Abstract
A woman in her 40s was referred for acute and chronic postprandial abdominal cramps on a background of relapsing remitting multiple sclerosis on ocrelizumab therapy as well as coeliac disease on a gluten-free diet, with a family history of ulcerative colitis. Initial colonoscopy demonstrated mild patchy colitis. The patient was trialled on mesalazine, which was ceased due to intolerance. Subsequently, she continued on mercaptopurine monotherapy for management of mild symptoms. Despite this, her symptoms rapidly progressed, with endoscopic and histological evidence of severe rectal-sparing pancolonic inflammation, consistent with severe ocrelizumab-induced colitis. This was refractory to intravenous methylprednisolone and intravenous cyclosporine rescue therapy, requiring surgical management with a subtotal colectomy and subsequent ileorectal anastomosis, after which she remained in clinical, endoscopic and histological remission.
Keywords: Inflammatory bowel disease, Gastrointestinal system, Gastrointestinal surgery, Multiple sclerosis, Neurology (drugs and medicines)
Background
Ocrelizumab is an anti-CD20 humanised monoclonal antibody approved for management of primary progressive and relapsing remitting multiple sclerosis (RRMS), and thought to be less immunogenic than rituximab (a chimeric anti-CD20 monoclonal antibody) due to its humanised form of the fragment crystallisable region of the antibody.1 2 Nonetheless, both ocrelizumab and rituximab have been associated with both macroscopic gastrointestinal inflammation and microscopic colitis. Studies in murine models suggest that this may be related to dysregulation of the gastrointestinal immune system and impaired integrity of the colonic mucosal barrier from regulatory T cell dysfunction related to B cell depletion.3 Five cases of ocrelizumab-induced colitis have previously been reported (table 1).3–7 However, we report the first case of colitis refractory to ciclosporin rescue therapy and suggest that an underlying genetic predisposition may increase the risk, and potentially severity, of colitis in the setting of ocrelizumab use.
Table 1.
Summary of case reports of ocrelizumab-induced colitis
| Cases requiring surgical management | Cases with medical management only | ||
| Sunjaya et al4 | Man in his 40s on ocrelizumab for MS, after having previously been treated with dimethyl fumarate. Patient presented with bloody diarrhoea several weeks after 1 dose of ocrelizumab. Case report did not specify timing from ocrelizumab induction to presentation. Severe proctosigmoiditis was seen on flexible sigmoidoscopy with ulceration. Case report did not comment on histology. No improvement with intravenous corticosteroids and hydrocortisone enemas, and progressed to a segmental sigmoid resection with an end colostomy and Hartmann’s pouch. | Barnes et al3 | Woman in her 50s on ocrelizumab for MS, no prior treatments for MS mentioned in case report. Patient presented with bloody diarrhoea 18 months after starting ocrelizumab, receiving a total of 3 doses 6 months apart. Deep punched out ulcerations of oesophagus were seen on oesophagogastroduodenoscopy and scattered deep ulcerations to the proximal transverse colon on flexible sigmoidoscopy. Histology showed patchy, moderately active chronic inflammation. Responded to 7 days of intravenous hydrocortisone and was discharged on an oral steroid wean. Follow-up showed endoscopic and histological remission on colonoscopy 9 months later. |
| Lee et al5 | Woman in her 40s on ocrelizumab for MS, after having previously been treated with alemtuzumab and teriflunomide. Patient presented with bloody diarrhoea after 2 doses 6 months apart. Case report did not specify timeframe from dosing to presentation. Abdominal X-ray was suspicious for Clostridium difficile infection. Treated with empiric oral vancomycin and intravenous metronidazole. The patient had a flexible sigmoidoscopy that showed nodular mucosa and plaques. Histology consistent with biological medication effect, negative for C. difficile. Failed to improve with intravenous hydrocortisone and progressed to a total colectomy with end ileostomy. | Tuqan et al6 | Woman in her 60s on ocrelizumab for MS, no prior treatments for MS mentioned in case report. Patient presented with bloody diarrhoea 14 months after starting ocrelizumab, receiving a total of 3 doses 6 months apart. Flexible sigmoidoscopy showed moderate inflammation and histology consistent with drug-induced colitis. Treated with oral mesalazine and budesonide with improving colitis on flexible sigmoidoscopy 3 months later. No comment was made on further follow-up to confirm remission. |
| Akram, Valasek and Patel7 | Man in his 60s on ocrelizumab for MS, after having previously been treated with interferon beta-1a and dimethyl fumarate. Patient presented with bloody diarrhoea 1 week after 1 dose of ocrelizumab. CT abdomen/pelvis with intravenous contrast showed pancolitis. Flexible sigmoidoscopy showed diffuse mucosal oedema, loss of vascular pattern and multiple small ulcers to the proximal extent of the examination. Histology confirmed moderately active colitis with mild architectural changes and mild plasmacytosis. Initially failed prednisolone weaning. Successful subsequent corticosteroid wean with the addition of extended release budesonide. Colonoscopy at week 50 showed mucosal healing. | ||
MS, multiple sclerosis.
Case presentation
The patient was a woman in her 40s with a 19-year history of difficult-to-treat RRMS, history of coeliac disease diagnosed serologically and histologically in her early 30s, variable adherence to a gluten-free diet and family history of ulcerative colitis in her biological father. Her RRMS was previously non-responsive to interferon beta-1a, pegylated interferon beta-1a and fingolimod, and she was given ocrelizumab for progressive RRMS symptoms, with a good clinical response. Ten months after started taking ocrelizumab, the patient presented with intermittent severe acute and chronic postprandial abdominal cramps associated with diarrhoea and underwent a gastroscopy and colonoscopy. The gastroscopy was unremarkable, with small bowel biopsies confirming no active coeliac disease. The colonoscopy, however, revealed non-specific patchy right colonic inflammation (figure 1). Histology demonstrated patchy mild active chronic colitis in samples from the caecum and ascending colon without the presence of granulomas. There was no evidence of small bowel involvement on colonoscopy or subsequent magnetic resonance enterography. Given the non-specific findings, the patient’s symptoms and inflammatory biomarkers were monitored closely. Six months later, her abdominal pain and diarrhoea persisted despite dietary modification, including adherence to a strict gluten-free diet. Further investigations revealed a mildly elevated calprotectin of 167 μg/mg and C reactive protein (CRP) of 9 mg/L, although bedside intestinal ultrasound demonstrated a normal bowel wall thickness throughout the colon and ileum without Doppler signal indicating no active colonic or ileal inflammation. A trial of mesalazine-MMX (Mezavant) 4.8 g daily was given due to increasing inflammatory biomarkers, which the patient discontinued due to severe diarrhoea, suspected to be an idiosyncratic reaction. On review 3 months later, after consultation with her treating neurologist, she was given mercaptopurine 50 mg daily for persistent symptoms due to colonic inflammation. Despite this, 1 month later she was admitted to the hospital with an acute worsening of symptoms.
Figure 1.
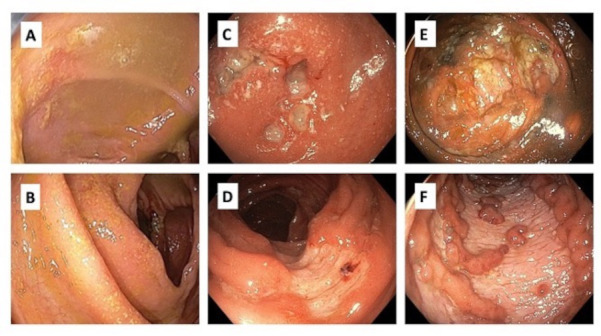
Endoscopic appearance of caecum (A, C, E) and descending colon (B, D, F). (A) Caecum at first colonoscopy, (B) descending colon at first colonoscopy, (C) caecum when presented with acute severe colitis, (D) descending colon when presented with acute severe colitis, (E) caecum at colonoscopy 7 days later after ciclosporin therapy, (F) descending colon 7 days later after ciclosporin therapy.
Investigations
On admission her CRP was 132, faecal calprotectin was >2200, and a colonoscopy revealed severe pancolonic inflammation, with deep punched out ulcers throughout the colon (figure 1). Histology confirmed pancolitis with relative rectal sparing, with multiple ulcers extending to the deep lamina propria and occasionally to the muscularis mucosa. There was a mixed inflammatory infiltrate with plasma cells, small lymphocytes and neutrophils throughout the interstitium, and multiple foci of cryptitis and crypt abscesses, with rare apoptotic bodies. Viral inclusions were not identified on light microscopy, immunohistochemistry for cytomegalovirus was negative and parasite were not identified. Vasculitis changes were not identified in the mucosal vessels. The features were not typical of immunotherapy-related colitis, given only rare apoptotic bodies were noted. Ocrelizumab-induced colitis was the favoured diagnosis given previous case reports demonstrating pancolitis with ulceration and mild architectural distortion and the relative paucity of apoptotic bodies, as seen in this case.3–7
Differential diagnosis
The primary differential diagnoses included inflammatory bowel disease (IBD), infective colitis, vasculitis, ischaemic colitis and drug-induced colitis. IBD was considered given the family history; however, the endoscopic and histological appearances were atypical, and the temporal relationship with ocrelizumab made this less likely. A negative infective screen and the absence of evidence of infective changes on histology excluded infectious colitis. A vasculitic process was considered unlikely as there were no other features of vasculitis clinically or histologically. The disease distribution, endoscopic and histological appearances were not suggestive of ischaemic colitis. The diagnosis of drug-induced colitis from ocrelizumab was made by exclusion of other causes, the temporal relationship between symptom onset and ocrelizumab commencement, the previous histological descriptions in the literature and the endoscopic and histological pictures.
Treatment
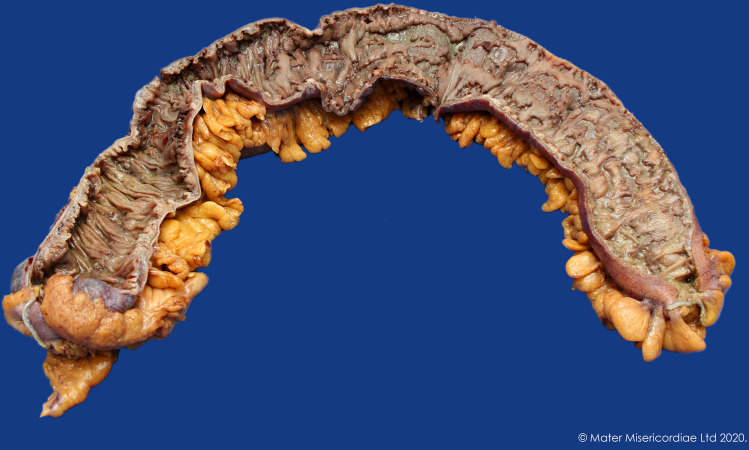
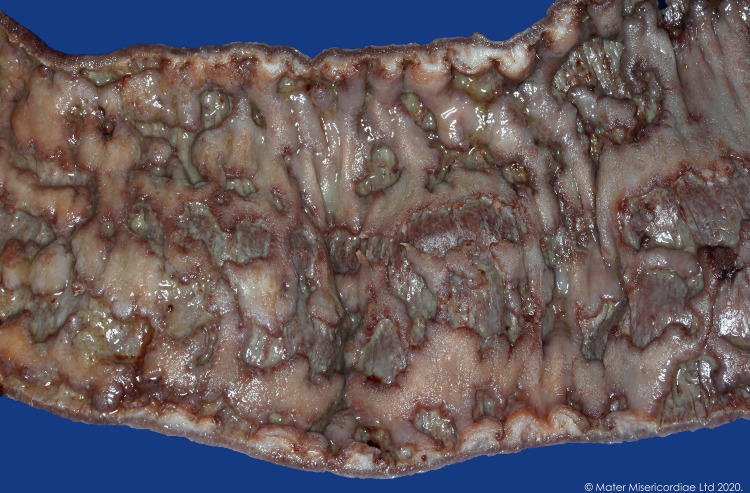
The patient completed 3 days of intravenous methylprednisolone 60 mg/day and then, based on a day 3 CRP of 72 and inadequate clinical response, was given intravenous ciclosporin rescue therapy (2 mg/kg) and oral prednisolone for steroid refractory disease. Following 4 days of intravenous ciclosporin, a repeat colonoscopy showed persistent, worsening, contiguous deep ulcers throughout the colon with rectal sparing, with some ulcers up to 2 cm in diameter (figure 1). Biopsies showed marked improvement in the acute inflammatory component, but ongoing moderate-severe chronic active colitis with multifocal ulceration in the left colon, and mild chronic colitis in the right colon and rectum. Given the persistent severe endoscopic appearance, a decision was made to proceed to colectomy with an end ileostomy (figures 2 and 3). Histology from the resected bowel macroscopically described the mucosa as ulcerated and microscopically identified extensive well-demarcated ulcers with confluent geographical areas, some areas involving the surface of the deep submucosa and the superficial muscularis propria in others. The base of each ulcer was made up of well-vascularised granulation tissue mixed with neutrophils and acute inflammatory slough. It was noted the neutrophil-rich crypt-based inflammation seen on previous biopsies was not identified, possibly representing treatment effect. The patient had an uncomplicated postoperative course, ocrelizumab was subsequently ceased, and the patient switched to teriflunomide for ongoing treatment of her RRMS. Eight months later, a flexible sigmoidoscopy of the sigmoid colon (up to 30 cm) showed normal bowel without evidence of mucosal inflammation. One month later, the patient underwent an uncomplicated ileorectal anastomosis.
Figure 2.
Colon resection.
Figure 3.
Colon resection segment.
Outcome and follow-up
Postoperatively the patient remains in remission 20 months after colectomy, without evidence of recurrent rectal or ileal inflammation. She has ongoing regular follow-up to monitor for any evidence of disease recurrence.
Discussion
We describe a unique case of ocrelizumab-induced severe pancolitis, non-responsive to intravenous corticosteroids and ciclosporin rescue therapy, with subsequent sustained clinical remission postcolectomy and interval ileorectal anastomosis. There have been multiple documented cases of colitis secondary to rituximab8; however, we have only identified five published cases of ocrelizumab-induced colitis, all in patients with multiple sclerosis; three cases responsive to medical therapy, and two cases requiring surgical management.3–7 Of the two cases requiring surgery, one described severe steroid refractory proctosigmoiditis, managed with a segmental sigmoid resection, end colostomy and Hartmann’s pouch4; and the other was a case of fulminant left-sided colitis, managed with a total colectomy and ileostomy.5 Medical treatment in these reported cases included oral and intravenous corticosteroids, with no previous reports of use of ciclosporin or other potent immunosuppressants. This is the first reported case undergoing ileorectal anastomosis, which was successful and uncomplicated.
It appears that colitis can occur after any dose of ocrelizumab, based on colitis occurring after the first, second or third dose of ocrelizumab in the published case reports. This suggests there should remain a high index of suspicion for ocrelizumab-induced colitis in patients presenting with bloody diarrhoea even if they have tolerated previous doses of ocrelizumab. Furthermore, as observed in this case, colitis can occur 10 months after treatment with ocrelizumab, suggesting there can be a long latency period before colitis develops.
Interestingly, although ocrelizumab is quite commonly used for B cell depletion in certain types of lymphoma and often as long-term maintenance therapy, our literature search did not identify any cases of colitis reported in this population, with all identified cases occurring in patients with multiple sclerosis (MS). This suggests that either an underlying genetic predisposition to autoimmunity or a lack of other chemotherapy to suppress the T-cell-mediated response could be a key predisposing factor. In our case, the patient had a strong history of autoimmune disease, with a personal history of MS and coeliac disease, along with a first-degree family member with ulcerative colitis, potentially increasing her risk of a more severe disease phenotype.
The other notable observation is the tendency towards an ulcerative colitis-like phenotype, with the majority of ocrelizumab-induced colitis cases noting left-sided disease with confluent inflammation. Significantly, in our case, colectomy in addition to cessation of ocrelizumab seems to have been curative, with no recurrence of disease 12 months postileorectal anastomosis and no further rectal inflammation. Given the ulcerative colitis-like phenotype, pre-existing management algorithms and predictive scores used for acute severe ulcerative colitis may provide clinical guidance; however, more data is needed to assess whether ciclosporin could be efficacious in this context. We would suggest that anti-TNF agents are contraindicated in the MS population, given the association with central and peripheral demyelination,9 and would not recommend these agents for refractory colitis. However, novel small molecules, such as janus kinase (JAK) inhibitors and sphingosine-1-phosphate receptor (S1PR) modulators, may present a viable alternative treatment to ciclosporin for steroid refractory ocrelizumab-induced colitis.
Ocrelizumab-induced colitis has only been observed in patients with an autoimmune predisposition. The colitis can be severe, with significant ramifications for the patient. From the data available, colectomy appears to be curative, although the potential role for immunosuppressants beyond corticosteroids, such as ciclosporin and other agents, in reducing colectomy risk, remains unclear. A notable observation from this case, and the previously published cases, is that all patients requiring surgical intervention were below the age of 50, with those over the age of 50 responding to corticosteroids. This suggests patients under the age of 50 presenting with ocrelizumab-induced colitis may have more severe disease and ultimately require surgical management. Given the rarity of this condition, there is a lack of clinical data, although in view of the apparent ulcerative colitis-like phenotype, rescue therapies commonly used in steroid refractory severe ulcerative colitis could be considered in the absence of indications for urgent surgical management, but remains an area for future research.
Patient’s perspective.
I was admitted to hospital for what was supposed to be a fairly routine colonoscopy to investigate some inflammation that existed. I was supposed to be in the hospital for one night and come home after the colonoscopy the next day. I ended up spending an entire month in the hospital, right as the COVID-19 pandemic began. As I started the preparation for the colonoscopy, the pain that I began to experience would continue relentlessly for the 2 weeks that followed and was at an excruciating level. It was above a ‘ten’ on the measurement scale. My memory during those 2 weeks is of different types of corticosteroids being administered to try and reduce the inflammation. This did not work, and it was decided that the only solution was to remove a massive amount of my large bowel, leaving me with an ileostomy bag for several months. This period was without a doubt the most traumatic of my life and took me a very long time to recover from, both physically and psychologically. If, in fact, it was the ocrelizumab that diseased my bowel, I am glad that some investigation is being done to prevent this from happening to anyone else.
Learning points.
Ocrelizumab therapy may be associated with colitis, which can present as acute severe colitis.
There may be a stronger predilection for developing severe colitis from ocrelizumab in individuals with a genetic predisposition to autoimmune disease.
Colitis can occur after any dose of ocrelizumab and may have a long latency period between treatment and development of colitis.
Early involvement of colorectal surgeons should be considered for surgical planning. Patients under the age of 50 may have a more severe disease phenotype requiring surgery; surgical resection seems to be curative and ocrelizumab-induced colitis may not respond to medical therapy.
The role for immunosuppression beyond corticosteroids remains unclear, but rescue therapy could be considered for steroid refractory disease in the absence of an indication for urgent surgery.
Acknowledgments
Dr Rohan Lourie, Dr Chris Gillespie, Dr Nicholas Tutticci and Dr Kerryn Green.
Footnotes
Contributors: Supervised by Y-KA and JB. Patient was under the care of Y-KA. Report was written by RM, RF, JB and Y-KA.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378:1779–87. 10.1016/S0140-6736(11)61649-8 [DOI] [PubMed] [Google Scholar]
- 2.Robak T, Robak E. New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies. BioDrugs 2011;25:13–25. 10.2165/11539590-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 3.Barnes A, Hofmann D, Hall L-A, et al. Ocrelizumab-induced inflammatory bowel disease-like illness characterized by esophagitis and colitis. Ann Gastroenterol 2021;34:447. 10.20524/aog.2021.0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunjaya DB, Taborda C, Obeng R, et al. First case of refractory colitis caused by ocrelizumab. Inflamm Bowel Dis 2020;26:e49. 10.1093/ibd/izaa057 [DOI] [PubMed] [Google Scholar]
- 5.Lee HH, Sritharan N, Bermingham D, et al. Ocrelizumab-Induced severe colitis. Case Rep Gastrointest Med 2020;2020:1–4. 10.1155/2020/8858378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuqan W, Siddiqi F, Ray A. S1766 Ocrelizumab-Induced Colitis: A Case Report. Am J Gastroenterol 2020;115:S913. 10.14309/01.ajg.0000709112.86831.18 [DOI] [Google Scholar]
- 7.Akram A, Valasek M, Patel D. P096 de novo colitis after ocrelizumab therapy. Gastroenterology 2020;158:S1–2. 10.1053/j.gastro.2019.11.043 [DOI] [Google Scholar]
- 8.Eckmann JD, Chedid V, Quinn KP, et al. De novo colitis associated with rituximab in 21 patients at a tertiary center. Clin Gastroenterol Hepatol 2020;18:252–3. 10.1016/j.cgh.2019.03.027 [DOI] [PubMed] [Google Scholar]
- 9.Kaltsonoudis E, Voulgari PV, Konitsiotis S, et al. Demyelination and other neurological adverse events after anti-TNF therapy. Autoimmun Rev 2014;13:54–8. 10.1016/j.autrev.2013.09.002 [DOI] [PubMed] [Google Scholar]