Abstract
In the current research context of precision treatment of malignant tumors, the advantages of immunotherapy are unmatched by conventional antitumor therapy, which can prolong progression-free survival and overall survival. The search for new targets and novel combination therapies can improve the efficacy of immunotherapy and reduce adverse effects. Since current research targets for immunotherapy mainly focus on lymphocytes, little research has been done on erythrocytes. Nucleated erythroid precursor stem cells have been discovered to play an essential role in tumor progression. Researchers are exploring new targets and therapeutic approaches for immunotherapy from the perspective of erythroid progenitor cells (EPCs). Recent studies have shown that different subtypes of EPCs have specific surface markers and distinct biological roles in tumor immunity. CD45+ EPCs are potent myeloid-derived suppressor cell-like immunosuppressants that reduce the patient’s antitumor immune response. CD45− EPCs promote tumor invasion and metastasis by secreting artemin. A specific type of EPC also promotes angiogenesis and provides radiation protection. Therefore, EPCs may be involved in tumor growth, infiltration, and metastasis. It may also be an important cause of anti-angiogenesis and immunotherapy resistance. This review summarizes recent research advances in erythropoiesis, EPC features, and their impacts and processes on tumors.
Keywords: tumor, erythroid progenitor cells, immunotherapy, anemia, EPCs
1. Introduction
The targeted tumor microenvironment (TME) has been recognized as a promising cancer surveillance and treatment approach in recent years [1,2]. The body’s internal environment influences the TME but is also adjusted and re-edited by the TME [3]. The TME includes tumor cells, peripheral immune cells, neovascularization, endothelial cells, fibroblasts, and extracellular matrix [4]. Erythroid progenitor cells (EPCs) in the TME can suppress T cells by producing reactive oxygen species (ROS), interleukin (IL)-10, and transforming growth factor β (TGF-β) in a paracrine and intercellular manner. Their ability to suppress T-cell immunosuppression is more robust than myeloid-derived suppressor cells (MDSCs) [5,6]. Erythrocytes are the most abundant blood cells in the peripheral blood, providing a stable supply of oxygen and an essential part of the immune system [7,8]. In addition, reduced erythrocyte volume can lead to anemia, which is common in patients with advanced malignancy. Numerous clinical studies have shown that anemia is an independent risk factor for poor prognosis in tumor patients. Therefore, erythroid cells and tumors are closely related. Since mature erythrocytes do not have a nucleus, they are almost unaffected by extracellular factors. It is difficult for medical interventions to rapidly and effectively influence erythrocytes. EPCs are the progenitor stem cells of the red lineage and play an essential biological role in tumor progression. Therefore, researchers have explored the relevance of EPCs to tumors, aiming to provide new targets for diagnosing and treating malignant tumors from the perspective of the erythroid lineage.
2. The role of erythroid cells in tumor immunity
As an essential component of the immune system [7,8], erythrocytes express various immune-related molecules and are involved in many immune responses and immune regulation. They also play an essential role in tumor immunity. Human erythroid complement receptor 1 (CR1) [9] on the surface of erythrocytes binds to C3b for immune adhesion clearance, prophagocytosis, and lymphocyte regulation. Erythrocytes also produce cytokines or specific signaling molecules to regulate the immune response. Erythroid cells form rose junctions with invasive pathogens (including tumor cells and bacteria) and facilitate their elimination as macrophages pass through the liver and spleen. Erythroid cells re-enter the bloodstream [10,11]. Thus, the immunity mediated by erythrocytes may prevent cancer cells from spreading through the bloodstream.
In addition, erythrocytes contain antioxidants that reduce the autotoxic effects of free radicals released by phagocytes during phagocytosis, and it also increases the phagocytic effect of phagocytes [12]. Erythrocytes express the Duffy chemokine antigen/receptor (DARC), which removes angiogenic chemokines from the prostate TME and thus regulates prostate tumor growth [13]. Consequently, erythrocytes can bind cytokines released by tumor cells [14]. Healthy human erythrocytes promote T-lymphocyte proliferation and protect lymphocytes from apoptosis [15]. CD58 on the erythrocyte membrane can interact with CD2 on the lymphocyte membrane to induce cytokine production by T lymphocytes and indirectly promote B lymphocyte proliferation and differentiation [16,17]. These results demonstrate the critical role of erythrocytes in regulating the immune system [14]. The cytoplasm of erythrocytes contains the Natural Killer Enhancing Factor (NKEF), which significantly enhances the killing effect of NK cells on K562 cells [18].
Previous studies have found that cancer cells alter the cytokine profile and immune function of erythrocytes. Researchers exposed erythrocytes to non-small-cell lung cancer cell lines to change the erythrocytes and their cytokine profile. It was found that in the presence of these altered erythrocytes, T-cell proliferation was stimulated to a more significant extent and was no longer protected by stimulus drive. It is driven to release a variety of cancer-related cytokines [14]. The multiple immune mechanisms of erythrocytes protect them from suppressive or adverse host responses, and erythrocytes are suitable as carriers for drug delivery [19]. Several oncology drugs delivered via nano-erythrocytes have shown promising antitumor efficacy [20,21,22] (Figure 1).
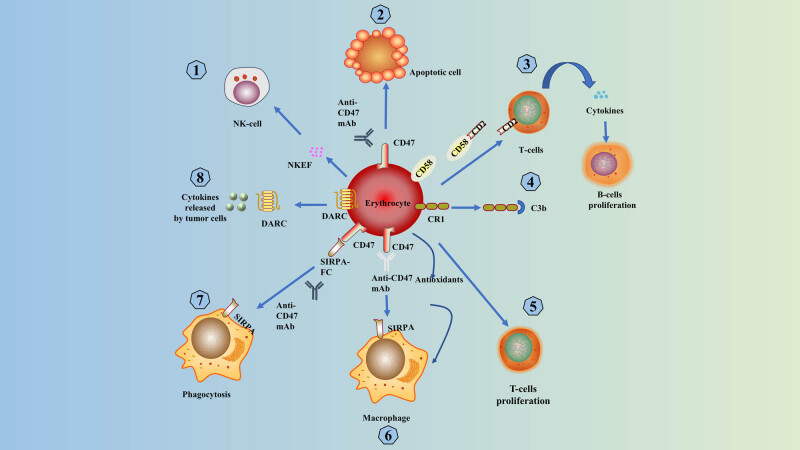
Figure 1.
Primary mechanisms of erythrocyte-mediated immunity: (1) NKEF in erythrocyte cytoplasm enhances the killing ability of NK-cells; (2) erythrocytes with CD47 unbound anti-CD47 mAb show apoptosis; (3) CD58 on the erythrocyte membrane can interact with CD2 on the lymphocyte membrane, thus inducing cytokine production by T lymphocytes and promoting the proliferation and differentiation of B lymphocytes; (4) erythrocytes complete IAC through CR1 binding to C3b; (5) erythrocytes in healthy humans can promote T-lymphocyte proliferation; (6) antioxidants contained in erythrocytes increase phagocytosis; (7) signal regulatory protein α interacts with CD47 to also promote phagocytosis of apoptotic cells [23]; and (8) erythrocytes expressed DARC can bind and scavenge cytokines released by tumor cells.
3. Cancer-associated anemia
Anemia is one of the most common complications of cancer, with 30–90% of cancer patients suffering from anemia [24]. Among hematologic tumors, the rate of anemia is high, and clinical studies have shown that approximately 45% of lymphoma patients develop anemia [25]. There is a rich network of blood vessels in the gastrointestinal mucosa or lung tissue. Cancer cells can destroy these blood vessels early, leading to chronic blood loss. Therefore, lung cancer, gastric, and early-stage colon cancer patients may have combined anemia. The prognosis is often poor. Many long-term clinical studies have shown that patients with malignant tumors present with anemia [26]. Statistically, patients with anemia have shorter survival times and a 65% increased overall risk of death compared to cancer patients without anemia [27]. Their quality of life and prognosis is significantly worse [28]. For instance, Caro et al. have found anemia substantially decreases the overall survival of patients with nasopharyngeal carcinoma and increases the risk of tumor metastasis [27].
3.1. Mechanisms of anemia in cancer patients
There are two forms of iron deficiency in cancer patients: absolute iron deficiency (AID) and functional iron deficiency (FID). Among more than 40% of oncology patients, FID is present [29]. AID is characterized by depleted iron stores and inadequate iron supply, whereas FID has adequate iron stores but insufficient iron supply to support erythropoiesis and other iron-dependent metabolic pathways [30,31]. FID is a significant cause of anemia in chronic disease, also known as cancer anemia or cancer-related anemia [32,33]. The leading cause of FID in cancer is the release of pro-inflammatory cytokines associated with cancer, such as IL-6, IL-1, tumor necrosis factor α (TNF-α), and interferon-γ (IFN-γ). These cytokines increase the synthesis of hepcidin, which decreases the amount of iron released into the bloodstream [32]. Myelosuppression by radiation, chemotherapy, and erythropoietin (EPO) stimulant agents for anemia can cause FID [30,32]. A common complication in cancer patients, chronic kidney disease, can cause FID by decreasing erythropoiesis and increasing hepcidin levels [34,35].
Patients with long-term tumors are often associated with chronic systemic inflammation, and multiple cytokines can lead to stalled maturation of early EPCs [36]. Tumor cells produce pro-inflammatory cytokines (e.g., TNF-α, IL-1, IL-6) that cause anemia, reduce erythrocyte lifespan, and alter energy metabolism by altering iron homeostasis, inhibiting erythropoiesis and blocking EPO synthesis, and affecting EPO activity [37]. It also inhibits EPO production and decreases the responsiveness of bone marrow hematopoietic stem cells (HSCs) and EPCs to EPO, leading to impaired erythropoiesis [38]. Recent studies have shown that TNF-α can upregulate PU.1 and GATA2 in HSPCs to inhibit erythroid differentiation and lead to anemia [39]. IFN-γ induces differentiation arrest in EPCs and shortens the lifespan of erythrocytes, which causes anemia [40]. In addition to the above-mentioned pro-inflammatory cytokines, elevated plasma VEGF concentrations stimulated increased EPO secretion and elevated circulating reticulocyte indices, which promote early EPC expansion in the bone marrow and spleen [41].
3.2. Mechanisms of the pro-tumor effect of anemia
The specific mechanism of anemia’s pro-tumor effect is unknown, and it may be multifaceted. Hypoxia may be one of the essential mechanisms. First, hypoxia may alter the intra- and extracellular distribution of chemotherapeutic drugs, enhance the expression of various drug resistance genes, and reduce the sensitivity of tumor cells to radiotherapy; second, hypoxia may activate angiogenesis, which enhances the invasiveness and metastatic risk of tumors and increases tumor survival. Furthermore, hypoxia promotes the production of cytokines and chemokines, which recruit tumor-promoting immune cells and weaken the tumor immune response [42,43,44]. All of the mechanisms mentioned above affect the treatment of tumors. Some studies have also found that anemia can increase the production of various growth factors and matrix-degrading enzymes and stimulate angiogenesis. The new blood vessels can provide nutrients and oxygen for tumor growth, the main pathway for tumor invasion and metastasis. Anemia can also impair immune function, weakening the anti-tumor immune response and significantly reducing the patient’s ability to defend against pathogenic infections. Studies have shown that the percentage of EPCs in peripheral blood is significantly increased in malignant tumor patients with anemia. EPCs in peripheral blood may promote tumor progression and metastasis by reducing immunity and releasing specific cytokines. We, therefore, consider that promoting the differentiation of EPCs, treating anemia, and reducing ineffective erythropoiesis may be a therapeutic strategy used to restore anti-tumor immunity and lessen the pro-tumor effects of EPCs.
4. The role of EPCs in tumors
The first report on tumors and EPCs was published in the 1980s by Geissler et al. They found that the percentage of EPCs in peripheral blood was significantly increased in patients with acute and chronic lymphocytic leukemia [45]. EPCs in peripheral blood increased dramatically in patients with combined anemia and negatively correlated with hemoglobin [46]. This may be due to the direct inhibition of erythroid differentiation by TME or some cytokines from tumor cells [46], as well as the significant decrease in EPO concentration in tumor patients, which blocks the differentiation pathway from EPCs to erythrocytes [46], increasing EPCs in peripheral blood and a decrease in mature erythroid cells. Hence, tumor patients present with anemia at the same time.
4.1. Surface markers of EPCs
Although EPCs were defined as early as 1970, few studies have detailed their cellular and molecular characteristics. According to the available studies, EPCs can express some surface markers, including IL-3R, FLT3, MPL, CD36, CD41, CD71, CD105, and CD235a [6,46,47,48,49,50]. After EPCs mature, CD71 [51] and CD45 [52] also disappear. Cells with CD71+CD235a+ and CD71+TER119+ have been defined by researchers as EPCs in human and mouse peripheral blood and spleen [46,47].
4.2. The role of CD45+ EPCs in tumors
In recent years, the role of EPCs in tumorigenesis and progression has attracted the attention of researchers. Research has reported an immunosuppressive cell of red lineage origin, CD45+ EPCs (CD45+CD71+CD235a+), similar to MDSCs [53], regulatory T cells (Tregs) [54], and tumor-associated macrophages [55]. CD45+ EPCs were significantly increased in patients with advanced tumors combined with anemia in peripheral blood. CD45+ EPCs produce arginase-2 [56], IL-10, and ROS [5], which inhibit T-cell proliferation and production of IFN-γ [57]. CD45+ EPCs also function as immunosuppressors in a direct cell-to-cell contact and paracrine manner. This has caused a decrease in antiviral, antibacterial, and antitumor immune responses in tumor patients and has contributed to tumor progression. Subsequently, researchers systematically analyzed the composition of immune cells in the spleen of tumor-bearing mice. They found that, in addition to elevated MDSCs and Tregs, EPCs were abundantly accumulated in the spleen. In mice bearing a tumor and aged 21–28 days, EPCs accounted for as much as 50% of the total spleen cell composition [46]. Moreover, EPCs expanded in the spleen, peripheral blood, and liver of tumor-bearing mice and cancer patients [5]. CD45+ EPCs can infiltrate murine and human tumors, and their abundance in TME is much higher than that of MDSCs or Tregs [5,6,46], and CD45+ EPCs also inhibit T cells more than MDSCs [5].
Previous studies have suggested that the spleen is the organ of origin of CD45+CD71+CD235+ EPCs. Hepatocellular carcinoma (HCC) could be a circulating origin or produced directly in HCC tissues [5]. The higher abundance of CD45+CD71+EPCs in HCC tissues, the more important their immunosuppressive capacity is, and they are closely associated with multiple prognostic factors. Therefore, immunofluorescence screening of CD45+CD71+ EPCs in tumor tissues may be a new clinical method to predict tumor recurrence after radical surgery for HCC [5].
The rapid proliferation of tumor cells leads to local hypoxia of the microenvironment. Under hypoxic conditions, CD45⁺ EPCs can regulate lipid metabolism and enhance energy metabolism in lymphoma cells via the AMPK–ACC–CPT1A pathway, which further promotes cell proliferation and inhibits apoptosis in lymphoma cells. Animal experiments have also shown that transplantation of CD45⁺ EPCs significantly increased resistance to antiangiogenic drugs, and ROS played a vital role in these processes. Therefore, CD45+ EPCs in lymphoma may enhance resistance to antiangiogenic drugs in tumors through ROS [58].
4.3. The role of CD45− EPCs in tumors
An EPC (also called Ter cells) with a phenotype of CD45−Ter119+CD71+ is enriched in the enlarged spleen of the cancerous host; CD45− EPCs are more mature EPCs than CD45⁺ EPCs. Since CD45− EPCs have no direct inhibitory effect on CD4+CD8+ T cells [5], they do not promote tumor progression by inhibiting antitumor immunity. It promotes tumor invasion and metastasis through the secretion of artemin into the blood [59,60,61] as artemin promotes the proliferation of surviving cancer cells and increases tumor cells’ invasiveness [59,62,63,64].
Recent studies have shown that increased serum artemin concentration and receptor expression are associated with poor prognosis in cancer patients. They have demonstrated that CD45− EPCs can promote tumor progression and metastasis through artemin [62]. Patients with pancreatic ductal adenocarcinoma have higher cell counts of CD45− EPCs in the spleen than patients with non-cancerous pancreatic tumors or benign pancreatic masses. Splenic CD45− EPCs activate the GFRα3-ERK signaling pathway by expressing artemin to promote PDAC cell proliferation and invasion. High splenic CD45− EPCs cell counts often indicate poor prognosis and are associated with tumor size and lymph node metastasis [62].
Until now, the clinical applications targeting Artemin and its signaling pathway are still relatively few. The specific mechanism by which artemin promotes tumor growth and distant metastasis also remains undetermined [59]. Han et al. found that inhibition of artemin secretion significantly hindered liver cancer growth and reduced the cancer-promoting ability of CD45− EPCs. In addition, the study found that deletion of GFR-α3 and RET resulted in deficient artemin signaling, including reduced phosphorylation of AKT and ERK. This provides us with a new perspective on treatment and its signaling pathway to inhibit the tumor-promoting effect of CD45− EPCs [65] (Figures 2 and 3).
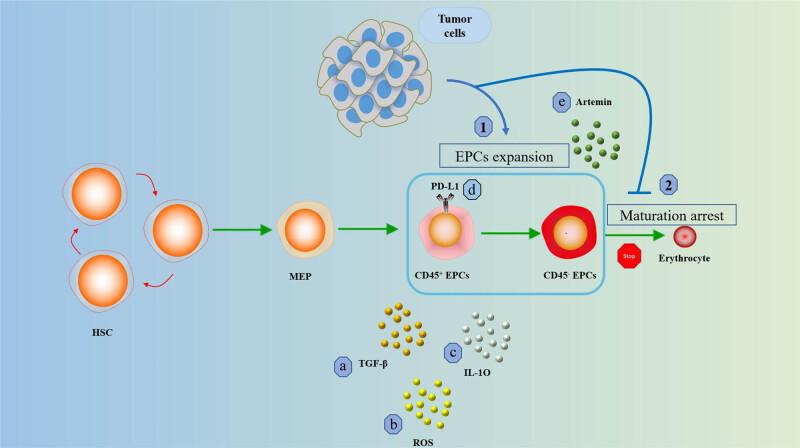
Figure 2.
The role of EPCs in cancer. (1) Tumors promote the expansion of EPCs and (2) tumors inhibit the maturation and differentiation of EPCs. Early CD45+ EPCs use (a) TGF-β, (b) ROS, (c) IL-10 and (d) PD-L1 regulates immune responses. More mature CD45− EPCs regulate cancer progression through (e) secretion of Artemin.
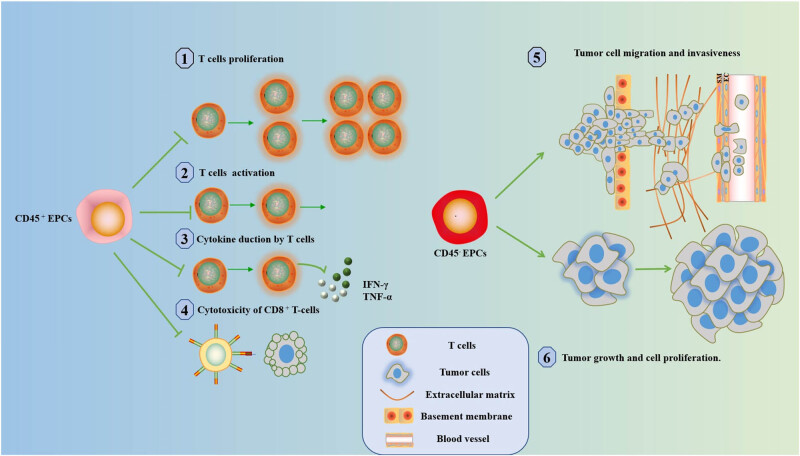
Figure 3.
The role of EPCs n cancer. CD45+ EPCs inhibit (1) T-cell proliferation, (2) T-cell activation, (3) IFN-γ and TNF-α production, and (4) CD8+ T-cell cytotoxicity. CD45− EPCs promote (5) tumor cell metastasis and invasion and (6) tumor growth and cell proliferation.
4.4. The immunomodulatory properties of CD71+ EPCs disappear during their maturation
Since CD71+ EPCs inhibit T-cell proliferation and IFN-γ production via ARG and ROS, ROS, ARG1, and ARG2 were highest in the early stage of CD71+ EPCs, so the immunosuppressive ability of CD71high CD235amid EPCs was strongest in the early stage. The immunomodulatory properties of CD71 EPCs were most effective in the earliest stage of their differentiation. With the differentiation and maturation of EPCs, CD71 on the surface of EPCs begins to decrease when it reaches Ortho-E and disappears after erythrocyte maturation. And the inhibitory effect of CD71+ EPCs on T cells is also completely lost, so the immunomodulatory properties of CD71+ EPCs are robust but transient, disappearing as they mature [56].
4.5. The radioprotective effect of CD31+ short-term EPCs
Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a member of the immunoglobulin immunoreceptor tyrosine inhibitory motif superfamily [66]. HSC expresses CD31 from early embryonic stages to late adulthood. CD31+ Lin-c-kit+ Sca-1− cells (CD31+ Sca-1−) can provide radioprotection, so CD31+ short-term EPCs may reduce the killing of tumor cells by radiotherapy [67].
5. Advances in the treatment of EPCs in tumors
With the in-depth research progress of EPCs, researchers began to explore the therapeutic value of EPCs. As early as 2007, Sasaki et al. exploited the property that EPCs can secrete angiogenic factors to promote angiogenesis in ischemic limbs [68]. Research by Mirmiran et al. using bifunctional transferrin (Tf) receptor one ligand-peptide, which targets the delivery of the characteristic gene into EPCs, was effective in treating the treatment of erythropoietic protoporphyria [69]. Wong et al. transformed CD36+ EPCs into a continuous cell line, CD36E. CD36E cells have EPC characteristics, of which approximately 27% of the cell population produces hemoglobin, which has potential application in tumors combined with anemia [70]. However, there are few experimental animal reports on tumor-targeted therapy for EPCs, and relevant clinical studies are still scarce.
In addition, Exosomes have been a hot research topic in recent years, and many exosome-based diagnostic and therapeutic approaches will soon enter clinical applications. Macrì et al. reported an EPC-derived vesicle (CD34+CD71low), which differed significantly between Diamond-Blackfan anemia (DBA) patients and the healthy population, may help to improve the diagnosis of DBA. Therefore, immunophenotypic analysis of extracellular vesicles derived from EPCs can be used to study the specific phenotype of erythroid differentiation [71].
Subsequently, Li et al. investigated the role of Danggui Buxue Tang in tumor therapy. They found that although Danggui Buxue Tang had no significant direct effect on tumor cell proliferation and apoptosis, it was able to promote the differentiation of EPCs and significantly attenuated the accumulation of EPCs, which enhanced the anti-tumor immune response and thus inhibited the progression of B16 melanoma [72]. This research provides a new strategy to inhibit malignant tumor progression and alleviate tumor-associated anemia treatment.
Hou et al. reduced tumor-induced CD45− EPCs in the spleen of tumor-bearing mice by localized ionizing radiation (IR) tumors and thus decreased artemin secretion. However, subsequent experiments revealed that IR alone was unable to reduce the tumor-induced accumulation of CD45− EPCs in the spleen of mice and required the mediation of type I/II IFN and CD8+ T cells. Intraperitoneally administered PD-L1 blocking antibodies also require the mediation of CD8+ T cells and IFN-γ to reduce the tumor-induced accumulation of CD45− EPCs. Moreover, the therapeutic effect of tumor patients was associated with a decrease in CD45− EPCs and artemin concentration. This research provides a new strategy for eliminating cancer-promoting EPCs [65].
Recently, Tan et al. reduced the frequency of EPCs in the spleen of an animal model of Lewis lung cancer by ultrasound-targeted microbubble and increased the frequency of T cells, especially CD8+ T cells. There was no pathological damage to the spleen, but this was insufficient to inhibit tumor progression. The researchers then combined UTMD with PDL-1 blockade treatment and found that the combination therapy inhibited tumor growth. Therefore, single immunotherapy and chemoradiotherapy may not be the best strategy for treating tumors. It is necessary to connect the value of targeted EPCs in enhancing immune response, improving the effectiveness of immunotherapy, and inhibiting tumor progression by finding appropriate treatment options [73].
6. Regulates differentiation and maturation of EPCs
With the established pro-tumor effects of EPCs, how to treat anemia and improve the prognosis of tumor patients will be a future research direction. Therefore, we believe that the pro-tumor effects of EPCs can be reduced by regulating erythropoiesis.
6.1. Erythropoiesis and its major regulatory pathways
Erythropoiesis is a complex process regulated by the interaction of multiple transcription factors and cellular molecules [74]. During this process, HSCs proliferate and differentiate into mature erythrocytes. HSC exists in a unique hematopoietic ecological niche [75]. In the early stages of erythropoiesis, HSCs differentiate into multipotent megakaryocyte-erythroid progenitor cells, followed by burst-forming unit erythrocytes (BFU-E) and colony-forming unit erythrocytes (CFU-E) [76]. During terminal erythropoiesis [77,78], CFU-E further differentiates into proto-erythrocytes (Pro-E). Pro-E decreases in volume, nuclei become concentrated, and they begin to produce specific proteins, such as hemoglobin, in large quantities. Pro-E then continues to differentiate according to the order of basophilic juvenile erythrocytes (Baso-E), poly-stained juvenile erythrocytes (Poly-E), and orthostained juvenile erythrocytes (Ortho-E). Finally, Ortho-E exits the nucleus and releases reticulocytes into the circulatory system [79]. It takes about 1 week for reticulocytes to mature into erythrocytes in healthy individuals [77]. As reticulocyte maturation proceeds, RNA, mitochondria, and ribosomes are degraded, and protein synthesis ceases [80]. Mature erythrocytes do not have a nucleus and have a biconcave disk shape. They have high concentrations of hemoglobin, which bind and transport O2.
The early stages of erythropoiesis are regulated by stem cell factor (SCF)/stem cell growth factor receptor (c-Kit), IL-3/IL-3 receptor (IL-3R) [81], and granulocyte-macrophage colony-stimulating factor (GM-CSF [81]) [77,82]. IL-3 regulates EPC self-renewal [83,84] and, together with activin A, induces mitosis and increases colony number [85]. Zinc finger transcription factor-2 (GATA-2), which is highly expressed in the early stages [86], promotes erythroid proliferation and inhibits erythroid differentiation [87,88]. Due to the switching mechanism of GATA, GATA-2 and GATA-1 are expressed at the early and late stages of erythropoiesis, respectively [86,89]. Zinc-finger transcription factor-1 (GATA-1) is erythropoiesis’s main transcriptional regulatory factor [90,91,92]. GATA-1 promotes erythroid differentiation in late erythropoiesis by positively regulating specific red lineage genes. GATA-1 also induces the expression of the anti-apoptotic protein Bcl-xL and erythropoietin receptor (EPO-R) together with transcription factor signaling and activator of transcription 5 (STAT5) [87,93]. During erythropoiesis, caspases cleave GATA-1 upon activation, blocking erythroid differentiation, and inducing cell death [94]. Chaperone heat shock protein 70 enters the nucleus after cystathionine activation and protects GATA-1 from caspase-3 cleavage, protecting early EPCs [94,95].
This phase of terminal erythropoiesis is dependent on iron metabolism and is regulated primarily by EPO [87,96] and SCF [81,97]. EPO binds to EPO-R and activates Janus kinase 2 (JAK2), and activation of JAK2 induces various signaling pathways, such as protein kinase B (AKT) and STAT5. This led to the activation of anti-apoptotic genes in the red lineage and enhanced the survival and proliferation of EPCs [98,99,100]. The binding between FAS and the FAS ligand (FASL) induces apoptosis in immature erythroid cells [87,101]. EPO interacts with membrane protein of death receptor family (FAS) and its ligand (FASL) to counteract the negative signal and prevent apoptosis of immature cells [102].
In addition, vitamin B12 and folic acid, trace elements (copper and iron), and hepcidin are essential for erythroid maturation. Peroxisome proliferator-activated receptor alpha and glucocorticoid receptor synergistically promote self-renewal of EPCs [103]. Regulators of human erythroid cell expansion (RHEX) can promote EPC expansion and erythroid differentiation [104]. Erythroid differentiation and maturation are also negatively regulated by members of the Tf and its cellular receptor (Tfr) [105,106,107] and TGF-β superfamily [108]. Long-chain non-coding RNA regulates erythroid differentiation by coordinating with chromatin accessibility [109]. A complex network of transcription factors and epigenetic regulators regulates erythropoiesis [90,110]. Positive and negative regulation of erythropoiesis is essential for maintaining erythroid homeostasis [87] (Figure 4).
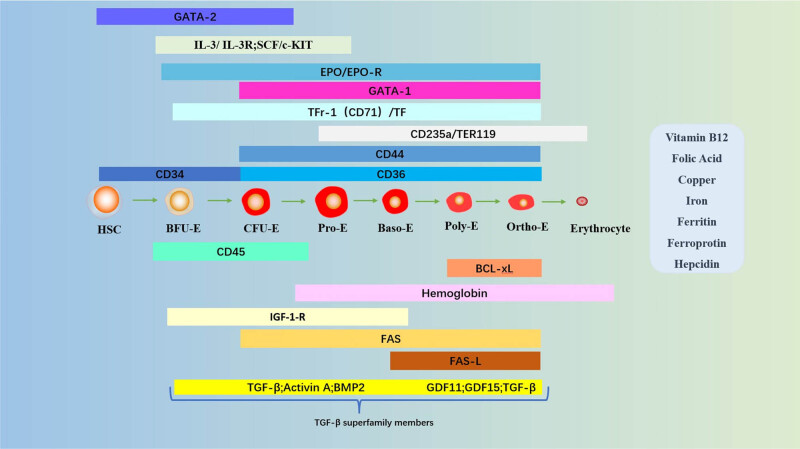
Figure 4.
The main pathways and molecules involved in regulating erythropoiesis. The different stages are shown: HSC, BFU-E, CFU-E, Pro-E, Baso-E, Poly-E, Ortho-E, and erythrocytes. Molecules involved: zinc finger factors that bind GATA sequences (GATA-1, GATA-2); IL-3; IL-3-R; SCF; c-Kit; EPO; EPO-R; Ter-119, glycophorin A-associated protein; CD235a, glycophorin A; CD44, cell surface adhesion molecule; CD34, transmembrane phosphoglycoprotein; CD36, platelet glycoprotein protein 4; CD45, common marker of leukocytes; BCL-xL, anti-apoptotic protein; hemoglobin; FAS; FAS-L; Tf; TfR-1 (or CD71), transferrin receptor 1; TGF-β; activin A; BMP-2, bone morphogenetic protein 2; GDF, growth differentiation factor. Vitamins, trace elements, and iron metabolism proteins necessary for erythropoiesis: vitamin B12, folic acid, copper, iron, ferritin, ferroprotin, hepcidin).
6.2. Promoting differentiation of EPCs into erythroid cells
As a result of dysregulated erythropoiesis in patients with advanced cancer, the expansion of EPCs effectively suppresses the antitumor immune response. Promote the differentiation of EPCs, reduce the production and accumulation of ineffective EPCs, and fundamentally reduce the inhibitory effect of EPCs on the immune response.
6.2.1. The role of TGF-β in tumors
The TGF-β superfamily regulates late erythrocyte maturation through the SMAD signaling pathway. Dysregulation of this signaling pathway may impair erythroid maturation and ineffective erythropoiesis [111–113]. V-domain immunoglobulins that inhibit T-cell activation enable the cells to produce TGF-β repeatedly [113]. TGF-β signaling has a myelosuppressive effect, blocking EPCs’ proliferation and accelerating their differentiation [114]. The activation of TGF-β and Smad3 is essential for the tumor-induced formation of CD45− EPCs in the spleen (also known as Ter cells) [62]. In TGF-β knockout tumor mice and Smad3-deficient mice, the expansion of CD45− EPCs was significantly reduced [59]. Therefore, activation of TGF-β and Smad3 contributed to the formation of Ter cells, resulting in more ineffective erythropoiesis.
The TGF-β superfamily includes TGF-β, activin, BMP, and GDF-11, and these cytokines play an essential role in erythropoiesis [115]. In particular, activin and GDF-11 are inhibitory on advanced erythropoiesis [116]. TGF-β not only plays a pro-differentiation function in erythropoiesis but is also a master regulator of numerous cellular functions, including cellular immunity [117–119]. Activin-A and BMP immune responses through effects on resistant and non-immune cell populations [120].
In cancer, TGF-β can exert pro-tumorigenic effects through various mechanisms, including immunosuppression [121,122]. All advanced tumors overproduce TGF-β. TGF-β promotes tumor growth, invasion, and metastasis in an autocrine and paracrine manner and inhibits anti-cancer drug sensitivity [123,124]. Excess TGF-β also leads to the proliferation of EPCs. Inhibiting the SMAD signaling pathway could rescue the proliferation of T cells and IFN-γ production suppressed by EPCs [5]. Studies have shown that ligand capture fusion protein (ACE-536) binding to GDF11 effectively inhibited Smad2/3 signaling, reducing anemia and ineffective erythropoiesis in mice with myelodysplastic syndrome [111]. Another study found that RAP-011, similar to ACE-536, improved inadequate erythrocyte production and corrected anemia in mice [112]. Preclinical studies have shown that some anti-TGF-β immunotherapies are effective for oncology treatment, specifically when combined with immune checkpoint inhibitors [125,126]. Therefore, immunotherapies targeting TGF-β activation or signaling may inhibit the tumor-promoting effects of EPCs and the tumor-promoting effects of TGF-β, thereby improving the efficacy of immunotherapies for various cancers.
6.2.2. The role of EPO in tumors
EPO maintains erythrocyte homeostasis by precisely regulating the number of erythrocytes through the degree of tissue oxygenation. Different levels of EPO also have various effects on erythropoiesis. When caspases are activated, cells with low sensitivity undergo apoptosis at low EPO levels. At mild EPO levels, cells are blocked or apoptotic during maturation, and at higher EPO levels, most cells survive and differentiate [127].
It was found that EPO-R-deficient mice develop severe anemia and impaired EPC function [128], making the role of EPO in erythropoiesis crucial. Malignant cells and stromal cells in the TME produce vascular endothelial growth factor, which stimulates EPO secretion by stromal cells in the spleen expressing platelet-derived growth factor-beta receptor [129], thereby increasing EPO production.
However, the mechanism by which EPO acts in cancer is controversial. On the one hand, studies in cell culture and animal models have shown that the EPO pathway promotes tumor cell activity, proliferation, metastatic potential, treatment tolerance, and intervention [130]. On the other hand, there are also studies showing that EPO has no direct stimulatory effect on tumor cell growth. EPO has no immediate stimulatory effect on tumor cell growth [131,132]. A recent study found that the application of anti-EPO antibodies to B16 tumor-bearing mice prevented the expansion of the red lineage. Treatment with this antibody also had some antitumor effect, slowing down the subcutaneous growth of B16 tumors. Thus, EPO and red lineage cells are new players in tumor–host interactions [6].
In addition, clinical trials have reported that recombinant human EPO to treat anemia in cancer patients during chemotherapy or radiotherapy increases cancer patient mortality. However, the mechanism was not precise [133,134]. A recent study reported that EPO enhanced the immunosuppressive effect of CD45− EPCs, which is detrimental to the antitumor development induced by local IR and blockade of programmed death-ligand 1 (PD-L1) [65], so EPO might indirectly affect T-cell function via Ter cells and their artemin production. Therefore, immunotherapies targeting EPO and its downstream signaling pathways could be strategies to reduce antitumor immunity.
6.2.3. Other regulations that promote EPC differentiation
Notch signaling pathways play critical roles in the proliferation, development, maintenance, and differentiation of stem cells and multicellular organisms [135] and link between angiogenesis and self-renewal of CSCs [136]. Previous studies have reported that γ-secretase inhibitors (GSI), which inhibit Notch signaling, induce erythroid differentiation, and promote hemoglobin production in erythroid leukemia cell lines [137]. Therefore, the combination of GSI with anti-cancer drugs may be a promising strategy for cancer treatment [136,138].
Oxygen plays a critical role in erythropoiesis, and hypoxia promotes the loss of the EPC surface marker CD71 and the appearance of the erythroid markers CD235a and CD239, thereby inhibiting the differentiation of EPCs and accelerating the maturation of EPCs [139]. It is because HIF-1α promotes the expression of the erythroid surface markers CD71 and CD235a, so HIF-1α plays a vital role in fostering erythroid differentiation. Hypoxia increases the expression of GATA-1. Overexpression of GATA-1 increases HIF-1α in cord blood CD34+ and K562 cells. So GATA-1 is also required for normal erythropoiesis [140]. In addition, caspase-1 is necessary for HSPC bone marrow differentiation, and caspase-1 promotes erythroid differentiation by cleaving the major erythroid transcription factorGATA1 [141].
Supplementation with iron, folic acid, and vitamin C can promote erythroid differentiation [142,143]. The fetal hemoglobin inducer MS-275 to reactivate the fetal hemoglobin-producing γ-globin gene can encourage the production of hemoglobin and erythroid differentiation in K562 cells [144]. Splenectomy can also inhibit the expansion of EPCs, but its clinical efficacy is controversial [59]. The aryl hydrocarbon receptor (AHR) plays an essential role in mammalian embryonic development. Antagonism of AHR signaling enhances the production of human embryonic stem cell (hESC)-derived erythroid cells and promotes terminal erythroid differentiation [145].
Adenovirus early region 2 binding factors (E2F-2), a transcription factor regulated by retinoblastoma, have a canonical function in promoting cell cycle progression [146,147]. In late erythroid maturation, high levels of E2F-2 expression promote nuclear cohesion and enucleation in terminal erythroid lineage cells [148]. Polycomb histone (PcG) is a crucial regulator of the terminal differentiation of HSCs [149–151]. The PcG gene BMI-1 regulates HSC self-renewal and promotes erythroid differentiation [152–154]. The mechanistic target of rapamycin (mTOR) is critical for cell growth (size) and proliferation [155]. Excessive activation of mTOR signaling interferes with cell cycle progression in Foxo3 mutant erythroid cells. Inhibiting mTOR signaling in vivo and ex vivo significantly enhanced Foxo3 mutant red lineage cells [156]. Because promotion and inhibition of erythroid cell proliferation have different effects on different diseases [47,157–160], further clinical studies are needed to investigate the appropriate therapeutic strategies (Figure 5 and Table 1).
Figure 5.
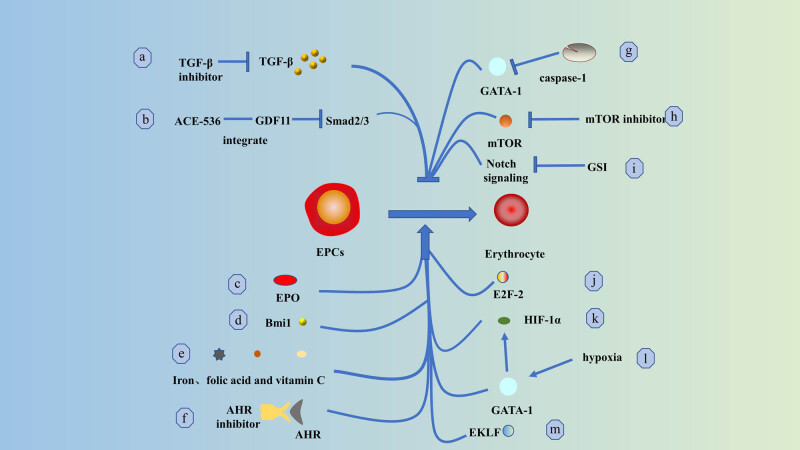
Critical modulations that promote differentiation of erythroid progenitors: (a) inhibition of TGF-β and (b) binding of GDF11 via ACE-536 effectively rescued differentiation arrest of EPCs; (c) EPO and (d) Bmi1 enhanced differentiation of EPCs; (e) iron, folic acid, and vitamin C also promoted differentiation of EPCs; (f) antagonism of AHR signaling improved hESC-derived erythrocyte production and enhanced terminal differentiation of EPCs; (g) caspase-1 promotes differentiation of EPCs by cleaving GATA1; (h) inhibition of mTOR signaling enhances maturation of EPCs; (i) inhibition of Notch signaling by GSI induces differentiation of EPCs and promotes hemoglobin production; (j) E2F-2 is expressed at high levels in (k). GATA-1 is essential for the differentiation and maturation of late EPCs; (l) hypoxia increases the expression of GATA-1 protein, and overexpression of GATA-1 increases the level of HIF-1α, which promotes the differentiation and maturation of EPCs; and (m) enhanced EKLF in late EKLF of EPCs may promote differentiation of terminal red lineage cells [161,162].
Table 1.
Regulatory pathways that promote the differentiation of EPCs
| Mechanisms | Effects | Ref |
|---|---|---|
| TGF-β inhibitor | ↑promote the differentiation of EPCs | [111–113] |
| ACE-536 | [111] | |
| EPO | [87,96,128] | |
| PcG, Bmi1 | [149–154] | |
| Iron, folic acid, and vitamin C | [142,143] | |
| AHR antagonist | [145] | |
| Caspase-1 | [141] | |
| mTOR inhibitor | [155] | |
| GSI | [137] | |
| E2F-2 | [146–148] | |
| HIF-1α | [140] | |
| Hypoxia | [139] | |
| EKLF | [148] | |
| GATA-1 | [90,91,92,93] | |
| MS-275 | [144] | |
| Resection of the spleen | [59] | |
| EKLF/KLF1 | [163] | |
| Long non-coding RNAs | [109] | |
| PPAR-α | [103] | |
| RHEX | [104] |
7. Conclusions
With the advancing research related to the role of EPCs in tumors, we are getting more explicit about the mechanisms by which EPCs affect tumors. EPCs are essential mechanisms of tumor growth, metastasis, and drug resistance. Further exploration of EPCs can help us gain insight into the immune microenvironment and thus identify potential targets for immunotherapy. Different subtypes of EPCs have different surface markers and expressions and play different roles in tumor immune evasion or progression. Moreover, high cell counts of EPCs always indicate poor prognosis and are also associated with tumor size and lymph node metastasis. Therefore, immunofluorescence screening of EPCs may be a novel clinical method to predict tumor recurrence. Clinical studies on EPCs are currently limited. Whether future therapeutic decisions can be made based on the expression of different phenotypes of EPCs still needs to be further explored. Previous studies have provided us with the thought that immunotherapy combined with radiotherapy and chemotherapy may be the most effective treatment modality. Besides, the treatment of cancer-related anemia is controversial. It will be a future research direction on how to treat anemia and improve the prognosis of oncology patients. As a result, addressing anemia and reducing EPCs’ pro-tumor function are crucial to improving the prognosis of cancer patients. Until now, that seems to be a promising immunotherapeutic strategy for patients with tumors combined with anemia. Finally, further information on artemisinin’s mechanism and signaling route is needed. The link between EPCs and malignancies and the TME are determined. This will open up new avenues for tumor diagnostics and therapy.
Footnotes
Funding information: The study was supported by NSFC (National Nature Science Foundation of China), No. 82172902 (Jing-Zhi Guan).
Author contributions: Conceptualization, H.Z., W.C., and J.-Z.G.; writing – original draft preparation, H.Z., W.C., and J.-Z.G.; writing – review and editing, H.Z., G.-Z.W., Y.-Y.W., W.C., J.-Z.G.; visualization, H.Z.; supervision, W.C., J.-Z.G.; funding acquisition, J.-Z.G. All authors have read and agreed to the published version of the article.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Contributor Information
Hao Zhang, Email: aizerfore@gmail.com.
Guang-zhi Wan, Email: w50099005@163.com.
Yu-ying Wang, Email: yuying199700@163.com.
Wen Chen, Email: 1607283686@qq.com.
Jing-Zhi Guan, Email: jzjz1970@hotmail.com.
References
- [1].Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021 Apr;11(4):933–59. [DOI] [PubMed]
- [2].Tang T, Huang X, Zhang G, Hong Z, Bai X, Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther. 2021 Feb 20;6(1):72. [DOI] [PMC free article] [PubMed]
- [3].Tarin D. Clinical and biological implications of the tumor microenvironment. Cancer Microenviron. 2012 Feb 3;5(2):95–112. [DOI] [PMC free article] [PubMed]
- [4].Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015 Mar 5;13:45. [DOI] [PMC free article] [PubMed]
- [5].Chen J, Qiao YD, Li X, Xu JL, Ye QJ, Jiang N, et al. Intratumoral CD45+CD71+ erythroid cells induce immune tolerance and predict tumor recurrence in hepatocellular carcinoma. Cancer Lett. 2021 Feb 28;499:85–98. [DOI] [PubMed]
- [6].Sano Y, Yoshida T, Choo MK, Jiménez-Andrade Y, Hill KR, Georgopoulos K, et al. Multiorgan signaling mobilizes tumor-associated erythroid cells expressing immune checkpoint molecules. Mol Cancer Res. 2021 Mar;19(3):507–15. [DOI] [PMC free article] [PubMed]
- [7].Bishlawy IM. Red blood cells, hemoglobin and the immune system. Med Hypotheses. 1999 Oct;53(4):345–6. [DOI] [PubMed]
- [8].Garratty G. Advances in red blood cell immunology 1960 to 2009. Transfusion. 2010 Mar;50(3):526–35. [DOI] [PubMed]
- [9].Liu D, Niu ZX. The structure, genetic polymorphisms, expression and biological functions of complement receptor type 1 (CR1/CD35). Immunopharmacol Immunotoxicol. 2009;31(4):524–35. [DOI] [PubMed]
- [10].Passantino L, Altamura M, Cianciotta A, Jirillo F, Ribaud MR, Jirillo E, et al. Maturation of fish erythrocytes coincides with changes in their morphology, enhanced ability to interact with Candida albicans and release of cytokine-like factors active upon autologous macrophages. Immunopharmacol Immunotoxicol. 2004;26(4):573–85. [DOI] [PubMed]
- [11].Hess C, Schifferli JA. Immune adherence revisited: novel players in an old game. N Physiol Sci. 2003 Jun;18:104–8. [DOI] [PubMed]
- [12].Forslid J, Hed J, Stendahl O. Erythrocyte enhancement of C3b-mediated phagocytosis by human neutrophils in vitro: a combined effect of the erythrocyte complement receptors CR1 and erythrocyte scavengers to reactive oxygen metabolites (ROM). Immunology. 1985 May;55(1):97–103. [PMC free article] [PubMed]
- [13].Shen H, Schuster R, Stringer KF, Waltz SE, Lentsch AB. The Duffy antigen/receptor for chemokines (DARC) regulates prostate tumor growth. FASEB J. 2006 Jan;20(1):59–64. [DOI] [PubMed]
- [14].Karsten E, Breen E, McCracken SA, Clarke S, Herbert BR. Red blood cells exposed to cancer cells in culture have altered cytokine profiles and immune function. Sci Rep. 2020 May 7;10:7727. [DOI] [PMC free article] [PubMed]
- [15].Porto B, Fonseca AM, Godinho I, Arosa FA, Porto G. Human red blood cells have an enhancing effect on the relative expansion of CD8+ T lymphocytes in vitro. Cell Prolif. 2001 Dec;34(6):359–67. [DOI] [PMC free article] [PubMed]
- [16].Antunes RF, Brandão C, Maia M, Arosa FA. Red blood cells release factors with growth and survival bioactivities for normal and leukemic T cells. Immunol Cell Biol. 2011 Jan;89(1):111–21. [DOI] [PubMed]
- [17].Shivam P, Jamal F, Kumari S, Bimal S, Narayan S, Das VNR, et al. Leishmania donovani: influence of anti-leishmanial therapy on expression of lymphocyte function-associated antigen-3 and its relevance to pathogenisis in visceral leishmaniasis. Hum Immunol. 2013 Dec;74(12):1575–8. [DOI] [PubMed]
- [18].Shau H, Gupta RK, Golub SH. Identification of a natural killer enhancing factor (NKEF) from human erythroid cells. Cell Immunology. 1993;147(1):1–11. [DOI] [PubMed]
- [19].Glassman PM, Hood ED, Ferguson LT, Zhao Z, Siegel DL, Mitragotri S, et al. Red blood cells: The metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv Drug Deliv Rev. 2021 Nov;178:113992. [DOI] [PMC free article] [PubMed]
- [20].Millán CG, Marinero MLS, Castañeda AZ, Lanao JM. Drug, enzyme and peptide delivery using erythrocytes as carriers. J Control Rel. 2004 Feb 20;95(1):27–49. [DOI] [PubMed]
- [21].Zelepukin IV, Yaremenko AV, Shipunova VO, Babenyshev AV, Balalaeva IV, Nikitin PI, et al. Nanoparticle-based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale. 2019 Jan 23;11(4):1636–46. [DOI] [PubMed]
- [22].Javed S, Alshehri S, Shoaib A, Ahsan W, Sultan MH, Alqahtani SS, et al. Chronicles of nanoerythrosomes: An erythrocyte-based biomimetic smart drug delivery system as a therapeutic and diagnostic tool in cancer therapy. Pharmaceutics. 2021 Mar 10;13(3):368. [DOI] [PMC free article] [PubMed]
- [23].Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood. 2012;119(23):5512–21. [DOI] [PubMed]
- [24].Rodgers GM, Becker PS, Blinder M, Cella D, Chanan-Khan A, Cleeland C, et al. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw. 2012 May;10(5):628–53. [DOI] [PubMed]
- [25].Yasmeen T, Ali J, Khan K, Siddiqui N. Frequency and causes of anemia in Lymphoma patients. Pak J Med Sci. 2019;35(1):61–5. [DOI] [PMC free article] [PubMed]
- [26].Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001 Jun 15;91(12):2214–21. [PubMed]
- [27].Zhang LL, Zhou GQ, Li YY, Tang LL, Mao YP, Lin AH, et al. Combined prognostic value of pretreatment anemia and cervical node necrosis in patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: A large-scale retrospective study. Cancer Med. 2017 Dec;6(12):2822–31. [DOI] [PMC free article] [PubMed]
- [28].Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascón P. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004 Oct;40(15):2293–306. [DOI] [PubMed]
- [29].Ludwig H, Müldür E, Endler G, Hübl W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 2013 Jul;24(7):1886–92. [DOI] [PMC free article] [PubMed]
- [30].Busti F, Marchi G, Ugolini S, Castagna A, Girelli D. Anemia and iron deficiency in cancer patients: role of iron replacement therapy. Pharm (Basel). 2018 Sep 30;11(4):E94. [DOI] [PMC free article] [PubMed]
- [31].Abiri B, Vafa M. Iron deficiency and anemia in cancer patients: the role of iron treatment in anemic cancer patients. Nutr Cancer. 2020;72(5):864–72. [DOI] [PubMed]
- [32].Grotto HZW. Anaemia of cancer: an overview of mechanisms involved in its pathogenesis. Med Oncol. 2008;25(1):12–21. [DOI] [PubMed]
- [33].Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005 Mar 10;352(10):1011–23. [DOI] [PubMed]
- [34].Ueda N, Takasawa K. Role of hepcidin-25 in chronic kidney disease: Anemia and beyond. Curr Med Chem. 2017;24(14):1417–52. [DOI] [PubMed]
- [35].Ueda N, Takasawa K. Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients. 2018 Aug 27;10(9):1173. [DOI] [PMC free article] [PubMed]
- [36].Prince OD, Langdon JM, Layman AJ, Prince IC, Sabogal M, Mak HH, et al. Late stage erythroid precursor production is impaired in mice with chronic inflammation. Haematologica. 2012 Nov;97(11):1648–56. [DOI] [PMC free article] [PubMed]
- [37].Means RT. Pathogenesis of the anemia of chronic disease: a cytokine-mediated anemia. Stem Cell. 1995 Jan;13(1):32–7. [DOI] [PubMed]
- [38].Adamson JW. The anemia of inflammation/malignancy: mechanisms and management. Hematology. 2008 Jan 1;2008(1):159–65. [DOI] [PubMed]
- [39].Manso BA, Krull JE, Gwin KA, Lothert PK, Welch BM, Novak AJ, et al. Chronic lymphocytic leukemia B-cell-derived TNFα impairs bone marrow myelopoiesis. Iscience. 2021 Jan 22;24(1):101994. [DOI] [PMC free article] [PubMed]
- [40].Libregts SF, Gutiérrez L, de Bruin AM, Wensveen FM, Papadopoulos P, van Ijcken W, et al. Chronic IFN-γ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011 Sep 1;118(9):2578–88. [DOI] [PubMed]
- [41].Greenwald AC, Licht T, Kumar S, Oladipupo SS, Iyer S, Grunewald M, et al. VEGF expands erythropoiesis via hypoxia-independent induction of erythropoietin in noncanonical perivascular stromal cells. J Exp Med. 2019 Jan 7;216(1):215–30. [DOI] [PMC free article] [PubMed]
- [42].Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021 Jan 20;21(1):62. [DOI] [PMC free article] [PubMed]
- [43].Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019 Nov 11;18(1):157. [DOI] [PMC free article] [PubMed]
- [44].Kim JY, Lee JY. Targeting tumor adaption to chronic hypoxia: implications for drug resistance, and how it can be overcome. Int J Mol Sci. 2017 Aug 25;18(9):E1854. [DOI] [PMC free article] [PubMed]
- [45].Geissler K, Hinterberger W, Bettelheim P, Neumann E, Grümayer ER, Radaszkiewicz T, et al. In vitro culture studies of granulocyte/macrophage and erythroid progenitor cells in lymphoproliferative disorders. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;52(6):553–61. [DOI] [PubMed]
- [46].Zhao L, He R, Long H, Guo B, Jia Q, Qin D, et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat Med. 2018 Oct;24(10):1536–44. [DOI] [PMC free article] [PubMed]
- [47].Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013 Dec 5;504(7478):158–62. [DOI] [PMC free article] [PubMed]
- [48].Mori Y, Chen JY, Pluvinage JV, Seita J, Weissman IL. Prospective isolation of human erythroid lineage-committed progenitors. Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):9638–43. [DOI] [PMC free article] [PubMed]
- [49].Psaila B, Barkas N, Iskander D, Roy A, Anderson S, Ashley N, et al. Single-cell profiling of human megakaryocyte-erythroid progenitors identifies distinct megakaryocyte and erythroid differentiation pathways. Genome Biol. 2016 May 3;17:83. [DOI] [PMC free article] [PubMed]
- [50].Sanada C, Xavier-Ferrucio J, Lu YC, Min E, Zhang PX, Zou S, et al. Adult human megakaryocyte-erythroid progenitors are in the CD34+CD38mid fraction. Blood. 2016 Aug 18;128(7):923–33. [DOI] [PMC free article] [PubMed]
- [51].Holm TM, Braun A, Trigatti BL, Brugnara C, Sakamoto M, Krieger M, et al. Failure of red blood cell maturation in mice with defects in the high-density lipoprotein receptor SR-BI. Blood. 2002 Mar 1;99(5):1817–24. [DOI] [PubMed]
- [52].Mello FV, Land MGP, Costa ES, Teodósio C, Sanchez ML, Bárcena P, et al. Maturation-associated gene expression profiles during normal human bone marrow erythropoiesis. Cell Death Discov. 2019;5:69. [DOI] [PMC free article] [PubMed]
- [53].Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012 Mar 22;12(4):253–68. [DOI] [PMC free article] [PubMed]
- [54].Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression – implications for anticancer therapy. Nat Rev Clin Oncol. 2019 Jun;16(6):356–71. [DOI] [PubMed]
- [55].Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017 Jul;14(7):399–416. [DOI] [PMC free article] [PubMed]
- [56].Grzywa TM, Sosnowska A, Rydzynska Z, Lazniewski M, Plewczynski D, Klicka K, et al. Potent but transient immunosuppression of T-cells is a general feature of CD71+ erythroid cells. Commun Biol. 2021 Dec 10;4(1):1384. [DOI] [PMC free article] [PubMed]
- [57].Elahi S, Vega-López MA, Herman-Miguel V, Ramírez-Estudillo C, Mancilla-Ramírez J, Motyka B, et al. CD71+ erythroid cells in human neonates exhibit immunosuppressive properties and compromise immune response against systemic infection in neonatal mice. Front Immunol. 2020;11:597433. [DOI] [PMC free article] [PubMed]
- [58].Wei X, Yang S, Pu X, He S, Yang Z, Sheng X, et al. CD45+ Erythroid Progenitor Cell Contribute to Antiangiogenic Drug Resistance Through Reactive Oxygen Species in Lymphoma. 2021 [cited 2021 Dec 30]. Available from: https://www.researchsquare.com/article/rs-41006/v1.
- [59].Han Y, Liu Q, Hou J, Gu Y, Zhang Y, Chen Z, et al. Tumor-induced generation of splenic erythroblast-like ter-cells promotes tumor progression. Cell. 2018 Apr 19;173(3):634–648.e12. [DOI] [PubMed]
- [60].Nakamura K, Smyth MJ. Aberrant erythropoiesis fuels tumor growth. Cell Res. 2018 Jun;28(6):611–2. [DOI] [PMC free article] [PubMed]
- [61].Grzywa TM, Justyniarska M, Nowis D, Golab J. Tumor immune evasion induced by dysregulation of erythroid progenitor cells development. Cancers (Basel). 2021 Feb 19;13(4):870. [DOI] [PMC free article] [PubMed]
- [62].Li TJ, Li H, Zhang WH, Xu SS, Jiang W, Li S, et al. Human splenic TER cells: A relevant prognostic factor acting via the artemin-GFRα3-ERK pathway in pancreatic ductal adenocarcinoma. Int J Cancer. 2021 Apr 1;148(7):1756–67. [DOI] [PubMed]
- [63].Song Z, Yang F, Du H, Li X, Liu J, Dong M, et al. Role of artemin in non-small cell lung cancer. Thorac Cancer. 2018 May;9(5):555–62. [DOI] [PMC free article] [PubMed]
- [64].Wang J, Wang H, Cai J, Du S, Xin B, Wei W, et al. Artemin regulates CXCR4 expression to induce migration and invasion in pancreatic cancer cells through activation of NF-κB signaling. Exp Cell Res. 2018 Apr 1;365(1):12–23. [DOI] [PubMed]
- [65].Hou Y, Liang HL, Yu X, Liu Z, Cao X, Rao E, et al. Radiotherapy and immunotherapy converge on elimination of tumor-promoting erythroid progenitor cells through adaptive immunity. Sci Transl Med. 2021 Feb 24;13(582):eabb0130. [DOI] [PMC free article] [PubMed]
- [66].Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–22. [DOI] [PubMed]
- [67].Baumann CI, Bailey AS, Li W, Ferkowicz MJ, Yoder MC, Fleming WH. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood. 2004 Aug 15;104(4):1010–6. [DOI] [PubMed]
- [68].Sasaki S, Inoguchi T, Muta K, Abe Y, Zhang M, Hiasa K, et al. Therapeutic angiogenesis by ex vivo expanded erythroid progenitor cells. Am J Physiol Heart Circ Physiol. 2007 Jan;292(1):H657–665. [DOI] [PubMed]
- [69].Mirmiran A, Schmitt C, Lefebvre T, Manceau H, Daher R, Oustric V, et al. Erythroid-progenitor-targeted gene therapy using bifunctional tfr1 ligand-peptides in human erythropoietic protoporphyria. Am J Hum Genet. 2019 Feb 7;104(2):341–7. [DOI] [PMC free article] [PubMed]
- [70].Wong S, Keyvanfar K, Wan Z, Kajigaya S, Young NS, Zhi N. Establishment of an erythroid cell line from primary CD36+ erythroid progenitor cells. Exp Hematol. 2010 Nov;38(11):994–1005.e1-2. [DOI] [PMC free article] [PubMed]
- [71].Macrì S, Pavesi E, Crescitelli R, Aspesi A, Vizziello C, Botto C, et al. Immunophenotypic profiling of erythroid progenitor-derived extracellular vesicles in diamond-blackfan anaemia: A new diagnostic strategy. PLoS One. 2015;10(9):e0138200. [DOI] [PMC free article] [PubMed]
- [72].Li C, Zhu F, Xu C, Xiao P, Wen J, Zhang X, et al. Dangguibuxue decoction abolishes abnormal accumulation of erythroid progenitor cells induced by melanoma. J Ethnopharmacol. 2019 Oct 5;242:112035. [DOI] [PubMed]
- [73].Tan X, Yi C, Zhang Y, Tang N, Xu Y, Liu Z. Ultrasound-targeted microbubble destruction alleviates immunosuppression induced by CD71+ erythroid progenitor cells and promotes PDL-1 blockade immunotherapy in the lewis lung cancer model. Front Oncol. 2021;11:768222. [DOI] [PMC free article] [PubMed]
- [74].Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014 Aug;20(8):833–46. [DOI] [PMC free article] [PubMed]
- [75].Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014 Jan 16;505(7483):327–34. [DOI] [PMC free article] [PubMed]
- [76].Jafari M, Ghadami E, Dadkhah T, Akhavan-Niaki H. PI3k/AKT signaling pathway: Erythropoiesis and beyond. J Cell Physiol. 2019 Mar;234(3):2373–85. [DOI] [PubMed]
- [77].Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb Perspect Med. 2013 Apr 1;3(4):a011601. [DOI] [PMC free article] [PubMed]
- [78].Li W, Guo R, Song Y, Jiang Z. Erythroblastic island macrophages shape normal erythropoiesis and drive associated disorders in erythroid hematopoietic diseases. Front Cell Dev Biol. 2020;8:613885. [DOI] [PMC free article] [PubMed]
- [79].Moras M, Lefevre SD, Ostuni MA. From erythroblasts to mature red blood cells: organelle clearance in mammals. Front Physiol. 2017;8:1076. [DOI] [PMC free article] [PubMed]
- [80].Broxmeyer HE. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. J Exp Med. 2013 Feb 11;210(2):205–8. [DOI] [PMC free article] [PubMed]
- [81].Bernstein ID, Andrews RG, Zsebo KM. Recombinant human stem cell factor enhances the formation of colonies by CD34+ and CD34+lin- cells, and the generation of colony-forming cell progeny from CD34+lin- cells cultured with interleukin-3, granulocyte colony-stimulating factor, or granulocyte-macrophage colony-stimulating factor. Blood. 1991 Jun 1;77(11):2316–21. [PubMed]
- [82].von Lindern M, Zauner W, Mellitzer G, Steinlein P, Fritsch G, Huber K, et al. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999 Jul 15;94(2):550–9. [PubMed]
- [83].Serke S, Huhn D. Effects of various recombinant human hemopoietic growth factors (rhEpo, rhG-CSF, rhGM-CSF, rhIl-3) on the growth of peripheral blood progenitor cells (BFU-E, CFU-GM). Blut. 1990 Jul;61(1):25–9. [DOI] [PubMed]
- [84].Lewis JL, Marley SB, Blackett NM, Szydlo R, Goldman JM, Gordon MY. Interleukin 3 (IL-3), but not stem cell factor (SCF) increases self-renewal by human erythroid burst-forming units (BFU-E) in vitro. Cytokine. 1998 Jan;10(1):49–54. [DOI] [PubMed]
- [85].Mizuguchi T, Kosaka M, Saito S. Activin A suppresses proliferation of interleukin-3-responsive granulocyte-macrophage colony-forming progenitors and stimulates proliferation and differentiation of interleukin-3-responsive erythroid burst-forming progenitors in the peripheral blood. Blood. 1993 Jun 1;81(11):2891–7. [PubMed]
- [86].Suzuki M, Kobayashi-Osaki M, Tsutsumi S, Pan X, Ohmori S, Takai J, et al. GATA factor switching from GATA2 to GATA1 contributes to erythroid differentiation. Genes Cell. 2013 Nov;18(11):921–33. [DOI] [PubMed]
- [87].Valent P, Büsche G, Theurl I, Uras IZ, Germing U, Stauder R, et al. Normal and pathological erythropoiesis in adults: from gene regulation to targeted treatment concepts. Haematologica. 2018 Oct;103(10):1593–603. [DOI] [PMC free article] [PubMed]
- [88].Moriguchi T, Yamamoto M. A regulatory network governing Gata1 and Gata2 gene transcription orchestrates erythroid lineage differentiation. Int J Hematol. 2014 Nov;100(5):417–24. [DOI] [PubMed]
- [89].Bresnick EH, Hewitt KJ, Mehta C, Keles S, Paulson RF, Johnson KD. Mechanisms of erythrocyte development and regeneration: implications for regenerative medicine and beyond. Development. 2018 Jan 10;145(1):dev151423. [DOI] [PMC free article] [PubMed]
- [90].Cantor AB, Orkin SH. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene. 2002 May 13;21(21):3368–76. [DOI] [PubMed]
- [91].Gutiérrez L, Caballero N, Fernández-Calleja L, Karkoulia E, Strouboulis J. Regulation of GATA1 levels in erythropoiesis. IUBMB Life. 2020 Jan;72(1):89–105. [DOI] [PubMed]
- [92].Simon MC, Pevny L, Wiles MV, Keller G, Costantini F, Orkin SH. Rescue of erythroid development in gene targeted GATA-1- mouse embryonic stem cells. Nat Genet. 1992 May;1(2):92–8. [DOI] [PubMed]
- [93].Gregory T, Yu C, Ma A, Orkin SH, Blobel GA, Weiss MJ. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood. 1999 Jul 1;94(1):87–96. [PubMed]
- [94].Ribeil JA, Zermati Y, Vandekerckhove J, Cathelin S, Kersual J, Dussiot M, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007 Jan 4;445(7123):102–5. [DOI] [PubMed]
- [95].Hermine O, Arlet JB, Ribeil JA, Guillerm F, Vandekerkhove J, Courtois G. HSP70, an erythropoiesis regulator that determines the fate of erythroblasts between death and differentiation. Transfus Clin Biol. 2013 May;20(2):144–7. [DOI] [PubMed]
- [96].Bhoopalan SV, Huang LJS, Weiss MJ. Erythropoietin regulation of red blood cell production: from bench to bedside and back. F1000Res. 2020;9:9. [DOI] [PMC free article] [PubMed]
- [97].Wu H, Klingmüller U, Besmer P, Lodish HF. Interaction of the erythropoietin and stem-cell-factor receptors. Nature. 1995 Sep 21;377(6546):242–6. [DOI] [PubMed]
- [98].Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. 2008 Dec;36(12):1573–84. [DOI] [PubMed]
- [99].Menon MP, Karur V, Bogacheva O, Bogachev O, Cuetara B, Wojchowski DM. Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J Clin Invest. 2006 Mar;116(3):683–94. [DOI] [PMC free article] [PubMed]
- [100].Ribeil JA, Arlet JB, Dussiot M, Cruz Moura I, Courtois G, Hermine O. Ineffective erythropoiesis in β-thalassemia. Sci World J. 2013 Mar 28;2013:e394295–11. [DOI] [PMC free article] [PubMed]
- [101].Testa U. Apoptotic mechanisms in the control of erythropoiesis. Leukemia. 2004 Jul;18(7):1176–99. [DOI] [PubMed]
- [102].Liu Y, Pop R, Sadegh C, Brugnara C, Haase VH, Socolovsky M. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood. 2006 Jul 1;108(1):123–33. [DOI] [PMC free article] [PubMed]
- [103].Lee HY, Gao X, Barrasa MI, Li H, Elmes RR, Peters LL, et al. PPAR-α and glucocorticoid receptor synergize to promote erythroid progenitor self-renewal. Nature. 2015 Jun 25;522(7557):474–7. [DOI] [PMC free article] [PubMed]
- [104].Verma R, Su S, McCrann DJ, Green JM, Leu K, Young PR, et al. RHEX, a novel regulator of human erythroid progenitor cell expansion and erythroblast development. J Exp Med. 2014 Aug 25;211(9):1715–22. [DOI] [PMC free article] [PubMed]
- [105].Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017 Jan 26;168(3):344–61. [DOI] [PMC free article] [PubMed]
- [106].Coulon S, Dussiot M, Grapton D, Maciel TT, Wang PHM, Callens C, et al. Polymeric IgA1 controls erythroblast proliferation and accelerates erythropoiesis recovery in anemia. Nat Med. 2011 Oct 23;17(11):1456–65. [DOI] [PubMed]
- [107].Aoto M, Iwashita A, Mita K, Ohkubo N, Tsujimoto Y, Mitsuda N. Transferrin receptor 1 is required for enucleation of mouse erythroblasts during terminal differentiation. FEBS Open Bio. 2019 Feb;9(2):291–303. [DOI] [PMC free article] [PubMed]
- [108].Blank U, Karlsson S. TGF-β signaling in the control of hematopoietic stem cells. Blood. 2015 Jun 4;125(23):3542–50. [DOI] [PubMed]
- [109].Ren Y, Zhu J, Han Y, Li P, Wu J, Qu H, et al. Regulatory association of long noncoding RNAs and chromatin accessibility facilitates erythroid differentiation. Blood Adv. 2021 Dec 14;5(23):5396–409. [DOI] [PMC free article] [PubMed]
- [110].Arlet JB, Ribeil JA, Guillem F, Negre O, Hazoume A, Marcion G, et al. HSP70 sequestration by free α-globin promotes ineffective erythropoiesis in β-thalassaemia. Nature. 2014 Oct 9;514(7521):242–6. [DOI] [PubMed]
- [111].Suragani RN, Cadena SM, Cawley SM, Sako D, Mitchell D, Li R, et al. Transforming growth factor-β superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat Med. 2014 Apr;20(4):408–14. [DOI] [PubMed]
- [112].Dussiot M, Maciel TT, Fricot A, Chartier C, Negre O, Veiga J, et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in β-thalassemia. Nat Med. 2014 Apr;20(4):398–407. [DOI] [PMC free article] [PubMed]
- [113].Söderberg SS, Karlsson G, Karlsson S. Complex and context dependent regulation of hematopoiesis by TGF-beta superfamily signaling. Ann N Y Acad Sci. 2009 Sep;1176:55–69. [DOI] [PubMed]
- [114].Zermati Y, Fichelson S, Valensi F, Freyssinier JM, Rouyer-Fessard P, Cramer E, et al. Transforming growth factor inhibits erythropoiesis by blocking proliferation and accelerating differentiation of erythroid progenitors. Exp Hematol. 2000 Aug;28(8):885–94. [DOI] [PubMed]
- [115].Naka K, Hirao A. Regulation of hematopoiesis and hematological disease by TGF-β family signaling molecules. Cold Spring Harb Perspect Biol. 2017 Sep;9(9):a027987. [DOI] [PMC free article] [PubMed]
- [116].Carrancio S, Markovics J, Wong P, Leisten J, Castiglioni P, Groza MC, et al. An activin receptor IIA ligand trap promotes erythropoiesis resulting in a rapid induction of red blood cells and haemoglobin. Br J Haematol. 2014 Jun;165(6):870–82. [DOI] [PMC free article] [PubMed]
- [117].David CJ, Massagué J. Contextual determinants of TGF-β action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018 Jul;19(7):419–35. [DOI] [PMC free article] [PubMed]
- [118].Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGF-β. Nat Rev Immunol. 2010 Aug;10(8):554–67. [DOI] [PMC free article] [PubMed]
- [119].Mullen AC, Wrana JL. TGF-β family signaling in embryonic and somatic stem-cell renewal and differentiation. Cold Spring Harb Perspect Biol. 2017 Jul 5;9(7):a022186. [DOI] [PMC free article] [PubMed]
- [120].Chen W, Ten Dijke P. Immunoregulation by members of the TGF-β superfamily. Nat Rev Immunol. 2016 Nov 25;16(12):723–40. [DOI] [PubMed]
- [121].Massagué J. TGF-β in cancer. Cell. 2008 Jul 25;134(2):215–30. [DOI] [PMC free article] [PubMed]
- [122].Huang JJ, Blobe GC. Dichotomous roles of TGF-β in human cancer. Biochem Soc Trans. 2016 Oct 15;44(5):1441–54. [DOI] [PMC free article] [PubMed]
- [123].Wang J, Sergina N, Ko TC, Gong J, Brattain MG. Autocrine and exogenous transforming growth factor beta control cell cycle inhibition through pathways with different sensitivity. J Biol Chem. 2004 Sep 17;279(38):40237–44. [DOI] [PubMed]
- [124].Derynck R, Turley SJ, Akhurst RJ. TGF-β biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021 Jan;18(1):9–34. [DOI] [PMC free article] [PubMed]
- [125].Groeneveldt C, van Hall T, van der Burg SH, Ten Dijke P, van Montfoort N. Immunotherapeutic potential of TGF-β inhibition and oncolytic viruses. Trends Immunol. 2020 May;41(5):406–20. [DOI] [PubMed]
- [126].Li S, Liu M, Do MH, Chou C, Stamatiades EG, Nixon BG, et al. Cancer immunotherapy via targeted TGF-β signalling blockade in TH cells. Nature. 2020 Nov;587(7832):121–5. [DOI] [PMC free article] [PubMed]
- [127].Ribeil JA, Arlet JB, Dussiot M, Moura IC, Courtois G, Hermine O. Ineffective erythropoiesis in β-thalassemia. Sci World J. 2013;2013:394295. [DOI] [PMC free article] [PubMed]
- [128].Munugalavadla V, Kapur R. Role of c-Kit and erythropoietin receptor in erythropoiesis. Crit Rev Oncol Hematol. 2005 Apr;54(1):63–75. [DOI] [PubMed]
- [129].Xue Y, Lim S, Yang Y, Wang Z, Jensen LDE, Hedlund EM, et al. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med. 2011 Dec 4;18(1):100–10. [DOI] [PubMed]
- [130].Pham TND, Ma W, Miller D, Kazakova L, Benchimol S. Erythropoietin inhibits chemotherapy-induced cell death and promotes a senescence-like state in leukemia cells. Cell Death Dis. 2019 Jan 8;10(1):22. [DOI] [PMC free article] [PubMed]
- [131].Patterson SD, Rossi JM, Paweletz KL, Fitzpatrick VD, Begley CG, Busse L, et al. Functional EpoR pathway utilization is not detected in primary tumor cells isolated from human breast, non-small cell lung, colorectal, and ovarian tumor tissues. PLoS One. 2015;10(3):e0122149. [DOI] [PMC free article] [PubMed]
- [132].Elliott S, Swift S, Busse L, Scully S, Van G, Rossi J, et al. Epo receptors are not detectable in primary human tumor tissue samples. PLoS One. 2013;8(7):e68083. [DOI] [PMC free article] [PubMed]
- [133].Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005 Sep 1;23(25):5960–72. [DOI] [PubMed]
- [134].Henke M, Laszig R, Rübe C, Schäfer U, Haase KD, Schilcher B, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003 Oct 18;362(9392):1255–60. [DOI] [PubMed]
- [135].Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409. [DOI] [PubMed]
- [136].Ghanbari-Movahed M, Ghanbari-Movahed Z, Momtaz S, Kilpatrick KL, Farzaei MH, Bishayee A. Unlocking the secrets of cancer stem cells with γ-secretase inhibitors: A novel anticancer strategy. Molecules. 2021 Feb 12;26(4):972. [DOI] [PMC free article] [PubMed]
- [137].Okuhashi Y, Itoh M, Arai A, Nara N, Tohda S. Gamma-secretase inhibitors induce erythroid differentiation in erythroid leukemia cell lines. Anticancer Res. 2010 Oct;30(10):4071–4. [PubMed]
- [138].Venkatesh V, Nataraj R, Thangaraj GS, Karthikeyan M, Gnanasekaran A, Kaginelli SB, et al. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018;5:5. [DOI] [PMC free article] [PubMed]
- [139].Bapat A, Schippel N, Shi X, Jasbi P, Gu H, Kala M, et al. Hypoxia promotes erythroid differentiation through the development of progenitors and proerythroblasts. Exp Hematol. 2021 May;97:32–46.e35. [DOI] [PMC free article] [PubMed]
- [140].Zhang FL, Shen GM, Liu XL, Wang F, Zhao YZ, Zhang JW. Hypoxia-inducible factor 1-mediated human GATA1 induction promotes erythroid differentiation under hypoxic conditions. J Cell Mol Med. 2012 Aug;16(8):1889–99. [DOI] [PMC free article] [PubMed]
- [141].Tyrkalska SD, Pérez-Oliva AB, Rodríguez-Ruiz L, Martínez-Morcillo FJ, Alcaraz-Pérez F, Martínez-Navarro FJ, et al. Inflammasome regulates hematopoiesis through cleavage of the master erythroid transcription factor GATA1. Immunity. 2019 Jul 16;51(1):50–63.e5. [DOI] [PMC free article] [PubMed]
- [142].Gonzalez-Menendez P, Romano M, Yan H, Deshmukh R, Papoin J, Oburoglu L, et al. An IDH1-vitamin C crosstalk drives human erythroid development by inhibiting pro-oxidant mitochondrial metabolism. Cell Rep. 2021 Feb 2;34(5):108723. [DOI] [PMC free article] [PubMed]
- [143].Gilreath JA, Rodgers GM. How I treat cancer-associated anemia. Blood. 2020 Aug 13;136(7):801–13. [DOI] [PubMed]
- [144].Voskou S, Phylactides M, Afantitis A, Melagraki G, Tsoumanis A, Koutentis PA, et al. MS-275 chemical analogues promote hemoglobin production and erythroid differentiation of K562 cells. Hemoglobin. 2019 Mar;43(2):116–21. [DOI] [PubMed]
- [145].Chen Y, Dong Y, Lu X, Li W, Zhang Y, Mao B, et al. Inhibition of aryl hydrocarbon receptor signaling promotes the terminal differentiation of human erythroblasts. J Mol Cell Biol. 2022 Jan 11;14:mjac001. [DOI] [PMC free article] [PubMed]
- [146].Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002 Jan;3(1):11–20. [DOI] [PubMed]
- [147].Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005 Apr 18;24(17):2810–26. [DOI] [PubMed]
- [148].Swartz KL, Wood SN, Murthy T, Ramirez O, Qin G, Pillai MM, et al. E2F-2 promotes nuclear condensation and enucleation of terminally differentiated erythroblasts. Mol Cell Biol. 2017 Jan 1;37(1):e00274–16. [DOI] [PMC free article] [PubMed]
- [149].Martin-Perez D, Piris MA, Sanchez-Beato M. Polycomb proteins in hematologic malignancies. Blood. 2010 Dec 16;116(25):5465–75. [DOI] [PubMed]
- [150].Schuringa JJ, Vellenga E. Role of the polycomb group gene BMI1 in normal and leukemic hematopoietic stem and progenitor cells. Curr Opin Hematol. 2010 Jul;17(4):294–9. [DOI] [PubMed]
- [151].Konuma T, Oguro H, Iwama A. Role of the polycomb group proteins in hematopoietic stem cells. Dev Growth Differ. 2010 Aug;52(6):505–16. [DOI] [PubMed]
- [152].Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003 May 15;423(6937):302–5. [DOI] [PubMed]
- [153].Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003 May 15;423(6937):255–60. [DOI] [PubMed]
- [154].Gao R, Chen S, Kobayashi M, Yu H, Zhang Y, Wan Y, et al. Bmi1 promotes erythroid development through regulating ribosome biogenesis. Stem Cell. 2015 Mar;33(3):925–38. [DOI] [PMC free article] [PubMed]
- [155].Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011 Jan;12(1):21–35. [DOI] [PMC free article] [PubMed]
- [156].Zhang X, Campreciós G, Rimmelé P, Liang R, Yalcin S, Mungamuri SK, et al. FOXO3-mTOR metabolic cooperation in the regulation of erythroid cell maturation and homeostasis. Am J Hematol. 2014 Oct;89(10):954–63. [DOI] [PMC free article] [PubMed]
- [157].Yang L, Shivakumar P, Kinder JM, Way SS, Donnelly B, Mourya R, et al. Regulation of bile duct epithelial injury by hepatic CD71+ erythroid cells. JCI Insight. 2020 Jun 4;5(11):135751. [DOI] [PMC free article] [PubMed]
- [158].Dunsmore G, Bozorgmehr N, Delyea C, Koleva P, Namdar A, Elahi S. Erythroid suppressor cells compromise neonatal immune response against bordetella pertussis. J Immunol. 2017 Sep 15;199(6):2081–95. [DOI] [PubMed]
- [159].Shim YA, Weliwitigoda A, Campbell T, Dosanjh M, Johnson P. Splenic erythroid progenitors decrease TNF-α production by macrophages and reduce systemic inflammation in a mouse model of T cell-induced colitis. Eur J Immunol. 2021 Mar;51(3):567–79. [DOI] [PubMed]
- [160].Dunsmore G, Koleva P, Ghobakhloo N, Sutton R, Ambrosio L, Meng X, et al. Lower abundance and impaired function of CD71+ erythroid cells in inflammatory bowel disease patients during pregnancy. J Crohns Colitis. 2019 Feb 1;13(2):230–44. [DOI] [PMC free article] [PubMed]
- [161].Pilon AM, Arcasoy MO, Dressman HK, Vayda SE, Maksimova YD, Sangerman JI, et al. Failure of terminal erythroid differentiation in EKLF-deficient mice is associated with cell cycle perturbation and reduced expression of E2F2. Mol Cell Biol. 2008 Dec;28(24):7394–401. [DOI] [PMC free article] [PubMed]
- [162].Tallack MR, Keys JR, Humbert PO, Perkins AC. EKLF/KLF1 controls cell cycle entry via direct regulation of E2f2. J Biol Chem. 2009 Jul 31;284(31):20966–74. [DOI] [PMC free article] [PubMed]
- [163].Gnanapragasam MN, McGrath KE, Catherman S, Xue L, Palis J, Bieker JJ. EKLF/KLF1-regulated cell cycle exit is essential for erythroblast enucleation. Blood. 2016 Sep 22;128(12):1631–41. [DOI] [PMC free article] [PubMed]