Abstract
Introduction
Isolated tricuspid valve surgery (TVS) may be associated with high morbidity and mortality. The aim of this study was to investigate the association of preoperative imaging and haemodynamic data derived from echocardiography (ECHO), cardiac magnetic resonance (CMR) and right heart catheterisation (RHC) with postoperative outcomes following TVS.
Methods
In a retrospective cohort study, patients who underwent isolated TVS at our institution between 2012 and 2020 were screened and followed up to 1 year. We only included those who had all three tests before surgery: ECHO, CMR and RHC. Patients with congenital heart disease, infective endocarditis and those who underwent concomitant valve or pericardial surgery were excluded. The primary outcome was a composite of mortality and congestive heart failure at 1 year. Time-to-event analyses at 1 year and Cox proportional hazards regression analyses were performed.
Results
A total of 60 patients were included (mean age of 60±14 years, 63% women), of whom 67% underwent TV repair. The primary outcome occurred in 16 patients (27%) with a 1-year mortality of 7%. It was associated with ECHO-derived right ventricular (RV) free wall strain and RHC-derived RV systolic and diastolic as well as mean pulmonary pressures. On multivariable Cox regression analysis, only RV systolic and diastolic pressures were significantly associated with the primary outcome at 1 year (HRs=5.9 and 3.4, respectively, p<0.05).
Conclusion
Baseline invasive haemodynamic assessment could have a strong association with clinical outcomes and help risk-stratify patients undergoing isolated TVS.
Keywords: Tricuspid Valve Insufficiency; Heart Failure, Systolic; Echocardiography; Magnetic Resonance Imaging; Cardiac Catheterization
WHAT IS ALREADY KNOWN ON THIS TOPIC
Isolated tricuspid valve surgery (TVS) is associated with a bad prognosis, and a presurgical risk stratification is an unmet need.
WHAT THIS STUDY ADDS
Haemodynamic parameters rather than imaging modalities are predictive of adverse outcomes following isolated TVS.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Invasive haemodynamic assessment through right heart catheterisation could be used in identifying high surgical risk patients undergoing isolated TVS.
Introduction
Tricuspid valve regurgitation (TR) is associated with increased mortality despite optimal medical treatment, even in the absence of right ventricular (RV) dysfunction or pulmonary hypertension.1 2 According to current valvular guidelines, a class I indication for tricuspid valve surgery (TVS) is only limited to patients undergoing left-sided surgery.3 Isolated TVS, a class IIa indication in patients with symptomatic right-sided heart failure,4 5 only represents 5%–10% of all TV surgeries.6–8 This is partly related to the relatively high-operative and long-term mortality reported with isolated TVS compared with other valvular surgeries.2 6–8 However, symptoms often occur with advanced stages of TR, and delays in surgical correction might explain the relatively poor outcomes of TVS.8–13 Hence, it is becoming increasingly relevant to better risk-stratify patients with severe TR by identifying preoperative anatomic, functional and haemodynamic parameters that could predict worse clinical outcomes after TVS to help guide timely interventions.3 14
While several diagnostic modalities are currently available to assess the tricuspid valve and right heart function before isolated TVS, the relative prognostic value of such testing remains uncertain. Therefore, we sought to investigate the association of preoperative imaging variables derived from echocardiography (ECHO) and cardiac magnetic resonance (CMR), as well as invasive haemodynamic variables derived from right heart catheterisation (RHC) with outcomes following isolated TVS.
Methods
Study population
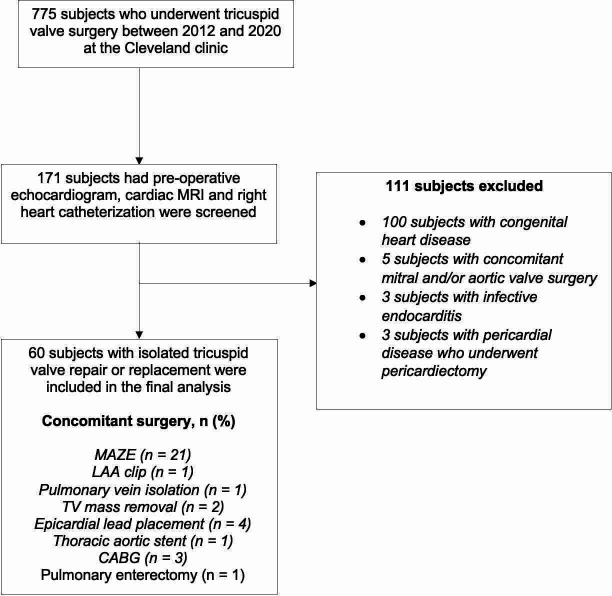
We conducted a retrospective cohort study using the Cleveland Clinic database for cardiovascular surgical operations, queried from January 2012 to January 2020 for patients undergoing isolated TVS, defined as TVS without concomitant left-sided valve surgery, and who were followed for at least 1 year. We only included those who had all three tests (ECHO, CMR and RHC) performed before surgery. Patients with congenital heart disease or infective endocarditis and those who underwent concomitant valve or pericardial surgery were excluded (figure 1).
Figure 1.
Study Consolidated Standards of Reporting Trials flowchart, showing the selection of the final population for the study analyses. Among 775 patients who underwent tricuspid valve (TV) surgery between 2012 and 2020 at the Cleveland clinic, 171 had available preoperative echocardiogram, cardiac MRI and right heart catheterisation. Among those, 111 subjects were excluded for having either congenital heart disease, concomitant valvular surgeries endocarditis or pericardiectomy. The final cohort of subjects analysed included 60 subjects. CABG, coronary artery bypass graft; LAA, left atrial appendage.
Study variables
Baseline patient characteristics, including demographics, comorbidities, medication use and laboratory data, were obtained from the electronic medical records. The aetiology of TR was determined by a review of the surgical report. Imaging variables were measured by ECHO and CMR, according to guidelines.15 16 For RV longitudinal and free wall strain measurement, we performed an offline analysis of speckle tracking ECHO using Velocity Vector Imaging (V.2.0; Siemens, Erlangen, Germany). The 30-day post-TVS ECHOs were also reviewed to assess the changes in RV dimensions (RV end-diastolic area and diameter and fractional area change (FAC)) following surgery. We defined reverse RV remodelling as a decrease in RV end-diastolic diameter, RV end-diastolic area or an increase in FAC at 30 days. CMR measurements included biventricular size and function and measures of TR severity (regurgitant volume and regurgitant fraction). RHC-derived haemodynamic data included central venous pressure, mean arterial pressure (MAP), RV and pulmonary artery (PA) systolic and diastolic pressures, pulmonary capillary wedge pressure, transpulmonary gradient and cardiac index (CI), and the following variables were calculated:
Cardiac power output=MAP×
Pulmonary effective arterial elastance=
Pulmonary arterial compliance=
Systemic elastance=0.9×
PA pulsatility index=
Study outcome
The primary outcome was a composite of mortality and congestive heart failure (CHF) at 1 year. CHF was defined as New York Heart Association classes III and IV symptoms on routine follow-up or hospitalisation for acute decompensated heart failure requiring diuresis with or without other interventions such as paracentesis and thoracentesis. The surgery date was chosen as the starting point of the observational period. Patients were followed up to 1 year or until developing the primary outcome.
Statistical analysis
Baseline characteristics were compared between study groups using a two-sided Student’s t-test or Kruskal-Wallis test as appropriate for continuous variables and a χ2 analysis of variance (ANOVA) test for categorical variables. Continuous variables are expressed as mean (SD) or median (IQR) and categorical variables as proportions.
The associations of different parameters derived from ECHO, CMR and RHC with the primary outcome were assessed through survival analyses with the Kaplan-Meier non-parametric method. As most included patients were in recent years of the study, we examined these associations at 1-year follow-up. To adjust for differences in baseline characteristics and calculate survival estimates, a multivariable Cox proportional hazard model was employed, and HRs with CI were computed. We adjusted for clinical characteristics using a clinical risk score, a score that has been developed and validated to predict mortality after TVS.17 Neither the Society of Thoracic Surgeons nor the European System for Cardiac Operative Risk Evaluation II has been fully validated for use following TVS. All analyses were done using STATA V.13.0 and R studio (V.1.3.1073).
Results
Of the 775 patients who underwent isolated TVS at our centre (mean age ~47 years, 59% men), and after applying the exclusion criteria, 60 patients (~8%) were finally included. Baseline preoperative characteristics related to the demographics, TR aetiology, surgical details, comorbidities, medications and biochemical data of the study population are presented in table 1. The mean age of the study population was 60±14 years, and 63% were women. Among all study patients, 68% underwent TV repair, with the predominant aetiology being annular dilation (61%) followed by leaflet pathology (cleft, flail, prolapse or torn leaflet; 25%). The most common concomitant procedure performed during surgery was MAZE (33%), followed by epicardial lead placement (5%) and coronary artery bypass grafting (5%). The median follow-up of the study patients was 5.8 months. After 1-year follow-up, 16 (27%) subjects had the primary outcome, with a 1-year mortality of 7%. There were no perioperative mortality or heart failure events. When comparing both groups of patients based on the primary outcome, there was no significant difference in age, gender, type of surgery (repair vs replacement) or baseline medications. However, a higher prevalence of prior stroke (19% vs 2%; p=0.024), atrial fibrillation (81% vs 43% vs; p=0.009) and CHF preoperatively (75% vs 5%; p=0.005), with lower mean levels of serum albumin (3.8 vs 4.1; p=0.045) were observed in patient who had the primary outcome compared with those who did not.
Table 1.
Baseline characteristics of patients undergoing isolated tricuspid valve surgery (TVS) according to the presence of primary outcome, a composite of mortality or congestive heart failure (CHF) at 1 year
| Total population (N=60) | No mortality or CHF (N=44) | Mortality or CHF (N=16) | P value† | |
| Age, mean (SD) | 60.9 (14.2) | 58 (15) | 63 (14) | 0.29 |
| Females | 38 (63%) | 27 (57%) | 13 (81%) | 0.08 |
| Surgery type, n (%) | ||||
| TV repair | 41 (68) | 31 (70) | 10 (62) | 0.23 |
| TV replacement | 19 (32) | 13 (30) | 6 (38) | |
| Aetiology* | ||||
| Annular dilation | 37 (61.7%) | 28 (63%) | 9 (56%) | 0.28 |
| Leaflet pathology | 15 (25%) | 13 (30%) | 2 (12.5%) | |
| Myxoma | 1 (1.6%) | 1 (2%) | 0 (0%) | |
| Carcinoid | 3 (5%) | 1 (2%) | 2 (12.5%) | |
| Redo TVS | 4 (6.7%) | 1 (2%) | 3 (19%) | |
| Concomitant surgery, n (%) | ||||
| MAZE | 20 (33%) | 16 (36%) | 4 (25%) | 0.31 |
| LAA clip | 1 (1.7%) | 0 (0%) | 1 (6%) | |
| Pulmonary vein isolation | 1 (1.7%) | 1 (2%) | 0 (0%) | |
| TV mass removal | 2 (3.3%) | 2 (4.5%) | 0 (0%) | |
| Epicardial lead placement | 3 (5%) | 2 (4.5%) | 1 (6%) | |
| Thoracic aortic stent | 1 (1.7%) | 0 (0%) | 1 (6%) | |
| CABG | 3 (5%) | 2 (4.5%) | 1 (6%) | |
| Pulmonary enterectomy | 1 (1.7%) | 1 (2%) | 0 (0%) | |
| Medical history, n (%) | ||||
| Coronary artery disease | 17 (28%) | 12 (27%) | 5 (31%) | 0.76 |
| Coronary bypass surgery | 8 (13%) | 5 (11%) | 3 (19%) | 0.46 |
| Transient ischemic attack/stroke | 4 (6%) | 1 (2%) | 3 (19%) | 0.024 |
| Peripheral arterial disease | 7 (11%) | 3 (7%) | 4 (25%) | 0.052 |
| CHF (New York Heart Association class III or IV) | 14 (22%) | 2 (5) | 12 (75) | <0.001 |
| Diabetes | 13 (21%) | 10 (23%) | 3 (19%) | 0.74 |
| Hyperlipidaemia | 16 (26%) | 11 (25%) | 5 (31%) | 0.63 |
| Hypertension | 34 (56%) | 25 (57%) | 9 (56%) | 0.97 |
| Chronic kidney disease | 11 (18%) | 7 (16%) | 4 (25%) | 0.42 |
| Dialysis | 6 (10%) | 5 (11%) | 0 (0%) | 0.16 |
| Atrial fibrillation | 32 (53%) | 19 (43%) | 13 (81%) | 0.009 |
| Pulmonary arterial hypertension | 8 (13%) | 3 (7%) | 3 (19%) | 0.18 |
| Permanent pacemaker | 2 (3%) | 1 (2%) | 0 (0%) | 0.21 |
| Chronic obstructive pulmonary disease | 5 (8%) | 2 (5%) | 3 (19%) | 0.078 |
| History of AV or MV surgery | 17 (28%) | 10 (23%) | 7 (44%) | 0.11 |
| Infective endocarditis | 3 (5%) | 1 (2%) | 1 (6%) | 0.45 |
| Liver cirrhosis | 6 (10%) | 3 (7%) | 3 (19%) | 0.18 |
| Body mass index, mean (SD) | 28.6 (6.7) | 28.1 (6.0) | 30.9 (8.5) | 0.16 |
| Medications | ||||
| Aspirin | 23 (38%) | 18 (41%) | 5 (31%) | 0.50 |
| P2Y12 inhibitors | 3 (5%) | 3 (7%) | 0 (0%) | 0.28 |
| Anticoagulation | 32 (53%) | 23 (52%) | 9 (56%) | 0.78 |
| ACE inhibitors or ARB | 21 (35%) | 17 (39%) | 4 (25%) | 0.33 |
| Spironolactone | 18 (30%) | 13 (30%) | 5 (31%) | 0.90 |
| Beta blockers | 37 (61%) | 27 (61%) | 10 (62%) | 0.94 |
| Calcium channel blockers | 11 (1%) | 7 (16%) | 4 (25%) | 0.42 |
| Loop diuretics | 29 (48%) | 18 (41%) | 11 (69%) | 0.056 |
| Thiazide diuretics | 10 (16%) | 7 (16%) | 3 (19%) | 0.79 |
| Digoxin | 4 (6%) | 2 (5%) | 2 (12%) | 0.27 |
| Antiarrhythmics | 4 (6%) | 4 (9%) | 0 (0%) | 0.21 |
| Statin | 23 (38%) | 17 (39%) | 6 (38%) | 0.94 |
| Baseline laboratory data | ||||
| Albumin, mean (SD) | 4.0 (0.51) | 4.1 (0.4) | 3.8 (0.5) | 0.045 |
| Creatinine, median (IQR) | 0.9 (0.8, 1.3) | 0.9 (0.8, 1.2) | 0.9 (0.80, 1.3) | 0.97 |
| GFR, mean (SD) | 69.7 (32.8) | 0.9 (0.6) | 1.1 (0.8) | 0.40 |
| ALP, mean (SD) | 112.3 (74.9) | 107.6 (69.2) | 133.1 (91.9) | 0.25 |
| ALT, mean (SD) | 23.2 (14.4) | 24.4 (15.9) | 20.3 (10.6) | 0.35 |
| AST, mean (SD) | 29.9 (14.5) | 28.5 (12.7) | 33.6 (19.4) | 0.24 |
| NT-proBNP, median (IQR) | 714 (211, 1284) | 714 (211, 1348) | 774 (301, 1309) | 0.66 |
| Total bilirubin, mean (SD) | 0.9 (0.7) | 68.0 (33.5) | 70.5 (31.4) | 0.79 |
| Haemoglobin, mean (SD) | 12.7 (2.0) | 12.8 (2.1) | 12.3 (1.8) | 0.41 |
| Haematocrit, mean (SD) | 38.0 (6.2) | 38.8 (5.5) | 37.6 (5.0) | 0.45 |
| Platelets, mean (SD) | 185.5 (69.4) | 191.5 (73.1) | 171.6 (49.0) | 0.32 |
*Leaflet pathology include cleft, flail, prolapse or torn leaflet.
†P values for continuous variables were calculated using t-tests (except for creatinine and NT-proBNP, in which Kruskal-Wallis test for medians was used).
ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; AV, aortic valve; CABG, coronary artery bypass graft; GFR, glomerular filtration rate; LAA, left atrial appendage; MV, mitral valve; NT-proBNP, N-terminal pro-brain natriuretic peptide; TV, tricuspid valve.
Tables 2 and 3 compare the baseline ECHO-derived, CMR-derived and RHC-derived data between the two groups. The median interval between all three preoperative modalities and surgery date was 25 days (IQR 5–68). According to the effective regurgitant orifice area classification of TR severity,18 23% of the population had severe, 6% massive and 69% torrential TR. Patients with the primary outcome had significantly worse apical RV strain (−8.3 vs −14.0; p=0.007). On the other hand, none of the variables measured on CMR were significantly different among the two groups. When looking at the haemodynamic data, the two groups had similar left-sided pressures; yet those who had the primary outcome had significantly higher right-sided pressures. Specifically, they had higher RV systolic (42.2 vs 32.8 mm Hg ; p=0.009), diastolic pulmonary pressure (21.5 vs 15.9 mm Hg, p=0.024) and mean PA pressure (30.8 vs 22.2 mm Hg; p=0.006). Table 4 shows the association of changes in RV dimensions at 30 days with the primary outcome. The majority of patients had signs of reverse RV remodelling evidenced by a decrease in RV end-diastolic area and diameter (70% and 78%, respectively), while 43% had an increase in FAC at 30 days. Although patients who did not have the primary outcome had a higher prevalence of reverse RV remodelling and a lower prevalence of significant (≥moderate) TR at 30 days, there was no significant difference between the two groups in terms of changes in RV dimensions or significant TR. Patients who had the primary outcome had higher prevalence of increased FAC compared with those who did not have the primary outcome (79% vs 42%, p=0.02). Only 12 subjects had follow-up ECHOs at 6 months and 10 subjects had follow-up ECHOs at 1 year, so we did not include results on RV remodelling beyond 30 days.
Table 2.
Baseline echocardiographic parameters of patients undergoing isolated tricuspid valve surgery according to the presence of the primary outcome, a composite of 1-year mortality or congestive heart failure (CHF)
| Echocardiographic parameters | No mortality or CHF (N=44) |
Mortality or CHF (N=16) |
P value* |
| Right ventricle | |||
| Moderate–severe RV dilation | 29 (66%) | 13 (81%) | 0.50 |
| Severe RV systolic dysfunction | 9 (20%) | 2 (12%) | 0.74 |
| RV basal strain, mean (SD) | −19.0 (11.1) | −17.2 (13.4) | 0.60 |
| Mid-RV strain, mean (SD) | −16.1 (9.2) | −12.8 (10.2) | 0.24 |
| Apical RV strain, mean (SD) | −14.0 (7.6) | −8.3 (5.1) | 0.007 |
| RV global longitudinal strain, mean (SD) | −13.1 (5.9) | −11.1 (6.5) | 0.25 |
| RV free wall strain, mean (SD) | −16.4 (8.0) | −12.8 (8.5) | 0.13 |
| RVSP (mm Hg), mean (SD) | 37.0 (10.5) | 34.4 (12.2) | 0.42 |
| RVOT VTI (cm/s), mean (SD) | 12.1 (5.0) | 19.7 (23.8) | 0.097 |
| RV base mid-systolic diameter (cm), mean (SD) | 5.2 (4.5) | 4.3 (1.3) | 0.42 |
| RV base end-systolic diameter (cm), mean (SD) | 3.9 (1.2) | 3.8 (1.2) | 0.69 |
| RV base end-diastolic diameter (cm), mean (SD) | 5.3 (1.2) | 5.1 (1.2) | 0.58 |
| RV end-diastolic area (cm2), mean (SD) | 35.5 (23.4) | 28.3 (14.9) | 0.26 |
| RV end-systolic area (cm2), mean (SD) | 21.5 (14.1) | 18.3 (10.9) | 0.42 |
| Fractional area change (%), mean (SD) | 0.4 (0.1) | 0.4 (0.1) | 0.44 |
| TAPSE (cm), mean (SD) | 61.2 (42.9) | 73.4 (1.3) | 0.71 |
| RA volume index (mL), mean (SD) | 2.5 (0.8) | 2.9 (1.0) | 0.12 |
| Inferior vena cava diameter (cm), mean (SD) | 12.1 (5.0) | 19.7 (23.8) | 0.097 |
| Hepatic vein pattern | |||
| Blunted systolic flow | 6 (14%) | 0 (0%) | 0.049 |
| Reversed systolic flow | 32 (72%) | 15 (93%) | |
| Tricuspid valve | |||
| Tricuspid inflow VTI, mean (SD) | 28.6 (10.2) | 35.4 (18.0) | 0.076 |
| Tricuspid regurgitation peak velocity (cm/s), mean (SD) | 250.2 (59.6) | 235.9 (54.8) | 0.41 |
| TR continuous VTI (cm), mean (SD) | 64.5 (24.4) | 69.9 (22.1) | 0.44 |
| VFR (mL/s), mean (SD) | 455.8 (420.8) | 313.4 (212.0) | 0.22 |
| Regurgitant volume (mL), mean (SD) | 116.9 (106.4) | 93.5 (61.6) | 0.43 |
| TR peak gradient (mm Hg), mean (SD) | 25.7 (11.5) | 23.3 (10.6) | 0.47 |
| TR mean gradient (mm Hg), mean (SD) | 14.1 (7.9) | 13.4 (6.5) | 0.74 |
| TR vena contracta (cm), mean (SD) | 1.3 (0.5) | 1.2 (0.5) | 0.31 |
| TR EROA complete (cm2) | 1.9 (1.6) | 1.4 (1.0) | 0.25 |
| New five point classification by EROA (>3) | 26 (59%) | 11 (68%) | 0.61 |
| New five point by vena contracta (>3) | 40 (90%) | 13 (81%) | 0.38 |
| Left ventricle | |||
| Left ventricular outflow track VTI (cm/s), mean (SD) | 17.5 (5.7) | 20.0 (5.3) | 0.15 |
| LV end-diastolic volume (cm3) mean (SD) | 82.5 (30.7) | 87.9 (37.5) | 0.59 |
| LVEF (%), mean (SD) | 57.4 (11.4) | 59.2 (10.0) | 0.58 |
*P values for continuous variables were calculated using t-tests and for categorical variables using χ2.
EROA, effective regurgitant orifice area; LVEF, left ventricular ejection fraction; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; VFR, volume flow rate; VTI, velocity time index.
Table 3.
Baseline haemodynamic and cardiac MRI parameters of patients undergoing isolated tricuspid valve surgery according to the presence of the primary outcome, a composite of 1-year mortality or congestive heart failure (CHF)
| No mortality or CHF (N=44) |
Mortality or CHF (N=16) |
P value* | |
| Right heart catheter data | |||
| Central venous pressure, mean (SD) | 13.2 (7.1) | 17.7 (8.2) | 0.058 |
| Mean arterial pressure, mean (SD) | 14.8 (7.1) | 18.0 (5.0) | 0.19 |
| RV systolic pressure (mm Hg), mean (SD) | 32.8 (9.9) | 42.2 (13.2) | 0.009 |
| RV diastolic pressure (mm Hg), mean (SD) | 10.1 (9.0) | 15.1 (8.3) | 0.078 |
| RV systolic work index (mm Hg mL/m2), mean (SD) | 4.8 (6.2) | 4.9 (1.6) | 0.96 |
| PA systolic pressure (mm Hg), mean (SD) | 32.5 (11.7) | 41.7 (14.6) | 0.023 |
| PA diastolic pressure (mm Hg), mean (SD) | 15.9 (6.9) | 21.5 (9.5) | 0.024 |
| PA mean pressure (mm Hg), mean (SD) | 22.2 (8.1) | 30.8 (11.0) | 0.006 |
| Pulmonary pulse pressure (mm Hg), mean (SD) | 16.6 (7.5) | 20.2 (7.6) | 0.13 |
| Pulmonary arterial elastance (mm Hg/mL), mean (SD) | 0.5 (0.2) | 0.7 (0.4) | 0.041 |
| Pulmonary arterial compliance (mL/mm Hg), mean (SD) | 4.6 (2.1) | 3.5 (1.9) | 0.13 |
| Pulmonary artery pulsatility index, mean (SD) | 1.7 (1.4) | 1.3 (0.6) | 0.31 |
| PCWP (mm Hg), mean (SD) | 13.9 (5.6) | 18.5 (8.7) | 0.030 |
| Transpulmonary gradient (mm Hg), mean (SD) | 7.9 (4.6) | 11.2 (4.4) | 0.030 |
| Pulmonary vascular resistance (Dynes·s/cm5), mean (SD) | 147.3 (101.7) | 215.5 (113.7) | 0.063 |
| Systemic elastance (mm Hg/mL), mean (SD) | 0.3 (0.2) | 0.5 (0.2) | 0.052 |
| Stroke volume index (mL/m2), mean (SD) | 38.4 (26.2) | 32.7 (9.9) | 0.51 |
| Cardiac index (L/min/m2), mean (SD) | 2.5 (0.7) | 2.3 (0.6) | 0.23 |
| Cardiac power output, mean (SD) | 35.4 (17.9) | 40.9 (12.7) | 0.39 |
| Cardiac MRI | |||
| RA area (cm2), mean (SD) | 38.3 (10.0) | 46.0 (14.8) | 0.38 |
| RV ejection fraction (%), mean (SD) | 50.5 (7.3) | 51.2 (9.5) | 0.76 |
| RV end-diastolic volume index (mL/m2), mean (SD) | 123.8 (35.8) | 129.4 (49.2) | 0.65 |
| RV end-systolic volume index (mL/m2), mean (SD) | 63.0 (19.7) | 67.7 (34.6) | 0.53 |
| RV stroke volume index (mL/m2), mean (SD) | 64.1 (22.0) | 61.8 (18.9) | 0.73 |
| PA net forward volume (mL), mean (SD) | 68.1 (24.3) | 58.4 (23.6) | 0.25 |
| TR regurgitant volume index (mL), mean (SD) | 55.5 (33.8) | 47.8 (32.6) | 0.44 |
| TR regurgitant fraction index (%), mean (SD) | 41.8 (16.5) | 37.0 (11.5) | 0.29 |
| LV end-diastolic volume (mL/m2), mean (SD) | 69.8 (22.3) | 75.0 (16.6) | 0.40 |
| LV end-systolic volume (mL/m2), mean (SD) | 31.5 (15.6) | 33.6 (14.2) | 0.64 |
| Stroke volume index (L/m2), mean (SD) | 38.6 (13.6) | 43.7 (7.4) | 0.16 |
| Cardiac index (L/min/m2), mean (SD) | 3.3 (3.8) | 2.8 (0.6) | 0.57 |
| LV ejection fraction (%), mean (SD) | 56.0 (8.5) | 58.5 (9.6) | 0.34 |
*P values for continuous variables were calculated using t-tests and for categorical variables using χ2.
LV, left ventricular; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; RA, right atrial; RV, right ventricular; TR, tricuspid regurgitation.
Table 4.
Change in echocardiographic parameters after tricuspid valve surgery and association with the primary outcome, a composite of mortality or congestive heart failure (CHF)
| Follow-up data | Total population (N=60) |
No mortality or CHF (N=44) |
Mortality or CHF (N=16) |
P value |
| Reverse RV remodelling at 30 days* | ||||
| Decrease in RV end-diastolic area | 42 (70%) | 32 (84%) | 10 (71%) | 0.30 |
| Decrease in RV end-diastolic diameter | 47 (78%) | 35 (92%) | 12 (86%) | 0.39 |
| Increase in FAC | 26 (43%) | 16 (42%) | 11 (79%) | 0.02 |
| ≥moderate TR at 30 days* | 7 (12%) | 3 (7%) | 3 (19%) | 0.08 |
*Reverse RV remodelling is defined as a decrease in RV end-diastolic diameter or RV end-diastolic area, or an increase in FAC at 30 days on echocardiogram.
FAC, fractional area change; RV, right ventricular; TR, tricuspid regurgitation.
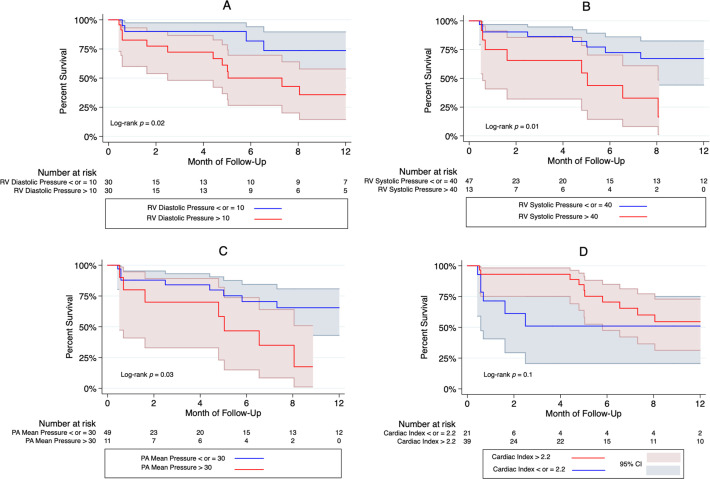
Regarding our time-to-event analyses, there was a significant difference at 1 year in the time to primary outcome based on RHC data (figure 2). Subjects with higher RV diastolic (log-rank p=0.02) and RV systolic pressures (log-rank p=0.01), as well as higher mean PA pressure (log-rank p=0.03), had a significantly higher likelihood of CHF and death at 1 year. Stratifying, according to RV free wall and global longitudinal strains, showed no difference in the likelihood of having the primary outcome at 5 months (online supplemental figure 1). In the multivariable-adjusted Cox regression analyses, only patients with baseline RV systolic pressure greater than 40 mm Hg (HR=3.64, 95% CI: 1.12 to 15.5; p=0.026) and RV diastolic pressure greater than 10 mm Hg (HR=3.18, 95% CI: 1.02 to 9.97; p=0.036) had a higher risk of having the primary outcome at 1 year (table 5). Among those who had an echocardiogram at 30 days (n=50), only 7 patients had ≥moderate TR. Compared with those who did not develop ≥moderate TR at 30 days, those who did have a higher risk of having the primary outcome (HR=4.7, 95% CI: 1.76 to 13.0; p=0.002) (data not shown).
Figure 2.
Kaplan-Meier curves for the association of different haemodynamic parameters with the primary outcome at 1-year follow-up, according to (A) right ventricular (RV) diastolic pressure, (B) RV systolic pressure, (C) pulmonary artery mean pressure and (D) cardiac index. Among the four invasive haemodynamic parameters studies, only RV systolic (>40 mm Hg) and diastolic pressures (>10 mm Hg) as well as mean pulmonary artery (PA) pressure (>30 mm Hg) were significantly associated with the composite outcome of death and congestive heart failure at 1 year. There was no association between cardiac index and the primary outcome. Shaded area indicate 95% CIs.
Table 5.
Univariate and multivariable Cox proportional hazard regression analyses of the primary outcome at 1 year, a composite of death and congestive heart failure (CHF)
| Patients who had the primary outcome | Unadjusted | Multivariable-adjusted model* | |||
| HR (95% CI) |
P value | HR (95% CI) |
P value | ||
| Right ventricular (RV) systolic pressure | |||||
| RV systolic pressure<40 mm Hg | 8 (16%) | 3.60 (1.33 to 9.69) | 0.011 | 3.64 (1.12 to 15.5) | 0.026 |
| RV systolic pressure40 mm Hg | 8 (61%) | ||||
| Right ventricular diastolic pressure | |||||
| RV diastolic pressure<10 mm Hg | 4 (12%) | 3.48 (1.11 to 10.8) | 0.031 | 3.18 (1.02 to 9.97) | 0.036 |
| RV diastolic pressure10 mm Hg | 12 (40%) | ||||
| Pulmonary artery mean pressure | |||||
| PA mean pressure<30 mm Hg | 9 (18%) | 3.05 (1.12 to 8.24) | 0.028 | 2.06 (0.54 to 7.85) | 0.10 |
| PA mean pressure30 mm Hg | 7 (64%) | ||||
*Adjusted for clinical risk score, comprised of age, gender, history of stroke, haemodialysis, chronic lung disease, ejection fraction, New York Heart Association class and reoperation) (17) and atrial fibrillation.
openhrt-2022-002124supp001.pdf (472.6KB, pdf)
Discussion
To our knowledge, this is the first study to simultaneously explore the prognostic value of anatomic and functional variables, as assessed by ECHO, CMR and invasive haemodynamic data obtained by RHC in patients undergoing isolated TVS. The main finding from our study is that right-sided pressures, rather than anatomic and functional parameters, are the factors associated with clinical outcomes after isolated TVS (figure 2). Specifically, elevated RV systolic and diastolic pressure preoperatively are the only factors associated with the primary outcome at 1 year on multivariable-adjusted Cox regression analyses.
Although severe TR is largely undertreated, its grim clinical implications have been increasingly recognised.19 ECHO remains the cornerstone of TV and RV assessment. However, its prognostic impact remains uncertain. Although some studies show that preoperative and intraoperative ECHO-derived LV function20 and RV dimensions10 21 may be associated with outcomes following TVS, most of these are limited by their small sample size and selection bias. Other larger-scale studies failed to show the predictive value of RV size. In a prospective analysis of 1292 patients with significant secondary TR, RV dilation (based on tricuspid annulus≥40 mm) was not associated with long-term survival.22 Similarly, qualitative measures of moderate–severe RV dilation were not associated with mortality after surgical TV replacement in a retrospective analysis of 189 patients with severe TR.23 In both of these studies, measures of RV systolic function were the only significant predictors of mortality after TVS. Notably, in the study by Topilsky et al, the only predictor of mortality was the right index of myocardial performance, which is strongly correlated with right heart haemodynamics.24
We previously showed at our institution that TVS has the potential in reversing RV remodelling and improving RV function,25 yet this study shows that reverse RV remodelling at 30 days was not associated with clinical outcomes. On the other hand, RV free wall strain has emerged as a valuable tool and has been previously shown to be an independent predictor of outcomes after isolated TVS.26 27 Similarly, in our study, RV global and RV free wall strains were the only ECHO parameters that were significantly different between the two study groups; however, their association with outcomes on time-to-event analysis was not statistically significant. Our study may be underpowered to detect a significant association of RV strain parameters given borderline p values from the survival analysis.
CMR is considered the gold standard in RV size and function assessment and has been increasingly used in valvular assessment. Data on the role of CMR before TVS are scarce. RV ejection fraction,28 RV mass index29 and TR severity by CMR30 have been shown to play a potential prognostic role, but none of the studies also included ECHO and RHC data. In our study, none of the imaging-derived parameters, whether by ECHO or CMR, were associated with the primary outcome following isolated TVS. This could be related to our patient population, which only included patients with severe symptomatic TR at a relatively advanced stage of the disease. Moreover, RV functional assessment by CMR might be affected by an increase in preload and in afterload, which is typical in severe TR patients.
Interestingly, and among a myriad of preoperative imaging and haemodynamic variables that we included, only right-sided pressures derived by RHC were associated with the primary outcomes. When studied in isolation, the value of invasive haemodynamic assessment in predicting outcomes following TV interventions31 32 has been previously explored. Similar to our findings, elevated right-sided pressures preoperatively,12 32 or postoperatively33 appear to be associated with adverse outcomes following TVS. Also, preoperative ECHO-based estimates of haemodynamic parameters have been shown to have prognostic value in patients undergoing TVS.22 24 34 35 Our study adds to the literature by showing that invasive haemodynamic data rather than imaging-derived anatomic or functional data provide the most prognostic value in patients undergoing TVS. It is important to note that while RHC-derived RV systolic pressure was associated with the study’s outcome, RVSP calculated by ECHO was not different between those who did and did not have the primary outcome. This is might be due to intraobserver/interobserver variability in the measurement of RVSP, a parameter that can challenge to accurately assess in many patients.
Furthermore, from a practical standpoint, identifying patients with a higher risk of worse clinical outcomes following TVS could allow for the more widespread use of novel transcatheter tricuspid valve interventions (TTVI). Several TTVI technologies targeting different structures of the tricuspid valve apparatus are currently available.36 For example, edge-to-edge repair using the TriClip system (Abbott Vascular, Santa Clara, California, USA), which effectively bridge the septal and one of the mural leaflets together is a valid alternative.37 Also, multiple devices targeting the annulus have been developed. For instance, the Cardioband system (Edwards Lifesciences, Irvine, California, USA) is a direct annuloplasty device.38 Transcutaneous TV replacement technologies are also being investigated with numerous available systems like the Lux-Valve (Ningbo Jenscare Biotechnology, Ningbo, China) or the Evoque valve (Edwards Lifesciences, Irvine, California, USA).39 40 Finally, the TricValve system (Products & Features GmbH, Vienna, Austria) that consists of two self-expanding biological valves that are implanted percutaneously into the superior and inferior vena cavae without disturbing the native tricuspid valve is a breakthrough system intended for patients with severely symptomatic TR and right heart failure with limited treatment alternatives.41
Several study limitations are worth noting. First, this was a single-centre retrospective study, and therefore, the analysis was subject to inherent biases, including ascertainment and selection bias, according to data availability from all three modalities. The centre experience with isolated TVS and its preoperative assessment using several modalities might not be generalisable to routine care of TR patients. Thus, these novel findings should be further validated in prospective clinical trials. The study is also subject to common limitations of observational design, where causality cannot be established. Significant loss to follow-up and relatively small sample size at follow-up beyond 1 year did not allow for longer-term survival analyses and might have not revealed significant associations of previously reported predictors of outcomes after TVS such as CMR-based parameters and speckle strain parameters. Moreover, the low event rates of the primary endpoints prohibited more extensive multivariable-adjusted regression analyses.
Conclusion
Routine use of preoperative invasive haemodynamic assessment could potentially have an important role in the risk stratification of patients undergoing isolated TVS. Our study shows that data derived from RHC, specifically RV systolic and diastolic pressures, rather than Echo and CMR-derived anatomical and functional data, are associated with postoperative mortality and CHF following isolated TVS.
openhrt-2022-002124supp002.pdf (76.5KB, pdf)
Acknowledgments
This paper was presented as an abstract at the ACC22 conference.
Footnotes
Twitter: @habib_layoun, @kassisMD
Contributors: EH, HL and SCH conceived and designed the study. EH, HL, JH, OAH and SCH collected, analysed and interpreted the data. EH, HL, JK, NK and SCH drafted and critically revised the manuscript. PCC, MH, AM, SDF, BG, HE, SU, GP, SK and SCH supervised the study. HL and SCH are responsible for the overall content and serve as guarantors. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. The data underlying this article cannot be shared publicly due to exclusivity of the data for the Cleveland Clinic Foundation and for the privacy of individuals who were included in the study.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Cleveland Clinic Institutional Review Board (#19-711, approved on 27 June 2019). Written informed consent was waived owing to the retrospective study design.
References
- 1.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405–9. 10.1016/j.jacc.2003.09.036 [DOI] [PubMed] [Google Scholar]
- 2.Fender EA, Zack CJ, Nishimura RA. Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart 2018;104:798–806. 10.1136/heartjnl-2017-311586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto CM, Nishimura RA, Bonow RO, et al. ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American heart association joint Committee on clinical practice guidelines. J Am Coll Cardiol 2020;2021:e25–197. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner H, Falk V, Bax JJ, et al. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;2017:2739–91. [DOI] [PubMed] [Google Scholar]
- 5.Zhan Y, Senapati A, Vejpongsa P, et al. Comparison of echocardiographic assessment of tricuspid regurgitation against cardiovascular magnetic resonance. JACC Cardiovasc Imaging 2020;13:1461–71. 10.1016/j.jcmg.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 6.Zack CJ, Fender EA, Chandrashekar P, et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol 2017;70:2953–60. 10.1016/j.jacc.2017.10.039 [DOI] [PubMed] [Google Scholar]
- 7.Axtell AL, Bhambhani V, Moonsamy P, et al. Surgery Does Not Improve Survival in Patients With Isolated Severe Tricuspid Regurgitation. J Am Coll Cardiol 2019;74:715–25. 10.1016/j.jacc.2019.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreyfus J, Flagiello M, Bazire B, et al. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J 2020;41:4304–17. 10.1093/eurheartj/ehaa643 [DOI] [PubMed] [Google Scholar]
- 9.Kim J-H, Kim H-K, Lee S-P, et al. Right ventricular reverse remodeling, but not subjective clinical amelioration, predicts long-term outcome after surgery for isolated severe tricuspid regurgitation. Circ J 2014;78:385–92. 10.1253/circj.CJ-13-0790 [DOI] [PubMed] [Google Scholar]
- 10.Kim Y-J, Kwon D-A, Kim H-K, et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation 2009;120:1672–8. 10.1161/CIRCULATIONAHA.109.849448 [DOI] [PubMed] [Google Scholar]
- 11.Singh SK, Tang GHL, Maganti MD, et al. Midterm outcomes of tricuspid valve repair versus replacement for organic tricuspid disease. Ann Thorac Surg 2006;82:1735–41. 10.1016/j.athoracsur.2006.06.016 [DOI] [PubMed] [Google Scholar]
- 12.Iscan ZH, Vural KM, Bahar I, et al. What to expect after tricuspid valve replacement? Long-term results. Eur J Cardiothorac Surg 2007;32:296–300. 10.1016/j.ejcts.2007.05.003 [DOI] [PubMed] [Google Scholar]
- 13.Bernal JM, Morales D, Revuelta C, et al. Reoperations after tricuspid valve repair. J Thorac Cardiovasc Surg 2005;130:498–503. 10.1016/j.jtcvs.2004.12.044 [DOI] [PubMed] [Google Scholar]
- 14.Taramasso M, Gavazzoni M, Pozzoli A, et al. Tricuspid Regurgitation: Predicting the Need for Intervention, Procedural Success, and Recurrence of Disease. JACC Cardiovasc Imaging 2019;12:605–21. 10.1016/j.jcmg.2018.11.034 [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of echocardiography and the European association of cardiovascular imaging. Journal of the American Society of Echocardiography 2015;28:1–39. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson 2013;15:35. 10.1186/1532-429X-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaPar DJ, Likosky DS, Zhang M, et al. Development of a risk prediction model and clinical risk score for isolated tricuspid valve surgery. Ann Thorac Surg 2018;106:129–36. 10.1016/j.athoracsur.2017.11.077 [DOI] [PubMed] [Google Scholar]
- 18.Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging 2017;18:1342–3. 10.1093/ehjci/jex139 [DOI] [PubMed] [Google Scholar]
- 19.Bruce CJ, Connolly HM. Right-sided valve disease deserves a little more respect. Circulation 2009;119:2726–34. 10.1161/CIRCULATIONAHA.108.776021 [DOI] [PubMed] [Google Scholar]
- 20.Bajzer CT, Stewart WJ, Cosgrove DM, et al. Tricuspid valve surgery and intraoperative echocardiography: factors affecting survival, clinical outcome, and echocardiographic success. J Am Coll Cardiol 1998;32:1023–31. 10.1016/s0735-1097(98)00355-6 [DOI] [PubMed] [Google Scholar]
- 21.Kim JB, Jung S-H, Choo SJ, et al. Clinical and echocardiographic outcomes after surgery for severe isolated tricuspid regurgitation. J Thorac Cardiovasc Surg 2013;146:278–84. 10.1016/j.jtcvs.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 22.Dietz MF, Prihadi EA, van der Bijl P, et al. Prognostic implications of right ventricular remodeling and function in patients with significant secondary tricuspid regurgitation. Circulation 2019;140:836–45. 10.1161/CIRCULATIONAHA.119.039630 [DOI] [PubMed] [Google Scholar]
- 23.Topilsky Y, Khanna AD, Oh JK, et al. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation 2011;123:1929–39. 10.1161/CIRCULATIONAHA.110.991018 [DOI] [PubMed] [Google Scholar]
- 24.Tei C, Nishimura RA, Seward JB, et al. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr 1997;10:169–78. 10.1016/S0894-7317(97)70090-7 [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee D, Nader S, Olano A, et al. Improvement in right ventricular systolic function after surgical correction of isolated tricuspid regurgitation. J Am Soc Echocardiogr 2000;13:650–4. 10.1067/mje.2000.103958 [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Lee H-J, Park J-B, et al. Preoperative right ventricular free-wall longitudinal strain as a prognosticator in isolated surgery for severe functional tricuspid regurgitation. J Am Heart Assoc 2021;10:e019856. 10.1161/JAHA.120.019856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prihadi EA, van der Bijl P, Dietz M, et al. Prognostic implications of right ventricular free wall longitudinal strain in patients with significant functional tricuspid regurgitation. Circulation 2019;12. 10.1161/CIRCIMAGING.118.008666 [DOI] [PubMed] [Google Scholar]
- 28.Park J-B, Kim H-K, Jung J-H, et al. Prognostic value of cardiac MR imaging for preoperative assessment of patients with severe functional tricuspid regurgitation. Radiology 2016;280:723–34. 10.1148/radiol.2016151556 [DOI] [PubMed] [Google Scholar]
- 29.Ahn Y, Koo HJ, Kang J-W, et al. Prognostic implication of right ventricle parameters measured on preoperative cardiac MRI in patients with functional tricuspid regurgitation. Korean J Radiol 2021;22:1253. 10.3348/kjr.2020.1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan Y, Debs D, Khan MA, et al. Natural History of Functional Tricuspid Regurgitation Quantified by Cardiovascular Magnetic Resonance. J Am Coll Cardiol 2020;76:1291–301. 10.1016/j.jacc.2020.07.036 [DOI] [PubMed] [Google Scholar]
- 31.Vijayaraghavan M, Prins KW, Prisco SZ, et al. Hemodynamic characteristics and outcomes of pulmonary hypertension in patients undergoing tricuspid valve repair or replacement.. CJC Open. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Meester P, Van De Bruaene A, Voigt J-U, et al. Outcome and determinants of prognosis in patients undergoing isolated tricuspid valve surgery: retrospective single center analysis. Int J Cardiol 2014;175:333–9. 10.1016/j.ijcard.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Liu Ju‐Hua, Chan D, et al. Prevalence, predictors and clinical outcome of residual pulmonary hypertension following tricuspid Annuloplasty. J Am Heart Assoc 2016;5:e003353. 10.1161/JAHA.116.003353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzzatti N, Iaci G, Taramasso M, et al. Long-Term outcomes of tricuspid valve replacement after previous left-side heart surgery. European Journal of Cardio-Thoracic Surgery 2014;46:713–9. 10.1093/ejcts/ezt638 [DOI] [PubMed] [Google Scholar]
- 35.Färber G, Tkebuchava S, Dawson R, et al. Minimally invasive, isolated tricuspid valve redo surgery: a safety and outcome analysis. Thorac Cardiovasc Surg 2018;66:564–71. 10.1055/s-0038-1627452 [DOI] [PubMed] [Google Scholar]
- 36.Layoun H, Schoenhagen P, Wang TKM, et al. Roles of cardiac computed tomography in guiding transcatheter tricuspid valve interventions. Curr Cardiol Rep 2021;23:114. 10.1007/s11886-021-01547-7 [DOI] [PubMed] [Google Scholar]
- 37.Nickenig G, Weber M, Lurz P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. The Lancet 2019;394:2002–11. 10.1016/S0140-6736(19)32600-5 [DOI] [PubMed] [Google Scholar]
- 38.Nickenig G, Weber M, Schueler R, et al. 6-Month Outcomes of Tricuspid Valve Reconstruction for Patients With Severe Tricuspid Regurgitation. J Am Coll Cardiol 2019;73:1905–15. 10.1016/j.jacc.2019.01.062 [DOI] [PubMed] [Google Scholar]
- 39.FL L, An Z, Ma Y, et al. Transcatheter tricuspid valve replacement in patients with severe tricuspid regurgitation. Heart 2021;107:1664–70. [DOI] [PubMed] [Google Scholar]
- 40.Fam NP, von Bardeleben RS, Hensey M, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE system: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv 2021;14:501–11. [DOI] [PubMed] [Google Scholar]
- 41.Figulla HR, Kiss K, Lauten A. Transcatheter interventions for tricuspid regurgitation - heterotopic technology: TricValve. EuroIntervention 2016;12:Y116–8. 10.4244/EIJV12SYA32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-002124supp001.pdf (472.6KB, pdf)
openhrt-2022-002124supp002.pdf (76.5KB, pdf)
Data Availability Statement
No data are available. The data underlying this article cannot be shared publicly due to exclusivity of the data for the Cleveland Clinic Foundation and for the privacy of individuals who were included in the study.