Abstract
Vibrio parahaemolyticus is a marine bacterium known to be a common cause of seafood gastroenteritis worldwide. The thermostable direct hemolysin (TDH) has been proposed to be a major virulence factor of V. parahaemolyticus. TDH causes intestinal fluid secretion as well as cytotoxicity in a variety of cell types. In this study, we investigated the interplay between the hemolysin's enterotoxic and cytotoxic effects by using both human and rat cell monolayers. As revealed by microspectrofluorimetry, the toxin causes a dose-dependent increase in intracellular free calcium in both Caco-2 and IEC-6 cells. This effect was reversible only when low toxin concentrations were tested. The TDH-activated ion influx pathway is not selective for calcium but admits ions such sodium and manganese as well. Furthermore, in the same range of concentration, the hemolysin triggers a calcium-dependent chloride secretion. At high concentrations, TDH induces a dose-dependent but calcium-independent cell death as assessed by functional, biochemical, and morphological assays.
Vibrio parahaemolyticus is the leading cause of gastroenteritis due to the consumption of seafoods worldwide. Although this microorganism persists as a health hazard in the Far East, where it was originally isolated (20), it has also been reported either as a source of human disease or as an environmental contaminant along the North American, African, and Mediterranean coasts (2, 4, 7). Although V. parahaemolyticus most often induces a self-limiting, watery diarrhea, it occasionally causes bloody diarrhea and, rarely, sudden cardiac arythmia (12). A protein secreted by V. parahaemolyticus, known as thermostable direct hemolysin (TDH), has received considerable attention in past decades as the main pathogenic factor. Although originally studied for its hemolytic property, TDH has been long suspected to be an enterotoxin involved in most cases of V. parahaemolyticus diarrhea. Additionally, an epidemiological role was also attributed to Trh, a TDH-related hemolysin (3, 13, 18). The link between TDH and secretory diarrhea was first demonstrated by Nishibuchi and coworkers (22), who, combining molecular genetics with an in vitro rabbit model, showed that only those strains expressing the TDH-encoding gene are able to induce intestinal chloride secretion. Using the same animal model, our group then found that TDH is one of the few enterotoxins produced by a human pathogen whose action is mediated by intracellular calcium (24). We later showed that TDH also raises the cytosolic free calcium concentration ([Ca2+]i) in nontransformed rat intestinal IEC-6 cells (5). TDH has also cardiotoxic (11) and cytotoxic (25) effects; the latter, in particular, have been only partially characterized.
In this in vitro study, we examined the interplay between the cytotoxicity of TDH and the toxin's capacity to induce fluid secretion, in view of the pathophysiological significance of such a link. We used both transformed and nontransformed cell lines in order to identify true epithelial alterations that are difficult to characterize in the whole animal intestine.
MATERIALS AND METHODS
Reagents.
Cell culture chemicals were obtained from GIBCO-Life Technologies (Milan, Italy). Fura-2/AM, SBFI/AM, BAPTA/AM, and Na4BAPTA were purchased from Molecular Probes (Eugene, Oreg.). Highly purified TDH as well as all other reagents of analytical grade were purchased from Sigma (St. Louis, Mo.). According to the manufacturer, 1 hemolytic unit (HU) is the amount of TDH that causes 50% lysis of a 1% suspension of human red blood cells in phosphate-buffered saline at pH 7.0 after incubation at 37°C for 2 h, followed by refrigeration for 12 to 24 h at 4°C. Endotoxin content of the TDH preparation was measured using a commercial kit (Gel-Clot Limulus amebocyte lysate assay; BioWhittaker, Walkersville, Md.). Endotoxin concentration was found to be <0.03 endotoxin units/ml.
Cell culture.
Cultures of IEC-6 cells were performed as previously described (24). Human Caco-2 intestinal cells were kindly donated by Mauro Rossi, University “Federico II,” Naples, Italy. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 25 mmol of glucose per liter and supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 2 mmol of l-glutamine per liter, 1% penicillin-streptomycin, and 1% sodium pyruvate. Cells were maintained in a humidified atmosphere of 5% CO2 in air at 37°C. Single-cell suspensions were obtained from 70 to 80% confluent cultures by incubation with 0.05% trypsin; cells were then seeded at 105 cells/cm2 onto either 13- or 25-mm-diameter glass coverslips or detachable polycarbonate microporous cell culture inserts (Snapwells, 12-mm diameter, 0.4-mm pore size; Costar, Cambridge, Mass.). Because vectorial electrolyte transport requires cells to grow in a polarized fashion with structured intercellular tight junctions, it was necessary to culture Caco-2 cells for 14 days before experiments (21).
Ussing chambers.
Experiments were conducted as previously described by Fasano et al. (6), with minor modifications. Snapwell inserts were mounted between the Perspex half-chambers of a modified Ussing chamber. Monolayers were bathed with Ringer solution (5 ml per half-chamber), kept at 37°C, and gassed with 5% CO2–95% O2. Ringer solution composition (millimoles per liter) was: NaCl, 53; KCl, 5; Na2SO4, 30.5; mannitol, 30.5; Na2HPO4, 1.69; NaH2PO4, 0.3; CaCl2, 1.25; MgCl2, 1.1; and NaHCO3, 25. In chloride-free experiments, chloride ions were replaced by an equivalent concentration of sulfate ions. Where appropriate, cell monolayers were preincubated with BAPTA/AM (17), a cell-permeant intracellular calcium buffer. Transepithelial potential difference (PD) was recorded with a voltmeter-amperometer (World Precision Instruments, Sarasota, Fla.), and electrical resistance (Rt) and short-circuit current (Isc) were calculated as previously described (24). The maximal changes in these parameters recorded after the addition of an agent were defined as peak differences, symbolized as ΔPD, ΔRt, and ΔIsc, respectively. According to Ohm's first law, these variables are related as follows: ΔIsc = ΔPD/ΔRt. Therefore, when resistance remains constant, an increase in ΔPD must be accompanied by an increase in ΔIsc. The net secretion of negatively charged ions (such as Cl−) from the serosal to the mucosal compartment results in an increase in Isc. Therefore, an Isc increase in the presence but not in the absence of chloride ions in the bathing buffer is considered a reliable indicator of the secretory effect of putative enterotoxins (24). To verify cell viability at the end of each experiment, 10−4 M epinephrine was added to the serosal side to check for a brisk response.
TEER measurements.
Monitoring of transepithelial electrical resistance (TEER) over a 48-h period was performed by placing the entire Snapwell into a resistance chamber (Snap-Endhom; World Precision Instruments) connected to a voltmeter (Millipore). A 1.0-cm2 planar electrode was used for all measurements. The screw cap allowed the planar electrode to remain at the same distance from the monolayers in repeated measurements.
LDH release assay.
Cytotoxicity was measured by cell lactate dehydrogenase (LDH) release as previously described (19). Briefly, culture supernatants were harvested in a 96-well flat-bottom assay plate. A substrate mixture consisting of 0.05 M l-(+)-lactic acid, 7 × 10−4 M p-iodonitrotetrazolium violet, and 3 × 10−4 M NAD in 0.2 M Tris buffer (pH 8.2) was added for 30 min at room temperature. Plates were read at 450 nm using an enzyme-linked immunosorbent assay reader (model DV990; Giò De Vita, Rome, Italy). Cytotoxicity calculations were based on the following formula; cytotoxicity (%) = 100 × (Asample − Aspontaneous)/(Atotal − Aspontaneous) where Asample is the optical density (OD) of the treated cells, Aspontaneous is the OD reading of untreated cells, and Atotal is the OD reading of treated cells lysed for maximal LDH release with Triton X-100 at a final concentration of 1%. The OD reading of blank wells is subtracted from all other readings.
Microspectrofluorimetry assay.
Fluorescent indicators were used to monitor changes in [Ca2+]i or [Na+]i as previously described (10, 24). Cells grown on no. 1 glass coverslips were incubated for 60 to 90 min in bicarbonate-buffered (pH 7.4), serum-free DMEM containing 2 μM fura-2/AM or SBFI/AM, under 5% CO2–95% O2 at 28°C. Indicator-loaded cells were transferred into HEPES-buffered (pH 7.4), serum-free DMEM, in which all fluorescence experiments were performed at 34 to 35°C. The divalent cation Mn2+ binds to fura-2 indicator, but unlike Ca2+, its binding quenches fura-2 fluorescence, and so loss of total fluorescence intensity becomes an indicator of increases of [Mn2+] (7). To monitor whether Mn2+ can enter cells after TDH challenge and quench the fluorescence of cytosolic fura-2, IEC-6 cells were bathed in medium containing 50 μM Mn2+. All measurements were performed on a Nikon Diaphot inverted microscope (40× CF Fluor objective; numerical aperture, 1.30; Nikon Corp.) coupled to a spectrofluorimeter (CM1T10I; SPEX Industries, Edison, N.J.) operating in the microfluorimetry mode. Data acquisition and analysis were performed with DM3000 software (SPEX Industries) running on a dedicated personal computer.
Scanning electron microscopy.
At the end of the 48-h TEER measurements, monolayers were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at room temperature for 20 min. Following postfixation in 1% OsO4 for 30 min, cells were dehydrated through graded ethanols, critical-point dried in CO2, gold coated by sputtering, and examined with a Cambridge 360 scanning electron microscope (Assing, Italy).
Statistical analysis.
Results are presented as means ± standard deviations. The data were analyzed using one-way analysis of variance, and P < 0.05 was considered statistically significant.
RESULTS
TDH induces a variation in ionic homeostasis in IEC-6 cells.
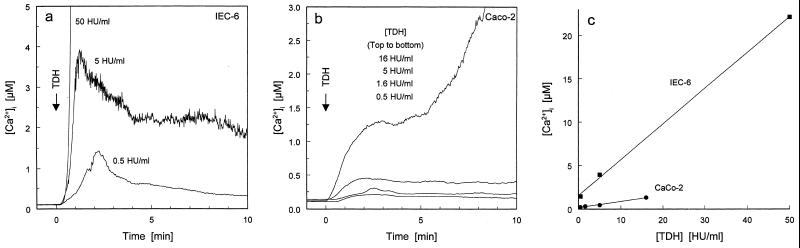
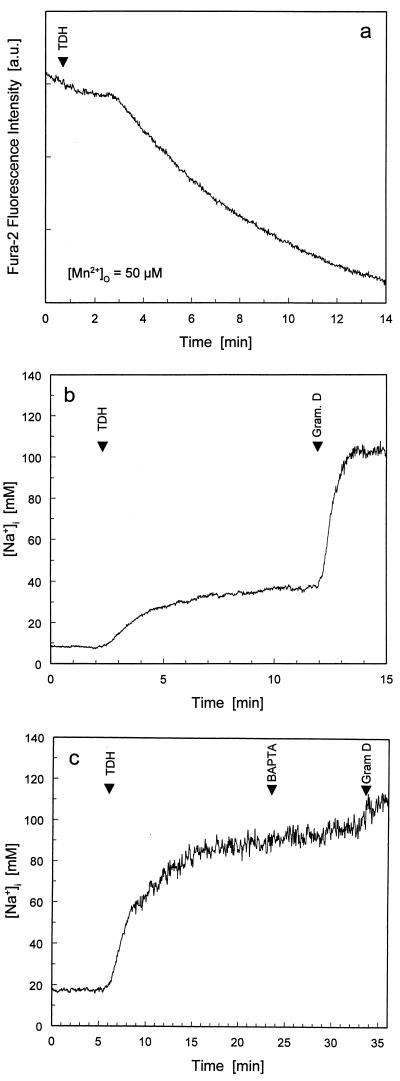
We recently reported that TDH causes the entry of calcium ions from the external medium (5) into rat nontransformed intestinal IEC-6 cells. To validate our experimental system, we assayed different doses of TDH for their effectiveness in increasing [Ca2+]i. Although all applied doses elevated [Ca2+]i, only the lowest toxin concentration caused a rapid and reversible response (Fig. 1a). Human transformed intestinal Caco-2 cells showed a qualitatively similar dose-response curve, but with a quantitative difference (Fig. 1b). A comparison between the responses in IEC-6 cells and Caco-2 cells is shown in Fig. 1c, where the highest [Ca2+]i reached in the first 3 min after TDH challenge is plotted against toxin concentration. These plots demonstrate that TDH is more effective at elevating [Ca2+]i in IEC-6 cells than in Caco-2 cells; hence, the former cell line was used for subsequent microspectrofluorimetry experiments. Previous observations on erythrocytes suggested that TDH-mediated ionic influx was not calcium specific (16). To check this hypothesis in our intestinal model, we monitored two cations, Mn2+ and Na+, for which fluorescent indicator methodology is well established. In the presence of extracellular Mn2+, TDH addition markedly accelerated the quenching of intracellular fura-2 fluorescence, suggesting that Mn2+ influx is promoted by the action of TDH (Fig. 2a). To monitor TDH-mediated changes in [Na+]i in IEC-6 cells, we used the fluorescent indicator for Na+, SBFI (9). Application of TDH at 0.5 HU/ml, a concentration that reversibly raised [Ca2+]i, increased [Na+]i by tens of millimolar above resting levels (the rise was between 30 and 80 mM) (Fig. 2b). Subsequent addition of gramicidin D (Fig. 2b), which forms high-conductance transmembrane pores that are highly selective for monovalent cations, allowed equilibration of [Na+]i with extracellular Na+ concentration ([Na+]o) (109 mM in DMEM). The TDH-induced rise in [Na+]i was completely unaffected when [Ca2+]o was lowered by chelation with Na4BAPTA (Fig. 2c). Furthermore, intracellular BAPTA loading, which is extremely effective in abolishing TDH-induced rises in [Ca2+]i (24), had no effect on the TDH-induced rise in [Na+]i (data not shown). Together, these findings indicate that TDH, besides causing a rise in intracellular free calcium concentration, can also induce influx of other divalent and monovalent cations—the latter occurring independently of [Ca2+]i or [Ca2+]o.
FIG. 1.
Dose-response study of the ability of TDH to raise [Ca2+]i in cultured intestinal cells. TDH at the concentrations indicated was bath applied to IEC-6 (a) and Caco2 (b) cells loaded with fura-2 indicator. The time course of change of [Ca2+]i was monitored through the Ca2+-sensitive fluorescence of fura-2. Panel c shows that IEC-6 cells are more susceptible to the [Ca2+]i-elevating effect of TDH. The graph in panel c was constructed by plotting the highest [Ca2+]i reached within 3 min of TDH application against the concentration of TDH used.
FIG. 2.
TDH-induced ion influx pathway is not specific for Ca2+. (a) Sum of fura-2 fluorescence emissions at 340- and 380-nm excitation in IEC-6 cells was monitored in the presence of 50 μM Mn2+ in the extracellular medium. After a typical latency, TDH application (0.5 HU/ml) induced rapid quenching of intracellular fura-2 fluorescence, indicating Mn2+ entry into the cells. Fluorescence intensity values are direct readings from the spectrofluorimeter and are thus expressed in arbitrary units (a.u.). (b) TDH elevates [Na+]i. TDH induces gradual elevation of [Na+]i. Addition of 10 μM gramicidin (Gram.) D, a Na+ ionophore, allowed [Na+]i to equilibrate with extracellular [Na+]. (c) TDH-induced rise in [Na+]i is not dependent on extracellular Ca2+. Addition of 2.5 mM Na4BAPTA to buffer extracellular [Ca2+] to <1 μM had no effect on the rise in [Na+]i induced by 0.5 HU of TDH per ml. This shows that TDH-induced Na+ influx is not dependent on the presence of extracellular Ca2+. All experiments were conducted with IEC-6 cells.
TDH causes chloride secretion and transepithelial electrical changes in Caco-2 monolayers.
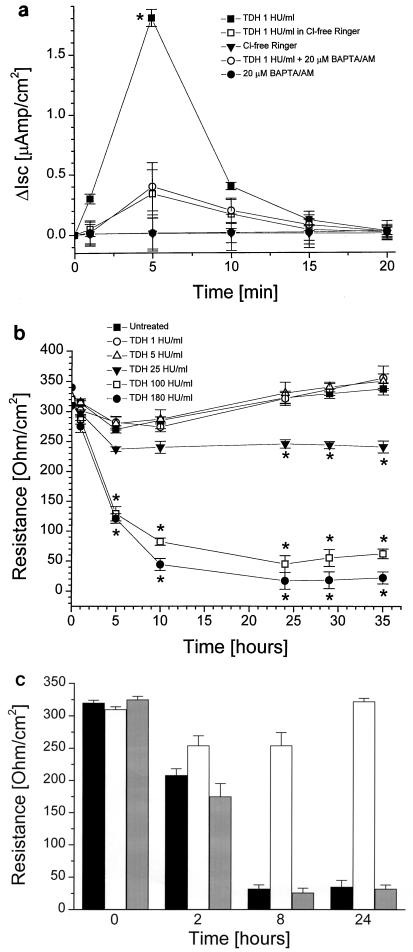
Unlike IEC-6 cells, 14-day-old Caco-2 cells form tight junctions in a functional epithelial barrier (21). Incubation with 1 HU of TDH per ml elicited a rapid, short-lived increase in Isc (Fig. 3a) when applied to the mucosal side of the monolayer. These electrical changes were both abolished when chloride was substituted with sulfate ions in the bathing buffer or when 20 μM BAPTA/AM was added 20 min before the toxin (Fig. 3a). These data provide evidence that at the above-cited dosage, TDH induces Ca2+-dependent chloride secretion by Caco-2 cell monolayers. To evaluate the functional integrity of the Caco-2 cell monolayers exposed to increasing toxin concentrations, TEER time courses at different TDH doses were constructed. As depicted in Fig. 3b, TDH concentrations that elicit chloride secretion and/or a reversible increase of [Ca2+]i did not decrease the monolayer's electrical resistance. In contrast, toxin concentrations that cause an irreversible increase of [Ca2+]i induced a rapid disruption of the integrity of the monolayer, as evidenced by a dramatic drop in the electrical resistance of the monolayer. Such damage is not prevented by preincubation with 20 μM BAPTA/AM (Fig. 3c).
FIG. 3.
Ussing chamber experiments on Caco-2 cell monolayers. (a) TDH (1 HU/ml) induced a rapid, short-lived rise in ΔIsc that was abolished either by preincubation with 20 μM BAPTA/AM or in CI−-free Ringer solution (n = 5). (b) Effect of increasing TDH concentrations on Caco-2 monolayer TEER (n = 5). (c) Preincubation with 20 μM BAPTA/AM, alone (white bars) or followed by treatment with TDH (100 HU/ml) (gray bars), caused monolayer disruption comparable to that induced by TDH (100 HU/ml), alone black bars (n = 5). Data differing significantly from control are marked by ∗.
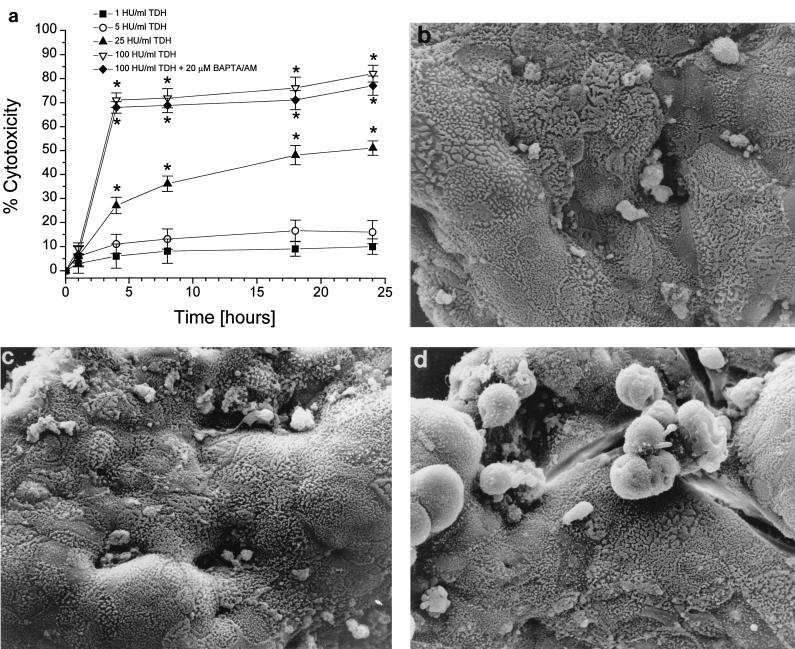
TDH induces cytotoxicity in CaCo-2 cells.
Low TDH concentrations induced LDH release that was not different from negative control values (data not shown). Increasing toxin doses proportionally induced a rapid death of cells, a phenomenon which was not counteracted by preincubation with 20 μM BAPTA/AM (Fig. 4a). Thus, buffering [Ca2+]i cannot counteract the permeabilizing effect of TDH. When a morphological analysis was performed by scanning electron microscopy, control Caco-2 cells adhered well to each other and showed numerous microvilli on the apical surface (Fig. 4b). Exposure to low toxin doses caused no significant change in the general morphology of the monolayer (Fig. 4c). In contrast, monolayers treated with 100 HU of TDH per ml showed significant rounding and detachment of cells (Fig. 4d).
FIG. 4.
LDH release measurements and scanning electron microscopy of Caco-2 cells. (a) TDH dose-response curve. While low TDH concentrations had no effect on the monolayer integrity, high toxin doses exhibited a calcium-independent cytotoxicity (n = 4). Micrographs show Caco-2 monolayers, untreated (b) or treated with either 1 (c) or 100 (d) HU of TDH per ml. While the lower toxin concentration caused no damage to the monolayer, the higher TDH concentration induced evident monolayer disruption and cellular structural changes. Data differing significantly from control are marked by ∗.
DISCUSSION
Although the clinical picture of V. parahaemolyticus infection is generally limited to mild gastroenteritis, more aggressive behavior of this agent has also been described (11, 12, 20). Hlady and Klotzy estimated that 8% of V. parahaemolyticus infections result in primary septicemia (10). Reports of invasive infections have also appeared in the Asian (15) and American (8) literature. The pathogenicity of V. parahaemolyticus has mostly been linked to TDH. The present paper adds novel information about the toxin's potential mechanism of action in the intestine and suggests a hypothesis about its role in human disease. We previously found that TDH acts as a calcium-dependent enterotoxin in rabbit intestinal mucosal preparations (24). We now know that the toxin is able to induce chloride secretion in Caco-2 cell monolayers, a functionally faithful and widely accepted model of the human intestinal epithelium. This direct effect on epithelial physiology is calcium mediated, as the BAPTA/AM experiments show. Electrophysiological, biochemical, and morphological evidence together show that a clear cytotoxic effect is observed only after challenging the monolayers with high toxin doses. Taken together, our results suggest that TDH acts as a porin (14) in the enterocyte's plasma membrane and allows the influx of multiple ionic species. When the number of TDH-generated porin channels is low, the cell's homeostatic capacity to counterbalance ion influx keeps the intracellular calcium concentration within a range compatible with the modulation of several functions, including cytoskeletal rearrangements (5) and ion secretion (24), without affecting cell viability. However, at high TDH concentrations, the number of channels could increase to overwhelm the cell's capacity to compensate, and the resulting massive ionic influx causes irreversible cell swelling and death (Fig. 4d). Therefore, cell death is the consequence of gross osmotic imbalance rather than the result of specific calcium signaling (26). These data support the idea that TDH, when locally present in high concentrations, may play a role in disrupting the epithelial barrier and in allowing vibrios to invade the host. Such high toxin concentrations could, for instance, develop in a stagnant intestinal loop where impaired luminal clearing and high concentrations of bile acids, which are known to enhance TDH production (23), could both raise the TDH concentration. Our data, however, do not exclude the possibility that a factor other than TDH (or the TDH-related toxin Trh) may contribute to the invasiveness of V. parahaemolyticus. This possibility arises from the recent work by Akeda et al., who, also using Caco-2 cells, showed that V. parahaemolyticus strains possess a cytotoxic factor that acts on the cell cytoskeleton in a calcium-independent fashion (1). TDH cytotoxicity is also calcium independent, as data from this paper and from other investigators (26) reveal. Confirming our previous results, we observed that TDH induced calcium flux into intestinal epithelial cells irrespective of the animal species, with nontransformed cells showing greater sensitivity to the toxin. The ion influx mechanism activated by TDH appears not to be specific for Ca2+. Mn2+ influx is allowed, and remarkably, Na+ influx is also activated by TDH in a calcium-independent manner. A similar cation influx was shown by Huntley and Hall (16), who studied the hemolytic process induced by TDH. These data, together with the results of our microspectrofluorimetry experiments, support the idea that TDH can act as an ionophore in the plasma membranes of red blood cells, enterocytes, and fibroblasts (data not shown) of different animal species (14). For the intestine, we propose the following molecular mechanism of action. When low toxin concentrations are present in the intestine, the limited amount of TDH can activate the nonspecific ion influx mechanism in enterocytes to only a limited extent. Ion-transporting ATPases in the enterocyte ultimately counteract the TDH-induced ion influx to prevent permanent damage to the cell. At high intraluminal concentrations, TDH could activate a massive, nonspecific ion influx through the enterocyte membrane. This ion influx would overwhelm the cell's extrusion mechanisms, leading to osmotic swelling, cell rounding, and death. The ensuing breach of the epithelial barrier enables the toxin and/or the bacteria to enter the bloodstream.
The results presented here not only extend our knowledge on the cytotoxic and enterotoxic properties of TDH but enable us to design further experiments to confirm the hypothesized mode of action of this virulence factor and its role in the pathogenesis of human gastroenteritis associated with consumption of seafood contaminated by V. parahaemolyticus.
ACKNOWLEDGMENTS
This work was supported by grants DK483373 to A.F. and GM46956 to J.P.Y.K. from the National Institutes of Health, 96.03.153 to F.R. from the Italian National Research Council (CNR), and 97.01187.PF49 from CNR Targeted Project “Biotechnology,” subproject 2.
REFERENCES
- 1.Akeda Y, Nagayama K, Yamamoto K, Honda T. Invasive phenotype of Vibrio parahaemolyticus. J Infect Dis. 1997;176:822–824. doi: 10.1086/517312. [DOI] [PubMed] [Google Scholar]
- 2.Barbieri E, Falzano L, Fiorentini C, Pianetti A, Baffone W, Fabbri A, Matarrese P, Casiere A, Katouli M, Kuhn I, Mollby R, Bruscolini F, Donelli G. Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl Environ Microbiol. 1999;65:2748–2753. doi: 10.1128/aem.65.6.2748-2753.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherwonogrodzky J W, Clark A G. The purification of the Kanagawa haemolysin from Vibrio parahaemolyticus. FEMS Microbiol Lett. 1982;15:175–179. [Google Scholar]
- 4.Eko F O, Udo S M, Antia-Obang O E. Epidemiology and spectrum of vibrio diarrheas in the lower cross river basin of Nigeria. Cent Eur J Public Health. 1994;2:37–41. [PubMed] [Google Scholar]
- 5.Fabbri A, Falzano L, Frank C, Donelli G, Matarrese P, Raimondi F, Fasano A, Fiorentini C. Vibrio parahaemolyticus-thermostable direct hemolysin modulates cytoskeletal organization and calcium homeostasis in intestinal cultured cells. Infect Immun. 1999;67:1139–1148. doi: 10.1128/iai.67.3.1139-1148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasano A, Verga M C, Raimondi F, Guandalini S. Effects of deconjugated bile acids on electrolyte and nutrient transport in the rabbit small intestine in vitro. J Pediatr Gastroenterol Nutr. 1994;18:327–333. doi: 10.1097/00005176-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Grynkiewicz G, Poenie M, Tsien R Y. A new generation of Ca+2 indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 8.Hally R J, Rubin R A, Fraimow H S, Hoffmann-Terry M L. Fatal Vibrio parahaemolyticus septicemia in a patient with cirrhosis: a case report and review of the literature. Dig Dis Sci. 1995;40:1257–1260. doi: 10.1007/BF02065534. [DOI] [PubMed] [Google Scholar]
- 9.Harootunian A T, Kao J P, Eckert B K, Tsien R Y. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989;264:19458–19467. [PubMed] [Google Scholar]
- 10.Hlady W G, Klontz K C. The epidemiology of Vibrio infections in Florida, 1981–1993. J Infect Dis. 1996;173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 11.Honda T, Goshima K, Takeda Y, Sugino Y, Miwatani T. Demonstration of the cardiotoxicity of the thermostable direct hemolysin (lethal toxin) produced by Vibrio parahaemolyticus. Infect Immun. 1976;13:163–171. doi: 10.1128/iai.13.1.163-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda T, Takeda Y, Miwatani T, Kato K, Nimura Y. Clinical features of patients suffering from food poisoning due to Vibrio parahaemolyticus, especially on changes in electrocardiograms. J Jpn Assoc Infect Dis. 1976;50:216–223. doi: 10.11150/kansenshogakuzasshi1970.50.216. [DOI] [PubMed] [Google Scholar]
- 13.Honda T, Ni Y, Miwatani T. Purification and characterization of a hemolysin produced by a clinical isolate of Kanagawa phenomenon-negative Vibrio parahaemolyticus and related to the thermostable direct hemolysin. Infect Immun. 1988;56:961–965. doi: 10.1128/iai.56.4.961-965.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda T, Ni Y, Miwatani T. The thermostable direct hemolysin of Vibrio parahaemolyticus is a pore-forming toxin. Can J Microbiol. 1992;38:1175–1180. doi: 10.1139/m92-192. [DOI] [PubMed] [Google Scholar]
- 15.Hsu G J, Young T, Peng M Y, Chang F Y, Chou M Y. Septicemia caused by Vibrio parahaemolyticus; a case report. Chung-Hua I Hsueh Tsa Chih (Taipei) 1993;52:351–354. [PubMed] [Google Scholar]
- 16.Huntley J S, Hall A C. Aspects of the haemolytic reaction induced by Kanagawa haemolysin of Vibrio parahaemolyticus. Toxicon. 1994;32:1397–1412. doi: 10.1016/0041-0101(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 17.Kao J P Y. Practical apects of measuring [Ca2+]i with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- 18.Kaper J B, Campen R K, Seidler R J, Baldini M M, Falkow S. Cloning of the thermostable direct or Kanagawa phenomenon-associated hemolysin of Vibrio parahaemolyticus. Infect Immun. 1984;45:290–292. doi: 10.1128/iai.45.1.290-292.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korzeniewski C, Callaewart D M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–319. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 20.Miwatani T, Takeda Y. Vibrio parahaemolyticus' epidemiology, ecology and biology. In: Miwatani T, Takeda Y, editors. Vibrio parahaemolyticus, a causative bacterium of seafood poisoning. Tokyo, Japan: Saiko; 1975. pp. 22–24. [Google Scholar]
- 21.Nath S K N, Desjeux J F. Human intestinal cell lines as in vitro tools for electrolyte transport studies with relevance to secretory diarrhoea. J Diarrhoeal Dis Res. 1990;8:133–142. [PubMed] [Google Scholar]
- 22.Nishibuchi M, Fasano A, Russell R G, Kaper J B. Enterotoxigenity of Vibrio parahaemolyticus with and without genes encoding thermostable direct hemolysin. Infect Immun. 1992;60:3539–3545. doi: 10.1128/iai.60.9.3539-3545.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osawa R, Yamai S. Production of thermostable direct hemolysin by Vibrio parahaemolyticus enhanced by bile acids. Appl Environ Microbiol. 1996;62:3023–3025. doi: 10.1128/aem.62.8.3023-3025.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raimondi F, Kao J P Y, Kaper J B, Guandalini S, Fasano A. Calcium dependent intestinal chloride secretion by Vibrio parahaemolyticus thermostable direct hemolysin in a rabbit model. Gastroenterology. 1995;109:381–386. doi: 10.1016/0016-5085(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 25.Sakazaki R, Tamura K, Nakamura A, Kurata T, Ghoda A, Kazuno Y. Enteropathogenic activity of Vibrio parahaemolyticus. In: Fujino T, Sakaguchi G, Sakazachi R, Takeda T, editors. International Symposium on Vibrio parahaemolyticus. Tokyo, Japan: Saikon; 1974. pp. 231–235. [Google Scholar]
- 26.Tang G Q, Iida T, Yamamoto K, Honda T. Ca2+-independent cytotoxicity of Vibrio parahaemolyticus thermostable hemolysin (TDH) on Intestine 407, a cell line derived from human embryonic intestine. FEMS Microbiol Lett. 1995;134:233–238. doi: 10.1111/j.1574-6968.1995.tb07943.x. [DOI] [PubMed] [Google Scholar]