Abstract
Background and Objectives
Deletions and duplications at 16p11.2 (BP4 to BP5; 29.5–30.1 Mb) have been associated with several neurodevelopmental and neuropsychiatric disorders including autism spectrum disorder, intellectual disability (ID), and schizophrenia. Seizures have also been reported in individuals with these particular copy number variants, but the epilepsy phenotypes have not been well-delineated. We aimed to systematically characterize the seizure types, epilepsy syndromes, and epilepsy severity in a large cohort of individuals with these 16p11.2 deletions and duplications.
Methods
The cohort of ascertained participants with the recurrent 16p11.2 copy number variant was assembled through the multicenter Simons Variation in Individuals Project. Detailed data on individuals identified as having a history of seizures were obtained using a semistructured phone interview and review of medical records, EEG, and MRI studies obtained clinically or as part of the Simons Variation in Individuals Project.
Results
Among 129 individuals with the 16p11.2 deletion, 31 (24%) had at least one seizure, including 23 (18%) who met criteria for epilepsy; 42% of them fit the phenotype of classic or atypical Self-limited (Familial) Infantile Epilepsy (Se(F)IE). Among 106 individuals with 16p11.2 duplications, 16 (15%) had at least one seizure, including 11 (10%) who met criteria for epilepsy. The seizure types and epilepsy syndromes were heterogeneous in this group. Most of the individuals in both the deletion and duplication groups had well-controlled seizures with subsequent remission. Pharmacoresistant epilepsy was uncommon. Seizures responded favorably to phenobarbital, carbamazepine, and oxcarbazepine in the deletion group, specifically in the Se(F)IE, and to various antiseizure medications in the duplication group.
Discussion
These findings delineate the spectrum of seizures and epilepsies in the recurrent 16p11.2 deletions and duplications and provide potential diagnostic, therapeutic, and prognostic information.
Recurrent copy number variants (CNVs, duplications and deletions) of 593 Kb at 16p11.2 (breakpoints 4 and 5, BP4-BP5) have been associated with several neurologic and non-neurologic manifestations. The first reported neurologic manifestations included autism spectrum disorder (ASD).1,2 Additional neurologic and psychiatric symptoms include schizophrenia and bipolar disorder (with duplications), developmental delay and hypotonia (with deletions), as well as seizures and intellectual disability (ID) with either deletions or duplications.3 An interesting early finding was that the head circumference in individuals with the duplication was smaller than those with the deletion,4,5 and similarly, the body mass index was higher in individuals with deletion.6
Many CNVs are associated with epilepsy, as an isolated finding or alongside other neurodevelopmental disorders.7-10 While the 16p11.2 region was not initially described as a seizure-associated locus, seizures were reported as early as 2007 in monozygotic twins with 16p11.2 microdeletion, who also experienced ID and abnormal aortic valve development,11 and epilepsy was reported in the initial cohorts of individuals with the 16p11.2 recurrent CNV.12,13 Recurrent deletions in other loci such as 15q11.2 and 16p13.11 have been identified in a cohort of patients with idiopathic (now referred to as genetic) generalized epilepsies, and one individual in this same cohort had the recurrent 16p11.2 deletion.14 In this study, we described the results of a systematic analysis of seizures and epilepsy phenotypes of a large cohort of clinically ascertained individuals with the recurrent 16p11.2 CNVs.
Methods
Ascertainment
The cohort was assembled through the multicenter Simons Variation in individuals Project, which started in 2010. Details on recruitment, ascertainment, and data collection methods have been previously published.15 Clinically ascertained participants were confirmed to have the same recurrent, approximately 600 kb, 16p11.2 BP4-BP5 deletion or duplication and no known additional pathogenic CNV or known monogenic disorders as assessed by whole-exome sequencing. Individuals received comprehensive phenotyping evaluations at 3 Simons Variations in Individuals Project (VIP) sites: Boston Children's Hospital, the University of California San Francisco, and the University of Washington. In addition, neuroimaging was performed at 2 other Simons VIP sites: Baylor College of Medicine and Children's Hospital of Philadelphia.
All individuals received a medical history interview by trained, licensed genetic counselors as part of the Simons VIP. Those with a reported history of seizures (31 with deletion and 17 with duplication) were then recontacted for a detailed seizure phenotype analysis via a semistructured phone interview performed by board-certified child neurologists (S.K. and A.P.) or child neurology trainees under their supervision (C.M. and A.R.). Medical records, EEG, and MRI results were reviewed where available. Data of interest included prevalence of seizures and epilepsy, age at seizure onset, seizure types, response to antiseizure medications (ASMs), seizure severity and burden, associated imaging findings, and nonepileptic events.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the local Institutional Review Boards of each participating institution. Written informed consent was obtained from all participating individuals or their legal guardians through the Simons VIP.
Definitions
Epilepsy was defined according to the International League Against Epilepsy (ILAE) terminology as 2 or more unprovoked seizures occurring greater than 24 hours apart or a single unprovoked seizure with data suggesting an increased risk of recurrence (i.e., epileptiform activity on EEG or specific MRI findings that would predispose to epilepsy).16
Seizures in the context of fever with the first occurrence in less than 1 year of age were not considered febrile seizures. While this is different from the current ILAE definition of febrile seizures, this age cutoff was chosen based on the fact that the first seizure with fever can be seen in different syndromes, for example, Self-limited (Familial) Infantile Epilepsy (Se(F)IE), with seizure onset between the ages of 3 and 12 months, and Dravet syndrome, with seizure onset between 1 month and 18 months of age but mostly before the age of 12 months.17 In addition, those started on ASMs after their first seizure without recurrence were not considered to experience epilepsy.
Epilepsy syndromes and seizure types were defined according to the ILAE operational classification of 2017.16 Classification of tonic-clonic seizures (TCSs) with unknown onset was given when no localizing features were reported at onset, and ictal or interictal EEG data were unavailable or insufficient to support a generalized mechanism.
Se(F)IE, previously benign infantile epilepsy (ilae.org, epilepsydiagnosis.org), is characterized by seizure onset between 3 and 20 months of age in a child with a normal perinatal history, neurologic examination, and development. Seizures can occur frequently and in clusters early on but typically remit within 1 year of onset. These seizures can be unprovoked or triggered by fever or illness; therefore, these individuals were included under this classification. We included the additional category of atypical Se(F)IE to describe individuals with a variation from the classical Se(F)IE. This was defined as the presence of 1 or more of the following: (1) absence of clusters, (2) seizure recurrence after remission (1 year after the last seizure), (3) later age of seizure remission (older than 2 years), and (4) early seizure onset (younger than 3 months).
Status epilepticus was defined as a continuous seizure longer than 5 minutes for TCSs, 10 minutes for focal seizures with impaired awareness, 15 minutes for absence status epilepticus, or a cluster of seizures longer than 15 minutes without return to baseline mental status then.
Intractable epilepsy was defined as a failure of 2 or more ASMs, given at adequate dosage, to achieve full seizure control. Treatment response was classified as a success if seizures resolved or improved (specified as partial success) or a failure if seizures did not improve.
Genetic Assessment
To confirm 16p11.2 deletions and duplications and to ensure that breakpoints are within the BP4 and BP5 low-copy repeats, all participants in the cohort were mapped using chromosomal microarrays. Methods were detailed in a prior publication.12
Data Availability
The original data that support the findings are available from the corresponding author on request.
Results
Individuals With 16p11.2 Deletions
I.1 General Characteristics
Among the 129 individuals with deletion initially surveyed, structured interviews revealed that 31 (24%) had at least 1 seizure, including 23 (18%) who met criteria for epilepsy. The remaining 8 individuals were not diagnosed with epilepsy; because 6 of them had a single seizure, with either normal interictal EEG or unavailable data, and 2 had a single seizure or seizure cluster, with normal EEGs, but were started immediately on medication such that epilepsy could not be confirmed. Ages of individuals with seizures ranged from 2 to 37 years during study enrollment. The male to female ratio was 15:16.
I.2 Seizure Characteristics
In the 31 patients with deletion and seizures, the age at first seizure ranged from the newborn period to 14 years (mean 18.4 months, median 7 months). Nineteen patients had seizure onset in the first year of life, including 2 with neonatal seizures. First-time seizures were associated with possible triggers in 11 individuals (fever in 7, nonspecific illness in 1, gastroenteritis in 2, and immunization in 1).
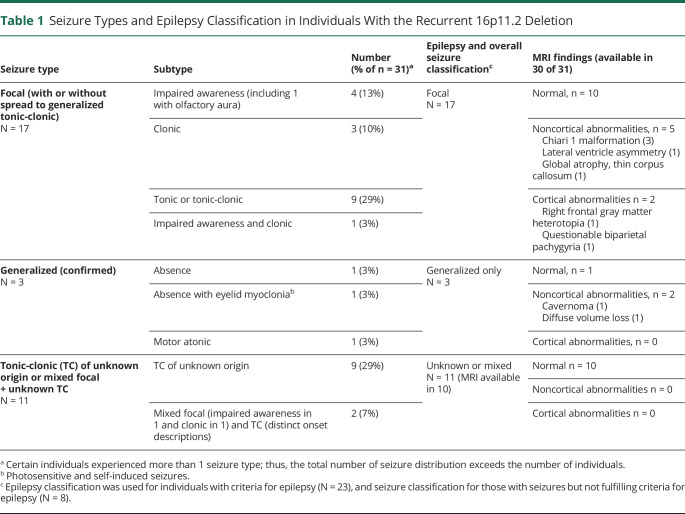
The most common seizure types were focal seizures alone or TCSs of unknown onset alone in 17 and 9 of the 31 individuals, respectively. Two individuals had a combination of focal seizures and TCSs of unknown onset (clinical onset differed from their focal seizures; thus, they were considered to have 2 seizure types). Confirmed generalized seizures were seen in 3. A breakdown of seizure subtypes is detailed in Table 1.
Table 1.
Seizure Types and Epilepsy Classification in Individuals With the Recurrent 16p11.2 Deletion
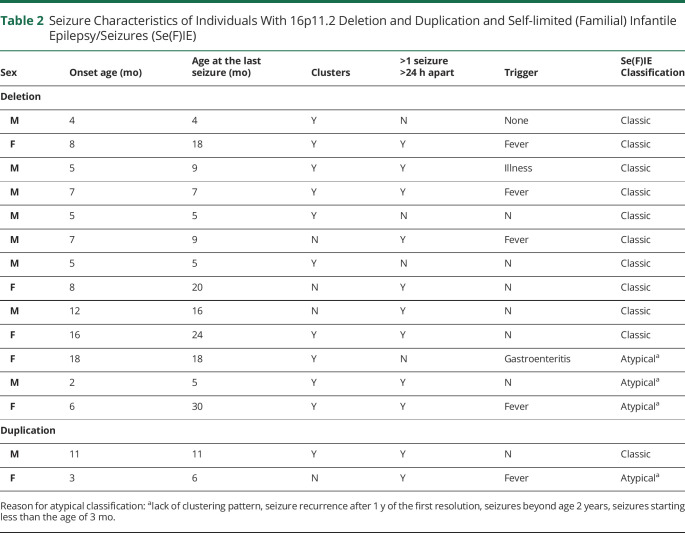
Se(F)IE was the most common epilepsy syndrome identified in the deletion cohort. Characteristics of these individuals are detailed in Table 2. A total of 13 individuals with deletion were found to experience Se(F)IE. This represents 42% of individuals with deletion and seizures (including those without epilepsy) and 57% of those with deletion and epilepsy. Ten individuals fit the criteria for classic Se(F)IE, while the remaining 3 were labeled as those with atypical Se(F)IE based on the criteria defined under methods.
Table 2.
Seizure Characteristics of Individuals With 16p11.2 Deletion and Duplication and Self-limited (Familial) Infantile Epilepsy/Seizures (Se(F)IE)
I.3 EEG Findings
EEG reports were available on 21 of the 31 individuals, and 2 additional EEGs were described as abnormal without additional details. Of those with available reports, 6 had normal EEG findings.
Of those with abnormal EEG findings, 3 had focal slowing only, 8 had interictal epileptiform activity only including focal (n = 4), generalized (n = 2), multifocal (n = 1), and mixed focal and generalized spikes (n = 1). One individual had both focal slowing and focal spikes. Three individuals experienced seizures captured on EEG, 2 with focal onset and no interictal abnormalities and 1 with a generalized seizure with eyelid myoclonia during photic stimulation and interictal generalized spike-wave complexes.
I.4 Treatment Response
ASM information was unavailable in 2 individuals. Eight were never started on ASMs, including 6 with seizures without epilepsy. One of the remaining 2 experienced Se(F)IE and eventually became seizure-free, and the second experienced focal epilepsy with ongoing seizures but was not treated. Of the 21 individuals who were started on ASMs, 7 achieved seizure freedom on monotherapy (including phenobarbital [PB] in 3, oxcarbazepine [OXC] in 1, carbamazepine [CBZ] in 1, lamotrigine [LTG] in 1, and topiramate [TPM] in 1). The first 5 of these 7 individuals experienced Se(F)IE.
A second medication was required in 11 individuals who were initially on either levetiracetam (LEV, 3), PB (3), ETX (1), OXC (1), valproic acid (VPA, 1), or CBZ (1), either due to side effects, lack of efficacy, or unclear reasons. The 3 individuals who did not respond to LEV monotherapy experienced Se(F)IE and became seizure-free on OXC. One of the individuals on PB also experienced Se(F)IE with controlled seizures but was switched to OXC for side-effect concerns. Of the remaining 8 individuals, 6 became seizure-free after addition of a second ASM (CBZ, VPA, LTG, OXC in 2, and PHT) and 2 continued to experience seizures despite the second ASM (CBZ for focal seizures added to PB, LTG for atypical absence seizures added to ETX); it is not known whether a third ASM was tried. Three additional individuals were placed on a third ASM. One experienced Se(F)IE, initially uncontrolled on LEV, and OXC was effective, but it is unclear why ZNS was added. This individual remained seizure-free. The second experienced focal epilepsy and was placed on PB, VPA, and LEV in an unknown order and achieved seizure freedom. The last individual was refractory to 6 ASMs (ETX, LTG, VPA, ZNS, LEV, and clonazepam).
Individuals With 16p11.2 Duplications
II.1 General Characteristics
Among the 106 individuals with duplications in the initially surveyed, structured interviews revealed that 17 (16%) experienced at least 1 seizure, including 12 (11%) who met criteria for epilepsy. Of those 12 patients, 1 was later excluded because a left middle cerebral artery stroke was found on MRI.
The ages of the 16 remaining individuals ranged from 1 to 59 years. The male to female ratio was 7:9.
II.2 Seizure Characteristics
Age at first seizure ranged from 1 to 10 years (mean = 36 months, median = 15 months). Eleven individuals (10%) fit criteria for epilepsy, while 4 did not because unprovoked seizures did not recur, including 1 who was started on medication after the unprovoked seizure.
The first seizure occurred in the first year of life in 7 individuals. First-time seizures were associated with fever in 3 individuals, all occurring between ages 1 and 5 years and initially diagnosed as febrile seizures. No other triggers were identified. None had a history of status epilepticus.
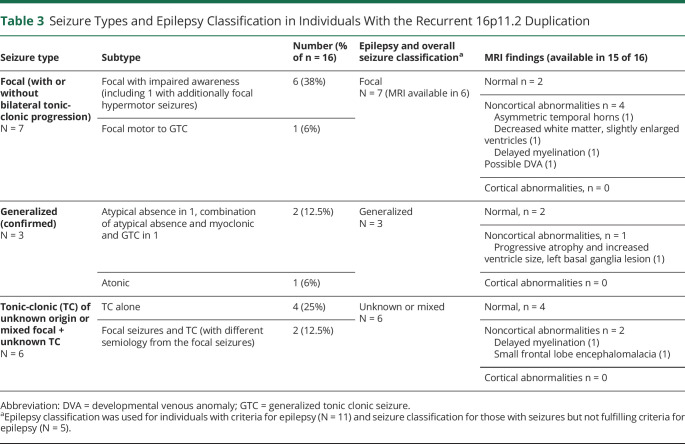
Seizure types are detailed in Table 3. Focal seizures alone occurred in 7 (44%), generalized alone in 3 (17%), TCSs of unknown onset in 4 (25%), and a combination of focal seizures and TCS of unknown onset (different semiology from the focal seizures) in 2 (12.5%) individuals. Two fit the criteria for Se(F)IE, 1 classic and 1 atypical (Table 2).
Table 3.
Seizure Types and Epilepsy Classification in Individuals With the Recurrent 16p11.2 Duplication
II.3 Electrographic Findings
EEG reports were available on 13 of the 16 individuals in the duplication and seizure cohort. Of them, 3 had normal EEGs.
Of the 10 with abnormal EEGs, 1 had slow posterior dominant rhythm only and 4 had interictal focal (n = 3) and multifocal spikes (n = 1). Three had slowing and interictal epileptiform activity (1 with generalized slowing and generalized spikes diagnosed as electrographic status epilepticus in sleep, 1 with generalized slowing and both generalized and centrotemporal spikes, and 1 with generalized and focal slowing and centrotemporal spikes). Two individuals had seizures on EEG, 1 with a focal seizure with otherwise no background abnormalities and 1 with a generalized seizure and interictal generalized spikes during hyperventilation. The frequency of the interictal epileptiform activity and pattern of sleep activation were unavailable on most of the individuals.
II.4 Response to Treatment
Four individuals were never started on an ASM, including the 2 individuals considered to experience classic or atypical Se(F)IE. Information was unavailable on 2. Of the 10 individuals who were started on ASMs, 4 achieved seizure freedom on monotherapy (including LEV in 2, LTG in 1, and VPA in 1). A second medication was required in 4 individuals either due to side effects (CLB in 1 and PB in 1) or a lack of efficacy (VPA in 1 and LEV in 1). These individuals achieved seizure freedom or remained seizure-free after the addition of VPA (1), OXC (1), ETX (1), and ZNS (1). One individual was on VPA but inadequately treated, and seizures persisted. The remaining individual was refractory to 6 medications, as well as VNS, with TPM, PHT, GBP, VPA, and CBZ being ineffective, and LTG partially effective.
Imaging Findings
III.1 Brain MRIs in the Deletion Group
MRIs of 14 individuals were directly reviewed in detail, MRI reports were available on an additional 16, and information was missing on 1.
Overall, 21 had normal MRIs (10 with focal, 1 with generalized, and 10 with unknown or mixed-type epilepsy). Noncortical abnormalities that are unlikely associated with seizures were seen in 7 individuals (5 with focal and 2 with generalized epilepsy). Cortical abnormalities were seen in 2 individuals with focal epilepsy, although relationship with seizures cannot be confirmed. These included right frontal gray matter heterotopia and questionable biparietal pachygyria (Table 1).
III.1 Brain MRIs in the Duplication Group
MRIs of 7 individuals were directly reviewed in detail, reports were available on an additional 8, and information was missing on 1.
Normal MRIs were seen in 8 individuals (2 with focal, 2 with generalized, and 4 with unknown or mixed-type epilepsy). Noncortical abnormalities that are unlikely associated with seizures were seen in 7 individuals (4 with focal, 1 with generalized, and 2 with unknown or mixed-type epilepsy). No cortical abnormalities were seen in the duplication group (Table 3).
Co-occurrence of Neurodevelopmental Disorders
IV.1 Deletion Group
Information on the presence or absence of (ASD) and/or ID was available on 28 individuals in this group, of which 9 (32%) had 1 or more neurodevelopmental disorder. ASD alone, ID alone, and both ASD and ID were seen in 2, 5, and 2 individuals, respectively.
Seizures and epilepsy characteristics were compared between individuals with a neurodevelopmental disorder (group A, n = 9) and those without, in the deletion group (group B, n = 19), excluding 3 individuals with no information on ID or ASD. In group A, the age of seizure onset was more variable, 5 days to 13 years (mean 36, median 7 months), compared with group B, where the range of seizure onset was 3 months to 4 years (mean 11.7, median 8 months). In group A, 7 (78%) experienced epilepsy, compared with 13 (69%) in group B. The most prevalent seizure type was focal seizures in both groups, 78% in group A and 53% in group B. Two individuals in group A and none in group B experienced pharmacoresistant epilepsy. Se(F)IE was present in 3 (33%) and 10 (53%) of individuals in the groups A and B, respectively. EEGs were normal in 1 individual in group A (11%) and showed interictal epileptiform activity in 5 (55%), while 5 individuals (26%) had normal EEGs (26%) and 3 (16%) had interictal epileptiform abnormalities in group B.
IV.2 Duplication Group
Information on the presence or absence of ASD and/or ID was available on 13 individuals in this group. Of them, 11(84%) experienced 1 or more neurodevelopmental disorder. ASD alone, ID alone, and both ASD and ID were seen in 3 (23%), 5 (38%), and 3 (23%) individuals, respectively.
The seizures and epilepsy characteristics were compared between individuals with a neurodevelopmental disorder (group C, n = 9) and those without in the duplication group (group D, n = 4), excluding the 4 individuals with no information on ID or ASD. In group C, the age of seizure ranged from 1 month to 10 years (mean 30, median 11.5 months), compared with group D where seizure onset ages were 11 and 30 months for 2 individuals. In group C, 8 (73%) had epilepsy, compared with 2 (100%), in group D. The most prevalent seizure type was focal seizures in group C, 73%, while in group D, 1 individual experienced focal seizures and the other experienced TCSs with unknown onset. The only individual in the duplication group who was pharmacoresistant belonged to group C. One individual from each group experienced Se(F)IE. EEGs were normal in 2 in group C (18%) and showed interictal epileptiform activity in 8 (73%) and interictal slowing in 1 (9%), while 1 individual in group D had a normal EEG. Data were unavailable on the second.
Nonepileptic Paroxysmal Events
One patient with a single seizure from the deletion group had dyskinesias in infancy.
Of all individuals who were initially screened for possible seizures but were not confirmed to experience seizures, (n = 40, 19 with deletions and 21 with duplications), at least 11 (27.5%) had documented nonepileptic paroxysmal events.
The symptoms seen in the deletion group included staring spells (n = 5), syncope (n = 1), choreiform movement disorder (n = 1), transient alternating hemiplegia (n = 1), and visual and auditory hallucinations (n = 1). In the duplication group, staring was seen in 2 individuals and syncope in 1.
Comparison of Epilepsy Characteristics Between the Deletion and Duplication Groups
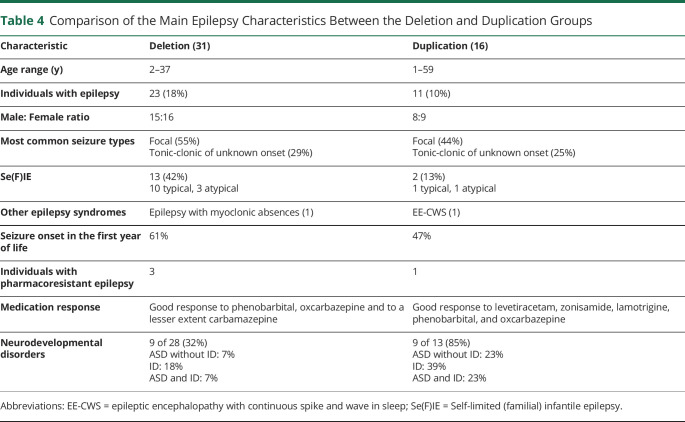
A direct comparison between the seizure and epilepsy phenotypes in the deletion and duplication groups is detailed in Table 4.
Table 4.
Comparison of the Main Epilepsy Characteristics Between the Deletion and Duplication Groups
Discussion
This is the largest series of individuals with 16p11.2 deletions and duplications with a systematic evaluation of seizure and epilepsy phenotypes.
The key findings of this study of a large, clinically ascertained cohort of individuals with 16p11.2 CNVs are that 24% and 18% of individuals with deletions and 15% and 10% of individuals with duplications have a history of a single seizure or epilepsy, respectively. In addition, epilepsy frequently manifested as Se(F)IE.
Subsets of this cohort have been included in prior publications that described the broad clinical and neuropsychiatric phenotype related to the recurrent 16p11.2 CNVs and reported on occurrence of seizures albeit not in detail.3,6 Our findings continue to show that the recurrent CNVs of 16p11.2 are highly associated with epilepsy. Occurrence of seizures and epilepsy is lower in our series compared with an earlier series from 2010, where seizures were reported in 40% of individuals with 16p11.2 deletions and 30% of individuals with 16p11.2 duplications.13 This may be related to differences in ascertainment, given higher likelihood of testing in patients identified earlier in the introduction of CNV evaluation in developmental delays, especially if they exhibited neurologic symptoms (e.g., seizures) or were more severely affected. The cohort was also smaller (16 individuals with deletion and 10 with duplication). Another factor could be the method of seizure and epilepsy diagnosis determination; in our cohort, we followed strict criteria to assign the diagnoses of seizures and epilepsy. Finally, additional genetic sequence abnormalities might not have been detected using array–comparative genomic hybridization technology.
There are likely additional factors at play regarding the neurologic, and, more specifically, the seizure and epilepsy phenotypes, given the phenotypic variability between individuals within each of the deletion and duplication groups. Despite this variability, some general patterns emerged from our analyses. Individuals displayed multiple seizure types, with predominance of focal seizures in both deletion and duplication groups. We did not identify a consistent epileptogenic locus.
In the 16p11.2 deletion group, we observed a high proportion of individuals with the specific epilepsy syndrome Se(F)IE as the predominant epilepsy feature. We refined our classification to account for some cases with atypical features. This syndrome was also seen in the duplication cohort but to a lesser extent.
Regarding treatment response, the effects were quite variable, although 3 medications seem to show a response pattern. PB, CBZ, and OXC had a positive effect on seizure control in the deletion cohort, and most of the responders experienced Se(F)IE. By comparison, there was no seizure improvement in the 6 individuals from the deletion cohort who were treated with LEV, 4 of whom experienced Se(F)IE. In both deletion and duplication groups, the response to ASMs was favorable, with only a few individuals remaining refractory to medications.
An interesting prior clinical observation connected 16p11.2 duplications to Benign Epilepsy with Centro-Temporal Spikes now classified as Childhood Epilepsy with Centro-Temporal Spikes (CECTS).18 In that series, 16p11.2 duplications were found to be enriched in individuals with CECTS, when compared with individuals with temporal lobe or generalized epilepsy. This was followed by an additional analysis of a large number of individuals with the recurrent 16p11.2 CNVs, which found that 3 of the 117 with a duplication and none of the 202 with deletions were on the CECTS spectrum. In our duplication cohort, 2 individuals had EEG findings of centrotemporal spikes, and only 1 of them had clear independent bilateral centrotemporal spikes with a horizontal dipole. Neither, however, had a clinical history suggestive of CECTS. One individual in the duplication cohort had EEG findings consistent with electrical status epilepticus in sleep and a clinical picture of epileptic encephalopathy with continuous spike and wave in sleep, which is on the severe end of the spectrum of epilepsies with focal central temporal spikes. While the numbers remain small, our findings align with that series.
Nonepileptic, paroxysmal events were also commonly seen in the evaluated participants who did not fit the criteria for seizures or epilepsy. The most commonly notable symptoms included staring spells, alternating hemiplegia, and movement disorders. All these symptoms can have seizure-like appearance. It is therefore important to recognize the presence of nonepileptic paroxysmal events in this population to avoid an overdiagnosis of seizures and unnecessary treatment.
There are at least 28 genes located in the 16p11.2 region. It remains unclear at this point, however, whether single genes are responsible for certain phenotypes. For a potential role in epilepsy, the most studied gene in this region is PRRT2. A loss-of-function of this gene can result in a range of phenotypes including Se(F)IE, infantile convulsions with paroxysmal kinesigenic dyskinesia (PKD), PKD in early adolescence, and other paroxysmal neurologic symptoms including episodic ataxia and hemiplegic migraines. The sequence variants in the PRRT2 genes are believed to lead to the neurologic phenotype via loss of function, similar to having a deletion.19 We can postulate that Se(F)IE syndrome seen in the deletion group is related, at least partly, to the loss-of-function of the PRRT2 gene. There is also a good response to OXC in the 16p11.2 deletion and an excellent response to CBZ (with a similar mechanism to OXC) in individuals with PRRT2 pathogenic variants.20 Heterozygous pathogenic variants in PRRT2 are not clearly associated with ASD, and therefore, the CNV phenotype definitely extends beyond this single gene and is likely related to other genes contained in the deleted or duplicated segment.
Imaging data in patients with 16p11.2 deletions and duplications have not provided a clear alternative explanation for the seizures for most of the patients in this cohort. It is important to note that one of the individuals with deletion and focal seizures had questionable pachygyria (location not reported), another with focal seizures had questionable increased cortical thickness, and one individual with a duplication and generalized seizures had possible focal polymicrogyria or malformation of cortical development, as well as gliosis in the right occipital region. These individuals were not excluded, given the uncertainty linking these findings to their epilepsy. MRI findings reported in the literature, including some of the individuals from the Simons Variation in Individuals Project cohort, showed an increased occurrence of patchy cortical anomalies in both deletion (abnormalities consistent with abnormally thick cortex) and duplication (abnormalities consistent with abnormally thin cortex) groups when compared with those in controls.26 It is therefore possible that migrational abnormalities are directly related to the 16p11.2 CNVs, but data are not enough to decisively conclude.
A significant correlation between the presence of a neurodevelopmental disorder and seizure characteristics is difficult to assess, given the small number of individuals in each subgroup. Some meaningful findings, however, merit further evaluation in a larger cohort. In particular, the presence of a neurodevelopmental disorder was more common in individuals with seizures and/or epilepsy in the duplication group (84%) compared with those in the deletion group (32%). In the deletion cohort, individuals with ID and/or ASD were slightly more likely to experience epilepsy (vs single seizure) compared with those without ID and/or ASD (78% vs 69%), but the significance is unclear. Se(F)IE was more common in individuals without ID and/or ASD (53% vs 33%). EEG abnormalities were more common in the ID and/or ASD group (55% vs 16%). Comparisons within the duplication cohort were difficult because one of the subgroups consisted only of 2 individuals, and no consistent differences were noted.
This descriptive study has limitations related to its retrospective nature and paucity of details on some individuals. Despite the fact that the semiology was reviewed from detailed clinic notes and directly through individual or parent interview whenever possible, assessing the exact seizure type remained challenging in certain cases. The reason was lack of ictal EEG data in most patients; thus, classification was based on seizure description. This was especially impactful in distinguishing generalized seizures from focal to bilateral TCS, possibly over-representing the occurrence of TCSs with unknown onset.
Another limitation concerns the ascertainment of epilepsy. Individuals who were started on treatment after their first seizure with no recurrence were not considered to experience epilepsy, thus the uncertainty as to whether these individuals would have gone on to experience more seizures without ASMs.
An additional limitation is the possibility of selection bias because the probands who were tested likely had an underlying condition leading to the testing, which could include seizures. However, data on a large cohort of seemingly unaffected individuals with 16p11.2 deletions have shown that there is higher penetrance of the CNV than previously established, specifically for neuropsychological performance.27 Other studies also showed high penetrance more generally for neurodevelopmental, psychiatric, and neurologic symptoms.28,29
Another limitation is the paucity of information on ASM dosage, leading to difficulty in assessing medication failure. In those where medication information was missing, it was not possible to completely rule out intractable epilepsy. We found 4 individuals who were found to be refractory (3 in the deletion and 1 in the duplication groups), and more detailed medication histories were available for those. This number could be an underestimation for the aforementioned reasons.
Despite these limitations, this descriptive series is the most detailed so far describing the seizure and epilepsy phenotypes related to 16p11.2 deletions and duplications. Our findings further underscore the importance of testing for CNVs in patients with epilepsy, particularly epilepsy in the first year of life, which may precede ASD and other neurodevelopmental disorders recognition. Prospective series with uniform testing for these indications may help refine the 16p11.2 epilepsy phenotype further.
Glossary
- ASD
autism spectrum disorder
- ASM
antiseizure medication
- CBZ
carbamazepine
- CECTS
childhood epilepsy with centrotemporal spike
- ID
intellectual disability
- ILAE
International League Against Epilepsy
- LEV
levetiracetam
- LTG
lamotrigine
- OXC
oxcarbazepine
- PB
phenobarbital
- PKD
paroxysmal kinesigenic dyskinesia
- Se(F)IE
Self-limited (Familial) Infantile Epilepsy
- TCS
tonic-clonic seizure
- TPM
topiramate
- VIP
Variations in Individuals Project
Appendix. Authors
Contributor Information
Alyssa Rosen, Email: rosenar@email.chop.edu.
Sudha Kilaru Kessler, Email: kesslers@email.chop.edu.
Kyle J. Steinman, Email: kylejs@uw.edu.
Sarah J. Spence, Email: sarah.spence@childrens.harvard.edu.
Melissa Ramocki, Email: melissa.ramocki@hotmail.com.
Elysa Jill Marco, Email: emarco@corticacare.com.
LeeAnne Green Snyder, Email: lgsnyder@simonsfoundation.org.
John E. Spiro, Email: jspiro@simonsfoundation.org.
Wendy K. Chung, Email: wkc15@cumc.columbia.edu.
Poduri Annapurna, Email: annapurna.poduri@childrens.harvard.edu.
Elliott H. Sherr, Email: sherre@neuropeds.ucsf.edu.
Study Funding
No targeted funding reported.
Disclosure
C. Moufawad El Achkar reports no disclosures relevant to the manuscript. A. Rosen reports no disclosures relevant to the manuscript. S.K. Kessler reports the following funding: research funding (payments to institution) from AHRQ/PCORI, FDA, UCB Pharma, SK Life, Esai, Acadia Pharmaceuticals, and Neuropace; consultant (payments to institution) for Epilepsy Study Consortium; and educational activities (payment to individual) from American Academy of Neurology. K.J. Steinman, S.J. Spencer, and M. Ramocki report no disclosures relevant to the manuscript. E.J. Marco is a consultant to Akili Interactive, Employee of Cortica Health care. L. Green Snyder, J.E. Spiro, W.K. Chung, A. Poduri, and E.H. Sherr report no disclosures relevant to the manuscript. Go to Neurology.org/NG for full disclosures.
References
- 1.Kumar RA, KaraMohamed S, Sudi J, et al. . Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17(4):628-638. doi: 10.1093/hmg/ddm376 [DOI] [PubMed] [Google Scholar]
- 2.Weiss LA, Shen Y, Korn JM, et al. . Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358(7):667-675. doi: 10.1056/NEJMoa075974 [DOI] [PubMed] [Google Scholar]
- 3.Steinman KJ, Spence SJ, Ramocki MB, et al. . 2 deletion and duplication: characterizing neurologic phenotypes in a large clinically ascertained cohort. Am J Med Genet A. 1120;11170(11):2943-2955. doi: 10.1002/ajmg.a.37820 [DOI] [PubMed] [Google Scholar]
- 4.McCarthy SE, Makarov V, Kirov G, et al. . Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41(11):1223-1227. doi: 10.1038/ng.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bijlsma EK, Gijsbers AC, Schuurs-Hoeijmakers JH, et al. . Extending the phenotype of recurrent rearrangements of 16p11.2: deletions in mentally retarded patients without autism and in normal individuals. Eur J Med Genet. 2009;52(2-3):77-87. doi: 10.1016/j.ejmg.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 6.D'Angelo D, Lebon S, Chen Q, et al. . Defining the effect of the 16p11.2 duplication on cognition, behavior, and medical comorbidities. JAMA Psychiatry. 2016;73(1):20-30. doi: 10.1001/jamapsychiatry.2015.2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consortium EK. Epi4K: gene discovery in 4,000 genomes. Epilepsia. 2012;53(8):1457-1467. doi: 10.1111/j.1528-1167.2012.03511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson H, Shen Y, Avallone J, et al. . Copy number variation plays an important role in clinical epilepsy. Ann Neurol. 2014;75(6):943-958. doi: 10.1002/ana.24178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mefford HC, Muhle H, Ostertag P, et al. . Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010;6(5):e1000962. doi: 10.1371/journal.pgen.1000962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mefford HC, Yendle SC, Hsu C, et al. . Rare copy number variants are an important cause of epileptic encephalopathies. Ann Neurol. 2011;70(6):974-985. doi: 10.1002/ana.22645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghebranious N, Giampietro PF, Wesbrook FP, Rezkalla SH. A novel microdeletion at 16p11.2 harbors candidate genes for aortic valve development, seizure disorder, and mild mental retardation. Am J Med Genet A 2007;143A(13):1462-1471. doi: 10.1002/ajmg.a.31837 [DOI] [PubMed] [Google Scholar]
- 12.Zufferey F, Sherr EH, Beckmann ND, et al. . A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J Med Genet. 2012;49(10):660-668. doi: 10.1136/jmedgenet-2012-101203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinawi M, Liu P, Kang SH, et al. . Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size. J Med Genet. 2010;47(5):332-341. doi: 10.1136/jmg.2009.073015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Kovel CG, Trucks H, Helbig I, et al. . Recurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsies. Brain. 2010;133(Pt 1):23-32. doi: 10.1093/brain/awp262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consortium SV. Simons Variation in Individuals Project (Simons VIP): a genetics-first approach to studying autism spectrum and related neurodevelopmental disorders. Neuron. 2012;73(6):1063-1067. doi: 10.1016/j.neuron.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 16.Fisher RS. Commentary: operational definition of epilepsy survey. Epilepsia. 2014;55(11):1688. doi: 10.1111/epi.12829 [DOI] [PubMed] [Google Scholar]
- 17.Cetica V, Chiari S, Mei D, et al. . Clinical and genetic factors predicting Dravet syndrome in infants with. Neurology. 2017;88(11):1037-1044. doi: 10.1212/WNL.0000000000003716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinthaler EM, Lal D, Lebon S, et al. . 2 600 kb Duplications confer risk for typical and atypical Rolandic epilepsy. Hum Mol Genet. 1120;23(22):6069-6080. doi: 10.1093/hmg/ddu306 [DOI] [PubMed] [Google Scholar]
- 19.Vlaskamp DRM, Callenbach PMC, Rump P, et al. . PRRT2-related phenotypes in patients with a 16p11.2 deletion. Eur J Med Genet. 2019;62(4):265-269. doi: 10.1016/j.ejmg.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 20.Ebrahimi-Fakhari D, Saffari A, Westenberger A, Klein C. The evolving spectrum of PRRT2-associated paroxysmal diseases. Brain. 2015;138(Pt 12):3476-3495. doi: 10.1093/brain/awv317 [DOI] [PubMed] [Google Scholar]
- 21.Kumar RA, Marshall CR, Badner JA, et al. . Association and mutation analyses of 16p11.2 autism candidate genes. PLoS One 2009;4(2):e4582. doi: 10.1371/journal.pone.0004582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haslinger D, Waltes R, Yousaf A, et al. . Loss of the Chr16p11.2 ASD candidate gene. Mol Autism. 2018;9:56. doi: 10.1186/s13229-018-0239-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosio Y, Berto G, Camera P, et al. . PPP4R2 regulates neuronal cell differentiation and survival, functionally cooperating with SMN. Eur J Cel Biol. 2012;91(8):662-674. doi: 10.1016/j.ejcb.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 24.Salman ED, Kadlubar SA, Falany CN. Expression and localization of cytosolic sulfotransferase (SULT) 1A1 and SULT1A3 in normal human brain. Drug Metab Dispos. 2009;37(4):706-709. doi: 10.1124/dmd.108.025767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCammon JM, Blaker-Lee A, Chen X, Sive H. The 16p11.2 homologs fam57ba and doc2a generate certain brain and body phenotypes. Hum Mol Genet. 2017;26(19):3699-3712. doi: 10.1093/hmg/ddx255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackmon K, Thesen T, Green S, et al. . Focal cortical anomalies and language impairment in 16p11.2 deletion and duplication syndrome. Cereb Cortex 2018;28(9):2422-2430. doi: 10.1093/cercor/bhx143 [DOI] [PubMed] [Google Scholar]
- 27.Stefansson H, Meyer-Lindenberg A, Steinberg S, et al. . CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505(7483):361-366. doi: 10.1038/nature12818 [DOI] [PubMed] [Google Scholar]
- 28.Crawford K, Bracher-Smith M, Owen D, et al. . Medical consequences of pathogenic CNVs in adults: analysis of the UK Biobank. J Med Genet 2019;56(3):131-138. doi: 10.1136/jmedgenet-2018-105477 [DOI] [PubMed] [Google Scholar]
- 29.Steinberg S, de Jong S, Mattheisen M, et al. . Common variant at 16p11.2 conferring risk of psychosis. Mol Psychiatry. 2014;19(1):108-114. doi: 10.1038/mp.2012.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data that support the findings are available from the corresponding author on request.