Abstract
Data obtained from long-term survey studies are valuable for assessing the population status and trends in critical populations of threatened species, like sea turtles. Akyatan Beach is one of the most important green turtle nesting beaches in the Mediterranean and has been monitored since 2006 without interruption. The beach is 22 km long and more than 100 m wide at some points, and both loggerhead and green turtles nest on the beach. However, loggerhead nesting is very limited compared to green turtles. A total of 3866 C. mydas nests were recorded over ten consecutive years at Akyatan Beach, with a mean of 387 ± 127 nests (range = 201–559). The average nesting density was 17.6 nests km-1 (range = 9.1–25.4 nests km-1). In the 3309 nests, a total of 355,259 eggs were counted. The overall mean clutch size was 112 ± 26.10 eggs. Of these eggs, 50.80% hatched (depredated nests included), and 78.07% of them were able to reach the sea. The overall mean hatching success was 73.07 ± 26.20%. The overall mean incubation duration was 51.4 ± 3.5 days. The clutch sizes and hatching success differed between years, and there was a significant decreasing trend in mean incubation duration over the ten years of the study. A total of 1585 green turtle nests (41.02% of nests) were totally or partially depredated by golden jackals and wild boars, while other predators depredated 20.5% of hatchlings. The nesting data obtained since 2006 showed strong annual fluctuations ranging from 170 (in 2007) to 562 (in 2006) with a slightly increasing but statistically insignificant trend (r = 0.94, p > 0.05). The main threats to the population were depredation by jackals and wild boars.
Keywords: Endangered species, Conservation, Sea turtles
BACKGROUND
Sea turtles are migratory species with a complex life history that spend most of their lives in the sea, and only females come to beaches to reproduce. They have different habitats during these complex life cycles. The creation of practical action plans to protect endangered sea turtles will be possible by obtaining detailed information about these different habitats and revealing the connections between these habitats.
In this respect, the Mediterranean is an area containing essential habitats for two species: the green turtle (Chelonia mydas) and the loggerhead turtle (Caretta caretta). It was defined as a regional management unit for both species. A total of 17 regional management units (RMUs) were identified for green turtles worldwide, and one of them is the Mediterranean region, which is listed in the “high threats” category (Wallace et al. 2010 2011). Recent studies reveal that the populations of these two species have increased both globally (Mazaris et al. 2017) and regionally (Casale et al. 2018).
The loggerhead and green turtles nesting in the Mediterranean prefer the coasts of Turkey, Greece, Libya, Egypt, and Tunisia as foraging and wintering grounds (Broderick et al. 2007; Casale and Margaritoulis 2010; Godley et al. 2003; Rees et al. 2008; Snape et al. 2016; Stokes et al. 2015). The nesting of green turtles in the Mediterranean is confined to Turkey, Northern Cyprus, Lebanon, Israel, and Egypt (Türkozan and Kaska 2010; Casale et al. 2018), and the average number of documented nests is 1500 nests/year in the Mediterranean (Casale and Margaritoulis 2010). Of the nesting grounds in the Mediterranean, Turkey, and Cyprus comprise almost 99% of the overall nesting activity (Kasparek et al. 2001; Türkozan and Kaska 2010; Casale et al. 2018). Recent studies using mtSTR described 3 to 4 management units for green turtle stocks in the Mediterranean (Tikochinski et al. 2018; Karaman et al. 2022).
Of the 13 major green turtle rookeries in the Mediterranean, six are located on Turkish coasts (Casale et al. 2018), and a total of 452–2051 green turtle nests are estimated annually in Turkey (Türkozan and Kaska 2010). Akyatan Beach has been regularly monitored since 2006, and a -1.2% change was reported in the nest numbers before 1999 and after 2000 monitoring programs (Casale et al. 2018). This decrease is noticeable since other crucial green turtle nesting beaches (Kazanlı and Samandağ, Turkey) provided a 71.4–279.1% increase in nest numbers (Casale et al. 2018). Since Akyatan Beach is a part of a strictly protected area and far from anthropogenic effects, we focused on the long term population parameters to identify possible causes of this decrease. A population size and nest density estimate not based on yearly long-term monitoring studies on nesting beaches may provide misleading results due to biased calculations caused by fluctuations in the number of nests (Sönmez et al. 2021). We, therefore, offered long-term data and ongoing nesting activities on the beach, which hosts about 20% of the total number of green turtle nests recorded in the Mediterranean.
MATERIALS AND METHODS
Study site
Akyatan Beach is located on the eastern Mediterranean coast of Turkey and within the Wildlife Development Area of 15,304 ha, which has held protection status by the Ministry of Forest and Agriculture since 1987. Akyatan Beach is between Akyatan Lagoon and the Mediterranean and is 22 km long (Fig. 1). Both green and loggerhead turtles nest on Akyatan beach, but green turtle nesting is denser. Conservation studies of sea turtles in Akyatan Beach have been regularly carried out since 2006 according to the protocol signed by the Ministry of Agriculture and Forestry, 7th Regional Directorate of Nature Conservation and National Parks, and WWF-Turkey.
Fig. 1.
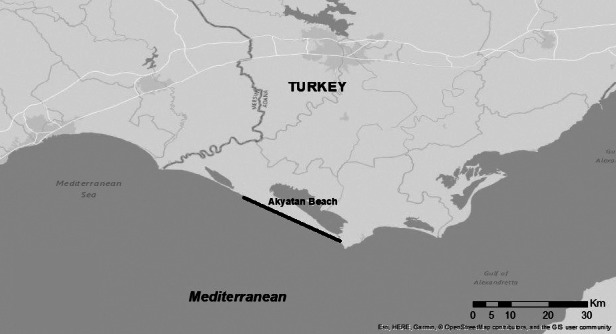
Akyatan Beach on the Turkish coast.
Nesting data
The nesting data for C. mydas were collected over ten nesting seasons (2012–2021) between June 1 and Sept. 15 each year (except for the 2014 and 2015 nesting seasons when the survey started on May 15th). About 14 km of the 22 km beach were monitored daily on foot, and the remaining 8 km were monitored once every three days (Fig. 1).
Adult emergence
The location of clutches within a nest and non-nesting emergences were determined by carefully using a metal rod. The distance of nests and the apex of non-nesting emergences from the sea were measured using a flexible tape measure in cm. All clutch locations were individually marked and recorded with GPS (Garmin Etrex 20), and non-depredated nests were caged with a wire mesh screen (72 × 72 cm, mesh size 9 cm) to prevent nest depredation by mammals. Depredated eggs were counted in the nests, moved to another location on the beach, and reburied. Clutches laid in areas at risk of flooding were relocated to a site with lower risk and the same dimensions of the original nest.
Hatchling emergence
The hatchlings reaching the sea were determined daily from hatchling tracks emanating from nests. Counted tracks were obscured by feet to avoid recounting. When the tracks were interrupted by predator tracks, such as golden jackal (Canis aureus) or wild boar (Sus scrofa) tracks, the hatchlings were assumed to be preyed upon before they reached the sea by those predators. Each nest was excavated carefully, by hand, or using a shovel 3 or 5 days after the first emergence of hatchlings. Nest contents were classified as hatched eggs/empty shells (hatchlings emerged), unhatched eggs (unfertilised eggs and eggs without visible embryos or blood formation), and dead embryos (developmentally delayed eggs, early embryo: embryo < 1 cm; middle embryo: embryo 1–2 cm; late embryo: embryo > 2 cm). The hatching success in the nests was calculated as the percentage of hatched empty shells/the total number of eggs in the entire clutch. Predated nests were not considered for the assessment of hatching success. When fragmented eggshells were found, eggshell pieces were reassembled to represent one egg. Also, incubation duration was defined as the number of days from the nesting date to the date of the first hatchling’s emergence onto the surface of the sand.
Estimates of population sizes
The total number of nesting females was estimated based on clutch frequency (CF) using the clutch frequency of 2.9 (range = 2.0–3.1) for green turtles in the Mediterranean (Broderick et al. 2002), since this information is not available for Akyatan Beach. We considered the remigration interval (RI) as three years for the green turtle populations in the Mediterranean region (Broderick et al. 2002). The total nesting female numbers were calculated with the following formula:
Total Nesting Female Numbers = Total Nest Number/CF * (Total years/RI) (Sönmez et al. 2021)
Also, the current female numbers in the last two nesting seasons (2020 and 2021) were calculated with the following formula:
Current Female Numbers = (Mean Nest Number/CF) * RI (Sönmez et al. 2021).
Statistical analysis
The data were not normally distributed according to Levene and Kolmogorov-Smirnov tests (p < 0.05). However, parametric tests could be used based on the central limit theorem regardless of the shape of our data in large sample sizes (Field 2013). We, therefore, used the One-Way ANOVA test to compare mean clutch size, depredated eggs, hatching success, and incubation duration among nesting seasons. The trend analysis for hatching success, incubation duration, and nest numbers across the years was performed by linear regression. All statistical analyses were performed using the IBM SPSS Statistics 20 software. All means are presented with SD (standard deviation).
RESULTS
Nests, eggs, and hatchlings
A total of 10,767 green turtle emergences were recorded, with 3866 (35.91%) clutches being successfully laid during ten consecutive reproduction seasons (2012–2021) on Akyatan Beach. The annual mean number of nests was 387 ± 127 (range = 201–559 nests), and the mean nest density was 17.57 nests km-1 (range = 9.14–25.41 nests km-1). Due to the risk of inundation of nests by seawater, 42 (1.09%) green turtle nests were relocated.
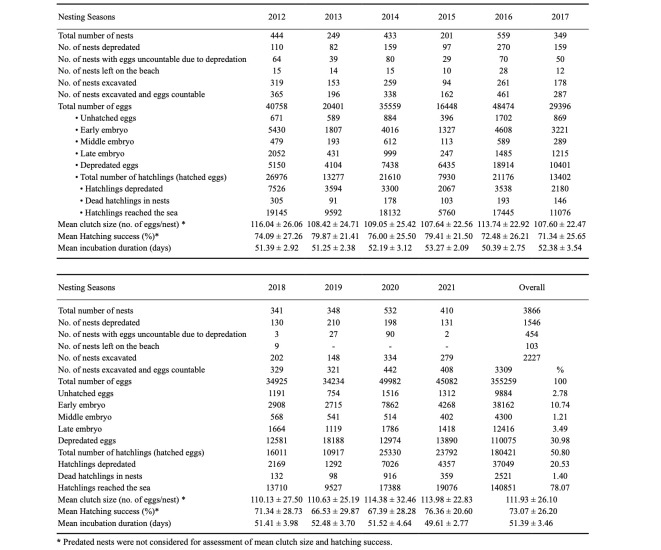
A total of 355,259 eggs were deposited in 3309 nests (excavated nests), and the mean clutch size excluding predated nests was 112 ± 26.10 eggs (range: 4–222) (Table 1). The clutch sizes were significantly different between the nesting seasons (one-way ANOVA, F = 3.105, p < 0.01) (Fig. 2a), and the 2012, 2016, 2020, and 2021 nesting seasons had larger mean clutch sizes (see Table 1 for details). Of the 355,259 eggs, 180,421 (50.80%) produced hatchlings, and 140,851 (78.07%) of them were able to reach the sea. When depredated nests were not included, the mean hatching success ranged from 66.53 ± 29.87% to 79.87 ± 21.41%, with an overall mean hatching success of 73.07 ± 26.20% (Table 1). The hatching success was significantly different between the nesting seasons (one-way ANOVA, F = 5.594, p < 0.0001) (Fig. 2b). The nesting seasons of 2019 and 2020 had lower mean hatching success (see Table 1 for details). The mean hatching success in 10 consecutive years showed a significant decreasing trend (r2 = 0.006, d.f. = 2164, p < 0.0001) (Fig. 3a). A total of 54,878 dead embryos were counted in 2227 nests, and of these dead embryos, 69.54% (38.162) were early-stage embryos, 7.84% (4.300) were mid-stage embryos and 22.62% (12.416) were late-stage embryos.
Fig. 2.
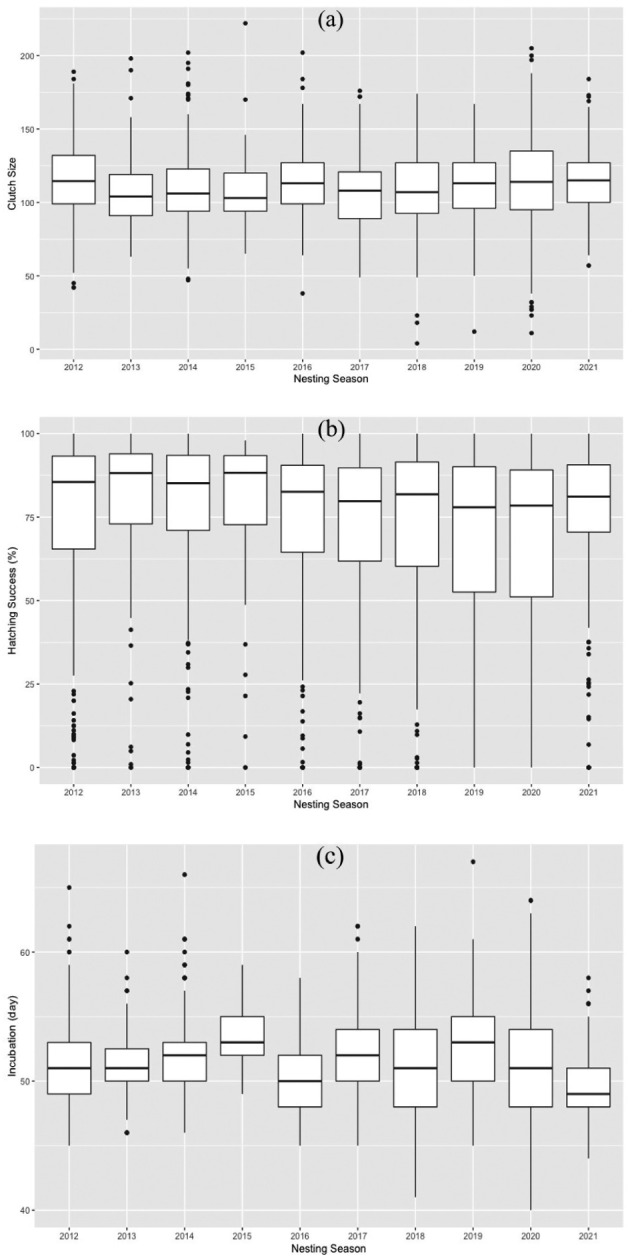
Interannual variation of green turtle (a) clutch size, (b) hatching success, and (c) incubation duration on Akyatan Beach for 10 years. Shown are medians (horizontal line), interquartile ranges (upper and lower box limits), range (vertical lines), and outliers (circles).
Fig. 3.
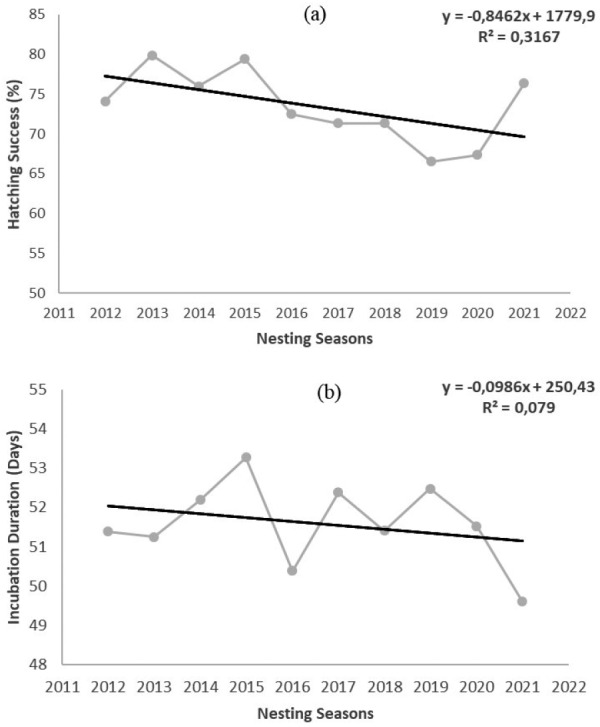
Temporal trend in hatching success (a) and incubation durations (b) of green turtle nests over 10 consecutive years on Akyatan Beach.
Table 1.
Biological data about Chelonia mydas at Akyatan Beach. Unhatched eggs = eggs without visible embryos or blood formation; developmentally delayed eggs, early embryo = embryo < 1 cm; middle embryo = 1 < embryo < 2 cm; late embryo = embryo > 2 cm)
The mean incubation durations ranged from 49.6 ± 2.77 to 53.3 ± 2.09 days, with an overall mean of 51.4 ± 3.46 days (Table 1). The incubation duration was significantly different during the breeding seasons (one-way ANOVA, F = 12.960, p < 0.0001) (Fig. 2c). The mean incubation durations in 10 consecutive years showed a significant decreasing trend (regression analysis, r2 = 0.007, d.f. = 1528, p < 0.001) (Fig. 3b).
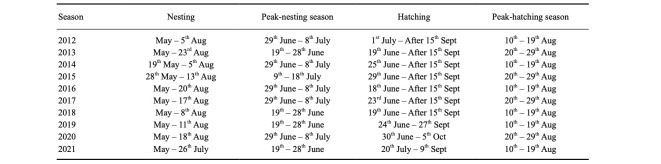
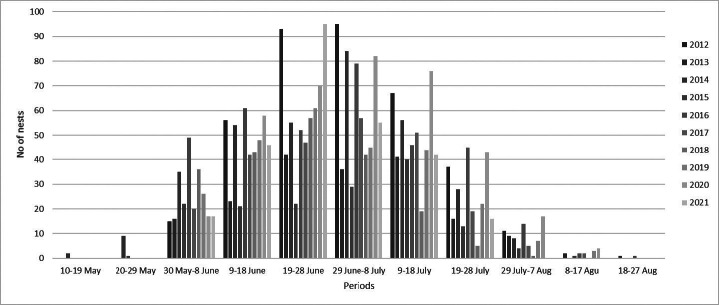
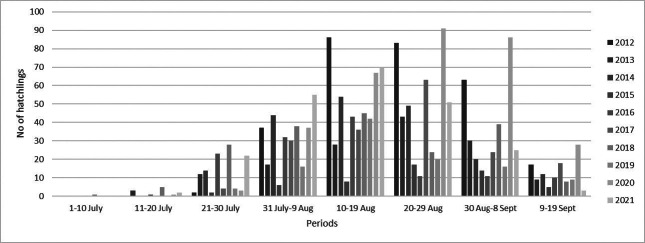
Three peak nesting periods were recorded according to the temporal distribution of green turtle nests between nesting seasons (Table 2). The first nest was recorded on the 19th of May, while the last nest was recorded on the 23rd of Aug. (Table 2). However, based on ten nesting seasons, the mean peak-nesting season on Akyatan Beach was between the 29th of June and the 8th of July (Fig. 4). Hatching starts during the third week of July (except in 2018, when it started in the first week of July), and there were two different periods for the peak-hatching season among nesting seasons (Table 2). The overall peak-hatching season was in the period of the 10th–19th Aug. (Fig. 5). The average distance of green turtle nests from the tide line was 43.17 ± 18.00 m (n = 3882, range 5.60–125.4; Fig. 6).
Table 2.
Dates for peak-nesting/hatching seasons, nesting and hatching season during 10 nesting seasons for green turtle in Akyatan Beach
Fig. 4.
Temporal distribution of green turtle nests on Akyatan Beach, Turkey (Data are grouped into 10 day bins).
Fig. 5.
Temporal distribution of green turtle hatching on Akyatan Beach, Turkey (Data are grouped into 10-day bins).
Fig. 6.
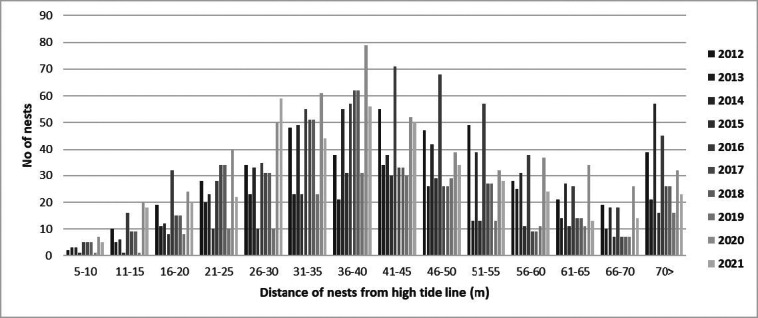
Spatial distribution of green turtle nests on Akyatan Beach.
A total of 1585 green turtle nests (41.02% of nests) were completely or partially depredated by golden jackals (Canis aureus) and wild boars (Sus scrofa). Of the 1585 nests, 1204 (75.96%) were depredated by golden jackals and 381 (24.04%) by wild boars. While the number of eggs depredated by golden jackals was counted in most cases, the number of eggs depredated by wild boars could not be counted. A total of 110,075 eggs were destroyed by golden jackals (Table 1). The number of depredated eggs significantly differed between the nesting seasons (one-way ANOVA, F = 32.438, p < 0.0001). The depredated eggs in 10 consecutive years showed a significant increasing trend (r2 = 0.009, d.f. = 1238, p < 0.0001). A total of 37,049 (20.53%) hatchlings were predated on their journey from the nest to the seawater edge (Table 1). Of the predated hatchlings, 36,242 (97.82%) were predated by jackals, 345 (0.93%) by crabs, 45 (0.12%) by seagulls, and 60 (0.16%) by wild boars. The fate of 357 (0.96%) hatchlings that were disoriented and entered the forest is unknown. Furthermore, 2521 (1.40%) hatchlings were found dead in the nests without predation (Table 1).
Nesting trend and abundance
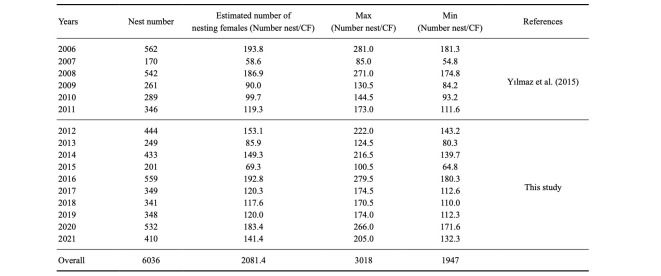
A total of 6034 green turtle nests were recorded during 16 consecutive years (2006–2021), with a mean of 377 ± 127.2 (range = 170–562) nests per year on Akyatan Beach. The number of nests showed a strong annual fluctuation ranging from 170 (in 2007) to 562 nests (in 2006), with a difference of 231% (Table 3). The yearly number of nests across 16 consecutive years showed a insignificant but increasing trend (regression analysis, r2 = 0.012, d.f. = 14, p > 0.05) (Fig. 7).
Fig. 7.
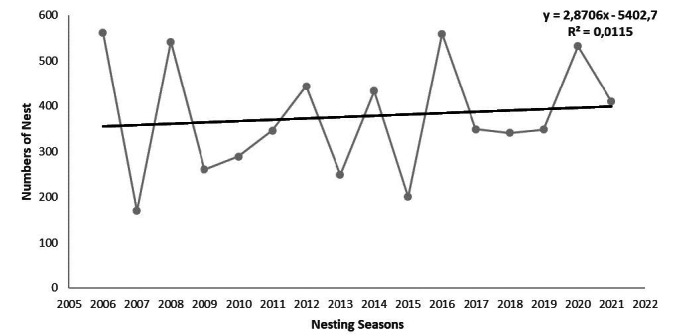
Temporal trend in the number of green turtle nests over 16 consecutive years on Akyatan Beach.
The total number of females nesting each year was estimated as 2081 (range = 1947–3018) (Table 3). The total mean number of females nesting on Akyatan Beach was 390 (range = 365–566). The number of females on Akyatan Beach in 2020–2021 was 487 (range = 456–707). The mean estimated population size (390 females) of nesting females for all years (2006–2021) was lower than for the current year (487 females) (2020–2021).
Table 3.
Number of nests recorded through the years and estimated number of females based on clutch frequency (CF) study in the Mediterranean region according to Broderick et al. (2002) (± SD)
Threats on the beach
During the research period, 12 green turtles were found stranded on the beach. Furthermore, seven adult green turtles were killed by golden jackals while nesting. Human disturbance on the beach at night was low due to its entry restriction its protected status as a Wildlife Development Area. The main anthropogenic threat was caused by several tractors illegally entering the beach, which compacted the sand, affecting hatchling emergence and leaving tracks that trapped hatchlings.
DISCUSSION
Nests, eggs, and hatchlings
Of the 13 major green turtle rookeries in the Mediterranean, six are located in Turkey, and these beaches comprise almost 80% of the overall nesting activity in the Mediterranean (Türkozan and Kaska 2010). The annual mean number of nests on Akyatan Beach (387 nests) constitutes almost 17.51–23.39% of the overall nesting in the Mediterranean, while it comprises 27.4–37.6% of the broad green turtle nesting activity along the Turkish coasts. The annual mean nest number of Akyatan is higher than other nesting colonies in the Mediterranean (see table S11; Casale et al. 2018) and therefore, still the most critical site for green turtles. Long-time surveys indicated a 47% increase in the number of nests laid by Mediterranean green turtle populations (Casale et al. 2018). However, the comparison of average nest numbers obtained due to the monitoring studies carried out before 1999 and the average number of nests in the monitoring studies carried out after 2000 provided a -1.2% change on Akyatan Beach (Casale et al. 2018). In contrast, Kazanlı and Samandağ nesting beaches (both in Turkey), two other essential nesting grounds in the Mediterranean, showed a remarkable increase (71.4–279.1%) during the same period. The decrease is surprising since Akyatan Beach is a strictly protected area, far from adverse anthropogenic effects. We believe that this could result from the shifting of adult females since the nest site fidelity of green turtles seems to be region specific rather than beach specific (Karaman et al. 2022). The interchange of nesting individuals between Akyatan-Sugözü and Samandağ-Syria was also reported with mark-recapture studies previously (Sönmez et al. 2017). On the other hand, nest numbers showed an increasing trend which is possibly the result of long term conservation efforts in the region.
The overall mean clutch size recorded for Akyatan Beach during ten consecutive reproduction seasons (112 eggs) is within the range for green turtle populations in the Mediterranean (Alagadi/North Cyprus: 116 eggs, Egypt: 101 eggs, Latakia/Syria: 108 eggs, Kazanlı/Turkey: 111 eggs, Akyatan/Turkey: 114 eggs, see table S15; Casale et al. 2018). The clutch size in green turtles increases with the body size of the female (Broderick et al. 2003). Bjorndal and Carr (1989) indicated that carapace lengths of females with a small mean clutch size in the 1976 nesting season were significantly shorter than all other years. The current study’s significantly different mean clutch sizes between the nesting seasons may be due to size differences of nesting females during those breeding seasons. However, since we have no measure of female size, we cannot precisely comment on this topic.
Hatching success in our study (73.07%) was higher than Egypt (53.7%) and lower than Alagadi/North Cyprus (84.2%), Latakia/Syria (80.0%), Kazanlı/Turkey (78.3%), Akyatan/Turkey (75.6%) and Samandağ/Turkey (81.1%) (see table S15; Casale et al. 2018). Also, the mean hatching success among the nesting seasons was determined to have significant differences, and showed a crucial decreasing trend over ten consecutive years. Hatching success can be affected by the nest’s location and its microhabitat (Zárate et al. 2013), temperature (Godley et al. 2001; Pike 2013; Hays et al. 2017; Türkozan et al. 2021), fungal infections (Limpus et al. 1983) and moisture (Wood and Bjorndal 2000). The hatching success is reduced when the temperature is higher (Godley et al. 2001; Pike 2013; Hays et al. 2017). Furthermore, Türkozan et al. (2021) reported that hatching success declines after conditions exceed the 33°C thermal thresholds for two-fifths of the incubation period in Akyatan Beach. But the hatching success was increased despite the reduced incubation duration caused by the higher temperature in the 2021 nesting season. The low mean incubation duration in the 2021 nesting season probably resulted from more depredated nests (65.65%) laid until May and June 15th, possibly with a high incubation duration.
Of the recorded embryonic deaths in nests, 69.54% were early-stage, 7.84% were mid-stage, and 22.62% were late-stage embryos. Compared to Yılmaz et al. (2015), while the early-stage embryos in our study were lower, mid-stage and late-stage were higher (early-stage embryos: 76.4%, mid-stage embryos 4.4%, and late-stage embryos: 19.2%). Environmental factors such as precipitation and temperature influence embryonic deaths (Rafferty et al. 2011). Booth and Dunstan (2018) found that the proportion of early embryo death was remarkable in two green turtle nests that experienced the highest nest temperature, lowest oxygen (PO2), and highest carbon dioxide (PCO2) during the first week of incubation. Also, the high temperatures experienced in the early stages of incubation might cause malformations, leading to increased mortality rates (Booth et al. 2020).
The overall mean incubation duration (51.39 ± 3.46) was in the range of values reported for green turtle populations in the Mediterranean (Alagadi/North Cyprus: 51.1 days, Egypt: 46.5 days, Kazanlı/Turkey: 52.2 days, Akyatan/Turkey: 52.9 days, and Samandağ/Turkey: 52.9 days, see table S15; Casale et al. 2018). Also, the incubation duration was determined to be significantly different between the nesting seasons. While variation in incubation durations is related to temperature profiles and sand characteristics on other nesting beaches (Yılmaz et al. 2015), the same nesting beach is affected by the temperature profile in different nesting seasons. Türkozan et al. (2021) found that incubation temperatures will significantly increase in future years in the Mediterranean region. This situation will further reduce the incubation durations of green turtle nests in the Mediterranean. The significant decrease in mean incubation durations over ten consecutive years found in our study supports this conclusion.
The overall peak-nesting season is between 29th June–8th July on Akyatan Beach (Fig. 3), with the first nesting event on 19th May and the last nesting event on 23rd Aug (Table 2). Yılmaz et al. (2015) reported the latest nest records as 7th Aug. 2008 for green turtles in Akyatan Beach. Broderick and Godley (1996) reported that the first nest was recorded on 31st May 1994, and the last nest on 25th Aug. 1993 for green turtles in Cyprus. For Caretta caretta, the earliest and the latest nest records were reported on 19th May 2001 and 14th Sept. 1984, respectively, in Greece (Margaritoulis 2005). Furthermore, Weishampel et al. (2005) reported that earlier nesting of C. caretta was correlated with sea surface warming. The peak nesting season for green turtle nests in the Mediterranean is between 19–28 June in Akyatan Beach (Yılmaz et al. 2015) and 12–19 June in Northern Cyprus (Broderick and Godley 1996). The first, last and peak-nesting season of sea turtle nests in the same or different populations may respond to climate change between nesting seasons. Patel et al. (2016) reported that the nesting season of the loggerhead turtle could shift to an earlier date by as much as 50 to 74 days in the Mediterranean by the year 2100 because of the rise in air and ocean temperatures.
Although non-depredated nests were caged with wire mesh in the morning survey, the predation rate (41.02% of nests) was high on Akyatan Beach. This predation rate is higher than the predation rate (33.4%) from the other study done on Akyatan Beach (Yılmaz et al. 2015). Also, the depredated eggs showed a significant increasing trend for ten consecutive years. This is because the nests were predated by wild boar (S. scrofa) despite being caged with wire mesh, especially in the 2012, 2014, 2016, 2017, and 2020 nesting seasons. Although the cages are placed at a depth of about 20 cm from the surface of the nests, the nests were predated by wild boar (S. scrofa). Engeman et al. (2016) demonstrated that cages and screens offer little protection against feral swine (S. scrofa) depredation. If other nutrients in the diet of the wild boar (S. scrofa) are included in the Wildlife Development Area during the nesting period, then the nest predation will reduce. While 75.96% of depredated nests were predated by golden jackals (C. aureus), 24.04% of departed nests were predated by wild boars (S. scrofa). Most of the nests depredated by golden jackals (C. aureus) were predated before being caged with wire mesh in the morning survey. Brown and Macdonald (1995) reported that 63.8% of green turtle nests were predated by canids (C. aureus and Vulpes vulpes) on Akyatan Beach. As in the study by Yılmaz et al. (2015), the golden jackal (C. aureus) continues to have the highest impact on egg predation at Akyatan Beach.
Nesting trend and abundance
Nest numbers during six consecutive nesting seasons (2006–2011) showed an insignificantly decreasing trend (Yılmaz et al. 2015), while longer-term data (2006–2021) identified an insignificant increasing trend with a mean of 377 nests (Fig. 7). The increasing nesting trend suggests that conservation efforts have been successful. Yılmaz et al. (2015) indicated an increase in green turtle nests in 6 consecutive nesting surveys at Samandağ and Kazanlı beaches. In other studies around the world, while the number of nests over 18 years showed a positive trend for the entire beach at Tortuguero, Costa Rica (Troëng and Rankin 2005), the number of nests over 14 years showed weak negative trends on Misali Island, Pemba (Giorno and Herrmann 2016). Recent studies showed that the Mediterranean and global green turtle populations suggest population increases in some regions (Stokes et al. 2014; Mazaris et al. 2017; Casale et al. 2018). The overall nesting female numbers (2006–2021) are lower than the current season (2020–2021). This discrepancy indicates either that the number of turtles nesting on Akyatan Beach has increased over the years or that the natality rate is higher than the mortality rate. A five consecutive years survey was carried out in 1994 and 1998 on Akyatan Beach for the first time, but nests of green turtles were not caged (Aureggi et al. 2000). In the study carried out in 2002, cages were placed on the nests for the first time (Oruç et al. 2002). A survey has been carried out on Akyatan Beach since 2006 (Yılmaz et al. 2015) and nests that have not undergone predation are caged. The reproductive maturation age of green turtles is estimated to be between 15 to 50 years (Limpus and Walter 1980; Limpus and Chaloupka 1997; Chaloupka et al. 2004; Lemm 2006). From recent surveys, we can deduce that the hatchlings come to Akyatan Beach after reaching sexual maturity. Despite the increase in the number of nesting females, nest numbers had an insignificantly increasing trend. The insignificant increase in nests means the yield for the 16-year consecutive conservation study despite the intensive predation of eggs and hatchlings by mammals on the Akyatan Beach. This shows once again that long-term survey studies are essential.
Threats on the beach
Golden jackals killed seven female green turtles during the 2012 and 2021 nesting seasons. Previous studies recorded the killing of nesting females by golden jackals (Peters and Verhoeven 1992; Akçınar et al. 2006). As in Yılmaz et al. (2015), the main threats were predation of eggs and hatchlings by jackals and eggs by wild boars. Since golden jackals and wild boars are protected as part of the Wildlife Development Area, removal and killing are not an option. In addition to the daytime survey, the night survey can be carried out mainly to prevent adult and nest predation during the nesting period. However, the 22 km long Akyatan Beach can complicate this survey. Providing additional natural nutrients which are included in the diet of golden jackals could reduce the depredation of adults, eggs, and hatchlings.
CONCLUSIONS
In conclusion, Akyatan Beach continues to be essential for the green turtle population in the Mediterranean. The clutch size, hatching success, and incubation duration differed significantly between the nesting seasons. Over ten consecutive years, the mean hatching success and incubation durations showed a significant decreasing trend. The first, last and peak-nesting seasons vary in the same or different populations. The golden jackal (C. aureus) predation of eggs continued to have the highest impact. The insignificant increasing trend in the green turtle nests during 16 consecutive surveys showed that conservation efforts were successful despite mammal predation of the nests. As well as the continuation of the surveys for the continuity of the population, studies about how global climate change affects the green turtle will contribute to the conservation biology of Mediterranean turtle populations.
Acknowledgments
The authors would like to thank all the volunteers. This study is part of an ongoing project funded by WWF Turkey through cooperation with the 7th Regional Directorate of Nature Conservation and National Parks of the Turkish Ministry of Agriculture and Forestry, Adana Office. The authors would like to thank anonymous reviewers and Dr. David Booth for their constructive comments on our MS.
Footnotes
Authors’ contributions: All authors contributed evenly to the study’s design and manuscript writing.
Competing interests: All authors declare that they have no conflict of interest.
Availability of data and materials: Not applicable.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Akçınar SC, Guclu O, Taskavak E, Turkozan O. 2006. Golden jackal predation on marine turtles in Goksu Delta, Turkey. In: Frick M, Panagopoulou A, Rees AF, Williams K (eds) 26th annual symposium on sea turtle biology and conservation. Island of Crete, Greece, 3–8 April 2006. Archelon, Athens, p. 120.
- Aureggi M, Gerosa G, Yerli SV. 2000. Five years of research at Akyatan beach (Turkey): one of the main nesting site for green turtle, Chelonia mydas, in the Mediterranean. Biogeographia 21:555–560. doi:10.21426/B6110008.
- Bjorndal KA, Carr A. 1989. Variation in clutch size and egg size in the green turtle nesting population at Tortuguero, Costa Rica. Herpetologica 45:181–189.
- Booth DT, Archibald-Binge A, Limpus CJ. 2020. The effect of respiratory gases and incubation temperature on early stage embryonic development in sea turtles. PLoS ONE 15(12):e0233580. doi:10.1371/journal.pone.0233580. . [DOI] [PMC free article] [PubMed]
- Booth DT, Dunstan A. 2018. A preliminary investigation into the early embryo death syndrome (EEDS) at the world’s largest green turtle rookery. PLoS ONE 13(4):e0195462. doi:10.1371/journal. pone.0195462. . [DOI] [PMC free article] [PubMed]
- Broderick AC, Glen F, Godley BJ, Hays GC. 2002. Estimating the number of green and loggerhead turtles nesting annually in the Mediterranean. Oryx 36:227–235. doi:10.1017/S0030605302000 431.
- Broderick AC, Godley BJ. 1996. Population and nesting ecology of the green turtle, Chelonia mydas, and the loggerhead turtle, Caretta caretta, in Northern Cyprus. Zool Middle East 13:27–46. doi:10.1080/09397140.1996.10637704.
- Broderick AC, Glen F, Godley BJ, Hays GC. 2003. Variation in reproductive output of marine turtles. J Exp Mar Biol Ecol 288:95–109. doi:10.1016/S0022-0981(03)00003-0.
- Broderick AC, Coyne MS, Fuller WJ, Glen F, Godley BJ. 2007. Fidelity and over -wintering of sea turtles. Proc Biol Sci 274(1617):1533–1538. doi:10.1098/rspb.2007.0211. . [DOI] [PMC free article] [PubMed]
- Brown L, Macdonald DW. 1995. Predation on green turtle Chelonia mydas nests by wild canids at Akyatan beach, Turkey. Biol Conserv 1:55–60. doi:10.1016/0006-3207(94)00020-Q.
- Casale P, Broderick AC, Camiñas JA, Cardona L, Carreras C, Demetropoulos A, Fuller WJ, Godley BJ, Hochscheid S, Kaska Y, Lazar B, Margaritoulis D, Panagopoulou A, Rees AF, Tomás J, Türkozan O. 2018. Mediterranean Sea turtles: current knowledge and priorities for conservation and research. Endang Species Res 36:229–267. doi:10.3354/esr00901.
- Casale P, Margaritoulis D. 2010. Sea turtles in the Mediterranean: distribution, threats and conservation priorities. IUCN, Gland, Switzerland, pp. 1–14.
- Chaloupka M, Limpus C, Miller J. 2004. Green turtle somatic growth dynamics in a spatially disjunct Great Barrier Reef metapopulation. Coral Reefs 23:325–335. doi:10.1007/s00338-004-0387-9.
- Engeman RM, Addison D, Griffin JC. 2016. Defending against disparate sea turtle nest predators: benefits to nesting success from eradicating invasive feral swine and caging nests from raccoons. Oryx 50(2):289–295. doi:10.1017/S0030605314000805.
- Field A. 2013. Discovering statistics using IBM SPSS statistics: And sex and drugs and Rock “N” Roll, 4th Edition, Sage, Los Angeles, London, New Delhi.
- Giorno T, Herrmann M. 2016. Nesting trends of the Green (Chelonia mydas) and Hawksbill (Eretmochelys imbricata) turtles on Misali Island, Pemba. Independent Study Project (ISP) Collection 2343. Available at: http://digitalcollections.sit.edu/isp_collection/2343.
- Godley BJ, Broderick AC, Glen F, Hays GC. 2003. Post-nesting movements and submergence patterns of loggerhead marine turtles in the Mediterranean assessed by satellite tracking. J Exp Mar Biol Ecol 287:119–134. doi:10.1016/S0022-0981(02)00547-6.
- Godley BJ, Broderick AC, Hays GC. 2001. Nesting of green turtles (Chelonia mydas) at Ascension Island, South Atlantic. Biol Conserv 97:151–158. doi:10.1016/S0006-3207(00)00107-5.
- Hays GC, Mazaris AD, Schofield G, Laloë J-O. 2017. Population viability at extreme sex-ratio skews produced by temperature -dependent sex determination. Proc R Soc B Biol Sci 284:20162576. doi:10.1098/rspb.2016.2576. . [DOI] [PMC free article] [PubMed]
- Kasparek M, Godley BJ, Broderick AC. 2001. Nesting of the green turtle, Chelonia mydas, in the Mediterranean: a review of status and conservation needs. Zool Middle East 24:45–74. doi:10.108 0/09397140.2001.10637885.
- Karaman S, Türkozan O, Carreras C, Yılmaz C, Sönmez B, Candan O, Ergene S, Ergene M, Uçar AH, Ulger C. 2022. Population genetic diversity of green turtles, Chelonia mydas, in the Mediterranean revisited. Mar Biol 169:77. doi:10.1007/s00227-022-04068-1.
- Lemm JM. 2006. Field guide to amphibians and reptiles of the San Diego region. University of California Press, Berkeley, California, USA, pp. 326.
- Limpus C, Chaloupka M. 1997. Nonparametric regression modelling of green sea turtle growth rates (southern Great Barrier Reef). Mar Ecol Prog Ser 149:23–34. doi:10.3354/meps149023.
- Limpus CJ, Mille JD, Baker V, McLachlan E. 1983. The hawksbill turtle Eretmochelys imbricata (L.) in north-eastern Australia: the Campbell Island rookery. Wildl Res 10:185–197. doi:10.1071/WR9830185.
- Limpus CJ, Walter DG. 1980. The growth of immature green turtles (Chelonia mydas) under natural conditions. Herpetologica 36:162–165.
- Margaritoulis D. 2005. Nesting activity and reproductive output of loggerhead sea turtles, Caretta caretta, over 19 seasons (1984–2002) at Laganas Bay, Zakynthos, Greece: The largest rookery in the Mediterranean. Chelonian Conserv Biol 4:916–929.
- Mazaris AD, Schofield G, Gkazinou C, Almpanidou V, Hays GC. 2017. Global sea turtle conservation successes. Sci Adv 3:e1600730. doi:10.1126/sciadv.1600730. . [DOI] [PMC free article] [PubMed]
- Oruç A, Karabacak Ö, Kaçar Ü. 2002. Akyatan deniz kaplumbağası yuvalama kumsalı değerlendirme raporu [Evaluation report for marine turtle nesting on Akyatan]. WWF-Turkey report, İstanbul.
- Patel SH, Morreale SJ, Saba VS, Panagopoulou A, Margaritoulis D, Spotila JR. 2016. Climate impacts on sea turtle breeding phenology in Greece and associated foraging habitats in the wider Mediterranean region. PLoS ONE 11:e0157170. doi:10.1371/journal.pone.0157170. . [DOI] [PMC free article] [PubMed]
- Peters A, Verhoeven KJF. 1992. Breeding success of the loggerhead, Caretta caretta, and the green turtle, Chelonia mydas, in the Göksu Delta, Turkey. Department of Animal Ecology, University of Nijmegen, Rapport No: 310.
- Pike DA. 2013. Climate influences the global distribution of sea turtle nesting. Glob Ecol Biogeogr 22:555–566. doi:10.1111/geb.12025.
- Rafferty AR, Santidrián Tomillo P, Spotila JR, Paladino FV, Reina RD. 2011. Embryonic death is linked to maternal identity in the Leatherback Turtle (Dermochelys coriacea). PLoS ONE 6:e21038. doi:10.1371/journal.pone.0021038. . [DOI] [PMC free article] [PubMed]
- Rees AF, Jony M, Margaritoulis D, Godley BJ. 2008. Satellite tracking of a green turtle, Chelonia mydas, from Syria further highlights importance of North Africa for Mediterranean turtles. Zool Middle East 45:49–54. doi:10.1080/09397140.2008.10638 306.
- Snape RTE, Broderick AC, Çiçek BA, Fuller WJ, Glen F, Stokes K, Godley BJ. 2016. Shelf life: neritic habitat use of a turtle population highly threatened by fisheries. Diversty Distrib 22:797–807. doi:10.1111/ddi.12440.
- Sönmez B, Elginöz E, Ilgaz M, Altınkaya H. 2021. Nesting activity of loggerhead turtles (2013–2020) and 20 years abundance trend (2001–2020) on Çıralı Beach, Turkey. Reg Stud Mar Sci 44:101758. doi:10.1016/j.rsma.2021.101758.
- Sönmez B, Türkecan O, Jded A. 2017. Long distance movement between nesting sites for two green turtles in the eastern Mediterranean. Marine Turtle Newsletter 153:7–8.
- Stokes KL, Broderick AC, Canbolat AF, Candan O, Fuller WJ, Glen F, Levy Y, Rees AF, Rilov G, Snape RTE, Stott I, Tchernov D, Godley BJ. 2015. Migratory corridors and foraging hotspots: critical habitats identified for Mediterranean green turtles. Diversty Distrib 21:665–674. doi:10.1111/ddi.12317.
- Stokes KL, Fuller WJ, Glen F, Godley BJ, Hodgson DJ, Rhodes KA, Snape RTE, Broderick AC. 2014. Detecting green shoots of recovery: the importance of long-term individual-based monitoring of marine turtles. Anim Conserv 17:593–602. doi:10.1111/acv.12128.
- Tikochinski Y, Bradshaw P, Mastrogiacomo A, Broderick A, Daya A, Demetropoulos A, Demetropoulos S, Eliades N-G, Fuller W, Godley B, Kaska Y, Levy Y, Snape R, Wright L, Carreras C. 2018. Mitochondrial DNA short tandem repeats unveil hidden population structuring and migration routes of an endangered marine turtle. Aquat Conserv Mar Freshw Ecosyst 28:788–797. doi:10.1002/aqc.2908.
- Troëng S, Rankin E. 2005. Long-term conservation efforts contribute to positive green turtle Chelonia mydas nesting trend at Tortuguero, Costa Rica. Biol Conserv 121:111–116. doi:10.1016/j.biocon.2004.04.014.
- Türkozan O, Almpanidou V, Yılmaz C, Mazaris AD. 2021. Extreme thermal conditions in sea turtle nests jeopardise reproductive output. Clim Change 167:30. doi:10.1007/s10584-021-03153-6.
- Türkozan O, Kaska Y. 2010. Turkey. In: Casale P, Margaritoulis D (Eds) Sea turtles in the Mediterranean: distribution, threats and conservation priorities. IUCN, Gland, pp. 257–293.
- Yılmaz C, Oruç A, Türkozan O. 2015. Marine turtles (Chelonia mydas and Caretta caretta) nesting along the eastern Mediterranean coast of Turkey: Results from six years of surveying. Herpetol J 25:197–204.
- Zárate P, Bjorndal KA, Parra M, Dutton PH, Seminoff JA, Bolten AB. 2013. Hatching and emergence success in green turtle Chelonia mydas nests in the Galápagos Islands. Aquat Biol 19:217–229. doi:10.3354/ab00534.
- Wallace BP, DiMatteo AD, Bolten AB, Chaloupka MY, Hutchinson BJ, Abreu-Grobois FA, Mortimer JA, Seminoff JA, Amorocho D, Bjorndal KA, Bourjea J, Bowen BW, Briseño Dueñas R, Casale P, Choudhury BC, Costa A, Dutton PH, Fallabrino A, Finkbeiner EM, Girard A, Girondot M, Hamann M, Hurley BJ, López-Mendilaharsu M, Marcovaldi MA, Musick JA, Nel R, Pilcher NJ, Troëng S, Witherington B, Mast RB. 2011. Global conservation priorities for marine turtles. PLoS ONE 6:1–14. doi:10.1371/journal.pone.0024510. . [DOI] [PMC free article] [PubMed]
- Wallace BP, DiMatteo AD, Hurley BJ, Finkbeiner EM. 2010. Regional management units for marine turtles: a novel framework for prioritising conservation and research across multiple scales. PLoS ONE 5:1–11. doi:10.1371/journal.pone.0015465. . [DOI] [PMC free article] [PubMed]
- Weishampel JF, Bagley DA, Ehrhart L. 2005. Earlier nesting by loggerhead sea turtles following sea surface warming. Glob Chang Biol 10:1424–1427. doi:10.1111/j.1529-8817.2003. 00817.x.
- Wood DW, Bjorndal KA. 2000. Relation of temperature, moisture, salinity and slope to nest site selection in loggerhead sea turtles. Copeia 1:119–128. doi:10.1643/0045-8511(2000)2000[0119:RO TMSA]2.0.CO;2.