Abstract
Neutrophils play an essential role in the defense against bacterial infections and orchestrate both the innate and adaptive immune responses. With their abundant numbers, diverse function and short life span, these cells are at the forefront of immune responses, and have gained attention in recent years because of their presence in tumor sites. Neutrophil involvement pertains to tumor cells’ ability to construct a suitable tumor microenvironment (TME) that accelerates their own growth and malignancy, by facilitating their interaction with surrounding cells through the circulatory and lymphatic systems, thereby influencing tumor development and progression. Studies have indicated both pro- and anti-tumor properties of infiltrating neutrophils. The TME can exploit neutrophil function, recruitment, and even production, thus resulting in pro-tumor properties of neutrophils, including promotion of genetic instability, tumor cell proliferation, angiogenesis and suppression of anti-tumor or inflammatory response. In contrast, neutrophils can mediate anti-tumor resistance by direct cytotoxicity to the tumor cells or by facilitating anti-tumor functions via crosstalk with T cells. Here, we summarize current knowledge regarding the effects of neutrophil heterogeneity under homeostatic and tumor conditions, including neutrophil phenotype and function, in cancer biology.
Keywords: Neutrophils in cancer, tumor microenvironment, TAN, neutrophil heterogeneity, neutrophils
Introduction
In humans and mice, 50%–70% and 10%–25% of circulating white blood cells are neutrophils, respectively1,2. This difference has been attributed to three factors: (1) evolutionary divergence between humans and mice over the course of approximately 10 million years, (2) different body sizes and lifespans, and (3) different microorganisms encountered in their respective environments3–5. Neutrophils are short-lived cells with an average half-life of 18.5 hours. Thus, to be constantly present in the circulation, they must be replenished in large quantities from the bone marrow (BM) through a process called granulopoiesis. In fact, the daily requirement is approximately 1011 neutrophils for humans and 107 for mice6,7. These notable properties led to the early insight that neutrophils are professional effector cells that perform a particular set of functions in immune defense. As the first cells recruited to inflammation sites, neutrophils are the primary players in innate immunity, mediating inflammation and combating bacterial infection. Moreover, they facilitate the bridging and activation of adaptive immunity1. Neutrophils are also recruited to tumor sites in response to various stimuli and perform complex context-dependent functions within the tumor vicinity. The importance of neutrophils in regulating tumor development and metastasis has only begun to be explored in the past decade, but numerous advancements have been made, as discussed in the sections below.
The tumor microenvironment (TME) is a complex system that consists of tumor cells, tumor extracellular matrix, fibroblasts, blood vessels, and immune cells. The cells in the TME together promote tumor progression by producing various growth factors, cytokines, and chemokines8. The roles of neutrophils in the TME have not been as extensively explored as those of other myeloid cells, such as dendritic cells (DCs) and macrophages9–11. However, increasing evidence demonstrates that neutrophils are indispensable in the function of the TME and underscore their essential roles in promoting tumor growth and metastasis, as well as in the orchestration of pathways leading to tumor resiliency. However, neutrophils have also been shown to possess tumor killing and suppressive properties12–14. The intrinsic heterogeneity of neutrophils, in which their plasticity can be reshaped by the TME or other immune surroundings, underlies these contradictory findings. Advanced technologies, such as high-dimensional transcriptomic datasets and single-cell resolution cell profiling, can reveal neutrophil heterogeneity within healthy tissues and diseased conditions15–18. In the following sections, neutrophil heterogeneity in physiological and tumor conditions is comprehensively discussed. First, we describe recent high-resolution analyses of neutrophil differentiation in the physiological state. We then provide an overview of heterogeneous neutrophil populations in tumor conditions. Subsequently, we emphasize current understanding of the dual opposite roles of neutrophils and the mechanisms underlying how neutrophils participate in tumor growth and metastasis.
Neutrophil differentiation, mobilization, and death
Neutrophil differentiation and development
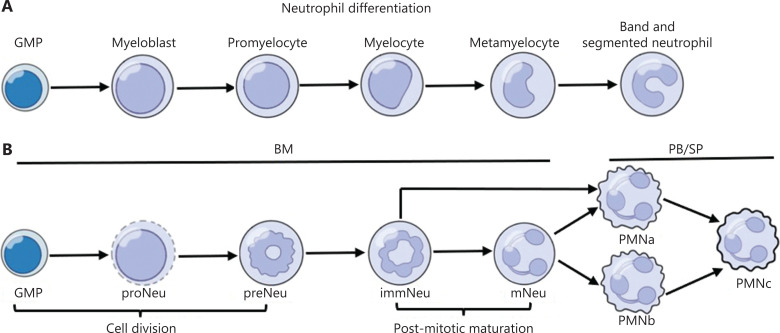
In the BM, neutrophil development starts from granulocyte monocyte progenitors (GMP). After lineage commitment, neutrophil differentiation progresses through the following stages: myeloblasts, promyelocytes, myelocytes, metamyelocytes, band cells, and segmented nucleus neutrophils19 (Figure 1A). Different stages of neutrophil differentiation are usually classified by morphological features including cell size, nuclear condensation, cell marker expression, differentiation properties, and protein content2. However, classifying neutrophil differentiation on the basis of appearance is subjective and may not represent the entire differentiation process or correlate with function. Thus, recent reports have used cellular marker expression, single-cell RNA sequencing (scRNA-seq), and proteomics to better define the stages of neutrophil differentiation.
Figure 1.
Neutrophil differentiation in steady state. (A) Traditional characterization of neutrophil development includes the following stages: GMP, myeloblast, promyelocyte, myelocyte, metamyelocyte, band and segmented neutrophil. (B) On the basis of trajectory analysis, the first 5 clusters, GMP, proNeu, preNeu, immNeu, and mNeu, originate primarily from the BM and represent neutrophil development in the BM. Neutrophil differentiation in BM is divided into 2 phases: cell division and post-mitotic maturation. The cell division stage is defined when GMP differentiate into proNeu and preNeu sequentially. The post-mitotic maturation stage encompasses immNeu, which give rise to non-proliferating mNeu. PMNa, PMNb, and PMNc are the major neutrophil subpopulations in the peripheral blood (PB) and spleen (SP). PMNa cells in the PB are derived from both mNeu and immNeu cells, whereas PMNb cells arise primarily from BM mNeu cells in the steady state.
Improving transcriptomic analysis for single cells has enabled studies of hematopoietic dynamics, and separated neutrophil precursors into early, intermediate, and late phases with specific signature genes and transcription factors20. Additionally, studies using cytometry by time-of-flight (CyTOF) and cell-cycle-based analysis have revealed that neutrophil development in the BM has 5 stages: the earliest committed neutrophil progenitor (proNeu1) develops into intermediate progeny (proNeu2), which then progresses to a committed proliferative neutrophil precursor (preNeu), which undergoes further differentiation into non-proliferating immature neutrophils (immNeu) and subsequently mature neutrophils (mNeu)21. Xie et al.16 have shown that, on the basis of analysis of the BM, peripheral blood, and spleen Gr1+ cells with scRNA-seq, neutrophils can be separated into 8 major populations (G0–G4 and G5a–G5c) with distinct molecular signatures. According to their analyses, the G0, G1, G2, G3, and G4 clusters correspond to BM GMP, proNeu, preNeu, immNeu, and mNeu, respectively, whereas G5a–G5c have polymorphonuclear morphology typical of PMNa, PMNb, and PMNc, the most differentiated neutrophils (Figure 1B). By using CyTOF and scRNA-Seq methods, Zhu et al.18 have identified a continuum of clusters in Ly6G expressing cells composed of 2 major populations: C1 and C2. Comparison of the C1 and C2 cells with the neutrophil subpopulations identified by Xie et al.16 has indicated that C1 and C2 are the concoction of G0/G1 and G2/G3, respectively. Furthermore, Giladi et al.15 have separated developing neutrophils into stage I and stage II. Comparison with the 8 neutrophil populations from Xie et al.16 has revealed that stage I is primarily G2–G4 cells, and stage II is a blend of G4 and G5 cells. Recently, Grieshaber-Bouyer et al.22 have identified 4 neutrophil clusters (P1–P4) and demonstrated that P1 cells correspond to preNeu. P1–P3 are most plentiful in the BM, and P4 is most plentiful in the circulation and spleen, in agreement with findings from the abovementioned studies on the neutrophil maturation process. Calzetti et al.17,23,24 have identified CD66b−CD64dimCD115− neutrophil-committed progenitor cells (NCPs) within the SSCloCD45dimCD34+ and CD34dim/− subsets of human BM and suggested that these NCPs are upstream of the previously described early neutrophil progenitors and proNeu, which generate human CD66b+ neutrophils exclusively. NCP clusters differ in the degree of maturation and eventually differentiate into 2 development routes, thus resulting in 4 subpopulations in total. These refined definitions of the development stages of neutrophils indicate that the differentiation of neutrophils is a complex and heterogeneous process.
Neutrophil mobilization
The release of neutrophils from the BM into the circulation is a tightly regulated process controlled by signaling pathways of the chemokine receptors CXC-chemokine receptor4 (CXCR4) and CXCR2 located on neutrophil precursors25. In steady state, osteoblasts and other BM stromal cells express CXCL12, which signals CXCR4+ neutrophils to remain inside the BM. During neutrophil maturation, CXCR4 expression is gradually downregulated, and the BM is consequently unable to retain neutrophils and releases them into the circulation. Moreover, the expression of CXCR2 is elevated on mature neutrophils, thus facilitating neutrophil emigration into the circulation through interaction with CXCL1 and CXCL2 produced by endothelial cells and megakaryocytes26.
Neutrophil aging and death
Neutrophils are discharged into the circulation according to circadian rhythms, and both circulating human and mouse neutrophils display different phenotypes throughout the day2,27, as evidenced by the fluctuating expression of CD62 L+ and CXCR2+ on newly released mature neutrophils in the circulation. Over time, in the circulation, neutrophils age and gradually lose expression of CD62 L, while CXCR4 and CD11b expression is upregulated, and the nuclei become hypersegmented2,28,29. These CXCR4+CD11b+ CD62 Llow aged neutrophils display enhanced activation of integrin and a remarkable ability to form neutrophil extracellular traps (NETs) in mouse venules during inflammation30. These phenotypic changes are caused by circadian cycling of the transcription factor brain and muscle Arnt-like protein 1 (BMAL1)27,29. BMAL1 upregulates the chemokine CXCL2, thus leading to CXCR2-induced diurnal changes in the transcription and migration of neutrophils in the circulation27,29. Aged neutrophils are believed to return to the BM or liver, and to be eliminated by macrophages at the end of their life cycle28. However, this mechanism alone cannot account for all neutrophil turnover. Therefore, knowledge regarding the destination of most aged neutrophils, and whether it relates to the uninterrupted replenishment of circulating neutrophils, remains lacking.
Both senescent neutrophils31 and neutrophils during infection32 can die in the vasculature or tissue and are then eliminated by Kupffer cells, a type of macrophages in the liver vasculature or other resident macrophages32. Most physiological neutrophils have been suggested to die via apoptosis, a non-inflammatory cell death. Previous studies have found that the removal of apoptotic neutrophils by both Kupffer cells and DCs appears to be controlled by regional interleukin-23 (IL-23), IL-17, and granulocyte colony-stimulating factor (G-CSF) signaling. The function of this cytokine axis promotes neutrophil maturation in the BM33 for replenishment and is down-regulated by liver X receptors31. Inflammatory lytic or violent cell death, such as pyroptosis, can be mediated in a caspase dependent or independent manner34. Necroptosis is a more lytic form of caspase-8 dependent programmed cell death that occurs when receptor interacting protein kinase-3 (RIPK3) and mixed lineage kinase domain-like protein (MLKL) pathways are triggered by bacteria such as Staphylococcus aureus35. Neutrophils may also die via several other lytic or inflammatory methods36,37. For instance, during NETosis, neutrophils burst and release their genetic matter to the extracellular space, releasing a web of DNA with toxic enzymes which can directly trap, contain, and kill microorganisms38. Neutrophils can also die via autophagy, necrosis, and phagocytosis36,37. Therefore, depending on the surrounding environment, different types of cell death occur, thus suggesting that neutrophil death involves a complex crosstalk between various intrinsic and extrinsic pathways.
Neutrophil recruitment in tissues and tumors
After neutrophils enter the bloodstream, they are prepared for entry into target tissues in response to inflammatory or chemoattracting cues. Extravasation of neutrophils across the vessel wall is a five-step process involving tethering, rolling, adhesion, crawling and transmigration, and is tightly regulated by adhesion molecules expressed on vascular endothelial cells, which interacts with the integrins expressed on neutrophils19. Neutrophils begin extravasation via tethering on the endothelium surface and subsequently roll along the vessel. For neutrophils to remain attached, the neutrophil ligand P-selectin glycoprotein ligand 1 (PSGL1) interacts with the endothelial cells adhesion molecules P-selectin and E-selectin39, thus allowing neutrophils to migrate along the endothelial surface. In addition, L-selectin expressed on circulating neutrophils facilitates secondary tethering40 and strengthens adhesion to the endothelium, thus enabling transmigration into the tissue39. The firm adhesion of rolling neutrophils is regulated primarily by the engagement of integrin lymphocyte function-associated antigen 1 (LFA-1) and its ligands intercellular adhesion molecule 1 (ICAM1) and ICAM2, which are expressed on the endothelium. Once firmly bound on the vascular endothelium, neutrophils transmigrate from the circulation to the inflamed tissue, where they exert various functions according to the inflammatory context or tissue41.
After neutrophil recruitment to tumor and cancer sites42, many cytokines, growth factors, and chemokines, including Il-17, IL-1β, G-CSF, CXCL1, and CXCL2, which participate in the maturation and mobilization of neutrophils, are frequently increased in the TME43,44. The underlying reason is that tumor cells, tumor-associated stromal cells, and tumor infiltrated immune cells contribute to elevated production of these cytokines and chemokines in tumor-bearing mice1. Tumor-derived G-CSF mobilizes neutrophils and facilitates their homing to non-neighboring tissues, thus enabling influx of tumor cells45. Tumor-associated macrophages (TAMs) produce IL-1β46, which promotes T cell secretion of IL-17, which in turn enhances G-CSF levels in the blood and facilitates the mobilization of neutrophils into the circulation47. Abnormal regulation of cytokines and chemokines by tumors or tumor-associated immune cells can disrupt the homeostasis of neutrophil preservation in, and release from, the BM.
As described above, neutrophils have high expression of CXCR1 and CXCR2, both of which interact with their ligands secreted by tumor cells, stromal cells, endothelial and immune cells in the TME, thus promoting their recruitment to tumor sites1,48,49. In addition to chemokines, tumor cells and tumor-infiltrating immune cells have been reported to secrete inflammatory cytokines implicated in the same pro-tumor function of neutrophils27,47,50. Tumor necrosis factor-alpha (TNFα) secreted from T cells increases neutrophil recruitment to tumor sites, and together with the high IL-17 levels in the TME, leads to tumor-promoting actions of the recruited neutrophils50. The tumor-secreted protease cathepsin C (CTSC) activates proteinase 3 (PR3) on neutrophil membranes, thus aiding in IL-1β processing and activation of nuclear factor κB, and leading to enhanced expression of IL-6 and CCL3 recruiting neutrophils to tumor sites51. Therefore, neutrophil recruitment to the TME entails upstream regulation of granulopoiesis and a complex network of chemokines and cytokines that traffic neutrophils to tumor sites.
Neutrophil heterogeneity
Neutrophil heterogeneity in physiological and inflammatory conditions
Neutrophils in the bloodstream and organs show considerable heterogeneity in phenotypes and functions, owing to differences in parameters including maturation, aging, activation state, and signals sent by tissues2,27. Xie et al.16 have identified 3 major clusters (G5a–G5c) of neutrophils in the peripheral blood and spleen. G5a neutrophils have high expression of Mmp8, S100a8, and other migration and inflammatory response related genes. G5b neutrophils express high levels of interferon-stimulated genes, such as Ifit3 and Isg15, thus facilitating interferon signaling, and the G5c subpopulation which had the highest aging score and therefore is suggested to be more aged or terminal than G5a and G5b. All 3 clusters are present during normal and inflamatory condition. Deerhake et al.52 have analyzed neutrophil heterogeneity in the lungs of mice infected with Cryptococcus neoformans (Cn), and identified 2 separate neutrophil populations in the lungs during acute Cn infection: one with an oxidative stress signature (Ox-PMN) and the other with elevated cytokine gene expression (Cyt-PMN)52. The authors have proposed that Ox-PMNs target the fungus and produce reactive oxygen species (ROS), whereas Cyt-PMNs have a longer lifespan, thereby allowing crosstalk with other immune cells such as DCs and alveolar macrophages, and facilitating a long term and specific anti-fungal response52. Thus, neutrophils carry distinct signatures which correspond to specialized functions, in conjunction with distinct differentiation routes together display heterogeneity in physiological and inflammatory conditions.
Neutrophil heterogeneity in cancer
The TME polarizes TAMs toward a pro-tumor (M2) phenotype from an original anti-tumor (M1) phenotype53. Like TAMs, tumor-associated neutrophils (TANs) can differentially polarize, thus resulting in a similar classification standard: TANs can be classified into an anti-tumorigenic (“N1”) or pro-tumorigenic (“N2”) phenotype54. TGF-β is an abundant and important cytokine in the vicinity of tumors, which induces neutrophils to adopt a N2 TAN phenotype. In contrast, blocking TGF-β in the TME induces a functional switch in associated neutrophils to an anti-tumoral phenotype54. N1 TANs have efficient tumor cell killing capability and high expression of immune activation related cytokines and chemokines, including CCL3, CXCL9, and CXCL10, which promote the recruitment and activation of CD8+ T cells. In contrast, N2 TANs promote pro-tumor function by producing large amounts of arginase 1 (Arg1)55, which downregulates the surrounding level of L-arginine, thus leading to T cell dysfunction and hindering anti-tumor responses mediated by T cells54. In summary, this framework of classifying TANs distinguishes their dual roles in tumor progression and explains the apparently contradictory findings from studies on neutrophil functions in cancer54.
The heterogeneity of circulating neutrophils can also be characterized according to sedimentation proprieties into high density neutrophil (HDNs) and low-density neutrophils (LDNs) under tumor conditions. HDNs consist of mature neutrophils, which are predominant in healthy individuals and have cytotoxic abilities toward tumor cells, whereas LDNs increase during tumor progression and have impaired neutrophil functions, immunosuppressive properties, and other characteristics opposite from those of mature HDNs. LDNs consist of 2 types of cells: immature myeloid-derived suppressor cells (MDSCs) and original HDNs, whose functional properties are converted by TGF-β-dependent signaling in the tumor mass56,57.
Single cell transcriptomic advances have permitted detailed characterization of the heterogeneity of neutrophils in the TME. ScRNA-seq data for splenic neutrophils and monocytes from a breast cancer mouse model have been compared with those for wild-type controls, and revealed that neutrophils form a continuum of phenotypes consisting of various unique clusters (C0, C2, C4, C5, C7, and C8). C0 is defined by high expression of genes, such as Camp17, indicative of mature neutrophils and high Ly6g expression; C2 significantly increases in mice carrying tumors and also has notable expression of immune regulating genes such as Il1β and Arg2; C4 and C5 overlap in marker genes including Cebpe and Retnlg; and C7 and C8 uniquely have high expression of cell cycle genes such as Tuba1b and Cdc20, thus suggesting existence of a neutrophil reservoir in the spleen, where neutrophils can be replenished58. ScRNA-seq analysis of infiltrating myeloid cells in human and mouse lung tumors has revealed that neutrophils establish a plethora of phenotypic states comprising 5 (hN1–hN5) cell subsets in humans and 6 (mN1–mN6) in mice. h/mN1 neutrophils have high expression of canonical neutrophil markers such as MMP8, MMP9, S100A8, S100A9, and ADAM8; h/mN2 neutrophils express high levels of interferon response genes such as IFIT1, IRF7, and RSAD2; hN3 and hN4 express high levels of CASS4 and CTSC, respectively; mN3 and mN4 express Cxcl3 and Pald1, respectively; h/mN5 produces the cytokines CCL3 and CSF1, as well as CSTB, CTSB, and IRAK2; and mN6 uniquely expresses Fcnb and Ngp. Moreover, 3 neutrophil subsets (mN4–6) have been identified, expressing the sialic acid binding Ig-like lectin F (SiglecF). Thus, the 6 subpopulations can be divided into 2 categories: SiglecFhi neutrophils, which are pro-tumor and contribute to the TME, and SiglecFlo neutrophils (mN1–3), which exist in healthy non-tumor lungs59.
On a cell surface marker level, CyTOF plus high-dimensional analysis of blood samples from melanoma-bearing patients without treatment has indicated that neutrophils in the blood cluster into 7 populations: the CD117+CD66b+CD38+ neutrophil progenitor hNep; the subpopulation of CD16dimCD62 Lbright band cells Cneut1; the terminally differentiated mature neutrophils Cneut2, with high expression of CD101, CD10, CD16, and CXCR2; the CXCR4+CD49d+CD62 Llo aged neutrophils; Cneut3, the only cluster expressing CD45RA+; Cneut4, which produces the lowest level of ROS among all subsets; the immature neutrophils Cneut5; and finally Cneut6, another subpopulation of band cells that express exclusively CD14+. Furthermore, the hNeP and Cneut1 populations display the strongest phagocytic efficiency in all clusters. The Cneut2 and Cneut5 populations show the most robust ROS producing ability when stimulated, whereas Cneut6 and Cneut4 have lower ROS production capability. More than 95% of neutrophils from healthy donors were Cneut2, whereas all other subsets combined composed ∼5%. The proportion of Cneut2 decreased to < 90% while the other clusters increased to greater than 10% of total neutrophils, as determined by flow cytometry, in patients with melanoma. Furthermore, Cneut2 and Cneut5 became the most prevalent neutrophil subsets in the blood of patients with melanoma. Investigation of the 7 clusters has revealed that during tumor progression, neutrophil heterogeneity in the blood is altered in response to tumor signaling60.
To classify PMNs in a mouse tumor model, Veglia et al.61 have described 3 subpopulations of PMNs from tumor bearing mice: the classical PMNs, which account for almost 95% of neutrophils in control spleen; the polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), which include immature neutrophils expressing PMN-MDSC-associated genes such as Ngp, Ltf, Cd177, Anxa1, Mmp8, S100a8, S100a9, Cebpe, Ltb4r1, and Cybb; and the activated, potentially immune suppressive PMN-MDSCs characterized by chemokines, chemokine receptors, and genes involved in cell activation, inflammation, and ER stress (Ccl4, Ccl3, Cxcl2, Cxcl3, Spp1, Il1b, Nfkbia, Socs3, Mif, Klf6, Atf3, Ptgs2, and Xbp1). The level of CD14 has been used to distinguish the 3 populations, given that CD14hi neutrophils correlate with higher immunosuppressive gene expression.
Wang et al.62 have analyzed scRNA-seq metadata of human PMNs from peripheral blood and tumor-infiltrating leukocytes in 5 patients with pancreatic ductal adenocarcinoma, and identified 6 populations of TANs including the non-cluster-specific TAN-0; the terminally differentiated pro-tumor subpopulation TAN-1, associated with poor prognosis; the inflammatory subpopulation TAN-2; the TAN-3 subpopulation newly migrated to the TME; the TAN-4 subpopulation, which preferentially expresses interferon-stimulated genes; and an undefined TAN-5 subpopulation. In addition, recent studies have used a high-resolution single-cell atlas to analyze non-small cell lung cancer infiltrating neutrophil population profiles. Four TAN subsets (TAN-1 to 4) and 2 normal adjacent tissue-associated neutrophils subsets (NAN-1 and NAN-2) have been identified, and extensive heterogeneity in phenotypes has been observed across subpopulations63. The TAN-1 cluster expresses high levels of CXCL1, CXCL2, CXCL8, ICAM1, and CD44, which regulate the activation, recruitment, and cell adhesion of neutrophils, and the formation of NETs; the TAN-2 cluster, which highly expresses MHC II-associated genes such as HLA-DRA, CD74, and HLA-DPB1, and therefore may have immunogenic antigen presenting functions and exhibit anti-tumor immunity; and the TAN-3 cluster, which exhibits pro-tumor characteristics and high expression of the lipid metabolism-associated genes PLIN2 and PLPP3, which promote tumor proliferation. TAN-3 also has high expression of PLAU, which encodes the plasminogen-activator urokinase (uPA), which activates extracellular matrix degrading proteases, and mediates tumor cell adhesion and migration by interacting with its cognate receptor, uPAR, expressed on tumor cells. Therefore, TAN-3 may play important roles in cancer cell proliferation and migration. Finally, the TAN-4 cluster highly expresses ribosomal-associated genes such as RPL10, RPS2, RPS18, and RPL3, thus suggesting an alterable transition to tumor endothelial cells. Additionally, the NAN-1 cluster highly expresses the alarmin S100A12, which mediates neutrophil proinflammatory functions; the NETosis co-factor PADI4; and the proangiogenic genes PROK2 and MMP9. The NAN-1 and NAN-2 clusters are similar, but NAN-2 has lower expression of S100A12, MME, and PROK2. These findings suggest that anti-tumoral and pro-tumoral neutrophils can co-exist in a given tumor isolate.
Collectively, neutrophils have different subpopulations with diverse properties in the spleen, blood, lung, and tumor tissues, thus highlighting the phenotypic and functional heterogeneity in tumor conditions. The nomenclature of neutrophil heterogeneity is complex, because each definition is defined on the basis of a certain limited dataset or model. Thus, these classifications must be compared and unified into a standardized naming system, given that the same “type” of TAN might have different names across reports or classification systems. We have summarized the current classifications in Table 1. However, a comprehensive analysis of current datasets is needed to unify the terminology of this complex classification system.
Table 1.
Neutrophil heterogeneity in cancer
| Species | Tumor | Populations | Signatures and/or functions | Reference |
|---|---|---|---|---|
| Mouse | AB12, LKR, and TC1 | N1 and N2 | N1: anti-tumor; N2: pro-tumor | 54 |
| 4T1 | LDN and HDN | HDNs: cytotoxic capability toward tumor cells; LDNs: impaired neutrophil function and immunosuppressive properties | 57 | |
| MMTV-PyMT transgenic mouse model of breast cancer | C0, C2, C4, C5, C7, and C8 | C0: Camp17 and Ly6g; C2: Il1β and Arg2; C4, C5: Cebpe and Retnlg; C7, C8: Tuba1b and Cdc20. | 58 | |
| LLC | Classical PMNs, PMN-MDSCs, and activated PMN-MDSCs | Classical PMNs: account for almost 95% neutrophils in control spleen; PMN-MDSCs: Ngp, Ltf, Cd177, Anxa1, Mmp8, S100a8, S100a9, Cebpe, Ltb4r1, and Cybb; activated PMN-MDSCs: Ccl4, Ccl3, Cxcl2, Cxcl3, Spp1, Il1b, Nfkbia, Socs3, Mif, Klf6, Atf3, Ptgs2, and Xbp1. potent immune suppressive activity | 61 | |
| KP1.9 | mN1–mN6 | mN1: Mmp8, Mmp 9, S100a8, S100a 9, and Adam8; mN2: Ifit1, Irf7, and Rsad2; mN3: Cxcl3; mN4: Pald1; mN5: Ccl3, Csf1, Cstb, and Irak2; mN6: Fcnb and Ngp | 59 | |
| Human | NSCLC | hN1–hN5 | hN1: MMP8, MMP9, S100A8, S100A 9, and ADAM8; hN2: IFIT1, IRF7, and RSAD2; hN3: CASS4; hN4: CTSC; hN5: CCL3, CSF1, CTSB, and IRAK2 | 59 |
| Melanoma | hNeP and Cneut1–Cneut5 | hNep: CD117+CD66b+CD38+ neutrophil progenitors; Cneut1: CD16dimCD62 Lbright band cell; Cneut2: terminally differentiated, mature neutrophils; Cneut3: CXCR4+CD49d+ | 60 | |
| CD62 Llo aged neutrophils; Cneut4: no specific features; Cneut5: immature neutrophils; Cneut6: CD16dimCD62 Lbright band cells | ||||
| Pancreatic ductal adenocarcinoma | TNA0–TNN5 | TAN-0: no cluster-specific distinctive features; TAN-1: terminally differentiated pro-tumor subpopulation; TAN-2: inflammatory subpopulation; TAN-3: transitional stage subpopulation; TAN-4: expression of interferon-stimulated genes; TAN-5: undefined subpopulation of low-quality cells | 62 | |
| NSCLC | TAN1–4/ NAN1–2 | TAN1: CXCL8, CXCL1, CXCL2, ICAM1, and CD44; TAN2: HLA-DRA, CD74, and HLA-DPB1; TAN3: PLIN2, PLPP, MAP1, LC3B, and PLAU; TAN4: RPL10, RPS2, RPS18, RPL3. NAN1: S100A12, PAD14, PROK2, and MMP9; NAN2: similar to NAN1 cluster, and decreased expression of S100A12, MME, and PROK2 | 63 |
Pro-tumoral neutrophil functions
Neutrophils have been suggested to facilitate tumor growth and malignancy, as evidenced by research indicating that Csf3-/- neutropenic mice present a modest tumor growth delay in an LLC1 tumor model and diminished urethane-induced lung carcinogenesis12. Mice implanted with B16 melanoma and treated with antibodies against Gr1 or G-CSF to deplete neutrophils show diminished myeloid cell infiltration, tumor growth, and angiogenesis13,64. These studies have demonstrated that neutrophils are crucial for fostering carcinogenesis. In this section, we discuss the pro-tumor effects of neutrophils through promoting DNA instability, tumor cell proliferation, angiogenesis, and inhibition of innate and adaptive immune responses.
Neutrophils induce DNA damage, thus leading to cancer occurrence
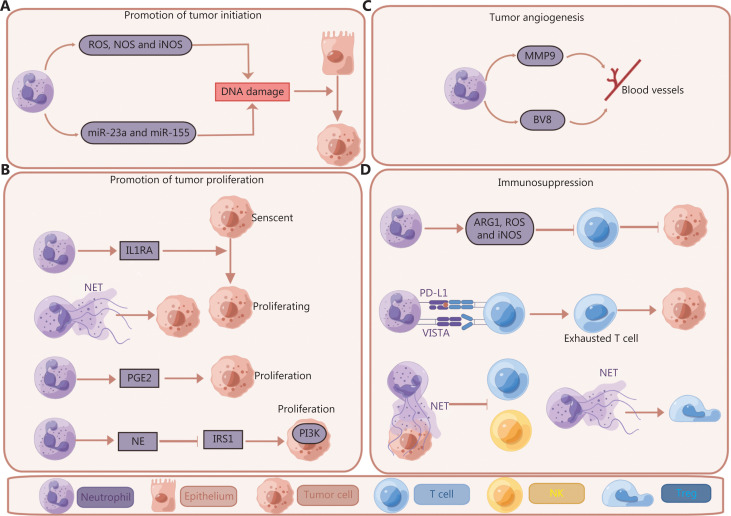
The direct procarcinogenic effect of neutrophils can be induced by the production and release of genotoxic DNA substances that exacerbate DNA instability65. Chronic colon inflammation in patients triggers neutrophils to release genotoxic factors such as ROS and chlorinating agents (HOCl), which cause G2/M checkpoint arrest and replication errors in colon epithelial cells66. Neutrophil-derived production of ROS has also been demonstrated to induce oxidative DNA damage in tissues such as the lungs and intestines, thereby increasing the mutational load and eventually leading to cancer development and progression67. A lung chemical carcinogenesis model has shown that ROS produced from neutrophils induced DNA damage in the lungs, thus promoting tumor formation under carcinogen co-treatment68. In a subcutaneous cancer mouse model, the inducible nitric oxide synthase (iNOS) and nitric oxide synthase (NOS) content produced by infiltrating neutrophils closely correlates with the number of hypoxanthine phosphoribosyl transferase (Hprt) mutations, which contribute to the burden of genetic abnormalities associated with tumor progression69. In inflammatory bowel disease, activated neutrophils in tissues release microparticles containing proinflammatory microRNAs, including miR-23a and miR-155, which lead to DNA double-strand breaks and consequently genomic instability in a ROS-independent manner. Thus, during inflammation, impaired tissue healing and higher mutation rates due to genomic instability in the epithelium result in tumor initiation and progression70 (Figure 2A).
Figure 2.
Neutrophils have protumor properties including causing DNA damage, and promoting tumor proliferation, angiogenesis, and immunosuppression. (A) Neutrophils cause DNA instability through genotoxic DNA substances including ROS, NOS, iNOS, and microRNAs such as miR-23a and miR-155, thus leading to tumor initiation and progression in multiple models (see text). (B) In a PTEN null prostate tumor model, neutrophils promote the proliferation of cancer cells by counteracting senescence with IL1RA production; anaplastic thyroid cancer conditioned medium induces TANs to release NETs in a mitochondrial DNA dependent manner, thereby promoting tumor proliferation. In a RAS-derived neoplasia zebrafish model, neutrophils produce PGE2, thus promoting proliferation of the pre-neoplastic cells in wounded tail fins. In a RAS-induced lung cancer model, neutrophil NE directly induces tumor cell proliferation by infiltrating into the endosomal compartment, degrading IRS-1, and activating PI3K signaling within tumor cells. (C) Neutrophils facilitate tumor angiogenesis via releasing MMP9 and BV8 in a transgenic mouse model with pancreatic islet cell carcinogenesis. (D) Immunosuppressive functions of neutrophils. Neutrophils produce Arg1, ROS, and iNOS, thus impairing the T cell-mediated anti-tumor response. In human hepatocellular carcinomas and gastric cancers, PD-L1+ neutrophils hinder the proliferation and activation of T cells, thus leading to the proliferation and progression of cancer cells. In a melanoma mouse model, VISTA expressed on neutrophils negatively regulates T cell-mediated antitumor immunity; NETs released in the TME form a protective layer on tumor cells and shield them from the cytotoxic activity of CD8+ T cells and NK cells. In a non-alcoholic steatohepatitis-hepatocellular carcinoma (NASH-HCC) model, tumor-induced NETs positively correlate with promotion of Treg differentiation in cancer by metabolic reprogramming of naïve CD4+ T-cells, thereby bolstering hepatocarcinogenesis.
Neutrophils promote tumor cell proliferation
Increasing evidence suggests that neutrophils play an important role in promoting tumor cell proliferation. For instance, in a RAS-derived neoplasia zebrafish model, neutrophils produce the trophic signal molecule prostaglandin E2 (PGE2), which increases the amount of pre-neoplastic cells in wounded tail fins71. Likewise, in RAS-induced lung cancer, neutrophil elastase (NE) has been found to directly cause tumor cell proliferation by infiltrating into endosomal compartments, degrading insulin receptor substrate-1 (IRS-1), and activating phosphoinositide 3-kinase (PI3K) signaling in tumor cells72. Furthermore, neutrophils have been found to counteract oncogene-induced senescence through inducing the expression of interleukin-1 receptor antagonist (IL-1RA) on tumors73. In anaplastic thyroid cancer, altering oxidative mitochondrial metabolism has been found to allow neutrophils to retain functionality and release NETs, thus promoting cancer cell proliferation74. Neutrophils can directly or indirectly lead to the proliferation of tumor cells (Figure 2B).
Neutrophils promote angiogenesis
Neutrophils have an important role in enhancing angiogenesis by producing matrix metalloprotease type 9 (MMP-9), vascular endothelial growth factor (VEGF), and (prokineticin 2) BV875–77. Tissue-infiltrating neutrophils are a major source of MMP9 secretion in vivo which can induce angiogenesis in the TME. Specifically, MMP-9 released by neutrophil tertiary granules78,79 breaks down the extracellular matrix and is followed by release of VEGF and increased angiogenesis75. In human breast cancer, neutrophils have also been found to produce cytokines, such as Oncostatin M, from the IL-6 family, which promotes the production of VEGF in cancer cells and increases angiogenesis and breast cancer cell detachment, thus aggravating invasive capacity80. In a transgenic mouse model with pancreatic islet cell carcinogenesis, VEGF-independent tumor angiogenesis is facilitated by Bv8 expressed by CD11b+Gr1+ neutrophils77 (Figure 2C). In summary, studies have revealed an essential role of neutrophils in angiogenesis during tumor initiation.
Immunosuppression
Neutrophils produce ROS, iNOS, Arg1, prostaglandins, and ligands of immune checkpoint inhibitory receptors, thereby suppressing innate and adaptive lymphoid cell functions1,7,64.
Neutrophil-derived ROS have been widely acknowledged to be key inhibitors of T cell activation during cancer, particularly in advanced tumors27. In a 4T1 breast tumor mouse model, tumor-induced oxidative neutrophils produce ROS, thus decreasing T cell activation even when glucose utilization is limited by the competitive inhibitor 2-deoxy-D-glucose81. Neutrophils can also hinder T cell anti-tumor activity by producing NO via iNOS, as demonstrated in a tumor-bearing KEP mouse model47. Neutrophil derived Arg1 has been observed to block T cell mediated anti-tumor cytotoxicity through depleting L-arginine in both mouse and human cancers54,82. In addition, TGFβ from tumors enhances Arg1 production by neutrophils54. In human hepatocellular carcinomas, the PD-L1+ neutrophils from patients effectively hinder T cell proliferation and activation through interacting with the ligand PD-1, but this process is partially prevented by blocking PD-L1. In human gastric cancer, GM-CSF expressed by tumors induces the activation of neutrophils and triggers the expression of PD-L1 through activation of the Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) signaling pathways. Furthermore, PD-L1+ neutrophils efficiently inhibit T cell activation and promote the proliferation and progression of human gastric cancer in vitro and in vivo, respectively83,84. V-Domain Ig Suppressor of T Cell Activation (VISTA) is expressed on several immune cells, including CD11b+ myeloid cells, naïve CD4+ and CD8+ T cells, CD4+ Foxp3+ Treg cells, and TCRγδ T cells, and negatively regulates T cell-mediated autoimmunity and antitumor immunity85. In a study using a transplantation mouse model with melanoma, VISTA has been found to enhance the effector functions of MDSCs and tolerogenic DC subsets. VISTA blockade increases the production and release of proinflammatory mediators and decreases their T cell-suppressive functions85.
Immunosuppressive neutrophils associated with tumors are generally termed MDSCs. MDSCs were first introduced in 2007 as a subpopulation of myeloid cells characterized by expression of CD11b and Gr1, and having an immature myeloid state. Their ability to restrain T cell, B cell, and natural killer (NK) cell functions renders them immunosuppressive—a characteristic enriched in tumor scenarios but rare in homeostatic conditions86,87. PMN-MDSCs preferentially use ROS, peroxynitrite, Arg1, and PGE2 to mediate immune suppression88. Indeed, PMN-MDSCs and normal neutrophils are difficult to distinguish because they share the same set of markers and identical morphology, thus leading to confusion89. Although PMN-MDSCs are often ascribed immature characteristics to distinguish them from fully differentiated neutrophils90, Gr1 and Ly6G are expressed on both mature and immature neutrophils, thus hindering the use of cell markers to separate mature neutrophils from their progenitors. Moreover, all CD11b+Gr1+ cells in mice with tumors were once believed to be MDSCs, but in fact not all CD11b+Gr1+ cells are immunosuppressive91. Evidence from an epithelial ovarian cancer mouse model has indicated that CD11b+Gr-1+ myeloid cells in the ascites are immunostimulatory rather than being immunosuppressive during advanced stages of cancer. Those myeloid cells constitute a population displaying homogeneity and morphological resemblance to neutrophils. Furthermore, like DCs, CD11b+Gr-1+ cells that are immunostimulatory can efficiently cross-prime cytotoxic T lymphocytes and suppress tumor progression in a subcutaneous injection tumor-bearing mouse model through adoptive transfer91. Hence, PMN-MDSCs should be classified as a subset of TANs with immunosuppressive properties based on established definitions. However, further elucidation is warranted.
In addition, previous studies have demonstrated that NETs influence lymphocyte cytotoxicity in primary tumors. In vivo and in vitro experiments have demonstrated that NETs organize into a protective layer around tumor cells, which physically blocks the cytotoxicity of CD8+ T cells and NK cells92. In non-alcoholic steatohepatitis-hepatocellular carcinoma (NASH-HCC), regulatory T-cells (Tregs) bolster hepatocarcinogenesis by impeding Th1-mediated cancer immunosurveillance and blocking tumor-infiltrating CD8+ T cells. Furthermore, NASH-induced NETs have been shown to positively correlate with promotion of Treg differentiation in cancer through metabolic reprogramming of naïve CD4+ T-cells, thus linking innate and adaptive immunity in the liver in NASH, and indicating that the metabolic changes induced by the pro-tumoral environment also have a critical role in altering immunity93 (Figure 2D).
Neutrophil antitumoral functions
Although most studies support that neutrophils are essential in promoting tumor progression, increasing evidence suggests that antitumoral neutrophil functions also exist. Previous studies have shown that neutrophils induce cell debridement14 and ROS-mediated cytotoxicity of tumor cells94,95, and proteases released from neutrophils have direct toxic effects on tumor cells96–98 and can directly eliminate them. Neutrophil-mediated killing of tumor cells can also occur through TNF-associated apoptosis inducing ligand (TRAIL) expression, and the release of Arg1 and tumor-derived TNFα99–101. A recent study has termed neutrophil-mediated cytotoxic activity, in which neutrophils mechanically destroy cancer cell plasma membranes and cause tumor cell death, “trogoptosis”100,102. In addition, neutrophils can indirectly crosstalk with T cells and facilitate tumor cell killing103–106. Therefore, in this section, we summarize the mechanisms underlying how neutrophils directly and indirectly kill tumor cells during tumor initiation and growth.
Direct tumor cytotoxicity by neutrophils
Neutrophils directly terminate tumor cells through cell-contact dependent mechanisms and the production of ROS14,107. In a PTEN-deficient uterine cancer mouse model, neutrophil-induced debridement of tumor cells, in certain situations, eliminates the cells in nascent tumors and thus prevents uterine epithelial carcinogenesis14. ROS-mediated killing is achieved via the transient receptor potential cation channel, subfamily M, member 2 (TRPM2), which elicits influx of a fatal amount of Ca2+ into target cells in an H2O2-dependent manner94. TRPM2 expression is increased in tumor cells undergoing epithelial-to-mesenchymal transition, thus leading to increased production of CXCL2 by tumor cells and neutrophil recruitment to tumor sites. Therefore, besides initiating an apoptotic cascade in the tumor, TRPM2 supports neutrophil recruitment to tumor sites94,95,97. Consequently, TRPM2 has 2 distinct anti-tumor functions: promoting neutrophil recruitment to tumor sites and activating the apoptotic cascade in the tumor cells themselves.
Neutrophil-induced death of cancer cells can also be triggered by TRAIL expression and Arg1 release99,100. In cytotoxic assays of neutrophils against the Jurkat human T cell leukemic cell line, neutrophils produce TRAILs and subsequently release them into the surrounding area, thus increasing apoptosis of tumor cells99. Arg1 derived from activated neutrophils or dead cells has been shown to induce ER stress in tumor cells, thereby leading to their apoptosis101. In Lewis lung carcinoma and fibrosarcoma transplantation mouse models, tumor cell-derived TNFα prompts neutrophils to express the hepatocyte growth factor receptor (HGFR; also known as MET). The interaction between MET and HGF in the TME promotes the recruitment of neutrophils and the production of NO, thereby leading to tumor cell death100. However, as seen in a transplantation melanoma mouse model, HGF-MET signaling in neutrophils also elicits an immunosuppressive phenotype that contributes to impaired mobilization of antitumor T cells and is characterized by diminished effectiveness of T cell adoptive transfer responses to immune checkpoint blockade therapies108. These findings suggest the contextual dependence of neutrophil involvement. Therefore, how MET expression affects neutrophil functions remains to be thoroughly explored in diverse tumor contexts (Figure 3A).
Figure 3.
Neutrophils have direct and indirect antitumor effects. (A) Neutrophils directly kill tumor cells through the production of H2O2, in a process involving TRPM2, an H2O2-dependent channel, thus causing lethal influx of Ca2+ into breast cancer cells. In Lewis lung carcinoma and fibrosarcoma transplantation mouse models, tumor-derived TNFα induces MET expression on neutrophils, which then interact with HGF and promote the production and release of NO by neutrophils, thus killing tumor cells. In cytotoxic assays of neutrophils against the Jurkat human T cell leukemic cell line, neutrophils express TRAIL on the cell surfaces and release it into the culture medium, thus increasing leukemia cell apoptosis. Arg1 derived from activated neutrophils or dead cells has been shown to induce apoptosis of the HeLa human cervical epithelial carcinoma cell line as well as the SF268 human glioblastoma cell line through activation of the ER stress pathway. Neutrophils kill cancer cells and attenuate tumorigenesis through releasing catalytically active NE, which proteolytically liberates the CD95 death domain (DD) and leads to breast cancer cell apoptosis. (B) In 3-methylcholathrene (3-MCA)-induced sarcomagenesis, neutrophils increase the production and release of IL-12 in macrophages, thereby promoting the polarization and IFN-γ production in a subset of CD4-CD8-TCRβ+ unconventional T cells and exerting an anti-tumor response. Cross-talk between N1 TANs and activated CD4+ and CD8+ T cells induces the expression of costimulatory molecules such as CD54, CD86, OX40 L, and 4-1BBL on the neutrophil surface, which in turn promote T cell activation and INF-γ production. (C) Neutrophils directly kill breast cancer target cells via Fc-mediated destruction of the cancer cell plasma membrane (trogoptosis). Destruction of antibody-opsonized cancer cells mediated by neutrophils is enhanced by blocking the CD47-SIRPα do not-eat-me checkpoint.
Increasing evidence indicates that neutrophil proteins have an essential role in antitumoral function. Recent studies have shown that neutrophils discharge catalytically active NE, thus directly killing cancer cells, and attenuating tumorigenesis through proteolytically freeing the CD95 death domain in tumor cells, which in turn interacts with histone H1 isoforms and selectively leads to cancer cell apoptosis96,109. In glioblastoma, neutrophils transfer myeloperoxidase (MPO)-containing granules into tumor cells, and consequently facilitate iron-dependent aggregation of lipid peroxides inside tumor cells. Either inhibiting or removing MPO restrains neutrophil-elicited tumor cell cytotoxicity97. Although purified lactoferrin has been suggested to have anti-tumor properties98, its role in the context of tumor interaction in vivo has not been fully explored.
Neutrophils indirectly induce tumor cell death via crosstalk with T cells
Neutrophils also elicit antitumor activities indirectly via crosstalk with T cells in the TME. During 3-methylcholathrene-induced sarcomagenesis, neutrophils enhance the secretion of IL-12 in macrophages. Consequently, IL-12 promotes polarization and interferon-γ (IFN-γ) production in a subset of CD4-CD8-TCRβ+ unconventional T cells, thereby initiating an anti-tumor immune response leading to tumor cell death103. Crosstalk between N1 TANs and activated T cells induces the expression of costimulatory molecules such as CD54, CD86, OX40 L, and 4-1BBL on neutrophil surfaces, thus promoting T cell activation and INF-γ production104. In addition, neutrophils have been shown to acquire an antigen presenting cell (APC) phenotype in early stage human lung cancers. These pseudo-APC “hybrid neutrophils” originate from immature CD11b+CD15hiCD10–CD16int/low cells, and promote the proliferation and activation of both CD4+ and CD8+ T cells104,105. Neutrophils have also been found to secrete chemokines such as CCL2, CCL3, CXCL1, CXCL2, and CXCL10, thus directly mediating and promoting the recruitment of T cells as well as other leukocytes106 (Figure 3B).
Neutrophils mediate tumor cell death via trogoptosis
Therapeutic antibodies mechanically destroy cancer cell plasma membranes at least partially through antibody-dependent cellular cytotoxicity (ADCC) by immune cells expressing Fc receptors, such as macrophages, NK cells, and neutrophils. The major routes through which NK cells and macrophages induce ADCC involve granule-dependent apoptotic and phagocytic mechanisms110,111. Neutrophils can be activated to destroy cancer cells via antibody-mediated cytotoxic activity, which is termed trogoptosis. Trogoptosis describes active and mechanical damage to cancer cell membrane integrity, thereby inducing a lytic and inflammatory type of cancer cell death. ADCC induced by neutrophils is unaffected by granule release and NADPH oxidase, and therefore differs from apoptotic pathways induced by NK cells, CTLs, and macrophage-mediated phagocytic cell death. Thus, trogoptosis is an anti-cancer cell destruction pathway used specifically by neutrophils and is not dependent on factors in the classical antimicrobial mechanisms. Neutrophil trogoptosis critically requires CD11b/CD18-dependent conjugate formation and the signaling downstream of the Fc receptors on neutrophils, including the activity of tyrosine kinase (Syk), phopshoinositol-3-kinase (PI3K), myosin light chain kinase (MLCK), and intracellular Ca2+. Furthermore, destruction of antibody-opsonized tumor cells by neutrophils is enhanced by blocking the CD47-SIRPα checkpoint102 (Figure 3C).
Neutrophils might appear to play dual or even opposite roles in tumor immunity and interactions. However, the underlying mechanisms responsible for this discrepancy are the tumor stage and the tumor context, which are essential factors affecting the roles of neutrophil functions in enhancing or suppressing cancer progression. The production of cytokines, chemokines, and growth factors found in the TME in different tumor stages may also contribute to the properties of neutrophils.
Neutrophils in tumor metastasis
Neutrophils are actively involved in, and have been reported to mediate, the following steps of cancer progression: cancer cell dissemination from the primary tumor, intravasation into the circulation or the lymphatic vascular system, prolongation of tumor cell survival in the blood circulation, extravasation into distant tissues and organs, and outgrowth of metastasis. Although previous studies have mostly supported the pro-metastatic role of neutrophils, an opposing role of anti-metastatic properties of neutrophils has also been reported44.
The pro-metastatic role of neutrophils
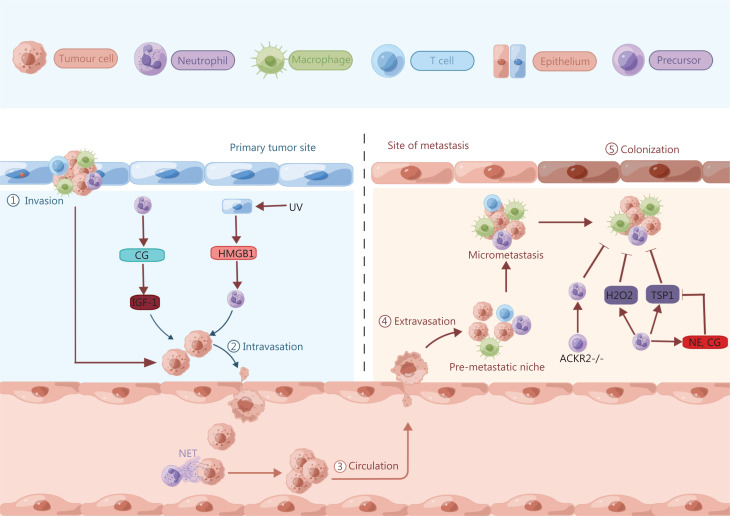
The initiation of the cancer metastatic cascade, e.g., dissemination from the primary tumor, intravasation, and priming of the premetastatic niche, has been overlooked in experimental metastasis models44. As described earlier, neutrophils are involved in promoting angiogenesis via secretion of MMP9, which degrades the extracellular matrix. This process provides more routes for cancer cells to disseminate from the primary site into the circulation to establish a distal seeding site44. Neutrophils also guide cancer cells to endothelial cells and facilitate their intravasation into the bloodstream. In a melanoma mouse model, UV-induced epithelial damage has been found to upregulate the level of high mobility group box 1 (HMGB1), thus leading to the recruitment of TLR4+ neutrophils to primary tumor sites, and subsequent expansion of the tumor cells toward blood vessels and entry into the blood stream112,113. The neutrophil-derived serine protease Cathepsin G (CG) promotes the migration of tumor cells via insulin-like growth factor 1 (IGF-1) activation. IGF-1 then enhances E-cadherin-mediated intercellular adhesion and tumor cell aggregation, thereby further facilitating tumor cell intravasation into blood vessels114 (Figure 4). Collectively, these findings suggest that neutrophils trigger cancer cell interactions with endothelial cells around the primary tumor site, thus resulting in the intravasation of cancer cells into the circulation and the formation of metastasis.
Figure 4.
Neutrophils are involved in metastatic progression. Neutrophils are actively involved in the following steps of cancer metastasis: dissociation from the primary tumor, intravasation into the circulation, extravasation into distant tissues and organs, and outgrowth of metastasis. In a melanoma mouse model, UV-damaged epidermal keratinocytes release HMGB1, thus recruiting and activating neutrophils in primary tumors, and subsequently promoting tumor angiogenesis and the ability of melanoma cells to migrate toward endothelial cells. The neutrophil-derived serine protease CG promotes MCF-7 cell migration via the activation of IGF-1, and IGF-1 then enhances E-cadherin-mediated intercellular adhesion and tumor cell aggregation, which in turn facilitate tumor cell intravasation into blood vessels. In a murine model of infection using cecal ligation and puncture, NETs support metastasis through sequestering disseminating cancer cells in the circulation and facilitating their seeding at distant anatomical sites. In a breast cancer model, tumor-entrained neutrophils produce H2O2, thereby preventing metastatic seeding in the lungs. In an experimental 4T1 metastasis model, neutrophils hinder the formation of metastasis through the upregulation of TSP1, in a manner systemically induced by tumor-secreted prosaposin. Neutrophils degranulate azurophilic granules, which release the serine proteases NE and CG, thus resulting in the proteolytic destruction of TSP1. ACKR2 deletion in neutrophil precursors enhances the expression of inflammatory chemokine receptors and mobilization, thus increasing neutrophils’ anti-metastatic activity in 4T1 breast cancer and B16F10 melanoma metastasis models.
Interestingly, as an abscopal result of tumor cell proliferation at the primary site, neutrophils can accumulate in distant organs before the arrival of disseminated cancer cells and form a region termed the premetastatic niche. For example, in a lung metastatic MMTV-polyoma middle T antigen (PyMT) mammary tumor mouse model, leukotrienes derived from neutrophils promote tumor cell colonization in distant organs through the selective expansion of the sub-pool of cancer cells with high potential for tumorigenesis. Genetic deletion or pharmacologic inhibition of the enzyme arachidonate 5-lipoxygenase (Alox5), which generates leukotrienes, suppresses the pro-metastatic activity of neutrophils and consequently decreases cancer metastasis. Additionally, metastatic progression is retarded in a MMTV-PyMT+-Ela2-Cre-DTA+ mouse model when lung neutrophils are specifically deleted in tumor-bearing mice115.
Increasing evidence demonstrates that NETs also support metastasis by sequestering disseminating cancer cells in the circulation and assisting in their settling in distant tissues116–121. In a metastatic breast cancer model, metastasis-supporting NETs proliferate around disseminated cancer cells in the lungs, thereby stimulating cancer cell migration and invasion in a feedback loop. Accordingly, intraperitoneal injection of DNase I-coated nanoparticles significantly decrease lung metastases in mice119. In addition, during ovarian cancer, the construction of NETs in the pre-metastatic niche plays an essential role in tumor cell seeding in the omentum. Genetic or pharmacologic inhibition of peptidylarginine deiminase 4 (PAD4), an enzyme critical for NET formation, decreases omental metastasis in mice122. Recent studies have also shown that NET-affiliated DNA interacts with the receptor coiled-coil domain containing protein 25 (CCDC25) on tumor cells, thus promoting the adhesion, motility, and growth of metastatic cancer cells in the liver123,124. Therefore, studies have provided new understanding of the molecular interactions between NETs and tumor cells.
The antimetastatic role of neutrophils
In contrast, reports have also shown that neutrophil accumulation and activation in the pre-metastatic niche decrease metastasis. Tumor-derived G-CSF and CCL2 induce the recruitment and activation of neutrophils in pre-metastatic lungs. Subsequently, these tumor-entrained neutrophils prevent metastatic seeding in the lungs through mediating H2O2 dependent killing of tumor cells125.
In addition to release of H2O2, neutrophils have been found to inhibit the formation of metastasis through upregulation of thrombospondin 1 (TSP1)126 in an experimental metastasis model100. Tumor cells secrete prosaposin systemically, which in turn induces expression of the anti-tumorigenic factor TSP1 in recruited neutrophils, thereby resulting in a metastasis-refractory microenvironment126. Furthermore, neutrophils degranulate azurophilic granules, which deliver the serine proteases NE and CG, thus leading to TSP1 proteolysis. Genetic depletion of these serine proteases protects TSP1 from degradation and inhibits lung metastasis127 (Figure 4).
In stark contrast to the findings from earlier studies, the recruitment and activation of neutrophils in the metastatic niche have been found to be essential for decreasing metastasis because of the corresponding killing of cancer cells128,129. Genetic deficiency in the atypical chemokine receptor 2 (ACKR2) promotes the expression of chemokine receptors on hematopoietic progenitors such as CCR1, CCR2, and CCR5. Activated anti-metastatic cytotoxic neutrophils are released from the BM in response to this increased expression, and they travel toward tumor cells with an anti-metastatic profile129. Accordingly, metastasis caused by transplantation with 4T1 breast cancer cell lines or by intravenous injection with B16F10 melanoma cell lines has been found to decrease in ACKR2 deficient mice (Figure 4). Thus, inactivating ACKR2 expression activates neutrophils’ anti-metastatic properties, and this method may serve as an innovative means of developing myeloid checkpoint therapies.
Conclusion
Neutrophils exert dual, apparently opposite, effects on tumor growth and metastasis. Neutrophils mediate DNA instability, and tumor cell proliferation and metastasis, and inhibit the innate and adaptive lymphocyte-mediated anti-tumor immune responses. In contrast, neutrophils engage in direct and indirect crosstalk with other immune cells, thus resulting in killing of tumor cells. These contradictory roles have been ascribed to the heterogeneity of neutrophils in distinct tumor contexts and interactions, thus leading to the differentiation of a variety of neutrophil subpopulations with opposing functions. Hence, a detailed examination of neutrophil heterogeneity in different tumor types and stages is necessary to enable inclusive and comprehensive classification of neutrophils, and to define the different clusters with specific gene signatures in cancer.
Neutrophil heterogeneity in cancer biology has attracted the attention of numerous researchers. Conventionally, neutrophil subpopulations have been divided on the basis of gradient centrifugation methods and polarization states. Single-cell resolution cell profiling has enabled definition of the transcription and protein profiles of different neutrophil subsets and supported a detailed elucidation of neutrophil heterogeneity in tumor conditions. However, different neutrophil subpopulations have diverse properties in different tumor tissues and contexts, and the definitions of neutrophil clusters vary across studies. Moreover, agreement is lacking regarding subpopulation surface markers, thus limiting knowledge regarding neutrophil heterogeneity in tumor conditions. Therefore, correlation analysis and experimental studies are required to reveal the neutrophils in tumor conditions in detail.
Under physiological conditions, neutrophil differentiation from undifferentiated proNeu to terminally differentiated mNeu in the BM progresses linearly. Neutrophil heterogeneity in steady state was believed to be persistent, on the basis of the existence of G5a and G5b. However, tumor-derived TGFβ directs neutrophils toward N2 with pro-tumoral properties, and TGFβ blockade converts N2 neutrophils to an antitumor N1 phenotype, thus indicating that neutrophil heterogeneity under tumor conditions is transient and plastic. The process of neutrophil development in the BM is tightly controlled by specific transcription factors. For instance, the CCAAT/enhancer-binding proteins (C/EBPs), including C/EBPα, C/EBPβ, and C/EBPε, have important roles in regulating neutrophil differentiation. C/EBPα drives CMP differentiation, and its disruption impairs GMP production, thus resulting in complete loss of mature neutrophils. C/EBPβ is highly expressed in G4 and G5a–G5c, and may play an essential role in mature neutrophils. C/EBPε is involved in GMP differentiation to myelocytes and has been suggested to play an important role in the terminal step of the neutrophil development. In addition, growth factor independent-1 (Gfi-1) plays an essential role in facilitating neutrophil differentiation27. Neutrophil heterogeneity under tumoral influence is modulated by differential transcription factor expression, which can be induced by tissue and tumor stimuli factors. In the tumor environment, tumor-derived TGFβ dictates the TAN phenotype and skews TAN differentiation toward the N2 pro-tumorigenic phenotype. In addition, the transition from HDNs to LDNs can also be driven by TGFβ. Tumor-derived TGFβ regulates the polarization and transition of neutrophils by activating the transcription factor SMAD. In contrast to TGFβ, tumor-derived IFN-γ and GM-SCF synergistically promote the differentiation of immature neutrophils into a subpopulation of APC-like hybrid neutrophils with anti-tumoral properties by downregulating the expression of transcription factors such as Ikaros105. This evidence indicates that neutrophil heterogeneity under physiological and pathological states may be a fixed programmed response potentially mediated by local or tumor-derived factors.
Although enormous achievements have elucidated the biology, functions, and heterogeneity of neutrophils, several questions remain to be addressed regarding the roles of neutrophils in tumors. Currently, processes revealing the functions of different neutrophil subsets in cancers are complex because the initial heterogeneity of neutrophils, compounded by the transformations and alterations in the TME, creates a complex landscape of neutrophil expression. Thus, targeting neutrophils to prevent tumor progression remains an appealing direction but has not yet been definitively achieved. Most researchers have concentrated on understanding the immunosuppressive properties or the protumor functions of neutrophils. Consequently, the anti-cancer functions of neutrophils are less understood, and more research is required to enable medical applications.
Another layer of complexity in this field is that similarities and differences exist in the results obtained from mouse or human origin tumor models, which are indeed distinct and have semi-conserved neutrophil properties. Although development of other higher animal models has been attempted, rodents remain the most common and feasible animal models used to study cancer biology.
Finally, the dual roles played by neutrophils in cancer are so profound that even neutrophil proteins such as NE and MPO can directly exert and assist in pro- or anti-tumor functions where the duality mirrors neutrophils themselves. Neutrophil death can affect tumor dynamics as well: on the one hand, NETs can directly nurture tumors and suppress immune cells; on the other hand, neutrophil apoptosis has anti-tumor properties by facilitating immune signaling. Therefore, when properly activated and regulated, neutrophils can initiate cytotoxicity toward tumor cells and unleash their anti-tumor potential, thus potentially enabling the development of new therapeutic strategies targeting cancer.
Grant support
All the figures in the manuscript were drawn in Figdraw. A.Y.H. was supported by NRSA Institutional Postdoctoral Training grant T32 (Grant No. 5T32HL066987-20). C.S. was supported by grants from the National Natural Science Foundation of China (Grant No. 82001661). F.X.M. and C.S. were supported by the Haihe Laboratory of Cell Ecosystem Innovation Fund (Grant No. HH22KYZX0019). F.X.M. was supported by the National Natural Science Foundation of China (Grant No. 82171756).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Song Chen, Alan Y. Hsu.
Collected the data: Song Chen, Qingyu Zhang, Lisha Lu, Jiajia Li, Jiali Zha, Fengxia Ma, Alan Y. Hsu.
Contributed data or analysis tools: Song Chen, Chunhui Xu.
Performed the analysis: Song Chen, Alan Y. Hsu.
Wrote the paper: Song Chen, Hongbo R. Luo, Alan Y. Hsu.
References
- 1.Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20:485–503. doi: 10.1038/s41568-020-0281-y. [DOI] [PubMed] [Google Scholar]
- 2.Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. 2019;19:255–65. doi: 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- 3.Doeing DC, Borowicz JL, Crockett ET. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol. 2003;3:3. doi: 10.1186/1472-6890-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol (Baltimore, MD: 1950) 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 5.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–24. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22:173–87. doi: 10.1038/s41577-021-00571-6. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Guo X, Ling J, Tang Z, Huang G, He L, et al. Nanomedicine-based cancer immunotherapies developed by reprogramming tumor-associated macrophages. Nanoscale. 2021;13:4705–27. [Google Scholar]
- 9.Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020;353:104119. doi: 10.1016/j.cellimm.2020.104119. [DOI] [PubMed] [Google Scholar]
- 10.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–82. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 12.Voloshin T, Gingis-Velitski S, Bril R, Benayoun L, Munster M, Milsom C, et al. G-CSF supplementation with chemotherapy can promote revascularization and subsequent tumor regrowth: prevention by a CXCR4 antagonist. Blood. 2011;118:3426–35. doi: 10.1182/blood-2010-11-320812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu R, Kujawski M, Pan H, Shively JE. Tumor angiogenesis mediated by myeloid cells is negatively regulated by CEACAM1. Cancer Res. 2012;72:2239–50. doi: 10.1158/0008-5472.CAN-11-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaisdell A, Crequer A, Columbus D, Daikoku T, Mittal K, Dey SK, et al. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell. 2015;28:785–99. doi: 10.1016/j.ccell.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giladi A, Paul F, Herzog Y, Lubling Y, Weiner A, Yofe I, et al. Single-cell characterization of haematopoietic progenitors and their trajectories in homeostasis and perturbed haematopoiesis. Nat Cell Biol. 2018;20:836–46. doi: 10.1038/s41556-018-0121-4. [DOI] [PubMed] [Google Scholar]
- 16.Xie X, Shi Q, Wu P, Zhang X, Kambara H, Su J, et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol. 2020;21:1119–33. doi: 10.1038/s41590-020-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwok I, Becht E, Xia Y, Ng M, Teh YC, Tan L, et al. Combinatorial single-cell analyses of granulocyte-monocyte progenitor heterogeneity reveals an early uni-potent neutrophil progenitor. Immunity. 2020;53:303–18.e5. doi: 10.1016/j.immuni.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhu YP, Padgett L, Dinh HQ, Marcovecchio P, Blatchley A, Wu R, et al. Identification of an early unipotent neutrophil progenitor with pro-tumoral activity in mouse and human bone marrow. Cell Rep. 2018;24:2329–41.e8. doi: 10.1016/j.celrep.2018.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 20.Velten L, Haas SF, Raffel S, Blaszkiewicz S, Islam S, Hennig BP, et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat Cell Biol. 2017;19:271–81. doi: 10.1038/ncb3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evrard M, Kwok IWH, Chong SZ, Teng KWW, Becht E, Chen J, et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018;48:364–79.e8. doi: 10.1016/j.immuni.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Grieshaber-Bouyer R, Radtke FA, Cunin P, Stifano G, Levescot A, Vijaykumar B, et al. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat Commun. 2021;12:2856. doi: 10.1038/s41467-021-22973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calzetti F, Finotti G, Tamassia N, Bianchetto-Aguilera F, Castellucci M, Canè S, et al. CD66b(-)CD64(dim)CD115(-) cells in the human bone marrow represent neutrophil-committed progenitors. Nat Immunol. 2022;23:679–91. doi: 10.1038/s41590-022-01189-z. [DOI] [PubMed] [Google Scholar]
- 24.Dinh HQ, Eggert T, Meyer MA, Zhu YP, Olingy CE, Llewellyn R, et al. Coexpression of CD71 and CD117 identifies an early unipotent neutrophil progenitor population in human bone marrow. Immunity. 2020;53:319–34.e6. doi: 10.1016/j.immuni.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–31. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capucetti A, Albano F, Bonecchi R. Multiple roles for chemokines in neutrophil biology. Front Immunol. 2020;11:1259. doi: 10.3389/fimmu.2020.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carnevale S, Ghasemi S, Rigatelli A, Jaillon S. The complexity of neutrophils in health and disease: focus on cancer. Semin Immunol. 2020;48:101409. doi: 10.1016/j.smim.2020.101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, A-González N, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–35. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019;50:390–402.e10. doi: 10.1016/j.immuni.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–32. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong C, Kidani Y, A-González N, Phung T, Ito A, Rong X, et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J Clin Invest. 2012;122:337–47. doi: 10.1172/JCI58393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98:1226–30. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- 33.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–94. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, et al. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep. 2018;22:2924–36. doi: 10.1016/j.celrep.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, He Z, Liu H, Yousefi S, Simon HU. Neutrophil necroptosis is triggered by ligation of adhesion molecules following GM-CSF priming. J Immunol. 2016;197:4090–100. doi: 10.4049/jimmunol.1600051. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence SM, Corriden R, Nizet V. How neutrophils meet their end. Trends Immunol. 2020;41:531–44. doi: 10.1016/j.it.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Figueroa E, Alvarez-Carrasco P, Ortega E, Maldonado-Bernal C. Neutrophils: many ways to die. Front Immunol. 2021;12:631821. doi: 10.3389/fimmu.2021.631821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 39.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–51. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994;180:1785–92. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–75. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lecot P, Sarabi M, Pereira Abrantes M, Mussard J, Koenderman L, Caux C, et al. Neutrophil heterogeneity in cancer: from biology to therapies. Front Immunol. 2019;10:2155. doi: 10.3389/fimmu.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osborne-Rivet L, Houssais JF. [Characterization by selective labelling and electrophoresis of non-ribosomal polysomal proteins from C11D LM (TK-) cells maintained in exponential growth for 28 h in the presence of actinomycin D (author’s transl)] Eur J Biochem. 1974;48:427–38. doi: 10.1111/j.1432-1033.1974.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 44.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–46. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 45.Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107:21248–55. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carmi Y, Rinott G, Dotan S, Elkabets M, Rider P, Voronov E, et al. Microenvironment-derived IL-1 and IL-17 interact in the control of lung metastasis. J Immunol. 2011;186:3462–71. doi: 10.4049/jimmunol.1002901. [DOI] [PubMed] [Google Scholar]
- 47.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–8. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mollica Poeta V, Massara M, Capucetti A, Bonecchi R. Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front Immunol. 2019;10:379. doi: 10.3389/fimmu.2019.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127–44. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–23. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39:423–37.e7. doi: 10.1016/j.ccell.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Deerhake ME, Reyes EY, Xu-Vanpala S, Shinohara ML. Single-cell transcriptional heterogeneity of neutrophils during acute pulmonary Cryptococcus neoformans infection. Front Immunol. 2021;12:670574. doi: 10.3389/fimmu.2021.670574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 54.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes JM, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–56. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- 56.Hsu BE, Tabariès S, Johnson RM, Andrzejewski S, Senecal J, Lehuédé C, et al. Immature low-density neutrophils exhibit metabolic flexibility that facilitates breast cancer liver metastasis. Cell Rep. 2019;27:3902–15.e6. doi: 10.1016/j.celrep.2019.05.091. [DOI] [PubMed] [Google Scholar]
- 57.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–73. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 58.Alshetaiwi H, Pervolarakis N, McIntyre LL, Ma D, Nguyen Q, Rath JA, et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci Immunol. 2020;5:eaay6017. doi: 10.1126/sciimmunol.aay6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–34.e10. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu YP, Eggert T, Araujo DJ, Vijayanand P, Ottensmeier CH, Hedrick CC. CyTOF mass cytometry reveals phenotypically distinct human blood neutrophil populations differentially correlated with melanoma stage. J Immunother Cancer. 2020;8:e000473. doi: 10.1136/jitc-2019-000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veglia F, Hashimoto A, Dweep H, Sanseviero E, De Leo A, Tcyganov E, et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J Exp Med. 2021;218:e20201803. doi: 10.1084/jem.20201803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Liu Y, Dai Y, Tang X, Yin T, Wang C, et al. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut. 2022 doi: 10.1136/gutjnl-2021-326070. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salcher S, Sturm G, Horvath L, Untergasser G, Fotakis G, Panizzolo E, et al. High-resolution sigle-cell atlas reveals diversity and plasticity of tissue-resident neutrophils in non-small cell lung cancer. Preprint at bioRxiv. 2022 doi: 10.1016/j.ccell.2022.10.008. Available from: https://www.biorxiv.org/content/10.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Guoqiang L, Sun M, Lu X. Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment. Cancer Biol Med. 2020;17:32–43. doi: 10.20892/j.issn.2095-3941.2019.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knaapen AM, Seiler F, Schilderman PA, Nehls P, Bruch J, Schins RP, et al. Neutrophils cause oxidative DNA damage in alveolar epithelial cells. Free Radic Biol Med. 1999;27:234–40. doi: 10.1016/s0891-5849(98)00285-8. [DOI] [PubMed] [Google Scholar]
- 66.Campregher C, Luciani MG, Gasche C. Activated neutrophils induce an hMSH2-dependent G2/M checkpoint arrest and replication errors at a (CA)13-repeat in colon epithelial cells. Gut. 2008;57:780–7. doi: 10.1136/gut.2007.141556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Canli Ö, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell. 2017;32:869–83.e5. doi: 10.1016/j.ccell.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Wculek SK, Bridgeman VL, Peakman F, Malanchi I. Early neutrophil responses to chemical carcinogenesis shape long-term lung cancer susceptibility. iScience. 2020;23:101277. doi: 10.1016/j.isci.2020.101277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandhu JK, Privora HF, Wenckebach G, Birnboim HC. Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. Am J Pathol. 2000;156:509–18. doi: 10.1016/S0002-9440(10)64755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Butin-Israeli V, Bui TM, Wiesolek HL, Mascarenhas L, Lee JJ, Mehl LC, et al. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J Clin Invest. 2019;129:712–26. doi: 10.1172/JCI122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Antonio N, Bønnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, et al. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015;34:2219–36. doi: 10.15252/embj.201490147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–23. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515:134–7. doi: 10.1038/nature13638. [DOI] [PubMed] [Google Scholar]
- 74.Cristinziano L, Modestino L, Loffredo S, Varricchi G, Braile M, Ferrara AL, et al. Anaplastic thyroid cancer cells induce the release of mitochondrial extracellular DNA traps by viable neutrophils. J Immunol (Baltimore, MD: 1950) 2020;204:1362–72. doi: 10.4049/jimmunol.1900543. [DOI] [PubMed] [Google Scholar]
- 75.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–8. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, et al. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol (Baltimore, MD: 1950) 2004;172:5034–40. doi: 10.4049/jimmunol.172.8.5034. [DOI] [PubMed] [Google Scholar]
- 77.Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A. 2008;105:2640–5. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mollinedo F, Schneider DL. Subcellular localization of cytochrome b and ubiquinone in a tertiary granule of resting human neutrophils and evidence for a proton pump ATPase. J Biol Chem. 1984;259:7143–50. [PubMed] [Google Scholar]
- 79.Mollinedo F, Manara FS, Schneider DL. Acidification activity of human neutrophils. Tertiary granules as a site of ATP-dependent acidification. J Biol Chem. 1986;261:1077–82. [PubMed] [Google Scholar]
- 80.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 81.Rice CM, Davies LC, Subleski JJ, Maio N, Gonzalez-Cotto M, Andrews C, et al. Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nat Commun. 2018;9:5099. doi: 10.1038/s41467-018-07505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–60. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y, et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:141. doi: 10.1186/s13046-015-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900–11. doi: 10.1136/gutjnl-2016-313075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu W, Dong J, Zheng Y, Zhou J, Yuan Y, Ta HM, et al. Immune-checkpoint protein VISTA regulates antitumor immunity by controlling myeloid cell-mediated inflammation and immunosuppression. Cancer Immunol Res. 2019;7:1497–510. doi: 10.1158/2326-6066.CIR-18-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133:2159–67. doi: 10.1182/blood-2018-11-844548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nature reviews. Immunology. 2021;21:485–98. doi: 10.1038/s41577-020-00490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mollinedo F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol. 2019;40:228–42. doi: 10.1016/j.it.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tomihara K, Guo M, Shin T, Sun X, Ludwig SM, Brumlik MJ, et al. Antigen-specific immunity and cross-priming by epithelial ovarian carcinoma-induced CD11b(+)Gr-1(+) cells. J Immunol. 2010;184:6151–60. doi: 10.4049/jimmunol.0903519. [DOI] [PubMed] [Google Scholar]
- 92.Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52:856–71.e8. doi: 10.1016/j.immuni.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 93.Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y, Huang Z, et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021;75:1271–83. doi: 10.1016/j.jhep.2021.07.032. [DOI] [PubMed] [Google Scholar]
- 94.Gershkovitz M, Caspi Y, Fainsod-Levi T, Katz B, Michaeli J, Khawaled S, et al. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. 2018;78:2680–90. doi: 10.1158/0008-5472.CAN-17-3614. [DOI] [PubMed] [Google Scholar]
- 95.Gershkovitz M, Fainsod-Levi T, Zelter T, Sionov RV, Granot Z. TRPM2 modulates neutrophil attraction to murine tumor cells by regulating CXCL2 expression. Cancer Immunol Immunother. 2019;68:33–43. doi: 10.1007/s00262-018-2249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui C, Chakraborty K, Tang XA, Zhou G, Schoenfelt KQ, Becker KM, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184:3163–77.e21. doi: 10.1016/j.cell.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun. 2020;11:5424. doi: 10.1038/s41467-020-19193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dong H, Yang Y, Gao C, Sun H, Wang H, Hong C, et al. Lactoferrin-containing immunocomplex mediates antitumor effects by resetting tumor-associated macrophages to M1 phenotype. J Immunother Cancer. 2020;8:e000339. doi: 10.1136/jitc-2019-000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koga Y, Matsuzaki A, Suminoe A, Hattori H, Hara T. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res. 2004;64:1037–43. doi: 10.1158/0008-5472.can-03-1808. [DOI] [PubMed] [Google Scholar]
- 100.Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522:349–53. doi: 10.1038/nature14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.García-Navas R, Gajate C, Mollinedo F. Neutrophils drive endoplasmic reticulum stress-mediated apoptosis in cancer cells through arginase-1 release. Sci. Rep. 2021;11:12574. doi: 10.1038/s41598-021-91947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018;23:3946–59.e6. doi: 10.1016/j.celrep.2018.05.082. [DOI] [PubMed] [Google Scholar]
- 103.Ponzetta A, Carriero R, Carnevale S, Barbagallo M, Molgora M, Perucchini C, et al. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell. 2019;178:346–60.e24. doi: 10.1016/j.cell.2019.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–80. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singhal S, Bhojnagarwala PS, O’Brien S, Moon EK, Garfall AL, Rao AS, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30:120–35. doi: 10.1016/j.ccell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 107.Mahiddine K, Blaisdell A, Ma S, Créquer-Grandhomme A, Lowell CA, Erlebacher A. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J Clin Invest. 2020;130:389–403. doi: 10.1172/JCI130952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O’Donnell JS, Szczepanski S, et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity. 2017;47:789–802.e9. doi: 10.1016/j.immuni.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 109.Hao L, Shan Q, Wei J, Ma F, Sun P. Lactoferrin: major physiological functions and applications. Curr Protein Pept Sci. 2019;20:139–44. doi: 10.2174/1389203719666180514150921. [DOI] [PubMed] [Google Scholar]
- 110.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–52. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Z, Yang C, Li L, Jin X, Zhang Z, Zheng H, et al. Tumor-derived HMGB1 induces CD62L(dim) neutrophil polarization and promotes lung metastasis in triple-negative breast cancer. Oncogenesis. 2020;9:82. doi: 10.1038/s41389-020-00267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507:109–13. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- 114.Morimoto-Kamata R, Yui S. Insulin-like growth factor-1 signaling is responsible for cathepsin G-induced aggregation of breast cancer MCF-7 cells. Cancer Sci. 2017;108:1574–83. doi: 10.1111/cas.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–7. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–58. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guglietta S, Chiavelli A, Zagato E, Krieg C, Gandini S, Ravenda PS, et al. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat Commun. 2016;7:11037. doi: 10.1038/ncomms11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68:1347–60. doi: 10.1002/hep.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zha C, Meng X, Li L, Mi S, Qian D, Li Z, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med. 2020;17:154–68. doi: 10.20892/j.issn.2095-3941.2019.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216:176–94. doi: 10.1084/jem.20181170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133–8. doi: 10.1038/s41586-020-2394-6. [DOI] [PubMed] [Google Scholar]
- 124.Dickson I. NETs promote liver metastasis via CCDC25. Nat Rev Gastroenterol Hepatol. 2020;17:451. doi: 10.1038/s41575-020-0345-1. [DOI] [PubMed] [Google Scholar]
- 125.Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–14. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Catena R, Bhattacharya N, El Rayes T, Wang S, Choi H, Gao D, et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov. 2013;3:578–89. doi: 10.1158/2159-8290.CD-12-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.El Rayes T, Catena R, Lee S, Stawowczyk M, Joshi N, Fischbach C, et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci U S A. 2015;112:16000–5. doi: 10.1073/pnas.1507294112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hagerling C, Gonzalez H, Salari K, Wang CY, Lin C, Robles I, et al. Immune effector monocyte-neutrophil cooperation induced by the primary tumor prevents metastatic progression of breast cancer. Proc Natl Acad Sci U S A. 2019;116:21704–14. doi: 10.1073/pnas.1907660116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Massara M, Bonavita O, Savino B, Caronni N, Mollica Poeta V, Sironi M, et al. ACKR2 in hematopoietic precursors as a checkpoint of neutrophil release and anti-metastatic activity. Nat Commun. 2018;9:676. doi: 10.1038/s41467-018-03080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]