Abstract
IMPORTANCE
Increasing evidence indicates that acute kidney injury (AKI) occurs frequently in children and young adults and is associated with poor short-term and long-term outcomes. Guidance is required to focus efforts related to expansion of pediatric AKI knowledge.
OBJECTIVE
To develop expert-driven pediatric specific recommendations on needed AKI research, education, practice, and advocacy.
EVIDENCE REVIEW
At the 26th Acute Disease Quality Initiative meeting conducted in November 2021 by 47 multiprofessional international experts in general pediatrics, nephrology, and critical care, the panel focused on 6 areas: (1) epidemiology; (2) diagnostics; (3) fluid overload; (4) kidney support therapies; (5) biology, pharmacology, and nutrition; and (6) education and advocacy. An objective scientific review and distillation of literature through September 2021 was performed of (1) epidemiology, (2) risk assessment and diagnosis, (3) fluid assessment, (4) kidney support and extracorporeal therapies, (5) pathobiology, nutrition, and pharmacology, and (6) education and advocacy. Using an established modified Delphi process based on existing data, workgroups derived consensus statements with recommendations.
FINDINGS
The meeting developed 12 consensus statements and 29 research recommendations. Principal suggestions were to address gaps of knowledge by including data from varying socioeconomic groups, broadening definition of AKI phenotypes, adjudicating fluid balance by disease severity, integrating biopathology of child growth and development, and partnering with families and communities in AKI advocacy.
CONCLUSIONS AND RELEVANCE
Existing evidence across observational study supports further efforts to increase knowledge related to AKI in childhood. Significant gaps of knowledge may be addressed by focused efforts.
Introduction
The pediatric acute kidney injury (AKI) research field has expanded during the past decade with international epidemiologic studies, long-term AKI follow-up studies, validation of AKI prediction models, and continued investigation of novel AKI biomarkers.1–9 The 26th Acute Disease Quality Initiative (ADQI XXVI) convened in Napa, California, in November 2021, the first Pediatric ADQI (pADQI) that specifically addressed the global burden of AKI in the child and young adult population, with focused attention paid to kidney development and AKI, prenatal or neonatal kidney physiology, dialysis devices engineered for children,10 and the unique aspects of pediatric AKI epidemiology. The pADQI was assembled to mirror the global workforce in pediatrics and used a public and patient-facing approach. The primary aim of the pADQI was to identify the work priorities across the pediatric AKI landscape of care, education, research, and advocacy.
Methods
The pADQI consensus meeting followed the established ADQI process, as previously described,11 to provide expert-based statements and interpretation of current knowledge for use by clinicians according to professional judgment and identify evidence care gaps to establish research priorities. We performed an objective scientific review and distillation of literature through September 2021 of (1) epidemiology, (2) risk assessment and diagnosis, (3) fluid assessment, (4) kidney support and extracorporeal therapies, (5) pathobiology, nutrition, and pharmacology, and (6) education and advocacy. The 6 workgroups were composed of representatives from 5 continents of varying age (median: 43 years; range, 24–74 years), gender (23 male, 23 female) and professional discipline (physicians, nurses, pharmacists, and nutritionists). In addition, a pediatric patient survivor of AKI and an expert in equity and care delivery were included in the pADQI and assisted all workgroups. The in-person meeting was conducted using workgroups and large group in mixed in-person and virtual (72% in person) formats. Work following the in-person meeting was completed virtually.
Using a modified Delphi method, each workgroup determined key questions supported by representative literature, or professional opinion when evidence was scarce, and presented their drafts iteratively to develop consensus statements (target of 2–3 per group) (main document), and to articulate a research agenda with a more comprehensive background (Table; eBackground and eBox in the Supplement). The consensus statements were categorized as recommendations or suggestions. The work product was finalized and approved by the entire group in 2 follow-up web-based meetings. This summary statement was based on existing evidence in the literature, thus institutional approval was not required. All members of pADQI consented to their inclusion in this article. The statements are presented herein as direct quotations.
Table.
Consensus-Derived Considerations to Address Knowledge Gaps for Children and Young Adults With Acute Kidney Injury
| Paradigm | Consensus | Knowledge gap and needed work |
|---|---|---|
| Epidemiology | • Stratified understanding of high risk: Neonates, heart disease, chronic kidney disease, sepsis, cystic fibrosis, transplant history, oncologic history, chronic liver disease |
• Middle- and low-income populations • Expanded AKI phenotypes |
| Diagnostics | • Biomarker-based delineation of standard vs high risk | • Integration of concurrent diagnostics • Differentiation of diagnostics over time |
| Fluid balance | • Use of percent fluid balance and anchor weight (ICU admission weight) | • Adjudicate “fluid overload” by illness state |
| KST | • Institute benchmarks and components of KST team • Need for pediatric specific extracorporeal therapies |
• KST team, quality, and cost optimization • KST integration of tandem therapies |
| Biopathology | • Pediatrics incorporated into research efforts • Importance of nephrotoxin stewardship programs • Promoting bench-bedside collaboration between preclinical, epidemiological and clinical trial researchers |
• Determination of sex as a biological variable related to AKI • Characterize medications affected by AKI across developmental spectrum • Identify the optimal nutritional interventions by age for AKI |
| Education and advocacy | • Differential approach based on national resources • Integration of electronic medical record/checklists |
• Governmentaland community partnerships • Cross-discipline health care training |
Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; KST, kidney support therapy.
The age spectrum of patients receiving the care of pediatric specialists ranged from extremely low birthweight neonates to young adults. Throughout this manuscript, the terms child, children, and pediatric reflect the entire age spectrum, unless specifically noted.
Group 1: What Will Drive the Continuing Refinement and Evolution ofAKI Epidemiology?
Consensus Statement 1A (Recommendation)
AKI epidemiology related to short-term outcomes is well-described in critically ill neonates and children from high-income countries; however, further study is needed in other health care contexts, such as low-middle income countries and in non-ICU and ambulatory settings and for socioeconomic and long-term outcomes.
Consensus Statement 1B (Suggestion)
An understanding of pediatric AKI epidemiology allows us to begin employing strategies to improve primary, secondary, and tertiary AKI prevention in at-risk patient groups.
Implementation of standardized criteria to define the presence and severity of AKI has facilitated a description of pediatric AKI epidemiology.11–14 Prospective multicenter studies provide insight into the epidemiology of AKI in critically ill neonates and children.1,2 Other studies describe AKI epidemiology in specific cohorts of children including sepsis,15 those receiving nephrotoxins,16 and cardiac surgical populations.17,18 However, residual knowledge gaps remain regarding the accurate description of AKI epidemiology in other health care contexts as the most data come from high-income countries, and few studies have described AKI epidemiology outside critical care settings.
Pediatric literature has described AKI and morbidity, mortality, and health resource use in critically ill children with a gradient-response association between AKI severity and the risk of worse outcomes.1,19,20 More recently, research has focused on assessing the medium and long-term sequalae of AKI, including longitudinal kidney function and its socioeconomic outcomes.16,21,22
A comprehensive description of pediatric AKI epidemiology reflects current practice and best available information (eFigure 1 in the Supplement). Given this, information should be up to date and readily available to clinicians and institutions in real time. A risk prediction study23 and a randomized clinical trial24 suggest a causal link between pediatric AKI and a limited scope of short-term outcomes, supporting prevention and mitigation of pediatric AKI and its complications as targets for improving outcomes. Pediatric AKI epidemiologic characteristics can be categorized as pre-event (eg, risk factors, key drivers, and predictors), peri-event (eg, demographic characteristics and etiology), and postevent (eg, outcomes and recovery). Studies of AKI epidemiology should explore the temporal nature of AKI, health contexts (eg, populations and locations) in which AKI has been inadequately described, and outcomes that are focused on patient, family, cost, and performance. Thus, by following epidemiologic trends, clinicians can help focus efforts to enhance public health surveillance, augment field investigations, clinical and preclinical studies, and further policy development to promote child health (eBox and eBackground in the Supplement).
Group 2: What Are the Unique Considerations for AKI Risk Stratification and Diagnosis in Children?
Consensus Statement 2A (Recommendation)
Validated tools which incorporate patient characteristics and exposure and also interface with the local health care environment should be utilized to estimate AKI risk in children, including assessment of objective measures of kidney fitness in at-risk children prior to a predictable or planned intervention.
Consensus Statement 2B (Suggestion)
Unique AKI phenotypes in children may overlap and change over time. Differentiating AKI phenotype(s) informs prognosis and has the potential to guide therapeutics.
Previous studies1,25–28 identified baseline patient characteristics and exposures that place children at increased risk for AKI, including but not limited to cancer therapy, cardiopulmonary bypass, extracorporeal membrane oxygenation, major surgical procedures, and a high nephrotoxic medication burden.1,26,29 Integrating diagnostic resources may optimize AKI risk assessment for high-risk diagnoses (eg, by using point-of-care testing and automated algorithms) or exposures (eg, by using the electronic medical record) could be updated continuously in real time30 (Figure 1A31).
Figure 1. Acute Kidney Injury (AKI) Risk Assessment and Dynamic Phenotyping.
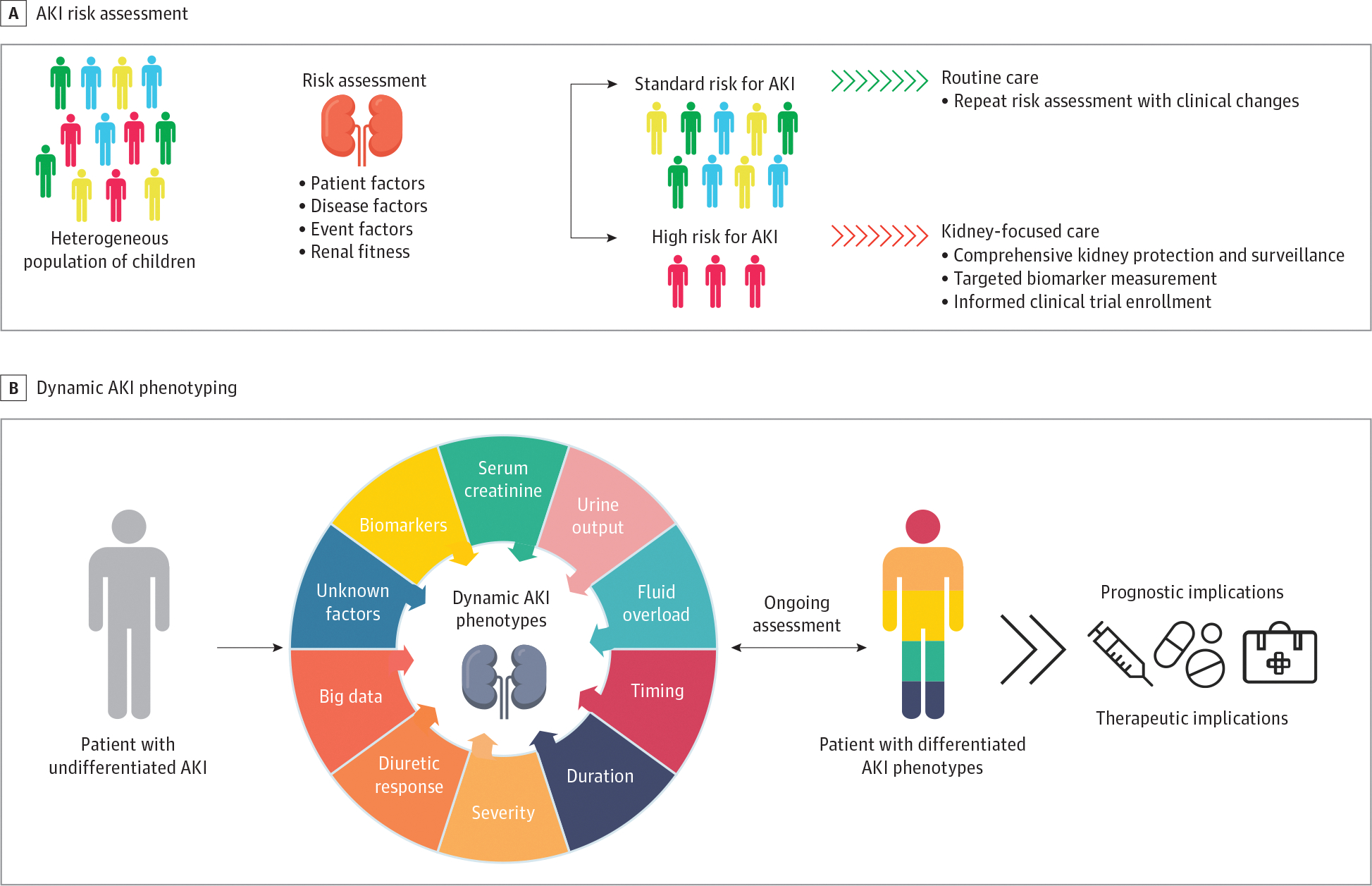
A, AKI risk assessment should occur in children with a potential kidney insult or any clinical changes to stratify patients into those at standard or high risk for AKI such that kidney-focused care can be implemented. B, Combined with individual susceptibility, multiple elements contribute to discernible AKI phenotypes in affected children that may have prognostic and therapeutic implications. Adapted from the 26th Acute Disease Quality Initiative31 with permission. These are open access images distributed under the terms of the Creative Commons Attribution License.
Prior to a planned exposure associated with increased AKI risk, ascertainment of kidney fitness using traditional measures of kidney function and damage, including serum creatinine and cystatin C levels, and urine albumin or protein level can be used to direct comprehensive kidney protection and surveillance and prevent AKI development.32 Preoperative values of candidate biomarkers may have utility in predicting AKI after cardiac surgery in adults and children.33–35 Kidney functional reserve values can predict postoperative AKI in adults.36 (Figure 1A31).
When available, biomarkers that indicate structural kidney injury may provide further insight into AKI risk and phenotype.37 For example, structural biomarker concentration elevation in the absence of functional biomarker elevation may indicate early kidney injury that is not readily reversible. Conversely, functional biomarker elevation alone may be indicative of decreased effective circulating volume and direct care toward restoration of volume. The terms functional AKI and structural AKI, to supplant the terms pre-renal AKI and intrinsic AKI were first proposed by the ADQI X Consensus Conference.38 Single center pediatric study and systematic reviews provide initial validation of these concepts, with structural AKI being associated with worse outcomes than functional AKI alone.39–41
Pediatric AKI is a heterogeneous disease with multiple etiologic factors, biochemical signatures, and clinical manifestations. Expert consensus and emerging data support the view that these elements combined with individual susceptibility contribute to unique, discernible AKI phenotypes in children with AKI (Figure 1B31). In particular, evidence supports the use of clinical features such as urine output,42,43 severity and duration of creatinine elevation,44,45 fluid overload,46 response to loop diuretics,47,48 and tubular injury biomarkers,39,41 to refine AKI diagnosis and reveal unique phenotypes. The concept of subclinical AKI is defined by an elevation in a urine tubular injury biomarker without elevation in functional marker. Subclinical AKI is associated with worse outcomes in children without AKI or with functional AKI.39,41 Although these studies examined static 1 or 2 factor-based AKI phenotypes, it is likely that more than 1 of these discernible phenotypes may be present in a child with AKI at a given time and that this unique phenotypic signature is dynamic (Figure 1B31). A dynamic diagnostic model to address a dynamic phenotype, akin to blood gas assessment in acute lung injury, may be required for AKI (eBox and eBackground in the Supplement).
Group 3: What Considerations Are Needed for Fluid Assessment in Sick Children?
Consensus Statement 3A (Recommendation)
Fluid balance is the difference between total input and output that can be expressed as “daily” and/or “cumulative” over a defined duration of time.
Consensus Statement 3B (Suggestion)
Fluid overload denotes a pathologic state of positive fluid balance associated with a clinically observable event(s), which may vary by age, case-mix, acuity, and phase of illness. No specific threshold of positive fluid balance alone can define fluid overload across all sick children.
As with AKI diagnosis, consistent use of consensus terminology is essential to further our understanding of fluid management and outcomes. Many terms have been used imprecisely to assess the impact of fluid volume on outcomes in sick children, including fluid balance, fluid accumulation, and fluid overload. Fluid overload has been conflated to describe both the degree of positive fluid balance within a patient and its association with adverse outcomes.49–55 Use of the term in this manner introduces bias into research and clinical practice, as it presumes any degree of positive fluid balance is synonymous with fluid overload and is therefore detrimental. Moreover, such use presumes that net fluid removal and targeting negative fluid balance is beneficial. Fluid overload is a distinct pathologic state of positive fluid balance with adverse consequences. Fluid balance is an objective calculation using specific methods. We suggest that the term percent cumulative fluid balance be used to describe the previously referred term, percent fluid overload,56 to separate the pathologic state from specific calculations. The terms daily fluid balance, cumulative fluid balance, and percent cumulative fluid balance describe fluid status of patients for purpose of clinical care and research (eTable 1 in the Supplement) more precisely.
The 2 methods most used to describe fluid balance are calculations from (1) cumulative fluid inputs and outputs and (2) changes in measured body weight.49–55,57–72 The weight change method has certain advantages because it accounts for unmeasured insensible losses, does not require an invasive bladder catheter, and is less resource intensive. A weight-change method is preferred in neonates to estimate fluid balance.58,73–77
Sick children are a heterogenous cohort of patients, and fluid overload may vary according to patient-specific susceptibilities (eg, by patient age, case-mix, phase, or severity of illness). Thus, no specific threshold can be recommended uniformly to define fluid overload in sick children. Interventions to mitigate or treat fluid overload will need to be patient-specific and consider the pathophysiologic context, the dynamics of the patient’s fluid balance, and the degree to which fluid balance affects the severity of the patient’s fluid overload or volume depletion. eFigure 2 in the Supplement provides a visual model of this precision approach (eBox and eBackground in the Supplement).
Workgroup 4: How Can Use of Kidney Support and Extracorporeal Therapies in Children Be Advanced?
Consensus Statement 4A (Recommendation)
A dedicated multidisciplinary team made up of kidney health care workers, patients, and families along with institutional investments of personnel, time, materials, and quality assurance/improvement systems are essential to a pediatric acute kidney support therapy (paKST) program.
Consensus Statement 4B (Suggestion)
Patient-centered goals of care, degree of kidney recovery, physiological stability, fluid balance, and global recovery and rehabilitation priorities inform decisions for de-escalation, liberation, transition, and follow-up to optimize hospital-based and lifelong outcomes.
A paKST program requires thoughtful planning and synchronization across the health care enterprise (eFigure 3 in the Supplement).28,78 Program development begins by identification of key stakeholders with a vested interest in paKST delivery and outcomes. A multidisciplinary team of kidney health care workers is responsible for fostering a culture of safety and transparency, navigating organizational barriers, facilitating shared decision-making, providing education, maintaining equipment, developing and maintaining policies, procedures, and guidelines, and managing quality improvement data.28,78–84 Patients and families are vital partners, identifying goals and advocating for effective and safe care. paKST device manufacturers and suppliers play an important role in working with pediatric paKST providers to design products that can be used safely and effectively in children, and to respond to the unique needs of a pediatric program. The care delivery model should be based on available resources with representation from multiple disciplines.28,78,79,82 Multiple blood purification and extracorporeal treatment modalities have been developed (eTable 2 in the Supplement). Programs must define their standards of high-quality delivery for the procedures they provide. Most quality metrics proposed by the ADQI XXII conference are relevant for children.85 If a program is unable to provide high-quality care in a particular area, the team must develop criteria and processes for transfer to an institution that provides such care, and should examine the components lacking in their program to see if they can be addressed. The education curriculum encompasses patient and team learning, is tailored to fit specific roles, and includes competency assessments.28,78,80,86,87 Quality assurance or improvement systems should be integrated into clinical practice, recognizing that cost, varied definitions, and lack of agreed-on benchmarks are known barriers to implementation.28,78–80,82–84,88–92 Previous work by Rewa et al80,89,90 and the 22nd ADQI conference28,80 has provided minimum programmatic standards that may be used for paKST.
While most children who require paKST for AKI will achieve liberation from dialysis, there is scant data to guide clinicians during kidney recovery.14,93–95 Current practices use estimates of the ability to maintain euvolemia and metabolic balance for decisions about timing of paKST liberation, transition, or de-escalation. Inability to execute timely transition from continuous paKST may negatively affect outcomes, especially in light of emerging data on effectiveness of the ICU Liberation Bundle.96–98 After paKST liberation, children are at risk for lifelong morbidity, including chronic kidney disease, but guidance on optimal follow-up practices is lacking (eBox and eBackground in the Supplement).3,99–101
Workgroup 5: How Do the Unique Pathobiology, Nutrition, and Pharmacology of the Developing Child and AKI Need to Be Integrated?
Consensus Statement 5A (Recommendation)
Successful pediatric translational AKI research programs include diverse teams using reverse translational approaches in partnership with clinical and epidemiological findings that prioritize development as a biologic variable. Sufficient support including pediatric specific government and industry funding along with meaningful partnerships among health professionals is necessary to understand and leverage the unique aspects of pediatric AKI to address kidney health and disease across the life course.
Consensus Statement 5B (Recommendation)
Patient centered outcomes such as functional status, quality of life, and optimal growth and development must drive the targeted nutritional interventions, optimizing short- and long-term nutrition, and incorporate measures of acute and chronic changes of anthropometrics, body composition, physical function, and metabolic control.
Engagement of diverse stakeholders across the preclinical and translational research realms is needed to disseminate findings and transform care. Most translational AKI models do not account for the additional complexity of pediatric AKI including the concept of development as a biologic variable (DABV).102 The association between premature birth and kidney function and maturation remain incompletely understood.103 Furthermore, the associations between a single AKI episode during kidney development and short-term and long-term outcomes remain unclear.104 Potential sex differences in AKI have prevented the inclusion of both males and females together in preclinical animal studies. In pediatrics, sex as a biologic variable is further confounded by DABV; hormone levels change throughout pubertal stages, and few clinical studies capture this information.105 Animal models of AKI that consider sex hormones and sex chromosomes could inform clinical evaluations sensitive to sex as a biologic variable and DABV. The association between AKI and chronic kidney disease has been established, however, there is a paucity of data on the systemic long-term impact of AKI.106,107 The potential associations between AKI with respect to DABV and long-term outcomes are unknown. Opportunities remain to use the unique aspects of pediatric patients, including but not limited to the lack of chronic comorbidities and ability to understand the impact of nephron endowment prior to nephron loss (Figure 231).
Figure 2. Development as a Biologic Variable Related to Acute Kidney Injury.
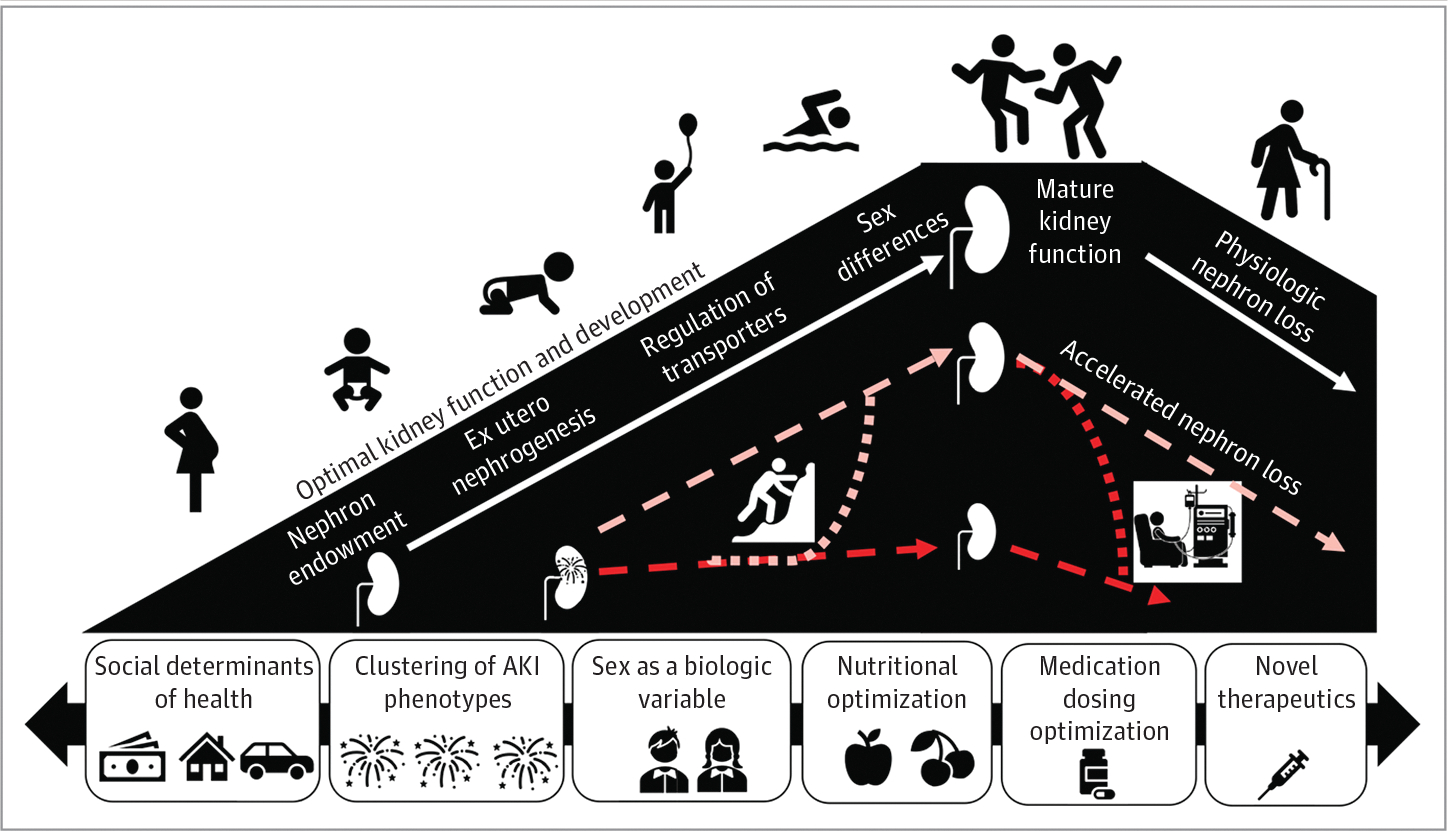
The left slope highlights important developmental stages critical to reach optimal kidney function. The right slope represents the normal age-related decrease of kidney function. Acute kidney injury (AKI) can occur at any phase along the developmental trajectory resulting in early kidney compromise and potentially accelerated nephron loss. The timing and degree of AKI likely affect the trajectory to recovery of normal kidney function or persistently decreased function. Research efforts must focus on uncovering and capitalizing on the ways to improve the outlook of an injured kidney. These efforts may include optimization of nutrition, limiting further injury, and novel therapeutics. There are factors that accelerate nephron loss and the trajectory toward kidney failure. The social determinants of health, the types or clusters of AKI, sex as a biologic variable, nutritional factors, and medication dosing are all components that affect the recovery or decrease of kidney function after AKI. Adapted from the 26th Acute Disease Quality Initiative31 with permission. These are open access images distributed under the terms of the Creative Commons Attribution License.
Malnutrition and protein energy wasting are prevalent and independently associated with mortality in children hospitalized with AKI, especially those requiring paKST, with prevalence ranging from 30% to 55%.108–111 Mortality is a crude end point to assess the importance of nutrition support in children with AKI. AKI survivors are at risk of worsened functional status, chronic ventilation, or dialysis after hospital discharge.111 Nutrition support and mobilization offer opportunities to improve functional outcomes in survivors through attenuation of muscle loss and promotion of muscle protein synthesis in critically ill children.112 The outcomes of malnutrition associated with somatic growth, body composition, development, immune function, metabolic derangements, and long-term physical and neurocognitive functioning require more precise measurement tools and targets.113 Nutritional therapeutic interventions are nuanced and include changes in feeding modality, composition (macronutrient or micronutrient) and volume of nutrition-related fluids which intersects with medical AKI management and metabolic control, which can be further complicated by institution of paKST. Nutritional needs are further affected by DABV.
Identifying and measuring appropriate outcome variables represent the first step to define the phenotypic nutritional morbidities in pediatric AKI, and thereby enable evaluation of nutritional intervention efficacy.114,115 We recommend program-based focused nutrition support for children with AKI and transitioning through acute kidney disease.116 Such a program would use evidence-based nutrition therapy adjusted in response to changes in objectively measured nutrition-related outcomes characterized by mitigating functional decline, a return to metabolic homeostasis, and facilitation of long-term physical and neurocognitive rehabilitative processes.
Physiologic derangement in acute illness, with susceptibility factors of DABV render kidney-eliminated and nephrotoxic medication use uniquely complex in sick children. High-value medications can be selected for detailed pharmacokinetic/pharmacodynamic/pharmaco-omics characterization to inform drug disposition, dosing, and monitoring decisions at the bedside for AKI and paKST (eBox and eBackground in the Supplement).117,118
Workgroup 6: Who Are the Key Stakeholders and What Is Required to Promote Education and Advocacy for AKI in Children?
Consensus Statement 6A (Recommendation)
Given the adverse immediate and lifelong outcomes for children with AKI, education and advocacy are essential, starting with the patient and family and expanding across health care teams, systems, and communities.
Consensus Statement 6B (Suggestion)
Customized AKI education and advocacy require a comprehensive, multidisciplinary, and patient/family centered focus, with AKI champions embedded at every level, embracing the complexities of diverse settings and individuals, which need to evolve over the pediatric life span.
Education may address the widespread gaps in AKI recognition.119 Lack of AKI awareness may be associated with disparities in AKI outcomes.120 Extending education to all stakeholders can reduce the risk of missing AKI or delaying care.121 Because delayed changes in clinical markers (serum creatinine level, urine output) may delay recognition of kidney injury, education requires awareness of risk factors. Education must be delivered in varied settings, different backgrounds of health care workers, and available resources with a flexible approach adjusted for the scenario and depth of knowledge needed (Figure 331).
Figure 3. The Role of Education and Advocacy in Pediatric Acute Kidney Injury (AKI).
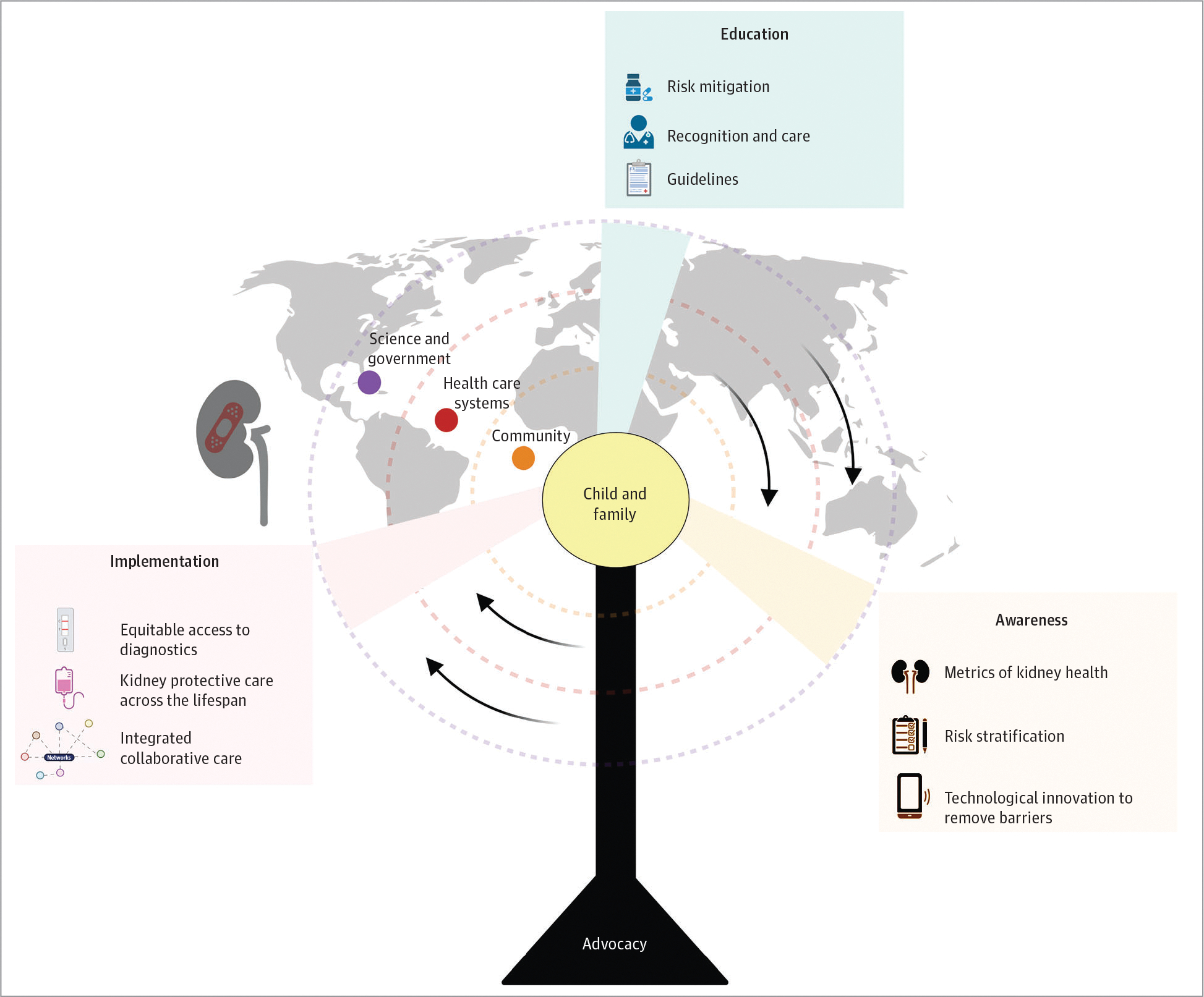
Education to improve AKI awareness and recognition in children rests on the pillar of advocacy and must engage key stakeholders in developing multidisciplinary context-appropriate customized approaches to improve AKI care globally and across all resource settings. Effective AKI education programs center the child and family, and expand across health care teams, systems, and communities with champions embedded at every level, health care systems, science, and government. AKI education must be a core competency for health care clinicians to ensure that AKI risk mitigation measures are in place to reduce AKI in community and hospital settings, to facilitate early recognition of AKI through technological innovation, and to appropriately manage AKI using context-specific guidelines that recognize the need for continuity of care and clinical follow-up. Implementation of an effective AKI education and advocacy program assures equitable access to diagnostics, emphasizes integrated multidisciplinary collaborative care, and recognizes the unique long-term health outcomes of AKI in children across the life span. Adapted from the 26th Acute Disease Quality Initiative31 with permission. These are open access images distributed under the terms of the Creative Commons Attribution License.
Education must include recognition of AKI risk, prevention and treatment of volume depletion, careful attention to fluid balance, avoidance or limitation of nephrotoxic medications, and blood pressure monitoring. Checklists, standardized screening and care protocols (care bundles), adapted to and used across varied settings, can enhance identification of patients with or at risk for AKI.122 Successful education programs also require strong advocacy efforts to engage different constituents, including patients and families, community members, physicians, allied health care professionals, industry affiliates, and governmental and nongovernmental organizations (eTable 3 in the Supplement). Education must be tailored to all members of the health care team, including patients and family, and should occur on micro (individual learners, training program curriculum, teaching modules, and alert systems for providers) and macro (health care system, communitywide educational programs, and governmental policy) levels.
AKI education must be an integral part of the curriculum of foundational and advanced education for all health care professionals, with continuing medical education for all practitioners. Programs should view AKI as a core competency metric.121,123 Specialists in AKI (including educators, researchers, and advocates) must use educational methods for those who access online free open-access medical education,124 available globally 24 hours per day. Patients and families may be unaware of the AKI episode or of the potential for long-term adverse outcomes. AKI education may help empower them to participate in healthy lifestyle modification and may improve follow-up, which has the potential to improve long-term outcomes.125,126 Adaptable and multifaceted educational tools, available in a variety of primary languages and for varying levels of health literacy, should support comprehensive care from admission through discharge, follow-up and potentially, readmission. Connectivity between patient and family with clinician may be essential in the AKI care continuum.
AKI diagnosis remains a significant challenge across areas with varying resource availability (low-income, war zones, and disaster areas), with the lack of appropriate laboratory supplies, adequate medical infrastructure, and personnel. A recent survey emphasized the importance of involving and educating governments about the implications of AKI.127 Fostering partnerships with industry and the international medical community will also be beneficial. Public-private partnerships and programs may have established platforms that can be used for earlier diagnosis of AKI (eBox and eBackground in the Supplement).128
Discussion
The pADQI consensus statements are expert-derived landscape definitions of the multifaceted and multidisciplinary approach needed to improve prediction, diagnosis, management, and follow-up care for AKI in children. The AKI story requires partnerships between patients and providers, across the medical landscape and across the ages of children. We have provided recommendations for current efforts and identified opportunities to address knowledge gaps (eBox in the Supplement).
Limitations
This study has limitations. The recommendations of the pADQI are based on existing evidence and consensus but a systematic review on all individual studies and pieces of data was not performed. We also acknowledge recent studies on AKI epidemiology, biomarkers, fluid accumulation, kidney support therapy, nutrition, pharmacology, and education are in progress or have been completed since the completion of pADQI and we could not account for their findings in our recommendations.
Conclusions
Nearly 2 decades of work in critical care nephrology has delivered a robust foundation on which to frame future AKI education, advocacy, research, and clinical practice. AKI in children is unique and is associated with long-lasting consequences. Improved outcomes in patients will require a comprehensive and cross-discipline approach requiring stakeholders across medicine, community, and government to partner with children and their families.
Supplementary Material
eBackground. Group Descriptions
eBox. Research Agenda for Pediatric Acute Kidney Injury
eFigure 1. An Evolving Approach to AKI Epidemiology
eTable 1. Definitions of Fluid Balance
eFigure 2. The Spectrum of Fluid Balance
eFigure 3. Multipronged Approach to Pediatric Acute Kidney Support Therapy
eTable 2. Blood Purification and Extracorporeal Treatment Modalities for Different Pathophysiological Conditions
eTable 3. Key Components of Education and Advocacy for Children With Acute Kidney Injury (AKI)</SI_Caption>
Key Points.
Question
What are the clinical care, education, research and advocacy-based priorities for acute kidney injury (AKI) in children and young adults?
Findings
In this consensus statement, a panel of 46 global experts developed pediatric AKI specific consensus statements regarding epidemiology, diagnostics, mechanical support, educational goals, and biologic and physiologic development of the growing child. In addition gaps in knowledge and areas for further research were identified.
Meaning
Focused and coordinated efforts, integrating AKI specialists and researchers with the general medical community and population, may improve short-term and long-term outcomes for children and young adults with AKI in the areas of disease awareness, diagnostics, recognition, management, and follow-up.
Funding/Support:
The in-person meeting was supported by educational grants from AM Pharma, Baxter, Biomerieux, BioPorto Diagnostics, Cincinnati Children’s Hospital, ExThera Medical, Leadiant, Lowell Therapeutics, MediBeacon, Medtronic, Nuwellis, Potrero, Res Seminars, Seastar Medical, and Stavro Medical.
Role of the Funder/Sponsor: The funders had no involvement in the selection of the panelists, design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. Representatives from the funders were present at the face-to-face meeting but did not participate in the panel discussions, voting, or final decisions.
Footnotes
Conflict of Interest Disclosures: Dr Goldstein reported receiving personal fees from BioPorto Diagnostics, grant support and personal fees from Medtronic Inc, NuWellis Inc, Leadiant, personal fees from MediBeacon Inc, stock options and personal fees from Baxter Healthcare, grant support and personal fees from SeaStar Medical, and grants from ExThera Medical outside the submitted work. Dr Akcan-Arikan reported receiving financial support for research from Bioporto research funds paid to her institution outside the submitted work. Dr Askenazi reported receiving personal fees from Baxter, Nuwellis, Medtronic, Seastar, and Bioporto, and financial support for research from Zorro-Flow outside the submitted work; in addition, Dr Askenazi reported having a patent for Zorro-Flow, an external urine collection device pending, and a patent for continuous renal replacement therapy (CRRT) advancements pending. Dr Bagshaw reported receiving personal fees from Baxter and BioPorto during the conduct of the study. Dr Barreto reported receiving financial support for research from FAST Biomedical Consultant and Wolters-Kluwer Consultant outside the submitted work. Dr Brophy reported receiving personal fees from UpToDate during the conduct of the study and American Board of Medical Specialities finance board. Dr Charlton reported receiving personal fees from Medtronics Carpediem Clinical Events Committee outside the submitted work. Dr Devarajan reported receiving grants from National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study; holding a patent as co-inventor on patents licensed to Abbott Diagnostics and BioPorto Inc, on use of neutrophil gelatinase-associated lipocalin as a biomarker of kidney injury. Dr Gist reported receiving financial support for research from Medtronic Speaker Honorarium and financial support for research from Bioporto Diagnostics consulting fees outside the submitted work. Dr Menon reported receiving personal fees from Nuwellis Inc outside the submitted work. Dr J. Morgan reported receiving personal fees from Medtronic Consultant outside the submitted work. Dr Mottes reported receiving financial support for research from Medtronic outside the submitted work. Dr Stanski reported receiving grants from National Center for Advancing Translational Sciences of the National Institutes of Health Institutional CT2 grant, 2UL1TR001425-05A1 and travel reimbursement from pADQI Travel funds to travel to consensus meeting (flight and hotel) during the conduct of the study; in addition, Dr Stanski reported holding a patent for PERSEVERE-II AKI Prediction Model pending. Dr Zappitelli reported receiving financial support for research from Bioporto Inc adjudicator/consultant for a study on neutrophil gelatinase-associated lipocalin and financial support for research from Baxter Inc Honorarium for a national talk on CRRT (not viewed by company ahead of time) outside the submitted work. Dr Kellum reported receiving personal fees from Dialco; being an employee of Spectral Medical and its wholly owned subsidiary Dialco outside the submitted work; and paid consultant for Astute Medical. Dr Ostermann reported receiving grants from Fresenius Research funding, grants from Baxter Research funding, and grants from Biomerieux Research funding during the conduct of the study. Dr Basu reported receiving personal fees from Bioporto Diagnostics outside of the submitted work. and personal fees from BioMerieux outside of the submitted work. during the conduct of the study; in addition, Dr Basu reported having a patent for Renal Angina Index pending outside of the submitted work. and a patent for olfactomedin-4 pending outside of the submitted work. No other disclosures were reported.
Group Information: All authors are members of the Pediatric ADQI Collaborative.
Contributor Information
Stuart L. Goldstein, Center for Acute Care Nephrology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Ayse Akcan-Arikan, Division of Critical Care Medicine and Nephrology, Texas Children’s Hospital, Baylor College of Medicine, Houston.
Rashid Alobaidi, Alberta Health Sciences University, Edmonton, Alberta, Canada.
David J. Askenazi, Children’s Hospital Alabama, Birmingham.
Sean M. Bagshaw, Alberta Health Sciences University, Edmonton, Alberta, Canada.
Matthew Barhight, Ann & Robert Lurie Children’s Hospital of Chicago, Northwestern University, Chicago, Illinois.
Erin Barreto, Mayo Clinic, Rochester, Minnesota.
Benan Bayrakci, Department of Pediatric Intensive Care Medicine, Life Support Center, Hacettepe University, Ankara, Turkey.
Orville N.R. Bignall, Nationwide Children’s Hospital, The Ohio State University, Columbus.
Erica Bjornstad, Children’s Hospital Alabama, Birmingham.
Patrick D. Brophy, Golisano Children’s Hospital, Rochester University Medical Center, Rochester, New York.
Rahul Chanchlani, McMaster University, Hamilton, Ontario, Canada.
Jennifer R. Charlton, University of Virginia, Charlottesville.
Andrea L. Conroy, Riley Children’s Hospital, Indiana University, Bloomington.
Akash Deep, King’s College London, London, United Kingdom.
Prasad Devarajan, Center for Acute Care Nephrology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Kristin Dolan, Mercy Children’s Hospital Kansas City, Kansas City, Missouri.
Dana Y. Fuhrman, Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
Katja M. Gist, Center for Acute Care Nephrology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Stephen M. Gorga, C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor.
Jason H. Greenberg, Yale University Medical Center, New Haven, Connecticut
Denise Hasson, Center for Acute Care Nephrology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Emma Heydari Ulrich, Alberta Health Sciences University, Edmonton, Alberta, Canada.
Arpana Iyengar, St John’s Academy of Health Sciences, Bangalore, Karnataka, India.
Jennifer G. Jetton, Stead Family Children’s Hospital, The University of Iowa, Iowa City.
Catherine Krawczeski, Nationwide Children’s Hospital, The Ohio State University, Columbus.
Leslie Meigs, Stead Family Children’s Hospital, The University of Iowa, Iowa City.
Shina Menon, Seattle Children’s Hospital, Seattle, Washington.
Jolyn Morgan, Center for Acute Care Nephrology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Catherine J. Morgan, Alberta Health Sciences University, Edmonton, Alberta, Canada.
Theresa Mottes, Ann & Robert Lurie Children’s Hospital of Chicago, Northwestern University, Chicago, Illinois.
Tara M. Neumayr, Washington University School of Medicine, St Louis, Missouri.
Zaccaria Ricci, University of Florene, Florence, Italy.
David Selewski, Medical University of South Carolina, Charleston.
Danielle E. Soranno, Riley Children’s Hospital, Indiana University, Bloomington.
Michelle Starr, Riley Children’s Hospital, Indiana University, Bloomington.
Natalja L. Stanski, Center for Acute Care Nephrology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Scott M. Sutherland, Lucille Packard Children’s Hospital, Stanford University, Stanford, California.
Jordan Symons, Seattle Children’s Hospital, Seattle, Washington.
Marcelo S. Tavares, Santa Casa de Belo Horizonte, Belo Horizonte, Minas Gerais, Brazil.
Molly Wong Vega, Division of Nephrology, Texas Children’s Hospital, Baylor College of Medicine, Houston.
Michael Zappitelli, The Hospital for Sick Children, Toronto, Ontario, Canada.
Claudio Ronco, Universiti di Padova, San Bartolo Hospital, Vicenza, Italy.
Ravindra L. Mehta, University of California, San Diego Health Sciences, San Diego.
John Kellum, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania.
Marlies Ostermann, Guys and St Thomas University, London, United Kingdom.
Rajit K. Basu, Ann & Robert Lurie Children’s Hospital of Chicago, Northwestern University, Chicago, Illinois.
REFERENCES
- 1.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(1):11–20. doi: 10.1056/NEJMoa1611391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jetton JG, Boohaker LJ, Sethi SK, et al. ; Neonatal Kidney Collaborative (NKC). Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1(3):184–194. doi: 10.1016/S2352-4642(17)30069-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson CH, Jeyakumar N, Luo B, et al. Long-term kidney outcomes following dialysis-treated childhood acute kidney injury: a population-based cohort study. J Am Soc Nephrol. 2021;32(8):2005–2019. doi: 10.1681/ASN.2020111665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg JH, Zappitelli M, Devarajan P, et al. ; TRIBE-AKI Consortium. Kidney outcomes 5 years after pediatric cardiac surgery: The TRIBE-AKI Study. JAMA Pediatr. 2016;170(11):1071–1078. doi: 10.1001/jamapediatrics.2016.1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madsen NL, Goldstein SL, Frøslev T, Christiansen CF, Olsen M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. 2017;92(3):751–756. doi: 10.1016/j.kint.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 6.Akcan-Arikan A, Gebhard DJ, Arnold MA, Loftis LL, Kennedy CE. Fluid overload and kidney injury score: a multidimensional real-time assessment of renal disease burden in the critically ill patient. Pediatr Crit Care Med. 2017;18(6):524–530. doi: 10.1097/PCC.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 7.Abbasi A, Mehdipour Rabori P, Farajollahi R, et al. Discriminatory precision of renal angina index in predicting acute kidney injury in children; a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1):e39. [PMC free article] [PubMed] [Google Scholar]
- 8.Gist KM, Goldstein SL, Wrona J, et al. Kinetics of the cell cycle arrest biomarkers (TIMP-2*IGFBP-7) for prediction of acute kidney injury in infants after cardiac surgery. Pediatr Nephrol. 2017;32(9):1611–1619. doi: 10.1007/s00467-017-3655-y [DOI] [PubMed] [Google Scholar]
- 9.Menon S, Goldstein SL, Mottes T, et al. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant. 2016;31(4):586–594. doi: 10.1093/ndt/gfv457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein SL, Vidal E, Ricci Z, et al. Survival of infants treated with CKRT: comparing adapted adult platforms with the Carpediem™. Pediatr Nephrol. 2022;37(3):667–675. doi: 10.1007/s00467-021-05180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta RL, Kellum JA, Shah SV, et al. ; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–1035. doi: 10.1038/sj.ki.5002231 [DOI] [PubMed] [Google Scholar]
- 14.Kidney International Supplements. KDIGO clinical practice guideline for acute kidney injury. March 2012. Accessed July 27, 2022. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf [Google Scholar]
- 15.Fitzgerald JC, Basu RK, Akcan-Arikan A, et al. ; Sepsis PRevalence, OUtcomes, and Therapies Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators Network. Acute kidney injury in pediatric severe sepsis: an independent risk factor for death and new disability. Crit Care Med. 2016;44(12):2241–2250. doi: 10.1097/CCM.0000000000002007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menon S, Kirkendall ES, Nguyen H, Goldstein SL. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr. 2014;165(3):522–7.e2. doi: 10.1016/j.jpeds.2014.04.058 [DOI] [PubMed] [Google Scholar]
- 17.Uber AM, Montez-Rath ME, Kwiatkowski DM, Krawczeski CD, Sutherland SM. Nephrotoxin exposure and acute kidney injury in critically ill children undergoing congenital cardiac surgery. Pediatr Nephrol. 2018;33(11):2193–2199. doi: 10.1007/s00467-018-4010-7 [DOI] [PubMed] [Google Scholar]
- 18.Blinder JJ, Asaro LA, Wypij D, et al. Acute kidney injury after pediatric cardiac surgery: a secondary analysis of the safe pediatric euglycemia after cardiac surgery trial. Pediatr Crit Care Med. 2017;18(7):638–646. doi: 10.1097/PCC.0000000000001185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hessey E, Morissette G, Lacroix J, et al. Healthcare utilization after acute kidney injury in the pediatric intensive care unit. Clin J Am Soc Nephrol. 2018;13(5):685–692. doi: 10.2215/CJN.09350817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland SM, Ji J, Sheikhi FH, et al. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. 2013;8(10):1661–1669. doi: 10.2215/CJN.00270113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hessey E, Perreault S, Dorais M, Roy L, Zappitelli M. Acute kidney injury in critically ill children and subsequent chronic kidney disease. Can J Kidney Health Dis. 2019;6:2054358119880188. doi: 10.1177/2054358119880188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjornstad EC, Marshall SW, Mottl AK, et al. Racial and health insurance disparities in pediatric acute kidney injury in the USA. Pediatr Nephrol. 2020;35(6):1085–1096. doi: 10.1007/s00467-020-04470-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgson LE, Selby N, Huang TM, Forni LG. The role of risk prediction models in prevention and management of AKI. Semin Nephrol. 2019;39(5):421–430. doi: 10.1016/j.semnephrol.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43(11):1551–1561. doi: 10.1007/s00134-016-4670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming GM, Sahay R, Zappitelli M, et al. The incidence of acute kidney injury and its effect on neonatal and pediatric extracorporeal membrane oxygenation outcomes: a multicenter report from the Kidney Intervention During Extracorporeal Membrane Oxygenation Study Group. Pediatr Crit Care Med. 2016;17(12):1157–1169. doi: 10.1097/PCC.0000000000000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blinder JJ, Goldstein SL, Lee VV, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143(2):368–374. doi: 10.1016/j.jtcvs.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 27.Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–561. doi: 10.2215/CJN.01900214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selewski DT, Askenazi DJ, Kashani K, et al. Quality improvement goals for pediatric acute kidney injury: pediatric applications of the 22nd Acute Disease Quality Initiative (ADQI) conference. Pediatr Nephrol. 2021;36(4):733–746. doi: 10.1007/s00467-020-04828-5 [DOI] [PubMed] [Google Scholar]
- 29.Zappitelli M, Moffett BS, Hyder A, Goldstein SL. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. 2011;26(1):144–150. doi: 10.1093/ndt/gfq375 [DOI] [PubMed] [Google Scholar]
- 30.Sandokji I, Yamamoto Y, Biswas A, et al. A time-updated, parsimonious model to predict AKI in hospitalized children. J Am Soc Nephrol. 2020;31(6):1348–1357. doi: 10.1681/ASN.2019070745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acute Disease Quality Initiative. Images. 2020. Accessed July 30, 2022. https://www.adqi.org/Images
- 32.Kashani K, Rosner MH, Haase M, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. 2019;14(6):941–953. doi: 10.2215/CJN.01250119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schunk SJ, Zarbock A, Meersch M, et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet. 2019;394(10197):488–496. doi: 10.1016/S0140-6736(19)30769-X [DOI] [PubMed] [Google Scholar]
- 34.Bennett MR, Pyles O, Ma Q, Devarajan P. Preoperative levels of urinary uromodulin predict acute kidney injury after pediatric cardiopulmonary bypass surgery. Pediatr Nephrol. 2018;33(3):521–526. doi: 10.1007/s00467-017-3823-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuhrman DY, Nguyen L, Hindes M, Kellum JA. Baseline tubular biomarkers in young adults with congenital heart disease as compared to healthy young adults: detecting subclinical kidney injury. Congenit Heart Dis. 2019;14(6):963–967. doi: 10.1111/chd.12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Husain-Syed F, Ferrari F, Sharma A, et al. Preoperative renal functional reserve predicts risk of acute kidney injury after cardiac operation. Ann Thorac Surg. 2018;105(4):1094–1101. doi: 10.1016/j.athoracsur.2017.12.034 [DOI] [PubMed] [Google Scholar]
- 37.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. ; TRIBE-AKI Consortium. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22(9):1748–1757. doi: 10.1681/ASN.2010121302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCullough PA, Bouchard J, Waikar SS, et al. Implementation of novel biomarkers in the diagnosis, prognosis, and management of acute kidney injury: executive summary from the tenth consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2013;182:5–12. doi: 10.1159/000349962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu RK, Wong HR, Krawczeski CD, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol. 2014;64(25):2753–2762. doi: 10.1016/j.jacc.2014.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57(17):1752–1761. doi: 10.1016/j.jacc.2010.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanski N, Menon S, Goldstein SL, Basu RK. Integration of urinary neutrophil gelatinase-associated lipocalin with serum creatinine delineates acute kidney injury phenotypes in critically ill children. J Crit Care. 2019;53:1–7. doi: 10.1016/j.jcrc.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 42.Kaddourah A, Basu RK, Goldstein SL, Sutherland SM; Assessment of Worldwide Acute Kidney Injury, Renal Angina and, Epidemiology (AWARE) Investigators. Oliguria and acute kidney injury in critically ill children: implications for diagnosis and outcomes. Pediatr Crit Care Med. 2019;20(4):332–339. doi: 10.1097/PCC.0000000000001866 [DOI] [PubMed] [Google Scholar]
- 43.Sutherland SM, Kaddourah A, Gillespie SE, et al. ; Assessment of the Worldwide Acute Kidney Injury, Renal Angina and Epidemiology (AWARE) Investigators. Cumulative application of creatinine and urine output staging optimizes the kidney disease: improving global outcomes definition and identifies increased mortality risk in hospitalized patients with acute kidney injury. Crit Care Med. 2021;49(11):1912–1922. doi: 10.1097/CCM.0000000000005073 [DOI] [PubMed] [Google Scholar]
- 44.Basu RK, Hackbarth R, Gillespie S, et al. Clinical phenotypes of acute kidney injury are associated with unique outcomes in critically ill septic children. Pediatr Res. 2021;90(5):1031–1038. doi: 10.1038/s41390-021-01363-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gist KM, Borasino S, SooHoo M, et al. Transient and persistent acute kidney injury phenotypes following the Norwood operation: a retrospective study. Cardiol Young. 2022;32(4):564–571. doi: 10.1017/S1047951121002560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gist KM, Selewski DT, Brinton J, Menon S, Goldstein SL, Basu RK. Assessment of the independent and synergistic effects of fluid overload and acute kidney injury on outcomes of critically ill children. Pediatr Crit Care Med. 2020;21(2):170–177. doi: 10.1097/PCC.0000000000002107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borasino S, Wall KM, Crawford JH, et al. Furosemide response predicts acute kidney injury after cardiac surgery in infants and neonates. Pediatr Crit Care Med. 2018;19(4):310–317. doi: 10.1097/PCC.0000000000001478 [DOI] [PubMed] [Google Scholar]
- 48.Penk J, Gist KM, Wald EL, et al. Furosemide response predicts acute kidney injury in children after cardiac surgery. J Thorac Cardiovasc Surg. 2019;157(6):2444–2451. doi: 10.1016/j.jtcvs.2018.12.076 [DOI] [PubMed] [Google Scholar]
- 49.Goldstein SL, Currier H, Graf Cd, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309–1312. doi: 10.1542/peds.107.6.1309 [DOI] [PubMed] [Google Scholar]
- 50.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653–658. doi: 10.1111/j.1523-1755.2005.67121.x [DOI] [PubMed] [Google Scholar]
- 51.Gorga SM, Sahay RD, Askenazi DJ, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy: a multicenter retrospective cohort study. Pediatr Nephrol. 2020;35(5):871–882. doi: 10.1007/s00467-019-04468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hassinger AB, Wald EL, Goodman DM. Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatr Crit Care Med. 2014;15(2):131–138. doi: 10.1097/PCC.0000000000000043 [DOI] [PubMed] [Google Scholar]
- 53.Hayes LW, Oster RA, Tofil NM, Tolwani AJ. Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care. 2009;24(3):394–400. doi: 10.1016/j.jcrc.2008.12.017 [DOI] [PubMed] [Google Scholar]
- 54.Hazle MA, Gajarski RJ, Yu S, Donohue J, Blatt NB. Fluid overload in infants following congenital heart surgery. Pediatr Crit Care Med. 2013;14(1):44–49. doi: 10.1097/PCC.0b013e3182712799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316–325. doi: 10.1053/j.ajkd.2009.10.048 [DOI] [PubMed] [Google Scholar]
- 56.Hoste EA, Maitland K, Brudney CS, et al. ; ADQI XII Investigators Group. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740–747. doi: 10.1093/bja/aeu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alobaidi R, Basu RK, DeCaen A, et al. Fluid accumulation in critically ill children. Crit Care Med. 2020;48(7):1034–1041. [DOI] [PubMed] [Google Scholar]
- 58.Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Patil N, Ambalavanan N. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr Nephrol. 2013;28(4):661–666. doi: 10.1007/s00467-012-2369-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bontant T, Matrot B, Abdoul H, et al. Assessing fluid balance in critically ill pediatric patients. Eur J Pediatr. 2015;174(1):133–137. doi: 10.1007/s00431-014-2372-9 [DOI] [PubMed] [Google Scholar]
- 60.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771–1776. doi: 10.1097/01.CCM.0000132897.52737.49 [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Wang J, Bai Z, et al. Early fluid overload is associated with acute kidney injury and PICU mortality in critically ill children. Eur J Pediatr. 2016;175(1):39–48. doi: 10.1007/s00431-015-2592-7 [DOI] [PubMed] [Google Scholar]
- 62.Lima L, Menon S, Goldstein SL, Basu RK. Timing of fluid overload and association with patient outcome. Pediatr Crit Care Med. 2021;22(1):114–124. doi: 10.1097/PCC.0000000000002547 [DOI] [PubMed] [Google Scholar]
- 63.Lombel RM, Kommareddi M, Mottes T, et al. Implications of different fluid overload definitions in pediatric stem cell transplant patients requiring continuous renal replacement therapy. Intensive Care Med. 2012;38(4):663–669. doi: 10.1007/s00134-012-2503-6 [DOI] [PubMed] [Google Scholar]
- 64.Mallory PP, Selewski DT, Askenazi DJ, et al. Acute kidney injury, fluid overload, and outcomes in children supported with extracorporeal membrane oxygenation for a respiratory indication. ASAIO J. 2020;66(3):319–326. doi: 10.1097/MAT.0000000000001000 [DOI] [PubMed] [Google Scholar]
- 65.Michael M, Kuehnle I, Goldstein SL. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol. 2004;19(1):91–95. doi: 10.1007/s00467-003-1313-z [DOI] [PubMed] [Google Scholar]
- 66.Selewski DT, Cornell TT, Blatt NB, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40(9):2694–2699. doi: 10.1097/CCM.0b013e318258ff01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selewski DT, Cornell TT, Lombel RM, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 2011;37(7):1166–1173. doi: 10.1007/s00134-011-2231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinitsky L, Walls D, Nadel S, Inwald DP. Fluid overload at 48 hours is associated with respiratory morbidity but not mortality in a general PICU: retrospective cohort study. Pediatr Crit Care Med. 2015;16(3):205–209. doi: 10.1097/PCC.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 69.Valentine SL, Sapru A, Higgerson RA, et al. ; Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network; Acute Respiratory Distress Syndrome Clinical Research Network (ARDSNet). Fluid balance in critically ill children with acute lung injury. Crit Care Med. 2012;40(10):2883–2889. doi: 10.1097/CCM.0b013e31825bc54d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Selewski DT, Askenazi DJ, Bridges BC, et al. The impact of fluid overload on outcomes in children treated with extracorporeal membrane oxygenation: a multicenter retrospective cohort study. Pediatr Crit Care Med. 2017;18(12):1126–1135. doi: 10.1097/PCC.0000000000001349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barhight MF, Brinton J, Stidham T, et al. Increase in chloride from baseline is independently associated with mortality in critically ill children. Intensive Care Med. 2018;44(12):2183–2191. doi: 10.1007/s00134-018-5424-1 [DOI] [PubMed] [Google Scholar]
- 72.Barhight MF, Brinton JT, Soranno DE, Faubel S, Mourani PM, Gist KM. Effects of hyperchloremia on renal recovery in critically ill children with acute kidney injury. Pediatr Nephrol. 2020;35(7):1331–1339. doi: 10.1007/s00467-020-04513-7 [DOI] [PubMed] [Google Scholar]
- 73.Selewski DT, Gist KM, Nathan AT, et al. ; Neonatal Kidney Collaborative. The impact of fluid balance on outcomes in premature neonates: a report from the AWAKEN study group. Pediatr Res. 2020;87(3):550–557. doi: 10.1038/s41390-019-0579-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Selewski DT, Akcan-Arikan A, Bonachea EM, et al. ; Neonatal Kidney Collaborative. The impact of fluid balance on outcomes in critically ill near-term/term neonates: a report from the AWAKEN study group. Pediatr Res. 2019;85(1):79–85. doi: 10.1038/s41390-018-0183-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Starr MC, Charlton JR, Guillet R, et al. ; Neonatal Kidney Collaborative Board. Advances in neonatal acute kidney injury. Pediatrics. 2021;148(5):e2021051220. doi: 10.1542/peds.2021-051220 [DOI] [PubMed] [Google Scholar]
- 76.van Asperen Y, Brand PL, Bekhof J. Reliability of the fluid balance in neonates. Acta Paediatr. 2012;101(5):479–483. doi: 10.1111/j.1651-2227.2012.02591.x [DOI] [PubMed] [Google Scholar]
- 77.Schmidt B, Roberts RS, Fanaroff A, et al. ; TIPP Investigators. Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: further analyses from the Trial of Indomethacin Prophylaxis in Preterms (TIPP). J Pediatr. 2006;148(6):730–734. doi: 10.1016/j.jpeds.2006.01.047 [DOI] [PubMed] [Google Scholar]
- 78.Mottes TA. CRRT Program Development. Critical Care Nephrology and Renal Replacement Therapy in Children. Springer; 2018:357–368. doi: 10.1007/978-3-319-90281-4_23 [DOI] [Google Scholar]
- 79.Mottes TA, Goldstein SL, Basu RK. Process based quality improvement using a continuous renal replacement therapy dashboard. BMC Nephrol. 2019;20(1):17. doi: 10.1186/s12882-018-1195-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rewa OG, Tolwani A, Mottes T, et al. Quality of care and safety measures of acute renal replacement therapy: workgroup statements from the 22nd acute disease quality initiative (ADQI) consensus conference. 2019;54:52–57. [DOI] [PubMed] [Google Scholar]
- 81.Mottes TA. Does your program know its AKI and CRRT epidemiology? the case for a dashboard. Front Pediatr. 2020;8:80. doi: 10.3389/fped.2020.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Askenazi D, Basu RK. Kidney support therapy in the pediatric patient: Unique considerations for a unique population. Semin Dial. 2021;34(6):530–536. doi: 10.1111/sdi.12978 [DOI] [PubMed] [Google Scholar]
- 83.Harer MW, Selewski DT, Kashani K, et al. Improving the quality of neonatal acute kidney injury care: neonatal-specific response to the 22nd Acute Disease Quality Initiative (ADQI) conference. J Perinatol. 2021;41(2):185–195. doi: 10.1038/s41372-020-00810-z [DOI] [PubMed] [Google Scholar]
- 84.Neyra JA, Kashani K. Improving the quality of care for patients requiring continuous renal replacement therapy. Semin Dial. 2021;34(6):501–509. doi: 10.1111/sdi.12968 [DOI] [PubMed] [Google Scholar]
- 85.Rewa OG, Tolwani A, Mottes T, et al. ; ADQI Consensus Meeting Members on behalf of ADQI XXII. Quality of care and safety measures of acute renal replacement therapy: workgroup statements from the 22nd acute disease quality initiative (ADQI) consensus conference. J Crit Care. 2019;54:52–57. doi: 10.1016/j.jcrc.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 86.Przybyl H, Androwich I, Evans J. Using high-fidelity simulation to assess knowledge, skills, and attitudes in nurses performing CRRT. Nephrol Nurs J. 2015;42(2):135–147. [PubMed] [Google Scholar]
- 87.Richardson A, Whatmore J. Nursing essential principles: continuous renal replacement therapy. Nurs Crit Care. 2015;20(1):8–15. doi: 10.1111/nicc.12120 [DOI] [PubMed] [Google Scholar]
- 88.Mottes T, Owens T, Niedner M, Juno J, Shanley TP, Heung M. Improving delivery of continuous renal replacement therapy: impact of a simulation-based educational intervention. Pediatr Crit Care Med. 2013;14(8):747–754. doi: 10.1097/PCC.0b013e318297626e [DOI] [PubMed] [Google Scholar]
- 89.Rewa O, Villeneuve PM, Eurich DT, et al. Quality indicators in continuous renal replacement therapy (CRRT) care in critically ill patients: protocol for a systematic review. Syst Rev. 2015;4:102. doi: 10.1186/s13643-015-0088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rewa OG, Eurich DT, Noel Gibney RT, Bagshaw SM. A modified Delphi process to identify, rank and prioritize quality indicators for continuous renal replacement therapy (CRRT) care in critically ill patients. J Crit Care. 2018;47:145–152. doi: 10.1016/j.jcrc.2018.06.023 [DOI] [PubMed] [Google Scholar]
- 91.Shen B, Xu J, Wang Y, Jiang W, Teng J, Ding X. Continuous renal replacement therapy quality control and performance measures. Contrib Nephrol. 2018;194:134–145. doi: 10.1159/000485611 [DOI] [PubMed] [Google Scholar]
- 92.Ruiz EF, Ortiz-Soriano VM, Talbott M, et al. ; University of Kentucky CRRT Quality Assurance Group. Development, implementation and outcomes of a quality assurance system for the provision of continuous renal replacement therapy in the intensive care unit. Sci Rep. 2020;10(1):20616. doi: 10.1038/s41598-020-76785-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uchino S, Bellomo R, Morimatsu H, et al. Discontinuation of continuous renal replacement therapy: a post hoc analysis of a prospective multicenter observational study. Crit Care Med. 2009;37(9):2576–2582. doi: 10.1097/CCM.0b013e3181a38241 [DOI] [PubMed] [Google Scholar]
- 94.Fröhlich S, Donnelly A, Solymos O, Conlon N. Use of 2-hour creatinine clearance to guide cessation of continuous renal replacement therapy. J Crit Care. 2012;27(6):744.e1–744.e5. doi: 10.1016/j.jcrc.2012.08.012 [DOI] [PubMed] [Google Scholar]
- 95.Schiffl H, Lang SM. Current approach to successful liberation from renal replacement therapy in critically ill patients with severe acute kidney injury: the quest for biomarkers continues. Mol Diagn Ther. 2021;25(1):1–8. doi: 10.1007/s40291-020-00498-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF Bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47(1):3–14. doi: 10.1097/CCM.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. 2017;45(2):171–178. doi: 10.1097/CCM.0000000000002149 [DOI] [PubMed] [Google Scholar]
- 98.Hopkins RO, Choong K, Zebuhr CA, Kudchadkar SR. Transforming PICU culture to facilitate early rehabilitation. J Pediatr Intensive Care. 2015;4(4):204–211. doi: 10.1055/s-0035-1563547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Starr MC, Hingorani SR. Prematurity and future kidney health: the growing risk of chronic kidney disease. Curr Opin Pediatr. 2018;30(2):228–235. doi: 10.1097/MOP.0000000000000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lebel A, Teoh CW, Zappitelli M. Long-term complications of acute kidney injury in children. Curr Opin Pediatr. 2020;32(3):367–375. doi: 10.1097/MOP.0000000000000906 [DOI] [PubMed] [Google Scholar]
- 101.Hessey E, Melhem N, Alobaidi R, et al. Acute kidney injury in critically ill children is not all acute: lessons over the last 5 years. Front Pediatr. 2021;9:648587. doi: 10.3389/fped.2021.648587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hukriede NA, Soranno DE, Sander V, et al. Experimental models of acute kidney injury for translational research. Nat Rev Nephrol. 2022;18(5):277–293. doi: 10.1038/s41581-022-00539-2 [DOI] [PubMed] [Google Scholar]
- 103.Charlton JR, Baldelomar EJ, Hyatt DM, Bennett KM. Nephron number and its determinants: a 2020 update. Pediatr Nephrol. 2021;36(4):797–807. doi: 10.1007/s00467-020-04534-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Askenazi DJ, Morgan C, Goldstein SL, et al. Strategies to improve the understanding of long-term renal consequences after neonatal acute kidney injury. Pediatr Res. 2016;79(3):502–508. doi: 10.1038/pr.2015.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bairey Merz CN, Dember LM, Ingelfinger JR, et al. ; participants of the National Institute of Diabetes and Digestive and Kidney Diseases Workshop on “Sex and the Kidneys”. Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol. 2019;15(12):776–783. doi: 10.1038/s41581-019-0208-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sato Y, Takahashi M, Yanagita M. Pathophysiology of AKI to CKD progression. Semin Nephrol. 2020;40(2):206–215. doi: 10.1016/j.semnephrol.2020.01.011 [DOI] [PubMed] [Google Scholar]
- 107.Selewski DT, Hyatt DM, Bennett KM, Charlton JR. Is acute kidney injury a harbinger for chronic kidney disease? Curr Opin Pediatr. 2018;30(2):236–240. doi: 10.1097/MOP.0000000000000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Castillo A, Santiago MJ, López-Herce J, et al. Nutritional status and clinical outcome of children on continuous renal replacement therapy: a prospective observational study. BMC Nephrol. 2012;13:125. doi: 10.1186/1471-2369-13-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lion RP, Vega MR, Smith EO, et al. The effect of continuous venovenous hemodiafiltration on amino acid delivery, clearance, and removal in children. Pediatr Nephrol. 2022;37(2):433–441. doi: 10.1007/s00467-021-05162-0 [DOI] [PubMed] [Google Scholar]
- 110.Vega MW, Juarez M, Lee JY, Srivaths P, Williams E, Akcan Arikan A. Quality improvement bedside rounding audits enhance protein provision for pediatric patients receiving continuous renal replacement therapy. Pediatr Crit Care Med. 2018;19(11):1054–1058. doi: 10.1097/PCC.0000000000001698 [DOI] [PubMed] [Google Scholar]
- 111.Smith M, Bell C, Vega MW, et al. Patient-centered outcomes in pediatric continuous kidney replacement therapy: new morbidity and worsened functional status in survivors. Pediatr Nephrol. 2022;37(1):189–197. doi: 10.1007/s00467-021-05177-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abraham AG, Mak RH, Mitsnefes M, et al. Protein energy wasting in children with chronic kidney disease. Pediatr Nephrol. 2014;29(7):1231–1238. doi: 10.1007/s00467-014-2768-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rees L Protein energy wasting; what is it and what can we do to prevent it? Pediatr Nephrol. 2021;36(2):287–294. doi: 10.1007/s00467-019-04424-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Verbruggen SC, Schierbeek H, Coss-Bu J, Joosten KF, Castillo L, van Goudoever JB. Albumin synthesis rates in post-surgical infants and septic adolescents; influence of amino acids, energy, and insulin. Clin Nutr. 2011;30(4):469–477. doi: 10.1016/j.clnu.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 115.Malagaris I, Herndon DN, Polychronopoulou E, et al. Determinants of skeletal muscle protein turnover following severe burn trauma in children. Clin Nutr. 2019;38(3):1348–1354. doi: 10.1016/j.clnu.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chawla LS, Bellomo R, Bihorac A, et al. ; Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi: 10.1038/nrneph.2017.2 [DOI] [PubMed] [Google Scholar]
- 117.Rodieux F, Wilbaux M, van den Anker JN, Pfister M. Effect of kidney function on drug kinetics and dosing in neonates, infants, and children. Clin Pharmacokinet. 2015;54(12):1183–1204. doi: 10.1007/s40262-015-0298-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nguyen TTL, Liu D, Ho MF, Athreya AP, Weinshilboum R. Selective serotonin reuptake inhibitor pharmaco-omics: mechanisms and prediction. Front Pharmacol. 2021;11:614048. doi: 10.3389/fphar.2020.614048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McGregor TL, Jones DP, Wang L, et al. Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: a retrospective observational study. Am J Kidney Dis. 2016;67(3):384–390. doi: 10.1053/j.ajkd.2015.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Macedo E, Cerdá J, Hingorani S, et al. Recognition and management of acute kidney injury in children: the ISN 0by25 Global Snapshot study. PLoS One. 2018;13(5):e0196586. doi: 10.1371/journal.pone.0196586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu KD, Goldstein SL, Vijayan A, et al. ; AKI!Now Initiative of the American Society of Nephrology. AKI!Now initiative: recommendations for awareness, recognition, and management of AKI. Clin J Am Soc Nephrol. 2020;15(12):1838–1847. doi: 10.2215/CJN.15611219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Macedo E, Hemmila U, Sharma SK, et al. ; ISN 0by25 Trial Study Group. Recognition and management of community-acquired acute kidney injury in low-resource settings in the ISN 0by25 trial: a multi-country feasibility study. PLoS Med. 2021;18(1):e1003408. doi: 10.1371/journal.pmed.1003408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.National Institute for Health and Care Excellence. Acute kidney injury: prevention, detection and management: NICE Guideline [NG148]. 2019. Accessed July 27, 2022. https://www.nice.org.uk/guidance/ng148 [PubMed]
- 124.Colbert GB, Topf J, Jhaveri KD, et al. The social media revolution in nephrology education. Kidney Int Rep. 2018;3(3):519–529. doi: 10.1016/j.ekir.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Silver SA, Goldstein SL, Harel Z, et al. Ambulatory care after acute kidney injury: an opportunity to improve patient outcomes. Can J Kidney Health Dis. 2015;2:36. doi: 10.1186/s40697-015-0071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harel Z, Wald R, Bargman JM, et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 2013;83(5):901–908. doi: 10.1038/ki.2012.451 [DOI] [PubMed] [Google Scholar]
- 127.Lunyera J, Kilonzo K, Lewington A, Yeates K, Finkelstein FO. Acute kidney injury in low-resource settings: barriers to diagnosis, awareness, and treatment and strategies to overcome these barriers. Am J Kidney Dis. 2016;67(6):834–840. doi: 10.1053/j.ajkd.2015.12.018 [DOI] [PubMed] [Google Scholar]
- 128.Finkelstein FO, Smoyer WE, Carter M, Brusselmans A, Feehally J. Peritoneal dialysis, acute kidney injury, and the Saving Young Lives program. Perit Dial Int. 2014;34(5):478–480. doi: 10.3747/pdi.2014.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eBackground. Group Descriptions
eBox. Research Agenda for Pediatric Acute Kidney Injury
eFigure 1. An Evolving Approach to AKI Epidemiology
eTable 1. Definitions of Fluid Balance
eFigure 2. The Spectrum of Fluid Balance
eFigure 3. Multipronged Approach to Pediatric Acute Kidney Support Therapy
eTable 2. Blood Purification and Extracorporeal Treatment Modalities for Different Pathophysiological Conditions
eTable 3. Key Components of Education and Advocacy for Children With Acute Kidney Injury (AKI)</SI_Caption>


