Abstract
A 31-year-old woman with transthyretin (TTR) amyloidosis secondary to a Thr60Ala mutation developed recurrent stroke-like episodes with fluctuating mental status. Evaluation for stroke and seizures was unrevealing. She was found to have leptomeningeal contrast enhancement on magnetic resonance imaging, which was confirmed to be CNS TTR amyloidosis on histopathology following brain and dura biopsy. While leptomeningeal disease has rarely been known to be associated with TTR amyloidosis, this is the first documented case of leptomeningeal disease secondary to a Thr60Ala mutation in the TTR gene. A literature review of TTR amyloidosis is presented with special focus on the treatment of leptomeningeal TTR amyloidosis.
Keywords: transthyretin amyloidosis, TTR amyloidosis, CNS involvement, leptomeningeal disease, plateau waves, stroke-like episodes
Introduction
Amyloidosis is a syndrome that develops following deposition of insoluble protein fibrils in locations that disrupt normal structure and function. 1 Besides the acquired forms, hereditary amyloidosis accounts for 3-5% of patients with amyloidosis according to amyloid registries at two university hospitals in Europe. The most common cause of hereditary amyloidosis is transthyretin (TTR). 2
Transthyretin is a homotetramer which is normally responsible for transporting thyroxine (T4) and retinol to cells in blood, and transporting thyroxine in the CSF.3,4 Transthyretin is predominantly synthesized in the liver, but is also made by the retinal pigment epithelial cells in the eye and choroid plexus cells in the brain. 5 There are currently over 140 known mutations known to cause TTR amyloidosis, and they are inherited in an autosomal dominant pattern. 6 Thr60Ala is relatively common in the United States and was the most frequently identified TTR mutation in one retrospective chart review, constituting 24% of all TTR mutations. 7
The clinical manifestation of TTR amyloidosis includes neuropathy, a condition which has previously been referred to as familial amyloid polyneuropathy (FAP). The most common presentation of FAP is a length-dependent sensory more than motor polyneuropathy as well as autonomic polyneuropathy, and the mean age of onset is 34 years. 8 Electrodiagnostic studies typically reveal an axonal sensorimotor polyneuropathy with sensory more than motor involvement, often with an associated carpal tunnel syndrome.1,9
The peripheral nervous system and heart are the primary organs involved in TTR amyloidosis 10 but other organs can also be involved – associations with ocular abnormalities, for example, were first described in the 1950s. 11 Vitreous opacification occurs in approximately 20% of families with TTR mutations and can be the initial manifestation of amyloidosis. 12
A rare manifestation of TTR amyloidosis is leptomeningeal disease, which was first described in 1980. 13 A recent review of 72 patients with leptomeningeal disease includes a total of 15 known causative TTR mutations with mean age of onset 44.9 years. 14 The most common mutations associated with leptomeningeal amyloidosis are C.113A>G (16.7%), c265T>C (16.7%), and c.148G>A (15.2%). 14 CNS symptoms include stroke, subarachnoid hemorrhage, dementia, ataxia, seizures, depression, and fluctuating levels of consciousness.13,15 The wide variety of clinical manifestations of amyloid leptomeningeal disease has been postulated to lead to delayed diagnosis and worse outcomes. 16
Case Report
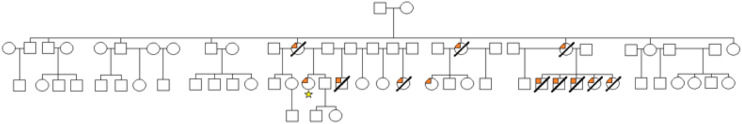
A 31-year-old woman with a known diagnosis of ATTRv amyloidosis was transferred to Columbia University Irving Medical Center for management of fluctuating mental status. She has an extensive family history of amyloidosis, including 13 individuals with amyloidosis and 11 of whom were deceased before age 41 years of age (Figure 1). She first developed multisystem symptoms related to amyloidosis at the age of 23. These symptoms include nausea and vomiting, gastroparesis, constipation, chest pain, palpitations with exertion, dizziness, and numbness and tingling in the hands and feet.
Figure 1.
Family pedigree of patient (starred) showing penetrance of transthyretin amyloidosis.
At the age of 23, she underwent evaluation for amyloidosis including an electrodiagnostic study with axonal sensorimotor polyneuropathy and echocardiogram with preserved ejection fraction. She had an abdominal fat pad biopsy with amyloidosis and TTR confirmed by immunohistochemistry, and her genetics testing disclosed a single copy of the Thr60Ala mutation without any additional TTR mutations. She was clinically diagnosed with amyloid cardiomyopathy based on her dyspnea at elevated heart rates, which was felt to be related to decreased diastolic filling time. She was enrolled in the APOLLO study (NCT01960348) with Patisiran 0.3 mg/kg every 3 weeks starting in 2016. While on Patisiran, her gastrointestinal and neuropathy symptoms were noted to improve.
Approximately 8 years later, she had a partial right nephrectomy for a right kidney mass, which was found to be a benign mixed epithelial tumor. The day after this procedure, she collapsed and was admitted to the hospital. She was found to have stroke-like symptoms with paralysis on the right side and inability to speak. She was unresponsive for 4 days and then spontaneously returned to near-baseline and was discharged. She had another stroke-like episode two months later during which she could not speak and had hemiplegia. She recovered slowly over two weeks, but further from her baseline. Imaging including MRI without contrast was negative for stroke.
After discharge she developed intermittent shaking spells with fluctuating mentation, for which she was hospitalized two months later. She had an electroencephalogram with diffuse delta slowing. She had a lumbar puncture with profile white blood cell count 1 per microliter, red blood cell count 3 per microliter, glucose 71 mg/dL, and elevated protein to 216 mg/dL and otherwise unremarkable findings, and an extensive workup for inflammatory, infectious, neoplastic, and paraneoplastic causes was negative. She was discharged home and was quickly readmitted for odd behavior including running down the street without reason as well as new depression. While hospitalized, she exhibited episodic agitation with physical aggression. She had a repeat lumbar puncture with elevated protein to 324 mg/dL and otherwise unremarkable findings. She received intravenous solumedrol for 5 days without improvement. She had an MRI brain with and without contrast which revealed new diffuse leptomeningeal enhancement. She had a whole-body CT scan which did not show a mass.
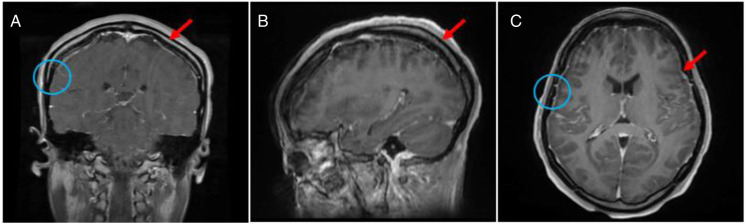
A month later, she was transferred to Columbia University Irving Medical Center for further management and workup of the agitation, fluctuating mental status episodes, and new leptomeningeal enhancement. Her exam was notable for inattention, normal language function, postural tremor in her bilateral fingers, hyperreflexia throughout without pathologic reflexes, and dysmetria. She again had a lumbar puncture with an elevated opening pressure of 27 cm H20 with elevated protein to 192 mg/dL, and otherwise unremarkable findings. She had a repeat MRI brain with and without contrast with persistent supratentorial and infratentorial linear leptomeningeal enhancement with extension along the upper cervical spinal cord (Figure 2). She had episodes of fluctuating mental status and was not found to have seizures on electroencephalogram. She was started on acetazolamide with the goal of lowering intracranial pressure and was started on an antipsychotic medication to improve agitation.
Figure 2.
T1-post-contrast MRI of the brain of the patient showing (A) coronal, (B) sagittal, and (C) axial views. The arrows reflect enhancement throught to be related to leptomeningeal disease. The circles reflect sites of dura and brain biopsy.
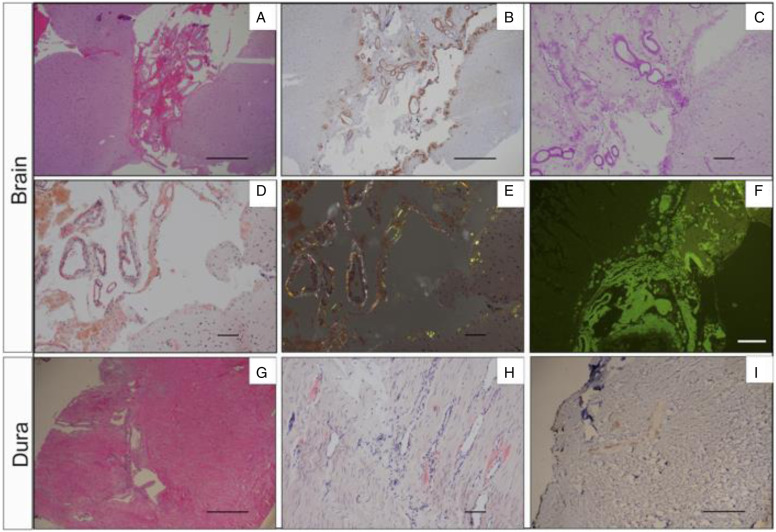
She had a right temporal craniotomy with brain and dural biopsy. The right temporal brain biopsy revealed thickened leptomeninges with eosinophilic amorphous depositions which stained positively for Congo red with apple-green birefringence under polarized light, Thioflavin-S fluorescence staining, and transthyretin (prealbumin) staining. The right temporal brain biopsy stained negatively for beta-amyloid staining. The right dural biopsy revealed thickened blood vessels with focal, patchy eosinophilic amorphous depositions, which stained positively for Congo red with apple-green birefringence in polarized light, and negatively for beta-amyloid stain (Figure 3). As there are no available proven treatments for leptomeningeal involvement by TTR amyloid, her family requested that she be discharged home with consideration to restart Patisiran for her systemic TTR amyloid disease. The patient signed informed consent to participate in the APOLLO clinical trial that was approved by the Columbia University IRB.
Figure 3.
Brain and dura biopsy. (A-F) Right temporal brain biopsy shows transthyrentin deposition in both leptomeninges and subpial brain parenchyma. (A). H&E image; (B). Transthyrentin (prealbumin) immunostaining; (C). PAS stain; (D). Congo red special strain; (E). Apple-green birefringence with polarized light; (F). Thioflavin immunofluorescence stain. (G-I) Right temporal dura biopsy displays transthyretin deposition in blod vessel walls. (G). H&E image; (H). Congo red special stain; (I). Beta-amyloid immunostaining. A, B, G, H, I: scale bar = 500μm; C, D, E, F: scale bar = 100μm.
Discussion
Transthyretin amyloidosis is a rare and fatal disease, affecting thousands of people worldwide. 17 Its recognition is important as untreated TTR amyloidosis can lead to multi-system involvement with cardiac conduction delay and cardiomyopathy, nephropathy, vitreous opacities, and rarely oculoleptomeningeal disease.18,19 Death is usually secondary to cardiac involvement. 1
Formerly, the mainstay of treatment of TTR amyloidosis was liver transplantation, which can be impactful as the vast majority of transthyretin is produced in the liver. 20 In fact, serum TTR Val30Met concentrations have been found to be reduced by over 90% following liver transplantation. 21 Clinically, liver transplantation has showed slowing of systemic symptomatology with enhanced survival of ATTRV30M patients. 22 With regards to neuropathy, liver transplantation has been shown to halt progression but has not been shown to be helpful in the recovery of current neuropathic abnormalities. 23 Interestingly, one retrospective survey which collected data from eight centers suggests that liver transplantation increases the incidence of ocular abnormalities in patients with ATTRV30M amyloidosis. This is thought to be because the frequency of severe ocular abnormalities, such as vitreous opacities and glaucoma, increases with progressive duration of disease given TTR is also produced in the retinal epithelium. 24 Most importantly, survival has improved as compared to pre-liver transplantation when the mean survival of patients with ATTRV30M patients was 7 to 10 years post-diagnosis. 8 Unfortunately, liver transplantation is not helpful for CNS manifestations of amyloidosis because TTR is also produced in the choroid plexus and retinal pigment of epithelial cells, 5 which is why this was not considered in this case. Moreover, CNS TTR deposition has been reported in TTR amyloidosis patients after liver transplantation. 25
Another form of treatment for TTR amyloidosis is tetramer stabilization, which prevents the accumulation of amyloid fibrils from occurring. Tafamidis is one such drug which has been shown to slow the progression of polyneuropathy and cardiomyopathy.26,27 Similar to liver transplantation, the impact on CNS disease is limited with studies mixed on whether the drug can attain levels high enough in the CSF to be therapeutic.28,29 The frequency of ocular findings in patients treated with Tafamidis has been shown to be lower, though not significantly lower, than in patients who are treated with supportive therapy or transplantation. The explanation for this is postulated to be due to Tafamidis’ limited ability to penetrate the CNS to a limited degree with individual variation in ocular penetration. 28 Diflunisal is another tetramer stabilizing medication in the NSAID class, 30 though it is similarly not known to have CNS applications. Recently, the Parkinson’s disease medication Tolcapone has been considered as a treatment for CNS disease in TTR amyloidosis. It is known to cross the blood-brain barrier, and has been found to bind with high affinity and specificity to the two T4-binding sites of TTR, which prevents formation of amyloid fibrils.31,32
RNA silencing therapies are a further treatment for TTR amyloidosis. This includes anti-sense oligonucleotides such as Inotersen 33 and small interfering RNAs like Patisiran. 34 These medications are designed for preferential uptake by the liver and do not cross the blood-brain-barrier. However, there is potential for treatment of CNS manifestations of TTR amyloidosis with intrathecal administration. A recent animal study showed that antisense nucleotides administered parenterally reduced hepatic TTR production, but not choroid plexus production. With intrathecal administration, however, there was significant CSF TTR concentration reduction. 35 There has been no demonstrated difference in the efficacy of these medications based upon a person’s particular TTR mutation.33,34 Amyloid-targeting medications are another group of treatments which include anti-Serum Amyloid P agents or anti-TTR antibodies.36,37
The pathogenic Thr60Ala mutation seen in this patient has not been previously described in the literature to be associated with leptomeningeal disease. The majority of the patient’s symptoms which led to her repeated hospitalizations can be explained by her leptomeningeal disease, including her fluctuating mental status. This presentation was most likely secondary to the plateau-wave phenomenon, 38 and is supported by the elevated opening pressure in one of her lumbar punctures. A palliative ventriculoperitoneal shunt was considered in this case but ultimately not pursued because of concern that it would clog with amyloid protein. The most commonly reported CNS symptom in ATTRV30M amyloidosis is transient focal neurological episodes, typically with temporary loss of function initially confused with stroke-like episodes like those seen in cerebral amyloid angiopathy.25,39 Recent studies suggest that certain CSF tests such as plasma neurofilament light chain might be useful in the future to monitor for CNS manifestations of amyloid, thereby preventing delays in diagnosis. 40
This patient’s presentation is unusual in that she developed both systemic and leptomeningeal disease earlier than is typical for TTR amyloidosis. Leptomeningeal disease secondary to TTR amyloidosis usually occurs 14 years following onset of systemic amyloid symptoms. The delay is likely due to the slow accumulation of amyloid in the leptomeningeal space following secretion by the choroid plexus. 40 Because current treatments have been effective in stabilizing systemic symptoms with prolonged survival, the incidence and importance of leptomeningeal disease secondary to amyloidosis is expected to increase. 8 Therefore, treatments for the CNS manifestations of amyloidosis will continue to grow in importance.
Footnotes
Author’s Note: In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that none of the authors have any competing interests.
ORCID iDs
Nathan Carberry https://orcid.org/0000-0001-9194-3247
Rachelle Dugue https://orcid.org/0000-0002-5179-4424
Michael Miller https://orcid.org/0000-0002-6350-5706
Tarini Goyal https://orcid.org/0000-0002-4436-3909
References
- 1.Simmons Z, Specht C. The neuromuscular manifestations of amyloidosis. J Clin Neuromuscu Dis. 2010;11:145-157. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson M, Schonland S, Yumlu S, et al. Hereditary apolipoprotein A1-associated amyloidosis in surgical pathology specimens. J Mol Diagn. 2009;11:257-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haggen G, Elliot W. Transport of thyroid hormones in serum and cerebrospinal fluid. J Clin Endocrinol Metab. 1973;37:415-422. [DOI] [PubMed] [Google Scholar]
- 4.Sekijima Y. Transthyretin (ATTR) amyloidosis: Clinical spectrum, molecular pathogenesis, and disease-modifying treatments. J Neurol Neurosurg Psychiatry. 2015;86:1036-1043. [DOI] [PubMed] [Google Scholar]
- 5.Shreiber G, Aldred A, Jaworowski A, Nilsson C, Achen, Segal MB. Thyroxine transport from blood to brain via transthyretin synthesis in the choroid plexus. Am J Physiol. 1990;258:338-345. [DOI] [PubMed] [Google Scholar]
- 6.Rowczenio DM, Noor I, Gillmore JD, et al. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum Mutat. 2014;35:2403-2412. [DOI] [PubMed] [Google Scholar]
- 7.Zhen DB, Swiecicki PL, Zeldenrust SR, Dispenzieri A, Mauermann M, Gertz M. Frequencies and geographic distributions of genetic mutations in transthyretin- and non-transthyretin-related familial amyloidosis. Clin Genet. 2015;88(4):396-400. [DOI] [PubMed] [Google Scholar]
- 8.Coelho T, Maurer MS, Suhr OB. THAOS: The transthyretin amyloidosis outcomes survey: Initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29:63-76. [DOI] [PubMed] [Google Scholar]
- 9.Li K, Kyle RA, Dyck PJ. Immunohistochemical characterization of amyloid proteins in sural nerves and clinical associations in amyloid neuropathy. Am J Pathol. 1992;141:217-226. [PMC free article] [PubMed] [Google Scholar]
- 10.Keppel S, Brannagan T, Helmke S, et al. Early-onset of transthyretin amyloidosis in a young Afro-Caribbean woman with Thr60Ala mutation. JACC: Case Rep. 2020;2:2063-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman HE, Thomas LB. Vitreous opacities diagnostic of familial primary amyloidosis. N Engl J Med. 1959;261:1267-1271. [DOI] [PubMed] [Google Scholar]
- 12.Kawaji T, Ando Y, Endo E, Nakamura M, Hirata A, Tanihara H. A case of vitreous amyloidosis without systemic symptoms in familial amyloidotic polyneuropathy. Amyloid. 2004;11:257-259. [DOI] [PubMed] [Google Scholar]
- 13.Goren H, Steinberg MC, Farboody GH. Familial oculoleptomeningeal amyloidosis. Brain. 1980;103:473-495. [DOI] [PubMed] [Google Scholar]
- 14.Quin Q, Wei C, Piao Y, et al. Current review of leptomeningeal amyloidosis associated with transthyretin mutations. Neurologist. 2021;26:189-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellie E, Camou F, Vital A, et al. Recurrent subarachnoid hemorrhage associated with a new transthyretin variant (Gly53Glu). Neurology. 2003;60:1625-1630. [DOI] [PubMed] [Google Scholar]
- 16.Parmann Y, Adams D, Obici L, et al. Sixty years of transthyretin familial amyloid polyneuropathy (TTR-FAP) in Europe: Where are we now? A European network approach to defining the epidemiology and management patterns for TTR-FAP. Curr Opin Neurol. 2016;29(suppl 1):S3-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: A model of medical progress for a fatal disease. Nat Rev Neurol. 2019;15(7):387-404. [DOI] [PubMed] [Google Scholar]
- 18.Andrade C. A peculiar form of peripheral neuropathy: Familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952;75:408-427. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda S, Hanyu N, Hongo M, et al. Hereditary generalized amyloidosis with polyneuropathy. Clinicopathological study of 65 Japanese patients. Brain. 1987;110:315-337. [DOI] [PubMed] [Google Scholar]
- 20.Holmgren G, Steen I, Ekstedt J, et al. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30). Clin Genet. 1991;40:242-246. [DOI] [PubMed] [Google Scholar]
- 21.Matsunami H, Makuuchi M, Kawasaki S, et al. A case of familial amyloid polyneuropathy treated with partial liver transplantation using a graft from a living related donor. Transplantation. 1995;60:301-303. [DOI] [PubMed] [Google Scholar]
- 22.Ericzon BG, Wilczek HE, Larsson M, et al. Liver transplantation for hereditary transthyretin amyloidosis: After 20 years still the best therapeutic alternative? Transplantation. 2015;99:1847-1854. [DOI] [PubMed] [Google Scholar]
- 23.Tashima K, Ando Y, Terazaki H, et al. Outcome of liver transplantation for transthyretin amyloidosis: Follow-up of Japanese familial amyloidotic polyneuropathy patients. J Neurol Sci. 1999;171:19-23. [DOI] [PubMed] [Google Scholar]
- 24.Buxbaum J, Brannagan T, Buades-Reines J, et al. Transthyretin deposition in the eye in the era of effective therapy for hereditary ATTRV30M amyloidosis. Amyloid. 2019;26(1):10-14. [DOI] [PubMed] [Google Scholar]
- 25.Maia LF, Magalhaes R, Freitas J, et al. CNS involvement in V30M transthyretin amyloidosis: Clinical, neuropathological, and biochemical findings. J Neurol Neurosurg Psychiatry. 2015;86:159-167. [DOI] [PubMed] [Google Scholar]
- 26.Coelho T, Maia LF, da Silva AM, et al. Long-term effects of Tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260:2802-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007-1016. [DOI] [PubMed] [Google Scholar]
- 28.Monteiro C, Martins da Silva A, Ferreira N, et al. Cerebrospinal fluid and vitreous body exposure to orally administered Tafamidis in hereditary ATTRV30M (p.TTRv50M) amyloidosis patients. Amyloid. 2018;25:120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvi F, Volpe R, Pastorelli F, et al. Failure of Tafamidis to halt progression of Ala36Pro TTR oculomeningovascular amyloidosis. J Stroke Cerebrovasc Dis. 2018;27:212-214. [DOI] [PubMed] [Google Scholar]
- 30.Sekijima Y, Tojo K, Morita H, Koyama J, Ikeda Si. Safety and efficacy of long-term Diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. Amyloid. 2015;22:79-83. [DOI] [PubMed] [Google Scholar]
- 31.Gomes J, Salvado M, Reig N, et al. Transthyretin stabilization activity of the catechol-O-methyltransferase inhibitor tolcapone (SOM0226) in hereditary ATTR amyloidosis patients and asymptomatic carriers: Proof-of-concept study. Amyloid. 2019;26:74-84. [DOI] [PubMed] [Google Scholar]
- 32.Pinheiro F, Varejao N, Esperante S, et al. Tolcapone, a potent aggregation inhibitor for the treatment of familial leptomeningeal amyloidosis. FEBS J. 2021;288:310-324. [DOI] [PubMed] [Google Scholar]
- 33.Adams D, Gonzalez-Duarte A, O’Riordan W, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11-21. [DOI] [PubMed] [Google Scholar]
- 34.Benson M, Waddington-Cruz M, Berk J, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22-31. [DOI] [PubMed] [Google Scholar]
- 35.Benson MD, Smith RA, Hung G, et al. Suppression of choroid plexus transthyretin levels by antisense oligonucleotide treatment. Amyloid. 2010;17(2):43-49. [DOI] [PubMed] [Google Scholar]
- 36.Bodin K, Ellmerich S, Kahan MC, et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468:93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higaki JN, Chakrabartty A, Galant NJ, et al. Novel conformation-specific monoclonal antibodies against amyloidogenic forms of transthyretin. Amyloid. 2016;23:86-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashi M, Handa Y, Kobayashi H, Kawano H, Ishii H, Hirose S. Plateau-wave phenomenon (I). Correlation between the appearance of plateau waves and CSF circulation in patients with intracranial hypertension. Brain. 1991;114(Pt 6):2681-2691. [DOI] [PubMed] [Google Scholar]
- 39.Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: Multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke. 2012;43(9):2324-2330. [DOI] [PubMed] [Google Scholar]
- 40.Sousa L, Coelho T, Taipa R. CNS involvement in hereditary transthyretin amyloidosis. Neurology. 2021;97(24):1111-1119. [DOI] [PubMed] [Google Scholar]