Abstract
Inflammatory back pain (IBP) is a characteristic clinical symptom of patients with axial spondyloarthritis (axSpA) that is caused by inflammation in the axial skeleton. In early disease stages the sacroiliac joints (SIJ) are most often affected, the spine usually at later stages. In many but clearly not all cases of axSpA new bone formation in form of syndesmophytes and ankylosis occur in the further course of the disease. Function and mobility may be impaired by both, inflammation and structural changes. In clinical trials outcome parameters most often used refer to pain, disease activity, function, mobility and global health but many researchers are also interested in radiographic progression in the axial skeleton of patients with axSpA. This viewpoint discusses the relevance of structural changes in the SIJ in comparison to the spine and in relation to functional outcomes and mobility.
Keywords: Spondylitis, Ankylosing; Magnetic Resonance Imaging; Inflammation; Tumor Necrosis Factor Inhibitors
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory rheumatic disease that manifests primarily in the axial skeleton, initially mostly in the sacroiliac joints (SIJ), later spreading to the spine in many but not all cases.1 The disease is characterised by inflammation and new bone formation in the axial skeleton and other entheseal sites.2 These pathologies are mostly assessed and detected by conventional radiography (CR) and MRI. Tumour necrosis factor inhibitors (TNFi), IL-17 antagonists (IL-17i) and Janus kinase inhibitors are shown to be efficacious in patients with axSpA including clinical signs and symptoms and reduction of axial inflammation as assessed by MRI.3–5 Another important outcome is radiographic damage, in axSpA usually appearing in form of syndesmophytes and ankylosis in the axial skeleton, for example, in the SIJ and the spine.
Disease modification in axSpA
The concept of disease modification, defined as retarding or stopping structural damage by antirheumatic drugs (DMARDs) originally coming from rheumatoid arthritis (RA) does not have a very solid basis in axSpA because randomised placebo-controlled trials are lacking and because only low rates of radiographic progression in the spine as assessed by CR and scoring with mSASSS6 have been reported for the bDMARDs TNFi and IL-17i7 8 while data on tsDMARDs are underway. However, a reduction of erosions and ankylosis in the SIJ as assessed by MRI during bDMARD and tsDMARD treatment has recently also been reported in axSpA patients.9–12 While studies looking at radiographic changes in the SIJ in early disease have already been published a while ago13 14 there is now a new report on such changes15 from the Swiss cohort which is well known because the authors have already provided more retrospective evidence that TNFi have decelerating effects on radiographic spinal progression in axSpA which is related to a decrease of inflammation as suggested by reduction of C reactive protein (CRP) levels.16
Indeed, the situation is quite complex. Therefore, it seems mandatory to put the data into perspective.17 About 15 years ago, we had asked the question: ‘what is the most important outcome parameter in ankylosing spondylitis?’ In this editorial, we challenged the view that radiographic progression is more or equally important as compared with disease activity, pain and function. Last year, we discussed the relevance of small changes in MRI erosion counts in patients with axSpA treated with bDMARDs and tsDMARDs mainly related to their relevance for patient-reported outcomes.18
The radiographic course of axSpA
The recent developments regarding radiographic progression in axSpA are a very good reason to think about the nature of structural damage and disease modification in this disease that affects the SIJ early in the course and extends later to the vertebral column where all three spinal segments can be involved. There are two main targets of disease modification in axSpA: (1) osteodestructive changes such as erosions and (2) osteoproliferative changes such as syndesmophytes and ankylosis. After the early hypothesis that the sequence of events is inflammation—erosion—fat metaplasia—bone formation19 the situation now seems to get more complicated because follow-up MRI studies have repeatedly shown that in the vast majority of cases the cause of new bone formation cannot be detected.20 21 Nevertheless, several factors predictive of bone formation have been identified showing that sex, smoking and almost all structural changes in the SIJ and the spine are predictive of radiographic progression as assessed by mSASSS,22 and there is strong evidence that spinal inflammation as assessed by MRI and increased CRP levels are also predictive23 while this is not the case for sacroiliac inflammation because, at least small changes are not specific for axSpA24 25 while more extensive inflammatory changes in the SIJ, in combination with HLA B27, were shown to predict development of structural changes in the SIJ.26 27
What about the natural course of radiographic progression in axSpA?
We do know that there is quite some variability in radiographic spinal changes in follow ups on both, the individual and the group level,28 however, persistently high disease activity as assessed by ASDAS is clearly associated with more radiographic (mSASSS) damage.29 An increase of one ASDAS unit was shown to lead to an increase of 0.72 mSASSS units within 2 years in that study, and patients with inactive disease had an mSASSS increase of about 5 points in 10 years.30
In cohort studies, about one-third of patients already have syndesmophytes at baseline, and mean mSASSS scores vary depending on disease duration. In patients with about 10 years of disease duration the mean mSASSS (range 0–72) is between 10 and 15. In patients treated with bDMARDs such as TNFi and IL-17i the mean change in mSASSS within 2 years has usually been below 1.6–8 Importantly, vertebral erosions play only a minor role in the development of structural spinal changes,31 32 and those seem to very rarely occur in nr-axSpA33—even though some patients classified as nr-axSpA have no definite structural changes in the SIJ but may have syndesmophytes (which may also be due to the limited reliability of the detection of such changes in the SIJ).
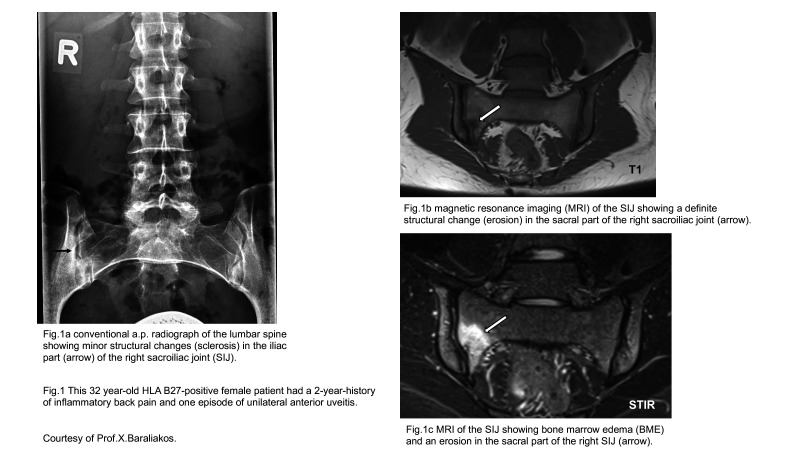
The situation for the SIJ is different for several reasons. It is currently increasingly discussed whether radiographic scoring should be replaced by MRI (figure 1) because it is at least as good regarding structural changes, much better in terms of active inflammation, and it does not require ionising radiation.34 However, the progression of radiographic changes in the SIJ by at least one grade after 2 years is known to only occur in a small percentage of patients with early axSpA, and an elevated CRP was a strong positive predictor of structural progression in the SIJ.35 36 Indeed, MRI scores for such structural SIJ changes are characterised by a floor effect indicating that the measure has a low limit for potential responses and many scores are near this limit. Thus, the range of SIJ MRI scores (data calculated from the original publication by Maksymowych)37 for fat metaplasia (3.8 at baseline and +0.5 change) and erosions (3.1 at baseline and −1.0 change) is 0–40, while the range of scores for backfill (3.0 at baseline and +0.2 change) and ankylosis (5.8 at baseline and +0.3 change) is 0–20.
Figure 1.
Comparison of a conventional radiograph and MRI scans of a young patient with axSpA.
Regarding erosions in the SIJ, there seems to be a natural tendency for improvement38 and only a slight increase within 5 years in an early cohort39 but there are short-term MRI data suggesting a benefit of bDMARD and tsDMARD therapy9–12.
Patients with axSpA may develop disease-related symptoms as early as in the third decade of life, and, taking into account the increased mortality rate, they will live for 40–50 years with the disease. What is the role of structural changes in the SIJ in this scenario? I think it is unlikely that this role is very important. In fact, we do know that all r-axSpA patients have or will develop such changes, while this is only the case in a few nr-axSpA patients: the transition rate from no definite change to definite change in the SIJ or from nr-axSpA to r-axSpA was reported to be limited to not more than 12% within 2 years in GESPIC,35 and even lower in other cohorts such as DESIR.39 Finally, as a matter of fact, there are patients progressing rapidly while others never will—and this is also true for the spine. Unfortunately, there are no solid predictors for rapid progression to date. The well reported and established differences in terms of radiographic progression between male and female patients with axSpA are beyond the scope of this editorial.
Nevertheless, there is no doubt that patients with r-axSpA are more likely to develop syndesmophytes than patients with nr-axSpA, and that is similar to those patients already having a syndesmophyte or more.
Furthermore, there has been an argument that patients with r-axSpA also respond better to bDMARD therapy than those with nr-axSpA. Indeed, this seemed actually to be the case in several early studies but this was more likely explained by wrong diagnoses and classification. The studies with certolizumab7 and bimekizumab40 41 have clearly shown that the response rates in r-axSpA and nr-axSpA are comparable—if appropriate inclusion criteria were used.
However, we do not know when erosions and ankylosis will occur in the SIJ and, more importantly, what is their prognostic significance? If at all, this is obviously rather limited . In addition, how frequently should we do follow-up X-rays or MRIs to know that and does it matter?
Of course, it is clear that axSpA patients with structural changes in the SIJ are much more likely to develop syndesmophytes42 but a decision to treat will always rely on the presence of active inflammation rather than on a higher likelihood to develop syndesmophytes because we do not know whether this works (in the absence of inflammation).
Another interesting question in this regard is the observation of increasing backfill in the SIJ43 in axSpA patients on DMARD treatment. What is this? A repair mechanism like in RA?44 The starting point for (more) ankylosis? In any case it is not clear what impact this observation has or will have for the patients.
In any case, it is now increasingly clear that, for diagnostic purposes, the combination of bone marrow oedema and erosions is the best option.45 The influence of age has to be taken into account when evaluating structural changes in SIJ,46 while the significance of other structural changes in the SIJ which also occur in the normal population is less clear.47
The important issue of replacing CR by low-dose CT in detail is beyond the scope of this editorial—even though there is increasing evidence that it does capture the whole spine (in contrast to CR and scoring with the mSASSS) and it is more sensitive to change.21
What about the consequences of structural changes related to function and mobility?
There is evidence that both, inflammation and structural changes in the spine, have an impact on function and mobility.48 In a 10-year study on TNFi-treated patients a with both, r-axSpA and nr-axSpA, physical function as assessed by the Bath AS functional index (BASFI) and the Bath AS spinal mobility score (BASMI) remained rather stable over time despite radiographic spinal progression, and no association between changes in mSASSS and BASFI was found, while syndesmophytes (mSASSS) seemed to exert more influence on spinal mobility (BASMI) over time. The data of that study showed that an increase of 20 and 12 mSASSS points over time would be responsible for an increase of one BASFI or one BASMI point, respectively.49 In a paper on the GESPIC cohort,50 statistical analyses adjusted for structural damage in the spine as assessed by mSASSS, disease activity (BASDAI) and gender, revealed an independent association of a sacroiliitis sum score with BASFI and BASMI. Change by one radiographic sacroiliitis grade in one joint was associated with BASFI/BASMI worsening by 0.10/0.12 points, respectively, independently of disease activity and structural damage in the spine. These data show that there is a minimal almost neglectable direct effect of structural SIJ changes on function and mobility.
Conclusions
The clinical value of detecting a decrease or increase of structural changes in the SIJ in much detail is not very clear at present, even though higher degrees of such changes are associated with more radiographic progression in the spine. Nevertheless, it is reassuring that anti-inflammatory therapies do rather decrease such changes in the SIJ—even though it seems very clear that the reduction of inflammation and bone formation in the spine is much more important from the patients’ point of view because of their impact on spinal function and mobility.
As previously mentioned, I would like to propose to get rid of the term nr-axSpA for diagnosing patients.51 This term should only be used for classification purposes. Especially in patients with syndesmophytes but no clear radiographic changes in the SIJ use of this term doesn’t make sense. Of course, SIJ need to be evaluated for more or less structural changes to assess the disease status of patients (according to their symptoms) but why should we determine a radiographic state in the SIJ methodologically known not to be very reliable that, in addition, is subject to change? Of interest, in a study on axSpA patients with a symptom duration of less than 2 years,52 nearly half of the patients already had structural changes in the SIJ—suggesting that the concept nr-axSpA has limited value for identifying early patients. Indeed, it seems more interesting to look at the time period between the onset of typical symptoms and the first appearance of structural changes in the SIJ, for example, to assess how fast structural changes develop. Finally, I think it’s time to replace CR of the SIJ by MRI since it is the better imaging technique to detect inflammatory and structural changes (figure 1) in the SIJ.50 53
Acknowledgments
I would like to thank Professor J. Sieper for his critical review of this editorial.
Footnotes
Correction notice: This article has been updated since it was first published online. The caption to figure 1 has been updated and reference 53 has been moved to the last paragraph.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Braun J, Sieper J. Ankylosing spondylitis. The Lancet 2007;369:1379–90. 10.1016/S0140-6736(07)60635-7 [DOI] [PubMed] [Google Scholar]
- 2.Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017;390:73–84. 10.1016/S0140-6736(16)31591-4 [DOI] [PubMed] [Google Scholar]
- 3.Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014;73:39–47. 10.1136/annrheumdis-2013-204231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 2013;382:1705–13. 10.1016/S0140-6736(13)61134-4 [DOI] [PubMed] [Google Scholar]
- 5.Deodhar A, van der Heijde D, Sieper J, et al. Safety and efficacy of Upadacitinib in patients with active ankylosing spondylitis and an inadequate response to nonsteroidal antiinflammatory drug therapy: one-year results of a double-blind, placebo-controlled study and open-label extension. Arthritis Rheumatol 2022;74:70–80. 10.1002/art.41911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Heijde D, Braun J, Deodhar A, et al. Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology 2019;58:388–400. 10.1093/rheumatology/key128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Heijde D, Baraliakos X, Hermann K-GA, et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis 2018;77:699–705. 10.1136/annrheumdis-2017-212377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun J, Haibel H, de Hooge M, et al. Spinal radiographic progression over 2 years in ankylosing spondylitis patients treated with secukinumab: a historical cohort comparison. Arthritis Res Ther 2019;21:142. 10.1186/s13075-019-1911-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maksymowych WP, Wichuk S, Dougados M, et al. Modification of structural lesions on MRI of the sacroiliac joints by etanercept in the EMBARK trial: a 12-week randomised placebo-controlled trial in patients with non-radiographic axial spondyloarthritis. Ann Rheum Dis 2018;77:78–84. 10.1136/annrheumdis-2017-211605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maksymowych WP, Claudepierre P, de Hooge M, et al. Structural changes in the sacroiliac joint on MRI and relationship to ASDAS inactive disease in axial spondyloarthritis: a 2-year study comparing treatment with etanercept in EMBARK to a contemporary control cohort in DESIR. Arthritis Res Ther 2021;23:43. 10.1186/s13075-021-02428-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maksymowych WP, Østergaard M, Landewé R. Impact of filgotinib on sacroiliac joint MRI structural lesions at 12 weeks in patients with active ankylosing spondylitis (TORTUGA trial). Rheumatology 2021;5:keab543. 10.1093/rheumatology/keab543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maksymowych WP, Baraliakos X, Lambert RG, et al. Effects of ixekizumab treatment on structural changes in the sacroiliac joint: MRI assessments at 16 weeks in patients with non-radiographic axial spondyloarthritis. Lancet Rheumatol 2022;4:e626–34. 10.1016/S2665-9913(22)00185-0 [DOI] [PubMed] [Google Scholar]
- 13.Dougados M, Maksymowych WP, Landewé RBM, et al. Evaluation of the change in structural radiographic sacroiliac joint damage after 2 years of etanercept therapy (EMBARK trial) in comparison to a contemporary control cohort (DESIR cohort) in recent onset axial spondyloarthritis. Ann Rheum Dis 2018;77:221–7. 10.1136/annrheumdis-2017-212008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rios Rodriguez V, Hermann K-G, Weiß A, et al. Progression of structural damage in the Sacroiliac joints in patients with early axial spondyloarthritis during long-term anti-tumor necrosis factor treatment: six-year results of continuous treatment with etanercept. Arthritis Rheumatol 2019;71:722–8. 10.1002/art.40786 [DOI] [PubMed] [Google Scholar]
- 15.Micheroli R, Kissling S, Bürki K, et al. Sacroiliac joint radiographic progression in axial spondyloarthritis is retarded by the therapeutic use of TNF inhibitors: 12-year data from the SCQM registry. RMD Open 2022;8:e002551. 10.1136/rmdopen-2022-002551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molnar C, Scherer A, Baraliakos X. Rheumatologists of the Swiss clinical quality management program. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss clinical quality management cohort. Ann Rheum Dis 2018;77:63–9. 10.1136/annrheumdis-2017-211544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun J, Sieper J. What is the most important outcome parameter in ankylosing spondylitis? Rheumatology 2008;47:1738–40. 10.1093/rheumatology/ken357 [DOI] [PubMed] [Google Scholar]
- 18.Braun J, Kiltz U, Baraliakos X. Significance of structural changes in the sacroiliac joints of patients with axial spondyloarthritis detected by MRI related to patients symptoms and functioning. Ann Rheum Dis 2022;81:11–14. 10.1136/annrheumdis-2021-221406 [DOI] [PubMed] [Google Scholar]
- 19.Sieper J, Appel H, Braun J, et al. Critical appraisal of assessment of structural damage in ankylosing spondylitis: implications for treatment outcomes. Arthritis Rheum 2008;58:649–56. 10.1002/art.23260 [DOI] [PubMed] [Google Scholar]
- 20.Baraliakos X, Heldmann F, Callhoff J, et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? a long-term observational study using MRI and conventional radiography. Ann Rheum Dis 2014;73:1819–25. 10.1136/annrheumdis-2013-203425 [DOI] [PubMed] [Google Scholar]
- 21.Stal R, Baraliakos X, van der Heijde D, et al. Role of vertebral corner inflammation and fat deposition on MRI on syndesmophyte development detected on whole spine low-dose CT scan in radiographic axial spondyloarthritis. RMD Open 2022;8:e002250. 10.1136/rmdopen-2022-002250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sepriano A, Ramiro S, van der Heijde D, et al. Biological DMARDs and disease modification in axial spondyloarthritis: a review through the lens of causal inference. RMD Open 2021;7:e001654. 10.1136/rmdopen-2021-001654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baraliakos X, Listing J, Rudwaleit M, et al. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther 2008;10:R104. 10.1186/ar2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baraliakos X, Richter A, Feldmann D, et al. Frequency of MRI changes suggestive of axial spondyloarthritis in the axial skeleton in a large population-based cohort of individuals aged <45 years. Ann Rheum Dis 2020;79:186–92. 10.1136/annrheumdis-2019-215553 [DOI] [PubMed] [Google Scholar]
- 25.Baraliakos X, Richter A, Feldmann D, et al. Which factors are associated with bone marrow oedema suspicious of axial spondyloarthritis as detected by MRI in the sacroiliac joints and the spine in the general population? Ann Rheum Dis 2021;80:469–74. 10.1136/annrheumdis-2020-218669 [DOI] [PubMed] [Google Scholar]
- 26.Bennett AN, McGonagle D, O'Connor P, et al. Severity of baseline magnetic resonance imaging-evident sacroiliitis and HLA-B27 status in early inflammatory back pain predict radiographically evident ankylosing spondylitis at eight years. Arthritis Rheum 2008;58:3413–8. 10.1002/art.24024 [DOI] [PubMed] [Google Scholar]
- 27.Arnbak B, Jensen TS, Schiøttz-Christensen B, et al. What level of inflammation leads to structural damage in the Sacroiliac joints? A four-year magnetic resonance imaging follow-up study of low back pain patients. Arthritis Rheumatol 2019;71:2027–33. 10.1002/art.41040 [DOI] [PubMed] [Google Scholar]
- 28.Baraliakos X, Listing J, von der Recke A, et al. The natural course of radiographic progression in ankylosing spondylitis--evidence for major individual variations in a large proportion of patients. J Rheumatol 2009;36:997–1002. 10.3899/jrheum.080871 [DOI] [PubMed] [Google Scholar]
- 29.Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis 2015;74:52–9. 10.1136/annrheumdis-2013-204055 [DOI] [PubMed] [Google Scholar]
- 30.Ramiro S, van der Heijde D, van Tubergen A, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455–61. 10.1136/annrheumdis-2014-205178 [DOI] [PubMed] [Google Scholar]
- 31.Baraliakos X, Listing J, Haibel H, et al. Vertebral erosions associated with spinal inflammation in patients with ankylosing spondylitis identified by magnetic resonance imaging: changes after 2 years of tumor necrosis factor inhibitor therapy. J Rheumatol 2013;40:1891–6. 10.3899/jrheum.120533 [DOI] [PubMed] [Google Scholar]
- 32.Ramiro S, van Tubergen A, van der Heijde D, et al. Brief report: erosions and sclerosis on radiographs precede the subsequent development of syndesmophytes at the same site: a twelve-year prospective followup of patients with ankylosing spondylitis. Arthritis Rheumatol 2014;66:2773–9. 10.1002/art.38775 [DOI] [PubMed] [Google Scholar]
- 33.Hebeisen M, Micheroli R, Scherer A, et al. Spinal radiographic progression in axial spondyloarthritis and the impact of classification as nonradiographic versus radiographic disease: data from the Swiss clinical quality management cohort. PLoS One 2020;15:e0230268. 10.1371/journal.pone.0230268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poddubnyy D, Diekhoff T, Baraliakos X, et al. Diagnostic evaluation of the sacroiliac joints for axial spondyloarthritis: should MRI replace radiography? Ann Rheum Dis 2022;81:ard-2022-222986–90. 10.1136/ard-2022-222986 [DOI] [PubMed] [Google Scholar]
- 35.Poddubnyy D, Rudwaleit M, Haibel H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis 2011;70:1369–74. 10.1136/ard.2010.145995 [DOI] [PubMed] [Google Scholar]
- 36.Dougados M, Sepriano A, Molto A, et al. Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5-year data of the DESIR cohort. Ann Rheum Dis 2017;76:1823–8. 10.1136/annrheumdis-2017-211596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen SJ, Wichuk S, Chiowchanwisawakit P, et al. Tumor necrosis factor inhibitor therapy but not standard therapy is associated with resolution of erosion in the sacroiliac joints of patients with axial spondyloarthritis. Arthritis Res Ther 2014;16:R100. 10.1186/ar4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen SJ, Weber U, Said-Nahal R, et al. Structural progression rate decreases over time on serial radiography and magnetic resonance imaging of sacroiliac joints and spine in a five-year follow-up study of patients with ankylosing spondylitis treated with tumour necrosis factor inhibitor. Scand J Rheumatol 2019;48:185–97. 10.1080/03009742.2018.1506822 [DOI] [PubMed] [Google Scholar]
- 39.Madari Q, Sepriano A, Ramiro S, et al. 5-Year follow-up of spinal and sacroiliac MRI abnormalities in early axial spondyloarthritis: data from the DESIR cohort. RMD Open 2020;6:e001093. 10.1136/rmdopen-2019-001093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deodhar A, Van der Heijde D, Gensler LS, et al. POS0939. Bimekizumab in patients with active non-radiographic axial spondyloarthritis: 24-week efficacy & safety from BE MOBILE 1, a phase 3 multicentre, randomized, placebo-controlled study. Ann Rheum Dis 2022;81:772–3. 10.1136/annrheumdis-2022-eular.2416 [DOI] [Google Scholar]
- 41.Baraliakos X, Deodhar A, van der Heijde D. Bimekizumab demonstrated sustained clinical responses to week 52 in phase 3 studies in psoriatic arthritis, non-radiographic axial spondyloarthritis and ankylosing spondylitis. L14, Acr 2022. [Google Scholar]
- 42.Maksymowych WP, Wichuk S, Chiowchanwisawakit P, et al. Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheumatol 2014;66:2958–67. 10.1002/art.38792 [DOI] [PubMed] [Google Scholar]
- 43.Maksymowych WP, Wichuk S, Chiowchanwisawakit P, et al. Fat metaplasia on MRI of the sacroiliac joints increases the propensity for disease progression in the spine of patients with spondyloarthritis. RMD Open 2017;3:e000399. 10.1136/rmdopen-2016-000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rau R, Wassenberg S, Herborn G, et al. Identification of radiologic healing phenomena in patients with rheumatoid arthritis. J Rheumatol 2001;28:2608–15. [PubMed] [Google Scholar]
- 45.Baraliakos X, Ghadir A, Fruth M, et al. Which magnetic resonance imaging lesions in the Sacroiliac joints are most relevant for diagnosing axial spondyloarthritis? A prospective study comparing rheumatologists' evaluations with radiologists' findings. Arthritis Rheumatol 2021;73:800–5. 10.1002/art.41595 [DOI] [PubMed] [Google Scholar]
- 46.Braun J, Baraliakos X. Different types of structural changes in the sacroiliac joints in axial spondyloarthritis-how important are joint shape variations? Rheumatology 2022. 10.1093/rheumatology/keac464. [Epub ahead of print: 12 Aug 2022]. [DOI] [PubMed] [Google Scholar]
- 47.Baraliakos X, Kuehn A, Tsiami S, et al. The influence of age on the prevalence of inflammatory and structural MRI lesions in the SIJ of patients with and without axSpA. Rheumatology 2022;9:keac505. 10.1093/rheumatology/keac505 [DOI] [PubMed] [Google Scholar]
- 48.Machado P, Landewé R, Braun J, et al. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis 2010;69:1465–70. 10.1136/ard.2009.124206 [DOI] [PubMed] [Google Scholar]
- 49.Poddubnyy D, Fedorova A, Listing J, et al. Physical function and spinal mobility remain stable despite radiographic spinal progression in patients with ankylosing spondylitis treated with TNF-α inhibitors for up to 10 years. J Rheumatol 2016;43:2142–8. 10.3899/jrheum.160594 [DOI] [PubMed] [Google Scholar]
- 50.Protopopov M, Proft F, Wichuk S, et al. Comparing MRI and conventional radiography for the detection of structural changes indicative of axial spondyloarthritis in the ASAS cohort. Rheumatology 2022;11:keac432. 10.1093/rheumatology/keac432 [DOI] [PubMed] [Google Scholar]
- 51.Deodhar A, Strand V, Kay J, et al. The term 'non-radiographic axial spondyloarthritis' is much more important to classify than to diagnose patients with axial spondyloarthritis. Ann Rheum Dis 2016;75:791–4. 10.1136/annrheumdis-2015-208852 [DOI] [PubMed] [Google Scholar]
- 52.Heuft-Dorenbosch L, Landewé R, Weijers R, et al. Combining information obtained from magnetic resonance imaging and conventional radiographs to detect sacroiliitis in patients with recent onset inflammatory back pain. Ann Rheum Dis 2006;65:804–8. 10.1136/ard.2005.044206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maksymowych WP, Lambert RGW, Østergaard M, et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI Working group. Ann Rheum Dis 2019;78:1550–8. 10.1136/annrheumdis-2019-215589 [DOI] [PubMed] [Google Scholar]