Abstract
Background
Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) promised to transform the management of peritoneal carcinomatosis (PC). Forty years since the introduction of the technique, published data from randomized controlled trials (RCTs) remain scarce. We assessed the cumulative comprehensive available evidence on the use of HIPEC in gastrointestinal (GI) and biliary tract malignancies and established the current benchmark for GI HIPEC research in both the prevention and treatment of peritoneal metastases.
Methods
RCTs were identified through a systematic search of Medline, Cochrane and Embase databases. Overall survival and progression-free survival were the outcomes of interest.
Results
The search resulted in 13 RCTs for gastric cancer (10 on prophylactic and 3 on therapeutic HIPEC), 4 for colorectal cancer (2 on prophylactic and 2 on therapeutic HIPEC), and 1 for pancreatic cancer. No RCTs were identified that included other types of GI or biliary tract cancers. Current randomized evidence does not support any overall survival benefit from the use of HIPEC in the adjuvant setting for gastric cancer or for colorectal cancer in any setting. Despite the survival benefit noticed in the treatment of PC from gastric cancer (risk ratio 0.85, 95% confidence interval 0.77-0.93; P<0.001), the results were derived from only 190 patients.
Conclusions
The current evidence from RCTs does not support the use of HIPEC in the treatment/prevention of PC in GI and biliary tract malignancies. HIPEC should continue to be considered experimental until level 1 evidence from properly designed international multicenter studies becomes available.
Keywords: Hyperthermic intraperitoneal chemotherapy, gastrointestinal cancer, gastric cancer, biliary tract cancer, colorectal cancer
Introduction
Peritoneal carcinomatosis (PC) is characterized by the presence of significant abdominal and constitutional symptoms, low treatment response rates, and a poor prognosis. The use of aggressive locoregional treatment combining cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) has been suggested to improve patients’ outcomes [1]. Cytoreductive surgery (CRS) consists of the complete removal of the macroscopic disease, while HIPEC involves chemotherapy in the peritoneal cavity, heated to a desirable temperature, ranging from 41.5-43°C, for 30-120 min, according to the investigator and the type of drug [2]. The rationale behind the procedure is to take advantage of the synergy between hyperthermia and local compartmental intra-abdominal chemotherapy, as well as to minimize the residual disease after the macroscopic resection [3]. The procedure has long been considered a “double-edged sword”, as the reduction in systemic toxicity is followed by a notable increase in postoperative morbidity [4]. For these reasons, the application of CRS and HIPEC in gastrointestinal (GI) malignancies has been a research topic of increasing interest.
Gastric cancer is the sixth most common cancer globally [5] and PC is the leading cause of death after a potential curative resection [6]. HIPEC has been investigated both for prevention of PC in high-risk patients (harboring serosal invasion and lymph node metastasis), and for treatment of patients with established PC [7].
Colorectal cancer is the fourth most common cancer [5]. PC in colorectal cancer can be detected synchronously or metachronously, and is associated with worse overall survival when compared to other metastatic sites [8]. Small HIPEC studies showed promising results in both PC prevention and treatment among patients with colorectal cancer, sparking a growing enthusiasm for the procedure [9].
Malignant peritoneal dissemination can also originate from appendiceal neoplasms, hepatobiliary, pancreatic and neuroendocrine tumors, and from pseudomyxoma peritonei (PMP). In view of its potential benefit in the PMP setting, CRS plus HIPEC has recently been proposed to represent the new standard of care for this tumor type [10].
The evolving investigational interest in CRS/HIPEC is also underscored by its experimental use in managing peritoneal sarcomatosis from GI tumors of stromal origin, as well as peritoneal mesotheliomas [11-13]. Despite the growing research interest in CRS/HIPEC across GI and biliary tract malignancies, high quality data from randomized controlled trials (RCTs) are still scarce. The scientific evidence associated with this topic remains far from level 1, with published meta-anlyses being mainly dominated by non-randomized cohorts, case-control and retrospective studies [14,15]. Considering all of the above-mentioned challenges and the need to shed light on the clinical evidence for CRS/HIPEC application from unbiased data, we performed a systematic review of the literature to summarize the existing comprehensive evidence from RCTs on the use of HIPEC in PC from GI and biliary tract malignancies.
Materials and methods
Data sources and selection
In December 2021, we performed a systematic search of Medline, Cochrane and Embase databases for RCTs of any duration and design comparing HIPEC treatment with any other therapy in patients who had either PC or a high risk of developing peritoneal metastases. The search string was as follows: (colorectal OR colon OR rectal OR gastric OR stomach OR appendiceal OR appendix OR pancreas OR biliary OR cholangeal OR gallbladder OR mesothelioma OR pseudomyxoma) AND (neoplasm* OR cancer* OR tumor*) AND (HIPEC OR IPHP OR IHC OR CHPP OR hyperthermic OR hyperthermic intraperitoneal chemotherapy OR hyperthermic intraperitoneal perfusion OR intraperitoneal hyperthermic chemoperfusion OR continuous hyperthermic peritoneal perfusion) AND (random*).
If no eligible RCTs for a specific cancer site were found, we reported on any randomized trial of HIPEC for this tumor type as an illustration of RCT construction feasibility. If no other RCTs were detected, a recent cohort study of interest was mentioned if available. All studies identified in our search were screened by 2 independent investigators (PF, NF) for eligibility, based on titles and abstracts. Any article identified as having the potential to fulfill our inclusion criteria underwent full-text evaluation. If no consensus on eligibility was reached between the 2 investigators, a third investigator (FK) was consulted. Forward and backward citation analysis supplemented the database search. Cohort and case-control studies, animal studies, as well as non-English studies, were excluded. The overall survival and progression-free survival were the outcomes of interest. This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline [16].
Data extraction
Two authors (PF, AG) independently extracted relevant data from included studies using a standardized extraction form. Any disagreement was resolved by consensus. Multiple records reporting on the same trial (e.g., at different time points of follow up) were considered as a single trial for all analyses. In case of doubly reported data, those from the most-informative publication and highest level of evidence were used. The data extracted included: first author, year of publication, chronological period of the study, study population, number of patients, details of experimental and control arm therapy, HIPEC technique, median follow up, survival rates, number of deaths, and number of disease progression incidences.
Statistical analysis
When the number of eligible RCTs permitted it, a meta-analysis was conducted, otherwise descriptive statistics were used. Overall survival was characterized by the proportion of patient deaths, as indicated from each study’s reported survival rates, while progression-free survival was calculated according to the proportion of disease progression events. Engauge Digitizer was used to calculate the survival rates at different time points from the Kaplan-Meier curves of the articles that studied the effect of HIPEC for treatment of PC in gastric cancer patients. For each outcome, a random-effects model was created, using the inverse variance method, to compare the risk ratio (RRs) and 95% confidence intervals (CIs) between patients who did and did not receive HIPEC. The fixed-effect model is also presented. Statistical heterogeneity was assessed using the I2 statistic. The statistical significance threshold was P<0.05. The risk of bias for each randomized trial was assessed by answering a series of quality questions regarding type of randomization, method of randomization concealment and description of withdrawals. All statistical analyses were performed using Stata version 14 (Stata Corp, College Station, Tex).
Results
Our search identified 2035 articles in total (426 Medline, 1098 Embase, 511 Cochrane) (Fig. 1) and their titles and abstracts were screened for eligibility. Twenty-three studies were retrieved for full-text review and, after exclusion of ineligible studies, 18 randomized trials were included in the review [6,17-33]. Reasons for exclusion were a non-randomized design, reporting of the same studies in different follow-up periods, the use of non-hyperthermic intraperitoneal chemotherapy, and a comparison between trial arms irrelevant to our study. The results will be presented according to the cancer site.
Figure 1.
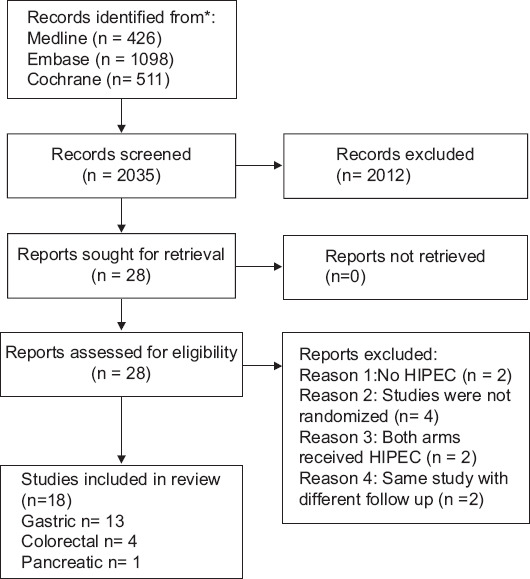
Review flow chart
HIPEC, hyperthermic intraperitoneal chemotherapy
Gastric cancer
Study demographics
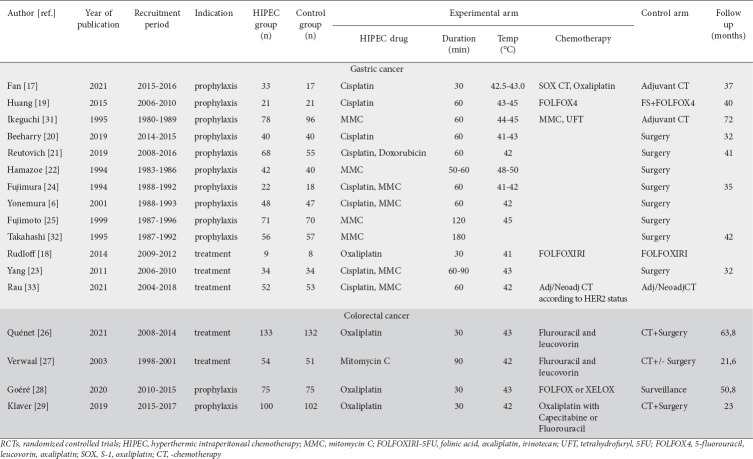
A total of 13 RCTs compared the use vs. non-use of HIPEC in gastric cancer PC [6,17-25,31-33]. The vast majority of the studies were conducted in China [17,19,20,23] and Japan [6,22,24,25,31,32]. Two studies originated from Europe [21,33] and one from the USA [18]. All studies but one randomized in 1:1 fashion [17], but only 3 studies described the exact type of randomization [20,23,33], 3 the randomization concealment [20,23,32], and 4 the withdrawal details [21,31-33]. Table 1 shows the basic characteristics of the RCTs.
Table 1.
Characteristics of RCTs that used HIPEC for prevention and treatment of peritoneal carcinomatosis in colorectal and gastric cancer
All the studies were greatly underpowered, as the sample size of patients randomized in each trial was particularly low, ranging from 17-274. A total of 1130 patients were included in the analysis (574 in the investigational and 556 in the control arm).
Only 3 studies enrolled patients with PC treated with CRS and HIPEC [18,23,33]. The rest of the studies recruited high-risk patients with locally advanced surgically resected gastric cancer, without PC (prophylactic HIPEC therapy). The HIPEC perfusate was kept at a temperature between 41°C and 45°C for 30-120 min, in most cases choosing the closed over the open technique. The HIPEC regimen was mitomycin C until 2001, but from then on cisplatin was the preferred chemotherapy solution. Fujimoto et al had the longest recruitment period (almost 9 years) and consequently collected the largest patient sample (n=141) compared to the rest of the studies [25], underscoring notable difficulties in patient recruitment. All the studies were single-center studies with recruitment periods ranging between 3 and 11 years, except for one multicenter study, which enrolled 105 patients over a recruitment period of 14 years [33].
Prophylactic HIPEC
Ten studies including a total of 940 patients (479 in the investigational and 461 in the control arms) compared the use of prophylactic HIPEC vs. no use in patients who had radical resection of their gastric cancer. In agreement with the NCI, NCCN and ESMO guidelines [34,35], adjuvant chemotherapy should be offered to all surgically resected high-risk gastric cancer patients (while observation is no longer a valid option). We divided the prophylactic HIPEC trials into 2 groups: (1) RCTs that compared HIPEC plus adjuvant chemotherapy vs. adjuvant chemotherapy alone (current standard of care); and (2) RCTs that compared prophylactic HIPEC vs. observation (surgery alone – outdated practice).
Adjuvant HIPEC/chemotherapy vs. adjuvant chemotherapy
Three studies [17,19,31] including a total of 266 patients (132 in the investigational and 134 in the chemotherapy alone arm) were identified. The combination of HIPEC plus chemotherapy for prophylaxis of peritoneal metastasis did not show any overall survival benefit compared with the use of adjuvant chemotherapy alone (RR 1.11, 95%CI 0.71-1.76; P=0.642; I2=31.7%) (Fig. 2), and no progression-free survival benefit (RR 0.90, 95%CI 0.61-1.31; P=0.570; I2=0%) (Fig. 3).
Figure 2.
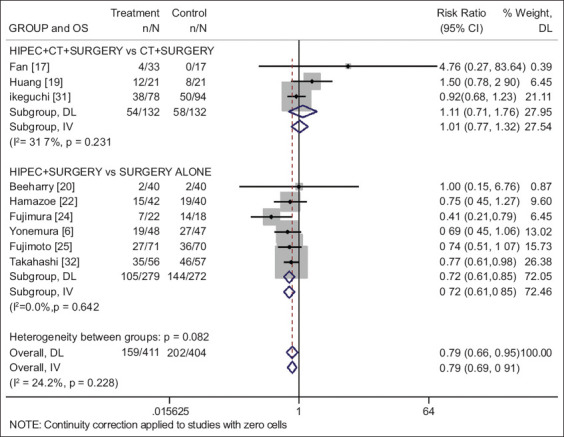
Forest plot of the overall survival for prophylactic use of HIPEC in gastric cancer, presented by group based on the use of CT (risk ratio values below 1 favor HIPEC and above 1 favor control)
HIPEC, hyperthermic intraperitoneal chemotherapy; OS, overall survival; CT, chemotherapy; DL, DerSimonian-Laird; IV, inverse variance
Figure 3.
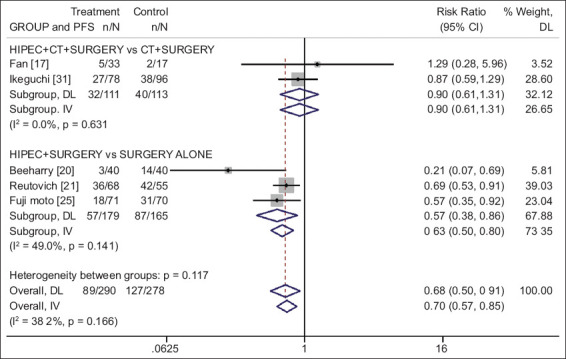
Forest plot of progression-free survival for prophylactic use of HIPEC in gastric cancer, presented by group based on the use of CT (risk ratio values below 1 favor HIPEC and above 1 favor control)
HIPEC, hyperthermic intraperitoneal chemotherapy; PFS, progression-free survival; CT, chemotherapy; DL, DerSimonian-Laird; IV, inverse variance
Adjuvant HIPEC vs. observation alone
Seven studies [6,18,20,21,24,25,32] including a total of 674 patients (347 in the investigational HIPEC and 327 in the observational arm) were identified. The use of surgery and adjuvant HIPEC vs. surgery alone was associated with a statistically significant survival benefit (RR 0.72, 95%CI 0.61-0.85; P<0.001; I2=0%) (Fig. 2), and progression-free survival benefit (RR 0.57, 95%CI 0.38-0.86; P=0.008; I2=49%) (Fig. 3).
HIPEC for treatment of PC
Three studies [18,23,33] including a total of 190 patients (95 in the investigational and 95 in the control arm) were identified. The studies indicated a nonsignificant trend for survival benefit in the first year (RR 0.80, 95%CI 0.62-1.02; P=0.07; I2=0%). The cumulative randomized evidence across trials reached statistical significance for 2-year (RR 0.86, 95%CI 0.75-0.99; P=0.036; I2=0%) and 3-year (RR 0.85, 95%CI 0.77-0.93; P<0.001; I2=0%) survival (Fig. 4). Nonetheless, the overall randomized sample size was much too small to draw certain conclusions regarding the use of HIPEC compared to no use.
Figure 4.
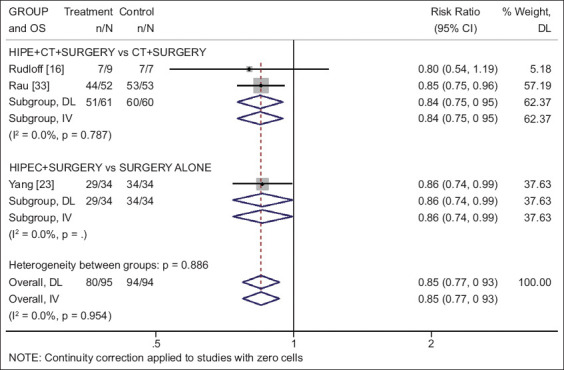
Forest plot of 3-year survival for treatment of peritoneal metastasis in gastric cancer, presented by group based on the use of CT (risk ratio values below 1 favor HIPEC and above 1 favor control)
HIPEC, hyperthermic intraperitoneal chemotherapy; OS, overall survival; CT, chemotherapy; DL, DerSimonian-Laird; IV, inverse variance
Colorectal cancer
Study demographics
Four randomized trials comparing HIPEC with a therapy without HIPEC for colorectal cancer were retrieved [26-29]. These studies were carried out in Europe (2 in France and 2 in The Netherlands). All the studies randomized in 1:1 fashion, described the method of randomization and allocation concealment, and reported the withdrawals. The sample size of patients randomized in each trial was low, ranging from 105-265 and the time of recruitment was from 3-6 years. Overall, 722 patients were included (362 in the investigational and 360 in the control arm). However, the 4 trials could not be analyzed together since they were based on 2 different treatment settings: 2 trials (PROPHYLOCHIP-PRODIGE 15 and COLOPEC) evaluated the prophylactic use of HIPEC for prevention of peritoneal metastases [28,29], while 2 trials (Verwaal’s and PRODIGE 7) focused on the therapeutic use of HIPEC in patients with colorectal cancer and peritoneal metastases [26,27]. Table 1 shows some basic characteristics of the trials. Although 3 of the trials were quite recent, the study by Verwaal et al was the oldest and differed in the HIPEC procedure [27]. Verwaal et al used mitomycin C as the perfusate for 90 min at a temperature of 41-42°C. The other 3 trials used an oxaliplatin-based regimen, while fluorouracil and leucovorin was administered right before the start of HIPEC. The procedure lasted for 30 min in each of these trials, at a temperature of 41-43°C. Systematic chemotherapy was used in every trial.
HIPEC for the treatment of PC
Two studies [26,27] that randomized a total of 370 patients (187 in the investigational and 183 in the control arm) were analyzed. Both studies indicated a nonsignificant trend for survival benefit. Curiously the best trend for survival outcome was reported in the trial of Verwaal et al, where mitomycin C (an agent no longer used in the treatment of metastatic colorectal cancer) was used for HIPEC [27]. In our analysis, cumulative randomized evidence across both trials did not show any statistical significance, either for overall survival (RR 0.88, 95%CI 0.67-1.16; P=0.380; I2=46.6%) or for progression-free survival (RR 0.98, 95%CI 0.88-1.09; P=0.746; I2=0%) from the use of HIPEC.
Prophylactic HIPEC
Two studies [28,29] that randomized a total of 352 patients (175 in the investigational and 177 in the control arm) for the use of prophylactic HIPEC vs. non-use, among patients who underwent resections of colorectal cancer, were analyzed. Analysis of comprehensive randomized data did not demonstrate any statistically significant difference from the use of prophylactic HIPEC vs. non-use, for either overall survival (RR 1.12, 95%CI 0.70-1.80; P=0.635; I2=0%) or progression free survival (RR 0.99, 95%CI 0.76-1.29; P=0.946; I2=0%).
In fact, another phase 2 trial is ongoing, part of the expected CAIRO6 trial, conducted to assess the feasibility and safety of perioperative chemotherapy and HIPEC compared to HIPEC and surgery alone, randomizing 40 patients to each group [36]. The CAIRO6 trial is currently the only randomized phase 3 trial expected to clarify the possible additive benefit of perioperative chemotherapy when combined with HIPEC, if substantial patient recruitment is achieved.
Appendiceal tumors and PMP
There were no randomized trials investigating the use vs. non-use of HIPEC exclusively for appendiceal tumors. Levine et al [37], presented a study of patients with mucinous appendiceal tumors with peritoneal involvement randomized to CRS and HIPEC plus mitomycin or oxaliplatin. Overall and disease-free survival were similar for both regimens, but mitomycin showed higher hematologic toxicity, with a significantly lower white blood cell count. In a later report assessing quality of life [38], oxaliplatin showed more favorable outcomes. The ongoing ICARuS clinical trial is expected to highlight the differences between HIPEC and EPIC (early postoperative intraperitoneal chemotherapy) in combination with CRS for colorectal and appendiceal cancer [39]. Two analyses of another randomized trial comparing high and low intra-abdominal pressure HIPEC in patients with PMP and colorectal cancer concluded that increased-pressure HIPEC is a feasible and safe method that increases the intraperitoneal distribution of cisplatin [40,41].
Malignant peritoneal mesothelioma (MPM)
No randomized studies on the role of HIPEC in the treatment of MPM were detected. As a non-randomized point of reference, a 2021 comparative study showed a clear survival advantage of CRS and HIPEC compared to CRS and postoperative intraperitoneal chemotherapy [42]. Nonetheless the non-randomized nature of the study exposed its outcomes to a large number of biases.
Pancreatic, hepatobiliary and other cancers
Padilla-Valverde et al recently described the pilot study of the only randomized trial applying HIPEC in pancreatic cancer [30]. The trial randomized 16 patients with ductal adenocarcinoma of the pancreas, recruited during 2018 and 2019, to receive either CRS plus HIPEC (n=10) or CRS alone (n=6) and has not yet presented any between-group differences in the early steps of the study. The search resulted in zero randomized trials for hepatobiliary cancers. The most recent retrospective case-control study suggested a survival benefit for HIPEC combined with radical surgery and capecitabine, in contrast to the same approach without HIPEC, with no increase in the complication rate [43]. No randomized studies were found for any other type of GI tumors.
Discussion
To our knowledge, this is the only article that attempts to comprehensively present the available randomized evidence for the use of HIPEC in all types of GI cancers. HIPEC combined with CRS indicated a significant survival benefit in the prophylactic setting of gastric cancer, only compared to surgery alone. However, in surgically resected high-risk gastric cancers, HIPEC alone is actually not recommendable, since adjuvant chemotherapy became a part of the standard of care in the management of these patients (NCI, NCCN and ESMO guidelines [34,35]). Thus, the adjuvant use of HIPEC alone cannot be recommended until superiority to the actual adjuvant systemic treatment can be documented. For any other cancer type, either no substantial survival benefit was proved or no randomized data were available. Nonetheless, it should be noted that even these nonsignificant trends are of interest, since patient populations with advanced neoplasmatic diseases also suffer from a variety of miscellaneous factors and commorbidities, making the evaluation of the survival benefit from multidisciplinary approaches more complex.
Unfortunately, after more than 40 years since the first HIPEC report, and in spite of its potential benefit, HIPEC use should still be still considered an exploratory treatment option. This may stem from the difficulties in organizing adequately powered studies, the complexity of conducting multicentric trials and the extremely long period of recruitment. Overall, 14 of the 18 RCTs analyzed were single-center and only 4 were multicenter; 94% (17/18) were single-nation and only one (6%) was a multinational study. The number of patients recruited per arm was less than 50 in half of the studies, while only 2 (11%) enrolled more than 100 patients per arm [26,29]. The mean time of recruitment in studies randomizing more than 50 patients per arm was 8 (range: 4-14) years.
Challenges in statistical planning were particularly evident in studies for PC prevention. For example, in studies of the prevention of PC from colorectal cancer, only 266 patients were randomized across the 3 available trials, which is far from representative. PC as first site of recurrence after surgery ranges from 17-25% [44,45]. To detect a 30% reduction in recurrence between the 2 arms at a study power of 80% and type 1 error a=0.05, more than 400 patients should have been randomized in each arm.
The timing of both data release and guidance delivery is not a redundant issue. For example, in gastric cancer the use of prophylactic HIPEC alone (despite a statistically significant benefit over non-use), cannot be considered a standard of care, as systemic adjuvant treatment is used with level 1 evidence, while observation alone is no more an ethical and valid option.
Bartlett et al comprehensively described the difficulties of conducting randomized trials for HIPEC [46]. While a multicenter design is necessary in order to recruit a desired number of eligible patients, it is also a source of population pollution due to varying institutional techniques and perioperative care. Aside from the difficulties involved in enrolling rare populations, recruiting patients in trials of aggressive procedures has proven difficult. A true bottleneck of HIPEC research is the heterogeneity of the study protocols, as is also evident in this review. Moreover, it is essential to highlight the prognostic impact of complete cytoreduction score and PC index on patients with PC and thus these factors should be taken seriously into account for more careful participant selection in future HIPEC RCTs [26,47,48].
As far as rarer malignancies are concerned, such as PMP and MPM, long periods of recruitment are additionally needed, so that sufficient patient accrual is achieved. In view of its potential benefit in the PMP setting, CRS plus HIPEC has been recently proposed to represent the new standard of care for this tumor type [10], but despite expert consensus, the level of evidence for this recommendation remains low until randomized data become available.
Trials assessing the benefit of HIPEC have been conducted, and the results of many ongoing randomized trials are also expected (Supplementary Table 1 (151.2KB, pdf) ). However, most of them continue to be single-country studies (China or Germany or France or Italy), not conducted in an international multicenter setting. At this moment, only a few of these studies appear promising, with estimated enrolment numbers ranging from 400 to more than 600 participants (NCT02960061, NCT02240524, NCT01882933).
Quality of life and toxicities are also a non-obsolete issue. An average of 3-12 months is required for the quality of life to improve and return to normal [49]. The most common complications are hematological complications, anastomotic leaks, bowel perforations and infectious complications. All the analyzed trials referred to postoperative morbidity, complications and/or toxicities, while it is also important to note that only 2 studies directly referred to quality of life assessment [29,33]. Complications should always be a matter of concern, since a short prolongation of survival (if any) means little if it is achieved at the expense of the quality of life.
Some potential limitations of the present study should be aknowledged. First, it cannot be excluded that some studies published in the English literature could have been missed in our systematic reasearch. Nonetheless, 3 major libraries (Medline, Embase and Cochrane) and abstracts from major conferences were scrutinized, so it is unlikely that any major randomized trial was overlooked, and other studies would probably not have any impact on the overall outcome. Secondly, a large proportion of the HIPEC literature is written in the Chinese or Japanese language, rendering it inaccessible. However, it is unlikely that important findings would not have been reported in the English literature or cited and discussed from scrutinized manuscripts. Finally, this meta-analysis has the limitation of being based on published data. Considering the extreme paucity of randomized patients available for analysis, it is highly unlikely that a meta-analysis of individual level data would have resulted in different outcomes.
In conclusion, the comprehensive randomized evidence available does not support the use of HIPEC in the treatment/prevention of PC in GI and biliary tract malignancies. HIPEC use should be considered investigational in any setting until evidence from properly designed international multicenter studies, of adequate statistical power, becomes available.
Summary Box.
What is already known:
Peritoneal metastases originating from gastrointestinal (GI) cancers are characterized by poor prognosis and low survival rates
Complete cytoreduction in combination with hyperthermic intraperitoneal chemotherapy (HIPEC) was hoped to be a valid option for the management of peritoneal carcinomatosis (PC)
Reliable level-1 evidence is necessary for the possible introduction of HIPEC in routine clinical practiceined
What the new findings are:
HIPEC indicates some promise in the treatment of PC from gastric cancer, but the existing trials contain an insufficient number of patients
Randomized trials for other GI and biliary tract malignancies are scarce and lack significant results
Biography
University of Ioannina, Greece; University of Ioannina School of Medicine, Greece; The Christie NHS Foundation Trust, Manchester, UK
Footnotes
Conflict of Interest: None
References
- 1.Neuwirth MG, Alexander HR, Karakousis GC. Then and now:cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol. 2016;7:18–28. doi: 10.3978/j.issn.2078-6891.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glehen O, Cotte E, Kusamura S, et al. Hyperthermic intraperitoneal chemotherapy:nomenclature and modalities of perfusion. J Surg Oncol. 2008;98:242–246. doi: 10.1002/jso.21061. [DOI] [PubMed] [Google Scholar]
- 3.Brücher BL, Piso P, Verwaal V, et al. Peritoneal carcinomatosis:cytoreductive surgery and HIPEC—overview and basics. Cancer Invest. 2012;30:209–224. doi: 10.3109/07357907.2012.654871. [DOI] [PubMed] [Google Scholar]
- 4.Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res. 1996;82:79–100. doi: 10.1007/978-1-4613-1247-5_6. [DOI] [PubMed] [Google Scholar]
- 5.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 6.Yonemura Y, Aretxabala de X, Fujimura T, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer:final results of a randomized controlled study. Hepatogastroenterology. 2001;48:1776–1782. [PubMed] [Google Scholar]
- 7.Seshadri RA, Glehen O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J Gastroenterol. 2016;22:1114–1130. doi: 10.3748/wjg.v22.i3.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 9.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran B, Baratti D, Yan TD, Kusamura S, Deraco M. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei) J Surg Oncol. 2008;98:277–282. doi: 10.1002/jso.21054. [DOI] [PubMed] [Google Scholar]
- 11.Salti GI, Ailabouni L, Undevia S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal sarcomatosis. Ann Surg Oncol. 2012;19:1410–1415. doi: 10.1245/s10434-012-2240-7. [DOI] [PubMed] [Google Scholar]
- 12.Hayes-Jordan A, Green HL, Lin H, et al. Complete cytoreduction and HIPEC improves survival in desmoplastic small round cell tumor. Ann Surg Oncol. 2014;21:220–224. doi: 10.1245/s10434-013-3269-y. [DOI] [PubMed] [Google Scholar]
- 13.van Oudheusden TR, Lemmens VE, Braam HJ, et al. Peritoneal metastases from small bowel cancer:Results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in The Netherlands. Surgery. 2015;157:1023–1027. doi: 10.1016/j.surg.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Narasimhan V, Tan S, Kong J, et al. Prognostic factors influencing survival in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for isolated colorectal peritoneal metastases:a systematic review and meta-analysis. Colorectal Dis. 2020;22:1482–1495. doi: 10.1111/codi.15003. [DOI] [PubMed] [Google Scholar]
- 15.Flood MP, Das AA, Soucisse ML, et al. Synchronous liver resection, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy for colorectal liver and peritoneal metastases:a systematic review and meta-analysis. Dis Colon Rectum. 2021;64:754–764. doi: 10.1097/DCR.0000000000002027. [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan B, Bu Z, Zhang J, et al. Phase II trial of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer after curative surgery. BMC Cancer. 2021;21:216. doi: 10.1186/s12885-021-07925-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin:results of the GYMSSA trial. J Surg Oncol. 2014;110:275–284. doi: 10.1002/jso.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang O, Lu X, Xu X, Shi Y. Fibrin-sealant-delivered cisplatin chemotherapy versus cisplatin hyperthermic intraperitoneal perfusion chemotherapy for locally advanced gastric cancer without peritoneal metastases:a randomized phase-II clinical trial with a 40-month follow-up. Cell Biochem Biophys. 2015;71:1171–1180. doi: 10.1007/s12013-014-0326-5. [DOI] [PubMed] [Google Scholar]
- 20.Beeharry MK, Zhu ZL, Liu WT, Yao XX, Yan M, Zhu ZG. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer:personal experience from a randomized case control study. BMC Cancer. 2019;19:932. doi: 10.1186/s12885-019-6125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reutovich MY, Krasko OV, Sukonko OG. Hyperthermic intraperitoneal chemotherapy in serosa-invasive gastric cancer patients. Eur J Surg Oncol. 2019;45:2405–2411. doi: 10.1016/j.ejso.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer . 1994;73:2048–2052. doi: 10.1002/1097-0142(19940415)73:8<2048::aid-cncr2820730806>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 23.Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer:final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–1581. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimura T, Yonemura Y, Muraoka K, et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer:randomized controlled study. World J Surg. 1994;18:150–155. doi: 10.1007/BF00348209. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529–534. [PubMed] [Google Scholar]
- 26.Quénet F, Elias D, Roca L, et al. UNICANCER-GI Group and BIG Renape Group. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7):a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:256–266. doi: 10.1016/S1470-2045(20)30599-4. [DOI] [PubMed] [Google Scholar]
- 27.Verwaal VJ, van Ruth S, Bree de E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 28.Goéré D, Glehen O, Quenet F, et al. BIG-RENAPE group. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15):a randomised, phase 3 study. Lancet Oncol. 2020;21:1147–1154. doi: 10.1016/S1470-2045(20)30322-3. [DOI] [PubMed] [Google Scholar]
- 29.Klaver CEL, Wisselink DD, Punt CJA, et al. COLOPEC collaborators group. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC):a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4:761–770. doi: 10.1016/S2468-1253(19)30239-0. [DOI] [PubMed] [Google Scholar]
- 30.Padilla-Valverde D, García-Santos E, Sanchez S, et al. Safety of perioperative hyperthermic intraperitoneal chemotherapy with gemcitabine in patients with resected pancreatic adenocarcinoma:a pilot study of the clinical trial EudraCT 2016-004298-41. J Gastrointest Oncol. 2021;12:S80–S90. doi: 10.21037/jgo-20-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeguchi M, Kondou A, Oka A, Tsujitani S, Maeta M, Kaibara N. Effects of continuous hyperthermic peritoneal perfusion on prognosis of gastric cancer with serosal invasion. Eur J Surg. 1995;161:581–586. [PubMed] [Google Scholar]
- 32.Takahashi T, Hagiwara A, Shimotsuma M, Sawai K, Yamaguchi T. Prophylaxis and treatment of peritoneal carcinomatosis:intraperitoneal chemotherapy with mitomycin C bound to activated carbon particles. World J Surg. 1995;19:565–569. doi: 10.1007/BF00294724. [DOI] [PubMed] [Google Scholar]
- 33.Rau B, Lang H, Königsrainer A, et al. 1376O - The effect of hyperthermic intraperitoneal chemotherapy (HIPEC) upon cytoreductive surgery (CRS) in gastric cancer (GC) with synchronous peritoneal metastasis (PM):A randomized multicentre phase III trial (GASTRIPEC-I-trial) Ann Oncol. 2021;32(5):S1040–S1075. [Google Scholar]
- 34.Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 35.Lordick F, Carneiro F, Cascinu S, et al. ESMO Guidelines Committee. Gastric cancer:ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005–1020. doi: 10.1016/j.annonc.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Rovers KP, Bakkers C, Nienhuijs SW, et al. Dutch Peritoneal Oncology Group and the Dutch Colorectal Cancer Group. Perioperative systemic therapy vs cytoreductive surgery and hyperthermic intraperitoneal chemotherapy alone for resectable colorectal peritoneal metastases:a phase 2 randomized clinical trial. JAMA Surg. 2021;156:710–720. doi: 10.1001/jamasurg.2021.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine EA, Votanopoulos KI, Shen P, et al. A multicenter randomized trial to evaluate hematologic toxicities after hyperthermic intraperitoneal chemotherapy with oxaliplatin or mitomycin in patients with appendiceal tumors. J Am Coll Surg. 2018;226:434–443. doi: 10.1016/j.jamcollsurg.2017.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moaven O, Votanopoulos KI, Shen P, et al. Health-related quality of life after cytoreductive surgery/HIPEC for mucinous appendiceal cancer:results of a multicenter randomized trial comparing oxaliplatin and mitomycin. Ann Surg Oncol. 2020;27:772–780. doi: 10.1245/s10434-019-08064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi AJ, Khan TM, Rehman SU, Nash GM, Hernandez JM. Early postoperative intraperitoneal versus hyperthermic intraperitoneal chemotherapy after optimal cytoreductive surgery for colorectal cancer with isolated peritoneal metastasis (ICARuS) Ann Surg Oncol. 2021;28:4100–4101. doi: 10.1245/s10434-021-10110-1. [DOI] [PubMed] [Google Scholar]
- 40.Reis ACV, Kusamura S, Azmi N, et al. Hemodynamic and respiratory implications of high intra-abdominal pressure during HIPEC. Eur J Surg Oncol. 2020;46:1896–1901. doi: 10.1016/j.ejso.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Kusamura S, Azmi N, Fumagalli L, et al. Phase II randomized study on tissue distribution and pharmacokinetics of cisplatin according to different levels of intra-abdominal pressure (IAP) during HIPEC ( NCT02949791) Eur J Surg Oncol. 2021;47:82–88. doi: 10.1016/j.ejso.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Wang T, Li H, Ye B, Zhang D. Value of cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy to treat malignant peritoneal mesothelioma. Am J Transl Res. 2021;13:10712–10720. [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S, Zhong Z, Yi W, et al. Effect of hyperthermic intraperitoneal perfusion chemotherapy combined with radical surgery and capecitabine on stage III gallbladder cancer. Can J Gastroenterol Hepatol. 2021;2021:4006786. doi: 10.1155/2021/4006786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roviello F, Marrelli D, de Manzoni G, et al. Italian Research Group for Gastric Cancer. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003;90:1113–1119. doi: 10.1002/bjs.4164. [DOI] [PubMed] [Google Scholar]
- 45.Wu F, Shi C, Wu R, Huang Z, Chen Q. Peritoneal recurrence in gastric cancer following curative resection can be predicted by postoperative but not preoperative biomarkers:a single-institution study of 320 cases. Oncotarget. 2017;8:78120–78132. doi: 10.18632/oncotarget.17696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartlett DL. HIPEC:the complexities of clinical trials. Ann Surg Oncol. 2008;15:1277–1279. doi: 10.1245/s10434-007-9768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faron M, Macovei R, Goéré D, Honoré C, Benhaim L, Elias D. Linear relationship of peritoneal cancer index and survival in patients with peritoneal metastases from colorectal cancer. Ann Surg Oncol. 2016;23:114–119. doi: 10.1245/s10434-015-4627-8. [DOI] [PubMed] [Google Scholar]
- 48.Bonnot PE, Piessen G, Kepenekian V, et al. FREGAT and BIG-RENAPE Networks. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study):a propensity score analysis. J Clin Oncol. 2019;37:2028–2040. doi: 10.1200/JCO.18.01688. [DOI] [PubMed] [Google Scholar]
- 49.Seretis C, Youssef H. Quality of life after cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies:a systematic review. Eur J Surg Oncol. 2014;40:1605–1613. doi: 10.1016/j.ejso.2014.08.477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.