29.Rhee JY, Ruthazer R, O’Connor K, Delmonico FL, Luskin RS, Freeman RB. The impact of variation in donation after cardiac death policies among donor hospitals: A regional analysis. Am J Transplant. 2011;11:1719-1726. [DOI] [PubMed] [Google Scholar]
- 30.Frontera JA. How i manage the adult potential organ donor: donation after cardiac death (Part 2). Neurocrit Care. 2010;12:111-116. [DOI] [PubMed] [Google Scholar]
- 31.Beach PR, Hallett AM, Zaruca K. Organ donation after circulatory death: vital partnerships. Am J Nurs. 2011;111:32-40. [DOI] [PubMed] [Google Scholar]
- 32.Dalle Ave AL, Shaw DM. Controlled donation after circulatory determination of death: ethical issues in withdrawing life-sustaining therapy. J Intensive Care Med. 2017;32:179-186. [DOI] [PubMed] [Google Scholar]
- 33.Pullicino P, Burke W, Mayer SA, Kossoff S. Withdrawal of life support in the neurologic intensive care unit. Neurology. Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology. 1999;53:2211. [DOI] [PubMed] [Google Scholar]
- 34.Truog RD, Campbell ML, Curtis JR, et al. Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College [corrected] of Critical Care Medicine. Crit Care Med. 2008;36:953-963. [DOI] [PubMed] [Google Scholar]
- 35.Fehnel CR, Armengol de la Hoz M, Celi LA, et al. Incidence and Risk Model Development for Severe Tachypnea Following Terminal Extubation. 2020. https://linkinghub.elsevier.com/retrieve/pii/S0012369220307704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prommer E. Organ donation and palliative care: can palliative care make a difference? J Palliat Med. 2014;17:368-371. [DOI] [PubMed] [Google Scholar]
- 37.Tunzi M, Spike JP. The role of patient comfort and “comfort measures only” in organ donation after cardiac death (DCD) After a Stroke. Am J Bioeth. 2014;14:39-41. [DOI] [PubMed] [Google Scholar]
- 38.Cooper JT, Chin LT, Krieger NR, et al. Donation after cardiac death: the university of wisconsin experience with renal transplantation. Am J Transplant. 2004;4:1490-1494. [DOI] [PubMed] [Google Scholar]
- 39.Boucek MM, Frizell R, Campbell D, et al. Pediatric heart transplantation after declaration of cardiocirculatory death. N Engl J Med. 2008;6:709-714. [DOI] [PubMed] [Google Scholar]
- 40.Suntharalingam C, Sharples L, Dudley C, Bradley JA, Watson CJE. Time to Cardiac Death After Withdrawal of Life-Sustaining Treatment in Potential Organ Donors. Am J Transplant. 2009;9:2157-2165. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez LA, Di Carlo A, Odorico JS, et al. Simultaneous pancreas-kidney transplantation from donation after cardiac death: successful long-term outcomes. Ann Surg. 2005;242:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scalea JR, Redfield RR, Rizzari MD, et al. When Do DCD Donors Die?: Outcomes and Implications of DCD at a High-volume, Single-center OPO in the United States. Ann Surg. 2016;263:211-216. [DOI] [PubMed] [Google Scholar]
- 43.Cook DA, Widdicombe N. Audit of Ten Years of Donation after Circulatory Death Experience in Queensland: Observations of Agonal Physiology following Withdrawal of Cardiorespiratory SupportAnaesth Intensive Care. SAGE Publications Ltd; 2018:400-403. [DOI] [PubMed] [Google Scholar]
- 44.Reich DJ, Munoz SJ, Rothstein KD, et al. Controlled non-heart-beating donor liver transplantation: A Successful Single Center Experience, with Topic Update. Transplantation. 2000;70:1159-1166. [DOI] [PubMed] [Google Scholar]
- 45.Kramer AH, Holliday K, Keenan S, et al. Donation after circulatory determination of death in western Canada: a multicentre study of donor characteristics and critical care practices. Can J Anesth Can Anesth. 2020;67:521-531. [DOI] [PubMed] [Google Scholar]
- 46.Edlow BL, Fins JJ. Assessment of covert consciousness in the intensive care unit: clinical and ethical considerations. J Head Trauma Rehabil. 2018;33:424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. Massachusetts Medical Society. 2019;380:2497-2505. [DOI] [PubMed] [Google Scholar]
- 48.Bernat JL. The boundaries of organ donation after circulatory death. N Engl J Med. 2008;359:669-671. [DOI] [PubMed] [Google Scholar]
- 49.Mandell MS, Zamudio S, Seem D, et al. National evaluation of healthcare provider attitudes toward organ donation after cardiac death. Crit Care Med. 2006;34:2952-2958. [DOI] [PubMed] [Google Scholar]
- 50.McIntyre A. Doctrine of Double Effect. In: Zalta EN, ed. Stanf Encycl Philos [Internet]. SpringMetaphysics Research Lab, Stanford University; 2019. https://plato.stanford.edu/archives/spr2019/entries/double-effect/ [Google Scholar]
- 51.Beller EM, Driel ML van, McGregor L, Truong S, Mitchell G. Palliative pharmacological sedation for terminally ill adults. Cochrane Database Syst Rev; 2015. [cited 2020 Sep 8]; Available from: https://www.readcube.com/articles/10.1002%2F14651858.CD010206.pub2.[Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edwards MJ. Opioids and benzodiazepines appear paradoxically to delay inevitable death after ventilator withdrawal. J Palliat Care. 2005;21:299-302. [PubMed] [Google Scholar]
- 53.Choubey AP, Siskind EJ, Ortiz AC, et al. Disparities in DCD organ procurement policy from a national OPO survey: A call for standardization. Clin Transplant. 2020;34:e13826. [DOI] [PubMed] [Google Scholar]
- 54.Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment; 2009. http://bmcneurol.biomedcentral.com/articles/10.1186/1471-2377-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi J, Campbell ML, Gélinas C, Happ MB, Tate J, Chlan L. Symptom assessment in non-vocal or cognitively impaired ICU patients: Implications for practice and future research. Heart Lung J Crit Care. 2017;46:239-245. [DOI] [PubMed] [Google Scholar]