Combining ADCs against HER2-positive breast cancers with therapies that induce cellular senescence may improve their therapeutic efficacy by facilitating a bystander effect against antigen-negative tumor cells.
Abstract
Antibody–drug conjugates (ADC) are antineoplastic agents recently introduced into the antitumor arsenal. T-DM1, a trastuzumab-based ADC that relies on lysosomal processing to release the payload, is approved for HER2-positive breast cancer. Next-generation ADCs targeting HER2, such as [vic-]trastuzumab duocarmazine (SYD985), bear linkers cleavable by lysosomal proteases and membrane-permeable drugs, mediating a bystander effect by which neighboring antigen-negative cells are eliminated. Many antitumor therapies, like DNA-damaging agents or CDK4/6 inhibitors, can induce senescence, a cellular state characterized by stable cell-cycle arrest. Another hallmark of cellular senescence is the enlargement of the lysosomal compartment. Given the relevance of the lysosome to the mechanism of action of ADCs, we hypothesized that therapies that induce senescence would potentiate the efficacy of HER2-targeting ADCs. Treatment with the DNA-damaging agent doxorubicin and CDK4/6 inhibitor induced lysosomal enlargement and senescence in several breast cancer cell lines. While senescence-inducing drugs did not increase the cytotoxic effect of ADCs on target cells, the bystander effect was enhanced when HER2-negative cells were cocultured with HER2-low cells. Knockdown experiments demonstrated the importance of cathepsin B in the enhanced bystander effect, suggesting that cathepsin B mediates linker cleavage. In breast cancer patient-derived xenografts, a combination treatment of CDK4/6 inhibitor and SYD985 showed improved antitumor effects over either treatment alone. These data support the strategy of combining next-generation ADCs targeting HER2 with senescence-inducing therapies for tumors with heterogenous and low HER2 expression.
Significance:
Combining ADCs against HER2-positive breast cancers with therapies that induce cellular senescence may improve their therapeutic efficacy by facilitating a bystander effect against antigen-negative tumor cells.
Introduction
Antibody–drug conjugates (ADC) are being rapidly incorporated into the antitumor armory. Ado-trastuzumab emtansine (T-DM1, Kadcyla), an ADC based on trastuzumab, is a therapeutic mAb that targets the tyrosine kinase receptor HER2. This was the first ADC approved for use against a solid tumor, namely HER2-positive breast cancer. As the design of ADCs refined over time, their antitumor activity has become markedly enhanced (reviewed in ref. 1). Recently, the next-generation ADC trastuzumab deruxtecan (T-DXd, ENHERTU) received accelerated approval for the treatment of patients with metastatic HER2-positive breast cancer who had received two or more prior anti–HER2-based regimens (2). Results reported at ESMO 2021 also showed a marked increase in progression-free survival (PFS) in patients in early, second, and subsequent lines of treatment (3). Additional ADCs targeting HER2 are currently in advanced stages of clinical development, such as [vic-]trastuzumab duocarmazine (SYD985), RC48, and ARX788 (1).
HER2 is generally associated with breast and gastric cancers because the corresponding gene (ERBB2) is amplified in approximately 15% of these tumors [source, cBioportal (4)]. However, it is also amplified in additional tumors. These include approximately 15% of urothelial and esophageal carcinomas; approximately 10% of pancreatic cancers and uterine carcinosarcomas; approximately 5% of prostate cancers, cervical squamous cell carcinomas, bladder urothelial carcinomas, uterine corpus endometrial carcinoma; and 3% of pediatric neuroblastomas, colorectal adenocarcinomas, bladder cancers, acral melanomas, and lung squamous carcinomas (4). Overall, it is estimated that 3% of all patients with cancer have tumors with amplifications of ERBB2 and may in turn potentially benefit from HER2-targeting therapies like ADCs (4).
In first-generation ADCs, such as T-DM1, the chemotherapeutic agent was bound to the antibody via an uncleavable linker (5). Thus, after binding to cell surface HER2 and subsequent internalization, T-DM1 required complete degradation in the lysosome to release its antimicrotubule payload, emtansine (6). Because emtansine cannot cross lipidic membranes, in principle, it only acts on the cell that has internalized T-DM1 (6). SYD985 is a next-generation ADC bearing a potent duocarmycin-hydroxybenzamide-azaindole (DUBA) payload that alkylates DNA by binding to its minor groove. This ADC was the focus of the phase III TULIP clinical trial reported at ESMO 2021 that showed that SYD985 increased PFS compared with standard “Physician's Choice” treatments for patients with HER2-positive metastatic breast cancer (7). Similar to other next-generation ADCs, SYD985 has a cleavable linker. This results in a payload that is more easily released from the targeting antibody, even outside the tumor cell (8). Most next-generation ADCs bear comparable peptide linkers designed to be cleaved by lysosomal proteases such as cathepsins (9). In addition, DUBA is able to permeate the plasma membrane upon release from the antibody, enabling it to act on neighboring cells that are negative for the target antigen. This so-called “bystander effect” increases the efficacy of ADCs such as SYD985, especially on tumors with heterogeneous expression of the target (1).
The term cellular senescence refers to a broad range of heterogeneous phenotypes that include cell-cycle arrest and an inflammation-promoting secretory phenotype. Senescence is triggered by a number of signals, including oncogene expression, replicative stress, or exposure to a variety of chemicals (10). The observation that many common antitumor therapies such as DNA-damaging agents (e.g., doxorubicin) and Cdk4/6 inhibitors (Cdk4/6i, e.g., palbociclib) induce senescence has led to increasing interest in this distinct cellular phenotype and its effect on antitumor efficacy (11).
One of the hallmarks of cellular senescence is the enlargement of the lysosomal compartment (12). In fact, the most commonly used marker of cellular senescence, beta-galactosidase (SA-β-gal) activity (13), is a surrogate marker of lysosomal enlargement (14). It is not clear whether this enlargement reflects increased lysosomal activity (15) or a hypertrophy of defective lysosomes caused by increasing reactive oxygen species levels (16).
Given the importance of lysosomal function in the mechanism of action of ADCs, in this article we present data aimed to clarify whether therapies inducing senescence and, hence, potentially increasing lysosomal function, affect the efficacy of ADCs targeting HER2.
Materials and Methods
Patient-derived tumor xenografts
PDTX118 and PDTX251M were collected at Vall d'Hebron University Hospital (Barcelona) following institutional guidelines. Our study was approved by Vall d'Hebron Institute of Oncology (VHIO) Animal Care and Use Committee in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients.
Cell lines
MCF7 (RRID:CVCL_0031), MCF10A (RRID:CVCL_0598), BT474 (RRID:CVCL_0179), SKBR3 (RRID:CVCL_0033), HEK293T (RRID:CVCL_0063), and MDA-MB-231 (RRID:CVCL_0062) cell lines were purchased from ATCC and maintained in DMEM:F12 medium (Gibco). MDA-MB-231/luc cells, MCF7-TetOff/p95HER2 cells (17), MCF10A-HER2 (18), and BT474-R3 (19) were maintained as described previously. HCC1954 (RRID:CVCL_1259) and T47D (RRID:CVCL_0553) cells were maintained in RPMI medium (Gibco). All media were supplemented with 10% FBS, 2 mmol/L l-glutamine, and 1% penicillin-streptomycin. Mycoplasma contamination was tested monthly using the MycoAlert Mycoplasma Kit (Lonza). Cells were not authenticated in-house and were not passaged more than 30 times.
Knockdown of CTSB or CTSL was performed with short hairpin RNAs (shRNA) from lentiviral MISSION shRNA Library (SIGMA). For detailed explanation, see Supplementary Materials and Methods.
Reagents
Doxorubicin (Aurovitas), trastuzumab (Roche), T-DM1 (Roche), and T-DXd (Daichii Sankyo) were obtained from a local pharmacy. SYD985 was obtained from Byondis B.V. Cdk4/6i palbociclib (LC Laboratories) and Ca-074Me (SIGMA) were purchased.
Senescence induction and cell count
Cells were counted, plated in 10 cm plates, and treated the next day with varying doses of doxorubicin (4 days) or Cdk4/6i palbociclib (5 days). In oncogene-induced senescence, p95HER2 expression was induced via doxycycline removal for 5 days. At days 4 and/or 5, when cells were fully senescent, cells were detached to remove nonviable cells and were counted again. Collected cells were seeded in two plates, one to analyze senescence-associated β-galactosidase (SA-β-gal) and the other to test sensitivity to ADCs. Only cultures with >80% of cells positive for SA-β-gal were used.
Where indicated, doxorubicin-treated cells were induced to express p95HER2 by removing doxycycline from the media and adding fresh media to the plates for 5 additional days.
SA-β-gal activity assay
SA-β-gal activity was detected by colorimetric Senescence β-Galactosidase Staining Kit (Cell Signaling Technology) following the manufacturer's indications. Chemiluminescent detection was performed using Galacto-Light Plus beta-Galactosidase Reporter Gene Assay System Kit (Applied Biosystems) but with a citric acid-sodium phosphate buffer at pH 5.5.
Stranded mRNA sequencing library preparation and sequencing
RNA was isolated with RNeasy Mini Kit (Qiagen). RNA sequencing (RNA-seq) libraries were prepared following the TruSeq Stranded mRNA LT Sample Prep Kit protocol (Illumina) and validated on Agilent 2100 Bioanalyzer with DNA 7500 assay (Agilent). The RNA-seq libraries were sequenced on HiSeq 2000 (Illumina).
RNA-seq data processing and analysis
RNA-seq reads were mapped against the human genome (GRCh38) using STAR (29) with ENCODE parameters. Genes were quantified using RSEM. The gene annotation file was downloaded from gencode release 28 and differential gene expression was performed with DESeq2 (RRID:SCR_015687).
Gene set enrichment analysis (GSEA) was performed using GSEA v4.2.1 provided by the Broad Institute (RRID:SCR_003199) using predefined gene sets. Senescence (FRIDMAN_SENESCENCE_UP; broadinstitute.org/gsea/) and senescence-associated secretory phenotype (SASP; ref. 20) signatures were obtained from published gene sets. The lysosomal signature was obtained from Kyoto Encyclopedia of Genes and Genomes database. Genes corresponding to these signatures are shown in Supplementary Table SI.
Senescence reversibility assay
When cells were senescent, we withdrew treatments and cultured cells in fresh media, or cultured in doxycycline supplemented media. Pictures were taken at indicated days.
Cell viability assays
Cells were seeded in 96-well plates and after 24 hours treated with T-DM1 or SYD985 at described concentrations. Seven days later, cells viability was determined by crystal violet staining.
Bystander effect assays
Cells were treated as described, collected, and plated in coculture with MDA-MB-231/luc cells at ratios of 5 or 10:1 in 96-well plates. The day after, cells were treated with T-DM1 (0.01, 0.03, or 0.82 nmol/L) or SYD985 (0.03 or 0.82 nmol/L) for 5 days or T-DXd (3.25 nmol/L) for 7 days. Bystander cell death was measured using Luciferase Assay System (Promega).
Bystander assays employing increasing concentrations of T-DM1 and SYD985 were also performed with MCF7-TetOff/p95HER2 cells overexpressing p95HER2.
qRT-PCR
qRT-PCR was performed using TaqMan Gene Expression Probes and TaqMan Universal Master Mix II with UNG (Applied Biosystems, RRID:SCR_005039) following the manufacturer's recommendations.
Western blotting
Cells were lysed and sonicated for protein extraction. Protein extracts were quantified, resolved in SDS-PAGE gels, transferred onto nitrocellulose membranes (GE Healthcare Biosciences), and subjected to immunoblot analysis. For detailed explanation and list of antibodies, see extended Materials and Methods.
Cathepsin B activity assay
To measure the proteolytic activity of cathepsin B (CTSB), we used the Cathepsin B Activity Fluorometric Assay Kit (Biovision) according to the manufacturer's instructions.
CTSB chemical inhibition
MCF7-TetOff/p95HER2 cells induced to express p95HER2 were seeded in 96-well plates and pretreated with the CTSB inhibitor CA-074-Me or vehicle. Next, MDA-MB-231/luc cells were plated onto MCF7-TetOff/p95HER2 cultures and cocultures were treated with different concentrations of SYD985 for 5 days. Bystander cell death was measured as described previously.
In vivo analysis
Tumor fragments were implanted into the fourth fat pad of 6–8 weeks old NOD/SCID mice (Janvier, RRID:IMSR_CRL:394). β-estradiol was added to drinking water. For PDTXSR1 and PDTX251M, mice were treated intravenously with SYD985 (10 mg/kg), via oral gavage with palbociclib (50 mg/kg, daily for 5 days), both or PBS. For the parental PDTX118 experiment, mice were treated intravenously with T-DM1 or SYD985 (both 10 mg/kg). Mice were sacrificed when tumor volume approached 1,200–2,000 mm3 or due to unacceptable toxicity. Tumor samples were then collected for analyses. Mice were treated in accordance with the Ethical Committee for the Use of Experimental Animals at the Vall d'Hebron Institute of Oncology.
IHC analysis
IHC for HER2 was performed as described previously (19). For CTSB, slides were incubated overnight with primary antibody from Abcam. Quantification of CTSB IHC was performed using the image software QuPath (University of Edinburgh, Edinburgh, Scotland) considering five image fields of representative tumors from each group.
Flow cytometry
Cells were harvested with accutase (Gibco), resuspended in blocking buffer, incubated with trastuzumab and with anti-human AlexaFluor-488 (Invitrogen). In the In vivo experiment, cells were incubated with EpCAM-AlexaFluor-647 (BioLegend). Cells were resuspended in Zombie Aqua viability marker and acquired on BD FACSCelesta (BD Biosciences). Flow cytometry data were analyzed with FlowJo software (BD BioSciences, RRID:SCR_008520).
Statistical analysis
For in vitro experiments, data are presented as average ± SD and were analyzed by two-sided Student t test. Results were considered statistically significant at P values <0.05. For in vivo experiments, mice were randomly assigned to treatment groups and we used two-way ANOVA with subsequent Bonferroni correction for analysis. We did not use statistical methods to predetermine sample size in animal studies, but we made efforts to use the minimum number of animals. Sample sizes of 3 to 4 mice per group were chosen and two flanks were implanted to reduce the number of animals. All experiments were repeated at least three times, except the in vivo experiments, and a single representative experiment is shown. All measurements were performed at least in triplicate.
Data availability statement
Data generated in this study are available within the article and Supplementary Data or can be facilitated upon request to the corresponding author.
Results
Diverse inducers of senescence cause an increased lysosomal phenotype
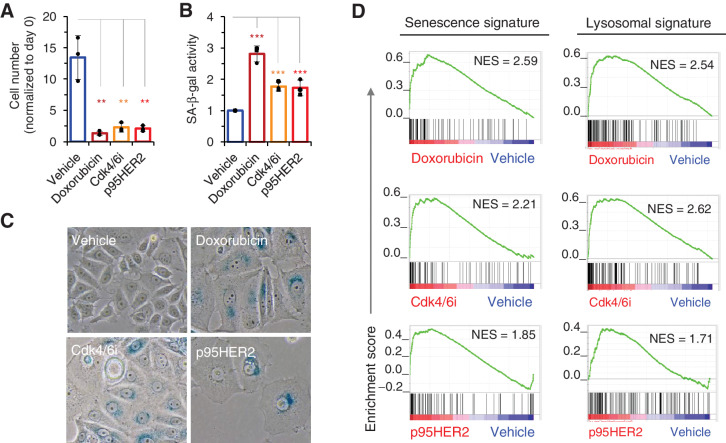
We have previously shown that expression of the oncoprotein p95HER2 induces a distinct senescence phenotype in MCF7 cells (17). We compared this phenotype with those induced by certain antitumor therapies, namely the DNA-damaging compound doxorubicin and the Cdk4/6i palbociclib. As expected, both p95HER2 and the antitumor treatments markedly inhibited cell proliferation (Fig. 1A), and increased the number of cells positive for SA-β-gal activity (Fig. 1B and C). Transcriptomic analyses confirmed an enrichment in a defined senescence signature in cells treated with doxorubicin or Cdk4/6i, comparable with that observed in cells expressing p95HER2 (Fig. 1D, left; Supplementary Table S1).
Figure 1.
Diverse senescence inducers increase lysosomal function. A, MCF7 cells were treated with vehicle, doxorubicin (50 nmol/L), the Cdk4/6i palbociclib (700 nmol/L) or induced to express p95HER2 for 4 or 5 days. Then, cells were counted; n = 3. B, SA-β-gal activity was quantified using Galacto-Light Plus β-Galactosidase Reporter Gene Assay System Kit (Applied Biosystems) from cells treated as in A. Results were normalized to those of cells treated with vehicle; n = 4. C, Representative pictures of SA-β-gal staining of MCF7 cells treated as in A. D, Transcriptomic profiles of cells treated as in A were determined by RNA-seq. Senescence and lysosomal GSEA signatures in treated cells were compared with that in cells treated with vehicle. P value corresponds to the NOM P value obtained by GSEA in the HALLMARK database. **, P < 0.01; ***, P < 0.001.
Despite these similarities, the senescence phenotypes induced by p95HER2 and those induced by the antitumor treatments showed marked differences. For example, while the expression of p95HER2 or treatment with doxorubicin induced a fully penetrant irreversible cell-cycle arrest (see also Supplementary Fig. S1A; ref. 17), the cell-cycle arrest induced by Cdk4/6i is transient, as cells resumed proliferation 1 week after the treatment was halted (Supplementary Fig. S1A). Furthermore, the induction of a secretory phenotype was clearly more pronounced in p95HER2-induced senescent cells than in therapy-induced senescent cells (Supplementary Fig. S1B). In fact, some of the components of the secretory phenotype induced during p95HER2-induced senescence, such as IL8, EPHA2, MMP1, or IL11, were not secreted by therapy-induced senescent cells (Supplementary Fig. S1C). These differences are likely due to upregulation of secreted components by p95HER2 signaling (see ref. 17). These p95HER2-induced secreted factors are added to the components of the senescence-associated secretory phenotype induced by other triggers. In agreement with this conclusion, while MMP1 is not secreted upon doxorubicin treatment, expression of p95HER2 in doxorubicin-induced senescent cells promoted its expression. In contrast, Lumican is expressed by senescent cells independently of p95HER2 expression (Supplementary Fig. S1D).
Notwithstanding these differences, transcriptomics revealed similar enrichments in lysosomal signatures across the various treatments (Fig. 1D), suggesting that the lysosomal enlargement is a common consequence of senescence induction. Given the relevance of lysosomal function for the efficacy of ADCs, we analyzed the effect of combining these with therapies that induce senescence.
Effect of therapy-induced senescence on sensitivity to anti-HER2 ADCs
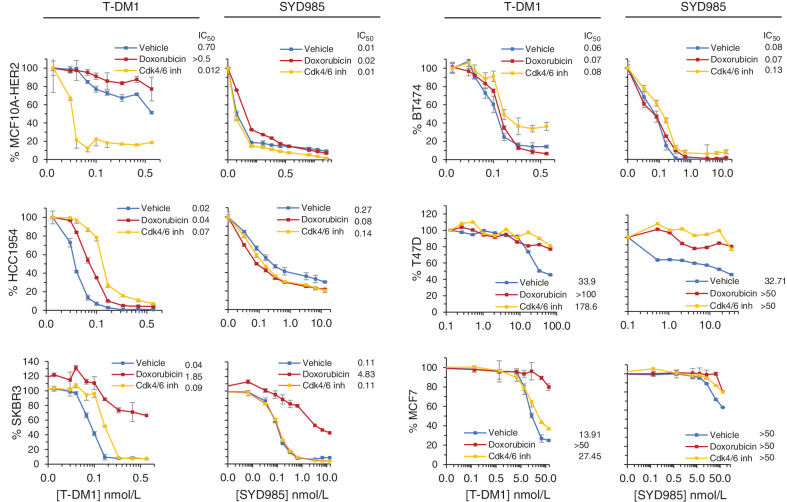
For subsequent analyses, we induced cellular senescence in a panel of breast cancer cell lines. Different concentrations of doxorubicin or Cdk4/6i were required to maximize the induction of cellular senescence (Supplementary Fig. S2A; Supplementary Table S2). The cell lines chosen expressed different levels of HER2 (Supplementary Fig. S2B), including cells with ERBB2 amplification and HER2 overexpression (defined as 3+ according to IHC semiquantitative analysis). In addition, we used cell lines that have historically been considered HER2-negative but express readily detectable level of HER2 (1+ by IHC analysis), now considered HER2-low. HER2-low tumors include 50% of all breast cancers (21), and can be potentially targeted with trastuzumab (22, 23). In all cases, treatment with doxorubicin or Cdk4/6i resulted in the inhibition of cellular proliferation and an increase in the number of SA-β-gal–positive cells, comparable to what was observed in Fig. 1 (Supplementary Fig. S2D).
The treatments with the inducers of senescence had little effect or decreased the sensitivity to the ADCs, irrespectively of the levels of HER2. In the majority of cases, treatments inducing senescence did not affect sensitivity to T-DM1 (Fig. 2). Only MCF10A cells overexpressing HER2 and treated with the Cdk4/6i showed a higher sensitivity to T-DM1 (Fig. 2). Similarly, the induction of cellular senescence did not increase sensitivity to SYD985 (Fig. 2).
Figure 2.
Effect of doxorubicin or Cdk4/6i on the sensitivity to T-DM1 or SYD985. The indicated cell lines were treated with a concentration of doxorubicin (ranging from 12.5 to 50 nmol/L, depending on cell line, for 4 days) or Cdk4/6i (700 to 3,000 nmol/L for 5 days), replated and treated with different concentrations of T-DM1 or SYD985 for 7 additional days without doxorubicin or Cdk4/6i. Then, cell numbers were estimated with the Crystal Violet staining assay.
To further assess the relevance of the lysosomal phenotype on the efficacy of ADCs, we used cells that are resistant to T-DM1 due to lysosomal dysfunction (24). Again, no improved response to SYD985 was observed in these cells upon treatment with Cdk4/6i (Supplementary Fig S2C). We concluded that in the majority of cases senescence induction does not affect cellular sensitivity to ADCs, despite the aforementioned lysosomal phenotype.
Some degree of lysosomal enlargement may precede the full cell-cycle arrest characteristic of cellular senescence. Because chemotherapeutics linked to ADCs are particularly active on proliferating cells, concentrations of doxorubicin or Cdk4/6i that induce a lysosomal enlargement but only a partial proliferation arrest may increase the activity of ADCs. Thus, we treated cells with different concentrations of doxorubicin or Cdk4/6i and measured lysosomal function using as a surrogate marker glucosamine (N-Acetyl)-6-sulfatase (GNS; ref. 25). Certain concentrations of doxorubicin or Cdk4/6i led to an increased expression of GNS, evidencing some degree of increased lysosomal activity, in cells still dividing, as judged by cell counting (Supplementary Fig. S3A and S3B). These concentrations of doxorubicin (10 and 25 nmol/L) or Cdk4/6i (50 and 100 nmol/L) did not lower the IC50 of SYD985 or T-DM1 (Supplementary Fig. S3C and S3D). We concluded that the lysosomal phenotype promoted by senescence-inducing therapies, even in cells that remain proliferative, does not lead to sensitization to HER2-directed ADCs.
Effect of cellular senescence on the bystander effect of ADCs
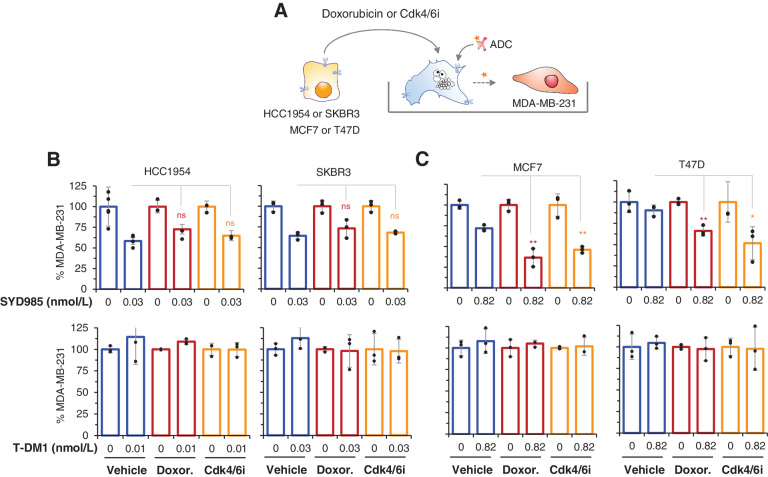
Next, we asked whether the induction of senescence could increase the bystander effect of SYD985. To address this possibility, we first induced senescence in cells expressing different levels of HER2 by treating them with doxorubicin or Cdk4/6i. Then, we cocultured these senescent cells with the HER2-negative cell line MDA-MB-231 expressing a luciferase reporter (Fig. 3A).
Figure 3.
Effect of doxorubicin or Cdk4/6i on the bystander effect of SYD985. A, Schematic showing the experimental procedure. The indicated cells were treated with doxorubicin or Cdk4/6i for 4 or 5 days, respectively. Then, cells were harvested and cococultured with MDA-MB-231 cells expressing luciferase and cocultures were treated with SYD985 or T-DM1 for 5 additional days. At the end of the experiment, MDA-MB-231/luc cells were quantified by luminescence. B and C, Luminescence, quantified as described in A, was determined at the end of the experiments and normalized to cells treated with vehicle. n ≥ 3. ns, nonsignificant; *, P < 0.05; **, P < 0.01.
Likely due to the elevated expression of the antigen, we observed a similar bystander effect of senescent cells expressing high levels of HER2 (HCC1954 and SKBR3 cells) compared with vehicle-treated controls on the killing of reporter MDA-MB-231/luc cells (Fig. 3B, upper graphs), as expected much higher than in HER2-low cells. Thus, the lysosomal phenotype induced by the treatments did not alter the bystander effect of senescent cells expressing high levels of HER2 treated with SYD985. This result indicated that, in cells expressing high levels of the target antigen, the lysosomal compartment does not limit the bystander effect of ADCs with a cleavable linker, as amplifying it by inducing senescence does not have an effect. As expected, T-DM1 had no bystander effect in similar assays (Fig. 3B, bottom graphs).
In contrast, cellular senescence amplified the bystander effect of SYD985 on HER2-negative cells cocultured with cells expressing low levels of HER2 (MCF7 and T47D cells; Fig. 3C, top graphs). We confirmed that this effect was independent from the trigger that induced senescence by analyzing the effect of oncogene-induced senescence. As shown in Supplementary Fig. S4A, p95HER2-induced senescent cells also amplified the SYD985 bystander effect. Again, T-DM1 did not have bystander effect on these cultures (Supplementary Fig. S4). A similar effect was observed when analyzing the bystander effect of T-DXd (Supplementary Fig S4B), arguing that the induction of cellular senescence can increase the antitumor activity of different ADCs with cleavable linkers.
We concluded that the bystander effect of ADCs with cleavable linkers and membrane permeable drugs can be increased by inducing cellular senescence in target cells with low levels of HER2 expression.
Role of cathepsins in the bystander effect
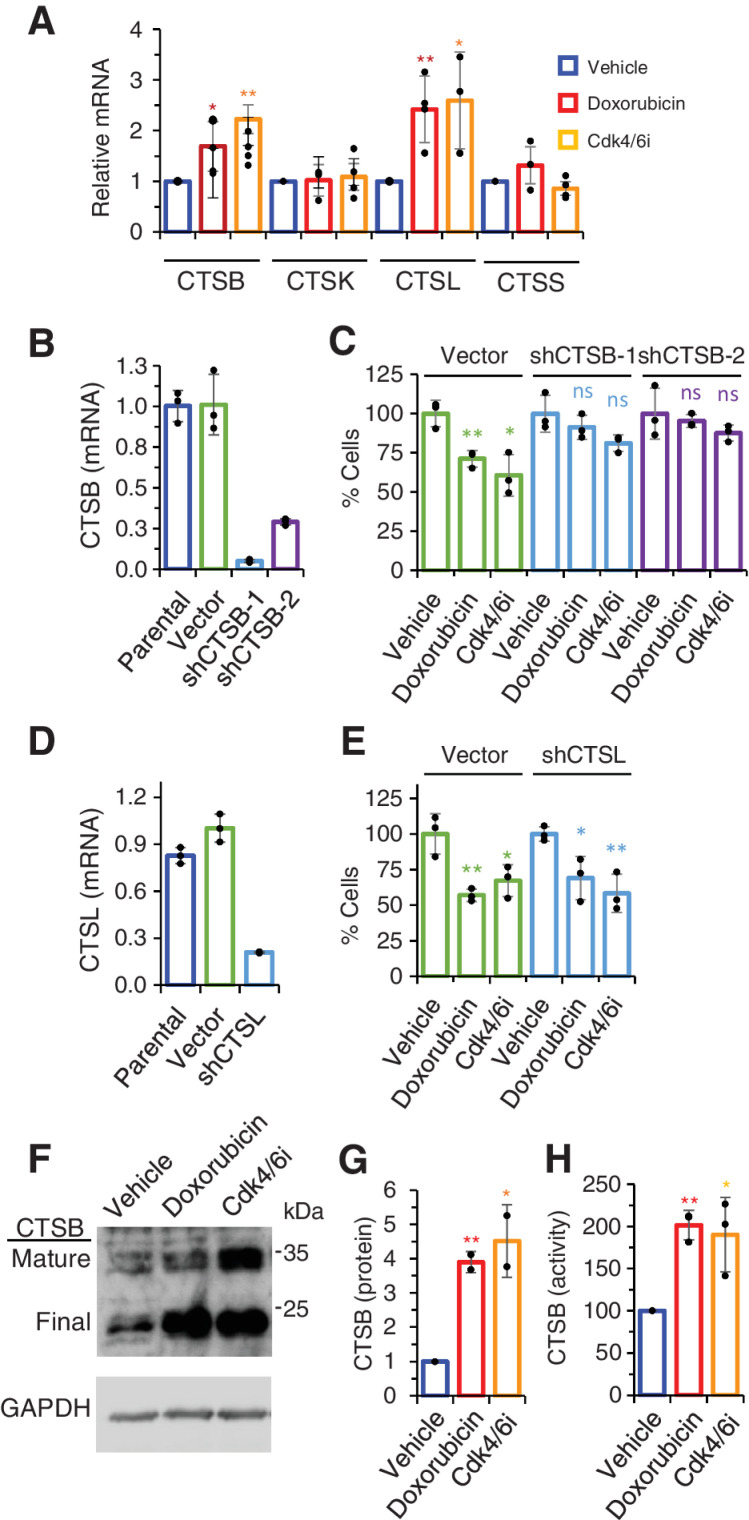
Cathepsins, particularly CTSB, have been previously shown to cleave the peptide linker of certain ADCs, including SYD985 (8, 26). Analysis of the transcriptome of senescent cells induced by doxorubicin or Cdk4/6i showed an upregulation of cathepsins B and L (CTSL) expression (Fig. 4A).
Figure 4.
Role of cathepsins on the bystander effect of SYD985. A, Levels of the indicated cathepsins were determined by qRT-PCR in MCF7 cells treated as in Fig. 1A. n ≥ 3. B, Levels of mRNA encoding CTSB in parental MCF7 cells or the same cells stably transfected with the empty vector or two different shRNA (sh) targeting CTSB. Results were normalized to parental cells. n = 3. C, As described in Fig. 3A, cocultures were performed to analyze the bystander effect of SYD985 upon knockdown of CTSB. n = 3. D and E, The role of cathepsin L on the bystander effect of SYD985 was analyzed as in B and C. F–H, MCF7 cells treated as indicated were lysed and lysates analyzed by Western blot analysis with anti-CTSB antibodies (F and G) or processed to determine CTSB activity with a specific assay (H). n = 3. *, P < 0.05; **, P < 0.01.
To determine the functional relevance of these cathepsins, we knocked down their expression with shRNAs and functionally characterized the resulting cells. While the knockdown of CTSB impaired the bystander effect of SYD985 (Fig. 4B and C), the knockdown of CTSL had no effect (Fig. 4D and E). Analysis of protein levels confirmed that the induction of senescence by the antitumor therapies upregulated CTSB (Fig. 4F and G) as well as increased its activity (Fig. 4H). Note that, based on previous reports (27), we identified the 34 and 24 kDa bands detected by Western blot as lysosomal active forms of CTSB, the mature precursor and the final form, respectively.
The p95HER2-induced senescence model further confirmed the relevance of CTSB, as treatment with the irreversible CTSB-specific inhibitor CA-074Me impaired the observed bystander effect (Supplementary Fig. S5A). The results of these experiments in MCF7 cells were successfully recapitulated in T47D cells, confirming the association between CTSB and bystander killing (Supplementary Fig. S5B–S5F).
Combination of therapy-induced senescence with ADCs
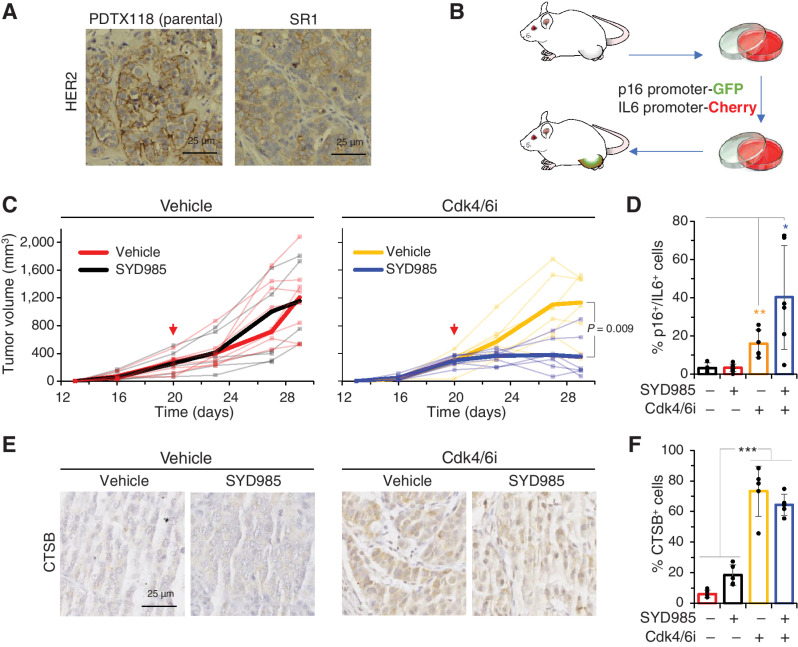
Using a patient-derived tumor xenograft (PDTX) sensitive to T-DM1 and SYD985 (Supplementary Fig. S6A), we previously generated several models of resistance to T-DM1 (19). While some of these models are sensitive to SYD985, one of them, SR1, shows resistance to both ADCs and displays a lower and more heterogenous expression of HER2 (Fig. 5A). This HER2 status resembles those in the coculture experiments shown in Fig. 3. We therefore selected PDTX SR1 as our model to test the efficacy of the combination in vivo.
Figure 5.
Effect of the combination Cdk4/6i-SYD985 on a PDTX resistant to SYD985. A, IHC analysis of HER2 in the parental PDTX or SR1, the corresponding resistant PDTX derived from it. B, Schematic showing the generation of SR1 tumors expressing reporters under the control of human p16 or IL6 promoters. Briefly, cells from SR1 were transiently cultured, infected with virus encoding the reporters as described in ref. 25, and reimplanted into immunodeficient mice. C, PDTXs expressing the reporters described in B were implanted into NOD.SCID mice and volumes were monitored periodically. As indicated by the arrow, control group (treated with vehicle) and Cdk4/6i group (treated with palbociclib at 50 mg/kg/day) were treated with 10 mg/kg of SYD985 in single dose fashion. Narrow lines represent the growth of individual tumors, wide lines represent averages. D, At the end of the experiment, the number of double-positive cells for IL6 and p16 was quantified from independent tumors by flow cytometry. n = 5. E and F, IHC analysis of CTSB in tumors treated as in C. Results from five different fields were quantified and the results are shown as averages ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To efficiently monitor cellular senescence in in vivo experiments, we recently generated PDTXs expressing GFP and Cherry reporters under the control of human p16 and IL6 promoters, respectively (Fig. 5B). Previous characterization of similar reporters showed that their simultaneous expression is a bone fide marker of cellular senescence (28).
While treatment with SYD985 or the Cdk4/6i had no effect on tumor growth, the combination had a significant antitumor effect (Fig. 5C). Analysis of the percentage of cells double positive for the GFP and Cherry reporters showed that, as expected, the number of double positive cells increased with Cdk4/6i treatment (Fig. 5D). Interestingly, the percentage of senescent cells in tumors treated with the combination was augmented to approximately 40%, further highlighting the preferential effect of DUBA on proliferating cells. Increased CTSB expression was also observed in vivo upon treatment with Cdk4/6i (Fig. 5E and F).
To further support the efficacy of the combination, we analyzed its effect on a PDTX primarily resistant to SYD985 (PDTX251M) considered HER2-high (Supplementary Fig. S6B and S6C). Although the mechanism behind this resistance is unknown, treatment with the Cdk4/6i also sensitized this model to SYD985 (Supplementary Fig. S6C).
Collectively, the results presented here show that the combination of therapies inducing senescence with ADCs bearing cleavable linkers is effective against heterogeneous tumors expressing low levels of the targeted antigen HER2 as well as against some primarily resistant tumors.
Discussion
The term senescence is used to define cells with diverse phenotypes that have in common a stable cell-cycle arrest and the ability to secrete a collection of molecules, which include several inflammatory factors (29). The only undisputed marker for cellular senescence, SA-β-gal activity, provides qualitative rather than exact results. While cells with the characteristic strong blue staining are undoubtedly considered senescent, there is no threshold of activity to clearly identify senescent cells with lower intensity of staining. Because of this shortcoming, the search for specific markers of senescent cells is actively ongoing (30).
The positivity of SA-β-gal activity likely does not identify a homogeneous phenotype but rather a variety of them. As described previously (31, 32), and as shown here, cell-cycle arrest induced by Cdk4/6 inhibition is largely reversible, in contrast with that induced by the oncogene p95HER2 or the severe DNA damage resulting from doxorubicin. Furthermore, as shown here, the SASP, which is considered a hallmark of cellular senescence, also varies markedly with different triggers of senescence.
In contrast with the SASP transcriptomic signature, the signature of increased lysosomal function is similarly enriched in senescent cells induced by the different triggers. This result likely reflects increased lysosomal activity, just as SA-β-gal does. In fact, the beta-galactosidase activity detectable at acidic pH, defined as SA-β-gal, is due to the action of a lysosomal beta-galactosidase encoded by GLB1 (14). While the hypertrophic lysosomes of senescent cells have been considered defective by some authors (16), the increased SA-β-gal activity, along with our transcriptomic analysis and the analysis of CTSB activity, indicates that it corresponds to increased lysosomal function. In summary, increased lysosomal function seems to constitute one of the most defining phenotypes of senescent cells.
Following the success of next-generation HER2-directed ADCs on HER2-amplified breast cancers, these therapies are now being tested in patients with HER2-low breast cancer with promising effects. While the first-generation ADC T-DM1 showed a poorer response in HER2-low tumors, ADCs with a cleavable linker such as T-DXd have demonstrated effectivity in these low HER2-expressing tumors in clinical trials (33), similar to the results obtained with SYD985 (8). Despite these positive results, all responses were transient, underscoring the need to improve the activity of these ADCs. Given the relevance of the lysosomal function in the mechanism of action of ADCs, the analysis of the efficacy of combinations of ADCs and therapies inducing senescence continues to be warranted.
The results presented here clearly show that lysosomal function is not a limiting factor of the efficacy of ADCs on target cells. This conclusion is firmly supported by the fact that the increase in the lysosomal function induced by different triggers of senescence does not affect the sensitivity of a variety of cells expressing different levels of HER2 to T-DM1 or SYD985. Furthermore, the lysosomal activity is also not limiting for the bystander effect of SYD985 on cells expressing high levels for HER2. However, the lysosomal activity of senescent cells, and the subsequent upregulation of the SYD985 valine-citrulline linker-cleaving enzyme CTSB (26), does enhance the bystander effect of SYD985 on cells expressing low levels of HER2. This conclusion, based on in vitro assays, was confirmed by using a SYD985-resistance PDTX model with low and heterogenous expression of HER2. This result opens the possibility for efficacious combinations of ADCs targeting HER2 together with therapies inducing senescence, particularly in breast and gastric tumors that express lower levels of HER2 defined by IHC (21, 34, 35). A future challenge to be tested in the clinic will be not only to confirm the potential efficacy of these combinations but also the toxicity profile and, based on that, define whether the strategy represents a positive balance to improve the treatment of patients. In addition, we showed that the combination of SYD985 and therapies inducing senescence is effective on some resistant models (Supplementary Fig. S6C), but not others (Supplementary Fig. S2C)
In conclusion, our data strongly support a strategy combining next-generation ADCs, carrying cleavable linkers and membrane permeable payloads, with therapies that induce cellular senescence specifically coupled to augmented lysosomal activity. This latter function is likely to drive an enhanced bystander effect in low target-expressing cells that may result in an overall increased efficacy of antitumor treatment.
Supplementary Material
Supplementary Tables
Cellular phenotypes induced by diverse senescence inducers
Effect of doxorubicin and Cdk4/6i on cells expressing different levels of HER2.
Effect of different concentration of doxorubicin or Cdk4/6i on the sensitivity to trastuzumab-based ADCs
Effect of different inducers of senescence on the bystander effect of ADCs.
Effect of knock-down of cathepsins on the bystander effect of SYD985.
Effect of ADCs, alone or in combination, on different PDTXs
Extended Materials and Methods and Supplementary figures with legends
Acknowledgments
This work was supported by Breast Cancer Research Foundation (BCRF-20-008), Instituto de Salud Carlos III (project reference numbers AC15/00062, CB16/12/00449 and PI19/01181), the EC under the framework of the ERA-NET TRANSCAN-2 initiative co-financed by FEDER, Fundación Mutua Madrileña and Asociación Española Contra el Cáncer. S. Duro-Sánchez is supported by the Spanish Ministerio de Universidades by the grant Formación de Profesorado Universitario (FPU20/05388). A. Esteve-Codina is funded by ISCIII /MINECO (PT17/0009/0019) and co-funded by FEDER. The authors acknowledge Alyson MacInnes for reviewing and editing the article.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Authors' Disclosures
S. Duro-Sánchez reports grants from Byondis B.V. during the conduct of the study. M. Lalinde-Gutiérrez reports grants from Byondis B.V during the conduct of the study. S. Pérez-Ramos reports grants from Byondis B.V. during the conduct of the study. S. Escrivá-de-Romaní reports grants from Byondis B.V. during the conduct of the study; grants and personal fees from AstraZeneca Daiichi Sankio, and F Hoffmann-La Roche Ltd; personal fees from Pfizer, Pierre-Fabre, Seagen, Novartis, and Kern; and grants from Byondis, Zymeworks, Synthon, MedSir, and Solti outside the submitted work. A. Pandiella reports personal fees from Daiichi Sankyo and Seagen outside the submitted work. J. Cortés reports personal fees from Roche, Celgene, Cellestia, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Merck Sharp & Dohme, GSK, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Elipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, Reveal Genomics, Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, AstraZeneca, Gilead, Novartis, and Samsung Bioepis and grants from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardanth Health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Puma C, and Queen Mary University of London outside the submitted work; in addition, J. Cortés has a patent for Pharmaceutical Combinations of A Pi3k Inhibitor And A Microtubule Destabilizing Agent (Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A) issued and a patent for Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy (Aleix Prat, Antonio Llombart, Javier Cortés. US 2019/ 0338368 A1) licensed. C. Saura reports personal fees from AstraZeneca, Gilead, Daiichi Sankyo, Seagen Int., Eisai Europe Ltd., Phillips Health Works, Pfizer Inc., Solti, Puma Biotechnology Inc., Pierre Fabre, Roche Farma, SA, and Novartis; and nonfinancial support from Lilly SAU, Pierre Fabre, AstraZeneca, Exeter Pharma LLC, AX's Consulting SARL, AX's Consulting SARL, and MediTech outside the submitted work. J. Arribas reports grants from Breast Cancer Research Foundation, Instituto de Salud Carlos III, ERA-NET TRANSCAN2, Asociación Española Contra el Cáncer, Byondis, and Fundación Mutua Madrileña during the conduct of the study and grants from Roche and Molecular Partners outside the submitted work; in addition, J. Arribas has a patent for EP22382294.1 issued, a patent for EP20382457.8 licensed to Mnemo Therapeutics, and a patent for EP2360187B1 licensed to Innovent. No disclosures were reported by the other authors.
Authors' Contributions
S. Duro-Sánchez: Conceptualization, investigation, methodology, writing–original draft, writing–review and editing. M. Nadal-Serrano: Conceptualization, investigation, methodology. M. Lalinde-Gutiérrez: Investigation. E.J. Arenas: Project administration. C. Bernadó Morales: Project administration. B. Morancho: Investigation, project administration. M. Escorihuela: Resources, investigation. S. Pérez-Ramos: Investigation. S. Escrivá-de-Romaní: Resources, investigation. L. Gandullo-Sánchez: Investigation. A. Pandiella: Resources, investigation, writing–review and editing. A. Esteve-Codina: Resources, investigation, writing–review and editing. V. Rodilla: Resources. F.A. Dijcks: Resources, writing–review and editing. W.H.A. Dokter: Conceptualization, resources, supervision, funding acquisition, visualization, writing–original draft, project administration, writing–review and editing. J. Cortés: Funding acquisition, investigation. C. Saura: Resources, investigation. J. Arribas: Conceptualization, resources, supervision, funding acquisition, visualization, writing–original draft, project administration, writing–review and editing.
References
- 1. Pegram MD, Miles D, Tsui CK, Zong Y. HER2-Overexpressing/amplified breast cancer as a testing ground for antibody-drug conjugate drug development in solid tumors. Clin Cancer Res 2019;26:775–86. [DOI] [PubMed] [Google Scholar]
- 2. Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020;382:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cortés J, Kim S-B, Chung W-P, Im S-A, Park YH, Hegg R, et al. LBA1 trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): results of the randomized phase III DESTINY-Breast03 study. Ann Oncol 2021;32:S1287–8. [Google Scholar]
- 4. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phillips GDL, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008;68:9280–90. [DOI] [PubMed] [Google Scholar]
- 6. Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res 2006;66:4426–33. [DOI] [PubMed] [Google Scholar]
- 7. Manich CS, O'Shaughnessy J, Aftimos PG, van den TE, Oesterholt M, Escrivá-de-Romaní SI, et al. LBA15 primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician's choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol 2021;32:S1288. [Google Scholar]
- 8. van der Lee MM, Groothuis PG, Ubink R, van der Vleuten MA, van Achterberg TA, Loosveld EM, et al. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol Cancer Ther 2015;14:692–703. [DOI] [PubMed] [Google Scholar]
- 9. Caculitan NG, dela CCJ, Ma Y, Zhang D, Kozak KR, Liu Y, et al. Cathepsin B is dispensable for cellular processing of cathepsin B-cleavable antibody–drug conjugates. Cancer Res 2017;77:7027–37. [DOI] [PubMed] [Google Scholar]
- 10. Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014;15:482–96. [DOI] [PubMed] [Google Scholar]
- 11. Wang B, Kohli J, Demaria M. Senescent cells in cancer therapy: friends or foes? Trends Cancer 2020;6:838–57. [DOI] [PubMed] [Google Scholar]
- 12. Robbins E, Levine EM, Eagle H. Morphologic changes accompanying senescence of cultured human diploid cells. J Exp Med 1970;131:1211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995;92:9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, et al. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006;5:187–95. [DOI] [PubMed] [Google Scholar]
- 15. Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radical Biol Med 2002;33:611–9. [DOI] [PubMed] [Google Scholar]
- 16. Park JT, Lee Y-S, Cho KA, Park SC. Adjustment of the lysosomal-mitochondrial axis for control of cellular senescence. Ageing Res Rev 2018;47:176–82. [DOI] [PubMed] [Google Scholar]
- 17. Angelini PD, Fluck MFZ, Pedersen K, Parra-Palau JL, Guiu M, Morales CB, et al. Constitutive HER2 signaling promotes breast cancer metastasis through cellular senescence. Cancer Res 2013;73:450–8. [DOI] [PubMed] [Google Scholar]
- 18. Parra-Palau JL, Morancho B, Peg V, Escorihuela M, Scaltriti M, Vicario R, et al. Effect of p95HER2/611CTF on the response to trastuzumab and chemotherapy. J Natl Cancer Inst 2014;106:dju291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nadal-Serrano M, Morancho B, Escrivá-de-Romaní S, Morales CB, Luque A, Escorihuela M, et al. The second generation antibody-drug conjugate SYD985 overcomes resistances to T-DM1. Cancers 2020;12:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muñoz-Espín D, Rovira M, Galiana I, Giménez C, Torres BL, Ribes MP, et al. A versatile drug delivery system targeting senescent cells. EMBO Mol Med 2018;10;e9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-low breast Cancer: pathological and clinical landscape. J Clin Oncol 2020;38:1951–62. [DOI] [PubMed] [Google Scholar]
- 22. Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res 2009;15:2010–21. [DOI] [PubMed] [Google Scholar]
- 23. Abdel-Latif GA, Al-Abd AM, Tadros MG, Al-Abbasi FA, Khalifa AE, Abdel-Naim AB. The chemomodulatory effects of resveratrol and didox on herceptin cytotoxicity in breast cancer cell lines. Sci Rep 2015;5:12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ríos-Luci C, García-Alonso S, Díaz-Rodríguez E, Nadal-Serrano M, Arribas J, Ocaña A, et al. Resistance to the antibody-drug conjugate T-DM1 is based in a reduction in lysosomal proteolytic activity. Cancer Res 2017;77:4639–51. [DOI] [PubMed] [Google Scholar]
- 25. Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. A gene network regulating lysosomal biogenesis and function. Science 2009;325:473–7. [DOI] [PubMed] [Google Scholar]
- 26. Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 2003;21:778–84. [DOI] [PubMed] [Google Scholar]
- 27. Mijanović O, Branković A, Panin AN, Savchuk S, Timashev P, Ulasov I, et al. Cathepsin B: a sellsword of cancer progression. Cancer Lett 2019;449:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Triana-Martínez F, Picallos-Rabina P, Silva-Alvarez SD, Pietrocola F, Llanos S, Rodilla V, et al. Identification and characterization of cardiac glycosides as senolytic compounds. Nat Commun 2019;10:4731–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, et al. Cellular senescence: defining a path forward. Cell 2019;179:813–27. [DOI] [PubMed] [Google Scholar]
- 30. González-Gualda E, Baker AG, Fruk L, Muñoz-Espín D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J 2021;288:56–80. [DOI] [PubMed] [Google Scholar]
- 31. Hu W, Sung T, Jessen BA, Thibault S, Finkelstein MB, Khan NK, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res 2015;22:2000–8. [DOI] [PubMed] [Google Scholar]
- 32. Vijayaraghavan S, Karakas C, Doostan I, Chen X, Bui T, Yi M, et al. CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin E negative cancers. Nat Commun 2017;8:15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast Cancer: results from a phase Ib study. J Clin Oncol 38:17s, 2020. (suppl; abstr 1887–96). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang H, Katerji H, Turner BM, Hicks DG. HER2-Low breast cancers. Am J Clin Pathol 2022;157:328–36. [DOI] [PubMed] [Google Scholar]
- 35. Trastuzumab deruxtecan DESTINY for some cancers. Cancer Discov 2020;10:898. doi: 10.1158/2159-8290.CD-ND2020-011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables
Cellular phenotypes induced by diverse senescence inducers
Effect of doxorubicin and Cdk4/6i on cells expressing different levels of HER2.
Effect of different concentration of doxorubicin or Cdk4/6i on the sensitivity to trastuzumab-based ADCs
Effect of different inducers of senescence on the bystander effect of ADCs.
Effect of knock-down of cathepsins on the bystander effect of SYD985.
Effect of ADCs, alone or in combination, on different PDTXs
Extended Materials and Methods and Supplementary figures with legends
Data Availability Statement
Data generated in this study are available within the article and Supplementary Data or can be facilitated upon request to the corresponding author.