Abstract
Objectives. To assess the effectiveness of vaccine-induced immunity against new infections, all-cause emergency department (ED) and hospital visits, and mortality in Indiana.
Methods. Combining statewide testing and immunization data with patient medical records, we matched individuals who received at least 1 dose of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines with individuals with previous SARS-CoV-2 infection on index date, age, gender, race/ethnicity, zip code, and clinical diagnoses. We compared the cumulative incidence of infection, all-cause ED visits, hospitalizations, and mortality.
Results. We matched 267 847 pairs of individuals. Six months after the index date, the incidence of SARS-CoV-2 infection was significantly higher in vaccine recipients (6.7%) than the previously infected (2.9%). All-cause mortality in the vaccinated, however, was 37% lower than that of the previously infected. The rates of all-cause ED visits and hospitalizations were 24% and 37% lower in the vaccinated than in the previously infected.
Conclusions. The significantly lower rates of all-cause ED visits, hospitalizations, and mortality in the vaccinated highlight the real-world benefits of vaccination. The data raise questions about the wisdom of reliance on natural immunity when safe and effective vaccines are available. (Am J Public Health. 2023;113(1):96–104. https://doi.org/10.2105/AJPH.2022.307112)
Strong and consistent evidence shows that mRNA vaccines BNT162b2 and mRNA-1273 and the Janssen vaccine JNJ-78436735 confer considerable protection to fully vaccinated individuals against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, severe illnesses requiring hospitalization, and mortality.1–6 However, vaccine effectiveness is not 100%, and the risk of breakthrough infections remains, especially with newer variants.7,8 Furthermore, data and opinions diverge on the extent of the waning immunity provided by the mRNA vaccines.9,10 While a population-based observational study suggested that immunity waned in individuals within 2 months after completing the 2-dose sequence of the BNT162b2 vaccine,11 a randomized clinical trial showed that 6 months after vaccination, the BNT162b2 vaccine’s effectiveness against SARS-CoV-2 infection remained strong at higher than 86%; its effectiveness against the severe disease was 96.7%.12
Natural immunity induced by SARS-CoV-2 infection also protects against reinfection. Systematic reviews of immunological evidence suggested that SARS-CoV-2‒specific immunity appeared soon after infection.13,14 Extensive observational studies confirmed the significantly reduced risk for subsequent infection by more than 80% for at least 6 to 12 months in individuals with previous infection.15–17 Data are mixed on the relative levels of protection conferred by vaccination versus infection.18–20 Less understood is the real-world time course of the protective effects of previous infection and vaccination against new infection acquisition and all-cause mortality and hospitalization in persons of different age groups. Unlike COVID-19‒specific outcomes used by earlier studies, all-cause emergency department (ED) visits, hospitalizations, and mortality cover a broader spectrum of health consequences of the disease.
In this observational cohort study, we leveraged public health immunization data and electronic medical record data from a statewide health information exchange and state health department to examine the incidence rates of SARS-CoV-2 infection, all-cause ED visit, hospitalization, and death in individuals who had been vaccinated compared with those with previous infections in a real-world population.
METHODS
We derived data used in this research from the Indiana Network for Patient Care (INPC), one of the largest health information networks in the United States.21 Briefly, the INPC is a central repository of clinical and administrative health data from 38 health systems representing 117 hospitals and 18 486 physician practices, commercial laboratories, and public health departments across Indiana. At the emergence of the pandemic, the Indiana Health Information Exchange expanded the INPC system to receive daily feeds of SARS-CoV-2 test results from all statewide testing locations and daily death records through the Indiana State Department of Health and Family Social Services Administration.22 Furthermore, all COVID-19 vaccine data contained in the Indiana immunization registry were combined with testing and outcomes data.
Study Design
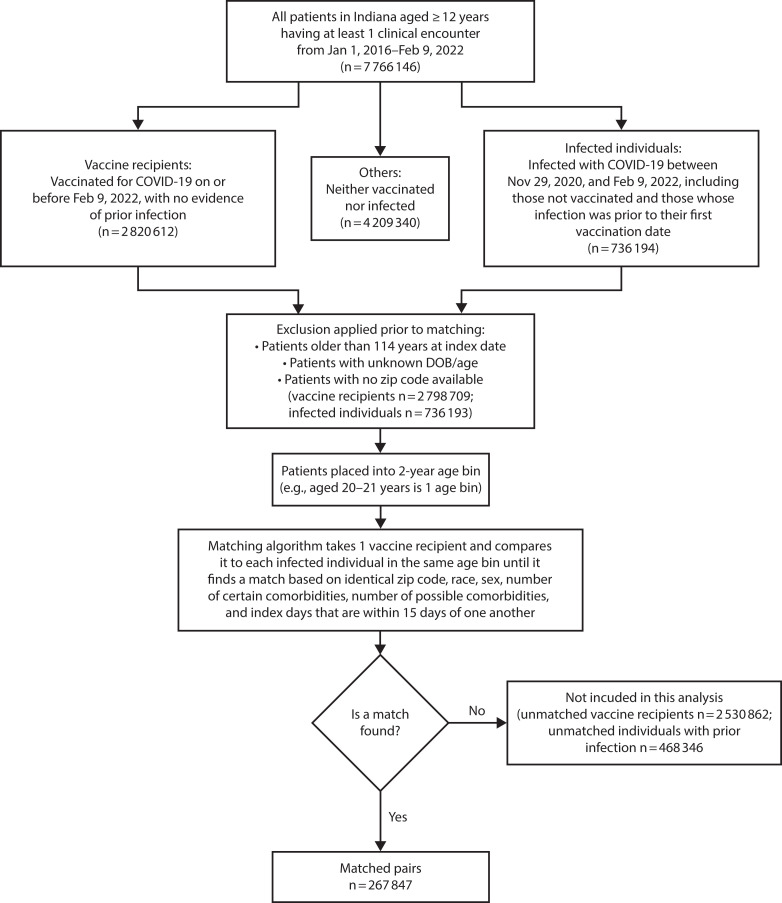
We derived the observational study cohort from the INPC and the Indiana statewide testing data. The cohort consisted of matched pairs of vaccine recipients and unvaccinated individuals with SARS-CoV-2 infections. See Figure A (available as a supplement to the online version of this article at https://ajph.org) for a schematic depiction of the comparison groups. Eligible participants were Indiana residents aged 12 years or older with at least 1 previously recorded health care encounter with the INPC between January 1, 2016, and February 9, 2022; the requirement of a previous encounter ensured a more complete capture of the characteristics of the study participants. Patient medical records in INPC and test data were linked, de-duplicated, and aggregated using a global algorithm.23 Vaccine data from the state immunization registry were imported into the health information exchange and integrated with laboratory test data.
Observation of infected participants started 30 days after the initial infection and ended at the end of follow-up or vaccination, whichever came first. Similarly, observation of vaccinated participants started 30 days after the initial vaccination and ended with the conclusion of follow-up or infection, whichever came first. We applied the 30-day time window of exclusion to both groups to ensure equal surveillance and comparability.
Matched Cohorts
A vaccine recipient’s index date was defined as 30 days after the first SARS-CoV-2 vaccination. In an individual with a previous SARS-CoV-2 infection, we defined the index date as 30 days after the initial infection. In both situations, the initial infection and vaccination represented the first point of viral exposure, whereas the 30-day window approximated the time of immunity development. We matched each vaccine recipient with an infected participant on the index date (+/− 15 days), age, gender, race/ethnicity, zip code, and the number of coexisting conditions that had been identified by the Centers for Disease Control and Prevention (CDC) as “conclusive” or “suggestive” risk factors for severe COVID-19 (https://bit.ly/3gTvA3w; complete lists of CDC-identified comorbid conditions also appear in the footnotes to Table 1). The construction of the matched cohort is depicted in Figure 1.
TABLE 1—
A Study of SARS-COV-2 Infection, Hospitalizations, and Mortality in Vaccinated and Infected Individuals: Demographic and Clinical Characteristics of the Matched Cohorts on the Index Date, Indiana, 2020‒2022
| Vaccinated (n = 267 847) | Unvaccinated With Previous Infection (n = 267 847) | P | |
| Race, no. (%) | > .99 | ||
| American Indian or Alaska Native | 53 (0.0) | 53 (0.0) | |
| Asian/Pacific Islander | 1 684 (0.6) | 1 684 (0.6) | |
| Black or African American | 21 044 (7.9) | 21 044 (7.9) | |
| Multiracial | 43 (0.0) | 43 (0.0) | |
| Other/unknown | 9 630 (3.6) | 9 630 (3.6) | |
| White | 235 393 (87.9) | 235 393 (87.9) | |
| Ethnicity, no. (%) | < .001 | ||
| Hispanic or Latino | 13 224 (4.9) | 16 733 (6.2) | |
| Not Hispanic or Latino | 220 367 (82.3) | 235 639 (88.0) | |
| Other/unknown | 34 256 (12.8) | 15 475 (5.8) | |
| Gender, no. (%) | > .99 | ||
| Female | 155 759 (58.2) | 155 759 (58.2) | |
| Male | 112 085 (41.8) | 112 085 (41.8) | |
| Unknown | 3 (0.0) | 3 (0.0) | |
| Age, y | > .99 | ||
| Mean (SD) | 38.9 (16.4) | 38.9 (16.4) | .98 |
| Median (IQR) | 37 (26‒51) | 37 (26‒51) | |
| 12–19, no. (%) | 31 454 (11.7) | 31 454 (11.7) | |
| 20–39, no. (%) | 115 511 (43.1) | 115 511 (43.1) | |
| 40–59, no. (%) | 87 199 (32.5) | 87 199 (32.5) | |
| 60–79, no. (%) | 31 249 (11.7) | 31 249 (11.7) | |
| 80–110, no. (%) | 2 434 (0.9) | 2 434 (0.9) | |
| CDC “certain” risk scorea | |||
| Mean (SD) | 0 (0.2) | 0 (0.2) | > .99 |
| Median (IQR) | 0 (0‒0) | 0 (0‒0) | |
| CDC “possible” risk scoreb | |||
| Mean (SD) | 0.1 (0.3) | 0.1 (0.3) | > .99 |
| Median (IQR) | 0 (0‒0) | 0 (0‒0) | |
| CDC sum risk scorec | |||
| Mean (SD) | 0.1 (0.4) | 0.1 (0.4) | > .99 |
| Median (IQR) | 0 (0‒0) | 0 (0‒0) |
Note. CDC = Centers for Disease Control and Prevention; IQR = interquartile range; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
CDC “certain” risk score conditions: cancer, chronic kidney disease, chronic obstructive pulmonary disease, heart conditions, sickle cell disease, solid organ transplant recipient, type 1 diabetes mellitus, type 2 diabetes mellitus.
CDC “possible” risk score conditions: asthma, cerebrovascular disease, hypertension, immunocompromised state, liver disease, neurologic conditions, obesity, other respiratory diseases, thalassemia.
CDC sum risk score: a sum of how many “certain” and “possible” conditions a person was flagged for.
FIGURE 1—
Construction of Matched Cohorts of Infected and Vaccinated Individuals: SARS-COV-2 Infection, Hospitalizations, and Mortality in Vaccinated and Infected Individuals, Indiana, 2020‒2022
Note. DOB = date of birth; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Outcome Events of Interest
The primary outcome events of interest were SARS-CoV-2 infection in those vaccinated or reinfection for the previously infected participants, all-cause ED visits, hospitalizations, and deaths. All outcome events in the study were identified and extracted from the INPC, and deaths were derived from the State of Indiana death records.
Statistical Analysis
Before comparing the outcome event rates, we examined the balance in demographic and clinical characteristics between the vaccine recipients and infection cases to ensure that the 2 groups were comparable and well matched. We used survival analyses to estimate the cumulative incidence rates of SARS-CoV-2 infection for the vaccinated and reinfection for those with previous infections. We similarly estimated the cumulative rates for hospitalization, ED visit, and death.
The index date (i.e., the time zero) represented 30 days after the initial exposure, either to the vaccine or the virus; protection from vaccine-induced and naturally acquired immunity would come after the index date. Matched pairs were censored when an infected participant received a vaccination. Time to mortality that was not observed before the end of the observation window, February 9, 2022, was censored (i.e., the patient was alive at the conclusion of the observation period). Time to infection or reinfection, ED visits, and hospitalization was censored at the time of the event, at the end of the observation window, or when the matched individual was censored, whichever came first. For vaccine recipients and individuals with previous infection, we computed the times from the index date to the outcome events or censoring. Cumulative incidence rates were calculated as , where is the estimated survival function. We used the log-rank test to perform comparisons of the cumulative incidence rates.
We conducted all analyses with R software version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). We considered P values less than .05 statistically significant.
RESULTS
From the INPC, we identified 2 798 709 unique vaccine recipients and 736 193 individuals with documented SARS-CoV-2 infection between November 29, 2020, and February 9, 2022. From these, we matched 267 847 vaccine recipients with the same number of infected participants (Figure 1). The demographic and clinical characteristics of the 2 groups of participants are presented in Table 1.
Infection, All-Cause Care Utilization, and Death
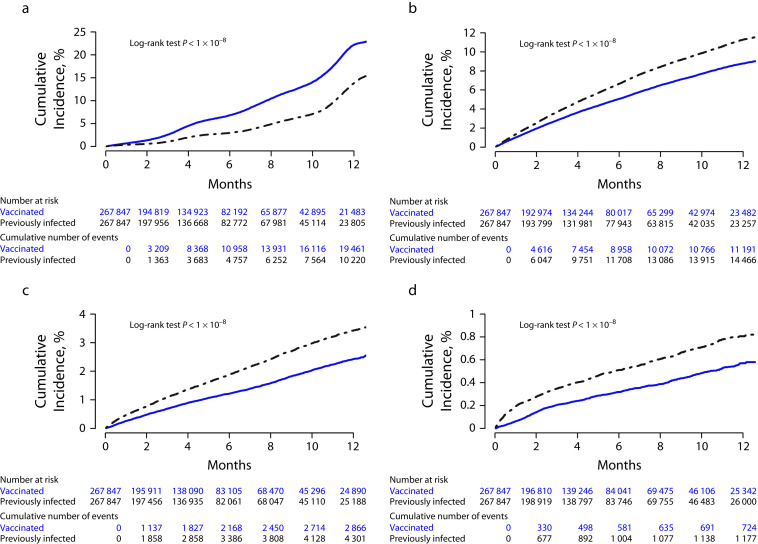
Cumulative incidence rates of events of interest were estimated and are presented graphically in Figure 2. Panel A shows a significantly higher cumulative incidence of infection or reinfection in vaccine recipients than those with previous infection (P < .001). Six months after the index date, the cumulative infection rate in the vaccinated was 6.7% (95% confidence interval [CI] = 6.6%, 6.9%), more than twice the rate in those with previous infections at 2.9% (95% CI = 2.9%, 3.0%).
FIGURE 2—
Cumulative Incidence Rates, in Vaccine Recipients and Individuals With Previous Infections, of (a) SARS-CoV-2 Infection or Reinfection, (b) Emergency Department Visit, (c) All-Cause Hospitalization, and (d) Death: SARS-COV-2 Infection, Hospitalizations, and Mortality in Vaccinated and Infected Individuals, Indiana, 2020‒2022
Note. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Figure B (available as a supplement to the online version of this article at https://ajph.org) shows that the cumulative incidence of all-cause ED visits was significantly lower in vaccinated individuals (P < .001). At 6 months, 6.6% (95% CI = 6.5%, 6.7%) of the individuals with previous infection and 5.0% (95% CI = 4.9%, 5.1%) of the vaccinated individuals had recorded ED visits. Figure C (available as a supplement to the online version of this article at https://ajph.org) shows that the all-cause hospitalization rate was also significantly lower in the vaccinated (P < .001). Six months after the index date, 1.9% (95% CI = 1.8%, 1.9%) of the previously infected individuals and 1.2% (95% CI = 1.1%, 1.3%) of the vaccinated had recorded hospitalization. Figure D (available as a supplement to the online version of this article at https://ajph.org) shows that the mortality rate was also significantly lower in the vaccinated (P < .001). Six months after the index date, mortality rates were respectively 0.51% (95% CI = 0.48%, 0.54%) in the previously infected and 0.32% (95% CI = 0.29%, 0.34%) in the vaccinated.
Age-Stratified Analysis
We performed additional analyses to examine the event rates in individuals of different age groups. Results are presented in Figures B through F (available as supplements to the online version of this article at https://ajph.org). While similar patterns generally held in all age strata, the magnitudes of the estimated incidence rates varied across age groups. In children aged 19 years or younger, vaccine effectiveness against new infections was considerably less than natural immunity acquired from earlier infections (Figure B, section A).
Compared with other age groups, children had the highest incidence rates of new infections. For instance, the 6-month cumulative incidence rates of infection were, respectively, 8.1% (95% CI = 7.6%, 8.5%) and 5.2% (95% CI = 4.8%, 5.5%) for the vaccinated and previously infected. Notably, despite the higher rate of infections observed in the vaccinated children, at 6 months, the rate of all-cause ED visits in the vaccinated children was significantly lower (5.0% for the vaccinated [95% CI = 4.6%, 5.3%] vs 6.9% for the previously infected [95% CI = 6.5%, 7.3%]). The rate of all-cause hospitalization was also lower in the vaccinated (0.3% for vaccinated [95% CI = 0.3%, 0.4%] vs 0.6% for the previously infected [95% CI = 0.5%, 0.8%]; Figure C, sections B and C). Mortality rates were extremely low (< 0.1%) in both vaccinated and previously infected children; the difference was not statistically significant (P = .5).
In adults aged 20 to 39 years, rates of incidence infection were higher among vaccine recipients (7.8%; 95% CI = 7.6%, 8.0%) than persons with previous infections (3.2%; 95% CI = 3.0%, 3.3%) at 6 months (Figure C, section A). However, the 6-month rate of all-cause ED visit was higher in those with previous infections (7.6%; 95% CI = 7.4%, 7.8%) than in the vaccinated (5.4%; 95% CI = 5.2%, 5.6%). Similarly, the 6-month rate of hospitalization was significantly higher in the previously infected (2.1%; 95% CI = 2.0%, 2.2%) than the vaccinated (1.2%; 95% CI = 1.1%, 1.3%). The mortality rate was also higher in the previously infected (0.08%; 95% CI = 0.07%, 0.10%) than the vaccinated (0.04%; 95% CI = 0.03%, 0.07%; Figure C, sections B‒D).
In adults aged 40 to 59 years, the cumulative incidence rate of infections was higher among the vaccinated (6.1%; 95% CI = 5.9%, 6.3%) than the previously infected (2.2%; 95% CI = 2.0%, 2.3%) at 6 months (Figure D, section A). However, the rate of ED visits was significantly lower in the vaccinated (4.6%; 95% CI = 4.4%, 4.8%) than the previously infected (5.4%; 95% CI = 5.2%, 5.6%). All-cause hospitalization rate was also lower in the vaccinated (1.0%; 95% CI = 0.9%, 1.1%) as compared with the infected (1.4%; 95% CI = 1.3%, 1.5%). All-cause mortality rate was similarly lower in the vaccinated (0.2%; 95% CI = 0.2%, 0.3%) than in the infected (0.3%; 95% CI = 0.3%, 0.4%; Figure D, sections B‒D).
In adults aged 60 to 79 years, the cumulative incidence of infections was higher in the vaccinated (3.2%; 95% CI = 2.9%, 3.5%) than the previously infected (1.9%; 95% CI = 1.7%, 2.1%) at 6 months (Figure E, section A, available as a supplement to the online version of this article at https://ajph.org). However, the rate of all-cause ED visits was lower in the vaccinated (4.6%; 95% CI = 4.3%, 4.9%) than the previously infected (5.7%; 95% CI = 5.3%, 6.0%). Rate of all-cause hospitalization was also lower in the vaccinated (2.4%; 95% CI = 2.2%, 2.6%) than in the previously infected (3.3%; 95% CI = 3.0%, 3.5%). All-cause mortality rate was similarly lower in the vaccinated (1.4%; 95% CI = 1.2%, 1.5%) than in the previously infected (2.2%; 95% CI = 2.0%, 2.4%; Figure E, sections B‒D).
Finally, in adults aged 80 years or older, the vaccinated had a lower rate of hospitalization at 6 months (6.2%; 95% CI = 4.9%, 7.4%) than the previously infected (7.6%; 95% CI = 6.3%, 9.0%). All-cause mortality rate was also lower in the vaccinated (8.7%; 95% CI = 7.3%, 10.1%) than the previously infected (12.9%; 95% CI = 11.2%, 14.5%; Figure F, sections C and D).
DISCUSSION
We compared the incidence rates of SARS-CoV-2 infections, all-cause ED visits, hospitalizations, and deaths in the vaccinated and previously infected individuals living in the state of Indiana by combining medical record data and comprehensive testing and vaccination data from a statewide health information exchange and the state department of health. The analysis included 267 847 pairs of vaccine recipients and individuals with previous infections, aged between 12 and 110 years, matched on age, gender, CDC-defined COVID-19 risk scores, and dates of initial exposure (to the vaccines or the virus itself).
The study data showed that vaccination provided superior protection against all-cause ED visits, hospitalizations, and all-cause mortality compared with the levels of protection conferred by previous SARS-CoV-2 infections. Previous studies have shown that mRNA vaccines are highly effective in preventing COVID-19‒related hospitalizations and mortality.3,24,25 However, to our knowledge, no studies have directly compared the real-world protective effects of recent (i.e., 6 months) natural and vaccine-induced immunity against all-cause mortality and hospitalization in a statewide population. The study showed that while people of all age groups benefited from vaccination, reduction in mortality was especially impressive in older adults aged 60 years or older.
Interestingly, at least in the study population and at time of this analysis, natural immunity appears more effective in preventing new infections, a finding that is also reported in an earlier observational study.26 Still, the significant reductions in all-cause health events (i.e., 24% reduction in ED visits, 37% reduction in hospitalization, and 37% reduction in mortality) in the vaccinated group are quite notable, especially considering the higher infection rate in the vaccine recipients during the same period. For states with large populations, a difference of such magnitudes could translate to hundreds or even thousands of lives saved.
Compared with COVID-19‒specific outcomes, all-cause hospitalization and mortality rates used in the current analysis may be more informative on the health consequences of SARS-CoV-2 infection and protective effects of vaccination.27 As the study indicates, the strong natural immunity acquired from a previous infection does not appear to fully compensate for the detrimental effects of the initial infection. Therefore, our findings reinforce the importance of vaccination as an essential public health measure to counter the health impacts of the SARS-CoV-2 pandemic. The significantly higher all-cause mortality observed in individuals with previous infection suggested that reliance on natural immunity to avoid negative SARS-CoV-2 health consequences is not a prudent strategy given the safe and readily available vaccines.
In this research, we have employed a matched cohort study design, an approach used by other large population-based vaccine-effectiveness studies.3 Compared with alternative methods, such as the test-negative design,28 matched cohorts directly emulate the structure of a clinical trial. Although the design provides no guarantee of a causal interpretation, estimation and inference are straightforward.29 The convenience of the analysis, however, comes at the expense of matching costs: among other things, many vaccinated and infected individuals were excluded from the analysis for lack of an appropriate match. We carefully selected the matching variables to minimize biases associated with excluding otherwise eligible participants. For the index date, we opted to use the date of the initial SARS-CoV-2 exposure plus 30 days to accommodate the temporal uncertainty in immunity development: previous studies showed that full immunity was conveyed by the vaccines 7 to 14 days after the second dose,30 whereas robust humoral and cellular immune response occur 5 to 15 days following the onset of symptoms, and antibodies peak within the first few weeks.13,31,32
Well-matched cohorts, however, do not preclude the possibility of remnant differences between the comparison groups, especially in characteristics not captured by the matching variables. For example, in the present context, one might suspect that the lower mortality among the vaccine recipients was attributable to their tendency for risk-averse behaviors, such as mask-wearing, hand sanitizing, and social distancing.33 But such an interpretation was not supported by the data showing a higher incidence of infection among vaccine recipients. In addition, the outcome of primary interest, all-cause mortality, is an objective metric that can be readily captured in both vaccinated and previously infected groups with equal accuracy. As a result, we contend that, despite the study’s observational nature, the comprehensive real-world data source, the large sample size, the temporally matched participant characteristics, and the consistent findings across different age groups lend credibility to the investigation.
While the findings related to the ED visits, hospital admissions, and deaths align with previous research,4,5,18,34 few real-world population-based studies have compared the effectiveness of protection against SARS-CoV-2 for natural infections and vaccinations.35,36 Although our results suggest that natural immunity provides greater protection against subsequent infections than vaccines, residual confounding attributable to health-seeking behavior may still have an impact on these results.37 If the rate of symptomatic testing for SARS-CoV-2 infection is greater among vaccinated individuals (a quantity unmeasured in our study), vaccine effectiveness would be underestimated.
The matched cohort design, while effective for comparing the relative proactive effects of natural and vaccine-induced immunity, presents significant challenges for examining the effects of different vaccines or vaccine doses, as well as their response to specific variants of SARS-CoV-2. In this research, we did not examine the differences among vaccine types, doses, and viral variants, which had distinct temporal patterns in the pandemic, to avoid an over-complication of the matching process. Notwithstanding this limitation, we showed that the all-cause mortality rate was 37% lower in vaccine recipients compared with individuals with previous infections 6 months after the index date. The reductions in ED visits and hospital admissions were respectively 24% and 37%. The findings highlight the real-world benefits of vaccination and allude to the health consequences of SARS-CoV-2 after the initial exposure.
ACKNOWLEDGMENTS
We would like to thank the Regenstrief Institute Inc for their support of faculty and staff effort.
CONFLICTS OF INTEREST
The authors have no conflicts of interest related to this study.
HUMAN PARTICIPANT PROTECTION
This study was reviewed and approved as exempt by the Indiana University institutional review board before data collection and analysis.
POST PUBLICATION UPDATE
March 16, 2023: When originally published, matched pair censoring on page 98 was described incorrectly as “when an infected participant received a vaccination or a vaccine recipient became infected.” Censoring only occurred when an infected participant received a vaccination. An erratum has since been issued, and this PDF has been updated to include the change.
REFERENCES
- 1.Bajema KL, Dahl RM, Prill MM, et al. Effectiveness of COVID-19 mRNA vaccines against COVID-19-associated hospitalization—five Veterans Affairs medical centers, United States, February 1‒ August 6, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1294–1299. doi: 10.15585/mmwr.mm7037e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston EH, Malani PN, Creech CB. The Johnson & Johnson Vaccine for COVID-19. JAMA. 2021;325(15):1575. doi: 10.1001/jama.2021.2927. [DOI] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson MG, Stenehjem E, Grannis S, et al. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care wettings. N Engl J Med. 2021;385(15):1355–1371. doi: 10.1056/NEJMoa2110362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grannis SJ, Rowley EA, Ong TC, et al. Interim estimates of COVID-19 vaccine effectiveness against COVID-19-associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance—nine states, June‒August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1291–1293. doi: 10.15585/mmwr.mm7037e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Natarajan K, Prasad N, Dascomb K, et al. Effectiveness of homologous and heterologous COVID-19 booster doses following 1 Ad.26.COV2.S (Janssen [Johnson & Johnson]) vaccine dose against COVID-19‒associated emergency department and urgent care encounters and hospitalizations among adults—VISION Network, 10 states, December 2021‒March 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):495–502. doi: 10.15585/mmwr.mm7113e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipsitch M, Krammer F, Regev-Yochay G, Lustig Y, Balicer RD. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2022;22(1):57–65. doi: 10.1038/s41577-021-00662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance—VISION Network, 10 states, August 2021‒January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott J, Richterman A, Cevik M. COVID-19 vaccination: evidence of waning immunity is overstated. BMJ. 2021;374(2320):n2320. doi: 10.1136/bmj.n2320. [DOI] [PubMed] [Google Scholar]
- 10.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626–636. doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas SJ, Moreira ED, Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Post N, Eddy D, Huntley C, et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2020;15(12):e0244126. doi: 10.1371/journal.pone.0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrotri M, van Schalkwyk MCI, Post N, et al. T cell response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2021;16(1):e0245532. doi: 10.1371/journal.pone.0245532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397(10283):1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen CH, Michlmayr D, Gubbels SM, Molbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397(10280):1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitale J, Mumoli N, Clerici P, et al. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italy. JAMA Intern Med. 2021;181(10):1407–1408. doi: 10.1001/jamainternmed.2021.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozio CH, Grannis SJ, Naleway AL, et al. Laboratory-confirmed COVID-19 among adults hospitalized with COVID-19-like illness with infection-induced or mRNA vaccine-induced SARS-CoV-2 immunity—nine states, January‒September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1539–1544. doi: 10.15585/mmwr.mm7044e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.León TM, Dorabawila V, Nelson L, et al. COVID-19 cases and hospitalizations by COVID-19 vaccination status and previous COVID-19 diagnosis—California and New York, May‒November 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):125–131. doi: 10.15585/mmwr.mm7104e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenai MB, Rahme R, Noorchashm H. Equivalency of protection from natural immunity in COVID-19 recovered versus fully vaccinated persons: a systematic review and pooled analysis. Cureus. 2021;13(10):e19102. doi: 10.7759/cureus.19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overhage JM. The Indiana health information exchange. In: Dixon BE, editor. Health Information Exchange: Navigating and Managing a Network of Health Information Systems. Vol. 1. Elsevier; 2016. pp. 267–279. [Google Scholar]
- 22.Dixon BE, Grannis SJ, McAndrews C, et al. Leveraging data visualization and a statewide health information exchange to support COVID-19 surveillance and response: application of public health informatics. J Am Med Inform Assoc. 2021;28(7):1363–1373. doi: 10.1093/jamia/ocab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grannis SJ, Overhage JM, McDonald CJ. Analysis of identifier performance using a deterministic linkage algorithm. Proc AMIA Symp. 2002:305–309. [PMC free article] [PubMed] [Google Scholar]
- 24.Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing COVID-19 hospitalizations in the United States. Clin Infect Dis. 2022;74(9):1515–1524. doi: 10.1101/2021.07.08.21259776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non–COVID-19 mortality risk—seven integrated health care organizations, United States, December 14, 2020–July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520–1524. doi: 10.15585/mmwr.mm7043e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazit S, Shlezinger R, Perez G, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) naturally acquired immunity vs. vaccine-induced immunity, reinfections versus breakthrough infections: a retrospective cohort study. Clin Infect Dis. 2022;75(1):e545–e551. doi: 10.1093/cid/ciac262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanga V, Chevinsky JR, Dimitrov LV, et al. Long-term symptoms among adults tested for SARS-CoV-2—United States, January 2020‒April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(36):1235–1241. doi: 10.15585/mmwr.mm7036a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol. 2016;184(5):345–353. doi: 10.1093/aje/kww064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Röltgen K, Powell AE, Wirz OF, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5(54):eabe0240. doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18):1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petherick A, Goldszmidt R, Andrade EB, et al. A worldwide assessment of changes in adherence to COVID-19 protective behaviours and hypothesized pandemic fatigue. Nat Hum Behav. 2021;5(9):1145–1160. doi: 10.1038/s41562-021-01181-x. [DOI] [PubMed] [Google Scholar]
- 34.Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence—25 US jurisdictions, April 4‒December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):132–138. doi: 10.15585/mmwr.mm7104e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott P, Haw D, Wang H, et al. Exponential growth, high prevalence of SARS-CoV-2, and vaccine effectiveness associated with the Delta variant. Science. 2021;374(6574):eabl9551. doi: 10.1126/science.abl9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165–2168. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]