ABSTRACT
Background
Plant-based diets are recommended for chronic disease prevention, yet there has been little focus on plant-based diet quality among participants of South Asian ancestry who consume a predominantly plant-based diet.
Objectives
We evaluated cross-sectional and prospective associations between plant-based diet quality and cardiometabolic risks among participants of South Asian ancestry who are living in the United States.
Methods
We included 891 participants of South Asian ancestry who completed the baseline visit in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. The prospective analysis included 735 participants who completed exam 2 (∼5 years after baseline). The plant-based diet quality was assessed using 3 indices: an overall plant-based diet index (PDI) that summarizes the consumption of plant foods, a healthy PDI (hPDI) that measures consumption of healthy plant foods, and an unhealthy PDI (uPDI) that reflects consumption of less healthy plant foods.
Results
At baseline, the PDI score was inversely associated with fasting glucose. We observed inverse associations between PDI and hPDI scores and HOMA-IR, LDL cholesterol, weight, and BMI (all P values < 0.05). Higher scores on the hPDI, but not PDI, were associated with lower glycated hemoglobin, higher adiponectin, a smaller visceral fat area, and a smaller pericardial fat volume. Each 5-unit higher hPDI score was associated with lower likelihoods of fatty liver (OR: 0.76; 95% CI: 0.64, 0.90) and obesity (OR: 0.88; 95% CI: 0.80, 0.97). There were no associations between uPDI scores and cardiometabolic risks. Prospectively, after covariate adjustment for baseline values, each 5-unit higher hPDI score was associated with an 18% lower risk of incident type 2 diabetes (OR: 0.82; 95% CI: 0.67, 1.00).
Conclusions
A higher intake of healthful plant–based foods was associated with a favorable cardiometabolic risk profile. Dietary recommendations to lower chronic disease risks among participants of South Asian ancestry should focus on the quality of plant-based foods.
Keywords: plant-based diets, cardiovascular disease, diabetes, South Asians, Asian Indians, cardiometabolic, diet, epidemiology
Introduction
Poor diet is a major, preventable risk factor for chronic disease and is a leading risk factor for deaths globally (1). Plant-based diets have been widely proclaimed to lower chronic disease risks (2), although existing evidence has been somewhat mixed, with some studies reporting no association and others reporting a lower risk of chronic disease (3). Previous studies have dichotomized plant-based diets as vegetarian or nonvegetarian based on the exclusion of some or all animal foods, and have not distinguished between the healthfulness of plant foods. From a public health standpoint, before promoting a vegetarian dietary pattern for chronic disease reduction, it is crucial to differentiate the quality of plant-based foods, as less healthy plant foods and healthy plant foods have opposing effects on cardiometabolic risks (4–7).
One of the fastest growing ethnic groups in the United States is people of South Asian ancestry, which includes people with ancestry from India, Pakistan, Bangladesh, Sri Lanka, Nepal, and Bhutan (8). A significant proportion (∼40%) of these are vegetarian due to their cultural traditions and religious beliefs (9). Despite this, people from South Asian countries have disproportionately higher rates of cardiovascular disease and type 2 diabetes (T2D) (10–12) and, on average, develop these conditions 10 years earlier than other racial and ethnic groups (12, 13). This paradox may partly be due to the healthfulness of the plant-based foods consumed by participants from South Asia. However, there is limited evidence regarding the associations between healthful and unhealthful plant-based diets and cardiometabolic risks among participants from South Asian countries living in the United States.
Given these important gaps in the literature, the goal of the current study was to examine both cross-sectional and prospective associations between healthy and unhealthy plant–based dietary patterns and cardiometabolic risks among participants in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. To capture the overall quality of a plant-based diet, we previously developed an overall plant-based diet index (PDI), a healthful PDI (hPDI), and an unhealthful PDI (uPDI). We adapted these indices to reflect foods consumed as part of the South Asian diet. We hypothesized that the hPDI scores but not uPDI scores would be favorably associated with cardiometabolic risks in a cohort of participants from South Asian countries living in the United States.
Methods
Study population
The MASALA study is a community-based, prospective, cohort study of South Asian men and women recruited from the San Francisco Bay area and the greater Chicago area. Detailed methods, study objectives, and a description of the MASALA cohort have been published previously (14). Briefly, to be eligible for the MASALA study, participants had to have South Asian ancestry (self-reported), have at least 3 grandparents who were born in the South Asian subcontinent, be aged 40–84 years, and have the ability to speak and/or read English, Hindi, or Urdu. The MASALA study excluded those with cardiovascular disease at baseline. The baseline clinical examination was conducted in 906 participants between October 2010 and March 2013. The second clinical examination was conducted between September 2015 and March 2018 (n = 749). As was done in prior studies (15), we excluded those with implausible energy intakes (<800 kcal/d or >4000 kcal/d for men; <500 kcal/d or >3500 kcal/d for women; n = 13) and those with missing data (n = 2). The current study includes 891 participants at baseline for the cross-sectional analysis and 735 participants at follow-up for the prospective analysis (Supplemental Figure 1). The MASALA study protocol was approved by the Institutional Review Boards of Northwestern University and the University of California, San Francisco. The current analysis protocol was approved by the Institutional Review Board of Brigham and Women's Hospital, Boston, MA. All participants provided written informed consent.
Dietary assessment and PDI scores
At baseline, we assessed dietary intakes over the past 12 months using a previously validated, ethnic-specific, semi-quantitative FFQ designed to assess dietary intakes of participants from South Asia (16). The FFQ consists of 163 items, with 61 items unique to the South Asian diet. For each food item, we computed the number of servings consumed per day from the frequency (day, week, month, year, never) and the serving size (average, small, large). For each item, the average serving size was provided. A small serving size was considered to be 0.5 of the average serving size, whereas a large serving size was 1.5 of the average. Foods were categorized into 19 predefined subgroups based on the similarity of their nutrient content, likeness, and their culinary use in the South Asian diet (Supplemental Table 1).
We previously developed 3 plant-based diet indices to reflect the quality of plant-based foods in a person's diet (17). In the current study, we adapted these indices to include foods consumed by participants from South Asia. Based on empirical evidence, the 20 food groups were categorized as healthy plant foods (whole grains, fruits, vegetables, herbs and spices, nuts, legumes, tea, and coffee), less healthy plant foods (refined grains, deep-fried snacks and pickles, potatoes, coconut, sugar-sweetened beverages, sweets, and desserts), and animal foods (animal fat, dairy, egg, fish or seafood, meat, miscellaneous animal foods, and milk-based desserts; Supplemental Table 1). Food groups (in servings/day) were ranked into quintiles (Q) and each quintile was assigned a score between 1 and 5. For the PDI, healthy and less healthy plant food groups were given positive scores (Q1 = 1, Q2 = 2, Q3 = 3, Q4 = 4, Q5 = 5), while animal food groups were given reverse scores (Q5 = 1, Q4 = 2, Q3 = 3, Q2 = 4, Q1 = 5). For the hPDI, we assigned positive scores to healthy plant food groups and reverse scores to less healthy plant food groups and animal food groups. For uPDI, we gave positive scores to less healthy plant food groups and reverse scores to healthy plant food groups and animal food groups. Because alcohol has different associations with various health outcomes, we did not include this as a food group, but adjusted for it in analyses. The 20 food group scores for an individual were summed to obtain the indices, with a theoretical range of 20–100, where higher scores indicate greater adherence to the diet index.
Ascertainment of cardiometabolic risk factors
Our primary outcome variables are cardiometabolic risk factors. After a 12-hour fast, participants visited the clinical field centers to obtain measures of cardiometabolic risk. At baseline, we obtained measures of subclinical atherosclerosis, measures of glycemia and dyslipidemia, anthropometry, blood pressure, and computed tomography (CT) body composition measures. At follow-up (∼5 years after baseline), we obtained measures of body weight, fasting glucose, glycated hemoglobin (HbA1c), and serum lipids.
Subclinical atherosclerosis
High-resolution B-mode ultrasonography (at University of California, San Francisco, the General Electric Vivid 7 ultrasound was used; at Northwestern, a Siemens Acuson Sequoia C256 was used) was conducted for measurements of right and left internal and common carotid artery intima media thicknesses using protocols described previously (18). Cardiac CT scans were performed using a cardiac-gated CT scanner (at the University of California, San Francisco, a Phillips 16D scanner or a Toshiba MSD Aquilion 64 was used; at Northwestern, a Siemens Sensation Cardiac 64 Scanner was used) using methods described previously (19). For each of the 4 major coronary arteries, Agatston scores were used to measure coronary artery calcium, and the sum of the unadjusted score was used (20).
Measures of glycemia and dyslipidemia
Fasting blood samples were obtained after a 12-hour fast. Participants who were not taking diabetes medications underwent a 75-g oral glucose tolerance test. At baseline and follow-up, fasting plasma glucose was measured using the hexokinase method (Ortho Clinical Diagnostics, Johnson & Johnson). Baseline fasting insulin was measured by sandwich immunoassay (Roche Elecsys 2010, Roche Diagnostics), and baseline insulin resistance was assessed by the HOMA-IR as fasting insulin (µIU/mL) × fasting glucose (mmol/L) ÷ 22.5 (21). The β-cell function (a surrogate measure of insulin sensitivity) was estimated at baseline using the oral disposition index, which was calculated as (Δinsulin0–30 ÷ Δglucose0–30) × (1 ÷ fasting insulin) (22). At baseline and follow-up, T2D was defined by the use of a glucose-lowering medication, fasting plasma glucose ≥7.0 mmol/L, and/or glucose ≥11.1 mmol/L at 2 hours after the challenge (23). We classified incident T2D as the presence of diabetes at follow-up in a participant who had no T2D at baseline.
At baseline, serum lipid values, including triglycerides and HDL cholesterol levels, were measured using enzymatic methods (Quest). LDL cholesterol was calculated using the Friedewald formula (24). Baseline high-sensitivity C-reactive protein (hsCRP) was measured using the BNII nephelometer (Siemens Healthcare Diagnostics). Serum total adiponectin was measured using Millipore Luminex adipokine panel A (EMD Millipore).
Body composition measures
Body weight, height, and waist circumference were measured using standardized methods (14). BMI was calculated as weight in kilograms divided by height in meters squared. Overweight was defined as a BMI ≥23.0 kg/m2 and obesity was defined as a BMI ≥27.5 kg/m2 (25). The abdominal visceral and subcutaneous fat areas were measured using CT scans of the abdomen using standardized protocols described previously (14). Noncontrast cardiac CT scans were used to quantify the pericardial fat volume and hepatic fat attenuation (14, 26). Fatty liver was defined as attenuation of <40 Hounsfield units (27).
Other cardiometabolic risk factors
Blood pressure was measured in a seated position using an automated blood pressure machine (V100 Vital Signs Monitor, GE Healthcare). The average of the last 2 readings was used to determine systolic and diastolic blood pressure. Hypertension was defined as the use of antihypertensive medication or blood pressure ≥140/90 mm Hg.
Assessment of covariates
At the baseline visit, all participants visited the clinical field center to provide informed consent and information on their personal history, demographics, socioeconomic status, medical history, family history, alcohol intake, and medication use. Intentional physical activity was assessed using the Typical Week's Physical Activity Questionnaire and quantified as total metabolic equivalent (MET) minutes per week (28). The sum of cultural traditional measures was assessed using a traditional cultural beliefs scale, consisting of 7 items, that was specifically developed for this cohort. The items were scored on a Likert scale, with lower scores representing stronger traditional South Asian beliefs (29). Total energy intake was assessed from the FFQ as kilocalories per day. All interviews were conducted by trained bilingual study staff in English, Hindu, or Urdu.
Statistical analysis
Baseline characteristics of study participants across quartiles of the 3 plant-based diet indices were compared using general linear regression adjusted for age, sex, and total energy for continuous variables and using the chi-square test for categorical variables. Tests for linear trend were conducted by assigning the median value to each quartile and treating this as a continuous variable in the regression model.
To quantify the associations between plant-based diet indices and cardiometabolic risk factors, we used multivariable general linear regression for continuous outcomes and logistic regression for categorical outcomes. For all linear models, we tested the assumptions of normality, linearity, and homogeneity by examining plots of residuals compared with predicted values and normal probability plots of residuals. Outliers were identified by a visual examination of the residual plots. When there was evidence of heteroscadasticity, we log-transformed the outcome variable and re-examined residual plots. In the first multivariable model, we adjusted for age, sex, study site, education, smoking status, alcohol, family history of diabetes, years lived in the United States, physical activity, diabetes medication use, cholesterol-lowering medication use, hypertension medication use, the sum of cultural traditional measures, and total energy. For all prospective analyses, we additionally adjusted for the baseline value of the cardiometabolic risk factor. Because BMI can be a potential mediator of the association between diet and the cardiometabolic risk, we adjusted for it separately in model 2. For all prospective associations, we adjusted for the baseline value of the covariates and the baseline value of the corresponding outcome measure. For all glycemia measures as an outcome, to minimize confounding due to prevalent disease, we excluded participants with T2D. For all linear associations, results are presented as the unit change (or the percentage change for log-transformed variables) in the outcome per 5-unit higher PDI, hPDI, or uPDI score. We tested for potential effect modifications by age and sex by including a cross-product term between these variables and the diet indices. Because these associations were not a priori, we corrected for multiple testing by setting the threshold for statistical significance to Pinteraction < 0.001 (0.05 ÷ [22 outcomes × 2 effect modifiers]). For all other statistical analyses, significance was set at a P value < 0.05. All statistical tests were 2-sided and performed using SAS, version 9.4 (SAS Institute).
Results
At baseline, compared with participants in quartile 1 of the PDI or hPDI scores, participants in quartile 4 had lived in the United States for fewer years, had stronger cultural tradition beliefs, were less likely to be current smokers and consume alcohol, and had lower BMIs and smaller waist circumferences. Participants with higher hPDI scores were likely to be older, female, and spend less time watching TV each week. Compared with participants with the lowest uPDI scores (quartile 1), participants with higher uPDI scores were likely to be younger, had lived in the United States for fewer years, had stronger cultural beliefs, and were less likely to consume alcohol (Table 1). Distributions of food groups (in servings/day) and baseline cardiometabolic risk factors by quartiles of the indices are shown in Supplemental Tables 2and3, respectively.
TABLE 1.
Baseline descriptive characteristics of MASALA participants by quartiles of plant-based diet indices1
| Quartiles | |||||
|---|---|---|---|---|---|
| Characteristic | 1 | 2 | 3 | 4 | P-trend |
| Overall plant-based diet index | |||||
| Participants, n | 211 | 229 | 211 | 240 | |
| Mean ± SEM | 51.1 ± 0.25 | 58.6 ± 0.12 | 63.8 ± 0.10 | 71.1 ± 0.24 | |
| Age, years | 54.8 ± 0.68 | 55.5 ± 0.62 | 54.7 ± 0.64 | 55.9 ± 0.63 | 0.41 |
| Sex, % female | 47.4 | 42.4 | 52.6 | 46.3 | 0.66 |
| Years lived in the US, years | 29.1 ± 0.69 | 26.6 ± 0.62 | 26.2 ± 0.64 | 26.3 ± 0.63 | 0.004 |
| Birth country | — | — | — | — | 0.09 |
| India | 74.4 | 83.8 | 87.7 | 88.3 | |
| Pakistan | 9 | 5.24 | 0.95 | 2.5 | |
| Bangladesh | 1.42 | 0.87 | 0 | 0 | |
| Nepal | 0.47 | 0 | 0.47 | 0.83 | |
| Sri Lanka | 2.84 | 0.87 | 0.47 | 0 | |
| Other | 11.9 | 9.22 | 10.41 | 8.37 | |
| Bachelor's degree or more, % | 84.4 | 86.0 | 88.6 | 91.7 | 0.01 |
| Questionnaire in Hindi or Urdu, % | 4.27 | 5.24 | 4.74 | 3.33 | 0.56 |
| Family income >$75,000, % | 74.5 | 73.4 | 75.6 | 70.9 | 0.49 |
| Sum of cultural traditions measures2 | 15.8 ± 0.45 | 14.1 ± 0.41 | 12.9 ± 0.43 | 13.3 ± 0.42 | <0.0001 |
| Smoking category, % | |||||
| Never | 79.1 | 80.4 | 82.9 | 90.0 | 0.001 |
| Former | 16.1 | 15.7 | 15.6 | 7.5 | |
| Current | 4.7 | 3.9 | 1.4 | 2.5 | |
| Tobacco pack-year consumption | 2.53 ± 0.42 | 1.68 ± 0.38 | 1.48 ± 0.40 | 0.50 ± 0.39 | 0.001 |
| Alcohol, % with 1+ drinks/week | 43.1 | 38.0 | 26.1 | 25.0 | <0.0001 |
| Physical activity, MET-min/week | 9970 ± 289 | 10113 ± 265 | 10343 ± 275 | 10159 ± 271 | 0.56 |
| TV watching, min/week | 565 ± 33 | 547 ± 30 | 536 ± 31 | 558 ± 31 | 0.85 |
| BMI, kg/m2 | 26.7 ± 0.29 | 26.1 ± 0.27 | 25.9 ± 0.28 | 25.4 ± 0.28 | 0.002 |
| Waist circumference, cm | 93.9 ± 0.71 | 92.9 ± 0.65 | 92.4 ± 0.67 | 91.1 ± 0.66 | 0.006 |
| Controlled hyperlipidemia, % | 77.2 | 78.2 | 84.7 | 77.9 | 0.53 |
| Hypertension, % | 37.9 | 45.4 | 37.4 | 39.2 | 0.75 |
| Diabetes categories, % | — | — | — | — | |
| Normal | 60.0 | 58.8 | 62.7 | 64.6 | 0.34 |
| Prediabetes | 18.1 | 20.6 | 17.7 | 15.0 | |
| Diabetes | 21.9 | 20.6 | 19.6 | 20.4 | |
| Family history of diabetes, % | 61.6 | 52.1 | 52.7 | 55.0 | 0.23 |
| Medication use, % | |||||
| Diabetes medication | 17.5 | 15.7 | 14.7 | 16.3 | 0.68 |
| Hypertension medication | 31.3 | 33.6 | 26.5 | 29.6 | 0.38 |
| Cholesterol-lowering medication | 32.7 | 31.0 | 25.1 | 29.2 | 0.25 |
| Cardiometabolic risk factors | |||||
| Triglycerides,3 mmol/L | 1.14 ± 1.01 | 1.12 ± 1.01 | 1.12 ± 1.01 | 1.15 ± 1.01 | 0.396 |
| LDL-C, mmol/L | 3.05 ± 0.059 | 2.95 ± 0.054 | 2.85 ± 0.056 | 2.74 ± 0.055 | <0.0001 |
| HDL-C, mmol/L | 1.32 ± 0.023 | 1.32 ± 0.021 | 1.31 ± 0.021 | 1.27 ± 0.021 | 0.099 |
| Fasting glucose,3 mmol/L | 2.12 ± 1.006 | 2.10 ± 1.006 | 2.10 ± 1.006 | 2.07 ± 1.006 | 0.02 |
| HbA1c, % | 6.16 ± 0.061 | 6.01 ± 0.056 | 6.08 ± 0.059 | 5.98 ± 0.057 | 0.08 |
| β-cell function3,4 | 1.32 ± 1.03 | 1.37 ± 1.03 | 1.39 ± 1.032 | 1.46 ± 1.03 | 0.04 |
| HOMA-IR4 | 1.58 ± 1.02 | 1.48 ± 1.02 | 1.47 ± 1.02 | 1.48 ± 1.02 | 0.06 |
| Subcutaneous fat area, cm2 | 245 ± 6.89 | 232 ± 6.42 | 235 ± 6.69 | 242 ± 6.49 | 0.80 |
| Visceral fat area, cm2 | 142 ± 3.79 | 133 ± 3.54 | 131 ± 3.60 | 130 ± 3.54 | 0.02 |
| Pericardial fat volume, cm3 | 61.5 ± 1.98 | 59.1 ± 1.84 | 58.0 ± 1.89 | 54.3 ± 1.86 | 0.01 |
| Hepatic fat attenuation, HU | 53.4 ± 0.738 | 55.0 ± 0.69 | 56.4 ± 0.703 | 56.2 ± 0.69 | 0.005 |
| C-reactive protein,3 mg/L | 1.18 ± 1.03 | 1.15 ± 1.03 | 1.13 ± 1.03 | 1.10 ± 1.03 | 0.13 |
| Adiponectin, mg/dL | 54.9 ± 1.02 | 53.9 ± 1.02 | 56.1 ± 1.02 | 54.8 ± 1.02 | 0.77 |
| Coronary artery calcium score | 2.24 ± 1.06 | 2.07 ± 1.06 | 2.26 ± 1.06 | 2.14 ± 1.06 | 0.80 |
| Common carotid IMT, mm | 0.937 ± 1.01 | 0.93 ± 1.006 | 0.937 ± 1.01 | 0.926 ± 1.01 | 0.32 |
| Internal carotid IMT, mm3 | 1.06 ± 1.01 | 1.07 ± 1.01 | 1.06 ± 1.01 | 1.05 ± 1.01 | 0.26 |
| Foods, servings/d | |||||
| Whole grains | 1.72 ± 0.07 | 1.88 ± 0.065 | 2.15 ± 0.07 | 2.18 ± 0.07 | <0.0001 |
| Fruits | 2.00 ± 0.10 | 2.40 ± 0.09 | 2.49 ± 0.09 | 2.48 ± 0.09 | 0.001 |
| Vegetables | 4.15 ± 0.14 | 4.50 ± 0.13 | 4.65 ± 0.14 | 4.79 ± 0.13 | 0.001 |
| Herbs and spices | 2.37 ± 0.10 | 2.89 ± 0.09 | 3.09 ± 0.09 | 3.39 ± 0.09 | <0.0001 |
| Tea and coffee | 2.13 ± 0.10 | 2.12 ± 0.09 | 2.06 ± 0.09 | 2.13 ± 0.09 | 0.91 |
| Nuts | 0.647 ± 0.044 | 0.745 ± 0.04 | 0.773 ± 0.042 | 1.06 ± 0.041 | <0.0001 |
| Legumes | 0.748 ± 0.049 | 1.05 ± 0.045 | 1.2 ± 0.046 | 1.64 ± 0.046 | <0.0001 |
| Vegetable oils | 0.133 ± 0.015 | 0.127 ± 0.014 | 0.089 ± 0.015 | 0.124 ± 0.015 | 0.40 |
| Sugar-sweetened beverages | 0.294 ± 0.034 | 0.330 ± 0.031 | 0.375 ± 0.032 | 0.358 ± 0.032 | 0.13 |
| Potatoes | 0.324 ± 0.031 | 0.307 ± 0.028 | 0.427 ± 0.029 | 0.328 ± 0.029 | 0.43 |
| Refined grains | 0.838 ± 0.051 | 0.786 ± 0.047 | 0.847 ± 0.049 | 1.037 ± 0.048 | 0.005 |
| Deep-fried snacks | 0.433 ± 0.034 | 0.447 ± 0.032 | 0.594 ± 0.033 | 0.766 ± 0.032 | <0.0001 |
| Sweets | 0.537 ± 0.042 | 0.574 ± 0.038 | 0.554 ± 0.04 | 0.648 ± 0.039 | 0.09 |
| Coconut | 0.051 ± 0.015 | 0.07 ± 0.013 | 0.084 ± 0.014 | 0.142 ± 0.014 | <0.0001 |
| Dairy | 3.78 ± 0.13 | 3.62 ± 0.12 | 3.47 ± 0.12 | 3.25 ± 0.12 | 0.002 |
| Animal fat | 0.603 ± 0.045 | 0.457 ± 0.041 | 0.434 ± 0.042 | 0.263 ± 0.042 | <0.0001 |
| Meat | 0.630 ± 0.029 | 0.396 ± 0.027 | 0.194 ± 0.028 | 0.001 ± 0.027 | <0.0001 |
| Egg | 0.418 ± 0.025 | 0.307 ± 0.023 | 0.186 ± 0.024 | 0.078 ± 0.023 | <0.0001 |
| Fish and seafood | 0.259 ± 0.015 | 0.16 ± 0.014 | 0.089 ± 0.015 | 0.019 ± 0.014 | <0.0001 |
| Miscellaneous animal foods | 0.285 ± 0.016 | 0.225 ± 0.015 | 0.166 ± 0.015 | 0.108 ± 0.015 | <0.0001 |
| Milk desserts | 0.193 ± 0.013 | 0.164 ± 0.012 | 0.158 ± 0.012 | 0.134 ± 0.012 | 0.001 |
| Healthy plant–based diet index | |||||
| Participants, n | 217 | 244 | 218 | 212 | |
| Mean ± SEM | 50.1 ± 0.29 | 59.2 ± 0.13 | 65.4 ± 0.11 | 73.2 ± 0.27 | |
| Age, years | 53.0 ± 0.64 | 54.2 ± 0.59 | 56.0 ± 0.62 | 58.0 ± 0.64 | <0.0001 |
| Sex % female | 28.6 | 46.7 | 55.1 | 58.0 | <0.0001 |
| Years lived in the US, years | 29.1 ± 0.67 | 26.7 ± 0.60 | 25.9 ± 0.63 | 26.4 ± 0.64 | 0.002 |
| Birth country | — | — | — | — | 0.0003 |
| India | 74.7 | 82.4 | 88.1 | 90.1 | |
| Pakistan | 7.83 | 5.74 | 2.29 | 1.42 | |
| Bangladesh | 0.46 | 0.82 | 0.92 | 0 | |
| Nepal | 0 | 0.82 | 0.46 | 0.47 | |
| Sri Lanka | 1.84 | 2.46 | 2.29 | 5.66 | |
| Other | 15.2 | 9.43 | 7.8 | 7.1 | |
| Bachelor's degree or more, % | 85.3 | 88.5 | 89.0 | 88.2 | 0.35 |
| Questionnaire in Hindi or Urdu, % | 3.69 | 6.56 | 4.59 | 2.36 | 0.33 |
| Family income >$75,000, % | 72.1 | 74.0 | 77.6 | 70.2 | 0.90 |
| Sum of cultural traditions measures2 | 14.7 ± 0.44 | 14.1 ± 0.40 | 14.0 ± 0.42 | 13.2 ± 0.44 | 0.03 |
| Smoking category, % | |||||
| Never | 71.0 | 84.0 | 86.7 | 91.5 | <0.0001 |
| Former | 21.2 | 15.2 | 11.5 | 6.1 | |
| Current | 7.8 | 0.8 | 1.8 | 2.4 | |
| Tobacco pack-year consumption | 3.210 ± 0.41 | 1.33 ± 0.37 | 1.04 ± 0.39 | 0.52 ± 0.40 | <0.0001 |
| Alcohol, % with 1+ drinks/week | 45.6 | 35.7 | 29.4 | 20.3 | <0.0001 |
| Physical activity, MET-min/week | 10102 ± 282 | 9934 ± 256 | 10013 ± 271 | 10570 ± 279 | 0.25 |
| TV watching, min/week | 640 ± 32 | 521 ± 29 | 517 ± 30 | 533 ± 31 | 0.02 |
| BMI, kg/m2 | 26.5 ± 0.29 | 26.5 ± 0.26 | 25.6 ± 0.28 | 25.3 ± 0.28 | 0.002 |
| Waist circumference, cm | 93.4 ± 0.69 | 93.8 ± 0.63 | 91.6 ± 0.66 | 91.1 ± 0.68 | 0.004 |
| Controlled hyperlipidemia, % | 75.9 | 77.6 | 80.8 | 83.9 | 0.03 |
| Hypertension, % | 37.5 | 40.9 | 36.7 | 45.3 | 0.20 |
| Diabetes categories, % | |||||
| Normal | 55.6 | 62.6 | 66.5 | 61.4 | 0.38 |
| Prediabetes | 23.6 | 15.6 | 16.1 | 16.2 | |
| Diabetes | 20.8 | 21.8 | 17.4 | 22.4 | |
| Family history of diabetes, % | 58.4 | 53.6 | 54.8 | 54.5 | 0.51 |
| Medication use, % | |||||
| Diabetes medication | 13.8 | 17.2 | 14.7 | 18.4 | 0.33 |
| Hypertension medication | 27.2 | 30.7 | 28.9 | 34.4 | 0.16 |
| Cholesterol-lowering medication | 27.2 | 27.5 | 27.5 | 36.3 | 0.50 |
| Cardiometabolic risk factors | |||||
| Triglycerides,3 mmol/L | 1.14 ± 1.01 | 1.12 ± 1.01 | 1.11 ± 1.01 | 1.16 ± 1.01 | 0.72 |
| LDL-C, mmol/L | 3.02 ± 0.058 | 2.90 ± 0.052 | 2.87 ± 0.055 | 2.78 ± 0.057 | 0.005 |
| HDL-C, mmol/L | 1.30 ± 0.022 | 1.30 ± 0.02 | 1.32 ± 0.021 | 1.30 ± 0.022 | 0.66 |
| Fasting glucose,3 mmol/L | 2.11 ± 1.01 | 2.10 ± 1.006 | 2.08 ± 1.01 | 2.09 ± 1.01 | 0.28 |
| HbA1c, % | 6.12 ± 0.06 | 6.11 ± 0.054 | 6.00 ± 0.057 | 5.99 ± 0.059 | 0.09 |
| β-cell function3,4 | 1.32 ± 1.03 | 1.41 ± 1.03 | 1.44 ± 1.03 | 1.39 ± 1.03 | 0.23 |
| HOMA-IR4 | 1.57 ± 1.02 | 1.56 ± 1.02 | 1.43 ± 1.02 | 1.43 ± 1.02 | <0.0001 |
| Subcutaneous fat area, cm2 | 244 ± 6.71 | 247 ± 6.17 | 239 ± 6.58 | 224 ± 6.66 | 0.04 |
| Visceral fat area, cm2 | 141 ± 3.67 | 141 ± 3.34 | 127 ± 3.56 | 125 ± 3.67 | <0.0001 |
| Pericardial fat volume, cm3 | 63.1 ± 1.94 | 59.4 ± 1.75 | 55.5 ± 1.86 | 54.2 ± 1.92 | 0.001 |
| Hepatic fat attenuation, HU | 53.4 ± 0.723 | 54.7 ± 0.652 | 56.1 ± 0.696 | 57.0 ± 0.712 | <0.0001 |
| C-reactive protein,3 mg/L | 1.21 ± 1.03 | 1.18 ± 1.03 | 1.08 ± 1.03 | 1.09 ± 1.03 | 0.006 |
| Adiponectin, mg/dL | 53.8 ± 1.02 | 53.4 ± 1.02 | 55.7 ± 1.02 | 56.9 ± 1.02 | 0.02 |
| Coronary artery calcium score | 2.21 ± 1.06 | 2.13 ± 1.06 | 2.27 ± 1.06 | 2.10 ± 1.06 | 0.71 |
| Common carotid IMT, mm | 0.939 ± 1.01 | 0.938 ± 1.01 | 0.93 ± 1.01 | 0.921 ± 1.01 | 0.03 |
| Internal carotid IMT, mm3 | 1.08 ± 1.01 | 1.062 ± 1.01 | 1.058 ± 1.01 | 1.041 ± 1.01 | 0.006 |
| Foods, servings/d | |||||
| Whole grains | 1.50 ± 0.067 | 1.97 ± 0.06 | 2.10 ± 0.06 | 2.38 ± 0.07 | <0.0001 |
| Fruits | 1.90 ± 0.094 | 2.17 ± 0.085 | 2.51 ± 0.09 | 2.84 ± 0.093 | <0.0001 |
| Vegetables | 3.78 ± 0.136 | 4.44 ± 0.123 | 4.67 ± 0.131 | 5.25 ± 0.135 | <0.0001 |
| Herbs and spices | 2.45 ± 0.093 | 3.01 ± 0.085 | 3.06 ± 0.090 | 3.26 ± 0.092 | <0.0001 |
| Tea and coffee | 2.01 ± 0.094 | 2.183 ± 0.085 | 2.23 ± 0.090 | 2.00 ± 0.093 | 0.951 |
| Nuts | 0.539 ± 0.042 | 0.744 ± 0.038 | 0.886 ± 0.040 | 1.09 ± 0.041 | <0.0001 |
| Legumes | 0.716 ± 0.047 | 1.122 ± 0.043 | 1.353 ± 0.046 | 1.581 ± 0.047 | <0.0001 |
| Sugar-sweetened beverages | 0.11 ± 0.015 | 0.106 ± 0.014 | 0.129 ± 0.015 | 0.13 ± 0.015 | 0.25 |
| Potatoes | 0.503 ± 0.033 | 0.351 ± 0.03 | 0.272 ± 0.031 | 0.237 ± 0.032 | <0.0001 |
| Refined grains | 0.385 ± 0.03 | 0.39 ± 0.027 | 0.312 ± 0.029 | 0.288 ± 0.03 | 0.008 |
| Deep-fried snacks | 1.236 ± 0.048 | 0.926 ± 0.043 | 0.803 ± 0.046 | 0.558 ± 0.047 | <0.0001 |
| Sweets | 0.610 ± 0.034 | 0.630 ± 0.031 | 0.587 ± 0.033 | 0.427 ± 0.034 | <0.0001 |
| Coconut | 0.849 ± 0.039 | 0.629 ± 0.035 | 0.504 ± 0.037 | 0.338 ± 0.038 | <0.0001 |
| Dairy | 0.115 ± 0.014 | 0.079 ± 0.013 | 0.117 ± 0.014 | 0.044 ± 0.014 | 0.006 |
| Animal fat | 3.46 ± 0.122 | 3.42 ± 0.111 | 3.73 ± 0.118 | 3.49 ± 0.121 | 0.50 |
| Meat | 0.624 ± 0.043 | 0.494 ± 0.039 | 0.357 ± 0.042 | 0.254 ± 0.043 | <0.0001 |
| Egg | 0.585 ± 0.03 | 0.313 ± 0.027 | 0.195 ± 0.029 | 0.093 ± 0.03 | <0.0001 |
| Fish and seafood | 0.363 ± 0.025 | 0.256 ± 0.023 | 0.195 ± 0.024 | 0.154 ± 0.025 | <0.0001 |
| Miscellaneous animal foods | 0.218 ± 0.016 | 0.14 ± 0.014 | 0.094 ± 0.015 | 0.061 ± 0.015 | <0.0001 |
| Milk desserts | 0.283 ± 0.016 | 0.205 ± 0.014 | 0.166 ± 0.015 | 0.119 ± 0.016 | <0.0001 |
| Unhealthy plant–based diet index | |||||
| Participants, n | 229 | 224 | 217 | 221 | |
| Mean ± SEM | 51.9 ± 0.24 | 59.2 ± 0.10 | 63.9 ± 0.09 | 70.4 ± 0.20 | |
| Age, years | 56.3 ± 0.64 | 55.6 ± 0.62 | 55.8 ± 0.64 | 53.4 ± 0.65 | 0.005 |
| Sex, % female | 53.2 | 45.1 | 43.3 | 46.2 | 0.12 |
| Years lived in the US, years | 28.1 ± 0.64 | 27.7 ± 0.63 | 27.0 ± 0.64 | 25.1 ± 0.65 | 0.002 |
| Birth country | — | — | — | — | 0.07 |
| India | 81.2 | 79.5 | 88.0 | 86.4 | |
| Pakistan | 5.68 | 6.7 | 3.23 | 1.81 | |
| Bangladesh | 0.44 | 0.89 | 0.46 | 0.45 | |
| Nepal | 0.87 | 0.45 | 0 | 0.45 | |
| Sri Lanka | 0.87 | 0.45 | 1.38 | 1.36 | |
| Other | 10.9 | 12.1 | 6.90 | 9.48 | |
| Bachelor's degree or more, % | 89.1 | 86.2 | 88.5 | 87.3 | 0.76 |
| Questionnaire in Hindi or Urdu, % | 1.31 | 5.36 | 6.45 | 4.52 | 0.08 |
| Family income >$75,000, % | 74.4 | 75.6 | 73.2 | 70.8 | 0.32 |
| Sum of cultural traditions measures2 | 15.0 ± 0.43 | 14.2 ± 0.42 | 14.3 ± 0.42 | 12.4 ± 0.43 | <0.0001 |
| Smoking category, % | |||||
| Never | 80.8 | 82.1 | 83.4 | 86.9 | 0.05 |
| Former | 14.9 | 14.7 | 12.9 | 11.8 | |
| Current | 4.4 | 3.13 | 3.69 | 1.36 | |
| Tobacco pack-year consumption | 1.56 ± 0.40 | 2.07 ± 0.39 | 1.15 ± 0.40 | 1.25 ± 0.41 | 0.35 |
| Alcohol, % with 1+ drinks/week | 39.3 | 35.7 | 31.8 | 24.4 | 0.0006 |
| Physical activity, MET-min/week | 10432 ± 273 | 10212 ± 267 | 10283 ± 273 | 9644 ± 278 | 0.07 |
| TV watching, min/week | 520 ± 31 | 574 ± 30 | 555 ± 31 | 559 ± 31 | 0.48 |
| BMI, kg/m2 | 26.1 ± 0.28 | 26.3 ± 0.27 | 25.9 ± 0.28 | 25.8 ± 0.29 | 0.39 |
| Waist circumference, cm | 93.0 ± 0.67 | 92.7 ± 0.66 | 92.7 ± 0.67 | 91.7 ± 0.69 | 0.22 |
| Controlled hyperlipidemia, % | 75.3 | 79.7 | 76.7 | 86.2 | 0.02 |
| Hypertension, % | 43.7 | 39.7 | 36.9 | 39.8 | 0.32 |
| Diabetes categories, % | |||||
| Normal | 60.1 | 62.8 | 57.7 | 65.6 | 0.54 |
| Prediabetes | 18.4 | 17.5 | 20.9 | 14.5 | |
| Diabetes | 21.5 | 19.7 | 21.4 | 19.9 | |
| Family history of diabetes, % | 58.3 | 55.8 | 52.7 | 54.2 | 0.32 |
| Medication use, % | |||||
| Diabetes medication | 17.5 | 13.0 | 17.1 | 16.7 | 0.87 |
| Hypertension medication | 30.6 | 30.4 | 30.9 | 29.4 | 0.83 |
| Cholesterol-lowering medication | 29.7 | 31.3 | 28.6 | 28.5 | 0.65 |
| Cardiometabolic risk factors | |||||
| Triglycerides,3 mmol/L | 1.12 ± 1.01 | 1.12 ± 1.01 | 1.14 ± 1.01 | 1.15 ± 1.01 | 0.14 |
| LDL-C, mmol/L | 2.98 ± 0.056 | 2.87 ± 0.055 | 2.90 ± 0.056 | 2.81 ± 0.057 | 0.06 |
| HDL-C, mmol/L | 1.34 ± 0.021 | 1.31 ± 0.021 | 1.28 ± 0.021 | 1.29 ± 0.022 | 0.06 |
| Fasting glucose,3 mmol/L | 2.11 ± 1.01 | 2.09 ± 1.01 | 2.11 ± 1.01 | 2.07 ± 1.01 | 0.035 |
| HbA1c, % | 6.11 ± 0.06 | 6.01 ± 0.06 | 6.05 ± 0.06 | 6.04 ± 0.06 | 0.47 |
| β-cell function3,4 | 1.34 ± 1.03 | 1.38 ± 1.03 | 1.42 ± 1.03 | 1.42 ± 1.03 | 0.18 |
| HOMA-IR4 | 1.52 ± 1.02 | 1.50 ± 1.02 | 1.50 ± 1.02 | 1.47 ± 1.02 | 0.31 |
| Subcutaneous fat area, cm2 | 236 ± 6.70 | 239 ± 6.34 | 241 ± 6.57 | 239 ± 6.71 | 0.78 |
| Visceral fat area, cm2 | 130 ± 3.62 | 139 ± 3.52 | 132 ± 3.60 | 133 ± 3.67 | 0.74 |
| Pericardial fat volume, cm3 | 57.2 ± 1.89 | 58.6 ± 1.84 | 57.2 ± 1.88 | 59.3 ± 1.93 | 0.57 |
| Hepatic fat attenuation, HU | 55.5 ± 0.706 | 55.3 ± 0.687 | 55.3 ± 0.703 | 55.1 ± 0.721 | 0.71 |
| C-reactive protein,3 mg/L | 1.13 ± 1.03 | 1.16 ± 1.03 | 1.11 ± 1.03 | 1.15 ± 1.03 | 0.89 |
| Adiponectin, mg/dL | 56.2 ± 1.02 | 53.2 ± 1.02 | 55.2 ± 1.02 | 54.9 ± 1.02 | 0.58 |
| Coronary artery calcium score | 2.21 ± 1.06 | 2.28 ± 1.058 | 2.07 ± 1.06 | 2.13 ± 1.06 | 0.46 |
| Common carotid IMT, mm | 0.925 ± 1.01 | 0.937 ± 1.01 | 0.934 ± 1.01 | 0.932 ± 1.01 | 0.46 |
| Internal carotid IMT, mm3 | 1.06 ± 1.01 | 1.05 ± 1.01 | 1.06 ± 1.01 | 1.07 ± 1.01 | 0.45 |
| Foods, servings/d | |||||
| Whole grains | 2.10 ± 0.068 | 2.02 ± 0.066 | 1.88 ± 0.067 | 1.94 ± 0.069 | 0.06 |
| Fruits | 2.71 ± 0.093 | 2.43 ± 0.09 | 2.27 ± 0.092 | 1.97 ± 0.094 | <0.0001 |
| Vegetables | 5.32 ± 0.131 | 4.77 ± 0.128 | 4.24 ± 0.131 | 3.74 ± 0.133 | <0.0001 |
| Herbs/spices | 3.22 ± 0.091 | 3.15 ± 0.089 | 2.90 ± 0.091 | 2.51 ± 0.093 | <0.0001 |
| Tea and coffee | 2.57 ± 0.088 | 2.18 ± 0.086 | 2.12 ± 0.088 | 1.54 ± 0.09 | <0.0001 |
| Nuts | 1.04 ± 0.041 | 0.882 ± 0.04 | 0.749 ± 0.041 | 0.572 ± 0.042 | <0.0001 |
| Legumes | 1.15 ± 0.05 | 1.16 ± 0.049 | 1.17 ± 0.05 | 1.29 ± 0.051 | 0.09 |
| Sugar-sweetened beverages | 0.141 ± 0.015 | 0.143 ± 0.014 | 0.110 ± 0.015 | 0.078 ± 0.015 | 0.002 |
| Potatoes | 0.200 ± 0.032 | 0.312 ± 0.031 | 0.380 ± 0.032 | 0.479 ± 0.032 | <0.0001 |
| Refined grains | 0.246 ± 0.029 | 0.346 ± 0.028 | 0.365 ± 0.029 | 0.43 ± 0.03 | <0.0001 |
| Deep-fried snacks | 0.521 ± 0.046 | 0.812 ± 0.045 | 0.964 ± 0.046 | 1.25 ± 0.047 | <0.0001 |
| Sweets | 0.325 ± 0.032 | 0.542 ± 0.031 | 0.646 ± 0.032 | 0.765 ± 0.033 | <0.0001 |
| Coconut | 0.400 ± 0.039 | 0.629 ± 0.038 | 0.614 ± 0.039 | 0.69 ± 0.04 | <0.0001 |
| Dairy | 0.028 ± 0.014 | 0.073 ± 0.013 | 0.137 ± 0.014 | 0.12 ± 0.014 | <0.0001 |
| Animal fat | 4.05 ± 0.117 | 3.51 ± 0.114 | 3.38 ± 0.117 | 3.11 ± 0.119 | <0.0001 |
| Meat | 0.48 ± 0.043 | 0.416 ± 0.042 | 0.449 ± 0.043 | 0.387 ± 0.044 | 0.203 |
| Egg | 0.407 ± 0.031 | 0.349 ± 0.03 | 0.272 ± 0.031 | 0.147 ± 0.031 | <0.0001 |
| Fish and seafood | 0.380 ± 0.024 | 0.311 ± 0.023 | 0.173 ± 0.024 | 0.093 ± 0.024 | <0.0001 |
| Miscellaneous animal foods | 0.227 ± 0.015 | 0.111 ± 0.015 | 0.115 ± 0.015 | 0.055 ± 0.015 | <0.0001 |
| Milk desserts | 0.224 ± 0.016 | 0.214 ± 0.015 | 0.178 ± 0.016 | 0.155 ± 0.016 | 0.001 |
Values are age, sex, and calorie-adjusted means (SEM) or percentages, calculated using linear regression for continuous variables and chi-square tests for categorical variables. Abbreviations: HbA1c, glycated hemoglobin; HU, Hounsfield units; IMT, intima media thickness; MASALA, Mediators of Atherosclerosis in South Asians Living in America; MET, metabolic equivalents of task.
Higher scores indicate weaker traditional cultural beliefs.
Values were log-transformed to obtain a normal distribution of the residuals and transformed back into geometric means.
The β-cell function was measured using the oral disposition index.
In fully adjusted models at baseline, each 5-unit higher PDI score was associated with a lower percentage difference in fasting glucose (−1.03 ± 0.35%; P < 0.01; Table 2). A 5-unit higher hPDI score, but not PDI score, was associated with a lower HbA1c (0.43% ± 0.14%; P < 0.01). Each 5-unit higher PDI or hPDI score was associated with a 3.46%–4.02% lower log-HOMA-IR value (P < 0.05). Although higher scores on the PDI and hPDI were associated with greater insulin sensitivity or β-cell function, these associations did not reach statistical significance. Higher scores on all 3 indices were associated with lower LDL cholesterol (P < 0.05). On one hand, each 5-unit higher hPDI score was associated with a 5.68% (2.25%) lower hsCRP concentration, but this association was attenuated and no longer significant after adjusting for BMI. On the other hand, hPDI scores were positively associated with adiponectin (2.32 ± 1.08 mg/dL; P < 0.05) even after BMI adjustment.
TABLE 2.
Cross-sectional associations between plant-based diet scores and measures of cardiometabolic risk among participants with South Asian ancestry, aged 40–84 years, in the MASALA study1
| PDI | hPDI | uPDI | ||||||
|---|---|---|---|---|---|---|---|---|
| n 2 | Model3 | Score | P value | Score | P value | Score | P value | |
| Subclinical measures of atherosclerosis | ||||||||
| Coronary artery calcium score4 | 884 | 1 | −3.25 ± 4.93 | 0.50 | −0.138 ± 4.19 | 0.97 | 0.447 ± 5.14 | 0.93 |
| 2 | −2.51 ± 4.93 | 0.60 | 1.10 ± 4.20 | 0.79 | 0.428 ± 5.12 | 0.93 | ||
| Common carotid IMT, mm3 | 890 | 1 | −0.002 ± 0.503 | 0.99 | −0.115 ± 0.427 | 0.79 | 0.472 ± 0.522 | 0.37 |
| 2 | 0.177 ± 0.499 | 0.72 | 0.104 ± 0.426 | 0.81 | 0.519 ± 0.517 | 0.32 | ||
| Internal carotid IMT, mm3 | 889 | 1 | −0.749 ± 0.667 | 0.26 | −0.759 ± 0.568 | 0.18 | 0.210 ± 0.694 | 0.76 |
| 2 | −0.598 ± 0.564 | 0.36 | −0.563 ± 0.564 | 0.32 | 0.229 ± 0.685 | 0.74 | ||
| Glycemia measures among nondiabetics | ||||||||
| Fasting glucose,4 mmol/L | 664 | 1 | −1.12 ± 0.347 | 0.001 | −0.592 ± 0.299 | 0.05 | −0.725 ± 0.354 | 0.04 |
| 2 | −1.03 ± 0.347 | 0.003 | −0.521 ± 0.298 | 0.08 | −0.668 ± 0.352 | 0.06 | ||
| HbA1c, % | 660 | 1 | −0.284 ± 0.163 | 0.08 | −0.476 ± 0.138 | 0.001 | 0.180 ± 0.165 | 0.28 |
| 2 | −0.224 ± 0.161 | 0.17 | −0.428 ± 0.137 | 0.002 | 0.224 ± 0.163 | 0.17 | ||
| β-cell function4,5 | 543 | 1 | 4.15 ± 2.42 | 0.09 | 2.44 ± 2.07 | 0.24 | 0.52 ± 2.52 | 0.83 |
| 2 | 3.73 ± 2.42 | 0.13 | 2.11 ± 2.07 | 0.31 | 0.15 ± 2.51 | 0.95 | ||
| HOMA-IR4 | 613 | 1 | −4.97 ± 1.87 | 0.006 | −5.24 ± 1.61 | 0.001 | −3.13 ± 1.95 | 0.10 |
| 2 | −3.46 ± 1.65 | 0.03 | −4.02 ± 1.42 | 0.004 | −1.68 ± 1.72 | 0.32 | ||
| Lipids | ||||||||
| Triglycerides,4 mmol/L | 888 | 1 | 0.465 ± 1.127 | 0.68 | −0.116 ± 0.962 | 0.90 | 1.11 ± 1.17 | 0.34 |
| 2 | 1.05 ± 1.11 | 0.35 | 0.557 ± 0.950 | 0.56 | 1.31 ± 1.15 | 0.25 | ||
| HDL-C, mmol/L | 888 | 1 | −0.003 ± 0.008 | 0.67 | 0.009 ± 0.007 | 0.19 | −0.003 ± 0.008 | 0.76 |
| 2 | −0.007 ± 0.008 | 0.34 | 0.004 ± 0.007 | 0.53 | −0.004 ± 0.008 | 0.64 | ||
| LDL-C, mmol/L | 881 | 1 | −0.088 ± 0.019 | <0.0001 | −0.045 ± 0.016 | 0.006 | −0.046 ± 0.020 | 0.02 |
| 2 | −0.081 ± 0.019 | <0.0001 | −0.040 ± 0.016 | 0.02 | −0.042 ± 0.020 | 0.04 | ||
| Inflammation and adipokines | ||||||||
| C-reactive protein,4 mg/L | 878 | 1 | −2.71 ± 2.64 | 0.29 | −5.68 ± 2.25 | 0.009 | −1.27 ± 2.74 | 0.64 |
| 2 | −0.67 ± 2.42 | 0.78 | −2.95 ± 2.07 | 0.15 | −0.84 ± 2.49 | 0.73 | ||
| Adiponectin, mg/dL | 869 | 1 | 1.00 ± 1.28 | 0.43 | 2.86 ± 1.09 | 0.009 | 0.09 ± 1.32 | 0.95 |
| 2 | 0.57 ± 1.27 | 0.65 | 2.32 ± 1.08 | 0.03 | −0.05 ± 1.31 | 0.97 | ||
| Body composition measures | ||||||||
| Weight, kg | 891 | 1 | −0.720 ± 0.280 | 0.01 | −0.750 ± 0.241 | 0.002 | −0.428 ± 0.295 | 0.15 |
| BMI, kg/m2 | 889 | 1 | −0.222 ± 0.102 | 0.03 | −0.279 ± 0.087 | 0.001 | −0.051 ± 0.107 | 0.63 |
| Waist circumference, cm | 889 | 1 | −0.514 ± 0.241 | 0.03 | −0.620 ± 0.205 | 0.003 | −0.264 ± 0.251 | 0.29 |
| 2 | −0.041 ± 0.142 | 0.77 | −0.084 ± 0.121 | 0.49 | −0.084 ± 0.147 | 0.57 | ||
| Subcutaneous fat area, cm2 | 812 | 1 | 0.92 ± 2.33 | 0.69 | −2.81 ± 1.97 | 0.16 | 2.04 ± 2.44 | 0.40 |
| 2 | 3.31 ± 1.74 | 0.06 | 1.09 ± 1.48 | 0.46 | 1.94 ± 1.83 | 0.29 | ||
| Visceral fat area, cm2 | 866 | 1 | −3.04 ± 1.31 | 0.02 | −4.55 ± 1.11 | <0.0001 | 0.62 ± 1.37 | 0.65 |
| 2 | −1.51 ± 1.08 | 0.16 | −2.55 ± 0.92 | 0.006 | 0.81 ± 1.12 | 0.47 | ||
| Pericardial fat volume, cm3 | 878 | 1 | −1.34 ± 0.658 | 0.04 | −2.26 ± 0.559 | <0.0001 | 0.636 ± 0.690 | 0.36 |
| 2 | −0.586 ± 0.570 | 0.30 | −1.31 ± 0.487 | 0.007 | 0.852 ± 0.594 | 0.15 | ||
| Hepatic fat attenuation, HU | 875 | 1 | 0.377 ± 0.246 | 0.13 | 0.751 ± 0.207 | 0.0003 | −0.095 ± 0.256 | 0.71 |
| 2 | 0.201 ± 0.229 | 0.38 | 0.511 ± 0.194 | 0.009 | −0.121 ± 0.238 | 0.61 | ||
Values represent multivariable-adjusted changes in cardiometabolic risk markers (β ± SE or % increase ± SE for log-transformed variables) for each 5-unit increase in plant-based diet scores, calculated using multivariable linear regression. Abbreviations: HbA1C, glycated hemoglobin; hPDI, healthy plant–based diet index; HU, Hounsfield units; IMT, intima media thickness; MASALA, Mediators of Atherosclerosis in South Asians Living in America; PDI, plant-based diet index; uPDI, unhealthy plant–based diet index.
Numbers of participants vary due to missing values for outcome variables or covariates or to outliers.
Multivariable-adjusted model 1 was adjusted for age, sex, study site, education (Bachelor's degree or higher, yes compared with no), smoking status (never, former, current), alcohol (yes compared with no), family history of diabetes (any first-degree biological relatives), years lived in the United States, physical activity (MET-min/week), total energy, diabetes medication use, cholesterol-lowering medication use (yes compared with no), hypertension medication use (yes compared with no), and the sum of cultural traditional measures using multivariable linear regression. Multivariable model 2 was additionally adjusted for BMI (kg/m2).
Values were log-transformed to obtain a normal distribution of the residuals. For outcomes that were log-transformed, values represent percentage increases in outcome variable for every 5-unit increase in the diet index score.
The β-cell function was measured using the oral disposition index.
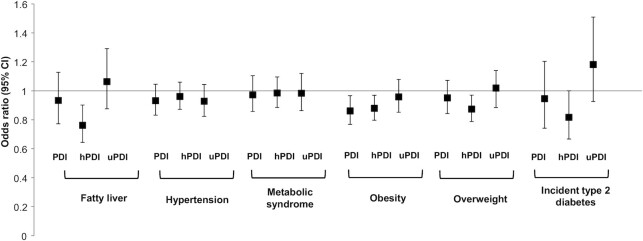
Higher scores on the PDI and hPDI were favorably associated with body composition and ectopic fat measures. For each 5-unit higher PDI or hPDI score, weight was lower by 0.72 kg (0.28 kg) and 0.75 kg (0.24 kg), respectively (P < 0.05). Each 5-unit higher PDI score was associated with a 0.22 kg/m2 (0.10 kg/m2) lower BMI, and each 5-unit higher hPDI score was associated with a 0.28 kg/m2 (0.09 kg/m2) lower BMI (P < 0.05). On one hand, when we examined the likelihoods of overweight (BMI ≥23 kg/m2) and obesity (BMI ≥27.5 kg/m2), each 5-unit higher PDI score was associated with a 14% lower likelihood (OR: 0.86; 95% CI: 0.77, 0.97) of obesity but was not associated with overweight (OR: 0.95; 95% CI: 0.84, 1.07). On the other hand, each 5-unit higher hPDI score was associated with a 13% lower likelihood (OR: 0.87; 95% CI: 0.79, 0.97) of overweight and a 12% lower likelihood (OR: 0.88; 95% CI: 0.80, 0.97) of obesity (Figure 1). After BMI adjustment, a higher hPDI score was associated with a smaller visceral fat area (−2.55 ± 0.92 cm2; P < 0.01), a smaller pericardial fat volume (−1.31 ± 0.49 cm3; P < 0.01), and higher hepatic fat attenuation (0.511 ± 0.194; P < 0.01), indicating less fat in the liver. For each 5-unit higher hPDI score, the likelihood of fatty liver was lower by 24% (OR: 0.76; 95% CI: 0.64, 0.90). We found no evidence for an association between any of the 3 plant-based diet scores and subclinical atherosclerosis, hypertension, or metabolic syndrome.
Figure 1.
Likelihood (ORs and 95% CIs) of fatty liver (n = 878; cases = 80), hypertension (n = 891; cases = 357), metabolic syndrome (n = 889; cases = 306), obesity (n = 891; cases = 270), overweight (n = 891; cases = 677), and incident type 2 diabetes (n = 45) per 5-unit increase in the PDI, hPDI, or uPDI score. The multivariable model was adjusted for age, sex, study site, education (Bachelor's degree or higher, yes compared with no), smoking status (never, former, current), alcohol (yes compared with no), family history of diabetes (any first-degree biological relatives), years lived in the United States, exercise (MET-min/week), total energy, diabetes medication use, cholesterol-lowering medication use (yes compared with no), hypertension medication use (yes compared with no), sum of cultural traditional measures, and BMI (except for obesity and overweight) using logistic regression (PROC LOGISTIC, SAS Institute). Fatty liver is defined as liver-spleen attenuation <40 Hounsfield units. Hypertension is defined using the National Cholesterol Education Program criteria as having blood pressure ≥140/90 mmHg or being on medication. Obesity is defined as a BMI ≥27.5 kg/m2. Overweight is defined as a BMI ≥23 kg/m2. Abbreviations: hPDI, healthy plant–based diet index; MET, metabolic equivalents of task; PDI, plant-based diet index; uPDI, unhealthy plant–based diet index.
In prospective analyses, for each 5-unit higher hPDI score, the likelihood of incident T2D (n = 45 cases) was lower by 18% (OR: 0.82; 95% CI: 0.67, 1.00). Although nonsignificant, each 5-unit higher uPDI score was associated with an 18% likelihood of incident T2D (OR: 1.18; 95% CI: 0.9, 1.51). We found no evidence of an association between baseline diet scores and changes in measures of fasting glucose, HbA1c, triglycerides, HDL cholesterol, or LDL cholesterol. However, each 5-unit higher PDI score at baseline was associated with less weight gain (−0.21 ± 0.11 kg; P = 0.05) at follow-up (Table 3).
TABLE 3.
Prospective associations between plant-based diet indices and change in cardiometabolic risk markers among participants with South Asian ancestry, aged 40–84 years, in the MASALA study1
| PDI | hPDI | uPDI | ||||||
|---|---|---|---|---|---|---|---|---|
| n 2 | Model3 | Score | P value | Score | P value | Score | P value | |
| Glycemia measures among nondiabetics | ||||||||
| Fasting glucose,4 mmol/L | 556 | 1 | −0.400 ± 0.321 | 0.21 | −0.348 ± 0.271 | 0.20 | 0.003 ± 0.322 | 0.99 |
| 2 | −0.374 ± 0.320 | 0.24 | −0.313 ± 0.270 | 0.25 | 0.015 ± 0.321 | 0.96 | ||
| HbA1c, % | 553 | 1 | 0.064 ± 0.170 | 0.71 | 0.034 ± 0.145 | 0.82 | 0.158 ± 0.170 | 0.36 |
| 2 | 0.064 ± 0.171 | 0.71 | 0.033 ± 0.145 | 0.82 | 0.158 ± 0.171 | 0.36 | ||
| Lipids | ||||||||
| Triglycerides,4 mmol/L | 733 | 1 | −0.629 ± 0.964 | 0.51 | −0.499 ± 0.821 | 0.54 | 0.598 ± 0.993 | 0.55 |
| 2 | −0.608 ± 0.966 | 0.53 | −0.413 ± 0.825 | 0.61 | 0.546 ± 0.993 | 0.58 | ||
| HDL-C, mmol/L | 732 | 1 | 0.005 ± 0.006 | 0.36 | 0.003 ± 0.005 | 0.52 | 0.004 ± 0.006 | 0.51 |
| 2 | 0.006 ± 0.006 | 0.31 | 0.004 ± 0.005 | 0.46 | 0.004 ± 0.006 | 0.46 | ||
| LDL-C, mmol/L | 728 | 1 | −0.027 ± 0.021 | 0.19 | −0.006 ± 0.017 | 0.74 | 0.005 ± 0.021 | 0.81 |
| 2 | −0.026 ± 0.021 | 0.20 | −0.007 ± 0.017 | 0.70 | 0.007 ± 0.021 | 0.74 | ||
| Body composition measures | ||||||||
| Weight, kg | 730 | 1 | −0.209 ± 0.106 | 0.05 | 0.004 ± 0.091 | 0.96 | −0.072 ± 0.109 | 0.51 |
Values represent multivariable-adjusted changes in cardiometabolic risk markers (β ± SE or % increase ± SE for log-transformed variables) for each 5-unit increase in plant-based diet scores, calculated using multivariable linear regression. Abbreviations: HbA1C, glycated hemoglobin; hPDI, healthy plant–based diet index; MASALA, Mediators of Atherosclerosis in South Asians Living in America; PDI, plant-based diet index; uPDI, unhealthy plant–based diet index.
Numbers of participants vary due to missing values for outcome variables or to outliers.
Multivariable-adjusted model 1 was adjusted for age, sex, study site, education (Bachelor's degree or higher, yes compared with no), smoking status (never, former, current), alcohol (yes compared with no), family history of diabetes (any first-degree biological relatives), years lived in the United States, physical activity (MET-min/week), total energy, diabetes medication use, cholesterol-lowering medication use (yes compared with no), hypertension medication use (yes compared with no), the sum of cultural traditional measures, and the baseline value of the corresponding cardiometabolic risk marker using multivariable linear regression. Multivariable model 2 was additionally adjusted for BMI (kg/m2).
Values were log-transformed to approximate a normal distribution of the residuals.
We found no evidence for an effect modification by age or sex on the association between plant-based diet indices and cardiometabolic risk markers (Pinteraction > 0.01).
Discussion
In this analysis of plant-based diet quality and cardiometabolic risks among South Asian adults living in the United States, we found that a healthy plant–based diet was associated with favorable measures of glycemic control, insulin resistance, a lower body weight, a lower BMI, favorable measures of adipokines and ectopic fat measures, and a lower incidence of T2D. However, we found no evidence for an association between an unhealthy plant–based diet and cardiometabolic risks. Our findings fill the current knowledge gap regarding the quality of plant-based foods in cardiometabolic risk prevention in an ethnic group with a high proportion of vegetarians and high cardiometabolic risks.
Vegetarianism has often been promoted as a healthy eating pattern, but there has been little to no focus on the quality of plant-based foods. While an overall plant-based diet was associated with some favorable measures of cardiometabolic risk in our cohort, those with greater adherence to a healthy plant–based diet had a far more favorable cardiometabolic risk profile. Although our study found no evidence for a higher cardiometabolic risk among those with the highest uPDI scores, many of these associations, albeit nonsignificant, were in the direction of a higher risk, especially for measures of ectopic fat and incident T2D. Importantly, compared to those in the lowest quartile of uPDI scores, participants in the highest quartile consumed fewer servings of fruits, vegetables, and nuts and more servings of potatoes, refined grains, deep-fried snacks, sweets, and coconut. These food groups have all been previously associated with a higher cardiometabolic risk (6, 30).
The strong, inverse associations between higher hPDI scores, but not PDI or uPDI scores, and ectopic fat measures, including the visceral fat area, hepatic fat attenuation, and the pericardial fat volume, are particularly striking. Ectopic fat accumulation in the abdomen, liver, and around the heart is a major risk factor for cardiometabolic diseases, independent of overall and central adiposity (31–34). Therefore, our finding of a strong, inverse association between a healthy plant–based diet and ectopic fat depots is significant for several reasons. First, compared to other ethnic groups, participants from South Asian countries have less favorable body composition profiles, including more visceral fat, more pericardial fat, and a higher prevalence of fatty liver (35), which may partially explain the disparities in cardiovascular risks by ethnicity (35–38). Second, given that a substantial proportion of participants from South Asia are vegetarian, for effective health promotion it is crucial to advocate for the consumption of healthy plant–based diets rather than the simple exclusion of animal foods. Third, because there are limited therapeutic options for lowering ectopic fat, consumption of a healthy plant–based diet remains among the strongest, modifiable risk factors for prevention of ectopic fat accumulation. Although our study is cross-sectional, our findings are similar to those in the Multi-Ethnic Study of Atherosclerosis cohort, where a higher dietary quality score based on the principles of a Mediterranean-type diet was associated with lower ectopic fat depots, including less visceral fat and less pericardial fat, and a lower prevalence hepatic steatosis, but not with subcutaneous fat measures (39). Future studies will need to confirm our findings with a prospective design.
While MASALA only accrued 45 incident T2D cases over an average of 5 years of follow-up, we were still able to identify that for each 5-unit higher hPDI score, the risk of incident T2D was lower by 18%. Importantly, the uPDI was associated, although nonsignificantly, with an 18% higher risk of T2D. The symmetry of these associations also supports the need to focus on the quality of plant-based foods for chronic disease reduction. This is of particular importance in a South Asian population, where the prevalence of T2D is high (11) and individuals often develop the disease at a younger age and a lower BMI (13).
It is possible that the degree of processing may have affected the observed associations. For instance, foods in the uPDI are all highly processed, while certain foods in the hPDI are unprocessed. When we accounted for the degree of processing in the hPDI by creating 2 subindices, a processed hPDI and an unprocessed hPDI, the findings were not materially different between these 2 subindices and the overall hPDI (Supplemental Tables 2 and 3). This may be because the quality of plant-based diets could be a more important indicator than the degree of processing, although our study was not designed to examine this research question. Additionally, a modified hPDI that included fish, poultry, and yogurt was not associated with LDL cholesterol and adiponectin, which is not surprising given how animal foods are consumed as part of the diet. Among participants from South Asia, consumption of processed meats is not high and, when animal foods are typically consumed, they replace vegetables.
Our findings are consistent with previous studies that examined the associations between vegetarianism and cardiometabolic risks among those from South Asia. In a random sample of 1038 people of Asian Indian descent in the United States, vegetarianism—defined as a primarily lacto-vegetarian diet without consumption of eggs, meat, fish, or poultry—was associated with lower odds of prevalent T2D but not metabolic syndrome or obesity (40). Our study is the first to document an inverse association between healthy plant–based diets and incident T2D among participants from South Asia. In an earlier analysis of the MASALA study, Gadgil et al. (41) identified a dietary pattern with consumption of fruits, vegetables, nuts, and legumes that is like the hPDI, and found this pattern to be associated with lower HOMA-IR values. More recently, in the MASALA study, Jin et al. (42) found that a vegetarian diet—defined as a diet without consumption of meat, fish, or poultry—was associated with lower BMIs; lower measures of visceral fat, total and LDL cholesterol, and fasting glucose; insulin resistance; and lower odds of fatty liver and coronary artery calcium (only in men). However, this study did not differentiate between the quality of plant-based foods. For example, there were no differences between vegetarians and nonvegetarians in the consumption of low-quality plant foods, such as refined grains, snacks, sugar, candy, jams, sugar-sweetened beverages, and starchy vegetables, or in the consumption of high-quality plant foods, such as fruits, nuts, and vegetables. In another Asian population, higher uPDI scores, but not hPDI scores, were associated with a higher risk of incident metabolic syndrome (HR: 1.44; 95% CI: 1.26, 1.64) (43). Similar to our findings, when examining individual components, higher adherence to the uPDI was associated with 46% higher odds (95% CI: 25%, 71%) of abdominal obesity in this group. Although not among participants from South Asia, a comprehensive meta-analysis found an inverse association between an overall PDI score and T2D, with the hPDI score having stronger, inverse associations with T2D (44).
In addition to its health benefits, a healthy plant–based diet is also environmentally sustainable, as it is in line with the universal healthy reference diet recommended by the EAT-Lancet commission on healthy diets from sustainable food systems (45). Like the universal healthy reference diet or the planetary health diet, the hPDI is based on increasing consumption of healthy foods, such as vegetables, fruits, whole grains, legumes, and nuts, and decreasing consumption of unhealthy foods, such as red meat, sugar, and refined grains. Importantly, like the planetary health diet, a diet that scores high on the hPDI not only focuses on healthy plant–based foods but also allows for moderate consumption of some animal foods. This is particularly useful in a South Asian population, where dairy consumption is common even among those who do not consume meat and where more than a third of the population consumes eggs, fish, or meat on a weekly basis (46). For example, in our study, those in the highest quartile of the hPDI scores consumed, on average, 0.09 servings per day of meat, while those in the lowest quartile consumed, on average, 0.59 servings of meat per day. Although the US Dietary Guidelines for Americans consider sustainability to be beyond their scope, it is critical to incorporate environmental sustainability to provide Americans with a more holistic recommendation. In fact, a recent study by Blackstone and colleagues (47) noted that among the 3 different diets recommended by the 2015–2020 Dietary Guidelines, the healthy vegetarian diet produced a 42%–84% lower environmental burden than the other 2 diets.
The strengths of the current study include the availability of dietary data, collected with a previously validated, ethnic-specific FFQ that captured foods unique to the South Asian diet. The MASALA study is the only prospective cohort of participants from South Asia in the United States with detailed data on subclinical atherosclerosis, body composition, and fasting blood measures. In addition to traditional risk factors, we were able to account for confounding due to ethnic-specific measures, such as adherence to cultural traditions. Still, our findings need to be interpreted in the context of a few limitations. First, although we adjusted for a comprehensive list of confounders, residual confounding remains a possibility given the observational nature of the study. Second, we did not have data on all cardiometabolic risk factors at follow-up. Third, we were limited in our power for the prospective analyses, as we only had 45 incident T2D cases. However, continued follow-up of the MASALA cohort will determine whether the uPDI is associated with a higher risk of incident T2D. At the same time, we will also be able to examine associations between hPDI scores, uPDI scores, and incident cardiovascular outcomes. Fourth, measurement errors are inevitable in collecting FFQ data. However, these are likely nondifferential in nature and will, therefore, result in attenuated associations. Fifth, although we collected dietary data at follow-up, we did not include information on changes in diet between the 2 cycles, as this was outside the scope of the present study. Finally, this study was conducted among a cohort of participants with South Asian ancestry who had a high socioeconomic status. It is not clear whether these findings extend to participants living in South Asia and participants of South Asian ancestry with a lower socioeconomic background.
In conclusion, we found that while consuming an overall plant-based diet was associated with lower cardiometabolic risks, the risks were much lower for those consuming a healthy plant–based diet. Future intervention and policy efforts to lower cardiometabolic risks in this high-risk population should focus on promoting a healthy plant–based diet, since it is associated with better health outcomes and is also environmentally sustainable.
Supplementary Material
Acknowledgements
The authors' responsibilities were as follows—SNB, FBH, and AMK: designed the research; NRK and AMK: provided essential materials; SNB, CMS, and UPG: analyzed data and performed the statistical analysis; SNB: wrote paper; SNB and AMK: share responsibility for the final content; and all authors: edited the manuscript and read and approved the final manuscript.
Author disclosures: FBH has received research support from the California Walnut Commission, honoraria for lectures from Metagenics and Standard Process, and honoraria from Diet Quality Photo Navigation, outside the submitted work. SNB is a scientific consultant for LayerIV for work outside the submitted work.
The authors report no potential conflicts of interest relevant to this manuscript.
Notes
The results of the current study were presented as a poster at the American Heart Association Epedemiology and Lifestyle 2019 scientific sessions Houston, TX, 5–8 March 2019.
The MASALA (Mediators of Atherosclerosis in South Asians Living in America) study was supported by NIH grants 1R01HL093009, 2R01HL093009, R01HL120725, and K24HL112827 and at the University of California, San Francisco, site with grants UL1RR024131, UL1TR001872, and P30DK098722.
The funding agencies had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Supplemental Figure 1 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn.
Abbreviations used: CT, computed tomography; HbA1C, glycated hemoglobin; hDPI, healthy plant–based diet index; hsCRP, high-sensitivity C-reactive protein; MASALA, Mediators of Atherosclerosis in South Asians Living in America; MET, metabolic equivalents of task; PDI, plant-based diet index; Q, quintiles; T2D, type 2 diabetes; uPDI, unhealthy plant–based diet index.
Contributor Information
Shilpa N Bhupathiraju, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Caleigh M Sawicki, Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Shatabdi Goon, Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Unjali P Gujral, Hubert Department of Global Health, Emory Global Diabetes Research Center, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Frank B Hu, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Namratha R Kandula, Division of General Internal Medicine and Geriatrics, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Alka M Kanaya, Division of General Internal Medicine, Department of Medicine, University of California, San Francisco, San Francisco, CA, USA.
Data Availability
Data described in the article, code book, and analytic code will not be made publicly available. Further information, including the procedures to obtain and access data from the MASALA (Mediators of Atherosclerosis in South Asians Living in America) study, is described at https://www.masalastudy.org/for-researchers.
References
- 1. GBD 2017 Risk Factor Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q. Association between plant-based dietary patterns and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA Intern Med. 2019;179(10):1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee Y, Park K. Adherence to a vegetarian diet and diabetes risk: a systematic review and meta-analysis of observational studies. Nutrients. 2017;9(6):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borch D, Juul-Hindsgaul N, Veller M, Astrup A, Jaskolowski J, Raben A. Potatoes and risk of obesity, type 2 diabetes, and cardiovascular disease in apparently healthy adults: a systematic review of clinical intervention and observational studies. Am J Clin Nutr. 2016;104(2):489–98. [DOI] [PubMed] [Google Scholar]
- 5. Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr. 2015;102(6):1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal Ket al. . Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2019; 59(7):1071–90. [DOI] [PubMed] [Google Scholar]
- 7. Hemler EC, Hu FB. Plant-based diets for cardiovascular disease prevention: all plant foods are not created equal. Curr Atheroscler Rep. 2019;21(5):18. [DOI] [PubMed] [Google Scholar]
- 8. South Asian Americans Leading Together (SAALT) . A demographic snapshot of South Asians in the United States. [Internet]. 2015. [Accessed 2021 May 24]. Available from: http://saalt.org/wp-content/uploads/2016/01/Demographic-Snapshot-updated_Dec-2015.pdff.
- 9. Singh PN, Arthur KN, Orlich MJ, James W, Purty A, Job JSet al. . Global epidemiology of obesity, vegetarian dietary patterns, and noncommunicable disease in Asian Indians. Am J Clin Nutr. 2014;100(Suppl 1):359S–64S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al Rifai M, Cainzos-Achirica M, Kanaya AM, Kandula NR, Dardardi Z, Joshi PHet al. . Discordance between 10-year cardiovascular risk estimates using the ACC/AHA 2013 estimator and coronary artery calcium in individuals from 5 racial/ethnic groups: comparing MASALA and MESA. Atherosclerosis. 2018;279:122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanaya AM, Herrington D, Vittinghoff E, Ewing SK, Liu K, Blaha MJet al. . Understanding the high prevalence of diabetes in U.S. South Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care. 2014;37(6):1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiu M, Austin PC, Manuel DG, Tu JV. Comparison of cardiovascular risk profiles among ethnic groups using population health surveys between 1996 and 2007. Can Med Assoc J. 2010;182(8):E301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011;34(8):1741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanaya AM, Kandula N, Herrington D, Budoff MJ, Hulley S, Vittinghoff Eet al. . Mediators of Atherosclerosis in South Asians Living in America (MASALA) study: objectives, methods, and cohort description. Clin Cardiol. 2013;36(12):713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merchant AT, Anand SS, Kelemen LE, Vuksan V, Jacobs R, Davis Bet al. . Carbohydrate intake and HDL in a multiethnic population. Am J Clin Nutr. 2007;85(1):225–30. [DOI] [PubMed] [Google Scholar]
- 16. Kelemen LE, Anand SS, Vuksan V, Yi Q, Teo KK, Devanesen Set al. . Development and evaluation of cultural food frequency questionnaires for South Asians, Chinese, and Europeans in North America. J Am Diet Assoc. 2003;103(9):1178–84. [DOI] [PubMed] [Google Scholar]
- 17. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi Let al. . Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kandula NR, Kanaya AM, Liu K, Lee JY, Herrington D, Hulley SBet al. . Association of 10-year and lifetime predicted cardiovascular disease risk with subclinical atherosclerosis in South Asians: findings from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. J Am Heart Assoc. 2014;3(5):e001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanaya AM, Kandula NR, Ewing SK, Herrington D, Liu K, Blaha MJet al. . Comparing coronary artery calcium among U.S. South Asians with four racial/ethnic groups: the MASALA and MESA studies. Atherosclerosis. 2014;234(1):102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanaya AM, Vittinghoff E, Lin F, Kandula NR, Herrington D, Liu Ket al. . Incidence and progression of coronary artery calcium in South Asians compared with 4 race/ethnic groups. J Am Heart Assoc. 2019;8(2):e011053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 22. Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJet al. . Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32(2):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55–60. [DOI] [PubMed] [Google Scholar]
- 24. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 25. WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- 26. Alman AC, Jacobs DR Jr, Lewis CE, Snell-Bergeon JK, Carnethon MR, Terry JGet al. . Higher pericardial adiposity is associated with prevalent diabetes: the Coronary Artery Risk Development in Young Adults study. Nutr Metab Cardiovasc Dis. 2016;26(4):326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EKet al. . Comparison of CT methods for determining the fat content of the liver. Am J Roentgenol. 2007;188(5):1307–12. [DOI] [PubMed] [Google Scholar]
- 28. Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8(6):805–13. [DOI] [PubMed] [Google Scholar]
- 29. Kanaya A, Ewing S, Vittinghoff E, Herrington D, Tegeler C, Mills Cet al. . Acculturation and subclinical atherosclerosis among U.S. South Asians: findings from the MASALA study. J Clin Exp Res Cardiol. 2014;1(1):102. [PMC free article] [PubMed] [Google Scholar]
- 30. Eyres L, Eyres MF, Chisholm A, Brown RC. Coconut oil consumption and cardiovascular risk factors in humans. Nutr Rev. 2016;74(4):267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DVet al. . Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51(6):1979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169(3):166–76. [DOI] [PubMed] [Google Scholar]
- 33. Kim TH, Yu SH, Choi SH, Yoon JW, Kang SM, Chun EJet al. . Pericardial fat amount is an independent risk factor of coronary artery stenosis assessed by multidetector-row computed tomography: the Korean Atherosclerosis Study 2. Obesity. 2011;19(5):1028–34. [DOI] [PubMed] [Google Scholar]
- 34. Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RSet al. . Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117(5):605–13. [DOI] [PubMed] [Google Scholar]
- 35. Shah AD, Kandula NR, Lin F, Allison MA, Carr J, Herrington Det al. . Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: results from the MASALA and MESA studies. Int J Obes (Lond). 2016;40(4):639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lear SA, Chockalingam A, Kohli S, Richardson CG, Humphries KH. Elevation in cardiovascular disease risk in South Asians is mediated by differences in visceral adipose tissue. Obesity. 2012;20(6):1293–300. [DOI] [PubMed] [Google Scholar]
- 37. Garg SK, Lin F, Kandula N, Ding J, Carr J, Allison Met al. . Ectopic fat depots and coronary artery calcium in South Asians compared with other racial/ethnic groups. J Am Heart Assoc. 2016;5(11):e004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flowers E, Lin F, Kandula NR, Allison M, Carr JJ, Ding Jet al. . Body composition and diabetes risk in South Asians: findings from the MASALA and MESA studies. Diabetes Care. 2019;42(5):946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah RV, Murthy VL, Allison MA, Ding J, Budoff M, Frazier-Wood ACet al. . Diet and adipose tissue distributions: the Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2016;26(3):185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Misra R, Balagopal P, Raj S, Patel TG. Vegetarian diet and cardiometabolic risk among Asian Indians in the United States. J Diabetes Res. 2018;2018:1675369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gadgil MD, Anderson CA, Kandula NR, Kanaya AM. Dietary patterns are associated with metabolic risk factors in South Asians living in the United States. J Nutr. 2015;145(6):1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jin Y, Kanaya AM, Kandula NR, Rodriguez LA, Talegawkar SA. Vegetarian diets are associated with selected cardiometabolic risk factors among middle-older aged South Asians in the United States. J Nutr. 2018;148(12):1954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim H, Lee K, Rebholz CM, Kim J. Plant-based diets and incident metabolic syndrome: results from a South Korean prospective cohort study. PLoS Med. 2020;17(11):e1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q. Association between plant-based dietary patterns and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA Intern Med. 2019;179(10):1335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willett W, Rockstrom J, Loken B, Springmann M, Lang T, Vermeulen Set al. . Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet North Am Ed. 2019;393(10170):447–92. [DOI] [PubMed] [Google Scholar]
- 46. National family health survey (NFHS-4). Ministry of Health and Family Welfare; 2015. [Google Scholar]
- 47. Blackstone NT, El-Abbadi NH, McCabe MS, Griffin TS, Nelson ME. Linking sustainability to the healthy eating patterns of the dietary guidelines for Americans: a modelling study. Lancet Planet Health. 2018;2(8):e344–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will not be made publicly available. Further information, including the procedures to obtain and access data from the MASALA (Mediators of Atherosclerosis in South Asians Living in America) study, is described at https://www.masalastudy.org/for-researchers.