Abstract
Cancer-related deaths are mainly caused by metastatic spread of tumor cells from the primary lesion to distant sites via the blood circulation. Understanding the mechanisms of blood-borne tumor cell dissemination by the detection and molecular characterization of circulating tumor cells (CTCs) in the blood of patients with cancer has opened a new avenue in cancer research. Recent technical advances have enabled a comprehensive analysis of the CTCs at the genome, transcriptome and protein level as well as first functional studies using patient-derived CTC cell lines. In this review, we describe and discuss how research on CTCs has yielded important insights into the biology of cancer metastasis and the response of patients with cancer to therapies directed against metastatic cells. Future investigations will show whether CTCs leaving their primary site are more vulnerable to attacks by immune effector cells and whether cancer cell dissemination might be the ‘Achilles heel’ of metastatic progression. Here, we focus on the lessons learned from CTC research on the biology of cancer metastasis in patients with particular emphasis on the interactions of CTCs with the immune system. Moreover, we describe and discuss briefly the potential and challenges for implementing CTCs into clinical decision-making including detection of minimal residual disease, monitoring efficacies of systemic therapies and identification of therapeutic targets and resistance mechanisms.
Keywords: Immunotherapy, Tumor Biomarkers, Tumor Escape, Tumor Microenvironment
Introduction
The Joint Research Centre (JRC) of the European Commission, in collaboration with the International Agency for Research on Cancer (IARC), has released the estimates of the burden of cancer in each of the European Union (EU-27) countries for 2020. A total of 2.7 million new cases of cancer (excluding non-melanoma skin cancers) and 1.3 million cancer-related deaths have been estimated for 2020 (https://www.iarc.who.int/fr/news-events/new-cancer-burden-estimates-for-2020-jrc-iarc-collaborations/). Although experimental studies in animal models have greatly enhanced our knowledge on the basic principles of cancer metastasis, the development in patients is sometimes difficult to model. For example, metastasis can occur in patients with estrogen receptor-positive (ER(+)) breast cancer more than 10 years after initial diagnosis and surgical resection of the primary tumor and this latency or dormancy period is not easy to mimic in mice that have a normal life span of approximately 2 years. Studies performed in patients with breast cancer indicated that even small tumors are able to release circulating tumor cells (CTCs) that extravasate into the bone marrow (and probably other organs) where they are called disseminated cancer cells (DTCs).1 Quantitative estimations have shown that these DTC-positive patients harbor at least one million tumor cells in their bodies despite the fact that they are called ‘metastasis-free’ by imaging procedures used for tumor staging. Interestingly, only half of these DTC-positive patients relapsed with 10 years of follow-up,2 suggesting that the host is able to control the outgrowth of a significant number of DTCs for many years. Factors promoting or suppressing circulation of CTCs and activation of dormant DTCs are subject of intense investigations. In this context, the immune system might play an important role as ‘gate keeper’.
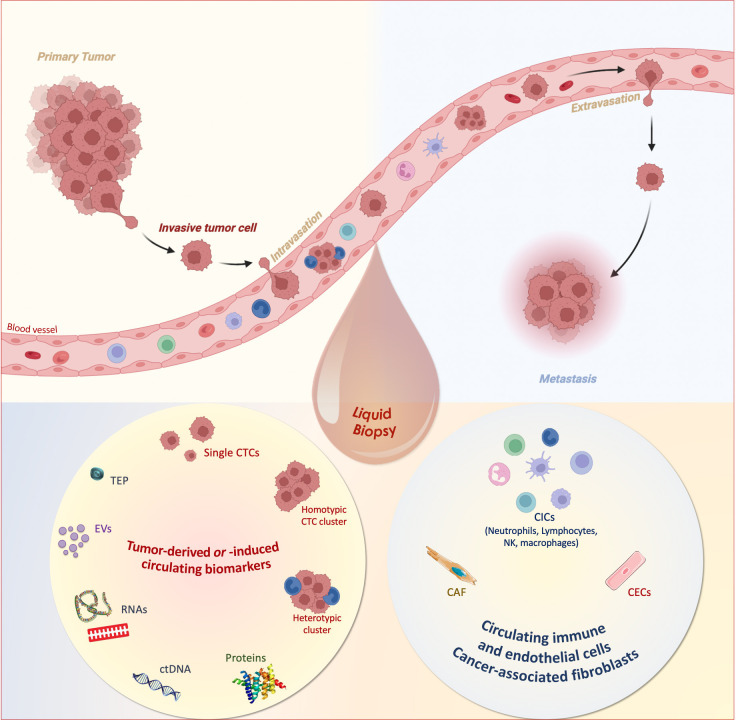
Technological advances over the past decade have allowed the detection and molecular characterization of CTCs in the blood of patients with cancer. Blood is easy to obtain and blood draws are much less invasive than needle biopsies of tissues. CTCs have constituted the core of the so-called liquid biopsy concept that has been introduced more than 10 years ago,3 and rapidly adapted to the analysis of tumor cell products such as cell-free nucleic acids or extracellular vesicles as well as immune cells4 (figure 1). Various clinical studies have indicated that CTC detection contributes to a better definition of the prognosis of the patients, enables the characterization of resistance mechanisms to therapy and tumor heterogeneity, the early detection of relapse in the metastatic setting and the detection of minimal residual disease in the earlier stages, which can contribute to a more personalized medicine in oncology. To get a comprehensive view of blood-borne cancer progression in the context of immunotherapies, we need to consider a broader definition of liquid biopsy including tumor-derived circulating biomarkers such as CTCs or cell-free DNA fragments and circulating components of the immune system (eg, immune cells, cytokines, interleukins) for each patient (figure 1).
Figure 1.
Tumor-derived or tumor-induced circulating biomarkers and circulating immune and endothelial cells as liquid biopsy for precision medicine. In a non-invasive sample, complementary circulating biomarkers can be isolated from the blood, counted and characterized. In the plasma or serum, extracellular vesicles (EVs), proteins, circulating cell-free tumor nucleic acids (circulating tumor DNA (ctDNA), non-coding and messenger RNAs), tumor-educated platelets (TEPs) can be found. In the cellular fraction, circulating tumor cells (CTCs; single, homotypic and heterotypic microemboli), circulating immune cells (CICs) and circulating endothelial cells (CECs) as well as cancer-associated fibroblasts (CAF) can be detected. The Figure was created with BioRender.com. NK, natural killer.
Personalized diagnostic and treatment strategies in cancer are highly based on the individual characteristics associated with malignant transformation and progression.5 To date, most patients are categorized at initial diagnosis, and treatment remains based on this initial classification while the tumor might undergo fundamental genotypic and functional changes. Recent research has confirmed that natural and therapy-induced selective pressure leads to clonal dynamics in cancers, leading to considerable heterogeneity and treatment resistance.6 7 In order to monitor these complex dynamic processes in patients with cancer, sequential monitoring of tumor composition in individual patients with cancer is required to adapt therapies to these changes. Today, these challenges may be addressed by the recent development of high-resolution, scalable blood-based detection methods of CTCs. These represent novel technologies to closely monitor the molecular composition of residual tumor burden following initial therapy. The additional benefit for patients consists in the fact that these procedures—as opposed to repetitive tissue biopsies—are minimal or non-invasive and are highly sensitive to monitor situations of low tumor load (‘minimal residual disease’, MRD) and discover tumor relapses prior to conventional imaging.8 9
In this review, we summarized and discussed the role of CTC research for better understanding of cancer metastasis with emphasis on tumor immune escape strategies, and how this knowledge can translate into novel strategies to improve the clinical outcome of patients with cancer.
CTCs capturing the metastatic cascade—tumor biology
CTCs can be derived from a primary and/or metastatic tumor lesion and circulate for a short time in the peripheral blood. Besides their value as diagnostic markers, the analyses of CTCs in patients with cancer (and experimental models) can contribute to understand the complexity of metastatic spread which may eventually lead to a favorable outcome of patients with solid malignancies.10 In this context, mathematical modeling might help to encompass the complex process of the metastatic cascade. Dujon et al used simulations (adaptation of the Drake equation) based on published breast cancer data and demonstrated that the survival of CTCs in the bloodstream is a key step in the complex process of the metastatic cascade.11
The detection of CTCs in lymph node-positive patients allows the analysis of tumor cell dissemination at a subclinical level,12 13 and contributes to a better understanding of the role of lymph nodes in the metastatic cascade.14 Monitoring of the genomic make up of CTCs by analysis of sequential blood samples from individual patients allows to get insights into the evolution of metastasis,15 including the role of intratumor heterogeneity. Moreover, the metabolic characterization of the CTCs has shed new light on the survival mechanisms of CTCs in the harsh blood microenvironment.16
Patients with tumor lesions in the brain lesions have a dismal prognosis. Despite the blood-brain barrier CTCs can enter the peripheral blood in these patients, which allows minimally invasive access to brain-derived tumor cells.17–19 Besides primary brain tumors, brain metastases cause significant harm to patients with cancer and they pose a major challenge to obtain personalized information because needle biopsy of these lesions is only possible in a few specialized clinical centers. Recent advances in the enrichment of CTCs from brain metastases by multiplexing of capture antibodies to different tumor-associated antigens have resulted in better capture rates20 and pilot studies on phenotypic characterization of CTCs in patients with brain metastases suggest that these cells might reflect the phenotype of the brain metastasis from the same patient. Nevertheless, experimental in vitro studies suggest that gene expression of tumor cells can change during the travel of CTCs in blood (eg, due to changes in oxygen levels).21
The study of CTC biology opens a new avenue for better understanding the process of blood-borne tumor cell dissemination. The harsh conditions in the blood require that CTCs are fit enough for survival and extravasation into a distant organ site. Interestingly, counter to previous findings in experimental mouse models22 this seems to be an essential step in the metastatic cascade,11 which is consistent with the fact that the CTC detection at initial diagnosis is a robust and significant factor associated with metastatic relapse in solid tumors, such as breast cancer. To survive in the blood after detachment from their tumor tissue of origin, CTCs must avoid undergoing anoikis, a special form of apoptosis. Numerous studies have shown that the epithelial-to-mesenchymal transition (EMT) favors the dissemination of single CTCs, while the role of EpCAM—one of the most prominent markers for enrichment of CTCs that is found also on metastasis-competent CTCs—in EMT is still not well understood.23 Overall, the most aggressive metastasis-competent CTCs seem to be those that have a high plasticity. Indeed, when we investigated the main characteristics of the unique existing series of colon CTC lines, which have surprisingly acquired only few mesenchymal features but expressed rather epithelial-related genes, suggesting that metastasis-initiator CTCs require a switch from EMT to mesenchymal-to-epithelial transition (MET).24
The role and importance of mesenchymal CTCs remain unsolved. Most data on the prognostic relevance of CTCs are based on assays using epithelial markers.25 26 Cells classified as ‘mesenchymal CTCs’ are frequently identified by unspecific markers also expressed on normal blood cells. Further characterization of mesenchymal CTCs is required to determine the degree of mesenchymal attributes of CTCs necessary to survive in the circulation or to initiate overt metastasis at distant sites and whether mesenchymal CTCs have a higher propensity to escape killing by from immune.
Although the majority of CTCs detectable in patients with cancer are single isolated cells, there is increasing evidence that the formation of aggregates among CTCs or clusters between CTCs and blood cells is important to metastatic progression27 28 (figure 1). For example, leukocytes in heterotypic clusters can epigenetically reprogram the attached neighbor CTCs, which results in enhanced CTC survival and induces proliferation of CTCs. Moreover, blood platelets can shield CTCs from destruction of immune cells, induce EMT programs in CTCs and help CTCs to extravasate. These insights derived from CTC analyses could lead to novel therapeutic strategies to prevent metastatic progression.
Functional CTC analyses depend on suitable models. During the past decade a few cell lines derived from CTCs of patients with different tumor entities could be established. However, these were exceptional cases where the CTC counts were very high at the start of the culture. Nevertheless, these models provide insights into CTC biology and in particular might help to identify pathways specific for metastasis-competent CTCs, discover new CTC markers and unravel mechanisms of drug resistance of these CTCs.10 29–31
Analysis of CTCs might also lead to new insights whether invasive diagnosis procedures like tumor biopsies or surgery might lead to the mobilization of CTCs during these procedures, and whether this potential mechanical tumor cell dissemination has an influence on the development of metastases. In prostate cancer, preliminary data suggest the possibility that tissue biopsy might lead to an increase in CTC concentrations in some patients associated with a higher rate of biochemical recurrence.32 Studies on larger and well-defined cohorts are needed to validate these provocative data and address the question whether tumor cells released into the blood by mechanical forces are able to survive, extravasate and form metastases at distant organs. Further analyses of CTCs in patients with cancer at the DNA, RNA and protein level offers now the opportunity to answer this question which has led to vivid controversial debates for several decades.
Immune escape mechanisms of CTCs
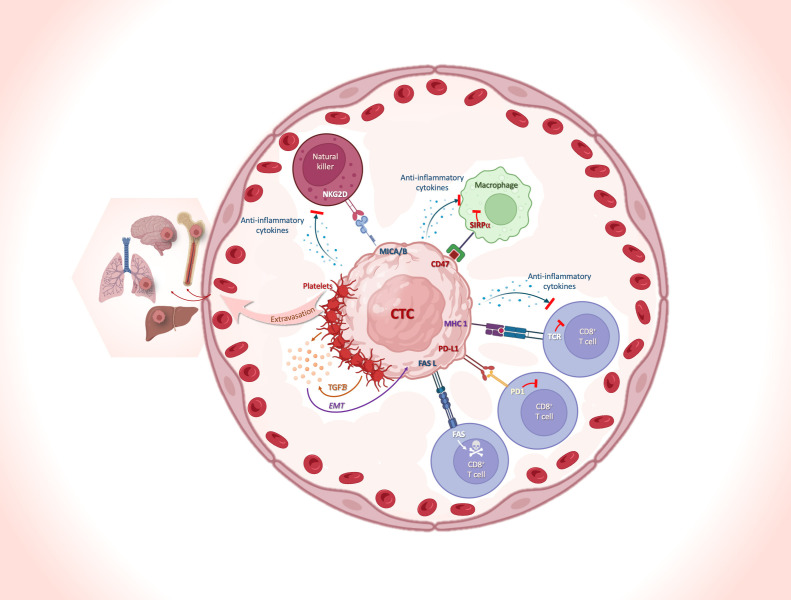
Blood is a hostile environment for CTCs. Although the primary tumor presumably sheds thousands of cells into the bloodstream every day,33 only a very small percentage of these cells survive in the bloodstream and become detectable as CTCs in a blood sample. Here, we will discuss reports dealing with the interaction of CTCs and immune cells mediating escape of CTCs from immune surveillance (figure 2) affecting both the innate and adaptive immune system. The recognition and elimination of CTCs via natural killer (NK)-cell-mediated lysis can be prevented by the interaction between NK-cell receptor D on NK cells and MICA/MICB on CTCs.34 T cell-mediated lysis can be inhibited by the interference with T-cell receptor recognition of MHC I molecules on CTCs, shielding by blood platelets that interfere at several levels (eg, induction of EMT or acquisition of a ‘pseudonormal’ phenotype conferring by the transfer of platelet-derived MHC I molecules),35 36 the expression of inhibitory immune-checkpoint proteins like programmed cell death-ligand 1 (PD-L1); the expression of CD47 (do not eat me signal) on CTCs37 38 that binds to its ligand signal-regulatory protein α expressed on macrophages and dendritic cells, inhibiting phagocytosis,39 and an altered expression of the apoptotic proteins FAS and/or FASL.40 In the following paragraphs, we will focus on escape mechanisms mediated by immune checkpoint inhibition as the most prominent mechanism for therapeutic interventions which has widely changed the therapeutic landscape in oncology.
Figure 2.
Immune-escape mechanisms of CTCs and collaboration with blood cells to facilitate their journey and optimize their survival in the peripheral blood. The schematic illustrates some of the main mechanisms of immune escape of CTCs, and the interactions between CTCs and immune cells in the peripheral blood. The interplay between NK cells and CTCs is shown, with the interaction between NKG2D on NK and MICA/MICB on CTCs, preventing recognition and elimination of the cells via NK-cell-mediated lysis. Different strategies of CTC escape: (1) the interference with TCR recognition of MHC I molecules; (2) the maintenance of the EMT phenotype resulting from TGFß secretion by the platelets recovering CTCs; (3) the expression of the inhibitory immune-checkpoint protein PD-L1; (4) the presentation of the ‘don’t eat me’ signaling receptor CD47 and (5) an altered expression of the apoptotic proteins FAS and/or FASL. The Figure was created with BioRender.com. CTC, circulating tumor cell; EMT, epithelial-to-mesenchymal transition; FASL, FAS ligand; MHC I, MHC class I; MICA, MHC I polypeptide-related sequence A; MICB, MHC I polypeptide-related sequence B; NK, natural killer; NKG2D, NK-cell receptor D (also known as NKG2-D type II integral membrane protein); PD-1, programmed cell-death protein 1; PD-L1, programmed cell death-ligand 1; SIRPα, signal-regulatory protein α; TCR, T-cell receptor; TGFβ, transforming growth factor β.
Releasing the brakes on antitumor immune responses by targeting immune checkpoint molecules is the primary focus of recent immunotherapeutic treatment strategies.41 Within the immunological synapse, a multitude of inhibitory receptors have been identified.42 Programmed cell death protein-1 (PD-1) and its ligand, PD-L1, have been one of the most prominent examples to antagonize immune escape mechanisms employed by tumor cells. PD-L1 limits immune effector functions by binding to its cognate receptor PD-1 expressed on tumor-specific T cells.43
CTC undergoing EMT appears to be associated with inferior clinical outcome,44 45 and there is increasing evidence for cross talk between EMT-inducing molecules and PD-L1.46–48 Interestingly, platelets are able to initiate and maintain EMT on CTCs by secretion of transforming growth factor-ß as well as support homing and outgrowth of DTCs in bone marrow (figure 2).49 PD-L1 expression by EMT-CTCs has been shown to be associated with poor survival in curatively patients with resected non-small cell lung cancer (NSCLC).50 Another recent study showed that cluster and single PD-L1+ EMT-CTCs subpopulations are of clinical significance in patients with metastatic breast cancer (MBC) and highlighted the importance of CTC phenotyping during treatment with eribulin.51 These data suggest that immune-checkpoint inhibitors might mitigate the additive effect of PD-L1 and EMT on clinical outcomes of patients with cancer and also how immune cells secreting EMT-inducing cytokines into the tumor microenvironment affect PD-L1 expression of tumor cells released into the circulation is subject of current investigations.52 53
The idea of disabling immune escape mechanisms on tumor cells is now being explored in clinical trials by using immune blockade inhibitors.43 Efforts to restore latent antitumor immunity focuses on antibody-based interventions targeting cytotoxic T-lymphocytes-associated protein 4 or PD-1 on T lymphocytes and its principal ligand, PD-L1, on tumor cells.41 54 Additional immunomodulatory antibodies tested in clinical trials will further expand the spectrum of immune checkpoint blockade.55 56 In view of the remarkable costs and the toxicity profiles of these therapies, predictive biomarkers able to discriminate responders from non-responders are urgently needed. In this context, CTC/PD-L1 assays should be tested as liquid biopsy for stratification and monitoring of patients with cancer undergoing immune checkpoint blockade. Our group has shown for the first time in 2015 that CTCs can express PD-L1 in patients with MBC: not all patients showed CTC-PD-L1+, not all CTCs expressed PD-L1 in the same patients.57 Subsequently, we evaluated in a prospective clinical trial the clinicopathological correlations and prognostic value of CTC-PD-L1+ in a new cohort of 72 patients with MBC. Unlike PD-L1+ tumor, CTC-PD-L1+ correlated to survival in MBC (clinical trial registration: NCT02866149).58 More recently, we performed a similar study with 54 patients with advanced NSCLC (clinical trial registration: NCT02866149) and showed again that the presence of CTC-PD-L1+ is associated with poor prognosis (progression free survival and overall survival) in this cohort of patients.59
Reappraisal of the role of PD-L1 expression by tumor tissue and by CTCs under anti-PD-1/PD-L1 treatment is necessary to evaluate its predictive value and potential role as a stratifying factor in strategies and trials for patients with MBC. Considering the detection of PD-L1 on CTCs, the current French clinical trial ALCINA2 (clinical trial registration: NCT02866149) focuses on the clinical relevance of liquid biopsy (CTC-PD-L1+, exosomes-PD-L1+, phenotype of immune cells) in patients with NSCLC in the context of immunotherapy. Liquid biopsy should allow classification of patients suitable for checkpoint inhibitor therapy before entering into clinical trials.60 How far CTCs can functionally take advantage of PD-L1 expression and which other mechanisms of immune escape are effective on CTCs/DTCs is currently under investigation.
Promotion of metastases by immune cells
It is well known that metastasis can be also supported by immune cells.61 Here, we will focus on reports that have included measurements of CTCs, describing metastasis-promoting tumor-immune cell interactions that can lead to the survival and proliferation of CTCs.
Regulatory T cells (Tregs) appear to also play an important role in tumor cell dissemination and formation of metastasis.62 More specifically, the expression of interleukin (IL)-1β and a concurrent increase in Treg frequency in the peripheral blood may be strong predictors of clinical outcome,63 and disease progression and occurrence of CTCs with recruitment of Tregs and myeloid-derived suppressor cells has been reported.62 64 Thus, suppression of the peripheral antitumor immune response might support CTC survival and promote metastatic progression. A possible link between Tregs and CTCs was indicated in MBC: CTC-positive patients had a higher frequency of CD4+ Tregs in the peripheral blood together with a stem-like CTC phenotype. Furthermore, CTCs in patients with breast cancer exhibit a downregulation in mTOR, PARP, myc genes, and among others upregulation of FOXO3.65 These genes are potentially involved in induction of immune tolerance (eg, downregulation of PARP can enable increased Treg activity).66 Recent improvement in CTC culturing and the establishment of CTC-derived cell lines31 67 might open new avenues for functional studies to unravel the influence of CTCs on the systemic Treg activation.45
Neutrophils appear to play an important role for promoting CTC dissemination and survival. CTCs directly adhere on top of neutrophils, indicating that CTCs use immune cells to hitchhike during extravasation.68 Multiple mechanisms have been proposed to be involved in this interaction, including the expression of the intercellular adhesion molecule-1 on CTCs and its binding to Mac-1 or β2-integrins on neutrophils,68 69 with cytokines such as IL-8 as presumable mediators in this crosstalk.70 71 Neutrophils can even actively entrap CTCs through the production of ‘neutrophil extracellular trap’ (NET), which describes a structure of extruded DNA and proteins on the surface of neutrophils. NETs can enhance CTC adherence and extravasation in target organs of metastatic spread.70 72 Interestingly, neutrophils form heterotypic clusters with CTCs and promote their survival and ability to grow out to overt metastases.27 This team identified cell–cell junction and cytokine-receptor pairs that define CTC-neutrophil clusters, representing key vulnerabilities of the metastatic process. Cluster formation might also have influences on MHC-mediated antigen presentation73 and provide therefore a more general way to promote survival of CTCs.
Clinical translation—implementation of CTCs into clinical decision-making
The clinical relevance of CTCs in patients with cancer has been extensively reviewed (eg,4 74 CTCs offer the advantage that information on all components of tumor cells including DNA, RNA and proteins can be obtained. Moreover, the analysis of intact tumor cells rather than fragments of circulating tumor DNA (ctDNA (released from apoptotic tumor cells seems to ensure that the information is really derived from viable tumor cells that can contribute to tumor progression. Here, we will provide a brief summary of the most prominent clinical applications and cite some reports that underline our statements. We apologize to all the authors who published excellent work in this area that we did not cite.
Early detection of cancer
Early detection of cancer is one of the key aims of blood biomarkers. Screening of populations at risk to develop cancer may help to identify and resect small malignant lesions before they have spread to regional lymph nodes or distant organs. Currently, this application is dominated by ultrasensitive methods to detect minute amounts of cell-free DNA in blood plasma,75 while the incidence of CTCs detectable at early cancer stages is usually too low using current technologies. However, increases in the sensitivity of CTC detection might be achieved by the analysis of larger blood volumes.7 For early detection of cancer, the future will be to combine different circulating biomarkers: tumor-derived or tumor-induced but also the immune cells.76
Monitoring of MRD—surveillance
After initial cancer diagnosis, many patients undergo surgical resection of their primary lesion and subsequent adjuvant radiotherapy or systemic therapy (eg, chemotherapy) aimed to eradicate locoregional or systemic spread of metastatic cells. CTC detection at initial diagnosis in a variety of solid tumors can help to determine the subsequent risk of developing metastatic relapse.77 78 Moreover, monitoring of patients with cancer (‘surveillance’) over time after initial adjuvant therapy by repeated blood analyses has opened new avenues for early detection of signs that indicate the presence of active MRD that poses a risk for overt metastatic relapse. CTC counts obtained 2 or 5 years after initial adjuvant therapy in breast cancer can identify patients with MRD who have a higher risk to develop metastatic relapse within the next 5 years of further follow-up.8 9 Since the life time of CTCs in blood is rather short (hours), these CTCs are not derived from the primary lesion resected many years ago but most likely are derived from small micrometastatic lesions. Further molecular analyses of these CTCs open a new avenue for deciphering the mechanisms that control the outgrowth of these lesions that may have been in a dormant or latent state for several years.
Monitoring of therapy efficacy in metastatic disease
Changes in CTC counts during therapy of patients with solid tumors such as breast cancer are associated with response to therapy. In breast cancer, CTC counts predicted progression of patients with metastatic disease much earlier and with high accuracy than the conventional blood serum proteins used as ‘tumor markers’ (eg, carcinoembryonic antigen (CEA)).79 Similar results were obtained in advanced prostate cancer where rapid declines in CTC counts during chemotherapy or antiandrogen therapies provided information on therapy responses independent from the standard serum marker prostate-specific antigen (PSA).80
Identification of therapeutic targets and resistance mechanisms
Besides quantitative assessment of CTCs, the molecular characterization of these cells opened a new avenue for the qualitative assessment of tumor burden in individual patients. Metastatic lesions are frequently not easy to probe by needle aspirations, which is a hurdle for getting personalized information of potential targets for therapy or resistance mechanisms. In contrast, blood samples can be easily obtained from every patient. Recent advances have made it possible to characterize CTCs at the DNA, RNA and protein level and obtain—in addition to ctDNA analyses which are restricted to genomic aberrations—a more comprehensive view of the alterations relevant to therapy.4 The advantage of CTCs over ctDNA in the context of immunotherapies is that cell surface proteins relevant for the interaction of tumor cells with immune cells such as MHC antigens and immune check point inhibitors such as PD-L1 can be assessed at the single cell level. Moreover, the direct cross talk in heterotypic clusters of CTCs and immune cells can be studied and it may be important for the response to immunotherapies. This cellular information together with the information derived from DNA analysis (eg, tumor mutational burden) will increase our knowledge about the biology of resistance mechanisms to immunotherapies.
Nevertheless, a considerable fraction of patient with metastatic disease has not enough CTCs for subsequent molecular analysis, which points again to the need to develop and validate technologies that increase the CTC capture rates.
Conclusions and perspectives
We have discussed how research on CTCs can capture the steps of the metastatic cascade in patients with cancer in particular with regard to the interface with immune cells, and how this CTC research has translated into clinical applications that might lead to an improved personalized management of patients with cancer at various stages of their disease. Although most of these applications are so far only for research use in clinical studies and are not part of the current recommendations for clinical use in routine diagnostics, recently completed and ongoing interventional studies4 will provide evidence on the clinical utility relevant for implementation into clinical decision-making. For the first time in this field, the METABREAST study (clinical trial registration: NCT01710605) proved the clinical utility of CTCs. In this interventional clinical trial, patients with first-line MBC were randomized between the clinician’s choice and CTC count-driven choice. In the CTC arm, patients with ≥5 CTC/7.5 mL received chemotherapy, whereas patients with <5 CTC/7.5 mL received endocrine therapy as first-line treatment. In conclusion, the CTC count is a reliable biomarker method for choosing between chemotherapy and endocrine therapy as the first-line treatment in hormone receptor-positive HER2− MBC.
Besides its potential value for improving patient’s care, CTC research offers unique opportunities to unravel the evolution of metastatic progression in patients with cancer. This knowledge will complement the valuable information derived from experimental studies. In particular, the interactions between CTCs and the cells of the immune system deserves intense future investigation. Many research groups have analyzed either CTCs or profiled immune cells in patients with cancer but studies assessing both the tumor and host response are under-represented. In this context, the lack of functional studies on CTCs might be overcome now by the establishment of conditions that allow in vitro cultures of CTCs and the availability of CTC-derived cell lines.31 67
Emerging evidence shows that the immune system may have a dual role on CTCs. On the one hand, CTCs released into the bloodstream might be more vulnerable for immune-mediated elimination, and recent studies have gained new insights into the question how CTCs can counteract immune-mediated killing. On the other hand, immune cells like neutrophils can support the survival and growth of CTCs and thereby support metastatic development. Other components of the ‘circulome’ such as platelets, endothelial cells or cancer-associated fibroblasts may also affect the survival and/or capacity to extravasate into distant tissues. Thus, the assessment of heterotypic clusters between CTCs and host cells is an exciting area of future investigations. Interestingly, recent data suggest that the release and survival of CTCs in the circulation undergoes circadian changes,81 82 which might have also important implications for the clinical use of CTCs as biomarkers. The future will tell us whether we identified the ultimate selective filter in cancer progression—CTCs releasing during the rest phase83—and more importantly, whether this nocturnal CTC dissemination is due to the role of sleep or circadian cycle.84
For the clinical implementation of CTCs as liquid biopsy marker, the harmonization and technical validation of the plethora of different technologies is essential. The European CANCER-ID network has carried out such studies85 which are now continued by the successor consortium called European Liquid Biopsy Society (www.elbs.eu) and will result into guidelines for the use of CTCs in cancer research. These activities are connected to other networks worldwide through the International Alliance for Liquid Biopsy Standardization.86
A challenge for CTC-based therapeutic decision-making is their infrequent detection. High-blood volume analysis could provide a solution, as exemplified in a recent report on monitoring response to (immuno)therapy in lung cancer using leukapheresis.87 Diagnostic leukapheresis has been applied in selected CTC research studies88 to obtain thousands of CTCs from individual patients with cancer but their use in large scale clinical studies is very challenging. In vivo CTC capture devices may become an alternative in the future89 but they are still at an experimental stage of development.
The availability of sufficient amounts of CTCs can also open new avenues for better understanding the response to immunotherapies. For example, besides correlative studies suggesting that PD-L1 expression on CTCs is more prognostically relevant than the expression on tumor tissue,58 90 it is not clear whether CTCs are more vulnerable to interventions targeting PD-L1 than cells in solid tumors. Platelet association, which may be more CTC specific, is also cited as providing immune protection. However, it is also unclear whether CTCs lacking platelet association are rapidly cleared by the innate immune system in patients with cancer. Future investigations may focus on these important questions. At present, there is a paucity of direct evidence and that we need more extensive analyses.
Taken together, the combined in-depth analysis of CTCs and circulating immune cells in relation to the changes occurring in the respective tumor microenvironment and clinical outcome of patients with cancer will provide new insights in the role of the immune system in metastatic progression.
Footnotes
Contributors: KP and CA-P designed and wrote together this review article.
Funding: CA-P and KP received funding from the European IMI research project CANCER-ID (115749-CANCER-ID), European Union Horizon 2020 Research and Innovation program under the Marie Skłodowska-Curie grant agreement No 765492 and ERA-NET EU/TRANSCAN 2 JTC 2016 PROLIPSY. CA-P is also supported by The National Institute of Cancer (INCa, http://www.e-cancer.fr) and SIRIC Montpellier Cancer Grant INCa_Inserm_DGOS_12553. KP also received funding from Deutsche Krebshilfe (Nr. 70112504), Deutsche Forschungsgemeinschaft (DFG) SPP2084 µBone and ERC Advanced Investigator Grant INJURMET (Nr. 834974).
Competing interests: KP and CA-P have received honoraria from Menarini and both authors have patent applications related to CTC technologies.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Braun S, Pantel K, Müller P, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 2000;342:525–33. 10.1056/NEJM200002243420801 [DOI] [PubMed] [Google Scholar]
- 2.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 2005;353:793–802. 10.1056/NEJMoa050434 [DOI] [PubMed] [Google Scholar]
- 3.Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 2010;16:398–406. 10.1016/j.molmed.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 4.Alix-Panabières C, Pantel K. Liquid biopsy: from discovery to clinical application. Cancer Discov 2021;11:858–73. 10.1158/2159-8290.CD-20-1311 [DOI] [PubMed] [Google Scholar]
- 5.Hahn WC, Bader JS, Braun TP, et al. An expanded universe of cancer targets. Cell 2021;184:1142–55. 10.1016/j.cell.2021.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marine J-C, Dawson S-J, Dawson MA. Non-Genetic mechanisms of therapeutic resistance in cancer. Nat Rev Cancer 2020;20:743–56. 10.1038/s41568-020-00302-4 [DOI] [PubMed] [Google Scholar]
- 7.Keller L, Pantel K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat Rev Cancer 2019;19:553–67. 10.1038/s41568-019-0180-2 [DOI] [PubMed] [Google Scholar]
- 8.Trapp E, Janni W, Schindlbeck C, et al. Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J Natl Cancer Inst 2019;111:380–7. 10.1093/jnci/djy152 [DOI] [PubMed] [Google Scholar]
- 9.Sparano J, O'Neill A, Alpaugh K, et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 2018;4:1700–6. 10.1001/jamaoncol.2018.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eslami-S Z, Cortés-Hernández LE, Thomas F, et al. Functional analysis of circulating tumour cells: the key to understand the biology of the metastatic cascade. Br J Cancer 2022;127:800–10. 10.1038/s41416-022-01819-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dujon AM, Capp J-P, Brown JS, et al. Is there one key step in the metastatic cascade? Cancers 2021;13:3693. 10.3390/cancers13153693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trapp E, Janni W, Schindlbeck C, et al. Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J Natl Cancer Inst 2019;111:380–7. 10.1093/jnci/djy152 [DOI] [PubMed] [Google Scholar]
- 13.Rack B, Schindlbeck C, Jückstock J, et al. Circulating Tumor Cells Predict Survival in Early Average-to-High Risk Breast Cancer Patients. 106. JNCI: Journal of the National Cancer Institute, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naxerova K, Reiter JG, Brachtel E, et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science 2017;357:55–60. 10.1126/science.aai8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joosse SA, Souche F-R, Babayan A, et al. Chromosomal aberrations associated with sequential steps of the metastatic cascade in colorectal cancer patients. Clin Chem 2018;64:1505–12. 10.1373/clinchem.2018.289819 [DOI] [PubMed] [Google Scholar]
- 16.Ubellacker JM, Tasdogan A, Ramesh V, et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 2020;585:113–8. 10.1038/s41586-020-2623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller C, Holtschmidt J, Auer M, et al. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med 2014;6:247ra101–247. 10.1126/scitranslmed.3009095 [DOI] [PubMed] [Google Scholar]
- 18.Riebensahm C, Joosse SA, Mohme M, et al. Clonality of circulating tumor cells in breast cancer brain metastasis patients. Breast Cancer Res 2019;21:101. 10.1186/s13058-019-1184-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garzia L, Kijima N, Morrissy AS, et al. A hematogenous route for medulloblastoma leptomeningeal metastases. Cell 2018;173:1549. 10.1016/j.cell.2018.05.033 [DOI] [PubMed] [Google Scholar]
- 20.Scharpenseel H, Hanssen A, Loges S, et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci Rep 2019;9:7406. 10.1038/s41598-019-43678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartkowiak K, Koch C, Gärtner S, et al. In vitro modeling of reoxygenation effects on mRNA and protein levels in hypoxic tumor cells upon entry into the bloodstream. Cells 2020;9. 10.3390/cells9051316. [Epub ahead of print: 25 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2:563–72. 10.1038/nrc865 [DOI] [PubMed] [Google Scholar]
- 23.Eslami-S Z, Cortés-Hernández LE, Alix-Panabières C. Epithelial cell adhesion molecule: an anchor to isolate clinically relevant circulating tumor cells. Cells 2020;9. 10.3390/cells9081836. [Epub ahead of print: 05 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balcik-Ercin P, Cayrefourcq L, Soundararajan R, et al. Epithelial-To-Mesenchymal plasticity in circulating tumor cell lines sequentially derived from a patient with colorectal cancer. Cancers 2021;13. 10.3390/cancers13215408. [Epub ahead of print: 28 10 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller L, Werner S, Pantel K. Biology and clinical relevance of EpCAM. Cell Stress 2019;3:165–80. 10.15698/cst2019.06.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner S, Keller L, Pantel K. Epithelial keratins: biology and implications as diagnostic markers for liquid biopsies. Mol Aspects Med 2020;72:100817. 10.1016/j.mam.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 27.Szczerba BM, Castro-Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019;566:553–7. 10.1038/s41586-019-0915-y [DOI] [PubMed] [Google Scholar]
- 28.Gkountela S, Castro-Giner F, Szczerba BM, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 2019;176:98–112. 10.1016/j.cell.2018.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou K, Cheong JE, Tarakkad Krishnaji S, et al. Inhibition of Wnt signaling in colon cancer cells via an oral drug that facilitates TNIK degradation. Mol Cancer Ther 2022. 10.1158/1535-7163.MCT-21-0801. [Epub ahead of print: 27 Oct 2022]. [DOI] [PubMed] [Google Scholar]
- 30.Smit DJ, Cayrefourcq L, Haider M-T, et al. High sensitivity of circulating tumor cells derived from a colorectal cancer patient for dual inhibition with Akt and mTOR inhibitors. Cells 2020;9. 10.3390/cells9092129. [Epub ahead of print: 20 Sep 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cayrefourcq L, Thomas F, Mazard T, et al. Selective treatment pressure in colon cancer drives the molecular profile of resistant circulating tumor cell clones. Mol Cancer 2021;20:30. 10.1186/s12943-021-01326-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joosse SA, Beyer B, Gasch C, et al. Tumor-Associated release of prostatic cells into the blood after transrectal ultrasound-guided biopsy in patients with histologically confirmed prostate cancer. Clin Chem 2020;66:161–8. 10.1373/clinchem.2019.310912 [DOI] [PubMed] [Google Scholar]
- 33.Chang YS, di Tomaso E, McDonald DM, et al. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A 2000;97:14608–13. 10.1073/pnas.97.26.14608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cluxton CD, Spillane C, O'Toole SA, et al. Suppression of natural killer cell NKG2D and CD226 anti-tumour cascades by platelet cloaked cancer cells: implications for the metastatic cascade. PLoS One 2019;14:e0211538. 10.1371/journal.pone.0211538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanikarla-Marie P, Lam M, Sorokin AV, et al. Platelet metabolism and other targeted drugs; potential impact on immunotherapy. Front Oncol 2018;8:107. 10.3389/fonc.2018.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Placke T, Örgel M, Schaller M, et al. Platelet-Derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 2012;72:440–8. 10.1158/0008-5472.CAN-11-1872 [DOI] [PubMed] [Google Scholar]
- 37.Steinert G, Schölch S, Niemietz T, et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res 2014;74:1694–704. 10.1158/0008-5472.CAN-13-1885 [DOI] [PubMed] [Google Scholar]
- 38.Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 2013;31:539–44. 10.1038/nbt.2576 [DOI] [PubMed] [Google Scholar]
- 39.Jaiswal S, Jamieson CHM, Pang WW, et al. Cd47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009;138:271–85. 10.1016/j.cell.2009.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruber I, Landenberger N, Staebler A, et al. Relationship between circulating tumor cells and peripheral T-cells in patients with primary breast cancer. Anticancer Res 2013;33:2233–8. [PubMed] [Google Scholar]
- 41.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56–61. 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- 42.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467–77. 10.1038/nri2326 [DOI] [PubMed] [Google Scholar]
- 43.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mego M, Gao H, Lee B-N, et al. Prognostic value of EMT-Circulating tumor cells in metastatic breast cancer patients undergoing high-dose chemotherapy with autologous hematopoietic stem cell transplantation. J Cancer 2012;3:369–80. 10.7150/jca.5111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med 2015;7:1–11. 10.15252/emmm.201303698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alsuliman A, Colak D, Al-Harazi O, et al. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer 2015;14:149. 10.1186/s12943-015-0421-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Gibbons DL, Goswami S, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014;5:5241. 10.1038/ncomms6241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Wang H, Zhao Q, et al. Pd-L1 induces epithelial-to-mesenchymal transition via activating SREBP-1c in renal cell carcinoma. Med Oncol 2015;32:212. 10.1007/s12032-015-0655-2 [DOI] [PubMed] [Google Scholar]
- 49.Leblanc R, Peyruchaud O. Metastasis: new functional implications of platelets and megakaryocytes. Blood 2016;128:24–31. 10.1182/blood-2016-01-636399 [DOI] [PubMed] [Google Scholar]
- 50.Manjunath Y, Upparahalli SV, Avella DM, et al. Pd-L1 expression with epithelial mesenchymal transition of circulating tumor cells is associated with poor survival in curatively resected non-small cell lung cancer. Cancers 2019;11. 10.3390/cancers11060806. [Epub ahead of print: 11 06 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polioudaki H, Mala A, Gkimprixi E, et al. Epithelial/Mesenchymal characteristics and PD-L1 co-expression in CTCs of metastatic breast cancer patients treated with eribulin: correlation with clinical outcome. Cancers 2020;12. 10.3390/cancers12123735. [Epub ahead of print: 11 12 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen EN, Gao H, Anfossi S, et al. Inflammation mediated metastasis: immune induced epithelial-to-mesenchymal transition in inflammatory breast cancer cells. PLoS One 2015;10:e0132710. 10.1371/journal.pone.0132710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mego M, Cholujova D, Minarik G, et al. CXCR4-SDF-1 interaction potentially mediates trafficking of circulating tumor cells in primary breast cancer. BMC Cancer 2016;16:127. 10.1186/s12885-016-2143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what's here, what's next? Curr Opin Immunol 2015;33:23–35. 10.1016/j.coi.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 55.Romagné F, André P, Spee P, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 2009;114:2667–77. 10.1182/blood-2009-02-206532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benson DM, Cohen AD, Jagannath S, et al. A phase I trial of the Anti-KIR antibody IPH2101 and lenalidomide in patients with relapsed/refractory multiple myeloma. Clin Cancer Res 2015;21:4055–61. 10.1158/1078-0432.CCR-15-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazel M, Jacot W, Pantel K, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol 2015;9:1773–82. 10.1016/j.molonc.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacot W, Mazel M, Mollevi C, et al. Clinical correlations of programmed cell death ligand 1 status in liquid and standard biopsies in breast cancer. Clin Chem 2020;66:1093–101. 10.1093/clinchem/hvaa121 [DOI] [PubMed] [Google Scholar]
- 59.Sinoquet L, Jacot W, Gauthier L, et al. Programmed cell death ligand 1-expressing circulating tumor cells: a new prognostic biomarker in non-small cell lung cancer. Clin Chem 2021;67:1503–12. 10.1093/clinchem/hvab131 [DOI] [PubMed] [Google Scholar]
- 60.David R. PD-L1 expression by circulating breast cancer cells. Lancet Oncol 2015;16:e321. 10.1016/S1470-2045(15)00074-1 [DOI] [PubMed] [Google Scholar]
- 61.Kitamura T, Qian B-Z, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol 2015;15:73–86. 10.1038/nri3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang H, Gebhardt C, Umansky L, et al. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer 2015;136:2352–60. 10.1002/ijc.29297 [DOI] [PubMed] [Google Scholar]
- 63.Wilke CM, Wu K, Zhao E, et al. Prognostic significance of regulatory T cells in tumor. Int J Cancer 2010;127:n/a–58. 10.1002/ijc.25464 [DOI] [PubMed] [Google Scholar]
- 64.Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med 2013;91:411–29. 10.1007/s00109-013-1021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hensler M, Vančurová I, Becht E, et al. Gene expression profiling of circulating tumor cells and peripheral blood mononuclear cells from breast cancer patients. Oncoimmunology 2016;5:e1102827. 10.1080/2162402X.2015.1102827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang P, Maruyama T, Konkel JE, et al. PARP-1 controls immunosuppressive function of regulatory T cells by destabilizing FOXP3. PLoS One 2013;8:e71590. 10.1371/journal.pone.0071590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koch C, Kuske A, Joosse SA, et al. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity. EMBO Mol Med 2020;12:e11908. 10.15252/emmm.201911908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spicer JD, McDonald B, Cools-Lartigue JJ, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 2012;72:3919–27. 10.1158/0008-5472.CAN-11-2393 [DOI] [PubMed] [Google Scholar]
- 69.Strell C, Lang K, Niggemann B, et al. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp Cell Res 2010;316:138–48. 10.1016/j.yexcr.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 70.Huh SJ, Liang S, Sharma A, et al. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res 2010;70:6071–82. 10.1158/0008-5472.CAN-09-4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong C, Slattery MJ, Liang S, et al. Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Mol Cell Biomech 2005;2:145–59. [PMC free article] [PubMed] [Google Scholar]
- 72.Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 2013;123:3446–58. 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin D, Shen L, Luo M, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther 2021;6:404. 10.1038/s41392-021-00817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol 2021;18:297–312. 10.1038/s41571-020-00457-x [DOI] [PubMed] [Google Scholar]
- 75.Heitzer E, Haque IS, Roberts CES, et al. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet 2019;20:71–88. 10.1038/s41576-018-0071-5 [DOI] [PubMed] [Google Scholar]
- 76.Alix-Panabières C. The future of liquid biopsy. Nature 2020;579:S9. 10.1038/d41586-020-00844-5 [DOI] [PubMed] [Google Scholar]
- 77.Riethdorf S, Müller V, Loibl S, et al. Prognostic Impact of Circulating Tumor Cells for Breast Cancer Patients Treated in the Neoadjuvant "Geparquattro" Trial. Clin Cancer Res 2017;23:5384–93. 10.1158/1078-0432.CCR-17-0255 [DOI] [PubMed] [Google Scholar]
- 78.Bidard F-C, Michiels S, Riethdorf S, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J Natl Cancer Inst 2018;110:560–7. 10.1093/jnci/djy018 [DOI] [PubMed] [Google Scholar]
- 79.Bidard F-C, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014;15:406–14. 10.1016/S1470-2045(14)70069-5 [DOI] [PubMed] [Google Scholar]
- 80.Pantel K, Hille C, Scher HI. Circulating tumor cells in prostate cancer: from discovery to clinical utility. Clin Chem 2019;65:87–99. 10.1373/clinchem.2018.287102 [DOI] [PubMed] [Google Scholar]
- 81.Cortés-Hernández LE, Eslami-S Z, Dujon AM, et al. Do malignant cells sleep at night? Genome Biol 2020;21:276. 10.1186/s13059-020-02179-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diamantopoulou Z, Castro-Giner F, Schwab FD, et al. The metastatic spread of breast cancer accelerates during sleep. Nature 2022;607:156–62. 10.1038/s41586-022-04875-y [DOI] [PubMed] [Google Scholar]
- 83.Thomas F, Dujon AM, Ujvari B, et al. Nocturnal circulating tumor cells: the ultimate selective filter in cancer progression? Med 2022;3:523–5. 10.1016/j.medj.2022.07.005 [DOI] [PubMed] [Google Scholar]
- 84.Dauvilliers Y, Thomas F, Alix-Panabières C. Dissemination of circulating tumor cells at night: role of sleep or circadian rhythm? Genome Biol 2022;23:214. 10.1186/s13059-022-02791-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neves RPL, Ammerlaan W, Andree KC, et al. Proficiency testing to assess technical performance for CTC-Processing and detection methods in CANCER-ID. Clin Chem 2021;67:631–41. 10.1093/clinchem/hvaa322 [DOI] [PubMed] [Google Scholar]
- 86.Connors D, Allen J, Alvarez JD, et al. International liquid biopsy standardization alliance white paper. Crit Rev Oncol Hematol 2020;156:103112. 10.1016/j.critrevonc.2020.103112 [DOI] [PubMed] [Google Scholar]
- 87.Tamminga M, Andree KC, van den Bos H, et al. Leukapheresis increases circulating tumour cell yield in non-small cell lung cancer, counts related to tumour response and survival. Br J Cancer 2022;126:409–18. 10.1038/s41416-021-01634-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fischer JC, Niederacher D, Topp SA, et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc Natl Acad Sci U S A 2013;110:16580–5. 10.1073/pnas.1313594110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim TH, Wang Y, Oliver CR, et al. A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nat Commun 2019;10:1478. 10.1038/s41467-019-09439-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sinoquet L, Jacot W, Quantin X, et al. Liquid biopsy and Immuno-Oncology for advanced nonsmall cell lung cancer. Clin Chem 2022. 10.1093/clinchem/hvac166. [Epub ahead of print: 02 Nov 2022]. [DOI] [PubMed] [Google Scholar]