Abstract
Evidence of sex-related disparities in the care and outcomes of patients with acute coronary syndrome (ACS) emerged >30 years ago, and yet the mechanisms behind these sex-specific differences remain unclear. In this Review, we discuss the current literature on differences between women and men in the clinical presentation, pathophysiology, evaluation, management, and outcomes of ACS. Although the symptoms of ACS and the benefits of therapy generally overlap between women and men, women continue to receive less-aggressive invasive and pharmacological therapy than men. In addition, young women in particular have worse short-term and long-term outcomes than men. To understand better the mechanisms behind these continued disparities, we have identified areas of future research that need to be urgently addressed in fields that range from clinical evaluation and management, to increasing representation of women in research.
Cardiovascular disease is the leading cause of death in women as well as in men in the USA, accounting for >20% of deaths in both sexes1. Each year, an estimated 390,000 women develop new and recurrent myocardial infarction (MI) and/or coronary heart disease in the USA alone2. Although deaths from cardiovascular disease have decreased over the past 3 decades, women in many parts of the world still have worse outcomes after acute coronary syndromes (ACS) than men (TABLE 1). Evidence of sex-related disparities in ACS prognosis emerged >30 years ago3; however, the mechanisms behind these differences remain unclear. These fundamental gaps in knowledge are compounded by the underrepresentation of women in cardiovascular clinical trials4. In this Review, we discuss the current, and sometimes conflicting, evidence for sex-related differences in the clinical presentation, pathophysiology, evaluation, management, and outcomes of ACS, and identify areas of future research that need to be urgently addressed.
Table 1 |.
Global trends in sex-based outcomes after acute coronary syndrome
| Country | Years | Outcome | Change in outcome over time in male patients | Change in outcome over time in female patients | Ref. |
|---|---|---|---|---|---|
| Denmark | 1978–2012 | 1-year mortality | From 50% to 9% | From 53% to 15% | 135 |
| Israel | 1981–1994 | 30-day mortality* | From 17% to 11% | From 24% to 15% | 136 |
| Japan | 1979–2008 | In-hospital mortality | From 16%‡ to 6% | From 23%‡ to 12% | 137 |
| Sweden | 1985–2004 | 28-day mortality | 69% reduction | 45% reduction | 138 |
| USA | 1992–2010 | 30-day mortality§ | From 18% to 15% | From 20% to 17% | 139 |
Age-adjusted mortality.
Value estimated from Figure in the cited paper.
Estimates are for an African-American population.
Presentation
Type of ACS
ACS refers to a spectrum of pathological events that leads to myocardial ischaemia and sometimes myocardial injury. ACS encompasses three types of conditions: ST-segment elevation myocardial infarction (STEMI), which is characterized by complete thrombosis of a coronary artery and myocardial necrosis; non-ST-segment elevation myocardial infarction (NSTEMI), which refers to partial thrombosis of a coronary artery and myocardial necrosis; and unstable angina, in which the artery is partially occluded and myocardial necrosis has not yet occurred. Among patients with ACS, fewer women present with STEMI and more present with unstable angina compared with men. An analysis of the GUSTO IIb trial5 showed that STEMI was significantly less frequent in women than in men (27.2% versus 37.0%; P <0.001). In the groups of patients with NSTEMI or unstable angina, women were more likely to have unstable angina than men5. The higher rate of STEMI in men than in women was later confirmed in a sample of 78,254 patients from the Get With The Guidelines–Coronary Artery Disease registry6.
Baseline risk factors
Women with ACS have long been described as being ‘older and sicker’ than their male counterparts. A seminal analysis of the GUSTO IIb trial5 showed that women with ACS were older and had higher rates of traditional risk factors such as diabetes mellitus, hypertension, and previous congestive heart failure compared with men with ACS (FIG. 1). These patterns have been confirmed by other studies from Australia7, Canada8, China9, South Korea10, the Middle East11, and the USA6,8, among others.
Figure 1 |. Sex-specific differences in baseline risk factors for ACS.
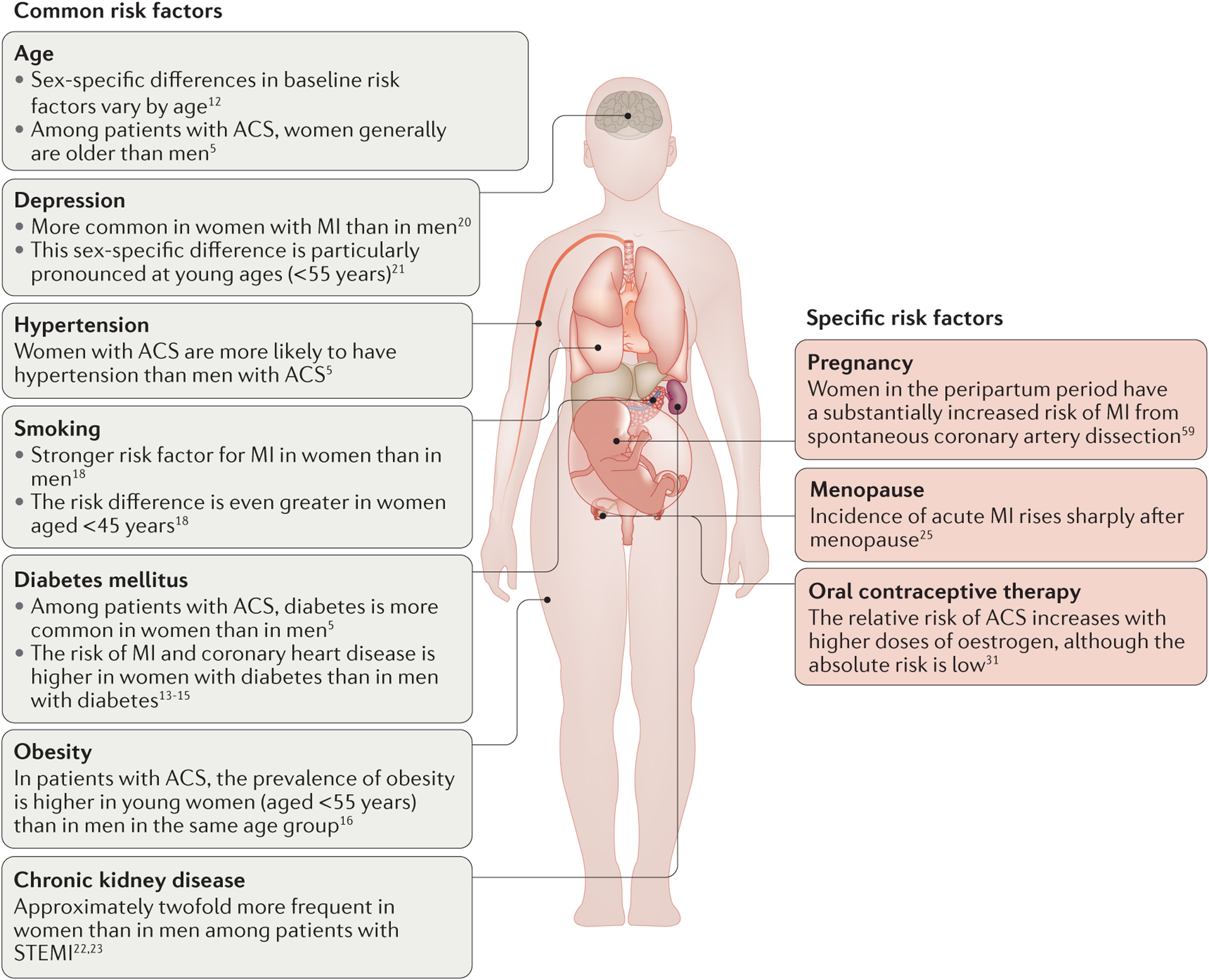
Women have higher rates than men of traditional risk factors such as diabetes mellitus, hypertension, and obesity, and have differential lifestyle and psychosocial determinants. In addition, women have sex-specific risk factors such as pregnancy and menopause. ACS, acute coronary syndrome; MI, myocardial infarction; STEMI, non-ST-segment elevation myocardial infarction.
Of note, differences in baseline risk factors seem to vary by age. A study from the US National Registry of Myocardial Infarction, a registry including >1 million patients with MI between 1994 and 2006, showed that women aged <65 years were more likely to present with a history of diabetes, heart failure, or stroke, and with a higher Killip class than men in the same age group12. These differences in presentation were less pronounced or absent altogether as patients aged.
Diabetes, in particular, carries a differential risk of ACS between women and men. The INTERHEART study13, a global, case–control study in >27,000 participants, showed that women with diabetes were 4.3-times more likely to develop an MI than women without diabetes. By contrast, in men with diabetes the risk of MI was 2.7-times higher than in men without diabetes13. This finding was confirmed in prospective cohort studies and in two randomized clinical trials, which showed that the risk of coronary heart disease was significantly higher in women with diabetes than in men with diabetes14,15. The reason for the differential risk of diabetes between women and men is unknown.
Several lifestyle and psychosocial factors also carry a differential risk of ACS in women and men, particularly in young women. Obesity is more common in young (aged <55 years) female patients with ACS than in their male counterparts (prevalence 51% versus 45%; P = 0.0004)16, although this difference does not seem to exist in older women17. Smoking is a stronger risk factor for MI in women than in men (relative risk (RR) 3.3 versus 1.9), and the difference in risk is even greater in women aged <45 years (RR 7.1 versus 2.3)18. Interestingly, smoking is the biggest risk factor for coronary plaque erosion, which is a particularly common mechanism of ACS in women19. Psychosocial factors such as depression also influence the relationship between patient sex and likelihood of ACS. Among patients with MI, depression is more common in women than in men20. This association is particularly pronounced in young women (aged <55 years), who are twice as likely to have depression compared with young men (prevalence 48% versus 24%)21.
Less traditional comorbidities also affect the likelihood of women developing ACS. In patients with STEMI, chronic kidney disease is approximately twofold more frequent in women than in men, and is associated with worse outcomes22,23. Menopause is thought to be an important risk factor in women because circulating oestrogen has a protective role on the vascular endothelium24. The incidence of acute MI rises sharply in women after menopause; however, the relationship between menopause, age, and cardiovascular events is difficult to unravel25. Although endogenous oestrogen seems to be protective, studies to examine the effect of exogenous oestrogen therapy on women after menopause show that hormone replacement therapy actually precipitates acute coronary events26,27. Of note, a controversial randomized, controlled trial by Schierbeck and colleagues found that women in early postmenopause might derive a cardiovascular benefit from hormone replacement therapy28. However, many investigators have questioned the methods and conclusions of this study29,30 and, therefore, the issue of exogenous oestrogen therapy in women in early postmenopause remains unresolved. Finally, oral contraceptive therapy has long been associated with an increased risk of venous thromboembolic events. A 15-year study of a large, Danish cohort showed that the absolute risk of MI in women taking hormonal contraception was low, but the relative risk was increased with larger doses of oestrogen, while being unaffected by progestin dose31.
Symptoms
The prevailing notion is that symptom presentation in women with acute MI is very different from that in men, and data from several large cohorts support this concept32. Specifically, the GRACE registry33 indicated that women were more likely to have atypical symptoms such as nausea than men. Similarly, a review of nine large cohort studies showed that the absence of chest pain was more common in women with ACS than in men (37% versus 27%)32. This difference was accentuated when only small, single-centre investigations were considered (30% versus 17%)32. Women are also more likely to present with pain in the upper back, neck, arm, and jaw, and with dyspnoea, weakness, and a sense of dread34–37.
Other reviews of sex-specific differences in ACS symptom presentation have yielded conflicting or inconclusive results, in part owing to small, heterogeneous studies that often had methodological concerns38–44. Data from the US National Registry of Myocardial Infarction indicated that women presented without chest pain more frequently than men (42.0% versus 30.7%; P <0.001); however, symptom presentation substantially over-lapped between women and men, rendering generalizations about sex-based differences in ACS symptoms difficult to make12,38. Therefore, although some studies have shown differing proportions of various symptoms in women and men with ACS, overall both sexes experience the same symptoms and, therefore, definitive conclusions on sex-specific differences in ACS symptom presentation cannot be drawn42.
Pathophysiology
Over the past 2 decades, mechanisms other than plaque rupture and thrombus formation have been increasingly recognized as the cause of a substantial proportion of ACS cases, especially in women45 (FIG. 2). Differing mechanisms of ACS that are more prevalent in women than in men include plaque erosion, coronary vasospasm, spontaneous coronary artery dissection, and stress-related (Takotsubo) cardiomyopathy.
Figure 2 |. Sex-specific differences in the pathophysiology of acute coronary syndrome.
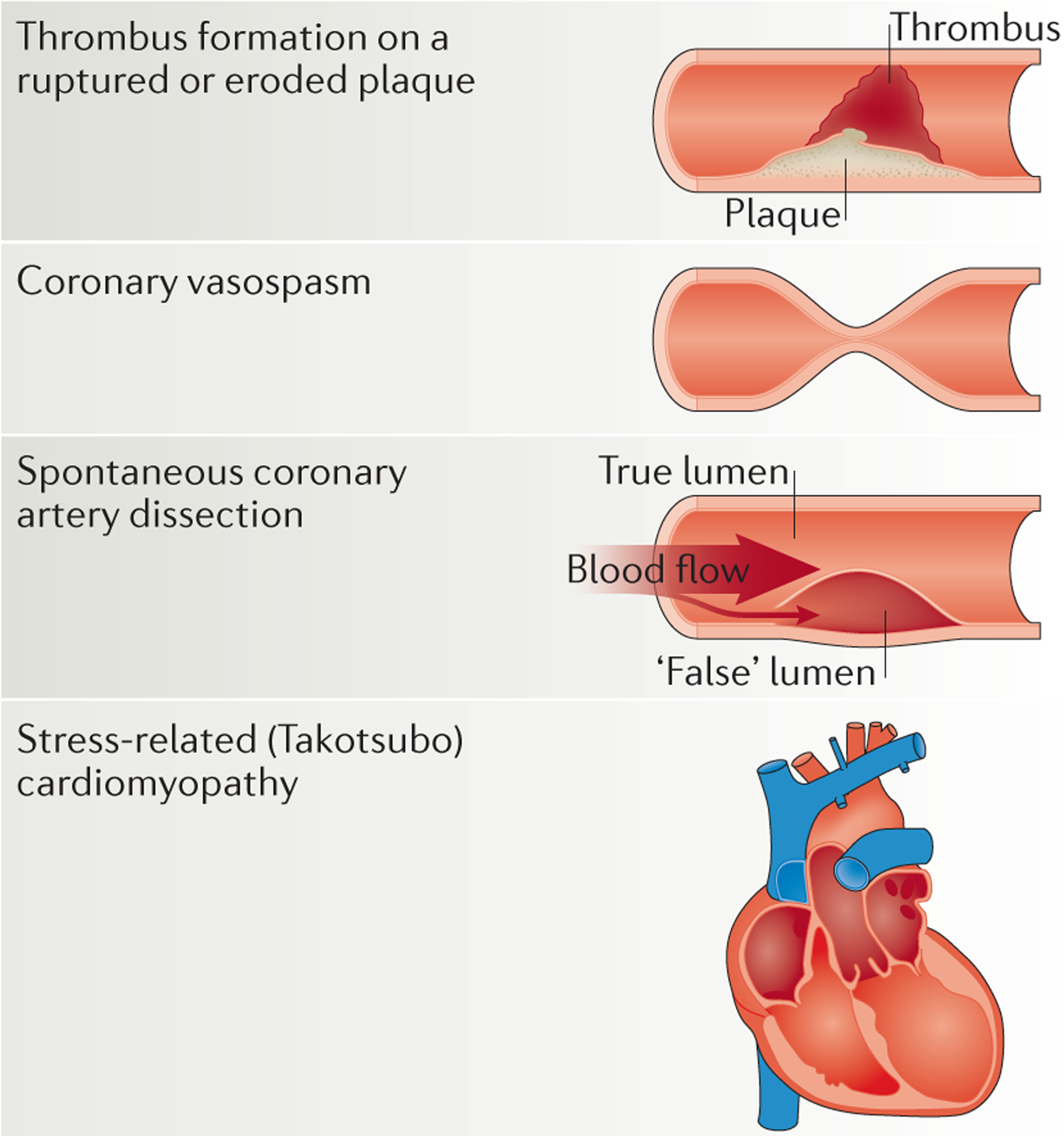
A substantial proportion of acute coronary syndrome cases, especially in women, are caused by mechanisms other than plaque rupture and thrombus formation45. Plaque erosion, coronary vasospasm, spontaneous coronary artery dissection, and stress-related (Takotsubo) cardiomyopathy are more prevalent in women than in men.
Plaque erosion and coronary vasospasm
ACS can be caused by coronary thrombosis with or without occlusion of the artery. Thrombosis, in turn, is caused most commonly by plaque rupture, but can also be caused by plaque erosion46. In patients with MI, plaque rupture is considerably more common in men than women47. The PROSPECT study48 showed that women had smaller coronary lumens, less plaque rupture, and differing coronary plaque characteristics (less necrotic core and calcium content) compared with men. Women were also more likely to have plaque erosions without true plaque rupture48.
The pathophysiology of plaque erosion is postulated to be related to a multitude of mechanisms, including endothelial dysfunction, leukocyte activation, and inflammation19. Plaque erosion might also be related to coronary vasospasm, given that vasospasm can lead to damage and erosions in the endothelium49. Coronary vasospasm probably occurs as a result of transient sympathovagal imbalance and decreased bioavailability of nitric oxide or other vasoactive substances50–52. ACS caused by coronary artery spasm disproportionately affects women53. Nonfatal acute MI occurs in 25% of the population with coronary vasospasm54, although the prognosis in these patients is better than in those with obstructive CAD55. Treatment of coronary vasospasm generally consists of therapy with calcium-channel blockers55.
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection is an uncommon, noniatrogenic, and nontraumatic cause of ACS. Spontaneous coronary artery dissection occurs when the layers of the coronary arterial wall separate leading to the formation of intramural haematoma, compression of the true lumen, and impairment of anterograde flow56,57. Spontaneous coronary artery dissection is often associated with systemic disease processes that predispose arterial beds to injury, such as fibro muscular dysplasia, systemic lupus erythematosus, and various connective tissue disorders58. Overall, this condition is much more common in women than in men: approximately 70% of reported cases of spontaneous coronary artery dissection to date have occurred in women59. Spontaneous coronary artery dissection also seems to be associated with hormonal changes during pregnancy, leading to a substantial burden of disease in women in the peripartum period. Spontaneous coronary artery dissection is increasingly being recognized as an important cause of STEMI in women, accounting for 11% of cases60. Furthermore, women aged <50 years seem to be at particular risk, given that 9% of female patients with ACS have spontaneous coronary artery dissection60. In terms of management, no prospective, randomized data are available to address how to treat patients with spontaneous coronary artery dissection58. A better understanding of the role of revascularization, antithrombotic medications, as well as other secondary prevention medications (such as β-blockers, angiotensin-converting-enzyme inhibitors, and statins) in patients with this condition is urgently needed. Overall in-hospital mortality for spontaneous coronary artery dissection ranges from 1% to 5%60–62. However, female patients, and particularly women in the postpartum period, have the worst prognosis63,64.
Stress-related (Takotsubo) cardiomyopathy
Stress-related cardiomyopathy, also known as Takotsubo cardiomyopathy or transient apical ballooning syndrome, is a condition in which sudden, severe, reversible, left ventricular dysfunction is triggered by an acute emotional stress65. Takotsubo cardiomyopathy has been shown to account for up to 3% of all ACS cases66,67; however, this cardiomyopathy is far more common in women than in men68 and has a marked prevalence in women in postmenopause, in which Takotsubo cardiomyopathy accounts for 6% of ACS cases69. Few data exist on treatment effectiveness because the heart failure usually resolves within weeks45. Prognosis is generally good for patients who survive the initial acute phase of heart failure, and whether outcomes differ by sex is unknown.
Evaluation and management
Diagnosis
Cardiac troponin, a component of the contractile apparatus of the myocardium, is a sensitive and specific marker of cardiac muscle injury that has become a corner stone of ACS diagnosis. Interestingly, troponin levels at ACS presentation are on average lower in women than in men70. Lower cut-off values for troponin levels in women have been proposed for the new high-sensitivity assays for troponin, which might improve detection of MI in women70. Nevertheless, despite these new diagnostic tools and thresholds for troponin, evidence suggests that diagnosis of ACS is missed more often in women than in men. In a study of 10,689 patients who presented to US emergency departments with ACS symptoms, women were slightly more likely to be discharged without hospitalization than men (3.4% versus 1.4%; P = 0.05)71. Additionally, data from the US National Registry of Myocardial Infarction indicated that young women (aged <60 years) were given a diagnosis other than ACS at the time of admission more frequently than young men72. Further research into sex-specific thresholds for troponin and other biomarkers might improve ACS diagnosis in women.
Delay in presentation
Substantial evidence suggests that women present to the hospital for ACS treatment later than men73–75. One study from Hong Kong showed that the median delay time from the onset of ACS symptoms to arrival at the hospital was 15.6 h for men, while the median delay for women was 53.7 h76. The delay in presentation might contribute to poorer outcomes in women77. The reasons for the greater delay in presentation in women than in men include lack of awareness, misinterpretation of symptoms, barriers to accessing care, fear, and embarrassment73,78,79.
Treatment
A preponderance of evidence suggests that, overall, evidence-based medications and invasive procedures are similarly effective in women and men with ACS80,81. However, women with ACS tend to receive fewer evidence-based medications and are less likely to have invasive interventions than their male counterparts6,82. The treatment strategies in both clinical trial and real-world settings are reviewed below.
Reperfusion therapy.
Results from clinical studies have shown that women and men with STEMI derive similar benefit from percutaneous coronary intervention (PCI) relative to fibrinolysis83,84. By contrast, the role of early invasive management in women with unstable angina or NSTEMI seems to be more nuanced. A meta-analysis of trials in the past 2 decades suggested that men with NSTEMI derive a survival benefit from early invasive therapy compared with conservative care85. By contrast, no significant benefit was found in women with NSTEMI85. However, if the results are limited to patients with positive ACS biomarkers (for example, elevated troponin levels) both women and men had improved outcomes with early invasive treatment86,87. Despite these data, multiple community-based studies show that women with ACS are significantly less likely to receive angiography and/or PCI than men with ACS88. Among patients with STEMI, a study in the USA showed that fewer women received reperfusion therapy with primary PCI or fibrinolytic therapy compared with men (56.3% versus 73.0%; P <0.0001), and this disparity persisted after adjustment for clinical factors6. Women with STEMI are also less likely to receive rapid reperfusion therapy compared with men with STEMI, either by fibrinolytic therapy (door-to-needle time <30 min; 28.3% versus 35.2%; P = 0.0005) or with primary PCI (door-to-balloon time <90 min; 39.0% versus 44.8%; P <0.0001)6. Substantial evidence has shown that such sex-specific disparities in STEMI reperfusion therapy are not unique to the USA89–92. Among patients with NSTEMI aged ≤65 years, fewer women receive coronary intervention compared with their male counterparts, irrespective of angiographic findings93. Therefore, women with either STEMI or NSTEMI seem to receive less-aggressive invasive care than men with these conditions.
Pharmacological therapies.
Most antiplatelet therapies have similar benefits in both women and men with ACS. Although the use of aspirin for primary prevention is of uncertain benefit in women94,95, the use of aspirin at the time of ACS is of clear benefit in both sexes96. Clopidogrel, an oral antiplatelet agent, has also been shown to reduce adverse outcomes in both women and men with ACS who have undergone PCI97. By contrast, women and men might respond differently to glycoprotein IIb/IIIa inhibitors, an intravenous antiplatelet therapy. A large meta-analysis showed that men had a reduction in the risk of death or recurrent ACS events when taking glycoprotein IIb/IIIa inhibitors, but women did not98. However, similar to the findings with early invasive intervention, when the analysis was limited to individuals with confirmed ACS (those with elevated troponin levels), the sex-associated effect disappeared, and both men and women in this high-risk group had improved outcomes98. The benefit of antiplatelet therapy in ACS must be weighed carefully against the risk of bleeding, which is higher in women than in men. A multitude of studies consistently show that women with ACS have higher bleeding risks than men with ACS99–103. This difference seems to be primarily attributable to sex-related differences in body surface area, drug metabolism, and pharmacokinetics.
Beyond antithrombotic therapy, a meta-analysis of β-blocker administration after MI showed similar benefits of these drugs in women and men104. Similarly, the long-term benefits of angiotensin-converting-enzyme inhibition after MI seem similar in both women and men, with a similar risk reduction for the composite of death, heart failure, and MI in both sexes (OR 0.71, 95% CI 0.65–0.77 and OR 0.79, 95% CI 0.67–0.93, respectively)105. The use of statin therapy is also similarly beneficial for women and men106,107 (RR ratio for major coronary events 0.77, 95% CI 0.64–0.94 versus RR ratio 0.74, 95% CI 0.69–0.79)107.
Despite a clear role for the above medications in the treatment of ACS, women still seem to receive less-aggressive medical therapy than men. A study of the ACC database of patients with ACS undergoing PCI between 2004 and 2006 showed that during hospitalization, fewer women received aspirin (adjusted OR 0.86, 95% CI 0.83–0.88) and glycoprotein IIa/IIIb inhibitors (adjusted OR 0.90, 95% CI 0.88–0.92) compared with men108. Similar disparities in therapy have been shown with thienopyridines and heparin8, β-blockers, and statins109. In many cases, the absolute differences in treatment between women and men were small6,33,110,111; however, the relationship consistently trended in the same direction, with women receiving less-aggressive care than men.
Outcomes
A substantial body of evidence supports a sex-related difference in short-term mortality after ACS, especially after STEMI. A meta-analysis including >136,000 patients from 11 randomized clinical trials of ACS showed that women with STEMI had worse 30-day outcomes than men, but women with NSTEMI or unstable angina had better outcomes112. Similarly, a study including 78,254 patients showed that sex-related differences in in-hospital mortality disappeared after adjustment for clinical factors; however, a disparity in patients with STEMI remained (10.2% versus 5.5%; P <0.0001)6. The reasons for the differing short-term prognosis by ACS type in women and men are unknown.
With regard to long-term outcomes, the evidence for a difference in mortality between female and male patients with ACS is conflicting. Some old studies suggest that no sex-based differences in long-term mortality exist113, whereas other studies suggest that the crude death rates are different, and that this difference is attenuated after adjustment for baseline clinical factors. For example, a review including 39 studies published between 1966 and 2012 showed that, compared with men, women had a higher unadjusted death rate after MI at both 5 and 10 years114. However, these differences were largely explained by sex-related differences in age, comorbidities, and treatment use114. Similarly, an analysis of a nationwide cohort in the Netherlands showed that long-term mortality differences between women and men were diminished, and even reversed, when baseline factors were taken into account115.
A growing body of literature suggests that sex-related outcomes differ by age82. Young women (aged <50 years) have a twofold greater early risk of death after acute MI than similarly aged men72. Young women (aged <65 years) with acute MI have a 22% higher risk of 30-day hospital readmission than young men, even after adjustment for confounders116. These differences persist over time such that the 2-year mortality after an ACS event is significantly higher in women than men among patients aged <60 years, but not among older patients (aged ≥70 years)117. Similar results from a large cohort in Sweden lend validity to these findings118. The age-dependence of the relationship between patient sex and outcomes after ACS seems to hold true for patients with STEMI and patients with NSTEMI in the short-term119 and in the long-term120,121.
The VIRGO study122, published in 2015, indicated that young women (aged <55 years) with STEMI received reperfusion therapy less frequently and were more likely to have reperfusion delays than similarly aged men. Longer door-to-needle and door-to-balloon times, along with lower prescription rates of discharge medications (statins, angiotensin-converting-enzyme inhibitors, and angiotensin-II-receptor blockers), in women than in men were also found among patients aged <45 years included in the AHA’s Get With The Guidelines–Coronary Artery Disease registry123. Similar results have been found in other studies124. Whether these differences in management contribute to the observed difference in outcomes between these two groups is unknown.
Women in clinical trials
Recommendations for ACS management in women are largely based on clinical trials that did not include an adequate number of women and that did not perform subgroup analyses by sex. In response to the widespread underrepresentation of women in clinical trials, both the NIH and the FDA issued mandates in 1993 for the inclusion of women in clinical trials125,126. Since then, although the absolute number of women in clinical trials has increased127, women remain markedly under-represented, particularly in ACS trials. Indeed, an analysis of randomized, controlled trials in ACS that were published between 1966 and 2000 showed that the enrolment of women increased from 20% in studies published in 1966–1990 to 25% in studies published in 1991–2000 (REF. 128). This level of enrolment was still far below the proportion of women with ACS reported for those years in the USA, where 43% of all patients with MI were women128. Similar initiatives to the 1993 US mandates for promoting participation of women in clinical trials have been developed by other countries such as Canada (TABLE 2), but unlike the NIH policies, the inclusion of women in clinical studies was not mandatory. In addition, clinical trial guidelines from several countries do not address patient sex specifically (TABLE 2).
Table 2 |.
Global policies on the inclusion of women in clinical trials
| Country | Organization | Policy | Ref. |
|---|---|---|---|
| Australia | National Health and Medical Research Council | The 2007 revised National Statement includes a statement of fair inclusion in clinical trials, but does not address patient sex specifically | 140 |
| Canada | Health Canada |
|
141 |
| Europe | European Medicines Agency (EMEA) |
|
142 |
| International | International Conference on Harmonization (ICH) |
|
143 |
| Japan | Ministry of Health Labour and Welfare |
|
144 |
| USA | NIH |
|
145 |
| USA | FDA |
|
146 |
The reasons for the underrepresentation of women in ACS trials are unknown, but are likely to be multifaceted. Possible reasons include concern about the safety of women of childbearing potential, for pregnant women, and for their fetuses, although this explanation cannot account for the low enrolment of old women in cardiovascular trials129. Further explanations might include a general unwillingness of women to volunteer to participate in studies, or a higher tendency for women to withdraw from studies before their completion compared with men. However, the adequate enrolment of women in hypertension trials discounts these claims, as does the enrolment of women in single-sex cardiovascular trials129. Other barriers to enrolling an adequate number of women in mixed-sex trials must be present and further research is needed to elucidate these barriers and how they can be addressed.
The NIH and the FDA mandates also stated that sex-specific analyses of drugs and treatments should be performed in clinical trials125,126 and yet, for example, not one of the trials included in a Cochrane review of ACS treatment strategies included data on sex-specific outcomes130,131. Beyond simply enrolling more female patients and performing more subgroup analyses by sex, leaders in the field have advocated for a more rigorous approach to understanding cardiovascular disease in women, in which studies are designed at the outset to have adequate power to analyse sex-based differences132. Such rigorously-designed studies will be necessary to address the plethora of unknowns that remain with regard to ACS management and outcomes in women.
Future directions
In BOX 1 we outline the questions that we believe need to be studied most urgently to address the underlying reasons for the sex-based disparities in ACS. These important, but unanswered, questions span the entire clinical spectrum of ACS, from pathophysiology and presentation, to management and outcomes.
Box 1 |. Urgent research questions about sex-based differences in ACS.
Presentation and baseline risk factors
Are sex-based differences in the symptoms of acute coronary syndromes (ACS) large enough to warrant a change in clinical practice?
Why does diabetes mellitus carry a greater relative risk of cardiovascular disease in women than in men?
Do psychological and behavioural risk factors such as depression have a larger role in the development of cardiovascular disease in women than in men?
Pathophysiology
Why is plaque erosion more common in women than in men, and how can plaque erosion be prevented?
Does microvascular dysfunction have a major role in the development of coronary artery disease or ACS in women?
Does the different pathophysiology of ACS in women correlate with differences in symptoms at presentation?
Evaluation and management
Why are young women with ACS more often misdiagnosed at the time of presentation than young men?
How should women with ACS and nonobstructive coronary artery disease on angiography be optimally managed?
How should spontaneous coronary artery dissection be managed medically; is there a role for revascularization?
What are the causes for women with ACS consistently receiving less-aggressive invasive and pharmacological therapy than men?
How can treatment rates in women be increased when appropriate?
Outcomes
Why do women have worse short-term outcomes than men after ST-segment elevation myocardial infarction, but not after non-ST-segment elevation myocardial infarction or unstable angina?
What are the mechanisms by which young, but not old, women with ACS have worse short-term and long-term outcomes than men in the same age group?
Representation in research
How can researchers be encouraged to include a greater proportion of women in cardiovascular trials?
How can the inclusion of prespecified, sex-based analyses be promoted?
Is there a role for incentive-based funding for the trials that include a representative proportion of women?
Beyond additional research on sex-related differences in ACS, advocacy and public policy that addresses sex gaps in ACS knowledge and care are also urgently needed. Although awareness of heart disease as the leading cause of death among women improved between 1997 and 2012, the rate of awareness in 2012 remained dismal at only 56% of all women surveyed133. Furthermore, the heart disease awareness level of African-American women in 2012 was similar to that of white women in 1997 (REF. 133), indicative of a dangerous and persistent racial disparity in awareness among women. Advocacy and educational programmes targeted to the general public, such as that of the Women’s Heart Alliance, are absolutely necessary, but are not sufficient. Evidence from the VIRGO study134 suggests that providers are 11% less likely to tell young women that they are at risk of heart disease compared with young men, and are also 16% less likely to discuss risk-factor modification with young women. This disparity highlights a cultural bias in medicine that must be directly addressed with both educational and policy tools. Potential interventions might include building educational courses on sex disparities into the medical school or residency curriculum, or recognizing providers who have high levels of prevention metrics in both women and men.
Conclusions
Women have more-varied pathophysiology underlying ACS events than men. In addition, cardiovascular risk factors have a differential effect in women, and some risk factors are more common in women than in men. This difference in pathophysiology and baseline risk factors, along with differential responses to therapy and sex-based variations in treatment patterns, might help to explain the differences in outcomes that are observed between women and men. More investigation into sex-related differences in ACS mechanisms and the clinical care of women with ACS is urgently needed.
Key points.
Women and men with acute coronary syndrome (ACS) tend to present with a similar constellation of symptoms, although at different rates
Women often have alternative mechanisms of ACS, such as spontaneous coronary artery dissection and vasospasm, beyond the plaque rupture most typically seen in men
Across the range of ACS, women generally receive less-aggressive invasive and pharmacological care than men
Sex-related outcomes after ACS vary by age; young women have worse short-term and long-term outcomes than men, but old women have similar outcomes to those of old men
Representation of women in clinical cardiovascular trials needs to increase in order to address the plethora of unknowns that remain about sex-related differences in ACS
Footnotes
Competing interests statement
The authors declare no competing interests.
FURTHER INFORMATION
Women’s Heart Alliance: https://fighttheladykiller.org
References
- 1.US Centers for Disease Control and Prevention. Health, United States, 2013 http://www.cdc.gov/nchs/data/hus/hus13.pdf (2013).
- 2.Mozaffarian D et al. Heart disease and stroke statistics — 2015 update: a report from the American Heart Association. Circulation 131, e29–e322 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Sorlie P & McNamara PM Prognosis after initial myocardial infarction: the Framingham study. Am. J. Cardiol 44, 53–59 (1979). [DOI] [PubMed] [Google Scholar]
- 4.Kim ES, Carrigan TP & Menon V Enrollment of women in National Heart, Lung, and Blood Institute-funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J. Am. Coll. Cardiol 52, 672–673 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Hochman JS et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N. Engl. J. Med 341, 226–232 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Jneid H et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation 118, 2803–2810 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Worrall-Carter L, McEvedy S, Wilson A & Rahman MA Gender differences in presentation, coronary intervention, and outcomes of 28,985 acute coronary syndrome patients in Victoria, Australia. Womens Health Issues 26, 14–20 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Poon S et al. Bridging the gender gap: Insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am. Heart J 163, 66–73 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Song XT, Chen YD, Pan WQ & Lu SZ & CRACE Investigators. Gender based differences in patients with acute coronary syndrome: findings from Chinese Registry of Acute Coronary Events (CRACE). Chin. Med. J. (Engl.) 120, 1063–1067 (2007). [PubMed] [Google Scholar]
- 10.Yu HT et al. Gender-based differences in the management and prognosis of acute coronary syndrome in Korea. Yonsei Med. J 52, 562–568 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shehab A et al. Gender disparities in the presentation, management and outcomes of acute coronary syndrome patients: data from the 2nd Gulf Registry of Acute Coronary Events (Gulf RACE-2). PLoS ONE 8, e55508 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canto JG et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 307, 813–822 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand SS et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur. Heart J 29, 932–940 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Peters SA, Huxley RR & Woodward M Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 57, 1542–1551 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Kappert K et al. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial (ONTARGET). Circulation 126, 934–941 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Dreyer RP et al. Gender differences in pre-event health status of young patients with acute myocardial infarction: a VIRGO study analysis. Eur. Heart J. Acute Cardiovasc. Care 5, 43–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Jimenez F et al. Prevalence and secular trends of excess body weight and impact on outcomes after myocardial infarction in the community. Chest 125, 1205–1212 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Njolstad I, Arnesen E & Lund-Larsen PG Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark Study. Circulation 93, 450–456 (1996). [DOI] [PubMed] [Google Scholar]
- 19.White SJ, Newby AC & Johnson TW Endothelial erosion of plaques as a substrate for coronary thrombosis. Thromb. Haemost 115, 509–519 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Parashar S et al. Impact of depression on sex differences in outcome after myocardial infarction. Circ. Cardiovasc. Qual. Outcomes 2, 33–40 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolderen KG et al. Depressive symptoms in younger women and men with acute myocardial infarction: insights from the VIRGO study. J. Am. Heart Assoc 4, e001424 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sederholm Lawesson S, Alfredsson J, Szummer K, Fredrikson M & Swahn E Prevalence and prognostic impact of chronic kidney disease in STEMI from a gender perspective: data from the SWEDEHEART register, a large Swedish prospective cohort. BMJ Open 5, e008188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sederholm Lawesson S et al. Gender difference in prevalence and prognostic impact of renal insufficiency in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart 97, 308–314 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarti S, Morton JS & Davidge ST Mechanisms of estrogen effects on the endothelium: an overview. Can. J. Cardiol 30, 705–712 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Mehta LS et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 133, 916–947 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Rossouw JE, Manson JE, Kaunitz AM & Anderson GL Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet. Gynecol 121, 172–176 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marjoribanks J, Farquhar C, Roberts H & Lethaby A Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev 7, CD004143 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Schierbeck LL et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 345 e6409 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Rossouw JE, Manson JE, Kaunitz AM & Stefanick ML Study had insufficient power to investigate safety. BMJ 345, e8146 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Marjoribanks J, Farquhar C, Roberts H & Lethaby A Trial does not change the conclusions of Cochrane review of long term hormone therapy for perimenopausal and postmenopausal women. BMJ 345, e8141 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW & Keiding N Thrombotic stroke and myocardial infarction with hormonal contraception. N. Engl. J. Med 366, 2257–2266 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Canto JG et al. Symptom presentation of women with acute coronary syndromes: myth versus reality. Arch. Intern. Med 167, 2405–2413 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Dey S et al. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart 95, 20–26 (2009). [DOI] [PubMed] [Google Scholar]
- 34.DeVon HA, Ryan CJ, Ochs AL & Shapiro M Symptoms across the continuum of acute coronary syndromes: differences between women and men. Am. J. Crit. Care 17, 14–24; quiz 25 (2008). [PMC free article] [PubMed] [Google Scholar]
- 35.Noureddine S, Arevian M, Adra M & Puzantian H Response to signs and symptoms of acute coronary syndrome: differences between Lebanese men and women. Am. J. Crit. Care 17, 26–35 (2008). [PubMed] [Google Scholar]
- 36.Arslanian-Engoren C et al. Symptoms of men and women presenting with acute coronary syndromes. Am. J. Cardiol 98, 1177–1181 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Lovlien M, Schei B & Gjengedal E Are there gender differences related to symptoms of acute myocardial infarction? A Norwegian perspective. Prog. Cardiovasc. Nurs 21, 14–19 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Canto JG, Canto EA & Goldberg RJ Time to standardize and broaden the criteria of acute coronary syndrome symptom presentations in women. Can. J. Cardiol 30, 721–728 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Coventry LL, Finn J & Bremner AP Sex differences in symptom presentation in acute myocardial infarction: a systematic review and meta-analysis. Heart Lung 40, 477–491 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Shin JY, Martin R & Suls J Meta-analytic evaluation of gender differences and symptom measurement strategies in acute coronary syndromes. Heart Lung 39, 283–295 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Chen W, Woods SL & Puntillo KA Gender differences in symptoms associated with acute myocardial infarction: a review of the research. Heart Lung 34, 240–247 (2005). [DOI] [PubMed] [Google Scholar]
- 42.DeVon HA & Zerwic JJ Symptoms of acute coronary syndromes: are there gender differences? A review of the literature. Heart Lung 31, 235–245 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Kyker KA & Limacher MC Gender differences in the presentation and symptoms of coronary artery disease. Curr. Womens Health Rep 2, 115–119 (2002). [PubMed] [Google Scholar]
- 44.Herlitz J, Bang A, Karlson BW & Hartford M Is there a gender difference in aetiology of chest pain and symptoms associated with acute myocardial infarction? Eur. J. Emerg. Med 6, 311–315 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Vaccarino V et al. Presentation, management, and outcomes of ischaemic heart disease in women. Nat. Rev. Cardiol 10, 508–518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virmani R, Kolodgie FD, Burke AP, Farb A & Schwartz SM Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol 20, 1262–1275 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Burke AP, Virmani R, Galis Z, Haudenschild CC & Muller JE 34th Bethesda Conference: task force #2 — what is the pathologic basis for new atherosclerosis imaging techniques? J. Am. Coll. Cardiol 41, 1874–1886 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Lansky AJ et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc. Imaging 5, S62–S72 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Falk E, Nakano M, Bentzon JF, Finn AV & Virmani R Update on acute coronary syndromes: the pathologists’ view. Eur. Heart J 34, 719–728 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Pozzati A, Pancaldi LG, Di Pasquale G, Pinelli G & Bugiardini R Transient sympathovagal imbalance triggers ‘ischemic’ sudden death in patients undergoing electrocardiographic Holter monitoring. J. Am. Coll. Cardiol 27, 847–852 (1996). [DOI] [PubMed] [Google Scholar]
- 51.Yoo SY & Kim JY Recent insights into the mechanisms of vasospastic angina. Korean Circ. J 39, 505–511 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Egashira K et al. Basal release of endothelium-derived nitric oxide at site of spasm in patients with variant angina. J. Am. Coll. Cardiol 27, 1444–1449 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Selzer A, Langston M, Ruggeroli C & Cohn K Clinical syndrome of variant angina with normal coronary arteriogram. N. Engl. J. Med 295, 1343–1347 (1976). [DOI] [PubMed] [Google Scholar]
- 54.Waters DD et al. Factors influencing the long-term prognosis of treated patients with variant angina. Circulation 68, 258–265 (1983). [DOI] [PubMed] [Google Scholar]
- 55.Walling A et al. Long-term prognosis of patients with variant angina. Circulation 76, 990–997 (1987). [DOI] [PubMed] [Google Scholar]
- 56.Saw J Spontaneous coronary artery dissection. Can. J. Cardiol 29, 1027–1033 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Vrints CJ Spontaneous coronary artery dissection. Heart 96, 801–808 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Yip A & Saw J Spontaneous coronary artery dissection — a review. Cardiovasc. Diagn. Ther 5, 37–48 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shamloo BK et al. Spontaneous coronary artery dissection: aggressive versus conservative therapy. J. Invasive Cardiol 22, 222–228 (2010). [PubMed] [Google Scholar]
- 60.Vanzetto G et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur. J. Cardiothorac. Surg 35, 250–254 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Mortensen KH, Thuesen L, Kristensen IB & Christiansen EH Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc. Interv 74, 710–717 (2009). [DOI] [PubMed] [Google Scholar]
- 62.DeMaio SJ Jr, Kinsella SH & Silverman ME Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am. J. Cardiol 64, 471–474 (1989). [DOI] [PubMed] [Google Scholar]
- 63.Thompson EA, Ferraris S, Gress T & Ferraris V Gender differences and predictors of mortality in spontaneous coronary artery dissection: a review of reported cases. J. Invasive Cardiol 17, 59–61 (2005). [PubMed] [Google Scholar]
- 64.Ito H et al. Presentation and therapy of spontaneous coronary artery dissection and comparisons of postpartum versus nonpostpartum cases. Am. J. Cardiol 107, 1590–1596 (2011). [DOI] [PubMed] [Google Scholar]
- 65.Akashi YJ, Nef HM, Mollmann H & Ueyama T Stress cardiomyopathy. Annu. Rev. Med 61, 271–286 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Azzarelli S et al. Clinical features of transient left ventricular apical ballooning. Am. J. Cardiol 98, 1273–1276 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Parodi G et al. Incidence, clinical findings, and outcome of women with left ventricular apical ballooning syndrome. Am. J. Cardiol 99, 182–185 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Gianni M et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur. Heart J 27, 1523–1529 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Sy F et al. Frequency of Takotsubo cardiomyopathy in postmenopausal women presenting with an acute coronary syndrome. Am. J. Cardiol 112, 479–482 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Daniels LB & Maisel AS Cardiovascular biomarkers and sex: the case for women. Nat. Rev. Cardiol 12, 588–596 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Pope JH et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N. Engl. J. Med 342, 1163–1170 (2000). [DOI] [PubMed] [Google Scholar]
- 72.Vaccarino V, Parsons L, Every NR, Barron HV & Krumholz HM Sex-based differences in early mortality after myocardial infarction. N. Engl. J. Med 341, 217–225 (1999). [DOI] [PubMed] [Google Scholar]
- 73.Moser DK et al. Reducing delay in seeking treatment by patients with acute coronary syndrome and stroke: a scientific statement from the American Heart Association Council on cardiovascular nursing and stroke council. Circulation 114, 168–182 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Nguyen HL et al. Age and sex differences and 20-year trends in prehospital delay in patients hospitalized with acute myocardial infarction (1986 to 2005). Circ. Cardiovasc. Qual. Outcomes 3, 590–598 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen HL, Saczynski JS, Gore JM & Goldberg RJ Age and sex differences in duration of prehospital delay in patients with acute myocardial infarction: a systematic review. Circ. Cardiovasc. Qual. Outcomes 3, 82–92 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaur R, Lopez V & Thompson DR Factors influencing Hong Kong Chinese patients’ decision-making in seeking early treatment for acute myocardial infarction. Res. Nurs. Health 29, 636–646 (2006). [DOI] [PubMed] [Google Scholar]
- 77.DeVon HA, Saban KL & Garrett DK Recognizing and responding to symptoms of acute coronary syndromes and stroke in women. J. Obstet. Gynecol. Neonatal Nurs 40, 372–382 (2011). [DOI] [PubMed] [Google Scholar]
- 78.Lichtman JH et al. Symptom recognition and healthcare experiences of young women with acute myocardial infarction. Circ. Cardiovasc. Qual. Outcomes 8, S31–S38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeVon HA Promoting cardiovascular health in women across the life span. J. Obstet. Gynecol. Neonatal Nurs 40, 335–336 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Antman EM et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). Circulation 110, e82–e292 (2004). [PubMed] [Google Scholar]
- 81.Anderson JL et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation 116, e148–e304 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Izadnegahdar M, Norris C, Kaul P, Pilote L & Humphries KH Basis for sex-dependent outcomes in acute coronary syndrome. Can. J. Cardiol 30, 713–720 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Tamis-Holland JE et al. Benefits of direct angioplasty for women and men with acute myocardial infarction: results of the Global Use of Strategies to Open Occluded Arteries in Acute Coronary Syndromes Angioplasty (GUSTO II-B) angioplasty substudy. Am. Heart J 147, 133–139 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Dolor RJ et al. Treatment Strategies for Women with Coronary Artery Disease (Agency for Healthcare Research and Quality (US), 2012). [PubMed] [Google Scholar]
- 85.Bavry AA et al. Invasive therapy along with glycoprotein IIb/IIIa inhibitors and intracoronary stents improves survival in non-ST-segment elevation acute coronary syndromes: a meta-analysis and review of the literature. Am. J. Cardiol 93, 830–835 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Glaser R et al. Benefit of an early invasive management strategy in women with acute coronary syndromes. JAMA 288, 3124–3129 (2002). [DOI] [PubMed] [Google Scholar]
- 87.O’Donoghue M et al. Early invasive versus conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. JAMA 300, 71–80 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Anand SS et al. Differences in the management and prognosis of women and men who suffer from acute coronary syndromes. J. Am. Coll. Cardiol 46, 1845–1851 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Lu HT et al. Sex differences in acute coronary syndrome in a multiethnic asian population: results of the malaysian national cardiovascular disease database-acute coronary syndrome (NCVD-ACS) registry. Glob. Heart 9, 381–390 (2014). [DOI] [PubMed] [Google Scholar]
- 90.de-Miguel-Balsa E et al. Accessibility to reperfusion therapy among women with acute myocardial infarction: impact on hospital mortality. J. Womens Health (Larchmt) 24, 882–888 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Kuhn L, Page K, Rahman MA & Worrall-Carter L Gender difference in treatment and mortality of patients with ST-segment elevation myocardial infarction admitted to Victorian public hospitals: a retrospective database study. Aust. Crit. Care 28, 196–202 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Leurent G et al. Gender differences in presentation, management and inhospital outcome in patients with ST-segment elevation myocardial infarction: data from 5000 patients included in the ORBI prospective French regional registry. Arch. Cardiovasc. Dis 107, 291–298 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Tavris D et al. Gender differences in the treatment of non-ST-segment elevation myocardial infarction. Clin. Cardiol 33, 99–103 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Antithrombotic Trialists C et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373, 1849–1860 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berger JS et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA 295, 306–313 (2006). [DOI] [PubMed] [Google Scholar]
- 96.[No authors listed.] Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. J. Am. Coll. Cardiol 12, 3A–13A (1988). [DOI] [PubMed] [Google Scholar]
- 97.Mehta SR et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 358, 527–533 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Boersma E, Harrington RA, Moliterno DJ, White H & Simoons ML Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. Lancet 360, 342–343 (2002). [DOI] [PubMed] [Google Scholar]
- 99.Subherwal S et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 119, 1873–1882 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehran R et al. A risk score to predict bleeding in patients with acute coronary syndromes. J. Am. Coll. Cardiol 55, 2556–2566 (2010). [DOI] [PubMed] [Google Scholar]
- 101.Mehta SK et al. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ. Cardiovasc. Interv 2, 222–229 (2009). [DOI] [PubMed] [Google Scholar]
- 102.Lansky AJ et al. Percutaneous coronary intervention and adjunctive pharmacotherapy in women: a statement for healthcare professionals from the American Heart Association. Circulation 111, 940–953 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Hess CN et al. Sex-based differences in outcomes after percutaneous coronary intervention for acute myocardial infarction: a report from TRANSLATE-ACS. J. Am. Heart Assoc 3, e000523 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bangalore S et al. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am. J. Med 127, 939–953 (2014). [DOI] [PubMed] [Google Scholar]
- 105.Flather MD et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. Lancet 355, 1575–1581 (2000). [DOI] [PubMed] [Google Scholar]
- 106.Heart Protection Study Collaborative, G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7–22 (2002). [DOI] [PubMed] [Google Scholar]
- 107.Cheung BM, Lauder IJ, Lau CP & Kumana CR Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. Br. J. Clin. Pharmacol 57, 640–651 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Akhter N et al. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). Am. Heart J 157, 141–148 (2009). [DOI] [PubMed] [Google Scholar]
- 109.Koopman C et al. Persisting gender differences and attenuating age differences in cardiovascular drug use for prevention and treatment of coronary heart disease, 1998–2010. Eur. Heart J 34, 3198–3205 (2013). [DOI] [PubMed] [Google Scholar]
- 110.Blomkalns AL et al. Gender disparities in the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: large-scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) National Quality Improvement Initiative. J. Am. Coll. Cardiol 45, 832–837 (2005). [DOI] [PubMed] [Google Scholar]
- 111.Gan SC et al. Treatment of acute myocardial infarction and 30-day mortality among women and men. N. Engl. J. Med 343, 8–15 (2000). [DOI] [PubMed] [Google Scholar]
- 112.Berger JS et al. Sex differences in mortality following acute coronary syndromes. JAMA 302, 874–882 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Johansson S et al. Sex differences in preinfarction characteristics and longterm survival among patients with myocardial infarction. Am. J. Epidemiol 119, 610–623 (1984). [DOI] [PubMed] [Google Scholar]
- 114.Bucholz EM et al. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation 130, 757–767 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koek HL et al. Short- and long-term prognosis after acute myocardial infarction in men versus women. Am. J. Cardiol 98, 993–999 (2006). [DOI] [PubMed] [Google Scholar]
- 116.Dreyer RP et al. Sex differences in the rate, timing, and principal diagnoses of 30-day readmissions in younger patients with acute myocardial infarction. Circulation 132, 158–166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vaccarino V, Krumholz HM, Yarzebski J, Gore JM & Goldberg RJ Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann. Intern. Med 134, 173–181 (2001). [DOI] [PubMed] [Google Scholar]
- 118.Redfors B et al. Trends in gender differences in cardiac care and outcome after acute myocardial infarction in Western Sweden: a report from the Swedish Web System for Enhancement of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). J. Am. Heart Assoc 4, e001995 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Champney KP et al. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart 95, 895–899 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kumbhani DJ et al. Influence of gender on long-term mortality in patients presenting with non-ST-elevation acute coronary syndromes undergoing percutaneous coronary intervention. Am. J. Cardiol 109, 1087–1091 (2012). [DOI] [PubMed] [Google Scholar]
- 121.Otten AM et al. Is the difference in outcome between men and women treated by primary percutaneous coronary intervention age dependent? Gender difference in STEMI stratified on age. Eur. Heart J. Acute Cardiovasc. Care 2, 334–341 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.D’Onofrio G et al. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: results from the VIRGO study. Circulation 131, 1324–1332 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bangalore S et al. Age and gender differences in quality of care and outcomes for patients with ST-segment elevation myocardial infarction. Am. J. Med 125, 1000–1009 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Davis M et al. Acute coronary syndrome in young women under 55 years of age: clinical characteristics, treatment, and outcomes. Clin. Res. Cardiol 104, 648–655 (2015). [DOI] [PubMed] [Google Scholar]
- 125.US Congress. National Institutes of Health revitalization act of 1993 National Institutes of Health; http://orwh.od.nih.gov/about/pdf/NIH-Revitalization-Act-1993.pdf (1993). [Google Scholar]
- 126.US Food and Drug Administration. Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs; notice. Fed. Regist 58, 39406–39416 (1993). [PubMed] [Google Scholar]
- 127.United States General Accounting Office. Women sufficiently represented in new drug testing, but FDA oversight needs improvement. GAO http://www.gao.gov/new.items/d01754.pdf (2001). [Google Scholar]
- 128.Lee PY, Alexander KP, Hammill BG, Pasquali SK & Peterson ED Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA 286, 708–713 (2001). [DOI] [PubMed] [Google Scholar]
- 129.Harris DJ & Douglas PS Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N. Engl. J. Med 343, 475–480 (2000). [DOI] [PubMed] [Google Scholar]
- 130.Johnson SM, Karvonen CA, Phelps CL, Nader S & Sanborn BM Assessment of analysis by gender in the Cochrane reviews as related to treatment of cardiovascular disease. J. Womens Health (Larchmt) 12, 449–457 (2003). [DOI] [PubMed] [Google Scholar]
- 131.Cucherat M, Bonnefoy E & Tremeau G Primary angioplasty versus intravenous thrombolysis for acute myocardial infarction. Cochrane Database Syst. Rev 3, CD001560 (2000). [DOI] [PubMed] [Google Scholar]
- 132.Bucholz EM & Krumholz HM Women in clinical research: what we need for progress. Circ. Cardiovasc. Qual. Outcomes 8, S1–S3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mosca L et al. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation 127,1254–1263 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leifheit-Limson EC et al. Sex differences in cardiac risk factors, perceived risk, and health care provider discussion of risk and risk modification among young patients with acute myocardial infarction: The VIRGO Study. J. Am. Coll. Cardiol 66, 1949–1957 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Karam Sadoon Alzuhairi PS et al. Incidence and outcome of first myocardial infarction according to gender and age in Denmark over a 35-year period (1978–2012). Eur. Heart J 1, 72–78 (2015). [DOI] [PubMed] [Google Scholar]
- 136.Gottlieb S et al. Mortality trends in men and women with acute myocardial infarction in coronary care units in Israel. A comparison between 1981–1983 and 1992–1994. Eur. Heart J 21, 284–295 (2000). [DOI] [PubMed] [Google Scholar]
- 137.Takii T et al. Trends in acute myocardial infarction incidence and mortality over 30 years in Japan: report from the MIYAGI-AMI Registry Study. Circ. J 74, 93–100 (2010). [DOI] [PubMed] [Google Scholar]
- 138.Lundblad D, Holmgren L, Jansson JH, Naslund U & Eliasson M Gender differences in trends of acute myocardial infarction events: the Northern Sweden MONICA study 1985–2004. BMC Cardiovasc. Disord 8, 17 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singh JA, Lu X, Ibrahim S & Cram P Trends in and disparities for acute myocardial infarction: an analysis of Medicare claims data from 1992 to 2010. BMC Med 12, 190 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Australian Government. National Statement on Ethical Conduct in Human Research (2007) - Updated May 2015 National Health and Medical Research Council; https://www.nhmrc.gov.au/guidelines-publications/e72 (2015). [Google Scholar]
- 141.Health Canada. Guidance Document: Considerations for Inclusion of Women in Clinical Trials and Analysis of Sex Differences http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/clini/womct_femec-eng.php (2013).
- 142.European Commission. Gender Mainstreaming in the 6th Framework Programme – Reference Guide for Scientific Officers/Project Officers ftp://ftp.cordis.europa.eu/pub/science-society/docs/gendervademecum.pdf (2003).
- 143.European Medicines Agency. Gender Considerations in the Conduct of Clinical Trials http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500059887.pdf (2005).
- 144.Japan Pharmaceutical Manufacturers Association. Pharmaceutical Administration and Regulations in Japan http://www.nihs.go.jp/mhlw/yakuji/yakuji-e_20110502-02.pdf (2011).
- 145.US Department of Health and Human Services. NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research – Amended, October, 2001. National Institutes of Health; https://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm (2001). [Google Scholar]
- 146.US Department of Health and Human Services. Guidance for Industry: M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals. Food and Drug Administration; http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073246.pdf (2010). [Google Scholar]


