Abstract
Objectives
To investigate low-grade inflammation in type 2 diabetes and explore associations to clinical aspects as well as microvascular and macrovascular complications.
Design
Cross-sectional analysis.
Setting
The outpatient diabetes clinic at the Department of Endocrinology at Aalborg University Hospital, Denmark.
Participants
100 participants with type 2 diabetes confirmed by a haemoglobin A1c (HbA1c)≥6.5% for a minimum of 1 year and 21 healthy controls.
Outcome measures
Serum levels of 27 inflammation-related biomarkers measured by immunoassay. Associations with microvascular and macrovascular complications, body weight, glycaemic control, medication and sex were investigated in the diabetes cohort.
Results
Serum levels of tumour necrosis factor (TNF)-α and eotaxin were elevated in type 2 diabetes (p<0.05), while interleukin (IL)-7 was decreased (p<0.001). IL-12/IL-23p40, IL-15, macrophage-derived chemokine (MDC) and C reactive protein (CRP) levels were increased with body weight (p<0.05), while eotaxin and TNF-α were increased with elevated HbA1c levels (p<0.04). Dipeptidyl peptidase-4 inhibitor therapy was associated with lower levels of induced protein-10, MDC and thymus and activation regulated chemokine (p<0.02), while females had higher levels of MDC (p=0.027). Individuals with ≥3 diabetic complications had elevated levels of IL-6, IL-10, IL-12/IL-23p40, IL-15 and CRP compared with those with ≤3 (p<0.05).
Conclusion
The level of low-grade inflammation in type 2 diabetes is associated with obesity, glycaemic regulation, therapeutical management, sex and complications. Our results underline the importance of addressing inflammatory issues in type 2 diabetes, as these may predispose for crippling comorbidities.
Keywords: DIABETES & ENDOCRINOLOGY, IMMUNOLOGY, Diabetic neuropathy
Strengths and limitations of this study.
Analysis of a broad palette of inflammatory biomarkers in serum in 100 participants with type 2 diabetes and 21 healthy controls.
High degree of heterogeneity of our cohort, which allows for generalisation to the population of type 2 diabetes.
Well-characterised cohort in regard to microvascular and macrovascular comorbidities.
The cross-sectional design is a limitation of the study and hinders any assumptions of causality.
This study is based on secondary analysis and thus inclusion and exclusion criteria were not designed specifically with the investigation of inflammatory biomarkers in mind.
Introduction
Tight glycaemic regulation is vital for balancing the existing energy demand in tissues by combining resources originating from the nutritional supply and release from internal storages. Low blood glucose is potentially life threatening, while long-term elevated levels have several metabolic consequences, including sorbitol production, mitochondrial dysfunction, and formation of advanced glycation end products.1 Chronic hyperglycaemia can be caused either by insulin deficiency, as seen in type 1 diabetes, or by a combination of generalised insulin resistance in peripheral tissues and insufficient insulin production resulting in type 2 diabetes. The latter is the most prevalent diabetes type accounting for up to 90% of the cases.2
The pathogenesis of type 2 diabetes is highly complex and multifactorial, and many aspects of the disease require further elucidation. However, it is clear that obesity along with a sedentary lifestyle is a substantial risk factor for development of insulin resistance and type 2 diabetes through stress-induced inflammation in adipose tissue leading to insensitivity of the insulin receptor.3 In recent years, the previous view on adipose tissue as a mere storage of fat has been disproved, and it is now accepted that especially visceral adipose tissue possesses important endocrine and inflammatory properties. As an example, adipocytes activated by expansion-associated hypoxia secrete cytokines and so-called adipokines, many of which are proinflammatory in nature.4 As the prevalence of both obesity and type 2 diabetes continue to rise worldwide,2 a better understanding of the inflammatory link between these lifestyle-associated conditions is crucial.
In addition to obesity-induced inflammation, excess glucose availability in diabetes causes alterations in normal homeostasis, facilitating the progression of proinflammatory cytokine release to the microenvironment. Low-grade systemic inflammation is thus regarded as an accompanying condition in type 2 diabetes.5 Increased levels of proinflammatory biomarkers such as interleukin (IL) 6 and C reactive protein (CRP) have been shown to be associated with an increased risk of type 2 diabetes development in several prospective studies.6 7 This suggests that the pathogenetic mechanisms in type 2 diabetes is influenced by systemic low-grade inflammation. It is, however, unclear whether this proinflammatory state remains during the course of the disease or if it increases or diminishes over time. In addition, standard medical treatment in type 2 diabetes such as statins and dipeptidyl peptidase-4 (DPP-4) inhibitors have immunomodulating properties and may thus influence the inflammatory response.8 9
The low-grade systemic inflammation in type 2 diabetes is clinically essential, because it is associated with the development and progression of long-term complications such as nephropathy, neuropathy and retinopathy.10–12 Moreover, low-grade inflammation is associated with cardiovascular disease in diabetes,13 which is the primary cause of morbidity and mortality in individuals with type 2 diabetes.14
The aim of this study was to investigate the level of low-grade systemic inflammation in a cohort of individuals with type 2 diabetes with varying disease duration. We hypothesised that individuals with type 2 diabetes exhibited higher levels of proinflammatory biomarkers than healthy controls, and accordingly, the primary endpoint was differences in circulating inflammatory biomarkers in healthy and people with type 2 diabetes. Furthermore, we hypothesised that levels of proinflammatory biomarkers in type 2 diabetes were associated with disease duration, obesity, glycaemic control, therapeutical management and presence of diabetes-related microvascular and macrovascular complications. The secondary endpoints were thus to investigate associations between inflammatory biomarkers and clinical characteristics of type 2 diabetes.
Methods
Study population
All individuals with type 2 diabetes scheduled for regular health visits at the outpatient diabetes clinic at the department of endocrinology at Aalborg University Hospital, Denmark were informed about the study and screened for eligibility after signing of the informed consent form, and 100 participants were included for cross-sectional analysis. Inclusion criteria included Northern European descent, age above 18 years, a verified diagnosis of type 2 diabetes with haemoglobin A1c (HbA1c)≥6.5% for a minimum of 1 year, and stable diabetes treatment. People with other endocrinological or neurological diseases were excluded. The primary outcome of the study was cardiac vagal tone and the results have been published elsewhere.15 The control cohort consisted of sex-matched healthy volunteers (n=21) recruited for a randomised controlled trial (N-20090008) likewise conducted by our research group.
Blood samples
Morning blood samples were drawn from the cubital vein after a fasting period of minimum 6 hours. For analysis of inflammatory biomarkers, blood was collected in EDTA tubes and centrifuged for 10 min at 1000g. Isolated serum was aliquoted in appropriate volumes and stored in a biobank at −80°C until the complete data set was collected. All samples were thawed just prior to analysis. Samples from both cohorts were analysed consecutively to minimise interplate variability. For analysis of HbA1c, blood was collected in lithium heparin tubes and analysed by routine biochemical procedures.
Inflammatory biomarkers
Biomarker concentrations in serum samples were analysed using the V-PLEX Neuroinflammation Panel 1 Human Kit (Meso Scale Diagnostics (MSD), Gaithersburg, Maryland, USA) on a MESO QuickPlex SQ 120 instrument (MSD) according to the manufacturer′s specifications. Sample values below the detection limit of the assay were assigned a value of the detection limit divided by √2.16 If more than 30% of the measured samples for any given biomarker were below the detection limit, the biomarker was excluded from the analysis. Likewise, samples with a coefficient of variation>30% between duplicate measurements were excluded from the analysis (online supplemental table 1). Biomarkers on the panel included: IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12/IL-23p40, IL-13, IL-15, IL-16, IL-17A, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, TNF-β, eotaxin, eotaxin-3, IFN-γ-induced protein (IP)-10, monocyte chemoattractant protein (MCP)−1, MCP-4, macrophage-derived chemokine (MDC), macrophage inflammatory protein (MIP)-1α, MIP-1β, thymus and activation regulated chemokine (TARC), and CRP.
bmjopen-2022-062188supp001.pdf (77.7KB, pdf)
Assessment of diabetic comorbidities
All participants in the type 2 diabetes cohort underwent investigations concerning common diabetic comorbidities: (1) peripheral neuropathy: signs of peripheral neuropathy was investigated by vibration perception threshold (VPT) at the dorsum of the first phalanx using a biothesiometer (Bio-Medical Instruments). The measurement was done three consecutive times bilaterally, and the final VPT was calculated as the mean value of both feet. Results above 18 V were considered abnormal and thus as signs of diabetic peripheral neuropathy. (2) Nephropathy: morning urine samples were collected by participants at home and handed over to study personnel for standard biochemical analysis. Diabetic nephropathy was defined as a urine albumin/creatinine ratio above 30 mg/g, which is a standard cut-off for early diabetic nephropathy and microalbuminuria. (3) Retinopathy: participants were asked if they had ever been diagnosed with proliferative or non-proliferative retinopathy. (4) Cardiac autonomic neuropathy (CAN): electrocardiographic recordings by the VAGUS device (Medicus Engineering Aps, Aarhus, Denmark) described in detail elsewhere15 were applied for evaluation of cardiac autonomic neuropathy. Recordings were made during rest, postural change, deep breathing and the Valsalva manoeuvre. Age-specific cut-off values were applied,17 and abnormal results in one or more exercises were considered as signs of cardiac autonomic neuropathy.
Data handling and statistics
Distribution of raw and log-transformed data was evaluated by Shapiro-Wilk test of normality. Pairwise comparisons among groups were achieved by independent samples t-test or Mann-Whitney U based on data distribution. Differences in inflammatory biomarkers between healthy and type 2 diabetes were investigated first by pairwise comparisons and second by a logistic regression model including age and body mass index (BMI) as confounders, as these factors were different between groups and known to influence systemic low-grade inflammation. For the volcano plot, the fold difference was calculated as the log2-ratio between two group means. Differences in inflammatory biomarkers between people with short-term and long-term disease duration were likewise investigated by a logistic regression model including age and BMI as confounders. Multiple logistic regression analyses were performed to investigate the association between clinical parameters and inflammatory biomarkers. The independent variables included obesity (BMI<30 vs BMI>30), blood glucose level (HbA1c<55 vs HbA1c>55), DPP-4 inhibitor therapy, glucagon-like peptide (GLP-1) receptor agonist therapy and sex. Additionally, two models were applied in which associations were adjusted for the remaining clinical variables, and total plasma cholesterol or statin therapy, all of which may have an impact on the systemic inflammatory status. Differences in inflammatory biomarkers between people with 0, 1, 2 or ≥3 comorbidities were investigated by a Bonferroni-corrected analysis of variance and subsequently the Dunn’s test. An α level of 0.05 was applied for all analyses. The STATA software (StataCorp LLC, V.15.1) was applied for all statistical analyses.
Patient and public involvement
Patients or members of the public were not included in the design, conduction, reporting or dissemination plans of this project.
Results
Study population
Two subjects in the type 2 diabetes group were excluded due to haemolysis of collected blood samples. Individuals in the diabetes group were older, had higher BMI and higher HbA1c compared with the healthy controls (p<0.001). On the contrary, healthy controls had higher total cholesterol (p<0.001), high-density lipoprotein (p=0.006), and low-density lipoprotein (p<0.001) compared with individuals in the type 2 diabetes cohort of which 66% were on lipid-lowering statin therapy. A full demographic overview can be found in table 1.
Table 1.
Demographic and clinical characteristics among groups
| Healthy (n=21) | Type 2 diabetes (n=98) | P value | |
| Basic demography | |||
| Age (years) | 52 (48–55) | 65 (56–71) | <0.001 |
| Sex (% of males) | 71 | 63 | 0.478 |
| BMI (kg/m2) | 25.6 (23.7–28.0) | 31.4 (27.5–34.5) | <0.001 |
| Current smokers (%) | 19 | 5 | 0.028 |
| Disease duration (y) | – | 10 (5–17) | – |
| Vital signs | |||
| Systolic BP (mm Hg) | 128 (119–137) | 137 (128–147) | 0.021 |
| Diastolic BP (mm Hg) | 76±11 | 77±9 | 0.720 |
| Pulse (beats/min) | 66±7 | 69.8±10 | 0.110 |
| Biochemistry | |||
| HbA1c | |||
| (mmol/mol) | 33 (33–37) | 55 (48–61.5) | <0.001 |
| (%) | 5.2 (5.2–5.5) | 7.2 (6.5–7.8) | <0.001 |
| Cholesterol (mmol/L) | 5.4±0.9 | 3.9±0.9 | <0.001 |
| eGFR (mL/min/1.73 m2) | |||
| >90 (%) | 62 | 46 | 0.197 |
| 40–90 (%) | 38 | 52 | 0.264 |
| <40 (%) | 0 | <1 | 0.507 |
| HDL (mmol/L) | 1.5 (1.3–1.8) | 1.2 (1.0–1.5) | 0.006 |
| LDL (mmol/L) | 3.3±0.9 | 1.9±0.7 | <0.001 |
| Triglycerides (mmol/L) | 1.1 (0.7–1.4) | 1.4 (1.0–2.0) | 0.023 |
| Diabetic comorbidities | |||
| Neuropathy (%) | – | 60 | – |
| Nephropathy (%) | – | 19 | – |
| Retinopathy (%) | – | 8 | – |
| CAN (%) | – | 40 | – |
| Medication | |||
| Antihypertensives (%) | – | 67 | – |
| DPP-4 inhibitor (%) | – | 18 | – |
| Metformin (%) | – | 78 | – |
| SGLT-2 inhibitor (%) | – | 23 | – |
| GLP-1 receptor agonist (%) | – | 23 | – |
| Statins (%) | – | 66 | – |
Boldface font indicates statistical significance (p<0.05). Antihypertensive medication includes ACE inhibitors, angiotensin II receptor antagonists, calcium channel blockers, beta blockers, diuretics and I1-imidazoline receptor antagonists. Results displayed as either mean±SD or median (1st–3rd quartiles) based on distribution of the data.
BMI, body mass index; CAN, cardiac autonomic neuropathy; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; GLP, glucagon-like peptide; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SGLT, sodium-glucose transport protein.
Inflammatory biomarkers in type 2 diabetes compared with healthy
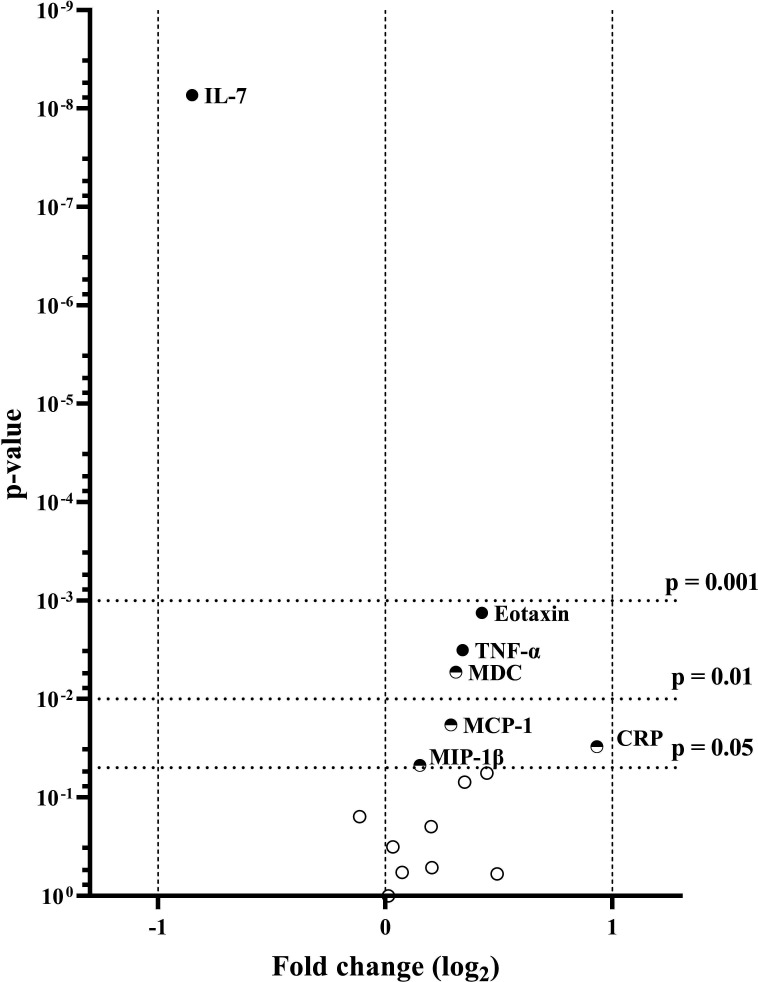
Serum levels of 27 inflammatory biomarkers were measured in individuals with type 2 diabetes and healthy controls. Eleven biomarkers were excluded from the statistical analyses due to being undetectable or of insufficient measurement quality due to low levels (online supplemental table 1). The remaining 16 biomarkers (IL-6, IL-7, IL-8, IL-10, IL-12/IL-23p40, IL-15, IL-16, IFN-γ, TNF-α, eotaxin, IP-10, MCP-1, MDC, MIP-1β, TARC, CRP) were measured in ≥95% of the samples. The concentrations of TNF-α (p=0.003) and CRP (p=0.030) were significantly higher in the type 2 diabetes cohort compared with the control cohort (figure 1). Similarly, four chemokines (eotaxin (p=0.001), MCP-1 (p=0.018), MDC (p=0.005) and MIP-1β (p=0.047)) showed elevated levels in the diabetes cohort. In contrast, the level of cytokine IL-7 was significantly lower in participants with type 2 diabetes compared with healthy controls (p<0.001). After adjustment for age and BMI, only IL-7, eotaxin and TNF-α remained significantly different. Serum concentrations of all measured biomarkers are presented in online supplemental table 2. When subdividing the type 2 diabetes cohort according to disease duration, only IL-10 was significantly different (p=0.008) between groups, even after adjustment for age and BMI, with a modestly increased levels found in subjects with disease duration above 10 years (table 2). Similarly, we investigated whether the presence of CAN (early or manifest) influenced the levels of inflammatory factors; however, none of these reached significant levels (data not shown).
Figure 1.
Volcano plot displaying pairwise comparisons of inflammatory factors in type 2 diabetes and healthy controls. Vertical dashed lines indicate threshold for twofold differences among groups. Horizontal dashed lines indicate p value thresholds of 0.05, 0.01 and 0.001, respectively. ●Significantly different after adjustment for age and BMI, ◓significantly different in the unadjusted model,○ above significance threshold in both models. Only significant analytes are labelled. BMI, body mass index; CRP, C reactive protein; IL, interleukin; MDC, macrophage-derived chemokine; MIP, macrophage inflammatory protein; TNF, tumour necrosis factor.
Table 2.
OR for associations between serum concentrations of inflammatory factors (cytokines (n=4), chemokines (n=6), proinflammatory cytokines (n=5), vascular injury (n=1)) in type 2 diabetes with short-term disease duration (<10 years, n=44) and long-term disease duration (>10 years, n=50) unadjusted and adjusted for age and BMI
| Unadjusted model | Adjusted model | ||||
| Or (95% CI) | P value | Or (95% CI) | P value | ||
| Cytokines | IL-7 | 1.03 (0.93 to 1.15) | 0.565 | 1.04 (0.93 to 1.18) | 0.466 |
| IL-12 /IL-23p40 |
1.00 (1.00 to 1.00) | 0.446 | 1.00 (0.99 to 1.01) | 0.717 | |
| IL-15 | 1.51 (0.74 to 3.10) | 0.256 | 1.32 (0.62 to 2.79) | 0.471 | |
| IL-16 | 1.00 (1.00 to 1.00) | 0.832 | 1.00 (1.00 to 1.00) | 0.813 | |
| Chemokines | Eotaxin | 1.00 (1.00 to 1.00) | 0.887 | 1.00 (1.00 to 1.00) | 0.687 |
| IP-10 | 1.00 (1.00 to 1.00) | 0.512 | 1.00 (1.00 to 1.00) | 0.244 | |
| MCP-1 | 1.00 (1.00 to 1.00) | 0.864 | 1.00 (1.00 to 1.00) | 0.523 | |
| MDC | 1.00 (1.00 to 1.00) | 0.810 | 1.00 (1.00 to 1.00) | 0.319 | |
| MIP-1β | 1.00 (0.99 to 1.01 | 0.992 | 1.00 (0.99 to 1.01) | 0.916 | |
| TARC | 1.00 (1.00 to 1.00) | 0.719 | 1.00 (1.00 to 1.00) | 0.260 | |
| Proinflammatory cytokines | IL-6 | 1.34 (0.79 to 2.27) | 0.271 | 1.21 (0.70 to 2.09) | 0.504 |
| IL-8 | 1.00 (0.94 to 1.06) | 0.983 | 1.00 (0.94 to 1.06) | 0.904 | |
| IL-10 | 111.85 (2.86 to 4377.78) | 0.012 | 103.97 (2.30 to 4699.58) | 0.017 | |
| IFN-γ | 1.02 (0.97 to 1.08) | 0.438 | 1.02 (0.96 to 1.09) | 0.447 | |
| TNF-α | 1.69 (0.70 to 4.12) | 0.246 | 1.78 (0.69 to 4.62) | 0.234 | |
| Vascular injury | CRP | 1.00 (1.00 to 1.00) | 0.697 | 1.00 (1.00 to 1.00) | 0.713 |
Boldface font indicates statistical significance (p<0.05).
BMI, body mass index; CRP, C reactive protein; IL, interleukin; TNF, tumour necrosis factor.
Inflammatory biomarkers in subgroups of type 2 diabetes
Obesity was significantly associated with concentration of five inflammatory biomarkers (IL-12/IL-23p40, IL-15, IFN-γ, MDC and CRP) (table 3—only analytes with p value below 0.05 shown). When adjusting for HbA1c, sex and total plasma cholesterol or statin use, IL-12/IL-23p40, IL-15 and CRP remained statistically significant associated with obesity. HbA1c was significantly associated with eotaxin and IL-12/IL-23p40 levels after adjusting for confounders, and levels of MDC were associated with sex with lower levels found in male subjects compared with females. Lower levels of IL-8, IP-10 and MDC were associated with DPP-4 inhibitor therapy, while higher levels of TNF-α were associated with GLP-1 receptor agonist therapy. Lastly, SGLT2 inhibitor therapy was associated with lower levels of MDC.
Table 3.
Multiple logistic regression analysis of serum concentrations between (A) type 2 diabetes+BMI<30 (n=40) and type 2 diabetes+BMI>30 (n=58), (B) type 2 diabetes with HbA1c<55(n=47) and type 2 diabetes with HbA1c>55 (n=51), (C) male type 2 diabetes (n=62) and female type 2 diabetes (n=36), (D) type 2 diabetes (n=80) and type 2 diabetes treated with DPP-4 inhibitors (n=18), (E) type 2 diabetes (n=75) and type 2 diabetes treated with GLP-1 receptor agonists (n=23), and (F) type 2 diabetes (n=75) and type 2 diabetes treated with SGLT2 inhibitor therapy (n=23) with overall R-squared value and effect size (95% CI) of BMI, HbA1c, sex, DPP-4 inhibitor therapy, GLP-1 receptor agonist therapy, or SGLT2 inhibitor therapy displayed
| Unadjusted model | Adjusted model 1 | Adjusted model 2 | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| A. Obesity | ||||||
| IL-12/IL-23p40 | 1.01 (1.00 to 1.02) | 0.003 | 1.01 (1.00 to 1.02) | 0.007 | 1.01 (1.00 to 1.02) | 0.007 |
| IL-15 | 2.32 (1.05 to 5.11) | 0.038 | 2.30 (1.02 to 5.15) | 0.043 | 2.30 (1.02 to 5.16) | 0.043 |
| IFN-γ | 1.12 (1.00 to 1.25) | 0.041 | 1.10 (0.99 to 1.23) | 0.087 | 1.10 (0.99 to 1.22) | 0.091 |
| MDC | 1.00 (1.00 to 1.00) | 0.029 | 1.00 (1.00 to 1.00) | 0.051 | 1.00 (1.00 to 1.00) | 0.049 |
| CRP | 1.00 (1.00 to 1.00) | 0.001 | 1.00 (1.00 to 1.00) | 0.001 | 1.00 (1.00 to 1.00) | 0.001 |
| B. Blood glucose | ||||||
| IL-8 | 1.08 (1.00 to 1.15) | 0.037 | 1.07 (0.99 to 1.14) | 0.076 | 1.07 (1.00 to 1.15) | 0.055 |
| TNF-α | 3.25 (1.21 to 8.73) | 0.019 | 2.64 (0.95 to 7.34) | 0.062 | 3.09 (1.11 to 8.58) | 0.031 |
| Eotaxin | 1.00 (1.00 to 1.01) | 0.031 | 1.00 (1.00 to 1.01) | 0.027 | 1.00 (1.00 to 1.01) | 0.025 |
| C. Sex | ||||||
| MDC | 1.00 (1.00 to 1.00) | 0.009 | 1.00 (1.00 to 1.00) | 0.021 | 1.00 (1.00 to 1.00) | 0.027 |
| D. DPP-4 inhibitor therapy | ||||||
| IL-8 | 0.88 (0.79 to 0.99) | 0.040 | 0.89 (0.79 to 1.00) | 0.052 | 0.89 (0.79 to 1.00) | 0.051 |
| IP-10 | 1.00 (1.00 to 1.00) | 0.013 | 0.99 (0.99 to 1.00) | 0.008 | 0.99 (0.99 to 1.00) | 0.008 |
| MDC | 1.00 (1.00 to 1.00) | 0.027 | 1.00 (1.00 to 1.00) | 0.011 | 1.00 (1.00 to 1.00) | 0.011 |
| TARC | 1.00 (1.00 to 1.01) | 0.004 | 1.00 (1.00 to 1.01) | 0.005 | 1.00 (1.00 to 1.01) | 0.005 |
| E. GLP-1 receptor agonist therapy | ||||||
| IL-8 | 1.08 (1.01 to 1.15) | 0.025 | 1.08 (0.99 to 1.17) | 0.069 | 1.08 (1.00 to 1.18) | 0.058 |
| IL-15 | 0.57 (0.23 to 1.41) | 0.226 | 0.29 (0.09 to 0.95) | 0.042 | 0.27 (0.08 to 0.92) | 0.036 |
| IL-16 | 1.00 (0.98 to 1.00) | 0.042 | 1.00 (0.98 to 1.00) | 0.077 | 1.00 (0.98 to 1.00) | 0.068 |
| TNF-α | 6.50 (2.07 to 20.42) | 0.001 | 4.60 (1.26 to 16.76) | 0.021 | 4.70 (1.25 to 17.69) | 0.022 |
| F. SGLT2 inhibitor therapy | ||||||
| MDC | 1.00 (1.00 to 1.00) | 0.014 | 1.00 (1.00 to 1.00) | 0.027 | 1.00 (1.00 to 1.00) | 0.033 |
Results presented as OR and 95% CI. Total plasma cholesterol, BMI, HbA1c, and sex were included in the adjusted model 1 as appropriate, while statin use, BMI, HbA1c and sex were included in the adjusted model 2 as appropriate. For simplicity, only analytes with p values below 0.05 in either model are shown. Bold font indicated statistical significance after Bonferroni adjustment (p<0.003).
BMI, body mass index; CRP, C reactive protein; DPP-4, dipeptidyl peptidase-4; GLP, glucagon-like peptide; HbA1c, haemoglobin A1c; IFN, interferon; IL, interleukin; IP, induced protein; MDC, macrophage-derived chemokine; TARC, thymus and activation regulated chemokine.
Diabetic comorbidities
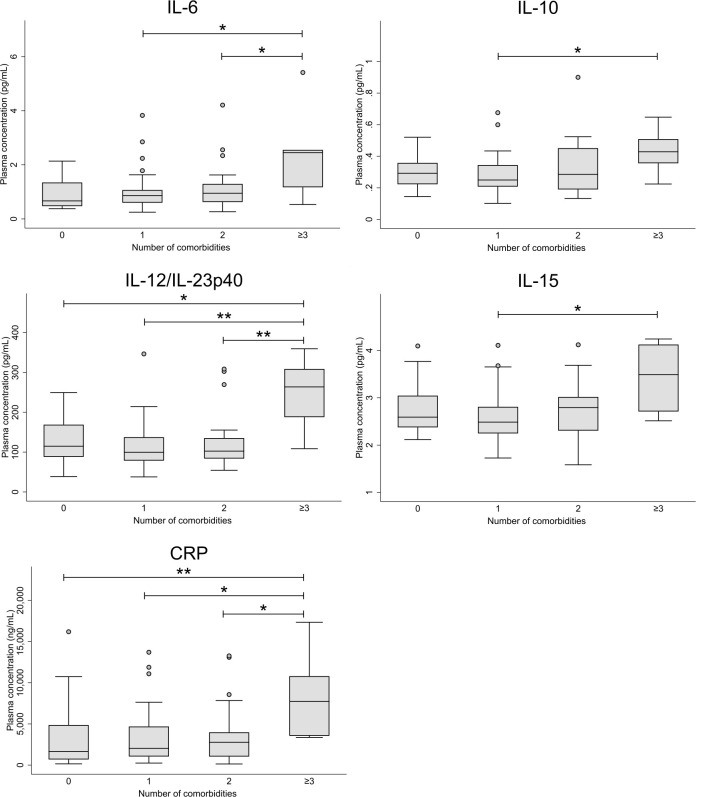
When subdividing the type 2 diabetes cohort into groups according to number of diabetic comorbidities, five biomarkers (IL-6, IL-10, IL12/IL-23p40, IL-15 and CRP) were significantly elevated in participants with three or more comorbidities compared with those with fewer or none (figure 2—only analytes with p values below 0.05 shown).
Figure 2.
Box plots displaying plasma concentrations of biomarkers in individuals with type 2 diabetes and 0 (n=20), 1 (n=43), 2 (n=28), or 3 or more (n=7) diabetic comorbidities (retinopathy, nephropathy, neuropathy, cardiac autonomic neuropathy). Only analytes with p values below 0.05 are shown. *P<0.05, **p<0.01. CRP, C reactive protein; IL, interleukin.
Discussion
In this study, we investigated the level of systemic low-grade inflammation in a cohort of individuals diagnosed with type 2 diabetes. Elevated levels of several inflammatory biomarkers were found in comparison to healthy controls, evident in both short-term and long-term disease duration. Moreover, in the type 2 diabetes cohort, obesity, hyperglycaemia and female sex were found to be associated with elevated levels of various inflammatory biomarkers. Lastly, we were able to establish a connection between the number of common diabetic comorbidities and elevated levels of inflammatory biomarkers.
Inflammatory biomarkers in type 2 diabetes compared with healthy
After adjustment for age and BMI, we showed that IL-7 was significantly decreased, while eotaxin and TNF-α was significantly increased in type 2 diabetes compared with healthy. The majority of research regarding IL-7 has been conducted in type 1 diabetes, where elevated levels are shown compared with healthy.18 IL-7 is highly involved in T cell function and proliferation, and a role of this cytokine in mediating expansion of insulin-producing β-cell-autoreactive T cells has been proposed thus implicating IL-7 in the pathogenesis of type 1 diabetes.19 The decreased levels in type 2 diabetes compared with healthy controls found in this study were somewhat surprising but may reflect the lack of T-cell activation the pathology of type 2 diabetes. Eotaxin has been linked to the development of atherosclerosis by facilitating monocyte infiltration in smooth muscle cells under the influence of proinflammatory mediators,20 and elevated levels of this chemokine have previously been reported in type 1 diabetes individuals with complications compared with individuals with no diabetic complications as well as healthy controls.21 Increased levels of CRP have previously been reported in adults with type 2 diabetes,13 22 but in our cohorts, the difference could be attributed to a skewed distribution of age and BMI in the two cohorts.
IL-10 is generally regarded as an anti-inflammatory cytokine with the ability to dampen the immune response, and previous data have shown downregulation of IL-10 in both type 2 diabetes and obesity per se.23 This contrasts our findings, which showed no differences in the overall diabetes cohort but an increase in individuals with long disease duration. This observation could reflect manifestations of compensatory mechanisms towards a long-term elevated inflammatory environment attempting to elicit an anti-inflammatory response. However, proinflammatory factors (eg, TNF-α) were elevated regardless of disease duration suggesting that any attempt of balancing the immune response remain challenging in the presence of type 2 diabetes.
Inflammatory biomarkers in subgroups of type 2 diabetes
Obesity and blood glucose regulation
In our type 2 diabetes cohort, obesity (BMI>30) was significantly associated with the levels of IL-12/IL-23p40 and CRP, while eotaxin and TNF-α levels were associated with glycaemic regulation (HbA1c). Previously it has been shown that TNF-α release is upregulated in connection with obesity and has been linked to the progression of insulin resistance.24 25 The fact that TNF-α was not associated with by obesity in our cohort is thus surprising. However, elevated levels of TNF-α in adipose tissue, but not in serum have previously been reported,26 which could also be the case in our cohort. In animal models, TNF-α antagonist treatment improves insulin resistance in obesity.27 A clinical study, however, failed to show the same effect in humans.28 Regarding eotaxin, this chemokine has been linked to the development of cardiovascular disease, which is likewise a complication to long-term hyperglycaemia, and our findings of increased levels in dysregulated individuals could therefore be a possible sign of atherosclerosis.20
Sex
We showed that the level of the chemokine MDC was associated with sex with higher levels seen in females compared with males. Different obesity-related inflammatory pathways between men and women with metabolic syndrome have previously been shown. Increased levels of proinflammatory mediators seem to facilitate low-grade systemic inflammation in males, while an insufficient anti-inflammatory milieu appears to be dominant in females.29 These findings suggest that any inflammation-modulating therapy in obesity should be differentiated according to sex and underlying mechanisms. In our type 2 diabetes cohort, however, this pattern was not recreated, indicating that the crucial factor may be aspects related to the metabolic syndrome rather than hyperglycaemia.
Therapeutical management
Lower levels of three chemokines (IL-8, IP-10 and MDC) were all associated with DPP-4 inhibitor therapy. DPP-4 inhibitor therapy is known to improve glycaemic control via prevention of breakdown of the incretin hormone GLP-1. In addition, several cytokines and chemokines are also substrates of the DPP-4 enzyme, and DPP-4 inhibitor therapy thus possesses immunomodulating properties possibly facilitating low-grade systemic inflammation in diabetes.9 Potentially this could explain why promising in vitro anti-inflammatory actions of DPP-4 inhibitors have failed to show convincingly results in humans.30 Surprisingly, we found lower levels of three DPP-4 substrates (IL-8, IP-10 and MDC) in connection with DPP-4 inhibitor therapy. Though seemingly in contrast with the expected result, similar observations have previously been reported e.g. lower levels of eotaxin in type 2 diabetes during DPP-4 inhibitor therapy.31 In our study, however, eotaxin levels were unaffected by DPP-4 inhibitor therapy, underlining the need for further research in the immunomodulating effects of these compounds.
GLP-1 receptor agonist therapy, which share the same pharmacodynamic endpoint as DPP-4 inhibitor therapy, is known to possess anti-inflammatory properties independent of improved glycaemic control.32 However, our results showed an approximately 25% increase in proinflammatory TNF-α levels in connection with GLP-1 receptor agonist therapy. This finding is unexpected and in contrast with a previous pilot study showing that liraglutide significantly decreased TNF-α levels in a type 2 diabetes cohort.33 Preclinical studies have likewise shown inhibitory effects of liraglutide on TNF-α expression.34 Other preclinical studies, however, have reported decreased proinflammatory effects of TNF-α through inhibition of the NK-κB pathway after GLP-1 receptor agonist therapy.35 If this is the case, this would neutralise the proinflammatory pathways caused by increased TNF-α levels seen in this study.
In our cohort, SGLT2 inhibitor therapy was associated with a decrease in MDC, known to facilitate and amplify type II immune response.36 The antidiabetic effects of SGLT2 inhibitors rely on the inhibition of renal reabsorption of glucose, but anti-inflammatory effects have also been reported including attenuation of IL-6 production37 and modulation of macrophage polarisation.38 The prospect of using the anti-inflammatory potential of SGLT2 inhibitors in various pathologies is currently receiving much attention.39
Additional subgroups
Apart from obesity, hyperglycaemia and sex, other factors such as current smoking status and specific medical therapy may likewise influence the level of inflammation in type two diabetes.40 41 In our cohort, only 5% were smokers, which is surprisingly low, giving the fact that smoking is a substantial risk factor for development of type 2 diabetes.40 The low number of current smokers may reflect selection or reporting bias or perhaps successful free smoking cessation programmes, as 40% of our participants reported to be previous smokers. This is, however, highly speculative. Nonetheless, the degree of a persistent proinflammatory effect of nicotine following smoking cessation is debated,42 and could potentially be influencing the results in the current study. Moreover, the high proportion of previous smokers could indicate that our cohort consisted of individuals with a high degree of determination and self-efficacy. Such selection bias is potentially also reflected in the median HbA1c of 55 mmol/mol, which is lower in comparison to other cohorts.13 43
In our cohort, 66% received lipid-lowering statin therapy, which is known to possess anti-inflammatory properties,8 which again could impact the level of investigated inflammatory biomarkers. Consequently, the reported elevated levels of several biomarkers compared with the healthy control cohort could be artificially low due to the anti-inflammatory effect of statins. Potentially this could explain why no proinflammatory biomarkers were increased in individuals with longer disease duration as these individuals were more likely to be on statin therapy.
Diabetic comorbidities
It has previously been established that low-grade systemic inflammation plays a role in progression of diabetic complications.10–12 We found that IL-6, IL-10, IL-12/IL-23p40, IL-15 and CRP were elevated in individuals with multiple diabetic comorbidities compared with those with fewer or none. In the literature, IL-6 elevation has in particular been associated with diabetic complications.44–47 Likewise, increased levels of CRP has previously been linked to development and severity of diabetic complications.45 48 In addition, the observed elevated levels of IL-10 were primarily found in subjects with longer disease duration, which could reflect that diabetes comorbidities typically become more prevalent with increasing exposure to glycaemic fluctuations and disease duration.11 Furthermore, IL-12 has previously been shown to be involved in the pathogenesis of several diabetic microvascular and macrovascular comorbidities.49 Interestingly, a study in obese and insulin-resistant IL-12 knockout mice showed that IL-12 disruption increased angiogenesis and restored peripheral blood flow perfusion through attenuation of oxidative stress and increased levels of angiogenic factors.50 In humans, a monoclonal antibody (Ustekinumab) targeting IL-12/IL-23p40 is currently used as a safe and effective treatment of psoriasis.51 Our data raise the intriguing possibility of applying this drug as a novel treatment option for diabetic microvascular and macrovascular complications but needs to be investigated in future randomised controlled trials. Finally, circulating levels of IL-15 have been shown to be influenced by fat mass and physical activity.52 Furthermore, IL-15 improve lipid deposition and insulin sensitivity by activation of the GLUT-4 transporter in skeletal muscles. Hence, IL-15 has been proposed as a novel therapeutic option for treating obesity and type 2 diabetes.53 The increased levels of IL-15 in individuals with three or more comorbidities found in this study seem to contradict the beneficial effects normally attributed to this cytokine, but as this is a cross-sectional study no conclusions of causality can be made.
Strengths and limitations
A major limitation of this study is the cross-sectional study design, which hinders any assumptions of the predictive potential of low-grade inflammation and clinical characteristics of type 2 diabetes. On this dataset, we tested for association between low grade inflammation in type 2 diabetes, and we selected á priori the anti-inflammatory markers, as they are part of the underlying pathogenesis. According to the study design, each of the serum markers were tested individually, and based on our unadjusted and adjusted models we suggest an association to the specific marker IL-10. As the manufacturer of the multiplex assay had defined division of serum markers into cytokines (n=4), chemokines (n=6), proinflammatory cytokines (n=5), vascular injury (n=1), we believe that Bonferroni’s correction is too conservative. The major strength of this study is the high degree of heterogeneity of our cohort, obtained by systematically screening all people in our outpatient diabetes clinic, thereby facilitating generalisation to the larger population of type 2 diabetes. However, selection bias in which individuals with low symptom burdens are more likely to participate cannot be ruled out. Contrary, a majority of patients with complications, who regard participation in a clinical trial as a possibility to receive extra attention from healthcare professionals, is likewise conceivable. It should also be noted that because this study is based on secondary analyses, the inclusion and exclusion criteria were not designed to exclude participants with comorbidities or medication use, which could impact the levels of the investigated inflammatory factors. Lastly, registration of retinopathy was restricted to participant recollection and reporting. Objective measures or consultation in patient records would have improved the validity of this outcome.
Conclusion
We showed that individuals with type 2 diabetes exhibit higher degrees of various inflammatory factors in serum, and that obesity and glycaemic dysregulation are associated with the level of specific inflammatory factors. Furthermore, a considerable increase in several inflammatory factors was seen in people with multiple diabetic comorbidities. Regarding medication, DPP-4 inhibitor therapy was associated with decreased levels of several chemokines, while increased TNF-α levels were observed in association with GLP-1 receptor agonist therapy. Taken together, our results show that individuals with type 2 diabetes have systemic low-grade inflammation. Although the cross-sectional nature of our study hinders the ability to look at the causality between systemic low-grade inflammation and diabetic complications, it is intriguing to speculate whether dampening of the inflammatory state could protect against development of comorbidities in type 2 diabetes.
Supplementary Material
Acknowledgments
We are grateful to Lene Holm Fruensgaard Pedersen for assistance in blood sample collection, and to Nina Wittorff Jensen, Rebekka Hanne Gerwig and Charlotte Gade Farcinsen Leth for expert technical assistance.
Footnotes
Twitter: @AWegeberg
Contributors: Study design and original idea by CB and BB. A-MLW collected the data. TO, A-MLW, FP, BB, JS and CB analysed and interpreted the data. TO wrote the first draft, but all authors contributed to the final manuscript. CB is the guarantor of the work, has full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding: CB received funding from the Talent Management Program, Aalborg University. AMW received funding from The Research Foundation of Health Science, Region of Northern Jutland.
Disclaimer: No funding source had any role in study design, data collection, data analysis, data interpretation, or the preparation of this article.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Deidentified participant data are available upon reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by North Denmark Region Committee on Health Research Ethics (N-20170045). Participants gave informed consent to participate in the study before taking part.
References
- 1.Ighodaro OM. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother 2018;108:656–62. 10.1016/j.biopha.2018.09.058 [DOI] [PubMed] [Google Scholar]
- 2.Federation ID . IDF diabetes atlas. 9th edn. Brussels, 2019. https://www.diabetesatlas.org [Google Scholar]
- 3.Prasad M, Chen EW, Toh S-A, et al. Autoimmune responses and inflammation in type 2 diabetes. J Leukoc Biol 2020;107:739–48. 10.1002/JLB.3MR0220-243R [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez ACdeO, Costa TF, Andrade ZdeA, et al. Wound healing - A literature review. An Bras Dermatol 2016;91:614–20. 10.1590/abd1806-4841.20164741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez LL, Garrie K, Turner MD. Type 2 diabetes - an autoinflammatory disease driven by metabolic stress. Biochim Biophys Acta Mol Basis Dis 2018;1864:3805–23. 10.1016/j.bbadis.2018.08.034 [DOI] [PubMed] [Google Scholar]
- 6.Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–34. 10.1001/jama.286.3.327 [DOI] [PubMed] [Google Scholar]
- 7.Thorand B, Löwel H, Schneider A, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA augsburg cohort study, 1984-1998. Arch Intern Med 2003;163:93–9. 10.1001/archinte.163.1.93 [DOI] [PubMed] [Google Scholar]
- 8.Albert MA, Danielson E, Rifai N, et al. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (Prince): a randomized trial and cohort study. J Am Med Assoc 2001;286:64–70. 10.1001/jama.286.1.64 [DOI] [PubMed] [Google Scholar]
- 9.Shao S, Xu Q, Yu X, et al. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol Ther 2020;209:107503. 10.1016/j.pharmthera.2020.107503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao B-Y, Zhang S-F, Li H-D, et al. Epigenetics and inflammation in diabetic nephropathy. Front Physiol 2021;12. 10.3389/fphys.2021.649587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care 2017;40:136–54. 10.2337/dc16-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nebbioso M, Lambiase A, Armentano M, et al. Diabetic retinopathy, oxidative stress, and sirtuins: an in depth look in enzymatic patterns and new therapeutic horizons. Surv Ophthalmol 2022;67:168–83. 10.1016/j.survophthal.2021.04.003 [DOI] [PubMed] [Google Scholar]
- 13.Alexandraki KI, Piperi C, Ziakas PD, et al. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J Clin Immunol 2008;28:314–21. 10.1007/s10875-007-9164-1 [DOI] [PubMed] [Google Scholar]
- 14.Maser RE, Mitchell BD, Vinik AI, et al. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes. Diabetes Care 2003;26:1895–901. 10.2337/diacare.26.6.1895 [DOI] [PubMed] [Google Scholar]
- 15.Wegeberg A-M, Lunde ED, Riahi S, et al. Cardiac vagal tone as a novel screening tool to recognize asymptomatic cardiovascular autonomic neuropathy: aspects of utility in type 1 diabetes. Diabetes Res Clin Pract 2020;170:108517. 10.1016/j.diabres.2020.108517 [DOI] [PubMed] [Google Scholar]
- 16.Croghan CW, Egeghy PP. Methods of dealing with values below the limit of detection using SAS. 5. South SAS User Gr, 2003. [Google Scholar]
- 17.Spallone V, Bellavere F, Scionti L, et al. Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr Metab Cardiovasc Dis 2011;21:69–78. 10.1016/j.numecd.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 18.Coulson DJ, Bakhashab S, Latief JS, et al. MiR-126, IL-7, CXCR1/2 receptors, inflammation and circulating endothelial progenitor cells: the study on targets for treatment pathways in a model of subclinical cardiovascular disease (type 1 diabetes mellitus). J Transl Med 2021;19:140. 10.1186/s12967-021-02785-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monti P, Bonifacio E. Interleukin-7 and type 1 diabetes. Curr Diab Rep 2014;14:518. 10.1007/s11892-014-0518-9 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Luk AOY, Ma RCW, et al. Independent predictive roles of eotaxin Ala23Thr, paraoxonase 2 Ser311Cys and β 3 -adrenergic receptor Trp64Arg polymorphisms on cardiac disease in type 2 diabetes-an 8-year prospective cohort analysis of 1297 patients. Diabet Med 2010;27:376–83. 10.1111/j.1464-5491.2010.02980.x [DOI] [PubMed] [Google Scholar]
- 21.Jamali Z, Nazari M, Khoramdelazad H, et al. Expression of CC chemokines CCL2, CCL5, and CCL11 is associated with duration of disease and complications in type-1 diabetes: a study on Iranian diabetic patients. Clin Lab 2013;59:993–1001. 10.7754/clin.lab.2012.120810 [DOI] [PubMed] [Google Scholar]
- 22.Malik A, Morya RK, Saha S, et al. Oxidative stress and inflammatory markers in type 2 diabetic patients. Eur J Clin Invest 2020;50. 10.1111/eci.13238 [DOI] [PubMed] [Google Scholar]
- 23.van Exel E, Gussekloo J, de Craen AJM, et al. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes. Diabetes 2002;51:1088–92. 10.2337/diabetes.51.4.1088 [DOI] [PubMed] [Google Scholar]
- 24.Hauner H, Bender M, Haastert B, et al. Plasma concentrations of soluble TNF-alpha receptors in obese subjects. Int J Obes 1998;22:1239–43. 10.1038/sj.ijo.0800773 [DOI] [PubMed] [Google Scholar]
- 25.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev 2003;14:447–55. 10.1016/S1359-6101(03)00052-2 [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995;95:2409–15. 10.1172/JCI117936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uysal KT, Wiesbrock SM, Marino MW, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 1997;389:610–4. 10.1038/39335 [DOI] [PubMed] [Google Scholar]
- 28.Bernstein LE, Berry J, Kim S, et al. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med 2006;166:902–8. 10.1001/archinte.166.8.902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ter Horst R, van den Munckhof ICL, Schraa K, et al. Sex-specific regulation of inflammation and metabolic syndrome in obesity. Arterioscler Thromb Vasc Biol 2020;40:1787–800. 10.1161/ATVBAHA.120.314508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teragawa H, Morimoto T, Fujii Y, et al. Effect of anagliptin versus sitagliptin on inflammatory markers: sub-analysis from the REASON trial. Diabetes Metab Syndr Obes 2020;13:4993–5001. 10.2147/DMSO.S282968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aso Y, Kase M, Sagara M, et al. Teneligliptin, a DPP-4 inhibitor, decreases plasma levels of inflammatory chemokines during a standard meal test in patients with type 2 diabetes. Am J Med Sci 2020;360:261–7. 10.1016/j.amjms.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 32.Bray JJH, Foster‐Davies H, Salem A, et al. Glucagon‐like peptide‐1 receptor agonists improve biomarkers of inflammation and oxidative stress: a systematic review and meta‐analysis of randomised controlled trials. Diabetes Obes Metab 2021;23:1806–22. 10.1111/dom.14399 [DOI] [PubMed] [Google Scholar]
- 33.Savchenko LG, Digtiar NI, Selikhova LG, et al. Liraglutide exerts an anti-inflammatory action in obese patients with type 2 diabetes. Rom J Intern Med 2019;57:233–40. 10.2478/rjim-2019-0003 [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Yi T, Cheng S, et al. Glucagon-like peptide-1 receptor agonist exendin-4 improves neurological outcomes by attenuating TBI- induced inflammatory responses and MAPK activation in rats. Int Immunopharmacol 2020;86:106715. 10.1016/j.intimp.2020.106715 [DOI] [PubMed] [Google Scholar]
- 35.Liu Y-T, He T, Li H-Q, et al. Liraglutide improves pancreatic islet β cell apoptosis in rats with type 2 diabetes mellitus by inhibiting the IKKε/NF-κB pathway. Eur Rev Med Pharmacol Sci 2021;25:4818–28. 10.26355/eurrev_202107_26395 [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Gray PA, Van Damme J, et al. Macrophage-derived chemokine (MDC). J Leukoc Biol 2000;68:400–4. 10.1189/jlb.68.3.400 [DOI] [PubMed] [Google Scholar]
- 37.Latva-Rasku A, Honka M-J, Kullberg J, et al. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double-blind, placebo-controlled study with 8-Week treatment in type 2 diabetes patients. Diabetes Care 2019;42:931–7. 10.2337/dc18-1569 [DOI] [PubMed] [Google Scholar]
- 38.Xu L, Ota T. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: focus on fat browning and macrophage polarization. Adipocyte 2018;7:121–8. 10.1080/21623945.2017.1413516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feijóo-Bandín S, Aragón-Herrera A, Otero-Santiago M, et al. Role of sodium-glucose co-transporter 2 inhibitors in the regulation of inflammatory processes in animal models. Int J Mol Sci 2022;23:5634. 10.3390/ijms23105634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang SA. Smoking and type 2 diabetes mellitus. Diabetes Metab J 2012;36:399–403. 10.4093/dmj.2012.36.6.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salazar JJ, Ennis WJ, Koh TJ. Diabetes medications: impact on inflammation and wound healing. J Diabetes Complications 2016;30:746–52. 10.1016/j.jdiacomp.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanbaeva DG, Dentener MA, Creutzberg EC, et al. Systemic effects of smoking. Chest 2007;131:1557–66. 10.1378/chest.06-2179 [DOI] [PubMed] [Google Scholar]
- 43.Amin K, Qadr SH, Hassan Hussein R, et al. Levels of cytokines and GADA in type I and II diabetic patients. Prim Care Diabetes 2020;14:61–7. 10.1016/j.pcd.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 44.Yao Y, Li R, Du J, et al. Interleukin-6 and diabetic retinopathy: a systematic review and meta-analysis. Curr Eye Res 2019;44:564–74. 10.1080/02713683.2019.1570274 [DOI] [PubMed] [Google Scholar]
- 45.Shelbaya S, Amer H, Seddik S, et al. Study of the role of interleukin-6 and highly sensitive C-reactive protein in diabetic nephropathy in type 1 diabetic patients. Eur Rev Med Pharmacol Sci 2012;16:176–82. [PubMed] [Google Scholar]
- 46.Herder C, Bongaerts BWC, Rathmann W, et al. Association of subclinical inflammation with polyneuropathy in the older population. Diabetes Care 2013;36:3663–70. 10.2337/dc13-0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González-Clemente J-M, Vilardell C, Broch M, et al. Lower heart rate variability is associated with higher plasma concentrations of IL-6 in type 1 diabetes. eur j endocrinol 2007;157:31–8. 10.1530/EJE-07-0090 [DOI] [PubMed] [Google Scholar]
- 48.Aryan Z, Ghajar A, Faghihi-Kashani S, et al. Baseline high-sensitivity C-reactive protein predicts macrovascular and microvascular complications of type 2 diabetes: a population-based study. Ann Nutr Metab 2018;72:287–95. 10.1159/000488537 [DOI] [PubMed] [Google Scholar]
- 49.Mishra M, Kumar H, Bajpai S, et al. Level of serum IL-12 and its correlation with endothelial dysfunction, insulin resistance, proinflammatory cytokines and lipid profile in newly diagnosed type 2 diabetes. Diabetes Res Clin Pract 2011;94:255–61. 10.1016/j.diabres.2011.07.037 [DOI] [PubMed] [Google Scholar]
- 50.Ali M, Mali V, Haddox S, et al. Essential role of IL-12 in angiogenesis in type 2 diabetes. Am J Pathol 2017;187:2590–601. 10.1016/j.ajpath.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marwaha AK, Tan S, Dutz JP. Targeting the IL-17/IFN-γ axis as a potential new clinical therapy for type 1 diabetes. Clin Immunol 2014;154:84–9. 10.1016/j.clim.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 52.Pérez-López A, Valadés D, Vázquez Martínez C, et al. Serum IL-15 and IL-15Rα levels are decreased in lean and obese physically active humans. Scand J Med Sci Sports 2018;28:1113–20. 10.1111/sms.12983 [DOI] [PubMed] [Google Scholar]
- 53.Duan Y, Li F, Wang W, et al. Interleukin-15 in obesity and metabolic dysfunction: current understanding and future perspectives. Obesity Reviews 2017;18:1147–58. 10.1111/obr.12567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062188supp001.pdf (77.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Deidentified participant data are available upon reasonable request to the corresponding author.