Abstract
Objectives
To determine the prevalence of possible sarcopenia and its association with other conditions in older adults in Bengbu, China.
Design, setting and participants
A cross-sectional study of 1082 community-dwelling Chinese people aged at least 60 years from March to June 2022.
Methods
Handgrip strength and information regarding associated conditions were collected. Possible sarcopenia was estimated based on handgrip strength with cut-off values (<28 kg in men; <18 kg in women) recommended by the Asia Working Group for Sarcopenia in 2019. Mann-Whitney U tests, χ2 tests and binary logistic regression analyses were used to explore relationships between possible sarcopenia and associated conditions.
Results
Possible sarcopenia was more prevalent in men (52.79%, n=246, age 79.43±7.33 years among men with possible sarcopenia) than in women (44.48%, n=274, age 78.90±7.71 years among women with possible sarcopenia). In men, possible sarcopenia positively correlated with high age (OR 2.658, 95% CI 1.758 to 4.019), physical inactivity (OR 2.779, 95% CI 1.646 to 4.691) and diabetes (OR 4.269, 95% CI 2.397 to 7.602), and negatively with hypertension (OR 0.586, 95% CI 0.384 to 0.893). The risk of possible sarcopenia in men decreased by 12.6% for every 1 kg/m2 increase of body mass index (OR 0.874, 95% CI 0.817 to 0.935). In women, possible sarcopenia positively correlated with high age (OR 3.821, 95% CI 2.677 to 5.455), physical inactivity (OR 2.185, 95% CI 1.488 to 3.210) and arthritis (OR 2.076, 95% CI 1.411 to 3.056).
Conclusion
Possible sarcopenia is prevalent in older adults and the factors affecting possible sarcopenia are different in men and women. Health education about these target factors can be considered as a potential measure to prevent possible sarcopenia.
Keywords: geriatric medicine, preventive medicine, primary care, public health
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study used the concept of low handgrip strength (<28 kg in men; <18 kg in women) in the latest guideline (Asia Working Group for Sarcopenia 2019) to assess possible sarcopenia.
The grouping of subjects by sex permitted additional insight into risk factors.
Because it was a cross-sectional study, cause-and-effect relationships could not be determined.
Introduction
As an independent disease in the 10th edition of International Classification of Diseases, sarcopenia has become an important public health issue.1 Sarcopenia is a geriatric syndrome characterised by loss of muscle mass and muscle strength and decreased physical function.2 Substantial evidence suggests that sarcopenia has an important impact on the health of older adults, and it often is associated with adverse outcomes such as illness, falls, reduced quality of life and even death.3 Sarcopenia is not only associated with ageing, but it can also result from a combination of chronic diseases including respiratory disease,4 diabetes5 and cancer.6 It is associated with environmental factors, and the risk of developing sarcopenia can be lowered by changes to physical activity and diet.7 8
In order to help predict the occurrence of sarcopenia in at-risk populations, the concept of ‘probable sarcopenia’ was introduced in the guideline of the European Working Group on Sarcopenia in Older People in 2018 (EWGSOP2). The guideline considered low muscle strength to be an indicator of probable sarcopenia.9 In 2019, the Asia Working Group for Sarcopenia (AWGS) updated its guideline first issued in 2014 and proposed the concept of ‘possible sarcopenia’, which was defined as the existence of low muscle strength with or without reduced physical performance.10 Both guidelines recommend using handgrip strength to assess muscle strength, but there are slight differences in threshold values used for diagnosis.9 10
The uses of these two international guidelines lead to differences in the reported prevalence of possible sarcopenia among different populations. One study determined that the prevalence of probable sarcopenia in a group of subjects in a Colombian community with a mean age 70.4±7.8 years was 46.5% based on threshold values from EWGSOP2.11 On the other hand, the values of the prevalence of probable sarcopenia in Swiss women (age 84.1±5.7 years) and men (age 82.6±5.2 years) were determined to be 26.3% and 28.0%, respectively.12 In a South Korean study, Kim and Won used the cut-off values recommended by the AWGS 2019 to screen for the possible sarcopenia and found a prevalence of 20.1% in men (age 76.4±3.9 years) and a prevalence of 29.2% in women (age 75.5±3.9 years).13
Despite renewed interest in the condition, a few studies have been performed to investigate the prevalence of possible sarcopenia and its relationship to various factors in Chinese populations. Therefore, the aims of this study were (1) to determine the prevalence of possible sarcopenia using the latest guideline (AWGS 2019) in a sample of older adults, aged 60 years and above, in Bengbu, China and (2) to explore the relationship between possible sarcopenia and its associated factors.
Methods
Sample
This was a cross-sectional, community-based study conducted in the city of Bengbu, China, from March 2022 to June 2022. Inclusion criteria for study participants were aged at least 60 years, ability to understand relevant issues and ability to provide informed consent. Exclusion criteria were inability to complete the handgrip strength measurement and lack of complete medical or demographic data.
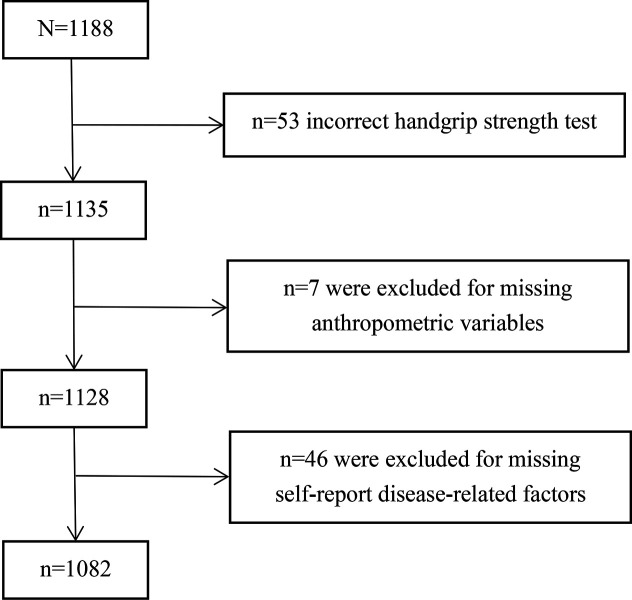
To ensure that the sample findings were valid for estimating the prevalence of possible sarcopenia in the general population, we calculated a minimum sample size of 792, assuming a prevalence of possible sarcopenia of 24.6%,13 at a 3% error rate and 95% CI. After considering the design effect as 1.5, the aim was to access a minimum sample size of 1188 individuals. Multistage random cluster sampling and random numbers table were conducted. First, all the streets were listed and seven streets were randomly grouped. Then, three communities were randomly grouped into each street. Finally, we contacted the leaders of the selected communities, and randomly recruited residents aged 60 years and above in each community to travel to nearby stalls for assessment. As a result, the final sample included 1082 elderly participants for a 91.08% response rate (figure 1).
Figure 1.
Participation of older adults.
Patient and public involvement
The older adults were not involved in the design, conduct, reporting or dissemination plans of our research.
Anthropometric measurements
All physical examinations were performed by trained medical students according to standardised procedures. Height and body weight were measured with a steel measuring tape and an electronic scale, respectively. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in metres. Waist circumference (WC) was measured from the middle point between the lower border of the rib cage and the iliac crest midaxillary at the end of a normal expiration with a soft measuring tape.
Assessment of possible sarcopenia
AWGS criteria (2019) define possible sarcopenia as the incidence of low muscle strength with or without reduced physical performance, therefore, in this study, low muscle strength was the only criterion used to define possible sarcopenia. Low muscle strength was defined as a handgrip strength of less than 28 kg in men and less than 18 kg in women. Handgrip strength was measured with an electronic hand dynamometer (EH101, https://www.senssun.com). Prior to use, the dynamometer was calibrated according to the manufacturer’s instructions. Each participant was asked to hold the dynamometer with the dominant hand with as much force as possible for 3 s. This process was repeated three times with 30 s between each trial, and the handgrip strength was taken as the maximum value from these three trials.10
Measurement of potential associated factors
Participants were sorted into two groups based on WHO age classification criteria: one group included participants that were aged 60–74 years and the other group included participants who were aged at least 75 years. Participants’ level of physical activity was determined using self-reported values. According to the latest WHO 2020 Guidelines on Physical Activity and Sedentary Behaviour,14 physical inactivity was defined as engagement in less than 150 min per week of moderate exercise, such as brisk walking, jogging or dancing, and the time of high intensity physical activity was multiplied by 2 to be translated into the time of moderate physical activity. Disease-related factors were assessed with a survey that asked the participants if they had been medically diagnosed with cancer, heart disease, hypertension, hyperlipidaemia, diabetes, respiratory diseases, arthritis or pain in the waist or lower extremities.
Statistical analysis
SPSS V.25.0 software (IBM) was used for data analyses. Continuous variables were expressed as mean±SD. Categorical variables were expressed as frequencies and percentages. The normality of the variables was verified using Kolmogorov-Smirnov tests. The male and female samples were divided into two groups: no sarcopenia (normal handgrip strength) or possible sarcopenia (weak handgrip strength: <28 kg in men; <18 kg in women). Student’s t-tests were applied to identify significant differences in normally distributed of continuous variables, while Mann-Whitney U tests were used for comparison of non-normally distributed of continuous variables between groups. The significance of differences in baseline characteristics were examined by using χ2 tests for categorical variables. The associated factors (age group, WC, BMI, physical inactivity, cancer, heart diseases, hypertension, hyperlipidaemia, diabetes, respiratory diseases, arthritis, pain in the waist or lower extremities) that were determined to reach the level of significance (p<0.05) were included as independent variables in separate binary logistic regression analysis models for males and females, with possible sarcopenia as the dependent variable.
Results
Data on handgrip strength and anthropometric measures were collected from 1082 adults aged 60 years and over (n=466 men, n=616 women; mean age 76.62±7.11 years). Of the participants, 484 (44.73%) were aged from 60 to 74 years, and 598 (55.27%) were aged at least 75 years.
Possible sarcopenia was determined according to the AWGS 2019 guidelines with gender-specific handgrip strength cut-off values. Of the 466 male participants, possible sarcopenia was identified in 246 (52.79%). Of the 616 female participants, possible sarcopenia was identified in 274 (44.48%). In both men and women, the majority of participants identified as having possible sarcopenia were aged 75 years and over.
Height and weight were significantly lower in the possible sarcopenia group than in the no sarcopenia group (both p<0.05). Among male participants, BMI was significantly lower in the possible sarcopenia group than in the group of no sarcopenia (p<0.05), but there was no statistically significant difference in BMI among female participants (p>0.05). Moreover, the possible sarcopenia group had a great number of participants who were classified as physically inactive (table 1).
Table 1.
Characteristics of participants with or without possible sarcopenia
| Overall sample (n=1082) |
Men, n=466 (43.07%) | Women, n=616 (56.93%) | |||||
| Possible sarcopenia (n=246; 52.79%) | No sarcopenia (n=220; 47.21%) |
P value | Possible sarcopenia (n=274; 44.48%) | No sarcopenia (n=342; 55.52%) | P value | ||
| Age (years) | 76.62±7.11 | 79.43±7.33 | 74.27±4.92 | <0.001 | 78.90±7.71 | 74.29±6.31 | <0.001 |
| Age group (n, %) | |||||||
| 60–74 years | 484 (44.73) | 78 (31.71) | 122 (55.45) | <0.001 | 82 (29.93) | 202 (59.06) | <0.001 |
| ≥ 75 years | 598 (55.27) | 168 (68.29) | 98 (44.55) | 192 (70.07) | 140 (40.94) | ||
| Height (cm) | 158.31±9.28 | 162.64±6.39 | 166.11±10.02 | <0.001 | 151.00±6.92 | 156.04±6.31 | <0.001 |
| Weight (kg) | 63.42±12.27 | 64.61±10.12 | 71.80±10.14 | <0.001 | 56.96±10.55 | 62.35±12.95 | <0.001 |
| WC (cm) | 90.83±9.95 | 91.26±10.01 | 91.95±7.92 | 0.204 | 89.83±10.76 | 90.59±10.35 | 0.553 |
| Handgrip strength (kg) | 22.91±8.57 | 21.49±5.21 | 35.42±5.11 | <0.001 | 13.85±3.72 | 23.14±4.13 | <0.001 |
| BMI (kg/m2) | 25.40±6.75 | 24.42±3.49 | 26.62±11.42 | <0.001 | 25.00±4.68 | 25.63±5.72 | 0.428 |
| Physical inactivity (n, %) | 270 (24.95) | 71 (28.86) | 28 (12.73) | <0.001 | 97 (35.40) | 74 (21.64) | <0.001 |
| Cancer (n, %) | 18 (1.66) | 6 (2.44) | 2 (0.91) | 0.204 | 6 (2.19) | 4 (1.17) | 0.319 |
| Heart diseases (n, %) | 345 (31.89) | 70 (28.45) | 76 (34.55) | 0.157 | 80 (29.20) | 119 (34.80) | 0.140 |
| Hypertension (n, %) | 599 (55.36) | 113 (45.93) | 127 (57.73) | 0.011 | 170 (62.04) | 189 (55.26) | 0.090 |
| Hyperlipidaemia (n, %) | 208 (19.22) | 46 (18.70) | 44 (20.00) | 0.723 | 48 (17.52) | 70 (20.47) | 0.355 |
| Diabetes (n, %) | 201 (18.58) | 56 (22.76) | 26 (11.82) | 0.002 | 54 (19.71) | 65 (19.01) | 0.836 |
| Respiratory diseases (n, %) | 93 (8.60) | 24 (9.76) | 8 (3.64) | 0.009 | 32 (11.68) | 29 (8.48) | 0.186 |
| Arthritis (n, %) | 349 (32.26) | 59 (23.98) | 55 (25.00) | 0.799 | 131 (47.81) | 104 (30.41) | <0.001 |
| Pain in the waist or lower extremities (n, %) | 534 (49.35) | 93 (37.80) | 89 (40.45) | 0.499 | 169 (61.68) | 184 (53.80) | 0.049 |
BMI, body mass index; WC, waist circumference.
In male participants, older adults with possible sarcopenia were significantly more likely than those without possible sarcopenia to have developed diabetes (22.76% vs 11.82%; p<0.05) and respiratory diseases (9.76% vs 3.64%; p<0.05). Conversely, participants with possible sarcopenia were significantly less likely to have developed hypertension (45.93% vs 57.73%; p<0.05). In female participants, older adults with possible sarcopenia were significantly more likely than those without possible sarcopenia to have developed arthritis (47.81% vs 30.41%; p<0.05), and pain in the waist or lower extremities (61.68% vs 53.80%; p<0.05) (table 1).
For male participants, a binary logistic regression analysis showed that the correlating variables age, BMI, physical inactivity, hypertension, diabetes and respiratory diseases explained whether a participant had possible sarcopenia or not to 23.6% (Nagelkerke’s R2=0.236, χ2 (6) = 90.767, p<0.0001) and the percentage accuracy in classification was 65.9%. Higher age (OR 2.658, 95% CI 1.758 to 4.019), physical inactivity (OR 2.779, 95% CI 1.646 to 4.691) and diabetes (OR 4.269, 95% CI 2.397 to 7.602) were risk factors for possible sarcopenia. Conversely, hypertension (OR 0.586, 95% CI 0.384 to 0.893) was a protective factor for possible sarcopenia. Moreover, the risk of possible sarcopenia decreased by 12.6% for every 1 kg/m2 increase of BMI (OR 0.874, 95% CI 0.817 to 0.935). Respiratory disease did not have a significant association with possible sarcopenia (table 2).
Table 2.
Binary logistic regression analysis of possible sarcopenia category by correlated variables in men
| Variables | Wald | Df | P value | OR | 95% CI | |
| Lower | Upper | |||||
| Age group (≥75 years vs 60–74 years) | 21.478 | 1 | <0.001 | 2.658 | 1.758 | 4.019 |
| Physical inactivity (yes vs no) | 14.640 | 1 | <0.001 | 2.779 | 1.646 | 4.691 |
| Hypertension (yes vs no) | 6.174 | 1 | 0.013 | 0.586 | 0.384 | 0.893 |
| Diabetes (yes vs no) | 24.289 | 1 | <0.001 | 4.269 | 2.397 | 7.602 |
| Respiratory diseases (yes vs no) | 2.659 | 1 | 0.103 | 2.169 | 0.855 | 5.501 |
| BMI | 15.378 | 1 | <0.001 | 0.874 | 0.817 | 0.935 |
| Constant | 9.647 | 1 | 0.002 | 14.990 | ||
Age groups, physical inactivity, hypertension, diabetes, respiratory diseases and BMI were simultaneously included in the model.
BMI, body mass index.
For female participants, a binary logistic regression analysis showed that the correlating variables age, physical inactivity, arthritis and pain in the waist or lower extremities explained whether a participant had possible sarcopenia or not to 18.5% (Nagelkerke’s R2=0.185, χ2 (4) = 91.593, p<0.0001), and the percentage accuracy in classification was 67.4%. Higher age (OR 3.821, 95% CI 2.677 to 5.455), physical inactivity (OR 2.185, 95% CI 1.488 to 3.210) and arthritis (OR 2.076, 95% CI 1.411 to 3.056) were risk factors for possible sarcopenia. However, pain in the lower extremities or waist did not have a significant association with possible sarcopenia (table 3).
Table 3.
Binary logistic regression analysis of possible sarcopenia category by correlated variables in women
| Variables | Wald | Df | P value | OR | 95% CI | |
| Lower | Upper | |||||
| Age group (≥75 years vs 60–74 years) | 54.498 | 1 | <0.001 | 3.821 | 2.677 | 5.455 |
| Physical inactivity (yes vs no) | 15.874 | 1 | <0.001 | 2.185 | 1.488 | 3.210 |
| Arthritis (yes vs no) | 13.733 | 1 | <0.001 | 2.076 | 1.411 | 3.056 |
| Pain in the waist or lower extremities (yes vs no) | 0.756 | 1 | 0.384 | 1.186 | 0.807 | 1.742 |
| Constant | 64.394 | 1 | <0.001 | 0.207 | ||
Age groups, physical inactivity, arthritis and pain in the waist or lower extremities were simultaneously included in the model.
Discussion
We investigated the prevalence of possible sarcopenia and its correlation with associated factors. We found that possible sarcopenia has a high prevalence in the community of Bengbu. The prevalence of 48.06% is higher than the prevalence of 38.5% found in adults in another study conducted in China.15 The reason for this discrepancy may be that the population in our study was older than the population (age 68.13±6.46 years) in the previous study. It should also be noted that the prevalence identified in our study is higher than that found in another study of similarly aged subjects (age 75.9±3.9) from the Asian country of South Korea.13 The reason for this discrepancy may be that the South Korean study used the calf circumference, SARC-F or SARC-CalF scales to screen participants prior to administering the handgrip strength test, whereas this study used handgrip strength test directly to identify possible sarcopenia. This difference suggests that many of the older adults in South Korea who were not identified as candidates for the handgrip strength test according to calf circumference, SARC-F or SARC-CalF scale criteria may have had lower handgrip strengths indicative of possible sarcopenia. Therefore, direct measurement of handgrip strength has important clinical value.
Our study found that possible sarcopenia is more common among men than women (52.79% in men and 44.48% in women), as did a study performed by Wearing et al12 (28% in men and 26.3% in women). Interestingly, Pang et al16 found that the prevalence of possible sarcopenia in men between the ages of 20 and 60 years (13%) is lower than that in women in the same age group (14.2%), but the relative prevalence in older adults over the age of 60 years is reversed (33.7% in men and 30.9% in women). A possible mechanism of pathogenesis of leading to differences in possible sarcopenia in men and women involves testosterone. Testosterone plays an important role in the development and maintenance of muscle mass and function and can increases muscle mass and muscle strength.17 18 Testosterone in men declines at a rate of 1% per year after the age of 30, and 40%–70% of men over the age of 70 have low testosterone levels.19 This may be one of the important reasons why the prevalence rate of older men is higher than that of women.
We found that high age and physical inactivity are positively associated with the prevalence of possible sarcopenia. This result is consistent with other studies that have identified the main cause of possible sarcopenia as age-related loss of muscle strength. A study of subjects from a Chinese population identified a 50.8% decrease in right handgrip strength in men aged 85–90 years compared with men aged 45–50 years and a 55.0% decrease in right handgrip strength in women.20 Several studies have reported that physical inactivity is the primary risk factor for decreased muscle strength.21 22 Tsekoura et al23 and Makizako et al24 confirmed that exercise intervention for older adults slows the decline of muscle strength with age. Nearly a quarter of the participants were physically inactive, which is another potential explanation for the high prevalence of possible sarcopenia observed in this study. The correlations of age and physical inactivity with possible sarcopenia suggests that encouraging more physical activity in older adults is particularly important to prevent or delay the onset and progression of sarcopenia.
The relationships of various factors to possible sarcopenia differed between the sexes. In men, diabetes was found to have a strong correlation with possible sarcopenia. Diabetes was associated with a 4.269-fold increase in the risk of possible sarcopenia in older men, but there was no correlation found in older women. Likewise, a longitudinal study in the UK showed an increase in probable sarcopenia after 8 years in men with diabetes, but not in women.25 However, Anagnostis et al26 reported that muscle strength was significantly lower in patients with type 2 diabetes mellitus than in subjects without diabetes, but a significant relationship only existed in women (standardised mean difference (SMD) for women −0.52, 95% CI −0.98 to −0.06, p=0.02; SMD for men −0.42, 95% CI −0.97 to 0.13, p=0.13). Thus, it is necessary to further investigate the existence of gender differences in the relationship between diabetes and possible sarcopenia.
We also found that hypertension is a protective factor for possible sarcopenia in men. Several studies showed that patients taking ACE inhibitors (ACEI) as therapy for hypertension had higher muscle strength than patients without hypertension and patients with hypertension who were not taking ACEI.27 28 Similarly, Ata et al pointed that ACEI therapy seems to have favourable effects on both hypertension and sarcopenia.29 The treatment reduces inflammation and endothelial dysfunction in hypertension30 and may improve skeletal muscle function by increasing muscle blood flow and glucose delivery.31 Although more than half of the participants in our study were hypertensive, the medications they were taking were not investigated, so the relationship between hypertensive medications and possible sarcopenia will be further explored in later studies.
We also found that the risk of possible sarcopenia in men decreased by 12.6% for every 1 kg/m2 increase of BMI. A study based on a Korean population showed that BMI was positively correlated with handgrip strength in both men and women, and the correlation was higher in men (β=0.976, r=0.378) than in women (β=0.190, r=0.134).32 Older adults with low BMI values may be underweight and at risk of malnutrition. For example, Granic et al33 found that a low protein intake (<1 g/kg) was associated with lower handgrip strength. A systematic review and meta-analysis showed that multinutrients significantly improved handgrip strength (n=6 studies; 780 participants; SMD 0.41; 95% CI 0.06 to 0.76; I2=79%), and nutritional supplementations with protein or amino acids was also associated with improved handgrip strength (n=7 studies; 535 participants; SMD=0.24; 95% CI 0.07 to 0.41; I2=16%).34 Therefore, early nutritional intervention for older patients with possible sarcopenia is an important strategy in decreasing the risk of progression to sarcopenia.
In our study, women with arthritis were found to have a higher risk of possible sarcopenia. There are more than 100 types of arthritis. Rheumatoid arthritis (RA) is the most common form, but other common types of arthritis include osteoarthritis and inflammatory arthritis.35 Several studies have shown that women are more likely to be diagnosed with RA than men,36–38 and adults with RA tend to have lower muscle masses or strengths compared with adults without RA.39–41 Notably, RA can cause joint pain and deformity, therefore, it is unclear to what extent decreases in handgrip strength in patients with RA reflect true low muscle strength and how much of the limited handgrip strength may be secondary to pain or deformity.42 Thence, other measurements should be considered to assess the possible sarcopenia in arthritis adults.
The major strength of our study is that it assessed possible sarcopenia using the latest guideline (AWGS 2019) and analysed two subgroups based on gender. However, several limitations should be mentioned. First, this study focused solely on a population of older adults in Bengbu, so findings may not be generalisable to other populations. Second, we did not investigate nutritional factors that may be related to possible sarcopenia; a nutrition survey will be added in future studies.
Conclusions
The prevalence of possible sarcopenia in older adults in Bengbu is high, and it is more common among men than women. Men with high age, physical inactivity, diabetes and no hypertension had a higher prevalence of possible sarcopenia. And BMI was also found to be an independent risk factor for possible sarcopenia in men. Women with high age, physical inactivity and arthritis had a higher prevalence of possible sarcopenia. Community healthcare institutions should pay attention to the screening of possible sarcopenia, especially among older men. Targeted health education should also be carried out to encourage older adults to actively participate in physical exercise.
Supplementary Material
Acknowledgments
We want to thank all the participants involved in the study. We would also like to thank the hardworking research personnel involved in the data collection making this study possible.
Footnotes
Contributors: JY: conceptualisation, methodology, data collection, writing, investigation, statistical analysis. YW: data collection, methodology, writing, investigation. LY: data collection, writing. MR: data collection, writing. LL: writing. HW: conceptualisation, methodology, statistical analysis and acted as a guarantor.
Funding: This work was supported in part by Major project of Humanities and Social Sciences in Universities of Anhui Province (SK2020ZD32), the 512 Talent Cultivation Programme of Bengbu Medical College (by51201208) and the Graduate Research Innovation Plan of Bengbu Medical College in 2021 (Byycx21023).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. The data are held at Physical fitness center of Bengbu Medical College.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethical approval was obtained by the ethics committees of Bengbu Medical College (Anhui, China; no.2018045). Participants gave informed consent to participate in the study before taking part.
References
- 1.Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an international classification of disease, tenth revision, clinical modification (ICD-10-CM) code. J Am Med Dir Assoc 2016;17:675–7. 10.1016/j.jamda.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working group on sarcopenia in older people. Age Ageing 2010;39:412–23. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller K. Sarcopenia. Wien Med Wochenschr 2019;169:157–72. 10.1007/s10354-018-0618-2 [DOI] [PubMed] [Google Scholar]
- 4.Bone AE, Hepgul N, Kon S, et al. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis 2017;14:85–99. 10.1177/1479972316679664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izzo A, Massimino E, Riccardi G, et al. A narrative review on sarcopenia in type 2 diabetes mellitus: prevalence and associated factors. Nutrients 2021;13:183. 10.3390/nu13010183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pamoukdjian F, Bouillet T, Lévy V, et al. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr 2018;37:1101–13. 10.1016/j.clnu.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 7.Nascimento CM, Ingles M, Salvador-Pascual A, et al. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med 2019;132:42–9. 10.1016/j.freeradbiomed.2018.08.035 [DOI] [PubMed] [Google Scholar]
- 8.Sieber CC. Malnutrition and sarcopenia. Aging Clin Exp Res 2019;31:793–8. 10.1007/s40520-019-01170-1 [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L-K, Woo J, Assantachai P, et al. Asian Working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020;21:300–7. 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Sousa Miguel Ángel, Pozo-Cruz JD, Cano-Gutiérrez CA, et al. High prevalence of probable sarcopenia in a representative sample from Colombia: implications for geriatrics in Latin America. J Am Med Dir Assoc 2021;22:859–64. 10.1016/j.jamda.2020.10.021 [DOI] [PubMed] [Google Scholar]
- 12.Wearing J, Konings P, de Bie RA, et al. Prevalence of probable sarcopenia in community-dwelling older Swiss people - a cross-sectional study. BMC Geriatr 2020;20:307. 10.1186/s12877-020-01718-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim M, Won CW. Sarcopenia in Korean Community-Dwelling Adults Aged 70 Years and Older: Application of Screening and Diagnostic Tools From the Asian Working Group for Sarcopenia 2019 Update. J Am Med Dir Assoc 2020;21:752–8. 10.1016/j.jamda.2020.03.018 [DOI] [PubMed] [Google Scholar]
- 14.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Li X, Xu M, et al. Sarcopenia prevalence and associated factors among older Chinese population: findings from the China health and retirement longitudinal study. PLoS One 2021;16:e0247617. 10.1371/journal.pone.0247617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang BWJ, Wee S-L, Lau LK, et al. Prevalence and associated factors of sarcopenia in Singaporean Adults-The Yishun study. J Am Med Dir Assoc 2021;22:885.e1–885.e10. 10.1016/j.jamda.2020.05.029 [DOI] [PubMed] [Google Scholar]
- 17.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 2005;90:678–88. 10.1210/jc.2004-1184 [DOI] [PubMed] [Google Scholar]
- 18.Storer TW, Basaria S, Traustadottir T, et al. Effects of testosterone supplementation for 3 years on muscle performance and physical function in older men. J Clin Endocrinol Metab 2017;102:583–93. 10.1210/jc.2016-2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin MJ, Jeon YK, Kim IJ. Testosterone and sarcopenia. World J Mens Health 2018;36:192–8. 10.5534/wjmh.180001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang G, Wu L. Handgrip strength references for middle-age and older Chinese individuals. J Am Med Dir Assoc 2020;21:286–7. 10.1016/j.jamda.2019.08.034 [DOI] [PubMed] [Google Scholar]
- 21.Ramsey KA, Rojer AGM, D'Andrea L, et al. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in older adults: a systematic review and meta-analysis. Ageing Res Rev 2021;67:101266. 10.1016/j.arr.2021.101266 [DOI] [PubMed] [Google Scholar]
- 22.Simsek H, Meseri R, Sahin S, et al. Prevalence of sarcopenia and related factors in community-dwelling elderly individuals. Saudi Med J 2019;40:568–74. 10.15537/smj.2019.6.23917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsekoura M, Billis E, Tsepis E, et al. The effects of group and home-based exercise programs in elderly with sarcopenia: a randomized controlled trial. J Clin Med 2018;7:480. 10.3390/jcm7120480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makizako H, Nakai Y, Tomioka K, et al. Effects of a multicomponent exercise program in physical function and muscle mass in Sarcopenic/Pre-Sarcopenic adults. J Clin Med 2020;9:1386. 10.3390/jcm9051386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Smith L, Hamer M. Gender-specific risk factors for incident sarcopenia: 8-year follow-up of the English longitudinal study of ageing. J Epidemiol Community Health 2019;73:86–8. 10.1136/jech-2018-211258 [DOI] [PubMed] [Google Scholar]
- 26.Anagnostis P, Gkekas NK, Achilla C, et al. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: a systematic review and meta-analysis. Calcif Tissue Int 2020;107:453–63. 10.1007/s00223-020-00742-y [DOI] [PubMed] [Google Scholar]
- 27.Di Bari M, van de Poll-Franse LV, Onder G, et al. Antihypertensive medications and differences in muscle mass in older persons: the health, aging and body composition study. J Am Geriatr Soc 2004;52:961–6. 10.1111/j.1532-5415.2004.52265.x [DOI] [PubMed] [Google Scholar]
- 28.Onder G, Penninx BWJH, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet 2002;359:926–30. 10.1016/S0140-6736(02)08024-8 [DOI] [PubMed] [Google Scholar]
- 29.Ata AM, Kara M, Ekiz T, et al. Reassessing sarcopenia in hypertension: StAR and ACE inhibitors Excel. Int J Clin Pract 2021;75:e13800. 10.1111/ijcp.13800 [DOI] [PubMed] [Google Scholar]
- 30.Silva IVG, de Figueiredo RC, Rios DRA. Effect of different classes of antihypertensive drugs on endothelial function and inflammation. Int J Mol Sci 2019;20:3458. 10.3390/ijms20143458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henriksen EJ, Jacob S. Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. J Cell Physiol 2003;196:171–9. 10.1002/jcp.10294 [DOI] [PubMed] [Google Scholar]
- 32.Lee YL, Lee BH, Lee SY. Handgrip strength in the Korean population: normative data and cutoff values. Ann Geriatr Med Res 2019;23:183–9. 10.4235/agmr.19.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granic A, Mendonça N, Sayer AA, et al. Low protein intake, muscle strength and physical performance in the very old: the Newcastle 85+ study. Clin Nutr 2018;37:2260–70. 10.1016/j.clnu.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veronese N, Stubbs B, Punzi L, et al. Effect of nutritional supplementations on physical performance and muscle strength parameters in older people: a systematic review and meta-analysis. Ageing Res Rev 2019;51:48–54. 10.1016/j.arr.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 35.Tang C-H. Research of pathogenesis and novel therapeutics in arthritis. Int J Mol Sci 2019;20:1646. 10.3390/ijms20071646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hitchon CA, Khan S, Elias B, et al. Prevalence and incidence of rheumatoid arthritis in Canadian first nations and Non-First nations people: a population-based study. J Clin Rheumatol 2020;26:169–75. 10.1097/RHU.0000000000001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis 2019;78:1463–71. 10.1136/annrheumdis-2019-215920 [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Liu Q, Lin C, et al. The prevalence of rheumatoid arthritis in middle-aged and elderly people living in Naqu City, Tibet, autonomous region of China. J Orthop Surg Res 2020;15:338. 10.1186/s13018-020-01883-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker JF, Von Feldt J, Mostoufi-Moab S, et al. Deficits in muscle mass, muscle density, and modified associations with fat in rheumatoid arthritis. Arthritis Care Res 2014;66:1612–8. 10.1002/acr.22328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekici R, Erden A, Güven SC, et al. Prevalence of sarcopenia and clinical implications in patients with newly diagnosed rheumatoid arthritis. Nutrition 2021;90:111353. 10.1016/j.nut.2021.111353 [DOI] [PubMed] [Google Scholar]
- 41.Tekgoz E, Colak S, Ozalp Ates FS, et al. Sarcopenia in rheumatoid arthritis: is it a common manifestation? Int J Rheum Dis 2020;23:1685–91. 10.1111/1756-185X.13976 [DOI] [PubMed] [Google Scholar]
- 42.Torii M, Hashimoto M, Hanai A, et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod Rheumatol 2019;29:589–95. 10.1080/14397595.2018.1510565 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. The data are held at Physical fitness center of Bengbu Medical College.