Abstract
Background
Immune checkpoint inhibitor (ICI) therapy can be complicated by gastrointestinal adverse events (AEs). Similarly, gastrointestinal AEs have been reported with the use of serine/threonine-protein kinase B-Raf (BRAF) and mitogen-activated protein kinase kinase (MEK) inhibitor therapy. We investigated the characteristics and management of gastrointestinal AEs related to sequential ICI and BRAF/MEK inhibitor therapy.
Methods
We identified 255 adult cancer patients who received both BRAF/MEK inhibitor therapy and ICI therapy between 2014 and 2021. Thirty-two eligible patients had gastrointestinal AEs after receiving both therapies and were categorized based on the order of their administration. Their clinical characteristics, evaluation, treatment and outcomes were compared.
Results
Of the 32 eligible patients, 18 (56.3%) received ICI therapy followed by BRAF/MEK inhibitors (early ICI group), and 14 (44.8%) received BRAF/MEK inhibitor therapy followed by ICI (early BRAF/MEK inhibitor group). Compared with the early BRAF/MEK inhibitor group, the early ICI group had higher rates of grade 3-4 diarrhea (50.0% vs. 14.3%, P=0.047) and grade 3-4 colitis (38.9% vs. 0%, P=0.010). The early ICI group had a later onset of colitis (347.5 vs. 84.5 days, P=0.011) and a higher rate of hospitalization at initial colitis presentation (100% vs. 71.4%, P=0.028). Patients in the early ICI group were more likely to have diarrhea or colitis recurrence (69.2% vs. 9.1%, P=0.019) and re-hospitalization for colitis (38.9% vs. 0%, P=0.010).
Conclusion
The sequential exposure of BRAF/MEK therapy after ICI may contribute to a more aggressive clinical profile of gastrointestinal toxicities that may warrant a more aggressive management strategy.
Keywords: Immune checkpoint inhibitor, BRAF inhibitor, MEK inhibitor, colitis, immune-related adverse event
Introduction
Diarrhea and colitis are common adverse events (AEs) associated with use of immune checkpoint inhibitors (ICIs), including inhibitors of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death-ligand 1 (PD-L1) [1-3]. Targeted therapies, such as inhibitors of serine/threonine-protein kinase B-Raf (BRAF) and selective mitogen-activated protein kinase kinase (MEK), have been used to treat patients with select cancers with activating mitogen-activated protein kinase pathway mutations [4-7]. Multiple combinations of BRAF and MEK inhibitors have been approved by the US Food and Drug Administration for use in patients with melanoma, lung, colon or thyroid cancers with BRAF mutations [6-7]. In clinical trials, gastrointestinal AEs, including diarrhea, abdominal pain, nausea and vomiting, were common, and were noted to be worse in patients who received BRAF and MEK inhibitors in combination than those who received either agent alone [6-8]. The mechanism by which BRAF and MEK inhibitors induce inflammation of the gastrointestinal tract remains unclear, and reports of severe colonic inflammation with ulceration secondary to these therapies are rare.
More recently, several clinical trials have investigated the combined use of BRAF/MEK inhibitors and ICIs to achieve more durable responses in patients with advanced cancers [9-12]. Multiple studies have documented cases of colonic inflammation in patients receiving these combination regimens, which in some instances led to severe presentations [13,14]. In one of these studies, colonic inflammation improved with the cessation of BRAF/MEK inhibitor therapy, which strongly suggests that BRAF and MEK inhibition influences the clinical course of colitis [13]. However, how the combined use of BRAF/MEK inhibitors and ICIs contributes to these gastrointestinal AEs and affects their severity in different settings remains unclear. In our study, we assessed the clinical characteristics, disease courses, and outcomes of patients who had gastrointestinal AEs after sequential exposure to BRAF/MEK inhibitors and ICIs.
Patients and methods
Patient selection and data collection
We obtained Institutional Review Board approval and screened 255 patients who received both BRAF/MEK inhibitor therapy and ICI therapy and were diagnosed with colitis between May 1, 2014, and March 1, 2021. Patients included in the study: 1) were older than 18 years; 2) had a cancer diagnosis and received both ICIs and BRAF/MEK inhibitors concurrently or sequentially; 3) had gastrointestinal symptoms of diarrhea or colitis, deemed to be related to gastrointestinal AEs, between the initiation of either ICI or BRAF/MEK inhibitor therapy and 3 months after its completion; and 4) had positive evidence of colitis on either endoscopy or abdominal imaging. These patients were further categorized into either an early ICI group (patients who received ICI therapy before BRAF/MEK therapy) or an early BRAF/MEK inhibitor group (patients who received BRAF/MEK therapy before ICI therapy). Patients with established alternative etiologies such as infection were excluded.
Patients’ demographic data (including age, sex and race), oncological data (including cancer type, stage and treatment), and medical comorbidities were extracted from electronic health records and endoscopy databases. Malignancy staging was assessed in accordance with the American Joint Committee on Cancer’s Cancer Staging Manual, 8th edition [15].
Evaluation of gastrointestinal AEs
Gastrointestinal AEs were assessed in terms of the duration and severity of diarrhea and colitis, based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0 [16]. When available, endoscopic findings on initial and repeat colonoscopies were noted. Endoscopic findings were categorized as ulcers, non-ulcerative inflammation (e.g., erythema, friability, erosions, inflammatory exudate, loss of vascular pattern, edema), or normal. Histologic findings were categorized as normal, acute active colitis (e.g., cryptitis, crypt abscess, apoptosis, eosinophilic infiltration, intraepithelial neutrophil infiltration), chronic active colitis (e.g., crypt architectural distortion, basal lymphoplasmacytosis, Paneth cell metaplasia), or microscopic colitis (e.g., intraepithelial lymphocytic infiltration, subepithelial collagen bands). These endoscopic and histologic categorizations were determined as we described previously [17]. Radiological findings of colitis from computed tomography, magnetic resonance imaging, X-ray, fluoroscopy and/or ultrasonography studies were reviewed and documented.
Treatment of gastrointestinal AEs and outcomes
The treatment of diarrhea and colitis included supportive measures, such as anti-diarrheal agents (e.g., loperamide, cholestyramine, mesalamine), and more aggressive approaches, such as corticosteroids, fecal microbiota transplantation (FMT), and selective immunosuppressive therapy (SIT) with infliximab or vedolizumab. The clinical outcomes of gastrointestinal AEs included the need for hospitalization or intensive care unit admission, the duration of hospital stay, symptom response (defined as resolution of diarrhea/colitis or improvement to CTCAE grade 1) and remission (defined as the maintenance of symptom response following completion of steroid taper), the recurrence of gastrointestinal symptoms, cancer therapy resumption, cancer outcomes, and death.
Statistical analysis
Categorical variables were summarized with percentages, and continuous variables were summarized with medians and interquartile ranges. The Fisher exact and chi-squared tests were used to assess associations between categorical variables. Continuous variables were compared using the Mann-Whitney U test. P-values of 0.05 or less were considered statistically significant. Statistical analysis was conducted using the SPSS (version 24.0; IBM) software.
Results
Patient characteristics
The patient selection flowchart is shown in Fig. 1. Of the 255 patients who received both ICI and BRAF/MEK inhibitor therapy during the study period, 223 did not meet the inclusion criteria; of the remaining 32 patients, 18 received ICI therapy before BRAF/MEK inhibitor therapy (early ICI group), and 14 received BRAF/MEK inhibitor therapy before ICI therapy (early BRAF/MEK inhibitor group). These 32 patients’ characteristics are provided in Table 1. Most patients in the early ICI group had malignant melanoma (94.4%), whereas patients in the early BRAF/MEK inhibitor group had either melanoma (64.3%) or endocrine tumors (35.7%) (P=0.018). Patients in the early ICI group had a higher median number of ICI therapy cycles before the onset of colitis, compared to the patients in the early BRAF/MEK inhibitor group (10 vs. 4, P=0.017). The ICIs and BRAF/MEK inhibitors the patients received are listed in Supplementary Tables 1 (129KB, pdf) and 2 (129KB, pdf) . Four patients developed ICI-mediated colitis before receiving any targeted therapy and later received BRAF/MEK inhibitor therapy; these patients were not included in the comparative analysis and were analyzed separately.
Figure 1.
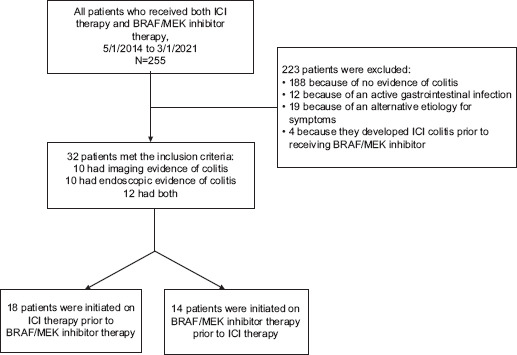
Flowchart showing the process of patient selection
ICI, immune checkpoint inhibitor
Table 1.
Patient characteristics
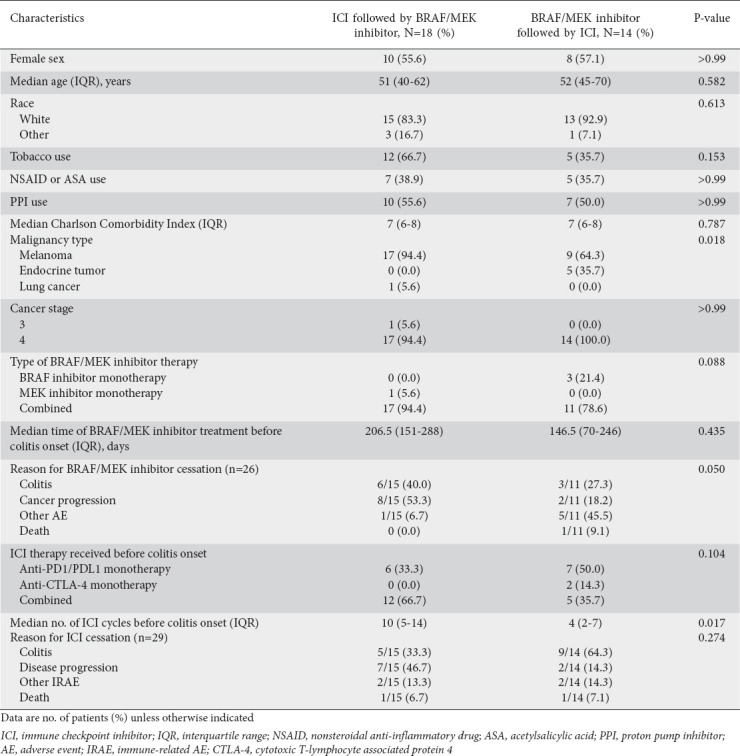
Characteristics of colitis presentation and treatment
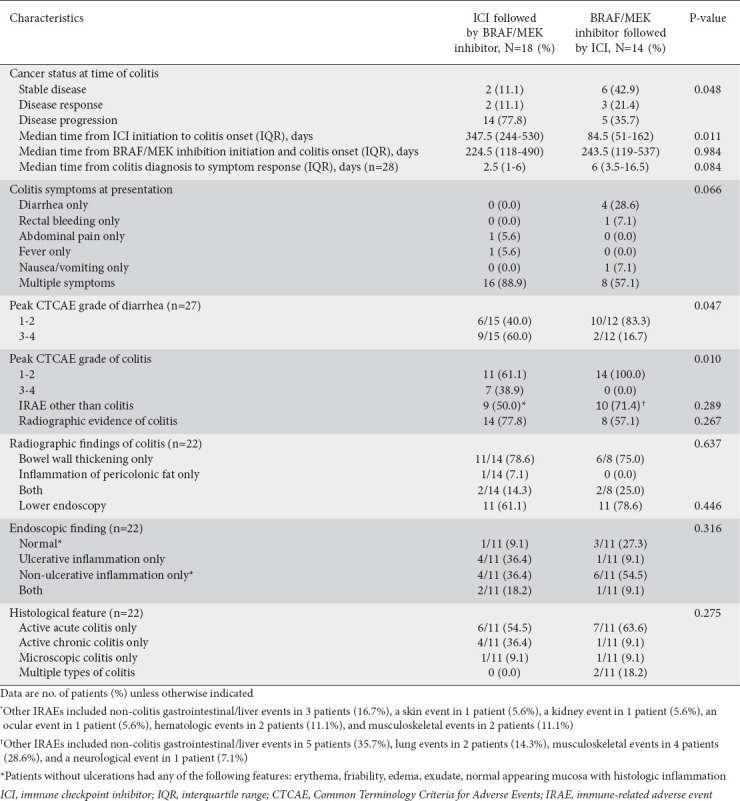
The characteristics of the patients’ colitis presentation are given in Table 2. Compared with the early BRAF/MEK inhibitor group, the early ICI group had a higher rate of cancer progression at the time of colitis onset (77.8% vs. 35.7%, P=0.048). Patients in the early ICI group were also more likely to have multiple colitis symptoms (88.9% vs. 57.1%, P=0.066) and abdominal pain (72.2% vs. 35.7%, P=0.072) at the initial presentation of colitis, and had significantly higher rates of grade 3-4 diarrhea (50.0% vs. 14.3%, P=0.047) and grade 3-4 colitis (38.9% vs. 0.0%, P=0.010). Of the 32 patients, 22 (68.8%) underwent radiologic evaluation for gastrointestinal symptoms; the early ICI and early BRAF/MEK inhibitor groups had similar rates of bowel wall thickening. Twenty-two (68.8%) patients underwent endoscopy; the early ICI group had a higher rate of ulceration than the early BRAF/MEK group (54.5% vs. 18.2%, P=0.182). Patients without ulcerations had reported any of the following features: erythema, friability, edema, exudate, or normal appearing mucosa with histologic inflammation. In both the early ICI and early BRAF/MEK inhibitor groups, the predominant histological finding was active acute inflammation (54.5% and 63.6%, respectively). However, 36.4% of the patients in the early ICI group had active chronic inflammation.
Table 2.
Characteristics of colitis presentation
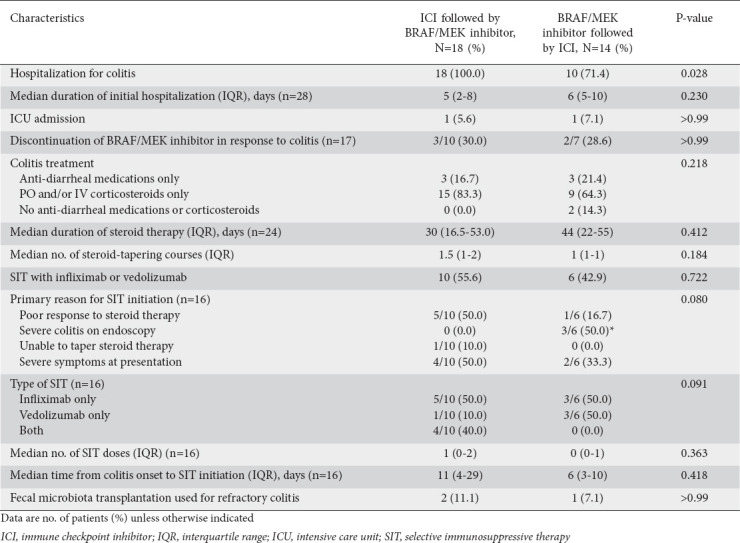
The characteristics of the patients’ colitis treatment are given in Table 3. All patients in the early ICI group, but only 71.4% of patients in the early BRAF/MEK inhibitor group, required hospitalization for initial colitis (P=0.028). However, the groups had similar median hospitalization durations (5 vs. 6 days, P=0.230) and rates of ICU admission (5.6% vs. 7.1%, P>0.99). In both groups, most patients received steroids (64.3% and 83.3%, respectively) and many patients (42.9% and 55.6%, respectively) received SIT; the groups had similar numbers of SIT doses. The early ICI group had a greater proportion of patients who initiated SIT owing to a poor response to steroid therapy (50.0% vs. 16.7%, P=0.080). The median durations of steroid therapy for the early ICI and early BRAF/MEK inhibitor groups were 30 and 44 days, respectively. One early ICI patient (7.1%) and 2 early BRAF/MEK inhibitor patients (11.1%) received FMT for refractory colitis.
Table 3.
Characteristics of colitis treatment
Colitis outcomes
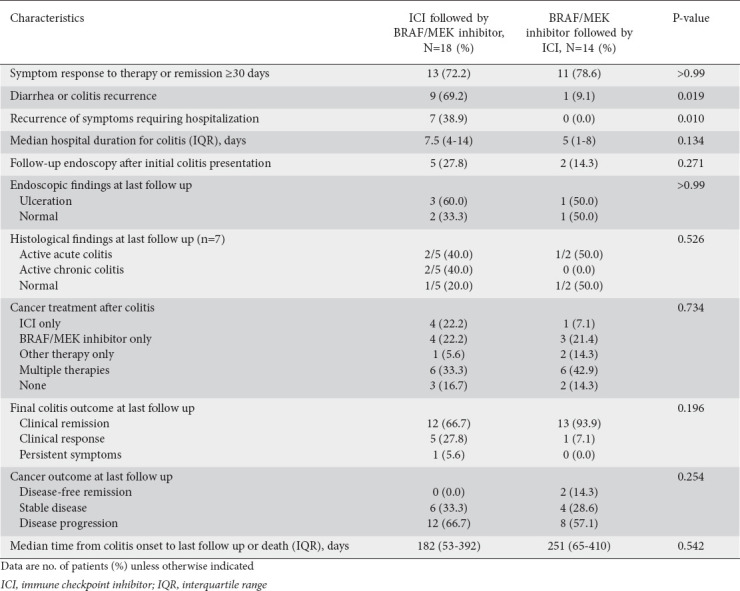
The characteristics of the patients’ colitis outcomes are given in Table 4. The early ICI and early BRAF/MEK inhibitor groups had similar rates of symptom response or remission (72.2% vs. 78.6%, P>0.99). The early ICI group had a higher rate of diarrhea or colitis recurrence (defined as the recurrence of colitis symptoms following the resolution of initial colitis symptoms; 69.2% vs. 9.1%, P=0.019) and a higher rate of hospitalization for colitis recurrence (38.9% vs. 0.0%, P=0.010). Among 7 patients who underwent repeat endoscopic evaluation, 5 were found to have persistent ulceration and/or active inflammation. Twenty-seven (84.4%) patients resumed cancer treatment after colitis resolution. Twenty (62.5%) patients had cancer progression at their final follow up.
Table 4.
Characteristics of colitis outcomes
Outcomes of excluded patients
The clinical characteristics of the 4 patients who developed colitis following ICI therapy and later received BRAF/MEK inhibitor therapy are presented in Supplementary Table 3 (129KB, pdf) . All 4 patients had melanoma and received ICI therapy that precipitated grade 1-2 colitis. All 4 patients achieved clinical remission of their ICI-mediated colitis with medical therapy and later received BRAF/MEK inhibitor therapy. None of these patients developed recurrence or complications of colitis after their initial presentation.
Discussion
Gastrointestinal AEs have been more frequently encountered with the increased use of ICIs and BRAF/MEK inhibitors for advanced cancers. In this study, we investigated the characteristics and management of gastrointestinal AEs related to sequential ICI and BRAF/MEK inhibitor therapies. We found that, compared to those who received BRAF/MEK inhibitors followed by ICIs, cancer patients who received ICIs followed by BRAF/MEK inhibitors had higher rates of high-grade diarrhea and colitis, colitis-related hospitalization and colitis recurrence. These findings suggest a unique pathologic process that can affect the clinical course of colitis and its management and outcomes.
Half of all malignant melanomas carry activating BRAF mutations, which cause a constitutional activation of the MAP kinase pathway that is important for the regulation of cellular proliferation [18]. BRAF inhibitors target the BRAF kinase, whereas MEK inhibitors act downstream of BRAF in the MAP kinase pathway. The mechanisms by which BRAF/MEK inhibitors can induce or exacerbate colonic inflammation are not clear; however, the MAP kinase pathway has been implicated in the proliferation, differentiation, and survival of gastrointestinal epithelium [19]. Furthermore, recent data suggest that MEK and its downstream effectors help regulate the claudin-dependent assembly of functional tight junctions in intestinal epithelial cells and that their dysregulation leads to gastrointestinal toxicity [20]. Gastrointestinal AEs associated with combination BRAF/MEK inhibitor therapy are common and include nausea, vomiting, constipation and diarrhea; however, only 2% of patients who receive combination BRAF/MEK inhibitor therapy develop grade 3-4 diarrhea [6]. This rate is similar to the incidence of grade 3-4 diarrhea (2.3%) reported in a meta-analysis of ICI use [21]. Case studies have described colitis induced by BRAF/MEK inhibitor therapy alone or in combination with ICI therapy and have suggested that withholding BRAF/MEK inhibitor therapy helps reduce colonic inflammation [13,14].
Few studies have investigated the effect of the sequential use of ICIs and BRAF/MEK inhibitors on immune-related gastrointestinal AEs. Our study suggests that the addition of BRAF/MEK inhibitors after ICI therapy results in a more severe presentation of colitis, probably owing to several factors. First, we found that the early ICI and early BRAF/MEK inhibitor groups differed in their predominant cancer types and stages, with the early ICI group having a larger proportion of patients who had cancer progression at the time of their colitis diagnosis and patients who stopped BRAF/MEK inhibitor therapy owing to cancer progression. Whether these findings are related to differences in the groups’ cancer types and/or treatment dosing is unclear; however, no differences were observed between the groups in terms of specific ICI or BRAF/MEK inhibitor use or overall cancer outcomes. Second, previous studies have highlighted that patients receiving combined CTLA-4 and PD-1/PD-L1 inhibitor therapy have a higher risk of gastrointestinal AEs than those receiving CTLA-4 or PD-1/PD-L1 inhibitor monotherapy [22,23]. In our study, a larger proportion of patients in the early ICI group than in the early BRAF/MEK group received combination CTLA-4 and PD-1/PD-L1 inhibitor therapy. This difference, though not statistically significant, may reflect an institutional practice pattern, and we speculate that varied therapeutic combinations may in fact alter the risk for gastrointestinal AEs.
We also found that patients who received ICIs before BRAF/MEK inhibitors had a relatively delayed initial presentation of colitis, albeit one with an increased severity. We hypothesize that, in these patients, the addition of BRAF/MEK inhibitors potentiates an ongoing, subclinical, ICI-mediated colonic inflammation that manifests as breakthrough severe symptoms. Previous in vivo studies have shown that the addition of BRAF inhibitors to CTLA-4 blockade can paradoxically potentiate T-cell expansion, thereby predisposing patients to an increased risk of toxicity [24]. More studies are needed to characterize this effect; however, our data suggest that it may be beneficial to evaluate and treat patients for both clinically significant and subclinical colonic inflammation before switching them between different cancer regimens, such as ICI and BRAF/MEK inhibitor therapy, that could trigger gastrointestinal toxicities. For example, it may be helpful to screen patients receiving ICI therapy for fecal biomarkers of inflammation (e.g., lactoferrin, calprotectin), to help identify patients at higher risk of developing colitis, before initiating BRAF/MEK inhibitor therapy. In our cohort, we identified 4 patients who initially developed ICI-mediated colitis, and then received BRAF/MEK inhibitors after colitis remission without further colitis recurrence afterwards. The medical treatment of colitis and possible resolution of therapy-related colonic inflammation may have alleviated these patients’ risk of AEs, as we observed in the main cohort. A future prospective study could be an effective way to test this hypothesis.
Mourad et al described several cases of colitis associated with MEK inhibitor monotherapy [13]. In those cases, colitis resolved after MEK inhibitor therapy was stopped. Other researchers have reported that patients receiving BRAF inhibitors in combination with MEK inhibitors have higher rates of gastrointestinal AEs than those receiving BRAF inhibitors alone [6]. For patients who have received ICIs, further studies to determine the incidences of gastrointestinal AEs among those receiving BRAF inhibitor monotherapy and those receiving MEK inhibitor monotherapy could help determine which subtype of targeted therapy would be the safest therapeutic alternative.
While ICI-mediated colitis is well-described in the literature, BRAF/MEK inhibitor-related colitis has been reported only in small case reports, which described similar endoscopic findings ranging from erythema to ulceration [12,13]. Subtle histological differences distinguish the 2 processes, as BRAF/MEK inhibitor-related inflammation typically does not display the intraepithelial lymphocytes or epithelial apoptotic bodies commonly seen in ICI-related inflammation, and presents with a higher CD4/CD8 ratio on immunostaining [25,26]. Additional clarification and characterization are needed to further differentiate the 2 etiologies on endoscopy and histology.
The current standard of care for patients with ICI-mediated colitis is 4-6 weeks of corticosteroids followed by SIT with infliximab or vedolizumab [27-30]. We previously showed that an earlier initiation of SIT and a greater total number of SIT infusions improved the outcomes of patients with ICI-mediated colitis.[23] More recently, ustekinumab and tofacitinib have been employed with favorable results in select patients with refractory ICI-mediated colitis [31,32]. On the basis of our data and our understanding of the underlying molecular mechanism of action, we hypothesize that patients who develop colitis after receiving ICIs followed by BRAF/MEK inhibitors also benefit from SIT in addition to the withholding of targeted therapy. Further study will elucidate the best strategy for treating patients who develop colitis after receiving these combination treatment regimens.
Several recent studies have elucidated the role that FMT has in treating Clostridioides difficile-associated colitis and inflammatory bowel disease through its modulation of the gut microbiome [33]. Studies in animal models have provided evidence that variation in the gut microbiome is associated with differences in response to ICI therapy [34,35]. Case studies have demonstrated the potential role of FMT in treating ICI-mediated colitis refractory to standard therapy [36], showing that FMT improved colitis symptoms and endoscopic healing. However, the long-term effects of FMT on gut bacterial taxa are unclear. In our cohort, 3 patients who received FMT for refractory colitis did not have further recurrence. More research is needed to assess the efficacy of FMT in patients with colitis who receive sequential ICI and BRAF/MEK inhibitor therapy.
Our study had several limitations. Firstly, because this was a retrospective cohort analysis of single-center data and had a limited sample size, the analysis was underpowered in terms of the impact of ethnicity and cancer type/status on outcomes. Secondly, although the types of ICIs or BRAF/MEK inhibitors used in the early ICI and early BRAF/MEK inhibitor groups did not differ significantly, individual types of these agents may have been under-represented in our small cohort. Furthermore, the impact of dosing differences of anti-neoplastic therapy used between the 2 groups could not be characterized in our analysis, given the high complexity of the patients’ overall clinical disease course. In addition, given its highly-selected patient population, this study had a risk of patient selection bias.
In conclusion, our findings characterize the gastrointestinal toxicities among patients who received combined ICI and BRAF/MEK inhibitor therapy for advanced malignancies and suggest that the sequence of these 2 categories of treatments may play a role in the clinical presentation and management of the gastrointestinal toxicities. Compared with those who received ICI therapy after BRAF/MEK inhibitors, patients who received BRAF/MEK inhibitor therapy after ICI had a more aggressive clinical profile, with higher rates of high-grade diarrhea and colitis, hospitalization, diarrhea and colitis recurrence, and re-hospitalization for gastrointestinal AEs. Large-scale prospective studies are necessary to corroborate our observations and provide further knowledge for the management of these challenging AEs.
Summary Box.
What is already known:
Immune-mediated diarrhea and colitis are among the most frequently encountered adverse effects (AEs) related to immune checkpoint inhibitor (ICI) therapy
Similarly, gastrointestinal AEs are also reported with the use of serine/threonine-protein kinase B-Raf (BRAF) and mitogen-activated protein kinase kinase (MEK) inhibitors
Despite the increased interest in the combined use of ICIs and BRAF/MEK inhibitors in patients with advanced cancers, data on the characteristics of gastrointestinal AEs in this population are quite limited
What the new findings are:
Our retrospective study provides insight into the general presentation and disease course of gastrointestinal AEs among patients who receive combinations of ICIs and BRAF/MEK inhibitors
In particular, we present novel findings of a more aggressive clinical profile of gastrointestinal AE in patients who receive BRAF/MEK inhibitor therapy following ICI therapy, compared with those who received BRAF/MEK inhibitor therapy first
Our findings suggest a unique pathologic process that can affect the clinical disease course of colitis and its management and outcomes
Acknowledgment
Medical editing of this paper was provided by Editing Services, Research Medical Library, at The University of Texas MD Anderson Cancer Center.
Biography
Baylor College of Medicine, Houston; The University of Texas Health Science Center at Houston; The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Footnotes
Conflict of Interest: Y. Wang: consulting fee for Sorriso, MabQuest, Azur Rx
References
- 1.Tang T, Abu-Sbeih H, Luo W, et al. Upper gastrointestinal symptoms and associated endoscopic and histological features in patients receiving immune checkpoint inhibitors. Scand J Gastroenterol. 2019;54:538–545. doi: 10.1080/00365521.2019.1594356. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Abu-Sbeih H, Mao E, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies:retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37. doi: 10.1186/s40425-018-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou F, Abu-Sbeih H, Ma W, et al. Association of chronic immune-mediated diarrhea and colitis with favorable cancer response. J Natl Compr Canc Netw. 2020;19:700–708. doi: 10.6004/jnccn.2020.7647. [DOI] [PubMed] [Google Scholar]
- 4.Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS):a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–615. doi: 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 5.Greco A, Safi D, Swami U, et al. Efficacy and adverse events in metastatic melanoma patients treated with combination BRAF plus MEK inhibitors versus BRAF inhibitors:a systematic review. Cancers (Basel) 2019;11:1950. doi: 10.3390/cancers11121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer:new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37:3183–3199. doi: 10.1038/s41388-018-0171-x. [DOI] [PubMed] [Google Scholar]
- 8.Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM):updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–1260. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 9.Gutzmer R, Stroyakovs.kiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150):primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835–1844. doi: 10.1016/S0140-6736(20)30934-X. [DOI] [PubMed] [Google Scholar]
- 10.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies:optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 11.Dummer R, Lebbé C, Atkinson V, et al. Combined PD-1, BRAF and MEK inhibition in advanced BRAF-mutant melanoma:safety run-in and biomarker cohorts of COMBI-i. Nat Med. 2020;26:1557–1563. doi: 10.1038/s41591-020-1082-2. [DOI] [PubMed] [Google Scholar]
- 12.Trojaniello C, Vitale MG, Ascierto PA. Triplet combination of BRAF, MEK and PD-1/PD-L1 blockade in melanoma:the more the better? Curr Opin Oncol. 2021;33:133–138. doi: 10.1097/CCO.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 13.Mourad N, Lourenço N, Delyon J, et al. PATIO group. Severe gastrointestinal toxicity of MEK inhibitors. Melanoma Res. 2019;29:556–559. doi: 10.1097/CMR.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 14.Minor DR, Puzanov I, Callahan MK, Hug BA, Hoos A. Severe gastrointestinal toxicity with administration of trametinib in combination with dabrafenib and ipilimumab. Pigment Cell Melanoma Res. 2015;28:611–612. doi: 10.1111/pcmr.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual, eighth edition. New York: Springer International Publishing; 2017. [Google Scholar]
- 16.National cancer institute common toxicity criteria for adverse events (CTCAE) version 5.0. [[Accessed 27 October 2022]]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf .
- 17.Wang Y, Abu-Sbeih H, Mao E, et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis. 2018;24:1695–1705. doi: 10.1093/ibd/izy104. [DOI] [PubMed] [Google Scholar]
- 18.Curti BD, Faries MB. Recent advances in the treatment of melanoma. N Engl J Med. 2021;384:2229–2240. doi: 10.1056/NEJMra2034861. [DOI] [PubMed] [Google Scholar]
- 19.Osaki LH, Gama P. MAPKs and signal transduction in the control of gastrointestinal epithelial cell proliferation and differentiation. Int J Mol Sci. 2013;14:10143–10161. doi: 10.3390/ijms140510143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients:a systematic review and meta-analysis. Oncoimmunology. 2017;6:e1344805. doi: 10.1080/2162402X.2017.1344805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi: 10.3389/fphar.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callahan MK, Masters G, Pratilas CA, et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunol Res. 2014;2:70–79. doi: 10.1158/2326-6066.CIR-13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelsomino F, Federico Di A, Tardio ML, et al. Drug-induced colitis on BRAF and MEK inhibitors for BRAF V600E-mutated non-small cell lung cancer:a case report. Invest New Drugs. 2022;40:190–193. doi: 10.1007/s10637-021-01166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JH, Pezhouh MK, Lauwers GY, Masia R. Histopathologic features of colitis due to immunotherapy with anti-PD-1 antibodies. Am J Surg Pathol. 2017;41:643–654. doi: 10.1097/PAS.0000000000000829. [DOI] [PubMed] [Google Scholar]
- 27.Brahmer JR, Lacchetti C, Schneider BJ, et al. National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy:American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puzanov I, Diab A, Abdallah K, et al. Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors:consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson JA. New NCCN guidelines:recognition and management of immunotherapy-related toxicity. J Natl Compr Canc Netw. 2018;16:594–596. doi: 10.6004/jnccn.2018.0047. [DOI] [PubMed] [Google Scholar]
- 30.Abu-Sbeih H, Ali FS, Wang X, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2019;7:93. doi: 10.1186/s40425-019-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas AS, Ma W, Wang Y. Ustekinumab for refractory colitis associated with immune checkpoint inhibitors. N Engl J Med. 2021;384:581–583. doi: 10.1056/NEJMc2031717. [DOI] [PubMed] [Google Scholar]
- 32.Esfahani K, Hudson M, Batist G. Tofacitinib for refractory immune-related colitis from PD-1 therapy. N Engl J Med. 2020;382:2374–2375. doi: 10.1056/NEJMc2002527. [DOI] [PubMed] [Google Scholar]
- 33.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2011;9:88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]
- 34.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Wiesnoski DH, Helmink BA, et al. Author Correction:Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2019;25:188. doi: 10.1038/s41591-018-0305-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


