Abstract
Olfaction, one of our five main qualitative sensory abilities, is the action of smelling or the capacity to smell. Olfactory impairment can be a sign of a medical problem, from a benign nasal/sinus problem up to a potentially serious brain injury. However, although clinicians (neurologists or not) usually test the olfactory nerves in specific clinical situations (for example, when a neurodegenerative disorder is suspected), they may omit such tests in many other situations. With the recent COVID-19 pandemic, the resurgence of anosmia has reminded us of the importance of testing this sensorineural function. We retrace here the main historical steps and discoveries concerning olfaction and anosmia.
Keywords: Olfaction, Anosmia, Infection, COVID-19
Abbreviations: ACE2, angiotensin-converting enzyme 2; ApoE4, apolipoprotein E4; BBB, blood-brain barrier; BMEC, brain microvascular endothelial cells; CHD7, chromodomain helicase DNA-binding protein 7; COVID-19, coronavirus disease 2019; CNS, central nervous system; CSF, cerebrospinal fluid; EEG, elektroenkephalogram (electroencephalogram); EOG, electro-olfactogram; FGF8, fibroblast growth factor 8; KAL1/ANOS1, Kall syndrome 1/Anosmic hypogonadism 1; KAL2/FGFR1, Kallmann syndrome 2/fibroblast growth factor receptor-1; MM, molecular mimicry; MRI, magnetic resonance imaging; CP, cribriform plate; OR7D4, olfactory receptor, family 7, subfamily D, member 4; ORF, Open Reading Frames; PARP9, Poly-ADP-Ribose-Polymerase Family Member 9; PROK2, prokineticin 2; QoL, quality of life; RBD, receptor-binding; RNA, ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SCN9A, sodium voltage-gated channel, alpha subunit 9; SLC12A6, Solute Carrier Family 12 Member 6; TMPRSS2, transmembrane serine protease 2
Graphical abstract
1. Introduction
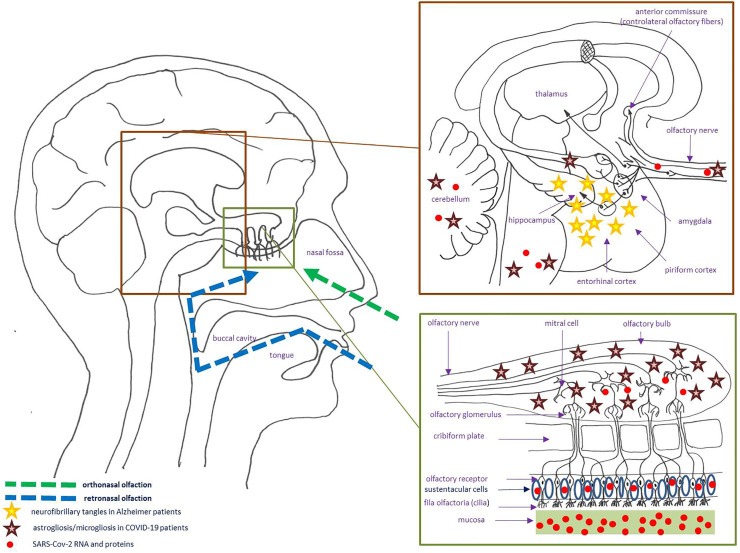
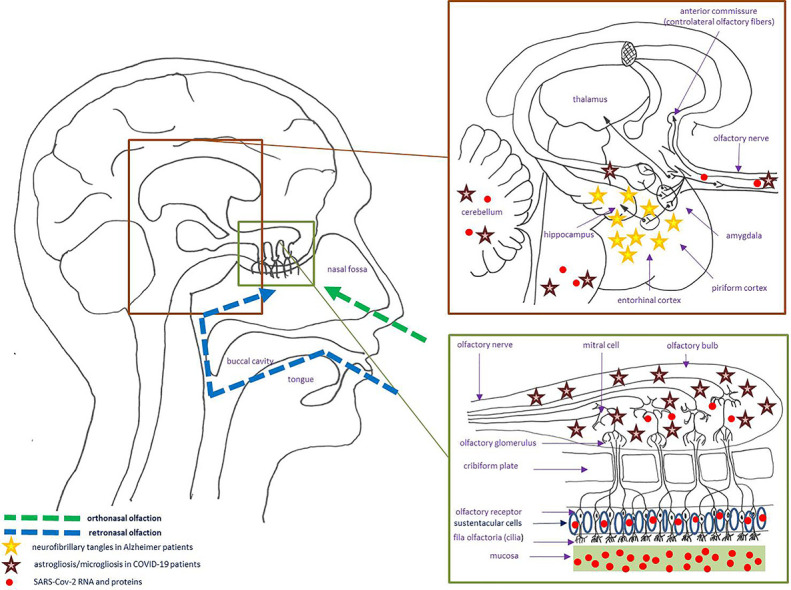
Since time immemorial, the basic survival mechanism of smell (‘olfaction’) alerts individuals to dangers such as fire or spoiled foods, but also greatly contributes to the sensation of flavor. When eating, smell is first perceived anteriorly (orthonasal olfaction), followed by the perception of ‘gustation’, before retronasal olfaction (referred to the oral cavity rather than to the olfactory epithelium); so patients with loss of smell regularly complain as having lost the sense of taste [1]. The first step in olfaction is the activation (by odorant molecules) of ciliated olfactory receptors (‘fila olfactoria’) located at the olfactory clefts. The pseudostratified columnar olfactory neuroepithelium (neural and supporting cells), characterized by its unique ability to regenerate, is lined with mucus largely produced by Bowman's glands [1]. The remaining part of the nasal cavity and pharynx is not covered by the olfactory neuroepithelium, but contribute to the detection of some molecules through the activation of the trigeminal, glossopharyngeal and vagus nerves. The “cribriform plate” (CP) is the horizontal portion of the ethmoid bone where filia olfactoria (fascicles of thin, unmyelinated axons of the bipolar olfactory receptor cells) go up through the CP to synapse in the olfactory bulbs (both a relay station and a complex center where sensory input is filtered and modified by neural elements intrinsic and extrinsic to the bulb). They are then transmitted along complex olfactory pathways, the signal reaches the “olfactory cortex” which has many axonal projections to other parts of the brain (Fig. 1 ) [1].
Fig. 1.
Schematic and simplified representation of the olfactory circuitry. Examples of the localization of some neuropathological changes due to Alzheimer's disease and COVID-19 are showed (stars) (COVID-19: coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2).
2. First anatomical descriptions of the nasal cavities and the basis of olfaction
The first detailed anatomical descriptions of the nasal fossae seems to date at least back to ancient Greece, mainly with Hippocrates of Kos (460 BCE–370 BCE) and Aristotle (384 BCE–322 BCE) [2]. Hippocrates observed the CP and thought that played a role in olfaction [3]. He described it as “soft like a sponge”, falsely considering this structure as cartilage [2]. He believed that the mucus observed inside the nose was secreted by the brain (he described as a “gland at the origin of all catarrhal troubles”) penetrate the nasal fossae (through this “spongious bone”) to be expelled from the body [4]: this was part of the “humoral theory” (“humorism”) of Hippocrates, later revived and expanded by Claudius Galenus (129–200) who was the first to describe this “spongious bone” as “cribrum” (meaning “sieve” in Latin) [5]. However, the ethmoid bone was clearly identified later, by Andreas Vesalius (1514–1564), Realdo Colombo (1510–1559), then Giovanni Filippo Ingrassia (1510–1580) who proposed the term “cribriform plate” [6]. Finally, Gabriele Fallopio (1510–1580) was the first to argue that the CP was not a separate ossicle, but an integral structure of the ethmoid bone [7].
Oribasius (320–403) clearly designated the “mammillary processes” (olfactive bulbs) as the “organ of olfaction” [8]. At that time, the catarrhal secretion of the nose was called “pituite”, a “large viscous sputum whose whitish tint and consistency was roughly reminiscent of the brain substance” [2]. So the nature of the brain was considered as being “pituitous”: this mucous was therefore seen as “feces of the brain” by Jean Fernel (1506–1558) [9]. As Jacobo Berengario da Carpi (1460–1530) before him, Vesalius disagreed with the Galen's theory: for him, the “secretions of the brain” percolated through the base of the skull (from the third ventricle, then crossing the pituitary gland), thinking it did not pass through CP but thought the “foramen lacerum” [10]. He also hypothesized that CP perforations could transmit air (for “cooling the brain”) and odors to the brain [10], unlike Nathaniel Highmore (1613–1685) who argued that the meninges (which are not perforated in the CP) prevented air from reaching the brain [11]. Finally, Vesalius confirmed the “mamillary processes” as being the seat of olfaction, without considering them as real cranial nerves: as he did not notice the fine nerve filaments connected to the olfactory mucosa, he called them “coesi” (“mutilated nerves”) [10]. It was probably Adriaan van den Spiegel (1578–1625) who definitely considered olfactive nerves as cranial nerves [12], before Thomas Willis (1621–1675) classified “nervi olfactorii” as “par primum” (first pair of cranial nerves) [13]. In fact, Willis clearly refuted Vesale's hypothesis, showing that when a liquid (milk or ink) is injected into the pituitary gland, it emerges in the jugular vein, not in the nasal cavities. However, Willis did not clearly understand the role of the CP, thinking that it also contributed to the resorption of cerebrospinal fluid (CSF) [13]. Finally, Conrad Victor Schneider (1614–1680) demonstrated that only branches of the olfactory nerves pass through the CP, not the nasal secretion produced by the mucous membrane of the nasal fossae (nowadays known as “Schneiderian membrane”) [14]. As described by Robert Bentley Todd (1809–1860) and William Bowman (1816–1892), the mucus secreted by Bowman's gland [15] protects the olfactory epithelium, allowing odors to dissolve so that they can be detected by olfactory receptor neurons corresponding to the ‘fila olfactoria’ described by Max Schultze (1825–1874) [16].
Julius Bernstein (1839–1917) highlighted that only “terminal organs” (fila olfactoria) have the capacity to experience the actions of odorants, the nerves being only a simple vector that transmit information and emotion to the brain [16], echoing the words of Jean-Jacques Rousseau (1712–1778) who considered that “smell is the sense of imagination” [17]. Paul Broca (1824–1880) defined olfaction as a “brutal sense”, stressing that it is usually more developed in the “brutal animals” with less intelligence (macrosmic animals), unlike humans (who are microsmic) [18]. Moreover, considering the olfactive nerves (olfactory bulbs) as true cranial nerves is probably not exact. First, olfactive nerves (like the optic nerves) are the only nerves that do not emerge from the brainstem (but are attached to the forebrain: limbic system). Secondly, the peripheral olfactory receptor neurons are situated in the olfactory epithelium (posterodorsal recess of the nasal cavity), whereas the central part of the main olfactory system comprises the olfactory bulb and the targets of its projections (olfactory tract) within brain structures implicated in memory formation and motivational aspects of behavior. Finally, the olfactory system is unique among the senses, in that receptors project directly to the cortex, the other senses relaying through the thalamus [1].
Since the middle of the 20th century, we know that olfactory receptor cells (derived from ectoderm and serving as the first-order neurons) can regenerate after they are damaged. Nagahara (1940) was the first to observe mitotic activity in the basal cells of the olfactory epithelium (he called “resting cells”) of adult mice [19], then Edwin William Schultz (1887–1971) demonstrated the regeneration of olfactory sensory neurons in monkeys after toxic damage [20,21]. Olfactory neurogenesis is necessary because of the vulnerability of the olfactory sensory neurons to environmental factors: based on the appearance of a new generation of neurons from stem cells, it was studied more intensively by neuroanatomists in the early 1970s [22]. Nowadays, we know the sensory olfactory neurons are continually replaced during adulthood from horizontal basal stem cells (able to regenerate all the cells of the olfactory epithelium, if damaged by trauma or toxins) in a neurogenic niche in the olfactory epithelium, but multipotent stem cells have also been found in the olfactory mucosa [22]. The extraordinary plasticity of the olfactory system could explain why olfactory training improves olfactory function in humans and may improve the olfactory recovery time to stimulate olfactory nerve regeneration (related to olfactory receptor and neurotrophic factor stimulation): so olfactory training may be an effective intervention for patients with olfactory dysfunction [23].
3. The concept of “anosmia”
Bernstein explained that two conditions are required for smell. First, there is the chemical condition due to chemical properties of the odorants acting on the olfactory receptors; secondly, there is also a mechanical condition, the regular renewal of a flow of air in the nasal cavities to maintain efficient olfaction: by stopping to breath, smell is suspended [16]. Bernstein also mentioned the experience of Ernst Heinrich Weber (1795–1878) who observed that, when the nasal cavities are full of a liquid (such as water or eau-de-cologne), there is no sense of smell [16]. The absence of the ability to smell is called “anosmia”, whereas “hyposmia” is only a decreased ability to smell. “Anosmia” comes from the Ancient Greek “an-” (meaning “absent”) and “-osmḗ” (meaning “odor”); it was also described under the term ‘chamesie” by Haly Abbas (930–994) or “olfactûs amissio” (“loss of olfaction”) by Daniel Sennert (1572–1637) [24]. During the 16th century, Fernel was one of the first to give details about the causes of anosmia, pointing out that the loss of smell can occur when “continuous stench”, especially in the case of “ozaena” (chronic atrophic rhinitis), as well as “when the duct of the nostrils and ethmoid bone, through which the spirit and smell ordinarily pass, is impeded by an outgrowth of flesh, or by a polyp or by a phlegmon, or by some defluxion” [9].
François Boissier de Sauvages (1706–1767) considered anosmia as a possible disease, unlike Philippe Pinel (1745–1826) who thought it was a pure symptom [24]. Based on the observations of Fernel, Théophile Bonet (1620–1689), Guillaume de Baillou (1538–1616) and Lorenz Heister (1683–1758), he classified anosmia in seven categories (Table 1 ) [25]. For him, it was mainly caused by nasal obstruction (“anosmia a polypo”) or destruction, especially due to rhinitis (“anosmia catarrhalis”) and other nasal infectious disorders, syphilitic (“anosmia syphilitica”) or not (“anosmia ab ozoena”), or due to various substances (“anosmia a siccitate” and “anosmia verminosa”) [25]. But he also reported on “anosmia paralytica” due to “obstruction and compression of the olfactory nerves”, without many details (Table 1) [25].
Table 1.
Classification of anosmia according to François Boissier de Sauvages.(1772).
| Type | Denomination | French definition | English translation |
|---|---|---|---|
| 1 | anosmia catarrhalis | “C'est celle qui accompagne le rhume ordinaire; & lorsque celle-ci est opiniâtre, elle subsiste après même qu'il est guéri.” | “It is what accompanies the common cold, and when it is stubborn, it remains even after it is cured.” |
| 2 | anosmia ab ozoena | “Anosmie causée par un ozène. Ceux qui puent du nez, soit à cause d'un ulcère qui ronge la membrane pituitaire, soit à cause de la putréfaction de la morve & de l'air qui séjournent trop longtemps dans les antres de Highmor & dans les autres sinus, ceux qui dissèquent les cadavres, qui vident les latrines, qui fréquentent les boucheries & les autres lieux où l'on respire de mauvaises odeurs, s'y habituent tellement, & en sont s'y affectés qu'ils ne sentent plus les autres, & perdent tout à fait l'odorat.” | “Anosmia caused by ozaena. Those who stink from the nose, either because of an ulcer that gnaws at the pituitary membrane, or because of putrefaction of mucus/snot and air that stagnates in the antrum of Highmore and in the other sinuses, those who dissect corpses, who empty latrines, who frequent butcher's shops and other places where bad smells are respired, get so used to and affected by them that they no longer smell others, and lose their sense of smell altogether.” |
| 3 | anosmia a polypo | “Anosmie cause par un polype. Lorsqu'il se forme un polype dans le nez, & qu'il croit au point de boucher les narines & d'affaisser le vomer; l'air ni les effluves odoriférans ne pouvant plus y entrer, il faut nécessairement que l'odorat se perde. » | “Anosmia caused by a polyp. When a polyp is formed in the nose, and grows to the point of blocking the nostrils and sagging the vomer; neither the air nor the odoriferous effluvium can enter, the sense of smell must necessarily be lost.” |
| 4 | anosmia syphilitica | “Anosmie vénérienne. C'est celui qui survient dans le troisième degré de la vérole, après que le dedans du nez est mangé par les ulcères qui s'y sont formés. Ces ulcères mangent non seulement les membranes, mais encore les cartilages, & détruisent entièrement l'organe de l'odorat. » | “Venereal anosmia. It is the one that occurs in the third degree of the great pox, after the inside of the nose is eaten by ulcers that have formed there. These ulcers eat not only the membranes, but also the cartilage, and completely destroy the organ of smell.” |
| 5 | anosmia verminosa | “Anosmie vermineuse. Plusieurs observations nous apprennent qu'il s'engendre des vers dans le nez, qui causent l'éternuement, la migraine, qui jettent le malade dans la fureur, & lui font entièrement perdent l'odorat. » | “Verminous anosmia. Several observations tell us that it generates worms in the nose, which cause sneezing, migraine, which throw the patient into rage, & make him entirely lose the sense of smell.” |
| 6 | anosmia a siccitate | “Anosmie causée par la sécheresse. Tout le monde sait que dans les fièvres & les maladies inflammatoires, la langue & la membrane pituitaire se dessèchent, lors surtout que la chaleur est considérable. Il n'est donc pas étonnant que ces maladies soient suivies du dégoût & de la perte d'odorat. Un homme qui voyage le vent en face, surtout en été, & qui respire la poussière qui s'élève des chemins, perd infailliblement l'odorat. La même chose arrive à ceux qui font un très grand usage du tabac, surtout de celui d'Espagne; & cela vient de ce que ces choses dessèchent les fibrilles nerveuses & les rendes insensibles aux impressions de dehors. On peut rapporter ici la perte de l'odorat, occasionnée par des calculs qui se forment dans les narines. » | “Anosmia caused by drought. Everybody knows that in fevers and inflammatory diseases, the tongue and pituitary membrane dry out, especially when the heat is considerable. It is therefore not surprising that these diseases are followed by disgust and loss of smell. A man who travels against the wind, especially in summer, and who breathes the dust that rises from the roads, unfailingly loses his sense of smell. The same thing happens to those who smoke a lot, especially tobacco from Spain; and this is because these things dry out the nervous fibrils and make them insensitive to sensations from the outside. We can report here the loss of sense of smell caused by stones forming in the nostrils.” |
| 7 | anosmia paralytica | “Anosmie paralytique. C'est celle qui accompagne les maladies soporeuses, & les différentes espèces de paralysies, & qui est occasionnée par l'obstruction et la compression des nerfs olfactifs.” | “Paralytic anosmia. It is the one that accompanies the soporific diseases and the different species of paralysis, and that is caused by the obstruction and compression of the olfactory nerves.” |
This is adapted from « Boissier de Sauvages F. V. Anosmia, perte d'odorat; Olfacûs amissio, Sennert; Chasemie, d'Haly-Abbas. In: Boissier de Sauvages F, ed. Nosologie méthodique, ou distribution des maladies en classes, en genres et en espèces, suivant l'esprit de Sydenham, & la méthode des botanistes. Lyon: JM Bruyset, 1772; pp 174–177″ [25] (translated from the French).
Many decades later, more details on “anosmia paralytica” were given by Hippolyte Cloquet (1787–1840) who compiled several cases written by earlier authors: loss of olfaction after the occurrence of a cerebral abscess (frontal lobe); lesion of the ethmoid bone; a case related to a tumor (“very hard stone”) of the brain; a case of a cerebral tumor compressing the olfactory nerves [24]. Cloquet highlighted that “the loss of olfaction is a necessary consequence of the absence of olfactory nerves” [24]. He also mentioned the possibility of “essential anosmia”, acquired or congenital, that is “always annoying”, unlike “symptomatic anosmia” which “disappears with the disease on which it depends” [24]. During the 19th century, anatomical knowledge grew, especially about the brain function in olfaction [18].
For two centuries, many scientists developed tests to assess olfaction in humans, such as Ernst Heinrich Weber (1795–1878) [26] or Hendrik Zwaademaker (1857–1930) and his “olfactometer” he used to diagnose “incomplete anosmia” [27]. Olfactory function may be tested by various psychophysical measurements (detection and recognition threshold tests, signal detection tests, quality discrimination tests, memory tests, sniffin’ stick test for orthonasal olfaction, retronasal olfactory stimulation using flavored aqueous solutions presented to the mouth, etc) and imaging (magnetic resonance imaging, MRI) or functional imaging (functional MRI) [1]. Electrophysiological measurements (electroencephalography, chemosensory event-related potentials) may also help to diagnose olfactory dysfunction. After Richard Caton (1842–1926) demonstrated that electrical potentials could be measured directly from the exposed surface of the cerebral cortex in animals (presented at the “forty-third annual meeting of the British Medical Association”, Edinburgh, 1875) [28], Hans Berger (1873–1941) recorded the first human “Elektrenkephalogramm” (EEG, as Berger coined it) in 1924 [29,30]. Interestingly, the phenomenon nowadays known as “Berger effect” (reactivity of alpha rhythms to eyes opening) may also be evoked by odorants [31]. However, if Ernst Fleischl von Marxow (1846–1891) observed that brain electrical activity may be influenced by odorants (response to ammonia presented to a rabbit's nose), scalp-recorded EEG by odorants was later demonstrated in humans in the 1960's [32,33], leading to the development of chemosensory event-related potentials [34]. Moreover, smelling produces a voltage change between the surface of the olfactory epithelium and any other point on the body (called “electro-olfactogram”, EOG), as first shown by David Ottoson (1918–2001) in the frog (1954) [35], many years after the works of Hosoya & Yoshida in the dog (1937) [36]; however, the first human EOGs were only published in 1969 [37]. EOG is a helpful tool to provide a complete picture of the processing of olfactory function, in combination with nasal endoscopy and air-dilution olfactometry [38].
Nowadays, we know that there are various etiologies for anosmia, with two main categories identified: “conduction injuries” (due to lesions of nose or nasal cavities) and “neuronal injuries” (due to lesions of olfactory neurons, olfactory bulbs or cerebral cortex) [1], as summarized in Table 2 . The most frequent causes of olfaction impairment are sinonasal diseases (7–56%), post-upper respiratory infection (18–45%), head trauma (8–20%), toxic exposure (2–6%) and congenital disorders (0–4%); olfactory disorders are idiopathic in up to 34% of cases [39].
Table 2.
Main conditions associated with olfactory dysfunction.
| Conduction injuries (nose and nasal cavities) | Neural injuries (olfactory neurons to cerebral cortex) | |
|---|---|---|
| Infection | - infectious rhinosinusitis (viral, bacterial or fungal) | -COVID-19 -AIDS (dementia) -Influenza -Rickettsia -Herpes simplex -neurosyphilis -meningitis |
| Medications | -rhinitis medicamentosa by using topical decongestants (oxymetazoline, phenylephrine, …) or oral medications (sympathetic amines, etc) -local anesthetics (cocaine, procaine, tetracaine) -intranasal saline solution (acethylchonine or acetyl-β-methylcholine, zinc sulfate, strychnine, etc) |
-anelgesics (antipyrine, codeine, morphine) -antimicrobials (griseofulvin, macrolides, neomycin, tetracyclines, lyncomycin, antivirals, etc) -myorelaxants -hypnotic agents -adrenal steroids (chronic use) -methotrexate ‑mercury/gold salts -cimetidine |
| Toxics | -cocaine -menthol, pepper, oil of peppermint, spices, etc |
-alcohol -heavy metal (mercury, nickel, cadmium, lead, manganese, etc) -acetone, acrylate, trichloroethylene, benzene, butylacetate, coke/coal |
| Inflammation | -chronic atrophic rhinitis (syphilis, leprosy, purulent sinusitis, radiotherapy, etc) -vasomotor rhinitis -inflammatory obstruction of the olfactory clefts |
-multiple sclerosis -Sjögren's syndrome |
| Tumor | -nasal polyposis -intranasal neoplasm (adenocarcinoma, leukemic infiltration, etc) -nasapharyngeal tumor (neurofibroma, schwannoma, etc) -osteoma, |
-neuro-olfactory tumor - frontal or temporal cerebral tumor, abscess or metastasis -parasagittal meningioma -tumor of the corpus callosum -para-optic chiasma tumor (aneurysm, craniopharyngioma, pituitary tumor) -osteoma |
| Allergy | -allergic rhinosinusitis (perennial, seasonal) | – |
| Nutritional/metabolic disorders | – | -chronic renal failure -abetalipoproteinemia -cirrhosis of liver ‑copper deficiency ‑zinc deficiency -vitamin deficiency (B1, B6, B12) -gout -diabetes -hypothyroidism -Addison's disease -Cushing's syndrome -Froelich's syndrome -panhypopituitarism -Whipple's disease |
| Degeneration | – | -Amyotrophic lateral sclerosis (ALS) -Guam ALS/dementia -Alzheimer's disease -Parkinson's disease -Huntington's disease -Korsakoff's syndrome |
| Traumatism | -deviated nasal septum | -head trauma -traumatic blow -haemorrhage |
| Genetic disorders | -cephalocele | -Kallman's syndrome -Down syndrome -familial dysautonomia -Refsum's disease |
| Iatrogen causes | -nasal surgery -radiotherapy |
-cranial or brain surgery -radiotherapy |
| Psychiatric disorders | -Munchausen's syndrome | -olfactory reference syndrome -schizophrenia/schizotypy -depression -anorexia nervosa -attention deficit disorder -hysteria |
| Miscelleanous | -Paget's disease -chronic obstructive pulmonary disease |
-hydrocephalus -stroke -migraine -seizure (temporal lobe epilepsy) -psychosis/depression -myasthenia gravis -aging |
4. Anosmia in neurology
More than simply playing a role in the enjoyment of food (by adding richness, complexity, and variety), olfaction also influences people's social behavior, plays a special role in emotions and in memory formation, and even may contribute to the choice of one's sexual partner [40]. As a consequence, impairing the ability to smell contributes to reduced quality of life (QoL) related to social interactions, eating, and feelings of wellbeing [40]. A reciprocal relationship has been noted between olfaction and depression: patients with depression have reduced olfactory performance, and patients with olfactory dysfunction present some symptoms of depression (that worsen with the severity of the loss of smell) [41]. So olfactory dysfunction has a negative impact on daily life (decrease in QoL and reduced body-related self-esteem) and is likely to predispose a person to a depressed mood [42], although depressive symptoms are not always severe (probably because of adaptation to the olfaction disorder in the long-term) [43]. Odor perception has also an important role in conditioning social and reproductive behaviors, with a role of some genes: precisely, odor perception between heterosexual partners may have an impact on depression and anxiety, possibly influenced by genetic variation in the OR7D4 (olfactory receptor, family 7, subfamily D, member 4) gene [44] coding for one of the most important odorant receptors on the plasma membrane of olfactory sensory neurons (responding to sex steroid-derived odors as androsterone andandrostadienone) in primates [45].
Global and local white matter network dysfunction of the brain have been found in patients with anosmia and intact structural integrity (without neurodegenerative disorder), these alterations being more frequent in patients with retronasal olfaction deficit [46]. Finally, impairment of olfaction is a common disorder in the general population, this risk increasing in the elderly (up to a quarter of patients over the age of 65 presenting impaired olfaction) [47,48]; but, anosmia is only present in 4–6% of the population [49,50]. Atrophy in the primary olfactory cortex (entorhinal cortex and amygdala) has been found in previous studies in young adults with anosmia/hyposmia, supporting the hypothesis of dysfunction and/or degeneration in areas critical to olfactory processing as a major cause of olfactory deficits in the older population [51]. Impaired olfaction may predict faster cognitive decline (with lower volume in the fusiform gyrus and the middle temporal cortex, including the hippocampus and entorhinal cortex) and indicate neurodegeneration in the brain among dementia-free older adults [52]. Recently, the “sniffing bead system” was specifically designed for screening olfactory function in older adults [53].
Olfactory dysfunction may be a clinical sign of many neurodegenerative disorders, including Alzheimer's disease, Huntington's disease, Parkinson's disease, vascular dementia, frontotemporal dementia, amyotrophic lateral sclerosis, progressive supranuclear palsy, Wilson's disease, idiopathic rapid eye movement sleep behavior disorder, etc. [54]. In some cases, olfactory dysfunction may be a preclinical sign of a neurodegenerative disorder, for example occurring many years (usually 4–8 years) before Parkinson's disease [55]. In such conditions, a key question remains: is neurodegeneration the basis for the perceptual differences in olfaction, or are disease-specific or other entities (respiratory infections or pollution) responsible for this association [54]? Most of the neurological causes of anosmia are neurodegenerative, but profound olfactory dysfunction is also observed in non-degenerative neurological disorders such as myasthenia gravis that seems to influence olfactory function to the same degree as that observed in a number of neurodegenerative diseases in which CNS cholinergic dysfunction has been documented [56]. Olfactory disorder, also common in many psychiatric disorders (depression, schizophrenia, bipolar disorder, etc) [57], may be observed in many other neurological diseases.
In 1821, Cloquet distinguished between “constitutional” and “acquired” anosmia [58], but Otto Charles Glaser (1880–1851) was probably the first to consider the possibility of hereditary cases of anosmia, writing that “'smell-blindness' is heritable” [59]. Congenital anosmia (absence of sense of smell from birth) may be divided into “syndromic congenital anosmia” and “isolated congenital anosmia” (when anosmia is the only symptom and for which no disease-causing gene was identified) [60]. Kallmann syndrome (characterized by hypogonadotroph hypogonadism and anosmia/hyposmia) is a classical cause of congenital syndromic anosmia, with many causative genes (KAL1/ANOS1, KAL2/FGFR1, FGF8, CHD7, PROK2, etc.; most mutations are inherited in X-linked, autosomal dominant, or autosomal recessive pattern, but many genes interact with each other in an oligogenic manner) [61], and anosmia may be also associated with Klinefelter syndrome (primary hypogonadism) [62], as it may be part of other syndromes such as Refsum disease (associated with retinis pigmentosa, deafness, demyelinating polyneuropathy, ataxia or ichtyosis) [63], congenital insensitivity to pain (SCN9A gene mutations causing disrupted synaptic signalling at the primary sensory axon terminal) or some ciliopathies [60]. In Down syndrome (trisomy 21), olfaction is severely impaired, usually appearing at a relatively young age: in such cases, olfactory performance correlates with cognitive performance, so the olfactory deficit may represent an early indicator of neurodegenerative events (similar to those in Alzheimer's disease) in this population [64].
Olfactory disturbance may also be the consequence of trauma, affecting either the peripheral or the central pathways of olfactory system, or even the secondary olfactory centers (such as orbitofrontal cortex): its incidences ranges from 4 to 60%, increasing with the severity of the head trauma [65]. Spontaneous recovery of olfactory function may occur over time, possibly due to a role of the subventricular neurogenesis and the increase in glomerular dopaminergic interneurons of the olfactory bulbs: despite no specific treatment, olfactory training may be a beneficial therapy in such conditions [66]. Finally, some studies also found that olfactory dysfunction frequently occurs in stroke patients (hyposmia and functional anosmia, more than complete loss of smell), suggesting the inclusion of olfactory assessment in clinical practice [67]. In contrast to congenital anosmia or anosmia due to age and neurodegenerative diseases, drug-induced disorders may regress after cessation of treatment [68]. Similarly, disorders following infection or head trauma seem to subside during the first year after injury, sometimes beyond [69].
5. Anosmia, infection and COVID-19
Olfactory functioning can be categorized as a range of normal (normosmic) to diminished (hyposmic) and absent (anosmic) ability to detect and correctly label odors. The main cause of chronic loss of smell remains upper respiratory infections, especially the common cold, influenza, pneumonia, or human immunodeficiency virus: such infections are associated with “dysosmia” (any distortion of the perception of smell, including “parosmia” and “phantosmia”), then, with time, sometimes anosmia [1]. Parosmia (also called “troposmia”) corresponds to the distortion of perceived odor quality, usually described as a “foul”, “rotten”, “sewage” or “burn” smell, most commonly elicited by some odorants (mainly gasoline, tobacco and coffee): its prevalence ranges between 2.1% [49] and 3.9% [70]. The origin of parosmia in unclear but could be explained by a “peripheral theory” (the loss of functioning olfactory neurons results in the inability to form a complete picture of the odorant) and a “central theory” (the integrative or interpretive centers in the brain form a distorted odor) [71]. Phantosmia is a phantom olfactory sensation (olfactory hallucination, or “phantom odor”), usually unpleasant, with no apparent olfactory stimulus: its prevalence is estimated to be 0.8% [49].
Aulus Cornelius Celsus (25 BCE-50 CE), based on Hippocrates observation of “coryza” (rhinitis inducing transient anosmia by nasal congestion), suggested that some cases of “phthisis” may be due to catarrh of the upper limbs [72]. In 1912, by producing experimental poliomyelitis following the application of the active poliovirus to the nasal mucous membrane, Simon Flexner (1863–1946) and Paul Franklin Clark (1882–1983) demonstrated that a microorganism may enter the body and reach the CNS through the nose [73]. Since then, many viruses (herpes simplex, influenza, etc) have been shown to have similar properties [74]. As with other respiratory viruses, coronaroviruses (named for the crown-like spikes on their surface) also have a propensity for neuroinvasion [75]. The spike proteins (S) are membrane-anchored trimers containing a receptor-binding (RBD) S1 segment (RBD binds to angiotensin-converting enzyme-2, or ACE2) and a membrane-fusion S2 segment: the binding of the S segment to the ACE2 receptor is correlated with coronavirus infectivity in the targeted tissue, governing clinical outcomes [76]. SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2) is responsible for the current coronavirus disease 2019 (COVID-19) pandemic, with 127,749,710 million patients diagnosed (2,794,174 deaths) worldwide by March 30th 2021 (https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6). Recently, a renewed interest in olfaction was observed following the observation of numerous cases of anosmia due to COVID-19: the first cases of anosmia and dysgeusia were observed in China, Italy, and Iran, before many cases were observed in other clusters [77]. Because SARS-CoV-2 virus causes reduction of smell and taste in a significant fraction of COVID-19 patients (incidence: 33.9–68%; prevalence: 86%) [78,79], there was evidence for considering dysosmia/anosmia as a symptom of COVID-19 infection; some patients also present solely with this symptom [80]. Observing that 44% of anosmic and 50% of hyposmic COVID-19 patients did not report having olfactory problems, a good tool to detect anosmia in such patients seems to be the “sniffin’ stick test” [80]. Parosmia and phantosmia were also reported in COVID-19, respectively in 22% and 21% of the patients in the study of Le Bon et al [81]. The prognosis for olfaction considered as favorable: anosmia/hyposmia usually persisted beyond 5 days (about 72.6% of anosmic patients recovered olfactory function within the first 8 days) [79], and most of the patients recovered by 30 days [82]; when olfactory dysfunction due to SARS-Cov-2 infection persists beyond 2 weeks, a therapy should be considered, especially olfactory training [83]. However, in the study of Le Bon et al, five weeks after developing sudden olfactory loss due to COVID-19, more than a third of patients displayed olfactory dysfunction according to psychophysical testing, suggesting potential peripheral neurosensory damage [81]. So anosmia may be persistent, and olfactory mucosa presenting with persistent loss of smell may reveal the presence of viral transcripts and of SARS-CoV-2-infected cells [84]. The pathogenic mechanism of this olfactory dysfunction remains unclear: postviral anosmia in the setting of upper respiratory tract infection is usually related to mucosal congestion and nasal obstruction (conductive olfactory loss) [85], but sinonasal symptoms are not frequent in COVID-19, suggesting that mechanisms other than sinonasal obstruction may play a role [86].
On 4 March 2020 (Beijing Ditan Hospital, China), the first study on neurological disease following SARS-CoV-2 virus infection was reported, some patients having positive CSF for SARS-CoV-2 (by gene sequencing), even though most patients with SARS-CoV-2 infection do not test positive for the virus in CSF [87]. To date, various neurological manifestations (other than anosmia/dysgeusia) have been described in COVID-19: headache, dizziness, impaired consciousness, cerebrovascular accident, acute necrotizing encephalopathy, meningo-encephalitis, acute inflammatory polyradiculoneuropathy, myalgia and psychiatric symptoms (depression, anxiety, insomnia) [88]. The exact mechanism of SARS-CoV-2 neuroinvasion is still unclear, but two main penetration routes were first suggested. In the “hematogenous route”, it was hypothesized that the spread of SARS-CoV-2 across the blood-brain barrier (BBB) could be the consequence of infection of the brain microvascular endothelial cells (or BMEC, lining the brain capillaries), via interactions of the S-protein of SARS-CoV-2 with ACE2 on the BMEC cell surface, facilitating the entry of virus into the CNS; the other hypothesis is the “trans-synaptic spread” through the olfactory nerve and/or the hypoglossal, facial, glossopharyngeal and vagus cranial nerve: the neuronal expression of ACE2 could facilitate SARS-CoV-2 infection through the uptake into dendrites and soma [88,89]. Two other mechanism were suspected: the “immune cell route” (infection of epithelial respiratory cells, then of the resident immune cells that could carry SARS-CoV-2 to various organs, including the CNS) and the “autoimmune mechanism” [88]. It was also proposed that the immune phenomena leading to multi-organ damage in some COVID-19 patients could be explained by molecular mimicry (MM): MM between the SARS-CoV-2 protein ORF7b (Open Reading Frames 7b) and OR7D4 could explain anosmia; in the same way, MM between the SARS-CoV-2 protein ORF1ab and PARP9 (Poly-ADP-Ribose-Polymerase Family Member 9) could explain leukopenia, MM between the SARS-CoV-2 nucleocapsid phosphor-protein and SLC12A6 (Solute Carrier Family 12 Member 6) could explain vascular damage [86]. Thus, once in the CNS, SARS-CoV-2 can reside either quiescent, or eventually be active leading to severe acute encephalitis (with neuroinflammation and prolonged neuroimmune activation) [89]. But, a question remains: are these neurological disorders directly due to viral invasion of the CNS, or can they be caused by indirect mechanisms [88]? The evidence of a causal relationship between SARSCoV-2 and autopsy brain findings remains equivocal (large and small infarcts, microhaemorrhages, focal parenchymal infiltrate of T-cells, etc), but this probably represents a combination of direct cytopathic effects mediated by SARS-CoV-2 replication or indirect effects due to respiratory failure, injurious cytokine reaction, reduced immune response and cerebrovascular accidents induced by viral infection [88]. According to Matschke et al., the neuropathological changes in patients with COVID-19 seem to be mild, with pronounced neuroinflammatory changes in the brainstem as the most common finding: in their study, SARS-CoV-2 RNA or proteins were detected in the brain of 21 (53%) of 40 examined patients, with SARS-CoV-2 viral proteins found in cranial nerves originating from the lower brainstem and in isolated cells of the brainstem (Fig. 1) [90].
For the olfactory system and COVID-19, we know that: a) the sustentacular cells (supporting cells) of the olfactory epithelium (expressing high levels of ACE2 and the cell surface-associated protease called “transmembrane protease serine 2” or “TMPRSS2”, allowing viral entry following binding of the viral spike protein to ACE2) are the primary target and entry point of SARS-CoV-2 (initiating a series of events leading to dysosmia/anosmia) (Fig. 1), b) some findings are consistent with an inflammatory olfactory neuropathy (prominent leukocytic infiltrates in the lamina propria, focal atrophy of the mucosa, and digestion chambers in the olfactory nerve fibers), and c) desquamation of the olfactory neuroepithelium leads to loss of olfactory cilia [91]. Thus, it was confirmed that SARS-CoV-2 can also enter the nervous system by crossing the neural-mucosal interface in olfactory mucosa: SARS-Cov-2 RNA and proteins were found in olfactory bulb, olfactory tubercle, brainstem and cerebellum (Fig. 1) [92]. Microbleeding or abnormal enhancement of the olfactory bulbs were reported on MR imaging of some COVID-19 patients (by using sequences with coronal thin-slice pre- and/or post‑gadolinium fat-suppressed T1WI in the anterior fossa of the cranium) [93]. Moreover, a few COVID-19 patients may experience more persistent olfactory dysfunction: in such patients, there is MRI evidence of the development of olfactory bulb atrophy [94]. As pointed out below, the olfactory dysfunction in Alzheimer's disease is well recognized, largely due to the accumulation of neurofibrillary tangles in central olfactory regions, especially the entorhinal cortex and hippocampus (this regions being considered to be among the first areas affected by the pathologic changes of classical Alzheimer's disease) (Fig. 1) [95]. It was also shown that people carrying one or two copies of the epsilon-4 allele of apolipoprotein E4 (ApoE4), a key associated genetic risk factor for late onset Alzheimer's disease, develop significant odor recognition deficits in comparison to those not carrying this haplotype [96]. So recently, as highlighted by some authors (and considering the high prevalence of anosmia in patients with mild-to-moderate forms of COVID-19), the question of whether SARS-CoV-2 infection will be associated with an increased risk and rate of future neurodegenerative disorders remains open and subject to speculation [89,97]. According to Manzo et al., a hypothesis could be that SARS-CoV-2 may be an increased risk factor for future dementia in anosmic patients with ApoE4 (higher than in ApoE4 patients with anosmia not induced by SARS-CoV-2), combined with virus-induced chronic modifications in the CNS, so these authors suggest a long-term follow-up of COVID-19 patients who develop olfactory dysfunction [97].
6. Conclusion
Centuries of researches have led to a better understanding of the anatomical bases and physiological mechanisms of olfaction, as well as the pathologies leading to olfactory dysfunction. Although the current COVID-19 pandemic has attracted considerable interest in anosmia, olfaction and its links with neurodegenerative disorders are not fully understood. Finally, we believe that physicians need to be aware of the olfactory deficits that may accompany or precede various disorders, neurological or not, and to consider assessment of olfactory function in clinical practice.
Authors contribution
SM and GS are responsible of the conceptualisation of the review and developed the original draft. JMV and GLM have extended the original draft. FD, AS and LC have reviewed and edited the final draft.
Funding
None.
Declaration of Competing Interest
There is no conflict of interest associated with this review.
Acknowledgements
None.
References
- 1.Doty R.L. 3rd ed. Wiley Blackwell; Hoboken, New Jersey: 2015. Handbook of Olfaction and Gustation. [Google Scholar]
- 2.Chauveau C. J.B. Baillière & Fils; Paris: 1912. Recherches sur l'histoire de l'anatomie et de la physiologie des fosses nasales depuis Hippocrates jusqu'à la période spécialistique. [Google Scholar]
- 3.Hirsch A. Gustavus Lange; Berolini: 1864. De Collectionis Hippocraticae Auctorum Anatomia. [Google Scholar]
- 4.Wright J. Lea & Febiger; Philadelphia and New York: 1914. A History of Laryngology and Rhinology. [Google Scholar]
- 5.Turliuc D.M., Sava A., Cucu A.I., Turliuc S., Dumitrescu A.M., Costea C.F. Cribriform plate and Galen's Cribrum Romanum. Revist Romana Anat. Clin. Antropol. 2016;15(1):123–126. [Google Scholar]
- 6.Cappello F., Gerbino A., Zummo G. Giovanni Filippo Ingrassia: a five-hundred year-long lesson. Clin. Anat. 2010;23(7):743–749. doi: 10.1002/ca.21038. [DOI] [PubMed] [Google Scholar]
- 7.Falloppio G. 1570. Mutinensis Physici Praeclarissimi, ac nostrotum temporum eximij Anatomici Expositio in librum Galeni de ossibus huic accesserunt observationes eiusdem authoris, Simonem Galignanum de Karera, Venetiis. [Google Scholar]
- 8.Oribasius . In: Oeuvres d'Oribase, texte grec, en grande partie inédit, colationné sur les manuscrits, traduit pour la première fois en français, avec une introduction, des notes, des tables et des planches (Bussemaker & Daremberg) Oribasius, editor. Imprimerie Impériale; Paris: 1858. De l'organe de l'odorat; pp. 306–309. [Google Scholar]
- 9.Fernel J. In: La pathologie. Fernel J., editor. La Veuve de Jean Le Boye; Paris: 1646. Les maladies & symptomes des narines, avec leurs causes et leurs signes; pp. 353–356. [Google Scholar]
- 10.Vesalius A. De humani corporis fabrica libri septem. J. Oporinum, Basel. 1553;7 [Google Scholar]
- 11.Highmore N. In: Corporis humani disquisitio anatomica: in qua sanguinis circulationem in quavis corporis particula plurimis typis novis, ac aenygmatum medicorum fuccicta dilucidatione ornatam prosequutus est Samuelis Broun, The Hague. Highmore N., editor. 1651. De Auro, Naso & Lingua; pp. 240–242. [Google Scholar]
- 12.Spiegel A. In: De humani corporis fabrica libri decem. Spiegel A., editor. 1632. De naso interno, sive olfactus instrumento; p. 390. [Google Scholar]
- 13.Willis T. Jo. Martyn & Ja; Allefry, London: 1664. Cerebri anatome: cui accessit nervorum descriptio et usus. [Google Scholar]
- 14.Schneider K.V. J Wilhelm; Witterberg: 1655. Liber de osse cribriformi, & sensu ac organo odoratus, morbis ad utrumq; spectantibus, de coryzâ, Haemorrhagiâ narium, polypo, sternutatione, amissione odoratus. [Google Scholar]
- 15.Todd R.B., Bowman W. In: The Physiological Anatomy and Physiology of Man. Todd R.B., Bowman W., editors. Blanchard & Lea; Philadelphia: 1857. Of smell. [Google Scholar]
- 16.Bernstein J. In: Les sens. Bernstein J., editor. F. Alcan; Paris: 1893. Le sens de l'odorat; pp. 245–252. [Google Scholar]
- 17.Rousseau J.J. In: Emile, ou l’éducation. Rousseau J.J., editor. J. Néaulme; La Haye: 1762. Livre second. L’âge de nature: de 2 à 12 ans (puer) p. 250. [Google Scholar]
- 18.Broca P. Anatomie comparée des circonvolutions cérébrales. Le grand lobe limbique et la scissure limbique dans la série des mammifères. Rev. Anthropol. 1878;1:385–498. [Google Scholar]
- 19.Nagahara Y. Experimentelle Studien über die histologischen Veränderungen des Geruchssorgans nach der Olfactoriusdurchschneidung. Jpn. J. Med. Sci. V Pathol. 1940;6:165–199. [Google Scholar]
- 20.Schultz E.W. Repair of the olfactory mucosa with special reference to regeneration of olfactory cells (sensory neurons) Am. J. Pathol. 1960;37:1–19. [PMC free article] [PubMed] [Google Scholar]
- 21.Schultz E.W. Regeneration of olfactory cells. Proc. Soc. Exp. Biol. Med. 1941;46:41–43. [Google Scholar]
- 22.Mackay-Sim A. Stem cells and their niche in the adult olfactory mucosa. Arch. Ital. Biol. 2010;148(2):47–58. [PubMed] [Google Scholar]
- 23.Kim B.Y., Park J.Y., Kim E.J., Kim B.G., Kim S.W. The neuroplastic effect of olfactory training to the recovery of olfactory system in mouse model. Int. Forum Allergy Rhinol. 2019;9(7):715–723. doi: 10.1002/alr.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cloquet H. In: Ophrésiologie, ou traité des odeurs, du sens et des organes de l'olfaction; avec l'histoire détaillée des maladies du nez et des fosses nasales, et des opérations qui leur conviennent. Cloquet H., editor. Méquignon-Marvis; Paris: 1821. Des lésions de l'olfaction; pp. 748–754. [Google Scholar]
- 25.Boissier de Sauvages F., Anosmia V. In: Nosologie méthodique, ou distribution des maladies en classes, en genres et en espèces, suivant l’esprit de Sydenham, & la méthode des botanistes. Boissier de Sauvages F., editor. JM Bruyset; Lyon: 1772. Perte d’odorat; Olfacûs amissio, Sennert; Chasemie, d’Haly-Abbas. [Google Scholar]
- 26.Weber E.H. Koehler; Leipzig: 1834. De pulsu, resorptione, auditu et tactu, F. [Google Scholar]
- 27.Zwaademaker H. On measurement of the sense of smell in clinical examination. Lancet. 1889;133(3435):1300–1302. [Google Scholar]
- 28.Caton R. The electric currents of the brain. Br. Med. J. 1875;2:278. [Google Scholar]
- 29.Jung R., Berger W. Fünfzig Jahre EEG. Hans Bergers Entdeckung des Elektrenkephalogramms und seine ersten Befunde 1924–1931. Arch. Psy. Nervenkr. 1979;227(4):279–300. doi: 10.1007/BF00344814. [DOI] [PubMed] [Google Scholar]
- 30.Berger H. Über das Elektrenkephalogram des Menschen. Arch. Psy. Nervenkr. 1929;87(1):527–570. [Google Scholar]
- 31.Gudziol H., Guntinas-Lichius O. Electrophysiologic assessment of olfactory and gustatory function. Handb. Clin. Neurol. 2019;164:247–262. doi: 10.1016/B978-0-444-63855-7.00016-2. [DOI] [PubMed] [Google Scholar]
- 32.Finkenzeller P. Gemittelte EEG-Potentiale bei olfactorischer Reizung. Pfügers Archiv. 1965;292:76–85. [PubMed] [Google Scholar]
- 33.Allison T., Goff W.R. Human cerebral evoked responses to odorous stimuli. Electroencephalogr. Clin. Neurophysiol. 1967;23(6):558–560. doi: 10.1016/0013-4694(67)90022-3. [DOI] [PubMed] [Google Scholar]
- 34.Osman A., Silas J. In: Handbook of Olfaction and Gustation. Doty R.L., editor. Wiley Blackwell; Hoboken New Jersey: 2015. Electrophysiological measurment of olfactory function; pp. 261–277. [Google Scholar]
- 35.Ottoson D. Sustained potentials evoked by olfactory stimulation. Acta Physiol. Scand. 1954;32(4):384–386. doi: 10.1111/j.1748-1716.1954.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 36.Hosoya Y., Yoshida H. Über die Bioelektrischen Erscheinungen an der Riechschleimhaut. Jap. J. Med. Sci. III Biophys. 1937;5:22. [Google Scholar]
- 37.Osterhammel P., Terkildsen K., Zilstorff K. Electro-olfactograms in man. J. Laryngol. Otol. 1969;83(7):731–733. doi: 10.1017/s0022215100070894. [DOI] [PubMed] [Google Scholar]
- 38.Knecht M., Hummel T. Recording of the human electro-olfactogram. Physiol. Behav. 2004;83(1):13–19. doi: 10.1016/j.physbeh.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Nordin S., Bramerson A. Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr. Opin. Allergy Clin. Immunol. 2008;8(1):10–15. doi: 10.1097/ACI.0b013e3282f3f473. [DOI] [PubMed] [Google Scholar]
- 40.Smeets M.A.M., Veldhuizen M.G., Galle S., Gouweloos J., de Haan A.J.A., Vernooij J., Visscher F., Kroeze J.H.A. Sense of smell disorder and health-related quality of life. Rehabil. Psychol. 2009;54(4):404–412. doi: 10.1037/a0017502. [DOI] [PubMed] [Google Scholar]
- 41.Kohli P., Soler Z.M., Nguyen S.A., Muus J.S., Schlosser R.J. The association between olfaction and depression: a systematic review. Chem. Senses. 2016;41(6):479–486. doi: 10.1093/chemse/bjw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kollndorfer K., Reichert J.L., Bruckler B., Hinterleitner V., Schopf V. Self-esteem as an important factor in quality of life and depressive symptoms in anosmia: a pilot study. Clin. Otolaryngol. 2017;42(6):1229–1234. doi: 10.1111/coa.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auinger A.B., Besser G., Liu D.T., Renner B., Mueller C.A. Long-term impact of olfactory dysfunction on daily life. Wien. Klin. Wochenschr. 2020 doi: 10.1007/s00508-020-01751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sookoian S., Burgueno A., Gianotti T.F., Marillet G., Pirola C.J. Odor perception between heterosexual partners: its association with depression, anxiety, and genetic variation in odorant receptor OR7D4. Biol. Psychol. 2011;86(3):153–157. doi: 10.1016/j.biopsycho.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Zhuang H., Chien M.S., Matsunami H. Dynamic functional evolution of an odorant receptor for sex-steroid-derived odors in primates. Proc. Natl. Acad. Sci. U. S. A. 2009;106(50):21247–21251. doi: 10.1073/pnas.0808378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen B., Akshita J., Han P., Thaploo D., Kitzler H.H., Hummel T. Aberrancies of brain network structures in patients with anosmia. Brain Topogr. 2020;33(3):403–411. doi: 10.1007/s10548-020-00769-2. [DOI] [PubMed] [Google Scholar]
- 47.Boesveldt S., Postma E.M., Boak D., Welge-Luessen A., Schopf V., Mainland J.D., Martens J., Ngai J., Duffy V.B. Anosmia - A clinical review. Chem. Senses. 2017;42(7):513–523. doi: 10.1093/chemse/bjx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doty R.L., Shaman P., Applebaum S.L., Giberson R., Siksorski L., Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 49.Landis B.N., Konnerth C.G., Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114(10):1764–1769. doi: 10.1097/00005537-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 50.Bramerson A., Johansson L., Ek L., Nordin S., Bende M. Prevalence of olfactory dysfunction: the Skovde population-based study. Laryngoscope. 2004;114(4):733–737. doi: 10.1097/00005537-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 51.Cerf-Ducastel B., Murphy C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res. 2003;986(1–2):39–53. doi: 10.1016/s0006-8993(03)03168-8. [DOI] [PubMed] [Google Scholar]
- 52.Dintica C.S., Marseglia A., Rizzuto D., Wang R., Seubert J., Arfanakis K., Bennett D.A., Xu W. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology. 2019;92(7):e700–e709. doi: 10.1212/WNL.0000000000006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Min H.J., Kim S.M., Han D.H., Kim K.S. The sniffing bead system, an olfactory dysfunction screening tool for geriatric subjects: a cross-sectional study. BMC Geriatr. 2021;21(1):54. doi: 10.1186/s12877-020-01871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doty R.L. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 2017;16(6):478–488. doi: 10.1016/S1474-4422(17)30123-0. [DOI] [PubMed] [Google Scholar]
- 55.Ross G.W., Petrovitch H., Abbott R.D., Tanner C.M., Popper J., Masaki K., Launer L., White L.R. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 2008;63(2):167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- 56.Doty R.L. Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis. 2012;46(3):527–552. doi: 10.1016/j.nbd.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carnemolla S.E., Hsieh J.W., Sipione R., Landis B.N., Kumfor F., Piguet O., Manuel A.L. Olfactory dysfunction in frontotemporal dementia and psychiatric disorders: a systematic review. Neurosci. Biobehav. Rev. 2020;118:588–611. doi: 10.1016/j.neubiorev.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Cloquet H. Méquignon-Marvis; Paris: 1821. Osphrésiologie, ou traité des odeurs, du et des organes de l'olfaction; avec l'histoire détaillée des maladies du nez et des fosses nasales, et des opérations qui leur conviennent. [Google Scholar]
- 59.Glaser O. Hereditary deficiencies in the sense of smell. Science. 1918;48(1252):647–648. doi: 10.1126/science.48.1252.647. [DOI] [PubMed] [Google Scholar]
- 60.Karstensen H.G., Tommerup N. Isolated and syndromic forms of congenital anosmia. Clin. Genet. 2012;81(3):210–215. doi: 10.1111/j.1399-0004.2011.01776.x. [DOI] [PubMed] [Google Scholar]
- 61.Stamou M.I., Georgopoulos N.A. Kallmann syndrome: phenotype and genotype of hypogonadotropic hypogonadism. Metabolism. 2018;86:124–134. doi: 10.1016/j.metabol.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cangiano B., Indirli R., Profka E., Castellano E., Goggi G., Vezzoli V., Mantovani G., Arosio M., Persani L., Borretta G., Ferrante E., Bonomi M. Central hypogonadism in Klinefelter syndrome: report of two cases and review of the literature. J. Endocrinol. Investig. 2020;44(3):459–470. doi: 10.1007/s40618-020-01324-3. [DOI] [PubMed] [Google Scholar]
- 63.Gibberd F.B., Feher M.D., Sidey M.C., Wierzbicki A.S. Smell testing: an additional tool for identification of adult Refsum’s disease. J. Neurol. Neurosurg. Psychiatry. 2004;75(9):1334–1336. doi: 10.1136/jnnp.2003.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cecchini M.P., Viviani D., Sandri M., Hahner A., Hummel T., Zancanaro C. Olfaction in people with down syndrome: a comprehensive assessment across four decades of age. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh R., Humphries T., Mason S., Lecky F., Dawson J., Sinha S. The incidence of anosmia after traumatic brain injury: the SHEFBIT cohort. Brain Inj. 2018;32(9):1122–1128. doi: 10.1080/02699052.2018.1483028. [DOI] [PubMed] [Google Scholar]
- 66.Marin C., Langdon C., Alobid I., Mullol J. Olfactory dysfunction in traumatic brain injury: the role of neurogenesis. Curr Allergy Asthma Rep. 2020;20(10):55. doi: 10.1007/s11882-020-00949-x. [DOI] [PubMed] [Google Scholar]
- 67.Wehling E., Naess H., Wollschlaeger D., Hofstad H., Bramerson A., Bende M., Nordin S. Olfactory dysfunction in chronic stroke patients. BMC Neurol. 2015;15:199. doi: 10.1186/s12883-015-0463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Welge-Lüssen A., Wolfensberger M. Reversible anosmia after amikacin therapy. Arch. Otolaryngol. Head Neck Surg. 2003;129(12):1331–1333. doi: 10.1001/archotol.129.12.1331. [DOI] [PubMed] [Google Scholar]
- 69.Reden J., Mueller A., Mueller C., Konstantinidis I., Frasnelli J., Landis B.N., Hummel T. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch. Otolaryngol. Head Neck Surg. 2006;132(3):265–269. doi: 10.1001/archotol.132.3.265. [DOI] [PubMed] [Google Scholar]
- 70.Nordin S., Brämerson A., Millqvist E., Bende M. Prevalence of parosmia: the Skovde population-based studies. Rhinology. 2007;45(1):50–53. [PubMed] [Google Scholar]
- 71.Leopold D. Distortion of olfactory perception: diagnosis and treatment. Chem. Senses. 2002;27(7):611–615. doi: 10.1093/chemse/27.7.611. [DOI] [PubMed] [Google Scholar]
- 72.Stegall J. John Churchill; London: 1837. The first four books of Aur. Corn. Celsus De Re Medica; with an ordo verborum and literal translation. [Google Scholar]
- 73.Flexner S., Clarck P.F. A note on the mode of infection in epidemic poliomyelitis. Proc. Soc. Exp. Biol. Med. 1912;10(1):1–2. [Google Scholar]
- 74.Doty R.L. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann. Neurol. 2008;63(1):7–15. doi: 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- 75.Bohmwald K., Galvez N.M.S., Rios M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mossel E.C., Huang C., Narayanan K., Makino S., Tesh R.B., Peters C.J. Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. J. Virol. 2005;79(6):3846–3850. doi: 10.1128/JVI.79.6.3846-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klopfenstein T., Kadiane-Oussou N.J., Toko L., Royer P.Y., Lepiller Q., Gendrin V., Zayet S. Features of anosmia in COVID-19. Med. Mal. Infect. 2020;50(5):436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meng X., Deng Y., Dai Z., Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am. J. Otolaryngol. 2020;41(5):102581. doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., Blecic S., El Afia F., Distinguin L., Chekkoury-Idrissi Y., Hans S., Delgado I.L., Calvo-Henriquez C., Lavigne P., Falanga C., Barillari M.R., Cammaroto G., Khalife M., Leich P., Souchay C., Rossi C., Journe F., Hsieh J., Edjlali M., Carlier R., Ris L., Lovato A., De Filippis C., Coppee F., Fakhry N., Ayad T., Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hornuss D., Lange B., Schroter N., Rieg S., Kern W.V., Wagner D. Anosmia in COVID-19 patients. Clin. Microbiol. Infect. 2020;26(10):1426–1427. doi: 10.1016/j.cmi.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le Bon S.D., Pisarski N., Verbeke J., Prunier L., Cavelier G., Thill M.P., Rodriguez A., Dequanter D., Lechien J.R., Le Bon O., Hummel T., Horoi M. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID-19: a prospective cohort study on 72 patients. Eur. Arch. Otorhinolaryngol. 2021;278(1):101–108. doi: 10.1007/s00405-020-06267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.D'Ascanio L., Pandolfini M., Cingolani C., Latini G., Gradoni P., Capalbo M., Frausini G., Maranzano M., Brenner M.J., Di Stadio A. Olfactory dysfunction in COVID-19 patients: prevalence and prognosis for recovering sense of smell. Otolaryngol. Head Neck Surg. 2021;164(1):82–86. doi: 10.1177/0194599820943530. [DOI] [PubMed] [Google Scholar]
- 83.Whitcroft K.L., Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020;323(24):2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- 84.Dias De Melo G., Lazarini F., Levallois S., Hautefort C., Michel V., Larrous F., Verillaud B., Aparicio C., Wagner S., Gheusi G., Kergoat L., Kornobis E., Cokelaer T., Hervochon R., Madec Y., Roze E., Salmon D., Bourhy H., Lecuit M., Lledo P.M. COVID-19-associated olfactory dysfunction reveals SARS-CoV-2 neuroinvasion and persistence in the olfactory system. BioRxiv. 2020 doi: 10.1101/2020.11.18.388819. [DOI] [Google Scholar]
- 85.Welge-Lüssen A., Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv. Otorhinolaryngol. 2006;63:125–132. doi: 10.1159/000093758. [DOI] [PubMed] [Google Scholar]
- 86.Kandemirli S.G., Altundag A., Yildirim D., Tekcan Sanli D.E., Saatci O. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad. Radiol. 2021;28(1):28–35. doi: 10.1016/j.acra.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun T., Guan J. Novel coronavirus and the central nervous system. Eur. J. Neurol. 2020;27(9) doi: 10.1111/ene.14227. [DOI] [PubMed] [Google Scholar]
- 88.Al-Sarraj S., Troakes C., Hanley B., Osborn M., Richardson M.P., Hotopf M., Bullmore E., Everall I.P. The spectrum of neuropathology in COVID-19. Neuropathol. Appl. Neurobiol. 2021;47(1):3–16. doi: 10.1111/nan.12667. [DOI] [PubMed] [Google Scholar]
- 89.Dhouib I.E. Does coronaviruses induce neurodegenerative diseases? A systematic review on the neurotropism and neuroinvasion of SARS-CoV-2. Drug Discov. Ther. 2020;14(6):262–272. doi: 10.5582/ddt.2020.03106. [DOI] [PubMed] [Google Scholar]
- 90.Matschke J., Lutgehetmann M., Hagel C., Sperhake J.P., Schroder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Puschel K., Krasemann S., Aepfelbacher M., Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vaira L.A., Hopkins C., Sandison A., Manca A., Machouchas N., Turilli D., Lechien J.R., Barillari M.R., Salzano G., Cossu A., Saussez S., De Riu G. Olfactory epithelium histopathological findings in long-term coronavirus disease 2019 related anosmia. J. Laryngol. Otol. 2020:1–13. doi: 10.1017/S0022215120002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brünink S., Greuel S., Lehmann M., Hassan O., Aschman T., Schumann E., Chua R. Lorenz, Conrad C., Eils R., Stenzel W., Windgassen M., Rößler L., Goebel H.H., Gelderblom H.R., Martin H., Nitsche A., Schulz-Schaeffer W.J., Hakroush S., Winkler M.S., Tampe B., Scheibe F., Körtvélyessy P., Reinhold D., Siegmund B., Kühl A.A., Elezkurtaj S., Horst D., Oesterhelweg L., Tsokos M., Ingold-Heppner B., Stadelmann C., Drosten C., Corman V.M., Radbruch H., Heppner F.L. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2020;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 93.Aragao M., Leal M.C., Cartaxo Filho O.Q., Fonseca T.M., Valenca M.M. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. AJNR Am. J. Neuroradiol. 2020;41(9):1703–1706. doi: 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsivgoulis G., Fragkou P.C., Lachanis S., Palaiodimou L., Lambadiari V., Papathanasiou M., Sfikakis P.P., Voumvourakis K.I., Tsiodras S. Olfactory bulb and mucosa abnormalities in persistent COVID-19-induced anosmia: a magnetic resonance imaging study. Eur. J. Neurol. 2021;28(1):e6–e8. doi: 10.1111/ene.14537. [DOI] [PubMed] [Google Scholar]
- 95.Price J.L., Davis P.B., Morris J.C., White D.L. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol. Aging. 1991;12(4):295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 96.Gilbert P.E., Murphy C. The effect of the ApoE epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J. Clin. Exp. Neuropsychol. 2004;26(6):779–794. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- 97.Manzo C., Serra-Mestres J., Isetta M., Castagna A. Could COVID-19 anosmia and olfactory dysfunction trigger an increased risk of future dementia in patients with ApoE4? Med. Hypotheses. 2021;147:110479. doi: 10.1016/j.mehy.2020.110479. [DOI] [PMC free article] [PubMed] [Google Scholar]