Abstract
Objectives
Delirium is a neuropsychiatric disorder that commonly occurs in elderly patients with cognitive impairment. The economic burden of delirium in Japan has not been well characterised. In this study, we assessed incremental medical costs of delirium in hospitalised elderly Japanese patients with cognitive impairment.
Design
Retrospective, cross-sectional, observational study.
Setting
Administrative data collected from acute care hospitals in Japan between April 2012 and September 2020.
Participants
Hospitalised patients ≥65 years old with cognitive impairment were categorised into groups—with and without delirium. Delirium was identified using a delirium identification algorithm based on the International Classification of Diseases 10th Revision codes or antipsychotic prescriptions.
Outcome measures
Total medical costs during hospitalisation were compared between the groups using a generalised linear model.
Results
The study identified 297 600 hospitalised patients ≥65 years of age with cognitive impairment: 39 836 had delirium and 257 764 did not. Patient characteristics such as age, sex, inpatient department and comorbidities were similar between groups. Mean (SD) unadjusted total medical cost during hospitalisation was 979 907.7 (871 366.4) yen for patients with delirium and 816 137.0 (794 745.9) yen for patients without delirium. Adjusted total medical cost was significantly greater for patients with delirium compared with those without delirium (cost ratio=1.09, 95% CI: 1.09 to 1.10; p<0.001). Subgroup analyses revealed significantly higher total medical costs for patients with delirium compared with those without delirium in most subgroups except patients with hemiplegia or paraplegia.
Conclusions
Medical costs during hospitalisation were significantly higher for patients with delirium compared with those without delirium in elderly Japanese patients with cognitive impairment, regardless of patient subgroups such as age, sex, intensive care unit admission and most comorbidities. These findings suggest that delirium prevention strategies are critical to reducing the economic burden as well as psychological/physiological burden in cognitively impaired elderly patients in Japan.
Keywords: Delirium & cognitive disorders, Dementia, Old age psychiatry
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study is the first in Japan to assess medical costs associated with delirium using a large nationwide database consisting of claims and discharge abstract data.
The study identified over 290 000 Japanese patients with cognitive impairment, with and without delirium.
This study did not limit patients by baseline characteristics such as departments, surgical procedures and comorbidities, thus providing a more generalisable view of the economic impact of delirium.
The study demonstrates that delirium is associated with significantly higher medical costs during hospitalisation, suggesting that prevention strategies may be critical to reducing the economic burden imposed by delirium.
This study only assessed a single episode of delirium during hospitalisation, potentially underestimating incremental costs associated with delirium beyond those captured in this cohort and time frame.
Introduction
Delirium is an acute neuropsychiatric disorder characterised by inattention and cognitive decline.1–3 Delirium is often observed in the elderly and in patients with cognitive impairment, including dementia,4 and is commonly observed in hospitalised patients such as intensive care unit (ICU), postoperative and palliative care patients.2 4 The incidence rate of delirium in the elderly ranges from 4% to 25% among hospitalised patients,5 from 15% to 53% among postoperative patients1 and is 80% among patients in the ICU.1
Patients with delirium often require additional resource use, which increases the burden on healthcare workers such as nurses.6–8 As a result, delirium poses a substantial burden on the healthcare system at large, as ongoing care requires additional medical resources. The presence of delirium may result in the administration of additional treatments, both pharmacological and non-pharmacological,4 frequent rehospitalisation and a greater risk of admission to long-term care.9 The presence of delirium has been shown to prolong hospital stays,10–12 which may potentially increase treatment costs and resource use. In fact, delirium following transcatheter and surgical aortic valve replacement resulted in a longer hospital stay and, consequently, an increase in hospitalisation costs.13
Dementia is one of the leading risk factors for delirium and often coexists with delirium among elderly patients.4 14 15 It has also been reported that patients with Alzheimer’s disease with delirium have a poorer trajectory of cognitive decline in the long term than those without delirium,16 17 and there has been evidence to show incremental medical cost of delirium in elderly patients with cognitive impairment in several populations.18 19 For instance, Fick et al reported incremental medical cost in a community-dwelling population with dementia from southeastern USA, comprising 2796 individuals over a period of 3 years.18 Boone et al reported additional medical costs for patients with postoperative neurocognitive disorders including delirium and dementia across 4285 hospitals in the USA.19 However, there is currently no published literature investigating the economic burden of delirium in Japan using a large-scale medical record database. Japan has the highest elderly population in the world, with almost 30% of the population aged 65 years and above.20 In addition, the number of hospitalised patients over 65 years old is increasing.21 Furthermore, 2.9%–12.5% of the ageing population in Japan is estimated to have dementia, which is increasing annually.22 Therefore, it is important to understand the economic burden of delirium in elderly patients with dementia in Japan. This study aimed to estimate the economic burden of delirium in hospitalised elderly patients with cognitive impairment in the Japanese population by means of a nationwide administrative database of acute care hospitals.
Methods
Study design and data source
This was a retrospective, cross-sectional, observational study evaluating medical costs of cognitively impaired elderly patients with and without delirium, using a nationwide administrative database (Medical Data Vision (MDV); Medical Data Vision Co, Ltd, Tokyo, Japan).23 The MDV database comprises anonymised administrative data of over 30 million patients from over 400 acute care hospitals, which covers approximately 24% of all acute care hospitals in Japan and contains claims and discharge abstract data acquired from inpatient and outpatient visits.23 The data used in the present study were collected between 1 April 2012 and 30 September 2020.
Patient characteristics were obtained from the discharge abstract data called Form 1. Data on treatments, procedures and prescriptions based on the Anatomical Therapeutic Chemical (ATC) classification system codes were obtained from the medical practice information field called Act Data. Disease diagnosis information based on the International Classification of Diseases 10th Revision (ICD-10) was obtained from the Disease Data field. Hospital scale information was obtained from the Patient Data field.
Patient and public involvement
This retrospective study did not involve patients in any phase, and the data presented here were obtained from an anonymised administrative hospital database.
Patient selection and characteristics
Patients were included if they were hospitalised for surgery or under an emergency, were ≥65 years of age at hospitalisation and had cognitive impairment. Cognitive impairment was defined as the presence of at least a diagnosis of dementia (ICD-10 codes F00–F03, F067, F107, G238), one prescription of an anti-dementia medication during hospitalisation (donepezil, galantamine, memantine or rivastigmine) or a low rank (I–IV and M) on the Dementia Scale—an observer-rated scale used to assess the degree of independence in activities of daily living (ADL) related to dementia (online supplemental table S1).24
bmjopen-2022-062141supp001.pdf (466.6KB, pdf)
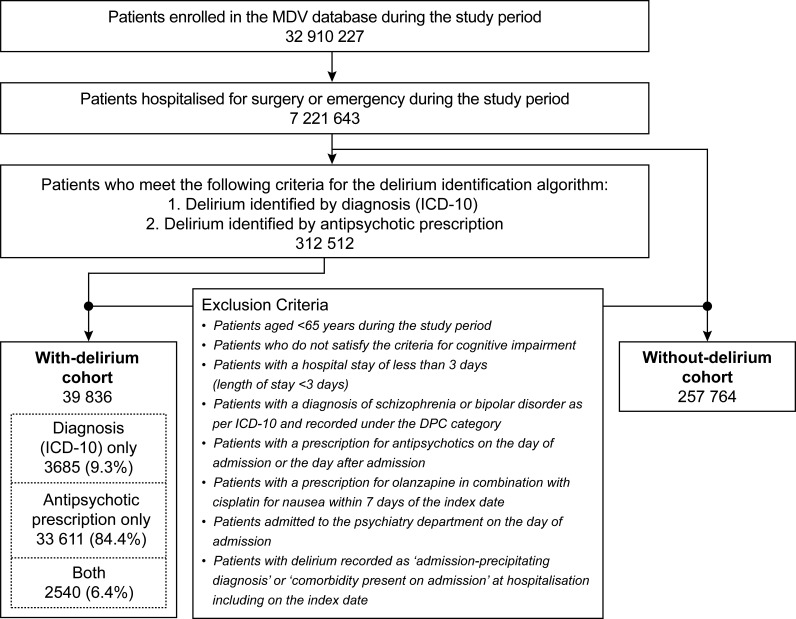
Patients with delirium were identified if they met the criteria for the delirium identification algorithm based on the algorithm previously proposed by Kim et al,25 which was modified to reflect with the clinical setting in Japan. Delirium was defined as having either a diagnosis of delirium (ICD-10 code, F05) or a prescription of at least one of five antipsychotic drugs (ATC code, N05A: quetiapine, haloperidol, perospirone, risperidone or olanzapine; online supplemental table S2), as recommended for the treatment of delirium by the Japanese Society of General Hospital Psychiatry.26 Prescriptions made within 1 week of hospitalisation were included. Patients were required to have a minimum hospital stay of 3 days with at least 2 days free from antipsychotic treatment after admission. This ‘2-day washout’ period was set to exclude patients who were prescribed antipsychotics for pre-existing conditions. Patients with other psychiatric conditions such as schizophrenia (ICD-10 codes F20–29) and bipolar disorder (ICD-10 codes F30–31) were excluded. Patients who had delirium recorded as ‘admission-precipitating diagnosis’ or ‘comorbidities present on admission’ on the index date or the day after were also excluded (figure 1). Patients prescribed olanzapine combined with cisplatin for nausea within 7 days from the index date were excluded.
Figure 1.
Patient selection flow chart. DPC, Diagnosis Procedure Combination; ICD-10, International Classification of Diseases, 10th Revision; MDV, Medical Data Vision.
Repeated episodes of hospitalisation were not evaluated, that is, only the first hospitalisation was evaluated if there was a record of multiple hospital admissions. The observation period was from the index date to the end of hospitalisation, defined as discharge, transfer to another hospital/nursing home or death.
The following information was collected from the administrative database for the groups with and without delirium: patient characteristics such as sex, age and ADL score (based on the Barthel Index27); comorbidities based on ICD-10 codes; inpatient departments; presence or absence of hospitalisation; type of surgery including type and duration of anaesthesia; numbers and classes of potentially inappropriate medications (PIMs; benzodiazepines, non-benzodiazepines, opioids, corticosteroids, H1-receptor antagonists, H2-receptor antagonists, antidepressants and anticholinergic drugs) that are thought to increase the risk of delirium, as identified based on the Beers criteria,28 the guidelines for medical treatment and its safety in the elderly from the Japan Geriatrics Society Working Group29 and the report by Noshiro et al30; duration of hospitalisation including ICU stay and patient outcomes such as death.
Outcomes
Total medical costs during hospitalisation (from index date to discharge date) were assessed for patients with and without delirium. The total medical expenses include the following: (1) drug cost, including formulations for internal and external use, and potions; (2) dispensary fee, including pharmacy charge and compounding fee such as for dispensing, prescription, narcotic/poisonous drug addiction, basic fee on receiving prescription and medication cost reduction; (3) surgical cost, including cost of surgery and anaesthesia; (4) treatment cost, including only treatment fee; (5) inspection cost, including pathological examination cost; (6) imaging cost, including image diagnosis; and (7) hospitalisation cost, including hospitalisation basic rate, specific hospital charge, diet therapy standard cost-sharing and life therapy standard cost-sharing.
Statistical analyses
In each group, outcome variables were summarised using standard descriptive statistics including mean, SD, median and IQR for continuous variables and the number and percentage of patients for categorical variables.
Total medical expenses were adjusted for patient characteristics and other confounders using a generalised linear model (GLM). Predefined covariates such as age, sex, ADL, presence or absence of 15 comorbidities (excluding dementia and AIDS/HIV from the 17 Charlson comorbidities; AIDS/HIV was excluded due to the lack of sufficient sample size during the study period), presence or absence of emergency hospitalisation, type and duration of anaesthesia during surgery, number of PIMs and ICU admission were included as covariates. Univariate analysis was performed with each covariate listed above.
Multicollinearity was evaluated using pairwise correlation coefficients and variance inflation factors (VIFs) for the multivariable linear regression framework were calculated prior to a quasi-likelihood analysis. Since there was no covariate with a VIF of >10, all covariates were included in the final model. For the GLM-adjusted total medical cost, missing values for the response variable and covariates were imputed (except in the subgroup analysis) by means of the multiple imputation method using the full conditional specification approach. Imputations were performed 100 times; the response variable was also included in the imputation model to reduce bias. To impute missing values, Bayesian regression models such as linear, discriminant function and logistic models were adopted for response variable and covariates, depending on the nature of the data.31 32 To address the non-normality and heteroscedasticity of the total medical cost, the quasi-likelihood method (QLM) was used with a logarithmic link function,33 34 and a dispersion parameter was introduced in the GLM. QLM allows for the variance function to be proportional to a power (exponent) of the mean (see online supplemental information for more details). The geometric least squares (LS) mean for total medical cost in each group, the geometric LS mean ratio between the two groups and its 95% CI were calculated.
Subgroup analyses based on patient characteristics, comorbidities and other covariates were performed using a similar GLM to investigate how total medical cost varied among the different subgroups. Statistical p value for the comparison between two groups in each subgroup was computed using a similar GLM used for the primary analysis, excluding the corresponding subgroup variable. Interaction for p values were computed in a similar manner but with the addition of an interaction term between the subgroup variable and the indicative variable of delirium (with or without delirium) to the primary analysis model. All analyses were performed using SAS V.9.4 (SAS Institute). For all statistical analyses, a two-sided p value of <0.05 was considered statistically significant. No corrections for multiple comparisons were performed.
Results
Patient attrition
A total of 7 221 643 patients hospitalised for either elective surgery or emergency during the study period were available in the MDV database.23 Subsequently, 312 512 patients were identified by the delirium identification algorithm. The final cohort of patients ≥65 years of age and with cognitive impairment comprised 39 836 patients with delirium and 257 764 patients without delirium (figure 1). In the group of patients with delirium, 3685 patients were identified by the ICD-10 criteria (F05) for delirium, 33 611 patients were identified by prescriptions of selected antipsychotics and 2540 patients were identified by both the ICD-10 criteria and prescriptions of antipsychotics.
Among the patients with delirium identified by the delirium identification algorithm (n=39 836), the most common diagnosis based on the ICD-10 criteria was delirium in 4093 patients (10.3%, under the code F05.9; online supplemental table S2), followed by delirium superimposed on dementia in 1027 patients (2.6%, code F05.1; online supplemental table S2). For the prescribed antipsychotics used for the delirium identification algorithm, the most common medication was haloperidol injection in 17 188 patients (43.1%), followed by risperidone solution in 12 081 patients (30.3%) and quetiapine tablet in 7489 patients (18.8%). The use of perospirone and olanzapine tablets was relatively uncommon (1.9% and 0.9%, respectively; online supplemental table S2).
Baseline characteristics
Patient demographics were comparable between the two groups (table 1), with a male population of 45.4% in the group with delirium and 40.1% in the group without delirium. Overall, 54.5% of patients with delirium and 51.4% of patients without delirium were aged ≥85 years. Moreover, 75.4% of patients with delirium and 68.4% of patients without delirium were dependent (ADL score 0–59). The proportion of patients with dementia diagnosed by the ICD-10 criteria was 53.6% in the group with delirium and 43.7% in the group without delirium. Additionally, 30.0% of patients with delirium were prescribed anti-dementia medications compared with 25.6% of patients without delirium (online supplemental table S1). More than 20% of patients across both groups had been prescribed ≥4 PIMs (with delirium group: 29.7%, without delirium group: 20.6%) (table 1).
Table 1.
Patient demographics and characteristics
| Number of patients | Number of patients with delirium | Number of patients without delirium |
| 39 836 | 257 764 | |
| Age (years), n (%) | ||
| Mean (SD) | 84.6 (7.0) | 84.1 (7.3) |
| 65–74 | 3623 (9.1) | 28 597 (11.1) |
| 75–84 | 14 491 (36.4) | 96 685 (37.5) |
| ≥85 | 21 722 (54.5) | 132 482 (51.4) |
| Sex, n (%) | ||
| Male | 18 104 (45.4) | 103 313 (40.1) |
| Female | 21 732 (54.6) | 154 451 (59.9) |
| ADL score (point), n (%) | ||
| Dependent group (0–59) | 30 048 (75.4) | 176 395 (68.4) |
| Independent group (60–100) | 9206 (23.1) | 78 154 (30.3) |
| Unknown | 582 (1.5) | 3215 (1.2) |
| Emergency hospitalisation, n (%) | ||
| Yes | 31 662 (79.5) | 189 328 (73.5) |
| Inpatient department*, n (%) | ||
| Internal medicine | 10 699 (26.9) | 72 910 (28.3) |
| Orthopaedics | 4842 (12.2) | 28 591 (11.1) |
| Gastroenterology | 4462 (11.2) | 25 993 (10.1) |
| Surgery | 4139 (10.4) | 19 011 (7.4) |
| Cardiology | 3890 (9.8) | 25 536 (9.9) |
| Neurosurgery | 2946 (7.4) | 23 876 (9.3) |
| Comorbidities†, n (%) (ICD-10 major category) |
||
| Circulatory disease | 25 456 (63.9) | 162 440 (63.0) |
| Endocrine, nutritional and metabolic diseases | 17 047 (42.8) | 110 282 (42.8) |
| Gastrointestinal disorders | 14 120 (35.4) | 83 928 (32.6) |
| Nervous system disorders | 14 016 (35.2) | 85 399 (33.1) |
| Respiratory disease | 12 325 (30.9) | 74 019 (28.7) |
| Mental and behavioural disorders | 11 492 (28.8) | 54 927 (21.3) |
| Surgery, n (%) | ||
| Yes | 17 994 (45.2) | 116 178 (45.1) |
| Type of surgery/anaesthesia | ||
| Surgery+no/local/light general anaesthesia | 10 050 (25.2) | 78 114 (30.3) |
| Surgery+general anaesthesia (<2 hours) | 4522 (11.4) | 25 203 (9.8) |
| Surgery+general anaesthesia (≥2 hours) | 3422 (8.6) | 12 861 (5.0) |
| Prescription of PIMs, n (%) | ||
| Yes | 18 370 (46.1) | 108 326 (42.0) |
| Number of PIMs (drugs) | ||
| 1 | 2146 (5.4) | 21 407 (8.3) |
| 2 | 2319 (5.8) | 20 086 (7.8) |
| 3 | 2070 (5.2) | 13 859 (5.4) |
| ≥4 | 11 835 (29.7) | 52 974 (20.6) |
| Duration of hospitalisation‡ (days) | ||
| Mean (SD) | 15.9 (11.6) | 14.2 (13.4) |
| Median | 14.0 | 12.0 |
| (Q1, Q3) | (9.0, 20.0) | (7.0, 18.0) |
| Duration of ICU stay (days) | ||
| Yes | 5942 (14.9) | 20 975 (8.1) |
| Mean (SD) | 3.2 (2.9) | 2.9 (2.9) |
| Median | 2.0 | 2.0 |
| (Q1, Q3) | (1.0, 4.0) | (1.0, 4.0) |
| Death, n (%) | ||
| Yes | 3574 (9.0) | 23 121 (9.0) |
| No | 36 262 (91.0) | 234 633 (91.0) |
*Top 6 of all selected departments are shown here.
†Top 6 of all selected comorbidities are shown here.
‡Duration of hospital stay (minimum, maximum): with delirium cohort (3 days, 495 days); without delirium cohort (3 days, 1357 days).
ADL, activities of daily living; ICD-10, International Classification of Diseases, 10th Revision; ICU, intensive care unit; PIMs, potentially inappropriate medications; Q, quartile.
Prognosis/hospitalisation
The median (IQR) duration of hospitalisation was 14 (9.0–20.0) days for patients with delirium and 12 (7.0–18.0) days for patients without delirium. Only 16.1% of patients with delirium were hospitalised for ≤1 week compared with 27.1% of patients without delirium. Median (IQR) duration of ICU stay was 2 (1.0–4.0) days in both groups; 14.9% of the patients with delirium and 8.1% of the patients without delirium were admitted to the ICU for at least 1 day (table 1 and online supplemental table S3).
Unadjusted medical costs in cognitively impaired elderly patients with and without delirium
The mean (SD) total medical cost per patient was 979 907.7 (871 366.4) yen in the group with delirium and 816 137.0 (794 745.9) yen in the group without delirium (table 2). In both groups, the largest contributor to the total medical cost was hospitalisation, followed by surgery (table 2). When categorised by patient characteristics, a similar pattern was observed; hospitalisation costs and surgical costs were the major contributors to total medical cost (online supplemental figure S1) in both groups. The subgroup of patients who underwent surgery and longer anaesthesia (≥2 hours) incurred the highest total cost across subgroups (online supplemental figure S1). When characterised by patient comorbidities, across most subgroups, hospitalisation cost emerged as the greatest contributor to total cost, followed by surgery. However, for patients with peripheral vascular disease, surgical cost was higher than hospitalisation cost (online supplemental figure S2).
Table 2.
Unadjusted medical costs in patients with cognitive impairment with and without delirium
| Patient cohort with delirium Mean±SD (JPY) per patient |
Patient cohort without delirium Mean±SD (JPY) per patient |
|
| N | 39 836 | 257 764 |
| Total | 979 907.7±871 366.4 | 816 137.0±794 745.9 |
| Hospitalisation cost | 528 760.0±351 385.0 | 445 497.1±347 548.9 |
| Surgical cost | 277 683.9±576 399.4 | 231 177.1±511 700.1 |
| Inspection cost | 66 846.6±90 615.6 | 54 202.6±49 425.2 |
| Drug cost | 53 420.9±159 390.4 | 41 097.3±182 713.4 |
| Imaging cost | 35 129.7±31 289.1 | 29 423.4±29 107.7 |
| Treatment cost | 16 951.5±72 122.6 | 13 843.1±84 341.6 |
| Dispensary cost | 1115.2±926.6 | 896.3±1036.2 |
JPY, Japanese yen; N, number of patients.
Adjusted medical costs in cognitively impaired elderly patients with and without delirium
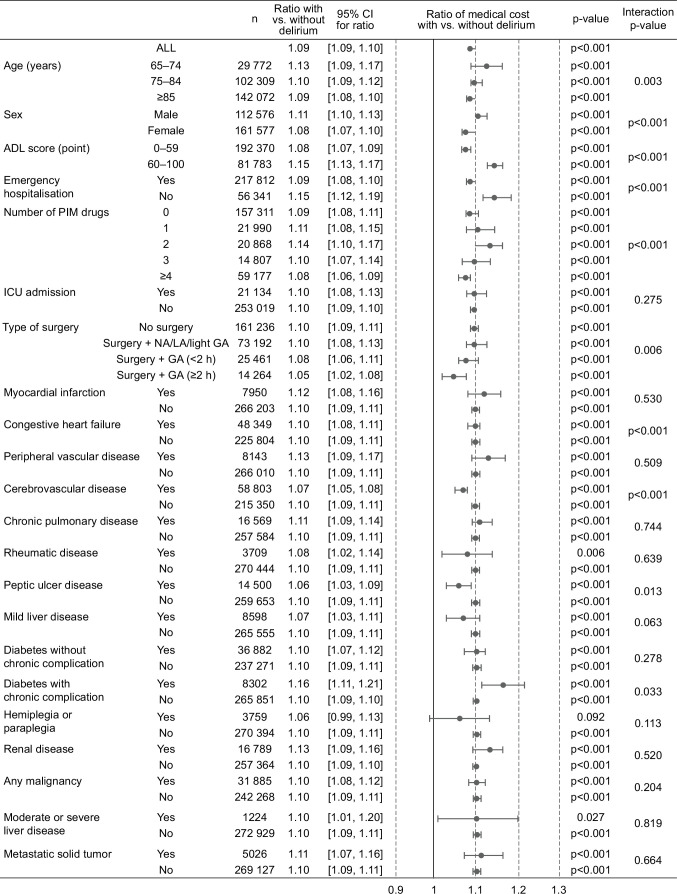
The adjusted total medical cost per patient was significantly greater in patients with delirium compared with patients without delirium (cost ratio=1.09, 95% CI: 1.09 to 1.10; p<0.001; table 3). When categorised by patient characteristics and comorbidities, patients with delirium incurred significantly higher costs compared with those without delirium in most of the subgroups except patients with hemiplegia or paraplegia (figure 2). Specifically, the increases in cost between those with delirium versus without delirium ranged from 5% to 16% across subgroups (figure 2). The greatest increase in cost was observed among patients having diabetes with chronic complications (cost ratio=1.16), patients who were independent (ADL score 60–100; cost ratio=1.15) and patients who had prescriptions of two PIMs (cost ratio=1.14). When the effect of each subgroup on adjusted cost ratio was assessed, significant interaction effects (figure 2) were observed for subgroups based on patient characteristics such as age (p=0.003), sex (p<0.001), ADL (p<0.001), emergency hospitalisation (p<0.001), PIM use (p<0.001) and surgery (p=0.006). The geometric LS mean ratios of the total medical costs from the univariate analysis were generally similar to those from the multivariable analysis, although only emergency hospitalisation was adjusted for in the multivariable analysis (online supplemental table S4).
Table 3.
Difference in the GLM-adjusted total medical cost
| n | Geometric LS mean (JPY) (SE) |
95% CI | Geometric LS mean ratio* |
95% CI for ratio | p-value | |
| Patients with delirium | 39 836 | 815 721.2 (1.0) | 810 206.1 to 821 273.9 | 1.09 | 1.09 to 1.10 | <0.001 |
| Patients without delirium | 257 764 | 745 295.0 (1.0) | 743 312.2 to 747 283.0 |
*Geometric LS mean ratio, with delirium/without delirium.
GLM, generalised linear model; JPY, Japanese yen; LS, least squares; n, number of patients.
Figure 2.
Adjusted medical cost categorised by patient characteristics and comorbidities. Since multiple imputation (MI) for missing values was not conducted for subgroup analyses due to time constraints, the total number of patients in each subgroup was not consistent with those in the main analysis where missing values were imputed using MI. ADL, activities of daily living; GA, general anaesthesia; ICU, intensive care unit; LA, light anaesthesia; n, number of patients; NA, no anaesthesia; PIM, potentially inappropriate medication.
Discussion
This study is the largest medical cost analysis of delirium in Japan to date, aimed at evaluating elderly patients with cognitive impairment in acute care hospitals. There was a 9% increase in total medical cost during hospitalisation in the patient group with delirium compared with the patient group without delirium. The total medical cost was consistently higher in the patient group with delirium than in the patient group without delirium, irrespective of patient characteristics, type of surgery and comorbidities (except patients with hemiplegia or paraplegia). There have been various reports of increased medical costs for patients with delirium. According to a systematic review, the additional cost of delirium is estimated to be in the range of US$806–US$24 509.35 A population-based retrospective study from 490 US hospitals reported an additional admission cost of US$2697 (23.7% increase) for patients with postoperative delirium after major urological cancer surgeries.36 Thus, the additional cost of delirium varies depending on the study duration and the target population, as well as the specific healthcare system in each country. Although the present study did not follow the medical cost of post-discharge period, additional medical cost during hospitalisation was observed in the patient group with delirium compared with the patient group without delirium, implying that the actual difference in medical costs for longer duration could be much larger. A study by Leslie et al, with a longer observation period, reported that the incremental healthcare costs due to delirium up to 1 year after discharge were nearly twofold higher for patients with delirium compared with patients without delirium.37 It has been previously reported that patients experiencing delirium have poorer prognosis even after hospital discharge,38–40 indicating prolonged utilisation of healthcare resources and consequent increase in treatment cost.
Previous studies have reported non-pharmacological interventions for the prevention of delirium in hospitalised elderly patients and patients with surgical treatments.41–45 Multicomponent non-pharmacological interventions for delirium have been implemented worldwide to reduce the incidence of delirium.46 In Japan, a systematic prevention programme reportedly decreased the incidence of delirium and improved clinical outcomes such as length of stay and incidence of falls.47 Pharmacological approaches to prevent delirium have also been studied.48 49 Effective delirium prevention strategies may contribute to reducing the incremental medical cost reported in the present study, as it has previously been reported that the prevention of delirium by multicomponent, targeted interventions decreased long-term nursing home costs.50 However, this must be further explored in larger dedicated studies.51
In the present study, we identified over 39 000 cognitively impaired elderly patients with delirium from a nationwide administrative database (MDV database23) using a delirium identification algorithm. The diagnosis of delirium by the ICD-10 criteria alone identified 9.3% of all patients identified by our algorithm. By contrast, 84.4% of patients with delirium were identified based on the prescription of antipsychotics. This result is consistent with the finding of a previous report from our research group52 as well as another study in Japan.53
Certain limitations to our study should be noted. The sensitivity and specificity of our modified delirium identification algorithm have not been validated in Japan.53 This requires that the algorithm be evaluated against the bedside assessment by an expert,25 which is usually feasible for single institutions but not for large-scale medical databases with more than 400 acute care hospitals, such as the one used in this study. Moreover, data on hypoactive delirium were not captured, because the included antipsychotics are used to treat hyperactive delirium. Data were limited to acute care hospitals and clinics registered under the Diagnosis Procedure Combination programme,54 thereby under-representing cases. Additionally, because the MDV database does not provide hospital identification data, we could not include the variability across hospitals as a random effect in the GLM. However, the variability across sites was included in the variability of error in the model (ie, we used a larger variability of error than that adjusted by the random effect). Therefore, the current results are considered adequately conservative. This study reports the costs pertaining to only one delirium-related hospitalisation, not considering recurrences, rehospitalisation or outpatient and rehabilitation costs. Finally, this study was not designed to investigate the causal link between the increase in cost and delirium.
In conclusion, this study demonstrated significantly higher medical costs associated with delirium among hospitalised elderly patients with cognitive impairment in Japan. The difference in medical cost was consistent regardless of patient characteristics and clinical settings, such as age, sex, ICU admission and most comorbidities, suggesting the economic burden of delirium is not attributed to specific patient characteristics and clinical settings. These findings suggest that delirium prevention strategies are important for reducing the economic burden of delirium for the cognitively impaired elderly in Japan.
Supplementary Material
Acknowledgments
The authors thank Shinya Miura, Hideaki Ogawa and Shinichiro Suzuki of CMIC Co, Ltd, for medical writing of the protocol and data analysis, and Hirokazu Kikuchi of CMIC Co, Ltd, for statistical modelling and data analysis support, which was funded by and performed under the guidance and approval of MSD KK, Tokyo, Japan. The authors also thank Machiko Abe of MSD KK, Tokyo, Japan, and Rezaul Karim Khandker and Geoffrey Johnson of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc, Rahway, New Jersey, USA, for advising on statistical analyses for cost comparisons. Editorial support, in the form of medical writing, assembling tables and creating high-resolution images based on authors’ detailed directions, collating author comments, copyediting, fact-checking and referencing, was provided by Annirudha Chillar and Varsha Sreenivasan of Cactus Life Sciences (part of Cactus Communications) and funded by MSD KK, Tokyo, Japan.
Footnotes
Contributors: SO, HS, KT, ZPQ, ST, AO and YO conceptualised the study. MI, NU, KO and SO planned the study design and data analysis. KO and YO designed the statistical analysis. HS, KT, ZPQ and ST contributed to the study design. KT, AO and YO provided advice on study design and contributed to the interpretation of the findings from the viewpoint of the clinical scientist, the physician and the epidemiologist, respectively. All authors contributed to interpretation of data and approved the final version of the manuscript. MI and SO are guarantors and accept full responsibility for the work.
Funding: This work was supported by MSD KK, Tokyo, Japan.
Disclaimer: The funder of the study was involved in the development of the study design, data analysis, data interpretation, writing of the manuscript and the decision to submit the manuscript for publication. All authors had full access to the study results.
Competing interests: MI, KO, NU, HS, KT, ST and SO are employees of MSD KK, Tokyo, Japan, a subsidiary of Merck & Co, Inc, Rahway, New Jersey, USA, and may own stock and/or stock options in Merck & Co, Inc, Rahway, New Jersey, USA. ZPQ was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, New Jersey, USA, at the time of the study and may have owned stock and/or stock options in Merck & Co, Inc, Rahway, New Jersey, USA. AO and YO have received funding from MSD KK, Tokyo, Japan, for research consulting.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. The Medical Data Vision database analysed in this study is not publicly accessible. According to the contract with Medical Data Vision Co, Ltd, the data cannot be shared with external researchers.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study used anonymised/de-identified data and therefore ethical review was not required, per the Ethical Guidelines for Epidemiological Research of the Japanese Ministry of Health, Labour and Welfare. Thus, no ethical or institutional review board approval was sought for this study.
References
- 1.Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry 2008;165:803–12. 10.1176/appi.ajp.2008.08020181 [DOI] [PubMed] [Google Scholar]
- 2.Hshieh TT, Inouye SK, Oh ES. Delirium in the elderly. Clin Geriatr Med 2020;36:183–99. 10.1016/j.cger.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 3.Oh ES, Fong TG, Hshieh TT, et al. Delirium in older persons: advances in diagnosis and treatment. JAMA 2017;318:1161–74. 10.1001/jama.2017.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. The Lancet 2014;383:911–22. 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006;35:350–64. 10.1093/ageing/afl005 [DOI] [PubMed] [Google Scholar]
- 6.Thomas N, Coleman M, Terry D. Nurses' experience of caring for patients with delirium: systematic review and qualitative evidence synthesis. Nurs Rep 2021;11:164–74. 10.3390/nursrep11010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonnell S, Timmins F. A quantitative exploration of the subjective burden experienced by nurses when caring for patients with delirium. J Clin Nurs 2012;21:2488–98. 10.1111/j.1365-2702.2012.04130.x [DOI] [PubMed] [Google Scholar]
- 8.Schmitt EM, Gallagher J, Albuquerque A, et al. Perspectives on the delirium experience and its burden: common themes among older patients, their family caregivers, and nurses. Gerontologist 2019;59:327–37. 10.1093/geront/gnx153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCusker J, Cole M, Dendukuri N, et al. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ 2001;165:575–83. 10.3928/00989134-20070201-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291:1753–62. 10.1001/jama.291.14.1753 [DOI] [PubMed] [Google Scholar]
- 11.Thomason JWW, Shintani A, Peterson JF, et al. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care 2005;9:R375–81. 10.1186/cc3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Boogaard M, Schoonhoven L, van der Hoeven JG, et al. Incidence and short-term consequences of delirium in critically ill patients: a prospective observational cohort study. Int J Nurs Stud 2012;49:775–83. 10.1016/j.ijnurstu.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 13.Potter BJ, Thompson C, Green P, et al. Incremental cost and length of stay associated with postprocedure delirium in transcatheter and surgical aortic valve replacement patients in the United States. Catheter Cardiovasc Interv 2019;93:1132–6. 10.1002/ccd.28014 [DOI] [PubMed] [Google Scholar]
- 14.Fong TG, Davis D, Growdon ME, et al. The interface between delirium and dementia in elderly adults. Lancet Neurol 2015;14:823–32. 10.1016/S1474-4422(15)00101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inouye SK. Delirium in older persons. N Engl J Med 2006;354:1157–65. 10.1056/NEJMra052321 [DOI] [PubMed] [Google Scholar]
- 16.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 2009;72:1570–5. 10.1212/WNL.0b013e3181a4129a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med 2012;172:1324–31. 10.1001/archinternmed.2012.3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fick DM, Kolanowski AM, Waller JL, et al. Delirium superimposed on dementia in a community-dwelling managed care population: a 3-year retrospective study of occurrence, costs, and utilization. J Gerontol A Biol Sci Med Sci 2005;60:748–53. 10.1093/gerona/60.6.748 [DOI] [PubMed] [Google Scholar]
- 19.Boone MD, Sites B, von Recklinghausen FM, et al. Economic burden of postoperative neurocognitive disorders among US Medicare patients. JAMA Netw Open 2020;3:e208931. 10.1001/jamanetworkopen.2020.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statistics of Bureau, Ministry of Internal Affairs and Communications . Population estimates by age (five-year groups) and sex, 2021. Available: https://www.e-stat.go.jp/en/stat-search/file-download?statInfId=000032191044&fileKind=4 [Accessed 15 Apr 2022].
- 21.Summary of patient survey, Ministry of health and welfare (Japanese), 2017. Available: https://www.mhlw.go.jp/toukei/saikin/hw/kanja/17/index.html [Accessed 02 Feb 2022].
- 22.Okamura H, Ishii S, Ishii T, et al. Prevalence of dementia in Japan: a systematic review. Dement Geriatr Cogn Disord 2013;36:111–8. 10.1159/000353444 [DOI] [PubMed] [Google Scholar]
- 23.Medical data vision (MDV) database, 2022. Available: https://www.mdv.co.jp/mdv_database/english/ [Accessed 02 Feb 2022].
- 24.Sakata N, Okumura Y, Fushimi K, et al. Dementia and risk of 30-day readmission in older adults after discharge from acute care hospitals. J Am Geriatr Soc 2018;66:871–8. 10.1111/jgs.15282 [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Lee J, Kim CA, et al. Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiol Drug Saf 2017;26:945–53. 10.1002/pds.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Committee on Treatment Strategy and Tactics . Clinical guideline for the treatment of delirium, 2nd edition (Japanese Society of General Hospital Psychiatry Practice Guidelines 1). Tokyo, Japan: Seiwa Shoten Publishers, 2015: 85–111. [Google Scholar]
- 27.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 28.The 2019 American Geriatrics Society Beers Criteria® Update Expert Panel . American Geriatrics Society 2019 updated AGS beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019;67:674–94. 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 29.Kojima T, Mizukami K, Tomita N, et al. Screening Tool for Older Persons' Appropriate Prescriptions for Japanese: Report of the Japan Geriatrics Society Working Group on "Guidelines for medical treatment and its safety in the elderly". Geriatr Gerontol Int 2016;16:983–1001. 10.1111/ggi.12890 [DOI] [PubMed] [Google Scholar]
- 30.Noshiro Y, Imai T, Sakai M. Relationship between the onset of delirium during hospitalization and the use of high-risk drugs for delirium. Med J Matsue City Hosp 2018;1:33–5. 10.32294/mch.22.1_33 [DOI] [Google Scholar]
- 31.Brand JPL . Development, implementation and evaluation of multiple imputation strategies for the statistical analysis of incomplete data sets, 1999. Available: https://repub.eur.nl/pub/19790/990408_BRAND,%20Jacob%20Pieter%20Laurens.pdf [Accessed 02 Feb 2022].
- 32.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 33.Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy 2004;9:197–204. 10.1258/1355819042250249 [DOI] [PubMed] [Google Scholar]
- 34.Blough DK, Ramsey SD. Using generalized linear models to assess medical care costs. Health Serv Outcomes Res Methodol 2000;1:185–202. 10.1023/A:1012597123667 [DOI] [Google Scholar]
- 35.Kinchin I, Mitchell E, Agar M, et al. The economic cost of delirium: a systematic review and quality assessment. Alzheimers Dement 2021;17:1026–41. 10.1002/alz.12262 [DOI] [PubMed] [Google Scholar]
- 36.Ha A, Krasnow RE, Mossanen M, et al. A contemporary population-based analysis of the incidence, cost, and outcomes of postoperative delirium following major urologic cancer surgeries. Urol Oncol 2018;36:341.e15–341.e22. 10.1016/j.urolonc.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 37.Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008;168:27–32. 10.1001/archinternmed.2007.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One 2011;6:e26951. 10.1371/journal.pone.0026951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leslie DL, Zhang Y, Holford TR, et al. Premature death associated with delirium at 1-year follow-up. Arch Intern Med 2005;165:1657–62. 10.1001/archinte.165.14.1657 [DOI] [PubMed] [Google Scholar]
- 40.Rudolph JL, Inouye SK, Jones RN, et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc 2010;58:643–9. 10.1111/j.1532-5415.2010.02762.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burry LD, Cheng W, Williamson DR, et al. Pharmacological and non-pharmacological interventions to prevent delirium in critically ill patients: a systematic review and network meta-analysis. Intensive Care Med 2021;47:943–60. 10.1007/s00134-021-06490-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669–76. 10.1056/NEJM199903043400901 [DOI] [PubMed] [Google Scholar]
- 43.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001;49:516–22. 10.1046/j.1532-5415.2001.49108.x [DOI] [PubMed] [Google Scholar]
- 44.Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing 2005;34:152–6. 10.1093/ageing/afi031 [DOI] [PubMed] [Google Scholar]
- 45.Vidán MT, Sánchez E, Alonso M, et al. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc 2009;57:2029–36. 10.1111/j.1532-5415.2009.02485.x [DOI] [PubMed] [Google Scholar]
- 46.Hshieh TT, Yang T, Gartaganis SL, et al. Hospital elder life program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry 2018;26:1015–33. 10.1016/j.jagp.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogawa A, Okumura Y, Fujisawa D, et al. Quality of care in hospitalized cancer patients before and after implementation of a systematic prevention program for delirium: the DELTA exploratory trial. Support Care Cancer 2019;27:557–65. 10.1007/s00520-018-4341-8 [DOI] [PubMed] [Google Scholar]
- 48.Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry 2019;76:526–35. 10.1001/jamapsychiatry.2018.4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu S, Cui Y, Shen J, et al. Suvorexant for the prevention of delirium: a meta-analysis. Medicine 2020;99:e21043. 10.1097/MD.0000000000021043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leslie DL, Zhang Y, Bogardus ST, et al. Consequences of preventing delirium in hospitalized older adults on nursing home costs. J Am Geriatr Soc 2005;53:405–9. 10.1111/j.1532-5415.2005.53156.x [DOI] [PubMed] [Google Scholar]
- 51.Rubin FH, Neal K, Fenlon K, et al. Sustainability and scalability of the hospital elder life program at a community hospital. J Am Geriatr Soc 2011;59:359–65. 10.1111/j.1532-5415.2010.03243.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ueda N, Igarashi M, Okuyama K, et al. Demographic and clinical characteristics of patients with delirium: analysis of a nationwide Japanese medical database. BMJ Open 2022;12:e060630. 10.1136/bmjopen-2021-060630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakakibara Y, Ochibe T, Amari S, et al. Study on the risk factors for postoperative delirium using the National health insurance claims database in Japan. Iryo Yakugaku 2019;45:195–207. 10.5649/jjphcs.45.195 [DOI] [Google Scholar]
- 54.Matsuda S, Fujimori K, Kuwabara K, et al. Diagnosis procedure combination as an infrastructure for the clinical study. Asian Pac J Dis Manage 2011;5:81–7. 10.7223/apjdm.5.81 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062141supp001.pdf (466.6KB, pdf)
Data Availability Statement
No data are available. The Medical Data Vision database analysed in this study is not publicly accessible. According to the contract with Medical Data Vision Co, Ltd, the data cannot be shared with external researchers.