Abstract
Objectives
The purpose of this study was systematically and quantitatively to assess the value of the neutrophil-to-lymphocyte ratio (NLR) for the diagnosis of neonatal sepsis by systematic review and meta-analysis.
Design
Systematic review and meta-analysis.
Methods
Eight major databases, including The Cochrane, PubMed, Embase, Web of Science, CNKI, Wanfang, China Biomedical Literature Database and VIP Database, were systematically searched for NLR diagnoses of neonatal sepsis from inception to June 2022. Two investigators independently conducted the literature search, screening, data extraction and quality evaluation with the Quality Assessment of Diagnostic Accuracy Studies-2 checklist. Statistical analysis was performed using Review Manager V.5.3, Stata V.16.0, R (V.3.6.0) and Meta-DISC V.1.4.
Results
A total of 14 studies comprising 1499 newborns were included in this meta-analysis. With a cut-off value ranging from 0.1 to 9.4, the pooled sensitivity of the NLR in the diagnosis of neonatal sepsis was 0.74 (95% CI: 0.61 to 0.83), the pooled specificity was 0.88 (95% CI: 0.73 to 0.95), the positive likelihood ratio (LR+) was 6.35 (95% CI: 2.6 to 15.47), the negative likelihood ratio (LR−) was 0.30 (95% CI: 0.19 to 0.46), the diagnostic OR (DOR) was 12.88 (95% CI: 4.47 to 37.08), area under the curve (AUC) was 0.87 (95% CI: 0.84 to 0.89). In the subgroup analysis of early-onset neonatal sepsis, the pooled sensitivity was 0.75 (95% CI: 0.47 to 0.91), the pooled specificity was 0.99 (95% CI: 0.88 to 1.00), the LR+ was 63.3 (95% CI: 5.7 to 696.8), the LR− was 0.26 (95% CI: 0.10 to 0.63), the DOR was 247 (95% CI: 16 to 3785) and the AUC was 0.97 (95% CI: 0.95 to 0.98).
Conclusions
Our findings suggest that the NLR is a helpful indicator for the diagnosis of early neonatal sepsis, but it still needs to be combined with other laboratory tests and specific clinical manifestations.
Keywords: INFECTIOUS DISEASES, NEONATOLOGY, Diagnostic microbiology
Strengths and limitations of this study.
We conducted a comprehensive search of each literature database and formulated detailed inclusion and ranking criteria to ensure the quantity and quality of the included literature.
Subgroup analyses were performed according to sepsis type, study area and cut-off value as described in the methodology section of this study.
Our included articles lack more multicentre and large sample studies.
There may be other clinical and statistical heterogeneity in the included studies.
Background
Neonatal sepsis is a systemic inflammatory response syndrome caused by a bacterial infection in the neonatal stage. The clinical manifestations gradually surface in the whole body of the inflammatory response and finally progress into organ failure, leading to death.1 Studies have shown that the morbidity of neonatal sepsis is 1%–20% in newborns and is also the third highest after premature delivery and neonatal encephalopathy (perinatal asphyxia and trauma).2 At present, neonatal sepsis is faced with insufficient diagnostic methods, resulting in the inability to guide clinical treatment in a timely manner, thereby affecting its therapeutic effect.
According to a survey, the global mortality rate of neonatal sepsis reached 1.0%–5.0%.3 Early and precise identification of neonatal sepsis is crucial for slowing the progression of the disease and decreasing mortality.4 Notwithstanding, there are many clinical biomarkers in the clinic for the diagnosis of neonatal sepsis, and due to the long time consumption, low diagnostic performance and the rapid progress of the disease, missed identification of neonatal sepsis delays diagnosis and treatment, increasing the risk of death.5
The accurate identification of neonatal sepsis is critical to provide sufficient treatment time and improve clinical outcomes. In contrast, the neutrophil-to-lymphocyte ratio (NLR) is an independent predictor in the clinic that has been widely used in various diseases, such as immune system diseases, tumours and cancers.6 Many studies have shown that the NLR is more reliable for diagnosing neonatal sepsis than neutrophil counts or lymphocyte counts alone. Nevertheless, there is still a dispute about diagnosing the effectiveness of neonatal sepsis.7 8
We assessed the accuracy as a biomarker for diagnosing neonatal sepsis in newborns by performing a systematic literature review and a meta-analysis, comparing the predictive value and providing a reference for the clinical diagnosis of neonatal sepsis.
Methods
The present meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement (PRISMA). For details, see PRISMA-(diagnostic test accuracy) DTA for abstracts and PRISMA-DTA.
Patient and public involvement
No patients were involved.
Data source
We searched the Cochrane, PubMed, Embase, Web of Science, CNKI, Wanfang, China Biomedical Literature Database and VIP Database for studies on the diagnostic accuracy of neonatal sepsis published before June 2022. We used a combination of subject words and free words to search the study and the following keywords: ‘Neutrophil and lymphocyte ratio’, ‘Infant’, ‘Newborn’, ‘Neonate’, ‘sepsis’, ‘septicemia’, ‘Neonatal Sepsis’. In addition, we checked the reference lists of each of the primary studies to identify additional publications. The retrieval format is shown in online supplemental additional file 1.
bmjopen-2021-060391supp001.pdf (133.8KB, pdf)
Study eligibility
Inclusion criteria: (1) The purpose of the study was to evaluate or explore the diagnostic value of the NLR in neonatal sepsis. (2) The case group included newborns with confirmed neonatal sepsis, and the control group included newborns with neonates without sepsis. (3) The diagnostic gold standard is blood culture. (4) It can directly or indirectly obtain the true positive, false positive, true negative and false negative values of the NLR in the diagnosis of neonatal sepsis. The language is English or Chinese.
Exclusion criteria: (1) Being able to be extracted from the full text. (2) Reviews, conference reports, individual cases and animal experiments. (3) A duplicated study.
Data extraction and quality assessment
Two authors (YX and YS) independently conducted the literature screening, data extraction and quality evaluation. In case of disagreement, the third author (WM) decided extracted data from the included literature, including the year of publication, country of origin, study design, author, publication year, newborn birth situation, study location, sample size, case and control numbers, cut-off value, true positive value, false positive value, false negative value, true negative value, sensitivity and specificity. We assess the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) checklist. We used Review Manager (V.5.3) for quality assessment.
Statistical analyses
Statistical heterogeneity was assessed using forest plots with 95% prediction interval, the tau-squared (τ2) value and I2 statistic. The 95% prediction interval was applied to estimate the effect size range in further studies.9 If there was heterogeneity between the studies, the source of the heterogeneity was further explored, and threshold effect and non-threshold effect analyses were carried out. Meta Disc V.1.4 software was used to analyse the threshold effect heterogeneity. For heterogeneity caused by non-threshold effects, we performed meta-regression analysis and sensitivity analysis to find the source of heterogeneity. At the same time, we performed subgroup analyses by cut-off value, neonatal birth status and type of sepsis to assess the stability of the results. The combined sensitivity, combined specificity, combined diagnostic OR (DOR), combined positive likelihood ratio (LR+), combined negative likelihood ratio (LR−) and its 95% CI were determined using Stata V.16.0. Simultaneously, summary receiver operating characteristic (SROC) curve analysis was performed. All studies are presented as a circle and plotted with the SROC curve. The summary point is represented by a dot which was surrounded by a 95% confidence region. The area under the SROC curve was calculated. At the same time, we assessed the bias of included studies by contour-enhanced funnel plots. If there was bias, we judged the stability of the results by the cut-and-fill method. We used Stata (V.16.0), R (V.3.6.0) and MetaDiSc (V.1.4) to perform the analyses.
Results
Identification of studies
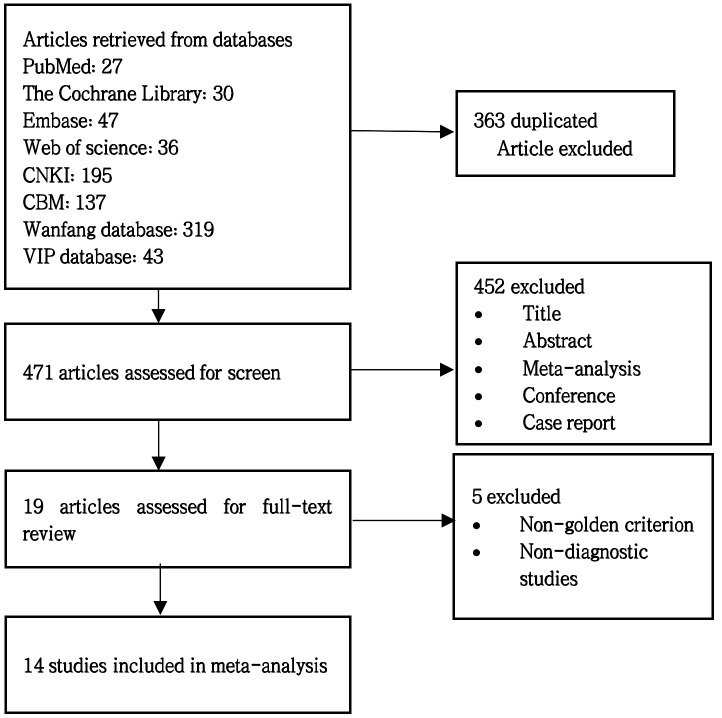
After checking duplicates and reading abstracts and excluding relevant literature according to the exclusion criteria, a final total of 14 studies were used for the current meta-analysis.10–23 The specific process is shown in figure 1. Of these, 783 neonates in the sepsis group and 716 neonates in the non-sepsis group were studied and evaluated. Online supplemental additional file 2 shows the significant characteristics of the selected studies. The baseline information included the following parameters: the number of patients, gestational age, regions, types of sepsis, disease diagnosis methods, study design and NLR cut-off value.
Figure 1.
Flowchart of study selection, inclusion and exclusion for the meta-analysis.
bmjopen-2021-060391supp002.pdf (91.6KB, pdf)
Quality of studies
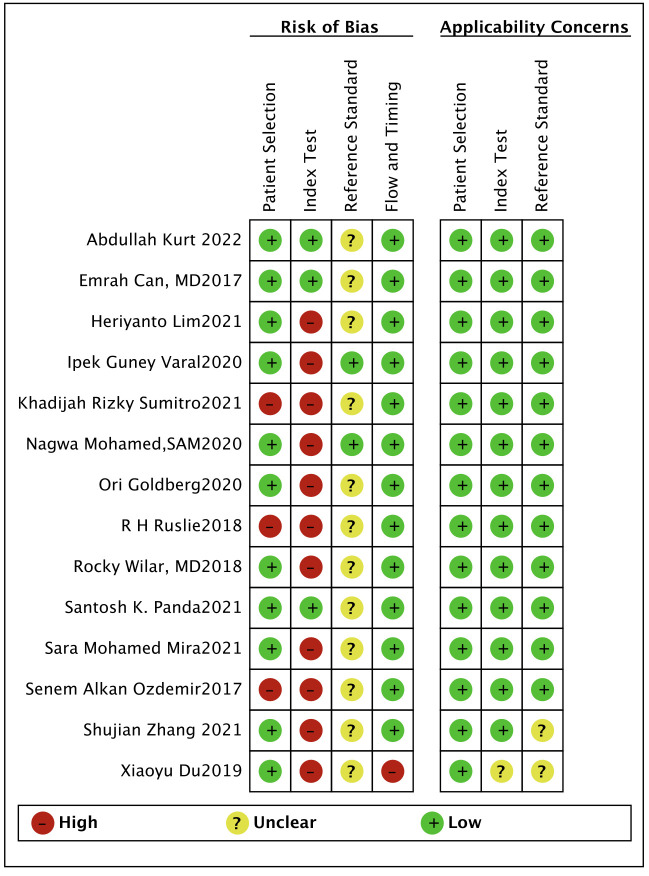
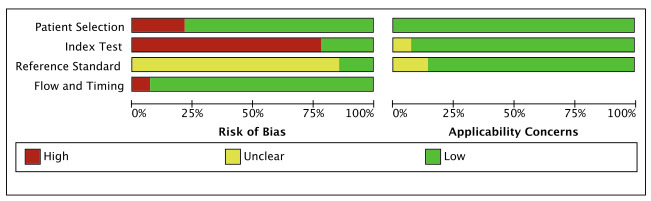
We imported the literature into Review Manager V.5.3 and used the QUADAS-2 tool to evaluate the quality of the 14 included references. According to the methodological evaluation results, the gold standard for the diagnosis of all patients is blood culture. For patient selection, three references were considered high risk. Since most studies do not specify a threshold in advance, there may be a risk of bias. Most articles did not mention whether the interpretation of the experimental results to be evaluated was performed without knowing the results of the gold standard, indicating that it is not clear whether the interpretation of the results will produce a risk of bias (figures 2 and 3).
Figure 2.
Risk of bias and applicability concerns summary.
Figure 3.
Risk of bias and applicability concerns graph.
Heterogeneity exploration
Since the heterogeneity of diagnostic meta-analysis is widespread, it is mainly composed of threshold effect heterogeneity and non-threshold effect heterogeneity. Through the combination of data, by combining the data we found that the results were highly heterogeneous. We first conducted a threshold effect test. By using MetaDiSc V.1.4, we found that the Spearman correlation coefficient was −0.037 (p=0.899) (p>0.05). It shows no threshold effect heterogeneity, so to further find the source of heterogeneity, we carried out meta-regression and sensitivity analysis. In the meta-regression analysis, we used the publication year (with 2019 as the cut-off), region, study type and neonatal birth status as variables for analysis. The meta-regression results show that articles in prospective studies are the main source of heterogeneity (p=0.01) (online supplemental additional file 3). Sensitivity analysis removes non-Asian, preterm and late-onset sepsis research results and shows that the region is the main source of heterogeneity (online supplemental additional file 4).
bmjopen-2021-060391supp003.pdf (105.7KB, pdf)
bmjopen-2021-060391supp004.pdf (113.2KB, pdf)
Data synthesis and subgroup analysis
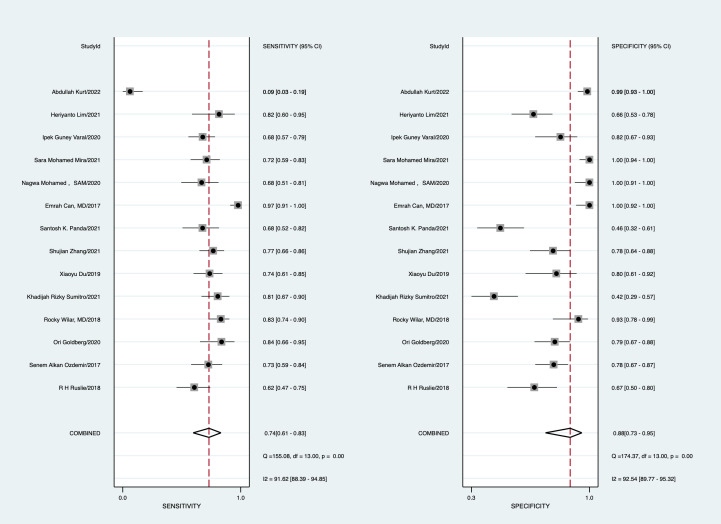
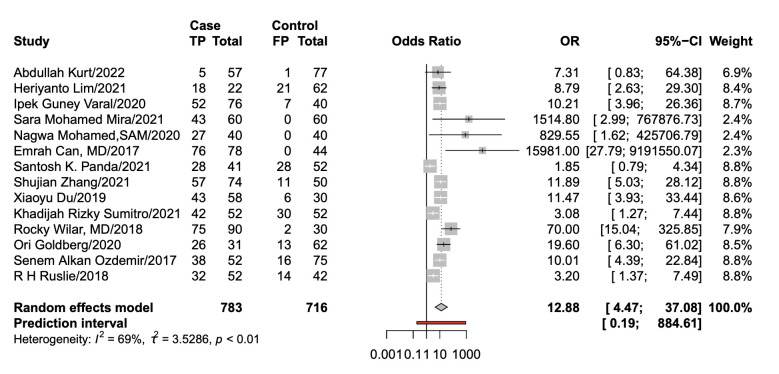
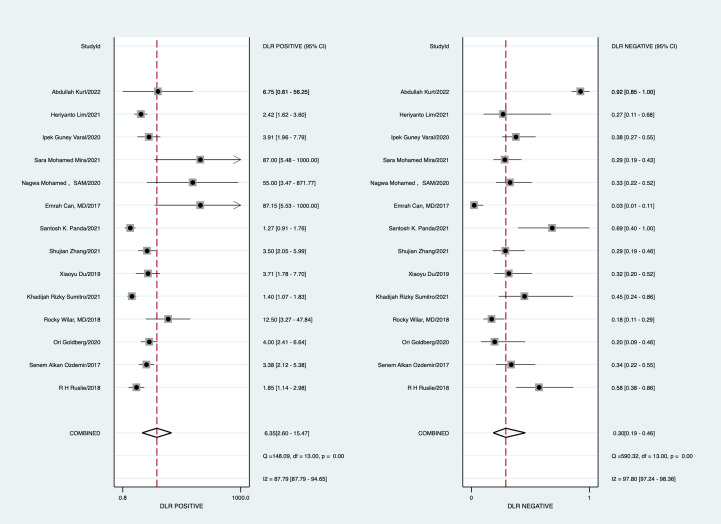
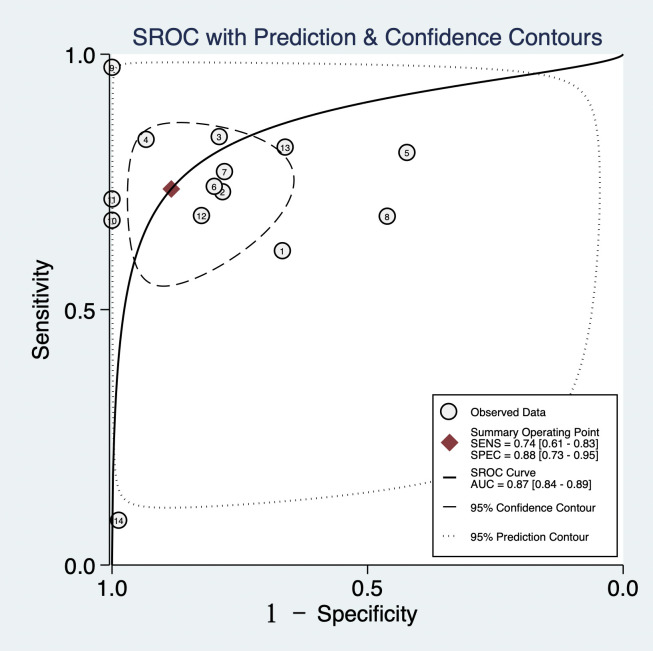
With a cut-off value ranging from 0.1 to 9.4, the pooled sensitivity and specificity of the NLR in the diagnosis of neonates were 0.74 (95% CI: 0.61 to 0.83) and 0.88 (95% CI: 0.73 to 0.95), respectively; LR+ was 6.35 (95% CI: 2.5 to 15.47), LR− was 0.30 (95% CI: 0.19 to 0.46), DOR was 12.88 (95% CI: 4.47 to 37.08) and area under the curve (AUC) was 0.87 (95% CI: 0.84 to 0.89) (figures 4–7).
Figure 4.
Forest plot of the pooled sensitivity and specificity.
Figure 5.
Forest plot of the pooled diagnostic OR. TP, True positive; FP, False positive.
Figure 6.
Forest plot of the pooled positive likelihood ratio (LR) and negative LR.
Figure 7.
Summary receiver operating characteristic (SROC) of the neutrophil-to-lymphocyte ratio for the diagnosis of sepsis. AUC, area under the curve;SEN, Sensitivity; SROC, Summary Receiver Operating Characteristic.
The results of the (Early⁃onset sepsis)EOS subgroup analysis showed that the pooled sensitivity and specificity of the NLR in the diagnosis of neonatal sepsis were 0.75 (95% CI: 0.47 to 0.91) and 0.99 (95% CI: 0.88 to 1.00); LR+ was 63.3 (95% CI: 5.7 to 696.8), LR− was 0.26 (95% CI: 0.10 to 0.63), DOR was 247 (95% CI: 16 to 3785) and the AUC was 0.97 (95% CI: 0.95 to 0.98). The results of the LOS subgroup analysis showed that the pooled sensitivity and specificity of the NLR in the diagnosis of neonatal sepsis were 0.60 (95% CI: 0.53 to 0.67) and 0.85 (95% CI: 0.80 to 0.90); LR+ was 3.71 (95% CI: 2.73 to 5.02), LR− was 0.41 (95% CI: 0.08 to 1.94), DOR was 11.14 (95% CI: 6.54 to 18.98) and the AUC was 0.85. Cut-off value: 0–2, pooled sensitivity and specificity were 0.74 (95% CI: 0.69 to 0.78) and 0.90 (95% CI: 0.71 to 0.97), respectively; LR+ was 7.1 (95% CI: 2.3 to 21.8), LR− was 0.29 (95% CI: 0.23 to 0.36), DOR was 25 (95% CI: 7 to 88), the AUC was 0.77. Cut-off value: 2–4, pooled sensitivity and specificity were 0.79 (95% CI: 0.72 to 0.85) and 0.62 (95% CI: 0.54 to 0.70); LR+ was 2.21 (95% CI: 1.24 to 3.92), LR− was0.33 (95% CI: 0.23 to 0.46), DOR was 6.73 (95% CI: 2.81 to 16.14) The AUC was 0.85. Cut-off value: >4, pooled sensitivity and specificity were 0.60 (95% CI: 0.53 to 0.67) and 0.91 (95% CI: 0.85 to 0.95); LR+ was 9.0 (95% CI: 0.3 to 270.24), LR− was 0.29 (95% CI: 0.03 to 2.68), DOR was 31.51 (95% CI: 0.81 to 1229.29) The AUC was 0.95 (online supplemental additional file 5).
bmjopen-2021-060391supp005.pdf (66.3KB, pdf)
Publication bias exploration
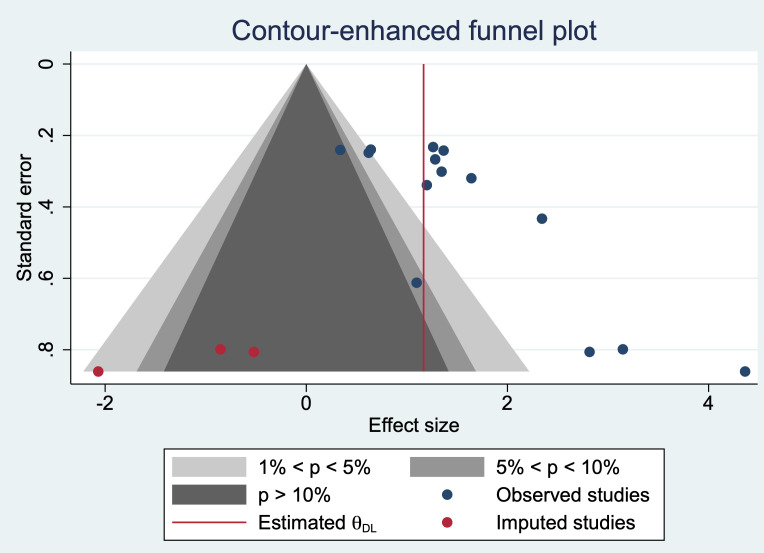
The contour-enhanced funnel plot results suggested that there was publication bias, and after our cut-and-fill method, the results showed that the stability of our meta-analysis results was not affected (figure 8).
Figure 8.
Contour-enhanced funnel plot of studies included in the meta-analysis.
Discussion
The early identification of neonatal sepsis remains challenging in the clinic, and the NLR is broadly used in diagnosing immune system diseases, tumours and cancers. However, the accurate diagnosis of neonatal sepsis is still questionable.24–26 For the first time, we conducted a meta-analysis and systematic review of the diagnostic performance of NLR in neonatal sepsis, which may provide a better reference value for the early diagnosis of neonatal sepsis and for NLR to diagnose neonatal sepsis, providing evidence-based evidence. The meta-analysis included all 14 studies from 7 nations, including 1499 patients with neonatal sepsis. Moreover, the results revealed that the combined AUC of the NLR in the diagnosis of neonatal sepsis was 0.874 (95% CI: 0.84 to 0.89), showing that the NLR is a helpful indicator for the diagnosis of early neonatal sepsis.
Omran et al found that NLR is closely related to neonatal sepsis. Within a few hours after neonatal sepsis, NLR can rapidly increase in a short time compared with (C-reactive protein)CRP. The use of NLR makes it possible to identify neonatal sepsis early27 can be used as an auxiliary diagnostic index for the diagnosis of neonatal sepsis,28 timely diagnosis and early appropriate antibiotic treatment. Seymour et al showed that in the ROC curve analysis of bacterial sepsis according to the Sepsis-2 standard, NLR showed a moderate AUC (0.68), which was significantly higher than that of CRP, lactate and (procalcitonin)PCT,29 30 suggesting that NLR has better diagnostic performance. Mahmoud et al found that when the cut-off value was 0.1, NLR showed the best specificity and negative predictive value for neonatal sepsis ((specificity)SPE was 99%, (Negative Predictive Value)NPV was 75%), compared with CRP and PCT, NLR showed higher specificity with better diagnostic power.19 A study by Alkan Ozdemir et al in the diagnosis of late-onset neonatal sepsis showed that NLR had a high sensitivity, specificity and accuracy of 0.73, 0.78, and 0.76, respectively, with an NLR cut-off value of 1.77.11 In the study of Wilar, it was found that the cut-off value of NLR was 1.5, and NLR could be used as a single laboratory index to diagnose neonatal sepsis,13 indicating that NLR could be a valuable indicator to exclude neonatal sepsis.
Subgroup analysis indicated that pooled sensitivity and specificity were higher for detecting the NLR in a group of early-onset neonatal sepsis. The results express the stability of the results. Neonatal early-onset sepsis mainly emphasises that the bacteria originate from intrauterine tissue and during delivery, and the spectrum of pathogenic bacteria is relatively concentrated.31 32 Streptococcus B and Escherichia coli are the most common pathogens of early-onset neonatal sepsis. In the future, more research can be incorporated to further verify the accuracy of the NLR diagnosis of early-onset sepsis.
Our study included homogeneous research as much as possible, but the included studies still had heterogeneity in which non-threshold effects can be explained to partial heterogeneity. The results of the meta-regression analysis indicated that the study type may be the main sources of heterogeneity (online supplemental additional file 3). The sensitive analysis results also indicate that the non-Asian region is the primary source of heterogeneity (online supplemental additional file 4). However, after removing all non-Asian articles, heterogeneity still existed, indicating this study’s heterogeneity is for other reasons.
In addition, several limitations of this study should be noted. (1) Although it is homogeneous to reduce the choice of bias applications, heterogeneity is still in the inclusive research. (2) The diagnosis of newborns will also have differences due to different researchers, resulting in false positive and false negative results for the diagnosis of neonatal sepsis, which leads to bias. (3) A part of the included research was a retrospective study, so there may be a selection of research objects. (4) The included research comes from different countries, and newborns have different immunity for different races and sexes. Therefore, it is necessary to carry out the same race, large sample, multicentre prospective clinical study to determine the value of the NLR in diagnosing neonatal sepsis in the future.
Conclusion
In summary, our findings suggest that the NLR is a helpful indicator for the diagnosis of early neonatal sepsis, but it still needs to be combined with other laboratory tests and specific clinical manifestations. However, it is limited to the research site and research type. Further research is needed to carry out multicentre prospective studies with multiple samples to verify the accuracy of NLR diagnosis and improve neonatal sepsis prognosis.
Supplementary Material
Footnotes
Contributors: YX and YS were the guarantors for the study and affirm that this manuscript is an honest, accurate and transparent account of the study being reported.YX wrote the manuscript. HL, YZ and WM performed the literature review. YX and YS performed the statistical analysis. WM and CW revised the text. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Lizhong D. Challenges in diagnosis and prevention of neonatal sepsis. Chin J Pediatr 2019;57:241–3. 10.3760/cma.j.issn.0578-1310.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1459–544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oza S, Lawn JE, Hogan DR, et al. Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000-2013. Bull World Health Organ 2015;93:19–28. 10.2471/BLT.14.139790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodska H, Valenta J, Pelinkova K, et al. Diagnostic and prognostic value of presepsin vs. established biomarkers in critically ill patients with sepsis or systemic inflammatory response syndrome. Clin Chem Lab Med 2018;56:658–68. 10.1515/cclm-2017-0839 [DOI] [PubMed] [Google Scholar]
- 5.Subspecialty Group of Neonatology, the Society of Pediatric, Chinese Medical Association, Professional Committee of Infectious Diseases, Neonatology Society, Chinese Medical Doctor Association . [Expert consensus on the diagnosis and management of neonatal sepsis (version 2019)]. Zhonghua Er Ke Za Zhi 2019;57:252–7. 10.3760/cma.j.issn.0578-1310.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 6.Gong W, Yang S, Yang X, et al. Blood preoperative neutrophil-to-lymphocyte ratio is correlated with TNM stage in patients with papillary thyroid cancer. Clinics 2016;71:311–4. 10.6061/clinics/2016(06)04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumarasamy C, Sabarimurugan S, Madurantakam RM, et al. Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer-a protocol for systematic review and meta-analysis. Medicine 2019;98:e14834. 10.1097/MD.0000000000014834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018;6:74. 10.1186/s40425-018-0383-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borenstein M. Research Note: In a meta-analysis, the I2 index does not tell us how much the effect size varies across studies. J Physiother 2020;66:135–9. 10.1016/j.jphys.2020.02.011 [DOI] [PubMed] [Google Scholar]
- 10.Ruslie RH, Tjipta DG, Samosir CT, et al. Bacterial pattern, and role of laboratory parameters as marker for neonatal sepsis[C]//IOP Conference Series: Earth and Environmental Science, 125. IOP Publishing, 2018: 012057. [Google Scholar]
- 11.Alkan Ozdemir S, Arun Ozer E, Ilhan O, et al. Can neutrophil to lymphocyte ratio predict late-onset sepsis in preterm infants? J Clin Lab Anal 2018;32:e22338. 10.1002/jcla.22338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg O, Amitai N, Chodick G, et al. Can we improve early identification of neonatal late-onset sepsis? A validated prediction model. J Perinatol 2020;40:1315–22. 10.1038/s41372-020-0649-6 [DOI] [PubMed] [Google Scholar]
- 13.Wilar R. Diagnostic value of eosinopenia and neutrophil to lymphocyte ratio on early onset neonatal sepsis. Korean J Pediatr 2019;62:217–23. 10.3345/kjp.2018.06723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumitro KR, Utomo MT, Widodo ADW. Neutrophil-to-lymphocyte ratio as an alternative marker of neonatal sepsis in developing countries. Oman Med J 2021;36:e214. 10.5001/omj.2021.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiaoyu D, Liang A. Changes and clinical significance of NLR, CRP and PCT in neonates with sepsis. Experimental and Laboratory Medicine 2019;37:110–2. [Google Scholar]
- 16.Zhang S, Luan X, Zhang W, et al. Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratio as predictive biomarkers for early-onset neonatal sepsis. J Coll Physicians Surg Pak 2021;30:821–4. 10.29271/jcpsp.2021.07.821 [DOI] [PubMed] [Google Scholar]
- 17.Panda SK, Nayak MK, Rath S, et al. The utility of the neutrophil-lymphocyte ratio as an early diagnostic marker in neonatal sepsis. Cureus 2021;13:e12891. 10.7759/cureus.12891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Can E, Hamilcikan Şahin, Can C. The value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for detecting early-onset neonatal sepsis. J Pediatr Hematol Oncol 2018;40:e229–32. 10.1097/MPH.0000000000001059 [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud N MSA, Baheeg G, Abdelhakeem M. Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio as new diagnostic markers for detection of early-onset neonatal sepsis in full-term newborns. MJMR 2019;30:150–5. 10.21203/rs.3.rs-126217/v1 [DOI] [Google Scholar]
- 20.Mira SM, Alkhalegy HA, Abd-Elraheem S. Neutrophil and platelet to lymphocyte ratio for detecting early-onset neonatal sepsis. IJMA 2021;3:1274–81. 10.21608/IJMA.2020.21844.1069 [DOI] [Google Scholar]
- 21.Varal I, Dogan P. Can neutrophil-lymphocyte ratio be a predictor of late-onset sepsis in preterm infants? Ann Med Res 2020;27:23. 10.5455/annalsmedres.2020.01.023 [DOI] [Google Scholar]
- 22.Lim H, Sukmawati M, Artana WD, et al. Validity of neutrophil lymphocyte count ratio in neonatal sepsis. Int J Health Sci 2021;5:53–61. 10.29332/ijhs.v5n2.1148 [DOI] [Google Scholar]
- 23.Kurt A, Tosun MS, Altuntaş N. Diagnostic accuracy of complete blood cell count and neutrophil-to-lymphocyte, lymphocyte-to-monocyte, and platelet-to-lymphocyte ratios for neonatal infection. Asian Biomedicine 2022;16:43–52. 10.2478/abm-2022-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakhuizen SE, de Haan TR, Teune MJ, et al. Meta-analysis shows that infants who have suffered neonatal sepsis face an increased risk of mortality and severe complications. Acta Paediatr 2014;103:1211–8. 10.1111/apa.12764 [DOI] [PubMed] [Google Scholar]
- 25.Shabuj KH, Hossain J, Moni SC, et al. C-reactive protein (CRP) as a single biomarker for diagnosis of neonatal sepsis: a comprehensive meta-analysis. Mymensingh Med J 2017;26:364–71. [PubMed] [Google Scholar]
- 26.Riché F, Gayat E, Barthélémy R, et al. Reversal of neutrophil-to-lymphocyte count ratio in early versus late death from septic shock. Crit Care 2015;19:1–10. 10.1186/s13054-015-1144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makkar M, Gupta C, Pathak R, et al. Performance evaluation of hematologic scoring system in early diagnosis of neonatal sepsis. J Clin Neonatol 2013;2:25–9. 10.4103/2249-4847.109243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omran A, Maaroof A, Mohammad MHS, et al. Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J Pediatr 2018;94:82–7. 10.1016/j.jped.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 29.Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017;376:2235–44. 10.1056/NEJMoa1703058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ljungström L, Pernestig A-K, Jacobsson G, et al. Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS One 2017;12:e0181704. 10.1371/journal.pone.0181704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of child health and human development neonatal research network, 2002-2003. Pediatr Infect Dis J 2005;24:635–9. 10.1097/01.inf.0000168749.82105.64 [DOI] [PubMed] [Google Scholar]
- 32.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol 2008;111:35–41. 10.1097/01.AOG.0000297311.33046.73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-060391supp001.pdf (133.8KB, pdf)
bmjopen-2021-060391supp002.pdf (91.6KB, pdf)
bmjopen-2021-060391supp003.pdf (105.7KB, pdf)
bmjopen-2021-060391supp004.pdf (113.2KB, pdf)
bmjopen-2021-060391supp005.pdf (66.3KB, pdf)
Data Availability Statement
No data are available.