Abstract
Cancer screening programs from majority of the low- and middle-income countries (LMICs) report screening coverage as the only performance indicator, and that too measured through population-based sample surveys. Such information is unreliable and has very little value in assessing programmatic quality and impact. Regular monitoring of key process and outcome indicators based on data collected through a robust information system is essential to ensure quality of a screening programme. Fragmented health systems, limited resources and absence of a culture of systematic evaluation are the major hindrances for most of the LMICs to build electronic information systems to manage screening. The COVID-19 pandemic has created an impetus for the countries to customize the freely available District Health Information Software (DHIS2) to collect electronic data to track the outbreaks and manage containment measures. In the present article we present Bangladesh as an exemplar LMIC that has a (DHIS2) based integrated health information system gradually upgraded to collect individual data of the participants to the national cervical cancer screening program. Such efforts paid rich dividends as the screening program was switched from opportunistic to a population-based one. Moreover, the electronic system could report impact of the pandemic on cancer screening on a monthly basis. The aggregate number of women screened in the year 2020 was 14.1% less compared to 2019. The monthly rate of screening during peak of the outbreak was only 5.1% of the previous year. The rate rapidly recovered as the program intensified screening in the hard-to-reach regions less affected by the pandemic and expanded the outreach services. Other LMICs may emulate Bangladesh example. Customizing the information system developed for pandemic surveillance to collect cancer screening data will help them build back the screening programs better.
Keywords: COVID-19, Cancer screening, Screening registry, Low- and middle-income countries, Bangladesh
1. Introduction
The coronavirus 2019 (COVID-19) induced health crisis slowed down or even suspended cancer screening activities in many countries and also deferred subsequent diagnostic and treatment services. Only a few high-income countries with effective screening registries have reported impact of the pandemic on cancer screening activities with quantitative estimates. Australian Breast cancer screening registry indicated that around 145,000 fewer screening mammograms were performed in January to June 2020 compared to the same period in 2018 (AIHW, 2020). The mid-June volumes of breast, colorectal and cervical cancer screening in Australia in 2020 remained 29%–36% lower than their pre-COVID-19 rates (Mast and Munoz del Rio, 2020). In the United Kingdom (UK) cancer screening programs were suspended in March 2020. As a result, the screening invitations could not be sent on time to nearly 3 million eligible people, creating a huge backlog. A 90% drop in the monthly number of colonoscopies was reported from the UK in April 2020 with a very significant increase in the colonoscopy waiting time by August 2020 (Greenwood and Swanton, 2021).
Very little is known about the impact of COVID-19 on cancer screening program in the low and middle income countries (LMICs), essentially because of the limited capability of the programs to collect data systematically. A recently published survey among the cancer screening program managers from 17 countries reported that screening was suspended for 30 days or longer in 13 (76.5%) countries and diagnostic services for the screen positives were suspended in nearly half (N = 9; 52.9%) of them (Villain et al., 2021). Compared to the pre-COVID days, the volume of cancer screening during August to September 2020 (when the survey was performed) was reported to be reduced by more than half by 11 of the 18 program managers. However, this information was only based on their perceptions and not backed by any real data. Ten participants expressed their intention to measure the impact of COVID on cancer screening more objectively. However, they were too busy mitigating the impact of the pandemic and all of them except one did not consider publishing such data as their immediate priority.
Cancer screening is a complex and resource-intensive public health initiative. Population-based screening significantly reduces mortality from breast, cervical, colorectal and lung cancers, when delivered within the framework of an organized program (Jansen et al., 2020; Zielonke et al., 2020; Fitzpatrick-Lewis et al., 2016). The benefits of screening (including saving of resources) for these selected cancers clearly outweigh the possible harms so long as quality is assured across the entire continuum of services. Quality assurance of a screening program requires systematic data collection from different service delivery points to estimate the key performance indicators (KPIs).
In the present review article, we describe the pivotal role of screening registries in implementing cancer screening programmes and how the COVID-19 pandemic has created an opportunity for the LMICs to build screening registries by leveraging the investments made to improve electronic health information systems to monitor the outbreaks. We have presented as a case study, how Bangladesh initiated the process of building a screening registry to manage their national cervical cancer screening programme in the pre-pandemic years, and used the same to objectively assess the impact of the pandemic on the programme.
2. Role of cancer screening registry
2.1. Definition & organization of screening registry
Cancer screening registry is a team or an organization that collects, utilizes and stores individual data in a centralized manner across the screening process to be used for program implementation as well as quality assurance (Anttila et al., 2015; Májek et al., 2019). The electronic database (also known as screening register) records contact and demographic information of the participants, screening test results, clinico-pathological information, and assigns a unique identifier to each individual. A fully functional screening register collecting individual level data can assess the process indicators (e.g., participation rate, screen positivity, participation rate to further assessment etc.), outcome indicators (e.g., precancer or cancer detection rates, positive predictive value etc.) and impact (e.g., downstaging of cancers, mortality reduction). Linkage to other databases is essential to fulfill the greatest potential of a screening register. Such databases may be a population register to identify the eligible population and send individual invitations or a population-based cancer registry (PBCR) to track the disease and/or mortality endpoints (Vale et al., 2019).
2.2. Challenges in establishing a screening registry
Though screening registry has a pivotal role in screening program management and quality assurance, establishing such a facility requires considerable organization of health systems. Significant investments are needed to develop the electronic system and establish robust linkages between the different service delivery points. Ideally, at least 10% of the program budget should be allocated to implement quality assurance, and a significant portion should be invested to support the screening registry (Perry et al., 2013). The expenditure is likely to be higher in the initial years. The information system needs to be compliant with national regulations and adequately address issues related to data privacy and security. Because of all these challenges, utilization of screening registries for program management has remained limited to the higher resourced countries.
3. Screening registries in limited resourced settings
3.1. Sub-optimal coverage and quality of cancer screening in the LMICs
Even though cancer screening is widely practiced in the LMICs, the coverage and quality of screening services are highly variable and often sub-optimal. Out of the 177 countries participating in a survey conducted by the World Health Organization (WHO) in the year 2015, 76% claimed to have breast cancer screening programs, and 79% to have cervical cancer screening programs (WHO, 2015). Less than 10% coverage for breast cancer screening was reported by 25% of the low-income countries. Nearly half of the low-income countries reported less than 10% coverage for cervical cancer. Many of the LMICs claiming to have a screening program did not even have an operational action plan or funding earmarked for implementation- the basic necessities to deliver quality-assured screening. The 90–70-90 global target (90% of the adolescent girls vaccinated against human Papillomavirus, 70% of the age-eligible women screened with a high-performance test and 90% of the precancers and cancers appropriately managed) stipulated by the WHO for the year 2030 to eliminate cervical cancer underscores the need to improve cancer screening in the LMICs, both in coverage and quality (WHO, 2020a).
3.2. Lack of screening registry as a major obstacle to evaluate programmes in the LMICs
Little efforts have been made till date to set up screening registries to improve the organization and quality of cancer screening in the LMICs and to monitor performance of the programmes. The performance indicator that is ever reported from these countries is screening coverage, which is almost always based on population surveys, and has poor reliability (Gakidou et al., 2008). The WHO STEP survey performed on a regular basis in each country aims to estimate cervical cancer screening coverage by asking every adult woman whether she has had a screening test for cervical cancer ever (WHO, 2021a). Accuracy of such self-reported information is very much dependent on the literacy status of the woman interviewed and her understanding of the screening process and is subject to memory bias. Moreover, screening coverage alone has very little value in understanding the performance and quality of a program. For example, Brazil has been reporting a cervical cancer screening coverage around 70% for many years without any appreciable decline in cervical cancer mortality being observed in the country (Basu et al., 2020). It is crucial to monitor other process and outcome indicators of quality mentioned earlier, to ensure that the screening performance is of adequate standard.
Some of the LMICs with relatively better organization of screening program routinely maintains paper-based records and use them to collect aggregate data periodically. The Ministry of Health of Morocco successfully evaluated the opportunistic breast cancer screening program in the country to demonstrate a reasonably high coverage of 63% in the year 2015 (Basu et al., 2018). However, paper-based records and collection of aggregate data do not permit tracking of the screen-positive individuals to ensure their compliance to further management - a key advantage of a screening registry maintaining individual records. The low compliance (only 34%) of the screen-positive women to undergo further assessment was responsible for the low detection rate of breast cancers in the Moroccan program. Systematic collection and reporting of individual data through a screening registry is essential to implement and manage a screening program efficiently. Lack of a programmatic mandate to implement quality assurance, fragmented health services and non-availability of dedicated resources are the major hindrances for the LMICs to establish screening registries.
3.3. Learning from other health programmes
The culture of using digital technologies and mobile communications to manage health programs is gradually being adopted by the resource-constrained countries in the recent years. Increasing number of LMICs are using individual-level electronic immunization registries (EIRs) to replace the paper-based aggregate reporting systems for vaccination programs or are adopting mHealth solutions on a range of maternal and child health outcomes (Dolan et al., 2019; Feroz et al., 2017). It is time to consider using similar technologies in managing and monitoring cancer screening programs as well. The COVID-19 pandemic has created opportunities for translating several pandemic-control measures to the benefit of cancer control; the investments made to improve health information systems is one such opportunity to be leveraged to build screening registries in the LMICs (Ginsburg et al., 2021).
4. Customization of COVID-19 surveillance system for cancer screening
4.1. Most LMICs have invested to build electronic surveillance system for COVID
Despite the health systems worldwide being overwhelmed by the COVID-19 outbreaks, the LMICs have shown great capacity to adapt and transform in response to the pandemic with tailored and innovative solutions and engaged communities (El Bcheraoui et al., 2020). Recognizing that strengthening the health system would be as important as focusing on vaccine, diagnostics and therapeutics to combat the pandemic, most of the LMICs have made significant vertical investments to improve health infrastructure and workforce, including that needed for disease surveillance (Usher, 2021). A COVID-19 reporting and monitoring system was rapidly set up at national and sub-national levels, since collecting data on real-time basis was crucial to monitor the outbreaks and provide valuable information for policy formulation, evaluation and adjustment. The information system built within a short period of time in resource constrained settings has already proven its success in disease tracking and subsequent implementation of risk-stratified isolation measures (Nachega et al., 2020).
4.2. Adopting COVID surveillance systems to set up screening registry
The experience and expertise to set up the COVID-19 surveillance system may be applied to other health programs like monitoring cancer screening. Mobile phone applications (apps) were successfully integrated with electronic COVID-19 databases in the LMICs to collect and utilize individual data for outbreak monitoring - a technology worth exploring to set up cancer screening information system (Verhagen et al., 2020). Recent data shows that cell phone penetration in the LMICs has exceeded 90% and the mobile internet connectivity is around 40%, and both the numbers are rising (Feroz et al., 2020). The vital records systems have been strengthened in many LMICs to allow people register births and deaths through smartphones during the outbreaks – a development that can also be of great benefit to future screening registries in these countries (BBC News, 2021). It is not at all uncommon to utilize the infrastructure created in response to a single disease for broader health gains. Utilization of the GeneXpert platforms originally procured for point-of-care tuberculosis testing to test for SARS-CoV-2 virus in many African nations is one such example. The governance structure, surveillance capabilities and workforce created to fight Ebola virus disease in DPR Congo were successfully applied in response to COVID-19 (WHO, 2020b).
Many of the LMICs (e.g., Rwanda, Nigeria, Ethiopia, China, India) developed their own system of collecting electronic data to track COVID-19 and disseminating them publicly (RBC, 2021; NCDC, 2021; EPHI, 2021; COVID 19 India, 2021). The WHO supported many limited resourced countries to strengthen their respective pandemic tracking and vaccine delivery systems by customizing the District Health Information Software (DHIS2), a web-based health information system originally developed by the University of Oslo, Norway (DHIS2, 2021; WHO, 2021b). More than 73 LMICs worldwide already use this open-source and free of charge integrated digital solution for collecting and managing data from multiple health programs like HIV or tuberculosis control, immunization, maternal and child health, adolescent health etc. (WHO, 2021c). Similar customization of the software is feasible to capture and manage cancer screening data. In fact, the “WHO cervical cancer toolkit” (published in 2019) recommended using the (DHIS2) platform to manage cervical cancer screening data, though the real-world usage of (DHIS2) for this purpose has been very limited (WHO, 2019).
5. Customization of (DHIS2) to collect cancer screening data – Bangladesh experience
Bangladesh launched the cervical cancer screening program in 2005 as a pilot and gradually scaled up the opportunistic program to all 64 districts by 2010 (Bhatla et al., 2021). In the year 2019, total 387,719 women aged between 30 and 60 years were screened with visual inspection with acetic acid (VIA) test and 4.1% of them tested positive. The positive women are referred to any of the 25 colposcopy centers across the country., The colposcopy clinic at the Bangabandhu Sheikh Mujib Medical University (BSMMU) in Dhaka is the largest of all, receiving around a quarter of all referrals generated across the country.
Bangladesh is one the few LMICs to take advantage of the freely available software to build a national cancer screening information system, and by doing so the country is in a position to measure the impact of COVID-19 on cancer screening program. Tackling the pandemic was an impetus for the national Government to ensure that the last mile health facilities are covered by the new health information system, which directly benefitted the screening program.
Supported by the United Nations International Children's Emergency Fund (UNICEF), the Government of Bangladesh launched an initiative in 2010 to transition from paper-based system to the (DHIS2) platform to collect and utilize routine health data from more than 13,000 community health clinics spread across primary and secondary levels. By 2019 Bangladesh became the largest (DHIS2) deployer in the world, with 98% of public health facilities covered by the new information system (DGHS, 2021). A dedicated (DHIS2) module was developed to manage data from the national cervical cancer screening program. A training of trainers programs was introduced to train large number of data managers on a continued basis. The system is being utilized to collect aggregate cancer screening performance data (number of women screened, number of women screened outside target age, screen positivity, treatment rate) on monthly basis from health facilities since 2013 (National Cervical and Breast Cancer Surveillance System, 2021; Nessa, 2018).
Bangladesh launched a pilot project in each division (region) of the country in 2018 to switch from opportunistic to population-based cancer screening. As part of the initiative, the (DHIS2) system was upgraded to collect individual data. The unique number issued to every citizen by the national Government is used as the unique identifier. The community health workers (female welfare assistants or female health volunteers) identify the eligible women through home visits, collect their address and contact details in paper forms and invite them to be screened. The data collected by the community workers is transferred to the online system from the paper forms at the community clinics. Screening is performed at the community clinics and the test results are also entered in the same system. The information system has enabled the community clinics to track the women non-compliant to screening. The pandemic was an impetus for the Government to expand the information system to the most distant primary care facilities, thus ensuring coverage of maximum number of the population. This greatly benefitted the screening programme as well. Further development of the system to link the colposcopy centers was initiated in 2019–20 but got delayed due to the pandemic. The colposcopy clinic at BSMMU maintains its own computerized record system and will continue to do so till the center is linked to the (DHIS2) system in the near future.
6. COVID-19 impact on cervical cancer screening programme in Bangladesh
6.1. Impact on cervical cancer screening
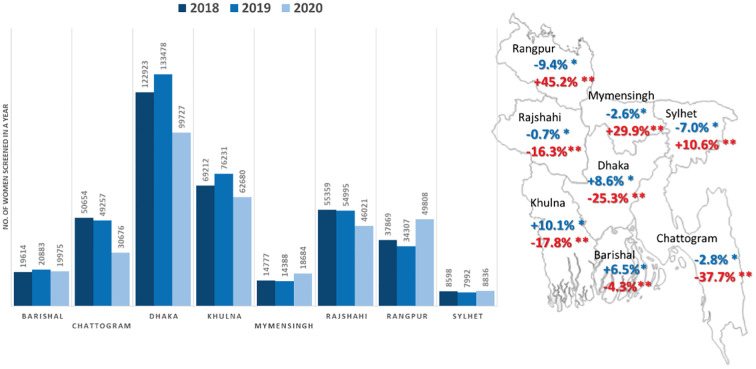
A nationwide lockdown was announced by the Government of Bangladesh from 23 March to 30 May 2020. During this period both screening and diagnostic services were suspended. We reviewed the number of women undergoing screening at all the eight divisions of the country by months for the calendar years 2018, 2019 and 2020. Compared to the year 2018, the overall number of women screened was 3.3% higher in 2019 with variations ranging between −9.4% and + 10.1% across different divisions (Fig. 1 ). The aggregate number of women screened in the year 2020 was 14.1% less compared to 2019. The most significant decrease was noted in Dhaka (25.3%) and Chattogram (37.7%) divisions, which were worst affected by the pandemic with highest number of deaths in the country. The restrictions in movement and limited access to the health facilities in these divisions continued well beyond the formal withdrawal of the lockdown. The divisions in the northern part of the country reported a significant overall increase in the total number of women screened in 2020 compared to the previous year due to two reasons. Firstly, the outbreaks were less severe in this predominantly rural region with many hard-to-reach areas. Secondly, the program used campaign modes to screen large number of women in a day at outreach clinics in the post-lockdown phase. The women preferred to attend these special clinics organized close to their doorsteps rather than visiting regular health facilities. The community mobilization efforts were intensified through the community workers in the campaign areas. A change in the protocol for managing the VIA positive women was made to incorporate ‘screen and treat’ approach. The strategic decision to adopt single visit approach significantly reduced the number of referrals to the colposcopy clinics. The (DHIS2) system was able to capture data from all the outreach clinics.
Fig. 1.
Number of women screened for cervical cancer in three consecutive years (2018,2019 and 2020) in different divisions of Bangladesh. The map shows percentage change in the number of women screened between 2018 and 2019, and 2019 and 2020.
[* Percentage change in 2019 compared to 2018 (shown in blue). ** Percentage change in 2020 compared to 2019 (shown in red)]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
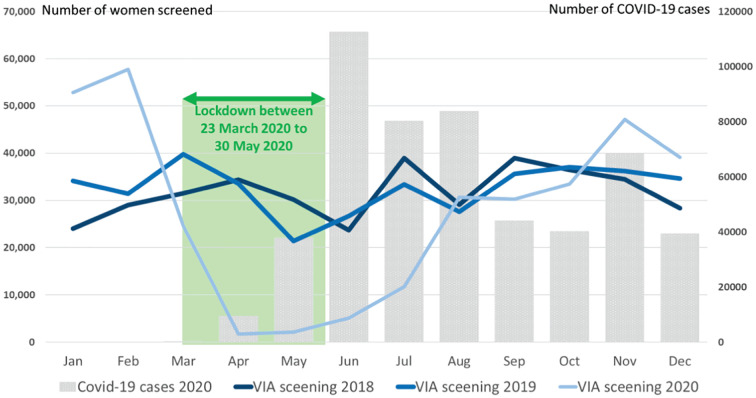
The impact of the pandemic on monthly number of women screened compared to the previous years (2018 and 2019) is shown in Fig. 2 . The number of women screened during the pre-COVID months (January and February) of 2020 was significantly higher compared to the previous two years. This was because of increasing adoption of population-based approach, in which the eligible women were systematically invited by the community health workers to attend screening. There was a significant drop in the monthly number of women undergoing screening after the COVID-19 outbreaks started in March and lockdown was imposed. The number of women screened in April 2020 was only 5.1% of the total number of women screened in the same month in the previous year. A gradual recovery in the number of women screened was observed since July 2020. Increase in the number of COVID infections in certain regions in November 2021 again led to a downward trend in the number of women screened in the subsequent month. The VIA positivity in the year 2020 ranged between 2.2% and 4.7% across the divisions, which was not significantly different from that observed in earlier years (e.g., 3.3% to 5.4% in 2019).
Fig. 2.
Number of women screened for cervical cancer in Bangladesh by months in 2018, 2019 and 2020; the bars show the number of COVID-19 cases detected by months in 2020.
6.2. Impact on referral of screen-positive women
The number of screen-positive women undergoing colposcopy at BSMMU colposcopy clinic also showed a drastic decline following the announcement of lockdown (Table 1 ). Overall, the number of patients undergoing colposcopy (first visit after a positive screening test) dropped by 27% in 2020 compared to the previous year. A reduction in referral to colposcopy was seen across all the divisions, ranging from 18% to 60%. The worst impact was seen during the period of May to August 2020 as screening was drastically reduced in the previous quarter due to lockdown.
Table 1.
Number of screen positive women referred from different divisions of Bangladesh undergoing colposcopy at Bangabandhu Sheikh Mujib Medical University in 2019 and 2020 (women undergoing repeat colposcopy or colposcopy after treatment were excluded).
| Referred from (division) | Period of the year (months) | No. of women undergoing colposcopy at BSMMU |
Percentage change between 2019 & 2020 | |
|---|---|---|---|---|
| 2019 | 2020 | |||
| Barisal | January–April | 2 | 5 | +150% |
| May–August | 3 | 0 | −100% | |
| September–December | 7 | 3 | −57% | |
| January–December | 12 | 8 | −33% | |
| Chattogram | January–April | 55 | 49 | −11% |
| May–August | 56 | 7 | −88% | |
| September–December | 88 | 39 | −56% | |
| January–December | 199 | 95 | −52% | |
| Dhaka & Mymensingh | January–April | 315 | 329 | +4% |
| May–August | 244 | 76 | −69% | |
| September–December | 335 | 328 | −2% | |
| January–December | 894 | 733 | −18% | |
| Khulna | January–April | 16 | 13 | −19% |
| May–august | 9 | 1 | −89% | |
| September–December | 17 | 3 | −82% | |
| January–December | 42 | 17 | −60% | |
| Rajsahi | January–April | 8 | 7 | −13% |
| May–August | 4 | 0 | −100% | |
| September–December | 10 | 2 | −80% | |
| January–December | 22 | 9 | −59% | |
| Rangpur | January–April | 7 | 9 | +29% |
| May–August | 6 | 0 | −100% | |
| September–December | 6 | 5 | −17% | |
| January–December | 19 | 14 | −26% | |
| Sylhet | January–April | 26 | 28 | +8% |
| May–August | 20 | 2 | −90% | |
| September–December | 31 | 13 | −58% | |
| January–December | 77 | 43 | −44% | |
| All divisions | January–April | 429 | 440 | +3% |
| May–August | 342 | 86 | −75% | |
| September–December | 494 | 393 | −20% | |
| January–December | 1265 | 919 | −27% | |
7. Conclusions
Although the COVID-19 pandemic induced a significant decrease in the number of screening tests, this effect was predominantly observed during the lockdown period with a rapid recovery up to the level of the previous years (2018 and 2019) in terms of the monthly number of screening tests after four months. A short disruption in cervical cancer screening should not lead to a significant increase of cervical cancer incidence since the screening program is aimed at detecting precancerous lesions, a principle fulfilled as long as the access to treatment is guaranteed, as in the case of the screen and treat approach implemented in Bangladesh. The rapid recovery of screening activities as well as the increase of screening tests in some regions of the country highlight the benefits of expanding population-based screening accompanied by proper screening registration to allow individual tracking of eligible populations to identify women overdue for screening. Bangladesh aims to gradually expand the population-based approach across the country and maintain the practice of screen and treat at least in the hard-to-reach regions.
Bangladesh has demonstrated that setting up a screening information system using the (DHIS2) technology is within reach of the LMICs. The pandemic created a demand to bolster health information systems in the country and the screening program reaped the benefit of it. Other LMICs should explore the possibility. The relevance of an electronic information system to manage and monitor screening programs will significantly increase in the post-pandemic situation. Only a systematic analysis of the pandemic impact will allow the program managers to plan manpower and fiscal resources to ‘build back the system better’. Having an information system capturing individual data will be very useful to adjust to the new situation and continue with screening activities as the outbreak waxes and wanes. However, implementation research is necessary to understand the local barriers and facilitators and also the effectiveness, acceptability, adoption, reach, cost and sustainability of an information system in the local context. Concerns about data privacy and data protection can be a major challenge that needs to be addressed.
With advancement in digital technology and the countries getting more conversant with adopting the technologic innovations, it is time for every country to consider establishing a robust and effective information system to manage the screening programs and monitor the same. After all, what gets measured gets done.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Credit authors statement
Dr. Partha Basu and Dr. Raul Murillo conceptualized the manuscript and data collection from Bangladesh. Dr. Ashrafunnessa collected and shared data from cancer screening programmes of Bangladesh. The data was analysed by Dr. Richard Muwonge, Dr. Li Zhang and Mr. Eris Lucas. All authors contributed to drafting the manuscript, reviewed the final version and agreed to the contents.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- AIHW Cancer Screening and COVID-19 in Australia. 2020. https://www.aihw.gov.au/reports/cancer-screening/cancer-screening-and-covid-19-in-australia/contents/did-fewer-people-screen-for-cancer-during-the-covid-19-pandemic Available from:
- Anttila A., Lönnberg S., Ponti A., Suonio E., Villain P., Coebergh J.W., von Karsa L. Towards better implementation of cancer screening in Europe through improved monitoring and evaluation and greater engagement of cancer registries. Eur. J. Cancer (Oxford, England: 1990) 2015;51(2):241–251. doi: 10.1016/j.ejca.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Basu P., Selmouni F., Belakhel L., Sauvaget C., Abousselham L., Lucas E., Muwonge R., Sankaranarayanan R., Khazraji Y.C. Breast Cancer screening program in Morocco: status of implementation, organization and performance. Int. J. Cancer. 2018;143(12):3273–3280. doi: 10.1002/ijc.31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu P., Zhang L., Hariprasad R., Carvalho A.L., Barchuk A. A pragmatic approach to tackle the rising burden of breast cancer through prevention & early detection in countries ‘in transition’. Indian J. Med. Res. 2020;152(4):343–355. doi: 10.4103/ijmr.IJMR_1868_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BBC News Measuring Africa's Data Gap: The Cost of Not Counting the Dead. 2021. https://www.bbc.com/news/world-africa-55674139 Available from:
- Bhatla N., Nessa A., Oswal K., Vashist S., Sebastian P., Basu P. Program organization rather than choice of test determines success of cervical cancer screening: case studies from Bangladesh and India. Int. J. Gynaecol. Obstetr.: Off. Organ Int. Feder. Gynaecol. Obstetr. 2021;152(1):40–47. doi: 10.1002/ijgo.13486. [DOI] [PubMed] [Google Scholar]
- COVID 19 India COVID 19 in India. 2021. https://www.covid19india.org Available from:
- DGHS DHIS - Interface for Collection of Nation-Wide Health Data. 2021. https://dghs.gov.bd/index.php/en/e-health/our-ehealth-eservices/84-english-root/ehealth-eservice/94-dhis-interface-for-collection-of-nation-wide-health-data Available from.
- DHIS2 DHIS2 project. 2021. https://dhis2.org/about/ Available from:
- Dolan S.B., Carnahan E., Shearer J.C., Beylerian E.N., Thompson J., Gilbert S.S., Werner L., Ryman T.K. Redefining vaccination coverage and timeliness measures using electronic immunization registry data in low- and middle-income countries. Vaccine. 2019;37(13):1859–1867. doi: 10.1016/j.vaccine.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bcheraoui C., Weishaar H., Pozo-Martin F., Hanefeld J. Assessing COVID-19 through the lens of health systems’ preparedness: time for a change. Glob. Health. 2020;16(1):112. doi: 10.1186/s12992-020-00645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPHI Ethiopian Public Health Institute. 2021. https://www.ephi.gov.et/index.php/public-health-emergency/novel-corona-virus-update Available from:
- Feroz A., Perveen S., Aftab W. Role of mHealth applications for improving antenatal and postnatal care in low and middle income countries: a systematic review. BMC Health Serv. Res. 2017;17(1):704. doi: 10.1186/s12913-017-2664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feroz A., Jabeen R., Saleem S. Using mobile phones to improve community health workers performance in low-and-middle-income countries. BMC Public Health. 2020;20(1):49. doi: 10.1186/s12889-020-8173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick-Lewis D., Ali M.U., Warren R., Kenny M., Sherifali D., Raina P. Screening for colorectal Cancer: a systematic review and meta-analysis. Clin. Colorectal Cancer. 2016;15(4):298–313. doi: 10.1016/j.clcc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Gakidou E., Nordhagen S., Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5(6) doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg O., Basu P., Kapambwe S., Canfell K. Eliminating cervical cancer in the COVID-19 era. Nat. Can. 2021;2:133–134. doi: 10.1038/s43018-021-00178-9. [DOI] [PubMed] [Google Scholar]
- Greenwood E., Swanton C. Consequences of COVID-19 for cancer care - A CRUK perspective. Nat. Rev. Clin. Oncol. 2021;18(1):3–4. doi: 10.1038/s41571-020-00446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen E., Zielonke N., Gini A., Anttila A., Segnan N., Vokó Z., Ivanuš U., McKee M., de Koning H.J., de Kok I., EU-TOPIA consortium Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur. J. Cancer (Oxford, England: 1990) 2020;127:207–223. doi: 10.1016/j.ejca.2019.12.013. [DOI] [PubMed] [Google Scholar]
- Májek O., Anttila A., Arbyn M., van Veen E.B., Engesæter B., Lönnberg S. The legal framework for European cervical cancer screening programmes. Eur. J. Pub. Health. 2019;29(2):345–350. doi: 10.1093/eurpub/cky200. [DOI] [PubMed] [Google Scholar]
- Mast C., Munoz del Rio A. Delayed Cancer Screenings—A Second Look. 2020. https://ehrn.org/articles/delayed-cancer-screenings-a-second-look Available from:
- Nachega J.B., Grimwood A., Mahomed H., Fatti G., Preiser W., Kallay O., Mbala P.K., Muyembe J.T., Rwagasore E., Nsanzimana S., Ngamije D., Condo J., Sidat M., Noormahomed E.V., Reid M., Lukeni B., Suleman F., Mteta A., Zumla A. From easing lockdowns to scaling-up community-based COVID-19 screening, testing, and contact tracing in Africa - shared approaches, innovations, and challenges to minimize morbidity and mortality. Clin. Infect. Dis. 2020;72(2):327–331. doi: 10.1093/cid/ciaa695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cervical and Breast Cancer Surveillance System Available from. 2021. https://cxbrcancersurveillance.mohfw.gov.bd/viacbe/dhis-web-commons/security/login.action
- NCDC COVID-19 Nigeria. 2021. http://covid19.ncdc.gov.ng/ Available from:
- Nessa A. Cervical Cancer screening program in Bangladesh. Bangladesh J. Obstet. Gynaecol. 2018;33:63–73. [Google Scholar]
- Perry N., Broeders M., de Wolf C., Tornberg S., Holland R., von Karsa L. In: European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis. Fourth edition, supplements. Perry N., Broeders M., de Wolf C., Tornberg S., Holland R., von Karsa L., editors. European Commission, Publications Office of the European Union; Luxembourg: 2013. Executive summary; pp. xiii–xx. [Google Scholar]
- RBC Get the Facts about Coronavirus: Take Steps to Care for Yourself and Help Protect Others in Your Home and Community. 2021. https://www.rbc.gov.rw/index.php?id=707 Available from:
- Usher A.D. Health systems neglected by COVID-19 donors. Lancet (London, England) 2021;397(10269):83. doi: 10.1016/S0140-6736(21)00029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale D.B., Anttila A., Ponti A., Senore C., Sankaranaryanan R., Ronco G., Segnan N., Tomatis M., Žakelj M.P., Elfström K.M., Lönnberg S., Dillner J., Basu P. Invitation strategies and coverage in the population-based cancer screening programmes in the European Union. Eur. J. Cancer Prevent. Off. J. Eur. Cancer Prevent. Organiz. (ECP) 2019;28(2):131–140. doi: 10.1097/CEJ.0000000000000426. [DOI] [PubMed] [Google Scholar]
- Verhagen L.M., de Groot R., Lawrence C.A., Taljaard J., Cotton M.F., Rabie H. COVID-19 response in low- and middle-income countries: Don’t overlook the role of mobile phone communication. Int. J. Infect. Dis.: IJID: Off. Public. Int. Soc. Infect. Dis. 2020;99:334–337. doi: 10.1016/j.ijid.2020.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain P., Carvalho A.L., Lucas E., Mosquera I., Zhang L., Muwonge R., Selmouni F., Sauvaget C., Basu P., IARC COVID-19 Impact Study Group Cross-sectional survey of the impact of the COVID-19 pandemic on cancer screening programs in selected low- and middle-income countries: study from the IARC COVID-19 impact study group. Int. J. Cancer. 2021 doi: 10.1002/ijc.33500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2015. Assessing national capacity for the prevention and control of noncommunicable diseases: report of the 2015 global survey. ISBN 978 92 4 156536 3. [Google Scholar]
- WHO 2019. https://www.who.int/ncds/surveillance/data-toolkit-for-cervical-cancer-prevention-control/en/
- WHO . World Health Organization; Geneva: 2020. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; p. 2020. [Google Scholar]
- WHO Building on Ebola response to tackle COVID-19 in DRC. 2020. https://www.afro.who.int/news/building-ebola-response-tackle-covid-19-drc Available from:
- WHO . World Health Organization; 2021. The WHO STEPwise approach to noncommunicable disease risk factor surveillance (STEPS) Available from: NCDs | STEPwise approach to surveillance (STEPS) (who.int) [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Country & Technical Guidance - Coronavirus disease (COVID-19) 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications Available from:
- WHO Analysis and use of health facility data. 2021. https://www.who.int/data/data-collection-tools/analysis-use-health-facility-data Available from:
- Zielonke N., Gini A., Jansen E., Anttila A., Segnan N., Ponti A., Veerus P., de Koning H.J., van Ravesteyn N.T., Heijnsdijk E., EU-TOPIA consortium Evidence for reducing cancer-specific mortality due to screening for breast cancer in Europe: a systematic review. Eur. J. Cancer (Oxford, England: 1990) 2020;127:191–206. doi: 10.1016/j.ejca.2019.12.010. [DOI] [PubMed] [Google Scholar]